Abstract
Recent work has indicated that photobiomodulation (PBM) may beneficially alter the pathological status of several neurological disorders, although the mechanism currently remains unclear. The current study was designed to investigate the beneficial effect of PBM on behavioral deficits and neurogenesis in a photothrombotic (PT) model of ischemic stroke in rats. From day 1 to day 7 after the establishment of PT model, 2-minute daily PBM (CW, 808 nm, 350 mW/cm2, total 294 J at scalp level) was applied on the infarct injury area (1.8 mm anterior to the bregma and 2.5 mm lateral from the midline). Rats received intraperitoneal injections of 5-bromodeoxyuridine (BrdU) twice daily (50 mg/kg) from day 2 to 8 post-stoke, and samples were collected at day 14. We demonstrated that PBM significantly attenuated behavioral deficits and infarct volume induced by PT stroke. Further investigation displayed that PBM remarkably enhanced neurogenesis and synaptogenesis, as evidenced by immunostaining of BrdU, Ki67, DCX, MAP2, spinophilin, and synaptophysin. Mechanistic studies suggested beneficial effects of PBM were accompanied by robust suppression of reactive gliosis and the production of pro-inflammatory cytokines. On the contrary, the release of anti-inflammatory cytokines, cytochrome c oxidase activity and ATP production in peri-infarct regions were elevated following PBM treatment. Intriguingly, PBM could effectively switch an M1 microglial phenotype to an anti-inflammatory M2 phenotype. Our novel findings indicated that PBM is capable of promoting neurogenesis after ischemic stroke. The underlying mechanisms may rely on: 1) promotion of proliferation and differentiation of internal neuroprogenitor cells in the peri-infarct zone; 2) improvement of the neuronal microenvironment by altering inflammatory status and promoting mitochondrial function. These findings provide strong support for the promising therapeutic effect of PBM on neuronal repair following ischemic stroke.
Keywords: photobiomodulation, ischemic stroke, neurogenesis, inflammation, mitochondrial function
Introduction
Stroke is considered to be the second leading cause of death, constituting a substantial economic burden for families and society (Ozturk, 2014). Ischemic stroke, the most common form of stroke, is induced by the occlusion of the cerebral vasculature, causing irreversible tissue infarction and abnormal cellular death (Andrabi et al., 2017). The photothrombotic stroke (PT) model is widely used as an experimental stroke model that simulates stroke conditions in humans by photothrombotic occlusion of cerebral microvessels (Pevsner et al., 2001; Schmidt et al., 2012). In this model, focal illumination of the exposed skull proceeds after administration of the photoactive dye, rose bengal. Illumination triggers a photochemical reaction produces reactive singlet oxygen that damages the vascular endothelium, generating a thrombotic lesion characterized by a necrotic core surrounded by a well-defined peri-infarct penumbral region consisting of surviving, but damaged, tissue. The size and the location of this lesion can be easily modulated by the intensity, the duration and the location of light delivery in concise, reproducible manner with a high rate of survival compared to other stroke models (Watson et al., 1985). In this process, local thrombosis is followed by free radical formation, disturbance of endothelial function, mitochondrial dysfunction, inflammatory response, neuronal injury, and subsequent behavioral deficits (Braeuninger and Kleinschnitz, 2009; Demyanenko et al., 2015; Schroeter et al., 2002; Shanina et al., 2005).
Neurogenesis in the adult brain may be involved in targeting and repairing damaged tissue in ischemic stroke (Plane et al., 2004; Wang et al., 2017), and the long-term survival of these nascent neurons plays a critical role in endogenous neurogenesis. Unfortunately, newly formed neurons struggle to survive long-term due to the prohibitive properties of the post-stroke local microenvironment, indicating that neurogenesis after stroke cannot fully perform its self-repair process in the presence of certain maladaptive endogenous factors (Thored et al., 2006; Wang et al., 2015). The inflammatory response is one facet of this deleterious microenvironment, and plays an important role in the pathological features of ischemic stroke. Brain injury activates inflammatory signaling and sets off a constellation of biochemical and molecular events that can culminate in neuronal cell death.
Giannakopoulou et al. found that long-term inflammation in the brain could induce neurogenesis giving concession to gliogenesis (Giannakopoulou et al., 2017). Neuroinflammation is characterized by activation of glial cells after ischemic stroke and is accompanied by the release of proinflammatory cytokines (Kim et al., 2014; Kreutzberg, 1996). There are two major phenotypes of activated microglia which respond to brain injuries: the M1 “proinflammatory” phenotype and the M2 “anti-inflammatory” phenotype. Previous studies, including our own, found that transformation between the two phenotypes of microglial polarization could confer benefits in neurodegenerative disorders (Hu et al., 2012; Lu et al., 2017a).
In addition to inflammation, mitochondrial dysfunction is another well-established factor in ischemia, owing to the key role of mitochondria in ATP production, Ca2+ homeostasis, the generation of reactive oxygen species (ROS), and modulation of apoptosis (Zuo et al., 2016). It has been reported that mitochondrial homeostasis and high-energy utility of neurons contributes to neuronal resistance to ischemia (Zuo et al., 2016). The morphology and permeability of mitochondria are affected by ischemic challenge, resulting in a loss of mitochondrial membrane potential and decreased ATP levels, contributing to neuronal injury and cell death (Landes and Martinou, 2011; Sharp et al., 2014). Notably, recent studies suggested that mitochondrial dysfunction is linked to the inhibition of adult neurogenesis (Voloboueva and Giffard, 2011).
While numerous studies have focused on promoting a favorable microenvironment by treatment with exogenous factors (Ahmed et al., 2016; Wang et al., 2017), the neurogenic properties and side effects of these factors remain to be established. PBM therapy is a well-documented non-drug-based therapy that could yield beneficial effects in an organism’s endogenous self-repair mechanisms (Eissa and Salih, 2017; Lu et al., 2017a). Numerous studies have indicated that the positive effects conferred by PBM result from decreasing inflammation, alleviating oxidative stress, and improving mitochondrial function (Ferraresi et al., 2015; Hamblin, 2017; Huang et al., 2013; Thunshelle and Hamblin, 2016). In addition, several studies indicated that PBM can upregulate brain derived neurotrophic factor, improve synaptogenesis, reduce long-term neurological deficits, and exert a positive effect on the differentiation of neural progenitor cells after experimental stroke, which suggested that PBM may have potential as a novel, non-invasive stroke treatment (Oron et al., 2006; Xuan et al., 2015; Xuan et al., 2014). In summary, we hypothesize that PBM can promote cortical neurogenesis by enhancing the proliferation and differentiation of neural progenitors in the peri-infarct zone, in part by improving the neuronal microenvironment.
Materials and Methods
Experimental design and drug administration
Male Sprague-Dawley rats (200–250 g) were used in the present work. Animals were randomly divided into 3 groups: (a) Normal animals without PT stroke; (b) PT stroke group; (c) PT stroke animals with PBM. All animal procedures complied with the guidelines of Institutional Animal Care and Use Committees. A strict blind procedure and analysis were conducted in this study. The researchers conducting behavioral tests, microscopic imaging and other evaluations were blinded to the experimental groups. PT stroke model was carried as described in our previous study (Ahmed et al., 2016). Brie y, the rats were anesthetized with an intraperitoneal (ip) injection of sodium pentobarbital (50 mg/kg) and placed in a stereotactic frame. Rose Bengal dye (0.1 mg/g, ip) was administrated to the animals for 5 minutes before the exposure of the skull. The periosteum was then gently removed, then the skull was illuminated for 15 minutes positioned onto the skull 1.8 mm anterior to the bregma and 2.5 mm lateral from the midline with a 6 mm diameter cold white light beam from a fiber optic cable. During the process of surgical operation, animals were placed on a heating pad, and rectal temperature was maintained at 37 ± 0.5 °C. Afterward, the skin was sutured using wound clips. For laser treatment, 2-minute daily laser irradiation (808 nm, 25 mW/cm2 at cerebral cortex tissue level, 350 mW/cm2 on the scalp), as reported in our previous work (Lu et al., 2017b), was applied using a Diode IR Laser System (808M100, Dragon Lasers) on the infarct injury area from day 1 to 7. The device information was shown in Table 1. All animals were restrained in a transparent DecapiCone (DCL-120, Braintree Scientific, Inc, MA, USA) for 2 minutes and returned to their home cage immediately after treatment. The only difference between animals in PT stroke group and PBM group is that the laser power was not turned on when PBM-untreated animals were secured under the fiber optic output. An experimental protocol is shown in Figure. 1.
Table 1.
Device Information
| Manufacturer | Changchun New Industries Optelectronics Tech, Cp., L |
| Model Indentifier | MDL – III – 808 – 500 mW |
| Wavelength (nm) | 808 ± 3.0 |
| Number of Emitters | 1 |
| Emitter Type | Diode IR Laser |
| Spatial Distribution of Emitters | Round LED Spot Light |
| Beam Delivery System | Fiberoptic |
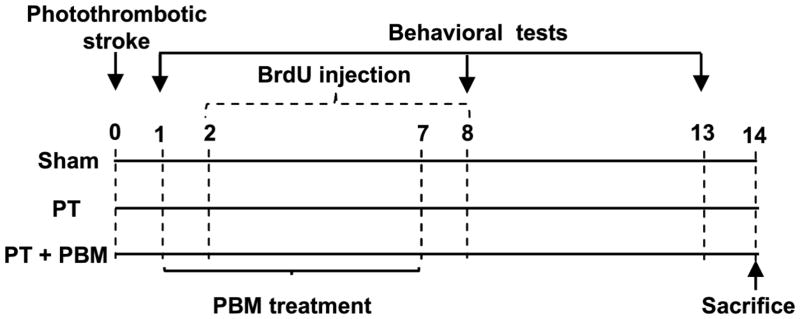
Fig. 1. Schematic diagram of the experimental protocols.
