Abstract
Background
Dopaminergic input to the prefrontal cortex (PFC) increases throughout adolescence and, by establishing precisely localized synapses, calibrates cognitive function. However, why and how mesocortical dopamine axon density increases across adolescence remains completely unknown.
Methods
We used a developmental application of axon-initiated recombination to label and track the growth of dopamine axons across adolescence in mice. We then paired this recombination with cell-specific knock-down of the netrin-1 receptor DCC to determine its role in adolescent dopamine axon growth. We then assessed how altering adolescent PFC dopamine axon growth changes the structural and functional development of the PFC by quantifying pyramidal neuron morphology and cognitive performance.
Results
Here we show, for the first time, that dopamine axons continue to grow from the striatum to the PFC during adolescence. Importantly, we discover that DCC, a guidance cue receptor, controls the extent of this protracted growth by determining where and when dopamine axons recognize their final target. When DCC-dependent adolescent targeting events are disrupted, dopamine axons continue to grow ectopically from the nucleus accumbens to the PFC and profoundly change PFC structural and functional development. This leads to alterations in cognitive processes known to be impaired across psychiatric conditions.
Conclusions
The prolonged growth of dopamine axons represents an extraordinary period for experience to influence their adolescent trajectory and predispose to or protect against psychopathology. DCC receptor signaling in dopamine neurons is a molecular link where genetic and environmental factors may interact in adolescence to influence the development and function of the prefrontal cortex.
Keywords: guidance cues, netrin-1, behavioral inhibition, cognitive flexibility, axon growth, axon-imitated recombination
Introduction
Adolescence is a period of heightened risk to develop psychiatric disease, suggesting that these disorders have a neurodevelopmental origin (1; 2). Adolescence is also an extended period of refinement to the structure and function of neurocircuitry. In particular, the prefrontal cortex (PFC) continues to develop until adulthood, and is among the final brain regions to fully mature (3). During adolescence, dopaminergic innervation to the rodent medial prefrontal cortex (mPFC) increases progressively, undergoing substantial modifications in fiber density, shape, and organization (4–7). In parallel to the adolescent development of mPFC dopamine connectivity, the structure and function of local circuitry is established (8; 9). Prolonged PFC dopamine development also occurs in primates, including humans (10–13), indicating that alterations in its course may lead to susceptibility or resilience to psychiatric disease (14; 15). However, the cellular and molecular events that govern the adolescent time course and trajectory of mesocortical dopamine innervation remain to be elucidated.
While the protracted increase in the density of mPFC dopamine innervation across adolescence has been assumed to result from dopamine axons that continue to grow to this region, this has not yet been experimentally validated. If indeed dopamine axons are still growing to the mPFC in adolescence, they may do so by utilizing mechanisms known to control axon growth early in life. During embryonic or early postnatal development, axons are known to grow toward their distant targets through a series of intermediate choice points. At each of these points, axons undergo target recognition events where they will stay, arborize, and form synapses; or continue to grow. These axon “decisions” are controlled by the spatiotemporal distribution of guidance cue proteins and their receptors (16). In adolescence, mesocortical dopamine axons may still be growing through intermediate choice points en route to their cortical target. Alternatively, the delayed increase in mPFC dopamine fiber density that occurs in adolescence may result from increased arborization of fibers that have already reached this region by this age. Whether guidance cues also mediate the targeting and/or arborization of axons during adolescent development remains to be explored.
Here, we applied axon-initiated viral tracing techniques, molecular recombination, and quantitative neuroanatomy to determine whether mesocortical dopamine axons are still growing to the PFC during adolescence or instead have already reached the cortex but are undergoing exaggerated arborization at this age. We then combined axon-initiated viral transduction with Cre-lox recombination to test whether the netrin-1 guidance cue receptor, DCC, mediates dopamine axon targeting or arborization in adolescence. We focused our study on the DCC protein because in both rodents and humans, DCC receptors are highly and conspicuously expressed by ventral tegmental area dopamine neurons across the lifetime (17–19). Furthermore, our work has implicated DCC signaling as a critical determinant of the extent of dopamine innervation to the cingulate and prelimbic subregions of the mPFC, specifically during adolescence (20). Finally, we determined whether subtle changes in the adolescent developmental profile of mPFC dopamine connectivity may have an impact on adult cognitive processes linked to a range of psychiatric conditions (21; 22).
Materials and Methods
Detailed descriptions of experimental procedures are provided in the Supplementary Materials and Methods.