PT model was induced on day 0, and on days 1 through 7, rats were treated with PBM (2-minute daily). BrdU (50 mg/kg, ip) was injected two times daily on days 2 through 8. Behavioral deficits were evaluated on days 1, 7 and 13. Rats were sacrificed on day 14, and whole brains were extracted and prepared for further analysis.
Tissue Preparation
For sample preparation, rats were sacrificed under anesthesia on day 14 after PT stroke. After perfusion with ice-cold saline, the rats were decapitated and the whole brains were then quickly removed. Brains were postfixed in 4% paraformaldehyde (PFA) at 4 °C overnight, and then cryoprotected with 30% sucrose. Afterwards, brains were immersed in OCT cryoprotectant and frozen overnight at −80 °C. A Cryostat (Leica RM2155, Nussloch, Germany) was used for cutting brain sections (25 μm). For homogenization of the brain, brain tissues from the peri-infarct regions and sham controls were dissected from the cortex and immediately frozen in liquid nitrogen. The tissues were homogenized as previously described (Zhao et al., 2016). In brief, a motor-driven Teflon homogenizer was used for homogenization in ice-cold homogenization medium (50 mM HEPES, pH 7.4, 150 mM NaCl, 12 mM β-glycerophosphate, 1% Triton X-100) with inhibitors of proteases and enzymes (Thermo Scientific, Rockford, IL). For the total protein fractions, the homogenates were vigorously mixed for 20 min and centrifuged for 30 min at 12,000×g at 4 °C. For mitochondrial and subcellular cytosolic fraction, tissue samples were homogenized in a buffer containing 10 mM HEPES (pH 7.9), 0.6% NP-40, 12 mM β-glycerophosphate with protease, and enzyme inhibitors. The homogenates were centrifuged for 20 min at 800 ×g, and the resulting supernatants were then centrifuged at 17,000×g for 20 min at 4 °C to yield cytosolic fractions in the supernatant and mitochondrial fractions in pellet. Protein concentrations were measured by Modi ed Lowry Protein Assay (Pierce, Rockford, IL).
5-Bromodeoxyuridine (BrdU) labeling
BrdU (5-bromodeoxyuridine, Carbosynth Ltd) was used to label proliferating cells. In the present study, 7 consecutive days of BrdU (50 mg/kg, ip) injections were performed every 12 h from day 2 after PT stroke.
Infarct Volume Assessment
Infarct volume of different groups were measured by Cresyl violet staining. In brief, the selected brain sections were stained with 0.01 % (w/v) cresyl violet for 10 min, followed by graded ethanol dehydration. The images of brain sections were acquired by AxioVision microscope system (Carl Zeiss, Germany). Image J (NIH) was applied to quantify the mean infarct area of each brain section. Infarct volume was calculated by the percentage of the total volume of the contralateral hemisphere per group (Ahmed et al., 2016).
Immunofluorescence Staining and Confocal Microscopy
Immunofluorescence staining was performed as previously described by our laboratory (Lu et al., 2017b). In brief, coronal brain sections (25 μm) were incubated with buffer containing 10% normal donkey serum and 0.1% Triton X-100 for 1 h at room temperature followed by incubation with the corresponding primary antibodies in the same buffer overnight at 4 ° C. The following primary antibodies were used: anti-BrdU, Ki67 (DSHB); anti-NeuN, MAP2, DCX (Santa Cruz); anti-synaptophysin, spinophilin (Abcam); anti-Iba1 and GFAP (Proteintech Group). After incubation with primary antibodies, the brain sections were washed three times at room temperature followed by incubation with proper secondary antibodies (Thermo Fisher Scientific) for 1 h at room temperature. Sections were then washed with PBS, mounted with water-based mounting medium, and coverslipped in Vectashield mounting medium with DAPI (Vector Laboratories). LSM510 Meta confocal laser microscope (Carl Zeiss) was used to capture the fluorescence images. The imaging areas were chosen from the peri-infarct penumbral region beneath the infarct surface for comparison of immunofluorescent intensity between groups. For quantitative analyses, the number of NeuN-positive cells per 150 μm length in the peri-infarct zone was counted in 4–5 areas selected on each slice. 3–4 slices (50 μm apart) were chosen from each rat.
Mitochondrial Cytochrome C Oxidase (CCO) Activity
As described previously (Zhao et al., 2016), CCO activity was measured by an activity assay kit (ab109911; Abcam Inc) using mitochondrial fractions. Briefly, the fractions were loaded in microtitre plate for 3 h at room temperature. After washing twice with provided solution 1, assay solution was added to each well and the mitochondrial cytochrome C oxidase activity was measured at 550 nm absorbance (Bio-Rad Benchmark Plus, Microplate Spectrophotometer). The data of relative CCO activity were quantified as percentage changes versus sham group.
Quantification of total ATP content
As previously described by our lab, an ATP quantification kit (Lu et al., 2017b), a kit ENLITEN® rLuciferase/Luciferin Reagent (FF2021, Promega, Madison, WI) was used to measure the level of ATP. Protein samples (30 μg) from total protein fractions were diluted in 100 μL of reconstituted rL/L reagent buffer (luciferase, D-luciferin, Tris-acetate buffer (pH 7.75), EDTA, magnesium acetate, bovine serum albumin, and dithiothreitol). Light emission from the reaction was examined in a standard microplate luminometer (PE Applied Biosystems) at 10-second intervals. The “background blank” was prepared, containing rL/L reagent and homogenization buffer, and the relative light units from “background blank” were subtracted from the sample light output in the assay. An ATP standard curve was used to determine the values of ATP, and the data of relative total ATP content were present as percentage changes versus sham group.
Pro-in ammatory cytokines assay
Indirect Enzyme-Linked-Immunosorbent-Assay (ELISA) was used to detect the levels of pro-inflammatory cytokines in each group (Lu et al., 2017a). Primary antibody sources were as follows: anti-IL-4, IL-10, IL-6, TNF-α (Proteintech Group), and IL-18 (Santa Cruz). The levels of pro-inflammatory cytokines were measured as the optical density at 450 nm on a spectrophotometer (Bio-Rad), and the values were calculated and presented as percentage changes versus sham group.
Western Blotting Analysis
Western blotting was performed as our previous described (Zhang et al., 2009). In brief, proteins (50 μg) were separated on sodium dodecyl sulfate-poly-acrylamide gel electrophoresis (SDS-PAGE) and transferred to PVDF membrane. PVDF membranes were then blocked and incubated with appropriate primary antibody at 4°C overnight. Primary antibody sources include: anti-CD32, CD86, iNOS, ARG, TGF-β, CD206, and β-actin from Proteintech Group. After 3 washes, membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies for 1 h at room temperature. The bound proteins were then captured on a digital imaging system and analyzed by Image J analysis software (Version 1.49; NIH, USA).
Cylinder test and Adhesive test
The cylinder test was used to evaluate the effect of PBM on the asymmetric forelimb preference (Ahmed et al., 2016). It was performed on days 1, 7 and 13 after the establishment of the PT stroke model. The device employed in this test was a transparent glass cylinder (diameter: 10 cm; height: 15 cm). In this cylinder, the rats were allowed to contact with the side of the cylinder with its left or right forelimb for 2 min. During this test, researcher counted the number of contacts between left or right paws and the wall of the transparent glass. Contralateral paw use was calculated according to the following formula: score = (contralateral paw use/total paw use) ×100. In addition to this behavioral tests, the adhesive removal test was performed to measure somatosensory deficits on days 1, 7 and 13 after PT stroke. In this test, the experimenter put a small adhesive strip (0.35 ×0.45 cm) on the front paw of the rats and then returned the animal to an empty cage. The maximum recorded time spent on removing the adhesive tape was 2 minutes.
Statistical Analysis
Statistical analysis was performed using one-way or two-way analysis of variance (ANOVA), followed by Student-Newman-Keuls post-hoc tests with SigmaStat 3.5 software to determine group differences. To determine the differences between normal control group and other groups, Dunnett’s test was performed for post-hoc analyses after ANOVA. A Student’s T test was adopted when only two groups were compared. Statistical significance was accepted at the 95% confidence level (P < 0.05). Data were expressed as means ± SE.
Results
PBM Significantly Alleviates Behavioral Deficits after PT Stroke
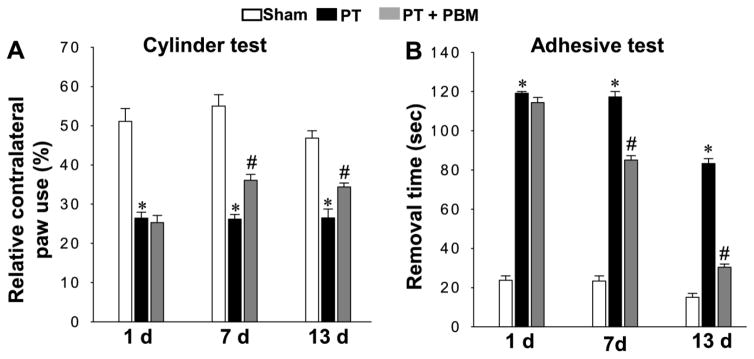
To investigate the effect of PBM on alleviating behavioral changes after PT stroke, we utilized the cylinder test, a behavioral test commonly applied to measure spontaneous forelimb use, on days 1, 7 and 13 after PT stroke. As shown in Fig. 2A, cylinder test results revealed that PT rats displayed a significant decrease in relative paw use contralateral to the damaged hemisphere, as compared to sham rats. Interestingly, PT stroke animals that underwent PBM displayed significant improvement in relative contralateral paw use compared with those without PBM. In addition, the adhesive test was performed to assess forepaw somatosensory activity. As shown in Fig. 2B, the time spent removing the adhesive tape was significantly increased in animals that underwent PT stroke compared to sham, indicating an impaired somatosensory cortex function. Notably, this behavioral deficit was significantly improved by PBM compared with PT group animals.
Fig. 2. Effect of PBM on behavioral deficits in PT stroke rats.
(A) Results of the cylinder test. The percentage of relative contralateral paw use was used to evaluate rats’ spontaneous forelimb use. (B) Results of the adhesive removal test. The time spent on removing a small rectangular adhesive strip was recorded and used for measuring stimulus-directed movement after PT. Data are expressed as mean ± SE (n = 8–10). *P < 0.05 versus sham group, #P < 0.05 versus PT.
PBM Decreases Cortical Infarct Size after PT Stroke and Increases the Number of Surviving Neurons after PT Stroke
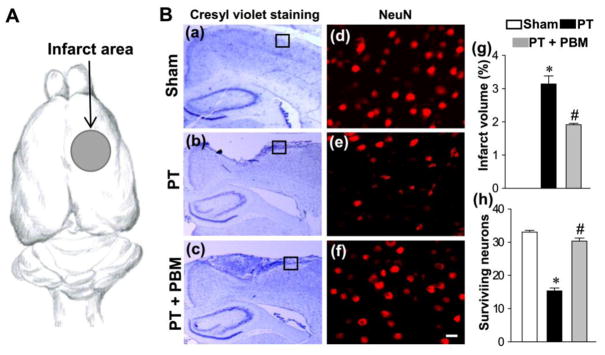
Next, we tested the effect of PBM on cortical infarct size and neuronal survival after PT. In the current work, the right somatosensory cortex (the dark area in Fig. 3A) was damaged by induction of the PT stroke model. To investigate the effect of PBM on the size of cortical infarct, coronal sections were collected for staining. As shown in Fig. 3B(a–c, and g), PT stroke generated an infarct injury in the sensorimotor, the size of which was significantly decreased in the animals with PBM, suggesting that PBM has the ability to repair the infarct injury after PT stroke. Sham animals did not have any sign of cortical infarct, as expected. To corroborate these findings, brain sections were immunostained for detection of surviving neurons using a mouse anti-NeuN antibody. As shown in Fig. 3B(d–f, and h), the number of NeuN positive cells in cortex from rats that underwent PT stroke demonstrated a significant loss compared with normal animals, and this effect was significantly reversed by PBM.
Fig. 3. Effect of PBM on the cortical infarct size and the number of surviving neurons.
(A) The area illuminated by cold fiber light was pointed out by an arrow presenting somatosensory cortex. (B) Representative images of cresyl violet staining (a–c) and confocal images of NeuN (d–f). The surviving neuros were counted from the locations in peri-infarct zone indicated by the boxes in (a–c). The surviving neurons (h) were counted from images like those in (d–f) taken from locations like those indicated by the boxes in (a–c). Infarct volume was calculated and expressed as the percentage of the total volume of the contralateral hemisphere per group in (g). The count of surviving neurons was analyzed in (h). All data are expressed as mean ± SE (n = 4–5). *P < 0.05 versus sham, #P < 0.05 versus PT. Scale bar =10 μm.
PBM Treatment Significantly Inhibits PT Stroke-induced Dendritic and Synaptic Injury
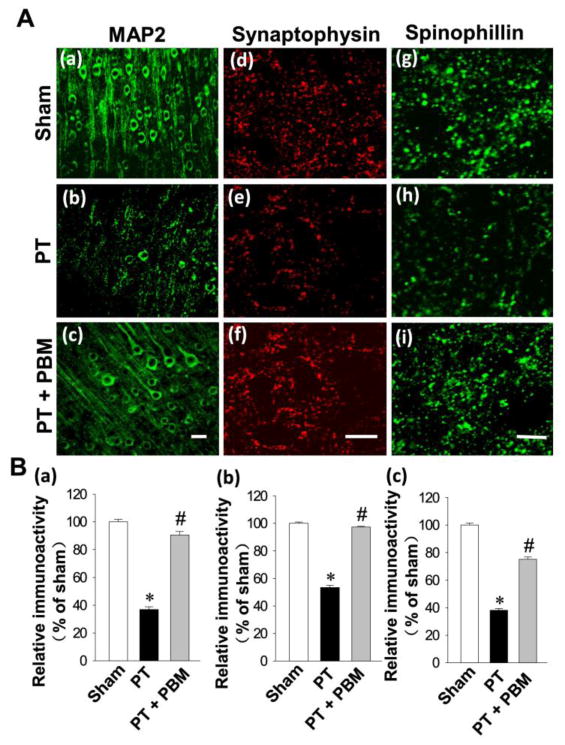
MAP2, a widely used neuronal dendritic marker, is a sensitive and early indicator for the assessment of neuronal injury (Kitagawa et al., 1989). We next investigated the expression of MAP2 in the cortex. As shown in Fig. 4A (a–c) and Fig. 4B (a), confocal microscopy and quantitative analyses demonstrated that the expression of MAP2 was significantly decreased in PT group compared with sham group, and this effect was significantly reversed by PBM. The expressions of synaptic proteins were further measured to determine the potential effects of PBM. As shown in Fig. 4A (d–i) and Fig. 4B (b, c), PT animals displayed decreased expressions of spinophilin (a postsynaptic marker) and synaptophysin (a presynaptic marker) that were significantly elevated in PBM treated animals, indicating that PBM provided effective protection against stroke-induced synaptic injury.
Fig. 4. Effect of PBM on PT stroke-induced neuronal injury in the infarct cortical region 14 days after PT stroke.
(A) Typical staining of MAP2 in healthy control animals (a), animals that underwent PT stroke without (b) or with PBM treatment (c). Representative confocal microscopy images of synaptophysin (d–f) and spinophilin (g–i) were taken from the infarct cortical region 14 days after PT stroke. (B) The fluorescent intensity of MAP2 (a), synaptophysin (b) and spinophilin (c) were quantified using Image J analysis software and expressed as percentage changes versus the respective control group. (C) The region in which neurogenesis is illustrated (a) and representative images from PT (b) and PBM treated rats (c) are displayed. All data are expressed as mean ± SE (n = 4–5). *P < 0.05 versus sham, #P < 0.05 versus PT. Scale bar =10 μm.
PBM Enhances Cortical Neurogenesis in PT Stroke Rats
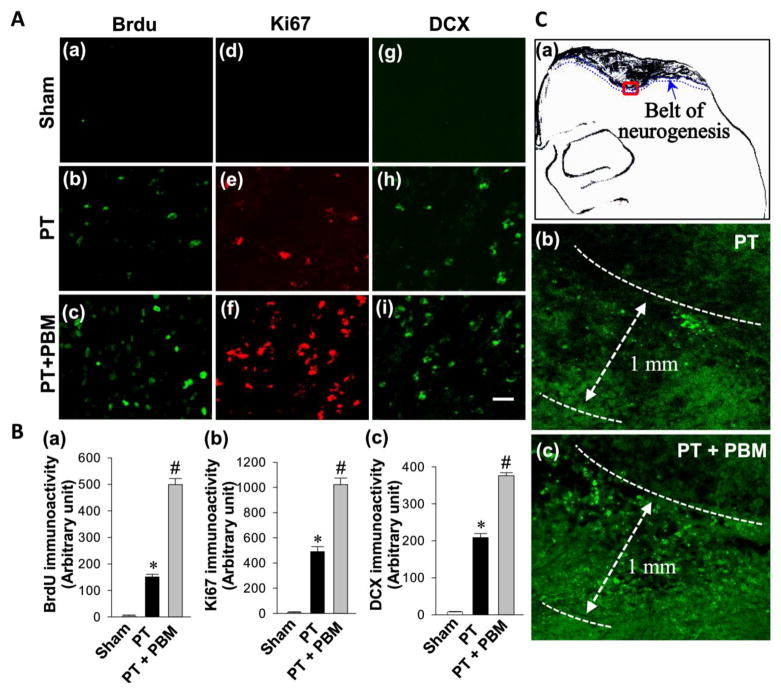
To investigate whether PBM could promote neurogenesis after PT stroke, brain sections were labeled with BrdU, Ki67 and DCX. As shown in Fig. 5A(a–c), representative confocal images revealed BrdU-positive cells were present in the cortex and concentrated in the peri-infarct region in PT group animals. When compared to the immunoactivity of BrdU in PT group, immunoactivity in PBM group was markedly increased. Fig. 5A presents the immunoreactivity of Ki67 (d–f) and DCX (g–i) revealing that PBM markedly increased the levels of Ki67 (a marker for stem cell proliferation) and DCX (a pre-mature neuronal marker and differentiation marker), as compared to PT group. Quantitative evaluation of BrdU, Ki67 and DCX were performed and shown in Fig. 5B. Intriguingly, as indicated in Fig. 5C(a), we observed that the increased neurogenesis was restricted to a regions (around 1.00 mm wide belt) beneath the infarct zone surface. As shown in Fig. 5C(b–c), PBM could significantly increase the quantity of BrdU-positive cells without changing the size of neurogenesis area at the examined time point (day 14 after PT). To distinguish whether this neurogenesis occurred directly in the cortex or occurred in the dentate gyrus (DG) or subventricular zone (SVZ), where the newly formed cells migrate to the injured cortex, confocal images of those three markers from DG and SVZ were also acquired. Interestingly, we did not find significant differences from those areas between groups (data not shown). These data clearly display that PBM could enhance cortical neurogenesis and maintain the survival of these newly formed neurons, as evidenced by significantly higher levels of neurogenesis markers after PBM in cortex.