Animals
Experimental procedures were performed according to the guidelines of the Canadian Council of Animal Care and approved by the McGill University/Douglas Mental Health University Institute Animal Care Committee. Mice were bred in the Douglas Mental Health University Institute Neurophenotyping center, maintained on a 12-h light–dark cycle (light on at 0800 h) and given ad libitum access to food and water unless noted. Dcc haploinsufficiency was achieved by crossing Dcclox/+ mice with DATCre mice (20; 23). Male mice were used in all experiments.
Axon-initiated recombination
To track the growth of dopamine axons during adolescence, we have adapted an axon-initiated viral transduction technique to specifically label midbrain neurons that terminate in the ventral striatum at the start of adolescence (24). At PND21, we injected a retrogradely transported virus expressing Cre recombinase (CAV-Cre, BioCampus Montpellier)(25) unilaterally at the level of the NAcc. We simultaneously injected a Cre-dependent enhanced yellow fluorescent protein (eYFP) virus DIO-eYFP (pAAV-Ef1a-DIO-EYFP-WPRE-pA, UNC Vector Core) into the ipsilateral VTA. CAV-Cre is preferentially taken up by axon terminals due to its internalization by the Coxsackievirus and adenovirus receptor (25), which is enriched at axon terminals (26). Thus, this recombination strategy limits eYFP labeling to VTA neurons with axons that terminate in the NAcc at PND21 (Figure 1A). Detailed information about the viruses, coordinates, and procedures are available in the Supplementary Materials and Methods.
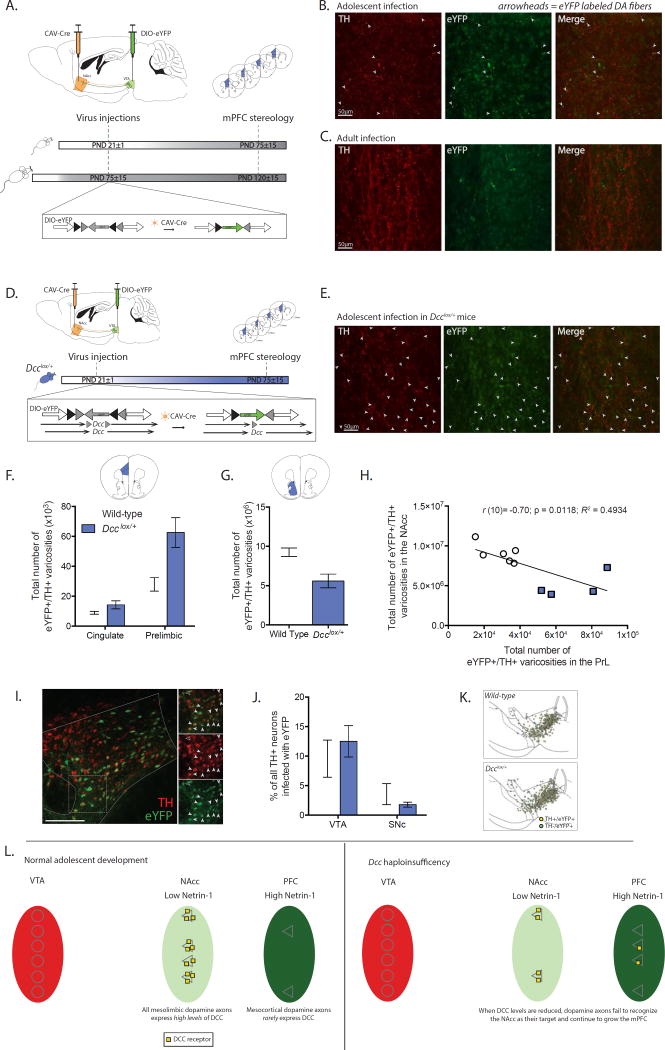
Figure 1. Mesocortical dopamine axons are still growing during adolescence; Dcc prevents the growth of mesolimbic dopamine axons to the mPFC by targeting them to the NAcc.