Fig. 5. LLI improves neurogenesis in the peri-infarct cortical region on day 14 after PT stroke.
(A) Representative confocal microscopy images of BrdU (a–c), Ki67 (d–f) and DCX (g–i) were taken from the cortical peri-infarct region on day 14 after PT stroke. (B) The immunoactivity associated with BrdU, Ki67 and DCX in each group were further quantified and shown in arbitrary unit (a, b and c respectively). (C) The increased neurogenesis was restricted to a regions (around 1.00 mm wide belt) beneath the infarct zone surface with the representative confocal microscopy of Brdu staining (a–c). All data are expressed as mean ± SE (n = 4–5). *P < 0.05 versus sham, #P < 0.05 versus PT. Scale bar = 20 μm.
PBM Rescues PT-induced Mitochondrial Dysfunction
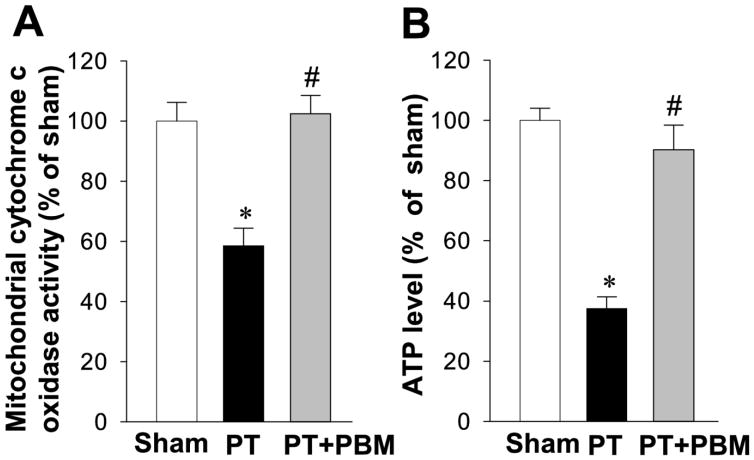
Our previous work has indicated that PBM could restore mitochondrial dynamics, suppress collapse of mitochondrial membrane potential, promote CCO activity, and increase synthesis of ATP in a streptozotocin-induced sporadic Alzheimer’s Disease rat model (Lu et al., 2017b). In this study, we investigated whether PBM can rescue PT-induced mitochondrial dysfunction. As shown in Fig. 6A, PT stroke significantly decreased CCO activity compared with that of normal animals, an effect ameliorated with PBM. To determine whether PBM could affect the level of cortical ATP production, we further measured the level of ATP. Our results showed that cortical ATP levels were significantly diminished in PT group compared with sham group. In contrast, the level of ATP in PBM group was significantly elevated in comparison with PT-induced stroke groups (Fig. 6B). These results suggested that PBM is effective in restoring mitochondria function by improving CCO activity and enhancing ATP synthesis in this PT stroke model.
Fig. 6. Effect of PBM on the activity of mitochondrial cytochrome c oxidase and the production of ATP in the cortical peri-infarct region.
Mitochondrial cytochrome c oxidase activity (A) and ATP production (B) in total protein samples were measured. The results of PT stroke group and PBM group are quantified as percentage changes versus sham. Values are expressed as mean±SE (n=5). *P<0.05 versus sham, #P<0.05 versus PT group.
PBM Suppresses PT Stroke-induced Gliosis and in ammation, and Alternates the Phenotype of Microglial Polarization
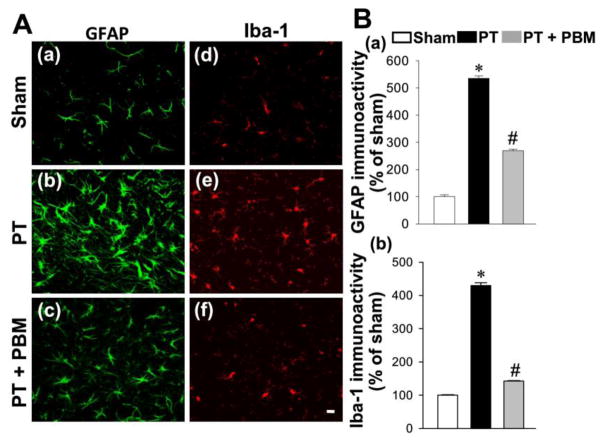
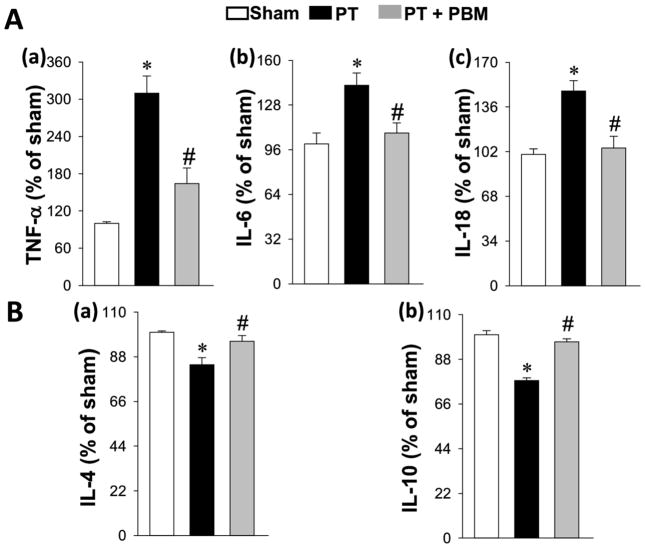
We next investigated the effect of PBM on inflammatory response in stroke, as neuroinflammation has been well documented to play an important role in the pathogenesis of stroke (Ahmed et al., 2016; Couch et al., 2017). As shown in Fig. 7A and B, representative microscopy and quantification analysis from peri-infarct cortex showed that the expressions of GFAP and Iba-1 in PT group were significantly elevated compared to the sham animals. However, the expressions of GFAP and Iba-1 in PBM treatment group were significantly decreased compared to the PT stroke animals. To determine the effect of PBM on inflammatory response, we measured the levels of specific pro-inflammatory cytokines and anti-inflammatory cytokines in the cortex protein. As shown in Fig. 8A(a–c), ELISA assays indicated that the elevated levels of pro-inflammatory cytokines IL-6, TNF-α and IL-18 after PT stroke were significantly ameliorated by PBM treatment. Intriguingly, the levels of anti-inflammatory cytokines, IL-1 and IL-10, were effectively elevated in PT animals treated with PBM compares with PT control group (Fig. 8B).
Fig. 7. PBM inhibits PT stroke-induced glial activation.
(A) Representative confocal microscopy of GFAP (a–c) and Iba-1 (d–f) staining taken from the peri-infarct cortical region. (B) The fluorescent intensity of GFAP (a) and Iba-1(b) was quantified and expressed as percentage changes versus the respective control group. Values are expressed as mean ± SE (n=4–5). *P<0.05 versus sham, #P<0.05 versus PT group.
Fig. 8. Effect of PB< on inflammatory cytokines.
(A) The levels of pro-inflammatory cytokines, TNF-a, IL-6 and IL-18 were measured and shown in (a–c). (B) The levels of anti-inflammatory cytokines, IL-4 and IL-10 were also were measured by ELISA assay (a–b). All data are expressed as mean ± SE (n = 4–5). *P < 0.05 versus sham, #P < 0.05 versus PT.
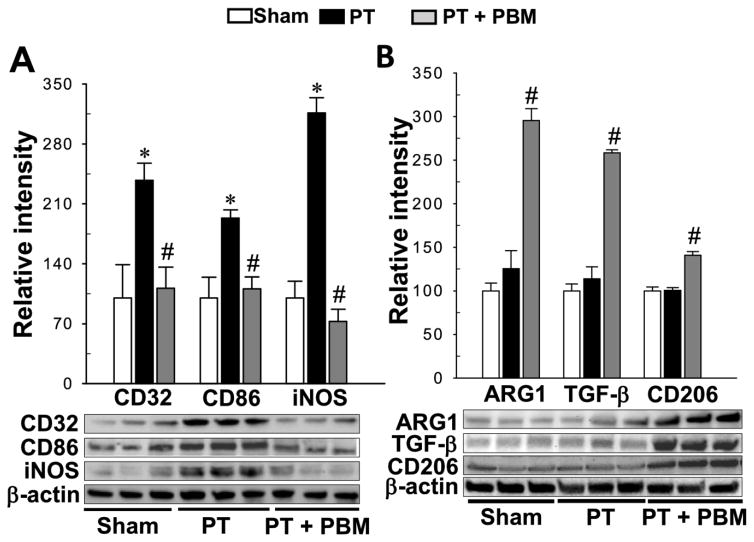
There are two different inflammation-related phenotypes of activated microglia, termed the M1 “pro-inflammatory” phenotype and the M2 “ anti-inflammatory” phenotype, which are related to producing pro-inflammatory factors and anti-inflammatory factors respectively (Durafourt et al., 2012; Lu et al., 2017a). We then investigated the effect of PBM on the transformation of two phenotypes of microglial polarization. As shown in Fig. 9A, Western blot analysis of M1 markers indicated a robust elevation of the expressions of CD32, CD86 and iNOS in the PT group compared with sham, an effect that was substantially mitigated by PBM treatment. Interestingly, the expressions of M2 markers (ARG1, TGF-β, CD206) in PBM groups were significantly elevated, as compared with PT group (Fig. 9B), demonstrating PBM treatment could transform microglial polarization from the M1 “pro-inflammatory” phenotype to the M2 “anti-inflammatory” phenotype.
Fig. 9. PBM shifts microglial polarization from M1 to M2 phenotype.
(A) Results of western blot analysis and quantitative analyses for M1 markers, CD32, CD86 and iNOS. (B) indicates the effect of LLI on M2 markers, ARG1, TGF-β and CD 206. All data are expressed as mean ± SE (n = 4–5). *P < 0.05 versus sham, #P < 0.05 versus PT.
Discussion
Using a well-established photothrombosis-induced ischemic stroke model, the current study first demonstrated the beneficial effect of PBM in promoting cortical neurogenesis. The key findings are that PBM markedly improved post-stroke cortical neurogenesis, and this effect was correlated with the improvement of the neuronal microenvironment and the proliferation and differentiation of neural progenitor cells in the peri-infarct zone. The underlying mechanism of PBM responsible for supporting neurogenesis and alleviating behavioral deficits may be related to the findings as illustrated in the current study: 1) restoration of mitochondrial function and providing the nascent neurons with more ATP, 2) suppression of reactive gliosis and neuroinflammation, 3) alteration of the microglial phenotype, as well as 4) enhancement of neural progenitor proliferation and differentiation. These findings indicate that PBM may provide functional recovery and promote neural repair following ischemic stroke, positioning it as a promising therapeutic option in stroke management.
A previous study suggested that the generation of new neurons in the adult brain primarily occurs in the subventricular zone (SVZ) and subgranular zone (SGZ) of the dentate gyrus, and that this process is stimulated in brain injury conditions (Jin et al., 2001; Liu et al., 1998; Parent et al., 1997; Shimazaki and Okano, 2008). Numerous evidence over the years has suggested that neurogenesis also occurred in the cortex (Gu et al., 2000; Jiang et al., 2001), and this cortical neurogenesis has been considered as a potential therapeutic target in stroke treatment. While numerous evidence indicated that though the adult brain may repair itself by neurogenesis after stroke, this effect is short-lived, unfortunately, as the ability of self-repair was dampened due to the harsh local microenvironment (Arvidsson et al., 2002; Thored et al., 2006). In the process of cellular proliferation, the cellular responses are governed by numerous and complex chemical and physical cues from their surrounding microenvironment (Kawamura et al., 2016). A great number of studies have investigated the effect of the microenvironment on cell proliferation. Studies found the creation of an optimal combination of cells with an appropriate microenvironment was important for promoting proliferation, migration, and differentiation (Kawamura et al., 2016; Trensz et al., 2016). In our study, we proposed that the effect of PBM on neurogenesis can be attributed to its improvement of the microenvironment which neurogenesis depends on for proper health, and the promotion of proliferation and differentiation of internal stem cells in the peri-infarct zone.
Findings from previous studies indicated that the milieu of inflammatory cytokines released by a cell is an important factor in creating an appropriate microenvironment for tissue repair (Kawamura et al., 2016; Yu et al., 2014). The inflammatory response plays an important role in the pathological process of ischemic stroke, and attenuated post-insult levels of pro-inflammatory cytokines may confer a protective effect to the brain (Esenwa and Elkind, 2016; Sieber et al., 2011). Cerebral ischemia is also usually accompanied with the release of pro-inflammatory cytokines (e.g. IL-1β, IL-6, TNF-α and IL-18), which initiates a serious neuroinflammatory attack to the brain (Winklewski et al., 2014; Yan et al., 2015). Evidence has demonstrated that PBM can decrease inflammation in diabetes, lung inflammation, skeletal muscle inflammation and crush injury to the rat sciatic nerve (Alcantara et al., 2013; Fukuoka et al., 2017; Mantineo et al., 2014; Miranda da Silva et al., 2015). Our previous study about the beneficial effect of PBM on AD suggested PBM can inhibit Aβ-induced reactive gliosis and neuroinflammation (Lu et al., 2017b). Furthermore, the effect of PBM on stroke has been widely investigated. Studies found PBM could improve behavioral outcomes in an embolic stroke model, middle cerebral artery occlusion (MCAO) model and in addition, there is some evidence demonstrating the beneficial effect of PBM on acute ischemic stroke in humans (Detaboada et al., 2006; Lampl et al., 2007; Lapchak et al., 2004; Zivin et al., 2009). In our study, we found PBM could decrease the level of reactive gliosis and pro-inflammatory cytokine release induced by PT stroke. Notably, we found PBM can promote the transformation from the M1 “pro-inflammatory” phenotype to the M2 “anti-inflammatory” phenotype. Previous studies have indicated that M1 microglia primarily release pro-inflammatory cytokines and M2 microglia primarily secrete anti-inflammatory factors which was in accordance with the results of the levels of inflammatory factors in our study (Barakat and Redzic, 2016; Choi et al., 2017; Yang et al., 2017; Yuan et al., 2017). Those results mentioned above indicated that the modulatory actions of PBM on gliosis, release of pro-/anti-inflammatory and transformation between the two phenotypes of activated microglia may contribute to decrease inflammatory damage and improve the neuronal microenvironment making it more amenable to neuronal health.
In addition to the inflammatory response, mitochondrial dysfunction contributes to the harsh microenvironment after stroke (Lapchak and Boitano, 2016; Miao et al., 2016; Narne et al., 2017). Evidence from numerous studies has indicated that depletion of ATP during stroke may play an essential role in the development of extensive cell death and brain injury (Dirnagl et al., 1999; Li et al., 2016; Moskowitz et al., 2010). Mitochondria are a major target of inflammation-related injury. It has been suggested that increased levels of inflammatory cytokines and ROS can lead to the impairment of mitochondrial function in neuroprogenitor cells (Voloboueva and Giffard, 2011). Mitochondria play a central role in the differentiation of progenitor cells into mature neurons and, as such, mitochondrial impairment can suppress this critical differentiation (Bernstein and Bamburg, 2003; Voloboueva and Giffard, 2011). In addition, it has been found that mitochondrial dysfunction can induce rapid mitochondrial membrane potential loss and subsequent neuronal death (Bolanos and Almeida, 2006; Voloboueva et al., 2007). Depletion of mitochondrial DNA has been demonstrated to induce the death of neuroprogenitor cells within a short time, corroborating the detrimental impact of mitochondrial damage on the well-being of neuroprogenitor cells (Fike et al., 2009). Consistent with this, studies have demonstrated that the preservation of mitochondrial function can confer protection to neurons against inflammatory injury (Voloboueva et al., 2007). The mechanisms behind this neuroprotection are multifactorial and not entirely elucidated, ranging from anti-inflammatory signaling pathways and attenuation of apoptotic factor release (Lu et al., 2017a; Zhao et al., 2016). Preservation of ATP production has, likewise, been shown to play a critical role in neuronal survival (Ahmed et al., 2016; Lu et al., 2017b). Lapchak et al. found that elevation of ATP induced by PBM had a correlation with the improvement of behavioral deficit after embolic strokes (Lapchak and De Taboada, 2010). Our previous work demonstrated PBM could rescue mitochondrial dysfunction and restore mitochondrial dynamics in STZ-induced Alzheimer’s disease (Lu et al., 2017b). In this study, we measured the level of ATP from total protein fractions and found PT stroke could induce cortical ATP depletion and, which was in accordance with previous evidence (Ahmed et al., 2016; Lapchak and De Taboada, 2010), and, interestingly, this loss was rescued by PBM. Modulated by the effects of PBM, mitochondrial cytochrome c oxidase (CCO) enzyme plays a critical role in inhibition of ATP losses (Detaboada et al., 2006; Eells et al., 2003). CCO is an enzyme which works in the cellular respiratory chain and regulates the production of ATP via oxidative phosphorylation (Medeiros and Jennings, 2002). The CuA and CuB complexes located in the center of CCO have been identified as the targets of PBM (Medeiros and Jennings, 2002). In our study, we measured the activity of CCO 14 days after PT stroke and results revealed that the activity of CCO in the PT stroke group had a significant decrease as compared to sham rats. PBM treatment, however was able to recover this when compared with PT stroke animals. In line with our findings on ATP, increasing activity of CCO was correlated with improved production of ATP (Pastore et al., 1994).