(A) Experimental strategy to label NAcc-projecting ventral tegmental area (VTA) dopamine neurons with eYFP from the start of adolescence (PND21±1) or during adulthood (PND75±15) using axon-initiated viral transduction. Six weeks later, at which point adolescent mice have reached adulthood, eYFP-positive dopamine axons in the mPFC were quantified. Inset: Viral recombination in midbrain neurons. (B) Representative micrographs of eYFP-positive dopamine fibers, indicated with arrowheads, which grew to the prelimbic subregion of the mPFC following viral injections in adolescent wild-type mice. (C) Representative micrographs from the prelimbic subregion of the mPFC of wild-type mice that received injections of the tracing viruses in adulthood. Although both TH-positive and eYFP-positive axons are present, eYFP-positive dopamine axons are exceedingly rare. (D) Infection strategy to combine axon-initiated viral transduction with conditional Dcc haploinsufficiency at the start of adolescence. Inset: In Dcclox/+ mice, the CAV-Cre virus also induces Dcc haploinsufficiency in labeled neurons. (E) Representative micrographs of eYFP-positive dopamine fibers, represented by arrowheads, in the prelimbic subregion of the mPFC of Dcclox/+ mice that received the viral injections at the start of adolescence. (F) Stereological quantification reveals that following adolescent injections (1) eYFP-positive dopamine varicosities are present in the mPFC in adult mice (i.e. dopamine axons continue to grow to from the NAcc into the mPFC during adolescence), and (2) the number of eYFP-positive dopamine varicosities is dramatically increased by Dcc haploinsufficiency (Two-way mixed-design ANOVA, main effect of genotype, F(1, 8) = 11.87, p = 0.0088; main effect of subregion, F(1, 8) = 38.03, p = 0.0003; genotype × subregion interaction, F(1, 8) = 7.262, p = 0.0273. n = 5 per group). (G) The number of eYFP-positive dopamine varicosities in the NAcc is dramatically reduced by Dcc haploinsufficiency (t(8) = 3.56, p = 0.0074) (H) Negative correlation between eYFP-positive dopamine varicosity number in the NAcc and PrL subregion of the mPFC. (I) Representative micrograph of eYFP infection in VTA. Inset: Co-labeled neurons indicated by closed arrowheads. A non-dopaminergic eYFP neuron is identified with an open arrowhead. (J) The percentage of eYFP-dopamine neurons does not differ between genotypes and in both genotypes there are significantly more eYFP-positive dopamine neurons in the VTA than in the substantia nigra (SNc) (Two-way mixed-design ANOVA, no interaction, F(1, 8) = 1.89, p = 0.20; no effect of genotype, F(1, 8) = 0.04644, p = 0.83; main effect of subregion, F(1, 8) = 23.84, p = 0.0012.) (K) Distribution map of stereological markers for TH-positive/eYFP-positive neurons in the VTA and SNc. (L) Model of adolescent dopamine axon growth. A subset of dopamine axons continue to grow from the NAcc into the mPFC during adolescence. DCC receptors within mesolimbic dopamine neurons promote target recognition events in the NAcc, preventing their ectopic growth into the mPFC.
Immunofluorescence
Stereology
Stereological quantification was performed as previously (4; 20; 28). The coefficient of error (CE) for stereological quantification of TH-positive varicosities was below 0.06 for all regions of interest in all sampled brains.
Axon structure analysis
Regions of interest were delineated according to the mouse brain atlas (29) at 5× magnification with a Leica DM4000B microscope. Individual eYFP-positive axon arbors were identified at 20× magnification. Neurolucida software (MicroBrightField) was used to trace the terminal arbors of selected axons at high magnification (40×), and to quantify arbor length, complexity, and varicosity density for each axon. Only eYFP-positive axons with intact arbors, defined as having all of the tips of their terminal branches within the section, were included in the analyses.
Western Blot
Bilateral punches of the mPFC, dorsal striatum and NAcc of PND75±15 male Dcclox/+ or Dcclox/+DATCre mice were processed for western blot as before (17; 30). Antibodies against Netrin-1 (1:750, Novus Biologicals, Littleton, CO, USA) and β-actin (1:15000, Sigma-Aldrich, Oakville, ON, Canada) were used.
Pyramidal neuron morphology
Brains of Dcclox/+ or Dcclox/+DATCre mice were processed for Golgi-Cox staining and the structure of mPFC pyramidal neurons was quantified using Neurolucida as previously (4; 20).