The effect of PBM on neuroprogenitor cells plays an important role in cortical neurogenesis. Previous studies indicated that PBM can increase neurogenesis by stimulating the the proliferation, differentiation, and migration of neuroprogenitor cells in DG and SVZ, which is accompanied by increased levels of brain derived neurotrophic factor (BDNF) and synaptogenesis (Xuan et al., 2015; Xuan et al., 2014). In our present work, the BrdU/DCX/Ki67-positive cells from DG and SVZ were not apparently observed, suggesting the PBM’s effects is specific in the peri-infarct zone in this PT stroke model. Although more evidence is needed to demonstrate the role of neuroprogenitor cells in this self-repair process, the presence of DCX and Ki67-positive cells in the peri-infarct area suggested that neuroprogenitor cells stimulated by PBM may play an important role in this self-repair via enhancing cortical neurogenesis. Synaptogenesis has been shown to be increased by PBM in traumatic brain injury models in mice (Xuan et al., 2015). In this study, the immunoactivity of spinophilin and synaptophysin were increased in an “expend” layer where neurogenesis was predominant, which suggested PBM can induce synaptogenesis between the newly formed neurons in the cortical area, although we cannot exclude the possibility that these increases in synaptic markers are partly from synaptogenesis of existing neurons.
Previous studies have indicated that several important parameters contribute to the biphasic dose response of PBM on tissue (Demidova-Rice et al., 2007; Oron et al., 2001; Xuan et al., 2016). Evidence suggests that a single dose can confer benefical effects on brain injury when applied at different time points (Oron et al., 2007; Xuan et al., 2015; Xuan et al., 2016). Other report have demonstrated that excessive repeated transcranial PBM therapy can induce neuroinflammation, nullifying its therapeutic action, which has led to numerous studies investigating the effect of single dose treatment strategy on stroke (Xuan et al., 2016). It should be noted that we did not find apparent improvement induced by a single dose from the behavioral test results in this model (data not shown). However, several studies have observed the neuroprotective effect of multiple doses on different models (Lu et al., 2017b; Salehpour et al., 2017; Xuan et al., 2015; Xuan et al., 2014). Therefore, in the present study, we have adapted a diode laser system by which the dose of PBM therapy was chosen based on our recent study as cortical dosage of 3 J per day using multiple doses (Lu et al., 2017b). It has been indicated that the 13.3 J dose received by cortex was not able to induce cortical temperature increase and histopathological damage according previous study (Chen et al., 2013). There are several excellent publications measuring the effect of different PBM parameters which suggest that the effect of PBM on animal may depend primarily on the total dose, the repetition regimen, wave modes, and wavelengths of laser (Ando et al., 2011; Wu et al., 2012; Xuan et al., 2016; Xuan et al., 2013)
Conclusions
Our current study demonstrated PBM, a well-documented non-drug based therapy, could alleviate behavioral deficits and promote cortical neurogenesis in the PT stroke model. Furthermore, the most important findings in this work is that PBM can shift the phenotype of microglial polarization from the M1 “pro-inflammatory” phenotype to the M2 “anti-inflammatory” phenotype. Although further in-depth mechanistic investigation about the effects of PBM on neurogenesis are still needed, the results of our study demonstrate that PBM can improve cortical neurogenesis after stroke and can improve functional outcomes in adult rats. We believe that this non-invasive laser treatment paradigm can shine a new light on the investigation of neurogenesis in stroke.
Highlights.
PBM alleviates behavioral deficits after PT stroke.
PBM decreases cortical infarct size and increases neuronal survival.
PBM promotes cortical neurogenesis after PT stroke.
PBM inhibits local inflammatory status and promotes mitochondrial function.
PBM promotes proliferation and differentiation of neuroprogenitor cells.
Acknowledgments
This study was supported by Research Grant NS086929 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, USA; an American Heart Association Grant-in-Aid 15GRNT25240004, and National Natural Science Foundation grants of China: 61575065 &11604104.
Footnotes
Statement of interest
The authors declare that there is no conflict of interest in the current study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed ME, Tucker D, Dong Y, Lu Y, Zhao N, Wang R, Zhang Q. Methylene Blue promotes cortical neurogenesis and ameliorates behavioral deficit after photothrombotic stroke in rats. Neuroscience. 2016;336:39–48. doi: 10.1016/j.neuroscience.2016.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcantara CC, Gigo-Benato D, Salvini TF, Oliveira AL, Anders JJ, Russo TL. Effect of low-level laser therapy (LLLT) on acute neural recovery and inflammation-related gene expression after crush injury in rat sciatic nerve. Lasers in surgery and medicine. 2013;45:246–252. doi: 10.1002/lsm.22129. [DOI] [PubMed] [Google Scholar]
- Ando T, Xuan W, Xu T, Dai T, Sharma SK, Kharkwal GB, Huang YY, Wu Q, Whalen MJ, Sato S, Obara M, Hamblin MR. Comparison of therapeutic effects between pulsed and continuous wave 810-nm wavelength laser irradiation for traumatic brain injury in mice. PloS one. 2011;6:e26212. doi: 10.1371/journal.pone.0026212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrabi SS, Parvez S, Tabassum H. Progesterone induces neuroprotection following reperfusion-promoted mitochondrial dysfunction after focal cerebral ischemia in rats. Disease models & mechanisms. 2017;10:787–796. doi: 10.1242/dmm.025692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nature medicine. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- Barakat R, Redzic Z. The Role of Activated Microglia and Resident Macrophages in the Neurovascular Unit during Cerebral Ischemia: Is the Jury Still Out? Medical principles and practice: international journal of the Kuwait University, Health Science Centre. 2016;25(Suppl 1):3–14. doi: 10.1159/000435858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein BW, Bamburg JR. Actin-ATP hydrolysis is a major energy drain for neurons. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2003;23:1–6. doi: 10.1523/JNEUROSCI.23-01-00002.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolanos JP, Almeida A. Modulation of astroglial energy metabolism by nitric oxide. Antioxidants & redox signaling. 2006;8:955–965. doi: 10.1089/ars.2006.8.955. [DOI] [PubMed] [Google Scholar]
- Braeuninger S, Kleinschnitz C. Rodent models of focal cerebral ischemia: procedural pitfalls and translational problems. Experimental & translational stroke medicine. 2009;1:8. doi: 10.1186/2040-7378-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, De Taboada L, O’Connor M, Delapp S, Zivin JA. Thermal effects of transcranial near-infrared laser irradiation on rabbit cortex. Neuroscience letters. 2013;553:99–103. doi: 10.1016/j.neulet.2013.07.049. [DOI] [PubMed] [Google Scholar]
- Choi JY, Kim JY, Park J, Lee WT, Lee JE. M2 Phenotype Microglia-derived Cytokine Stimulates Proliferation and Neuronal Differentiation of Endogenous Stem Cells in Ischemic Brain. Experimental neurobiology. 2017;26:33–41. doi: 10.5607/en.2017.26.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couch Y, Akbar N, Davis S, Fischer R, Dickens AM, Neuhaus AA, Burgess AI, Rothwell PM, Buchan AM. Inflammatory Stroke Extracellular Vesicles Induce Macrophage Activation. Stroke. 2017 doi: 10.1161/STROKEAHA.117.017236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demidova-Rice TN, Salomatina EV, Yaroslavsky AN, Herman IM, Hamblin MR. Low-level light stimulates excisional wound healing in mice. Lasers in surgery and medicine. 2007;39:706–715. doi: 10.1002/lsm.20549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demyanenko SV, Panchenko SN, Uzdensky AB. Expression of neuronal and signaling proteins in penumbra around a photothrombotic infarction core in rat cerebral cortex. Biochemistry Biokhimiia. 2015;80:790–799. doi: 10.1134/S0006297915060152. [DOI] [PubMed] [Google Scholar]
- Detaboada L, Ilic S, Leichliter-Martha S, Oron U, Oron A, Streeter J. Transcranial application of low-energy laser irradiation improves neurological deficits in rats following acute stroke. Lasers in surgery and medicine. 2006;38:70–73. doi: 10.1002/lsm.20256. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends in neurosciences. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- Durafourt BA, Moore CS, Zammit DA, Johnson TA, Zaguia F, Guiot MC, Bar-Or A, Antel JP. Comparison of polarization properties of human adult microglia and blood-derived macrophages. Glia. 2012;60:717–727. doi: 10.1002/glia.22298. [DOI] [PubMed] [Google Scholar]
- Eells JT, Henry MM, Summerfelt P, Wong-Riley MT, Buchmann EV, Kane M, Whelan NT, Whelan HT. Therapeutic photobiomodulation for methanol-induced retinal toxicity. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:3439–3444. doi: 10.1073/pnas.0534746100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eissa M, Salih WHM. The influence of low-intensity He-Ne laser on the wound healing in diabetic rats. Lasers in medical science. 2017 doi: 10.1007/s10103-017-2230-x. [DOI] [PubMed] [Google Scholar]
- Esenwa CC, Elkind MS. Inflammatory risk factors, biomarkers and associated therapy in ischaemic stroke. Nature reviews. Neurology. 2016;12:594–604. doi: 10.1038/nrneurol.2016.125. [DOI] [PubMed] [Google Scholar]
- Ferraresi C, Kaippert B, Avci P, Huang YY, de Sousa MV, Bagnato VS, Parizotto NA, Hamblin MR. Low-level laser (light) therapy increases mitochondrial membrane potential and ATP synthesis in C2C12 myotubes with a peak response at 3–6 h. Photochemistry and photobiology. 2015;91:411–416. doi: 10.1111/php.12397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fike JR, Rosi S, Limoli CL. Neural precursor cells and central nervous system radiation sensitivity. Seminars in radiation oncology. 2009;19:122–132. doi: 10.1016/j.semradonc.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuoka CY, Simoes A, Uchiyama T, Arana-Chavez VE, Abiko Y, Kuboyama N, Bhawal UK. The Effects of Low-Power Laser Irradiation on Inflammation and Apoptosis in Submandibular Glands of Diabetes-Induced Rats. PloS one. 2017;12:e0169443. doi: 10.1371/journal.pone.0169443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannakopoulou A, Lyras GA, Grigoriadis N. Long-term effects of autoimmune CNS inflammation on adult hippocampal neurogenesis. Journal of neuroscience research. 2017;95:1446–1458. doi: 10.1002/jnr.23982. [DOI] [PubMed] [Google Scholar]
- Gu W, Brannstrom T, Wester P. Cortical neurogenesis in adult rats after reversible photothrombotic stroke. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2000;20:1166–1173. doi: 10.1097/00004647-200008000-00002. [DOI] [PubMed] [Google Scholar]
- Hamblin MR. Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS biophysics. 2017;4:337–361. doi: 10.3934/biophy.2017.3.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Li P, Guo Y, Wang H, Leak RK, Chen S, Gao Y, Chen J. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke. 2012;43:3063–3070. doi: 10.1161/STROKEAHA.112.659656. [DOI] [PubMed] [Google Scholar]
- Huang YY, Nagata K, Tedford CE, McCarthy T, Hamblin MR. Low-level laser therapy (LLLT) reduces oxidative stress in primary cortical neurons in vitro. Journal of biophotonics. 2013;6:829–838. doi: 10.1002/jbio.201200157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Gu W, Brannstrom T, Rosqvist R, Wester P. Cortical neurogenesis in adult rats after transient middle cerebral artery occlusion. Stroke. 2001;32:1201–1207. doi: 10.1161/01.str.32.5.1201. [DOI] [PubMed] [Google Scholar]
- Jin K, Minami M, Lan JQ, Mao XO, Batteur S, Simon RP, Greenberg DA. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:4710–4715. doi: 10.1073/pnas.081011098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura R, Hayashi Y, Murakami H, Nakashima M. EDTA soluble chemical components and the conditioned medium from mobilized dental pulp stem cells contain an inductive microenvironment, promoting cell proliferation, migration, and odontoblastic differentiation. Stem cell research & therapy. 2016;7:77. doi: 10.1186/s13287-016-0334-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, Kawabori M, Yenari MA. Innate inflammatory responses in stroke: mechanisms and potential therapeutic targets. Current medicinal chemistry. 2014;21:2076–2097. doi: 10.2174/0929867321666131228205146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa K, Matsumoto M, Niinobe M, Mikoshiba K, Hata R, Ueda H, Handa N, Fukunaga R, Isaka Y, Kimura K, et al. Microtubule-associated protein 2 as a sensitive marker for cerebral ischemic damage--immunohistochemical investigation of dendritic damage. Neuroscience. 1989;31:401–411. doi: 10.1016/0306-4522(89)90383-7. [DOI] [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends in neurosciences. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Lampl Y, Zivin JA, Fisher M, Lew R, Welin L, Dahlof B, Borenstein P, Andersson B, Perez J, Caparo C, Ilic S, Oron U. Infrared laser therapy for ischemic stroke: a new treatment strategy: results of the NeuroThera Effectiveness and Safety Trial-1 (NEST-1) Stroke. 2007;38:1843–1849. doi: 10.1161/STROKEAHA.106.478230. [DOI] [PubMed] [Google Scholar]
- Landes T, Martinou JC. Mitochondrial outer membrane permeabilization during apoptosis: the role of mitochondrial fission. Biochimica et biophysica acta. 2011;1813:540–545. doi: 10.1016/j.bbamcr.2011.01.021. [DOI] [PubMed] [Google Scholar]
- Lapchak PA, Boitano PD. A novel method to promote behavioral improvement and enhance mitochondrial function following an embolic stroke. Brain research. 2016;1646:125–131. doi: 10.1016/j.brainres.2016.04.039. [DOI] [PubMed] [Google Scholar]
- Lapchak PA, De Taboada L. Transcranial near infrared laser treatment (NILT) increases cortical adenosine-5′-triphosphate (ATP) content following embolic strokes in rabbits. Brain research. 2010;1306:100–105. doi: 10.1016/j.brainres.2009.10.022. [DOI] [PubMed] [Google Scholar]
- Lapchak PA, Wei J, Zivin JA. Transcranial infrared laser therapy improves clinical rating scores after embolic strokes in rabbits. Stroke. 2004;35:1985–1988. doi: 10.1161/01.STR.0000131808.69640.b7. [DOI] [PubMed] [Google Scholar]
- Li M, Zhou ZP, Sun M, Cao L, Chen J, Qin YY, Gu JH, Han F, Sheng R, Wu JC, Ding Y, Qin ZH. Reduced Nicotinamide Adenine Dinucleotide Phosphate, a Pentose Phosphate Pathway Product, Might Be a Novel Drug Candidate for Ischemic Stroke. Stroke. 2016;47:187–195. doi: 10.1161/STROKEAHA.115.009687. [DOI] [PubMed] [Google Scholar]
- Liu J, Solway K, Messing RO, Sharp FR. Increased neurogenesis in the dentate gyrus after transient global ischemia in gerbils. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1998;18:7768–7778. doi: 10.1523/JNEUROSCI.18-19-07768.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Dong Y, Tucker D, Wang R, Ahmed ME, Brann D, Zhang Q. Treadmill Exercise Exerts Neuroprotection and Regulates Microglial Polarization and Oxidative Stress in a Streptozotocin-Induced Rat Model of Sporadic Alzheimer’s Disease. Journal of Alzheimer’s disease: JAD. 2017a;56:1469–1484. doi: 10.3233/JAD-160869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Wang R, Dong Y, Tucker D, Zhao N, Ahmed ME, Zhu L, Liu TC, Cohen RM, Zhang Q. Low-level laser therapy for beta amyloid toxicity in rat hippocampus. Neurobiology of aging. 2017b;49:165–182. doi: 10.1016/j.neurobiolaging.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantineo M, Pinheiro JP, Morgado AM. Low-level laser therapy on skeletal muscle inflammation: evaluation of irradiation parameters. Journal of biomedical optics. 2014;19:98002. doi: 10.1117/1.JBO.19.9.098002. [DOI] [PubMed] [Google Scholar]
- Medeiros DM, Jennings D. Role of copper in mitochondrial biogenesis via interaction with ATP synthase and cytochrome c oxidase. Journal of bioenergetics and biomembranes. 2002;34:389–395. doi: 10.1023/a:1021206220851. [DOI] [PubMed] [Google Scholar]
- Miao Y, Zhao S, Gao Y, Wang R, Wu Q, Wu H, Luo T. Curcumin pretreatment attenuates inflammation and mitochondrial dysfunction in experimental stroke: The possible role of Sirt1 signaling. Brain research bulletin. 2016;121:9–15. doi: 10.1016/j.brainresbull.2015.11.019. [DOI] [PubMed] [Google Scholar]
- Miranda da Silva C, Peres Leal M, Brochetti RA, Braga T, Vitoretti LB, Saraiva Camara NO, Damazo AS, Ligeiro-de-Oliveira AP, Chavantes MC, Lino-Dos-Santos-Franco A. Low Level Laser Therapy Reduces the Development of Lung Inflammation Induced by Formaldehyde Exposure. PloS one. 2015;10:e0142816. doi: 10.1371/journal.pone.0142816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010;67:181–198. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narne P, Pandey V, Phanithi PB. Interplay between mitochondrial metabolism and oxidative stress in ischemic stroke: An epigenetic connection. Molecular and cellular neurosciences. 2017;82:176–194. doi: 10.1016/j.mcn.2017.05.008. [DOI] [PubMed] [Google Scholar]
- Oron A, Oron U, Chen J, Eilam A, Zhang C, Sadeh M, Lampl Y, Streeter J, DeTaboada L, Chopp M. Low-level laser therapy applied transcranially to rats after induction of stroke significantly reduces long-term neurological deficits. Stroke. 2006;37:2620–2624. doi: 10.1161/01.STR.0000242775.14642.b8. [DOI] [PubMed] [Google Scholar]
- Oron A, Oron U, Streeter J, de Taboada L, Alexandrovich A, Trembovler V, Shohami E. low-level laser therapy applied transcranially to mice following traumatic brain injury significantly reduces long-term neurological deficits. Journal of neurotrauma. 2007;24:651–656. doi: 10.1089/neu.2006.0198. [DOI] [PubMed] [Google Scholar]
- Oron U, Yaakobi T, Oron A, Hayam G, Gepstein L, Rubin O, Wolf T, Ben Haim S. Attenuation of infarct size in rats and dogs after myocardial infarction by low-energy laser irradiation. Lasers in surgery and medicine. 2001;28:204–211. doi: 10.1002/lsm.1039. [DOI] [PubMed] [Google Scholar]
- Ozturk S. Epidemiology and the global burden of stroke--situation in Turkey. World neurosurgery. 2014;81:e35–36. doi: 10.1016/j.wneu.2012.10.074. [DOI] [PubMed] [Google Scholar]