Behavioral testing
Behavioral Inhibition
Mice were food restricted to 1.5 g food per day for the duration of the task in order to maintain a body weight that was 85% of the initial free feeding weight. We used a modified mouse Go/No-Go Task which was optimized for our operant equipment (31) to test behavioral inhibition, with chocolate-flavored dustless precision pellets (BioServ, Inc., Flemington, NJ, USA) as our operant reinforcer. After training, mice underwent 10 daily sessions of the Go/No-Go Task. This task required the mice to respond to a lighted ‘Go’ cue or inhibit their response to this cue when presented in tandem with an auditory ‘No-Go’ cue (Figure 4A). In the ‘Go’ trials, mice had to respond to the illuminated nose poke hole in the 3-second timeframe during which the cue light was on in order to receive a reward. This was counted as a ‘Hit’ in our analysis. In the ‘No-Go’ trials, an 80 dB tone was paired with the 3-second cue light to signal that the mouse should withhold from responding. If mice responded during the 3- second ‘No-Go’ trial, an ITI was initiated and no reward was dispensed. This was counted as a ‘Commission Error’ in our analysis. However, if mice withheld from responding for the 3 second duration of the tone/light ‘No-Go’ cue, a reward was dispensed. A randomized, variable pretrial period of 3–9 seconds preceded each trial and the number of premature responses was recorded. Within each session, the number of ‘Go’ and ‘No-Go’ trials were given in an approximately 1:1 ratio and presented in a randomized order. Each session lasted 30 minutes and consisted of 30–50 ‘Go’ and 30–50 ‘No-Go’ trials. Detailed information on the task and training is available in the Supplementary Materials and Materials
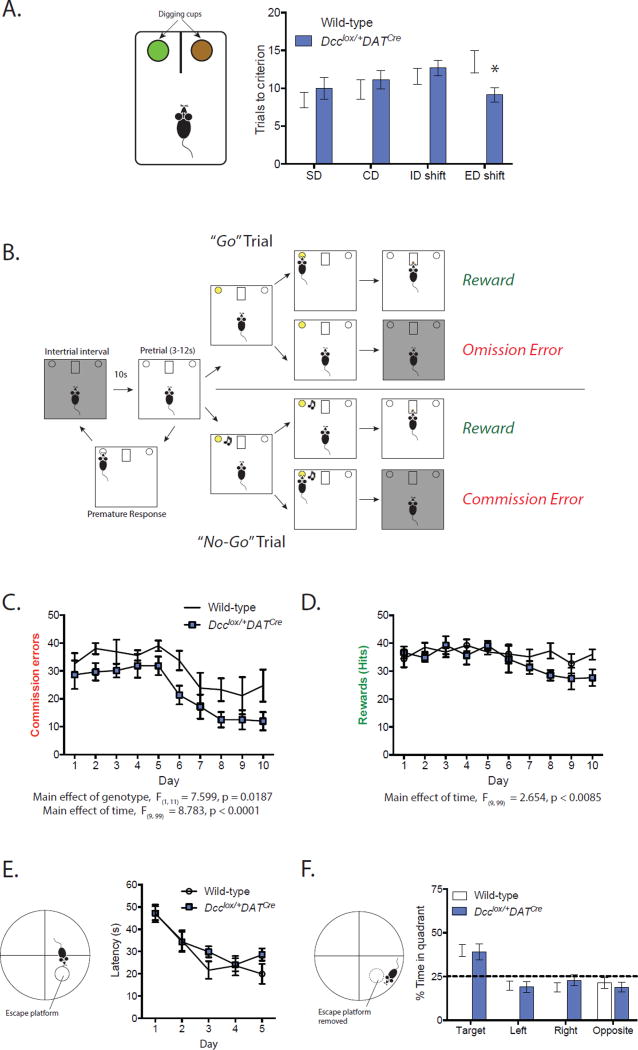
Figure 4. DCC-dependent rerouting of mesolimbic dopamine axons to the mPFC improves cognitive control.