- Parent JM, Yu TW, Leibowitz RT, Geschwind DH, Sloviter RS, Lowenstein DH. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1997;17:3727–3738. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastore D, Greco M, Petragallo VA, Passarella S. Increase in <--H+/e- ratio of the cytochrome c oxidase reaction in mitochondria irradiated with helium-neon laser. Biochemistry and molecular biology international. 1994;34:817–826. [PubMed] [Google Scholar]
- Pevsner PH, Eichenbaum JW, Miller DC, Pivawer G, Eichenbaum KD, Stern A, Zakian KL, Koutcher JA. A photothrombotic model of small early ischemic infarcts in the rat brain with histologic and MRI correlation. Journal of pharmacological and toxicological methods. 2001;45:227–233. doi: 10.1016/s1056-8719(01)00153-8. [DOI] [PubMed] [Google Scholar]
- Plane JM, Liu R, Wang TW, Silverstein FS, Parent JM. Neonatal hypoxic-ischemic injury increases forebrain subventricular zone neurogenesis in the mouse. Neurobiology of disease. 2004;16:585–595. doi: 10.1016/j.nbd.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Salehpour F, Ahmadian N, Rasta SH, Farhoudi M, Karimi P, Sadigh-Eteghad S. Transcranial low-level laser therapy improves brain mitochondrial function and cognitive impairment in D-galactose-induced aging mice. Neurobiology of aging. 2017;58:140–150. doi: 10.1016/j.neurobiolaging.2017.06.025. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Hoppen M, Strecker JK, Diederich K, Schabitz WR, Schilling M, Minnerup J. Photochemically induced ischemic stroke in rats. Experimental & translational stroke medicine. 2012;4:13. doi: 10.1186/2040-7378-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeter M, Jander S, Stoll G. Non-invasive induction of focal cerebral ischemia in mice by photothrombosis of cortical microvessels: characterization of inflammatory responses. Journal of neuroscience methods. 2002;117:43–49. doi: 10.1016/s0165-0270(02)00072-9. [DOI] [PubMed] [Google Scholar]
- Shanina EV, Redecker C, Reinecke S, Schallert T, Witte OW. Long-term effects of sequential cortical infarcts on scar size, brain volume and cognitive function. Behavioural brain research. 2005;158:69–77. doi: 10.1016/j.bbr.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Sharp WW, Fang YH, Han M, Zhang HJ, Hong Z, Banathy A, Morrow E, Ryan JJ, Archer SL. Dynamin-related protein 1 (Drp1)-mediated diastolic dysfunction in myocardial ischemia-reperfusion injury: therapeutic benefits of Drp1 inhibition to reduce mitochondrial fission. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2014;28:316–326. doi: 10.1096/fj.12-226225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazaki T, Okano H. [Mammalian neural stem cells]. Tanpakushitsu kakusan koso. Protein, nucleic acid, enzyme. 2008;53:311–317. [PubMed] [Google Scholar]
- Sieber MW, Claus RA, Witte OW, Frahm C. Attenuated inflammatory response in aged mice brains following stroke. PloS one. 2011;6:e26288. doi: 10.1371/journal.pone.0026288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thored P, Arvidsson A, Cacci E, Ahlenius H, Kallur T, Darsalia V, Ekdahl CT, Kokaia Z, Lindvall O. Persistent production of neurons from adult brain stem cells during recovery after stroke. Stem Cells. 2006;24:739–747. doi: 10.1634/stemcells.2005-0281. [DOI] [PubMed] [Google Scholar]
- Thunshelle C, Hamblin MR. Transcranial Low-Level Laser (Light) Therapy for Brain Injury. Photomedicine and laser surgery. 2016;34:587–598. doi: 10.1089/pho.2015.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trensz F, Lucien F, Couture V, Sollradl T, Drouin G, Rouleau AJ, Grandbois M, Lacraz G, Grenier G. Erratum to: Increased microenvironment stiffness in damaged myofibers promotes myogenic progenitor cell proliferation. Skeletal muscle. 2016;6:37. doi: 10.1186/s13395-016-0109-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voloboueva LA, Giffard RG. Inflammation, mitochondria, and the inhibition of adult neurogenesis. Journal of neuroscience research. 2011;89:1989–1996. doi: 10.1002/jnr.22768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voloboueva LA, Suh SW, Swanson RA, Giffard RG. Inhibition of mitochondrial function in astrocytes: implications for neuroprotection. Journal of neurochemistry. 2007;102:1383–1394. doi: 10.1111/j.1471-4159.2007.4634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LL, Li J, Gu X, Wei L, Yu SP. Delayed treatment of 6-Bromoindirubin-3′-oxime stimulates neurogenesis and functional recovery after focal ischemic stroke in mice. International journal of developmental neuroscience: the official journal of the International Society for Developmental Neuroscience. 2017;57:77–84. doi: 10.1016/j.ijdevneu.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Yang L, Wang Y. Enhanced differentiation of neural stem cells to neurons and promotion of neurite outgrowth by oxygen-glucose deprivation. International journal of developmental neuroscience: the official journal of the International Society for Developmental Neuroscience. 2015;43:50–57. doi: 10.1016/j.ijdevneu.2015.04.009. [DOI] [PubMed] [Google Scholar]
- Watson BD, Dietrich WD, Busto R, Wachtel MS, Ginsberg MD. Induction of reproducible brain infarction by photochemically initiated thrombosis. Annals of neurology. 1985;17:497–504. doi: 10.1002/ana.410170513. [DOI] [PubMed] [Google Scholar]
- Winklewski PJ, Radkowski M, Demkow U. Cross-talk between the inflammatory response, sympathetic activation and pulmonary infection in the ischemic stroke. Journal of neuroinflammation. 2014;11:213. doi: 10.1186/s12974-014-0213-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Xuan W, Ando T, Xu T, Huang L, Huang YY, Dai T, Dhital S, Sharma SK, Whalen MJ, Hamblin MR. Low-level laser therapy for closed-head traumatic brain injury in mice: effect of different wavelengths. Lasers in surgery and medicine. 2012;44:218–226. doi: 10.1002/lsm.22003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan W, Agrawal T, Huang L, Gupta GK, Hamblin MR. Low-level laser therapy for traumatic brain injury in mice increases brain derived neurotrophic factor (BDNF) and synaptogenesis. Journal of biophotonics. 2015;8:502–511. doi: 10.1002/jbio.201400069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan W, Huang L, Hamblin MR. Repeated transcranial low-level laser therapy for traumatic brain injury in mice: biphasic dose response and long-term treatment outcome. Journal of biophotonics. 2016;9:1263–1272. doi: 10.1002/jbio.201500336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan W, Vatansever F, Huang L, Hamblin MR. Transcranial low-level laser therapy enhances learning, memory, and neuroprogenitor cells after traumatic brain injury in mice. Journal of biomedical optics. 2014;19:108003. doi: 10.1117/1.JBO.19.10.108003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan W, Vatansever F, Huang L, Wu Q, Xuan Y, Dai T, Ando T, Xu T, Huang YY, Hamblin MR. Transcranial low-level laser therapy improves neurological performance in traumatic brain injury in mice: effect of treatment repetition regimen. PloS one. 2013;8:e53454. doi: 10.1371/journal.pone.0053454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan T, Chopp M, Chen J. Experimental animal models and inflammatory cellular changes in cerebral ischemic and hemorrhagic stroke. Neuroscience bulletin. 2015;31:717–734. doi: 10.1007/s12264-015-1567-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Xu S, Qian Y, Xiao Q. Resveratrol regulates microglia M1/M2 polarization via PGC-1alpha in conditions of neuroinflammatory injury. Brain, behavior, and immunity. 2017;64:162–172. doi: 10.1016/j.bbi.2017.03.003. [DOI] [PubMed] [Google Scholar]
- Yu B, Zhang X, Li X. Exosomes derived from mesenchymal stem cells. International journal of molecular sciences. 2014;15:4142–4157. doi: 10.3390/ijms15034142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J, Ge H, Liu W, Zhu H, Chen Y, Zhang X, Yang Y, Yin Y, Chen W, Wu W, Lin J. M2 microglia promotes neurogenesis and oligodendrogenesis from neural stem/progenitor cells via the PPARgamma signaling pathway. Oncotarget. 2017;8:19855–19865. doi: 10.18632/oncotarget.15774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang QG, Raz L, Wang R, Han D, De Sevilla L, Yang F, Vadlamudi RK, Brann DW. Estrogen attenuates ischemic oxidative damage via an estrogen receptor alpha-mediated inhibition of NADPH oxidase activation. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:13823–13836. doi: 10.1523/JNEUROSCI.3574-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao N, Zhuo X, Lu Y, Dong Y, Ahmed ME, Tucker D, Scott EL, Zhang Q. Intranasal Delivery of a Caspase-1 Inhibitor in the Treatment of Global Cerebral Ischemia. Molecular neurobiology. 2016 doi: 10.1007/s12035-016-0034-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zivin JA, Albers GW, Bornstein N, Chippendale T, Dahlof B, Devlin T, Fisher M, Hacke W, Holt W, Ilic S, Kasner S, Lew R, Nash M, Perez J, Rymer M, Schellinger P, Schneider D, Schwab S, Veltkamp R, Walker M, Streeter J. Effectiveness and safety of transcranial laser therapy for acute ischemic stroke. Stroke. 2009;40:1359–1364. doi: 10.1161/STROKEAHA.109.547547. [DOI] [PubMed] [Google Scholar]
- Zuo W, Yang PF, Chen J, Zhang Z, Chen NH. Drp-1, a potential therapeutic target for brain ischaemic stroke. British journal of pharmacology. 2016;173:1665–1677. doi: 10.1111/bph.13468. [DOI] [PMC free article] [PubMed] [Google Scholar]