(A) Dcclox/+DATCre mice required fewer trials than their wild-type littermates in the extradimensional (ED) part of the attentional set shifting task, indicating superior behavioral flexibility (Two-way mixed design ANOVA, genotype × task interaction, F(3, 72) = 2.749, p = 0.049; no effect of genotype, F(1, 24) = 0.01038, p = 0.9197; no effect of task, F(3, 72) = 2.123, p = 0.1048. Significant difference between genotypes during the ED shift, Bonferroni post hoc, p <0.05. Dcclox/+DATCre n = 14; Wild-type n = 12). (B) Diagram of the Go/No-Go task we adapted for mice. (C) Dcclox/+DATCre mice make significantly fewer commission errors than wild-type mice. (Two-way mixed design ANOVA, main effect of genotype, F(1, 11) = 7.599, p = 0.0187; main effect of time, F(9, 99) = 8.783, p < 0.0001; no interaction, F(9, 99) = 0.3734, p = 0.94. Dcclox/+DATCre n = 6; Wild-type n = 7). (D) There are no differences between the genotypes on the number of ‘Hits’ (Two-way mixed design ANOVA, no effect of genotype, F(1, 11) = 1.998, p = 0.185; main effect of time, F(9, 99) = 2.654, p < 0.0085; no interaction, F(9, 99) = 1.473, p = 0.1686. Dcclox/+DATCre n = 6; Wild-type n = 7). (E) No differences between genotypes in spatial learning in the Morris Water Maze. (Two-way mixed design ANOVA, main effect of day, F(4, 64) = 14.67, p < 0.0001; no effect of genotype, F(1, 16) = 1.972, p = 0.1793; no interaction, F(4, 64) = 0.7876, p = 0.5375. Dcclox/+DATCre n = 10; Wild-type n = 8). (F) No differences in the amount of time spent in the target quadrant between genotypes during the probe test in the Morris Water Maze. (Two-way mixed design ANOVA, main effect of quadrant, F(3, 48) = 12.73, p < 0.0001; no effect of genotype, F(1, 16) = 0.7938, p = 0.3862; no interaction, F(3, 48) = 0.2690, p = 0.8474. Dcclox/+DATCre n = 10; Wild-type n = 8).
Other behaviors
The Morris Water Maze, Attentional Set-Shifting Task, and Elevated Plus Maze, were performed as previously (20; 32). These tasks, and the Open Field procedure, are described in detail in the Supplementary Materials and Materials.
Data Analysis
Neuroanatomical data were analyzed using two-way mixed-design ANOVAs with genotype as a between-subjects factor and subregion as a within-subjects factor. Correlations were calculated using the Pearson correlation coefficient with one-tailed analysis. To quantify the complexity of individual dopamine axons, we adapted the Axonal Complexity Index (ACI) from developmental studies using Xenopus tadpoles (33). To quantify the complexity of mPFC neuron dendritic arbors, we used the Dendritic Complexity Index (DCI) (34). Western blot data were compared using Student’s t-tests. Behavioral data were analyzed using either a Student’s t-test (EPM and OF), or two-way mixed design ANOVA with genotype as a between-subjects factor and day (Go/No-Go, MWM training), task (ASST), or quadrant (MWM test) as a within-subjects factor. ANOVAs were followed by post-hoc Bonferroni tests where appropriate. All statistical analyses were carried out using Prism software (GraphPad).
Results
Dopamine axons continue to grow to the mPFC during adolescence
To determine if dopamine axons are still growing to the mPFC in adolescence we used axon-initiated recombination to limit eYFP expression to VTA neurons that have reached the NAcc by the start of adolescence (Figure 1A) (24). If the axons of these neurons continue to grow to the mPFC during adolescence, we should observe eYFP-positive dopamine axons in the adult mPFC. We targeted the NAcc because dopamine axons that arrive to the mPFC early during development grow through the developing striatum on their way to the cortex (5; 35). Furthermore, although the density of dopamine axon innervation to the NAcc achieves adult levels by ~PND20 in rodents (36), dopamine activity and connectivity in this region undergoes an exuberant period of dynamic changes in adolescence (37; 38). This is in contrast to the paucity of changes occurring in dopamine connectivity in other limbic targets at this age (39; 40).
Remarkably, we find eYFP dopamine axons in the cingulate and prelimbic subregions of the mPFC in adult mice that received dual viral infection in early adolescence. The presence of these fibers indicates that dopamine axons indeed continue to grow to the mPFC from the NAcc across adolescence (Figure 1B, F; see below). To our knowledge, this is the first evidence of axonal growth in the adolescent brain.
To exclude the possibility that these eYFP-positive dopamine axons are collaterals of fibers innervating the NAcc, we performed the same axon-initiated viral tracing experiment in adult wild-type mice (PND 75±15; Figure 1A, S1A). eYFP-positive dopamine axons in the cingulate or prelimbic subregions of the mPFC are absent or negligible in mice that received viral injections in adulthood and were euthanized 6 weeks later (Figure 1C, S1B). This in line with reports showing that (a) the vast majority of dopamine axons do not send collaterals between the NAcc and the mPFC (24; 41–45), and (b) dopamine axon growth into the mPFC is complete by PND 60 in rodents (5; 6).
DCC receptors within dopamine neurons prevent their ectopic growth to the mPFC in adolescence
In the NAcc and dorsal striatum Netrin-1 expression is low. However, DCC receptors are highly and exclusively expressed by dopamine axons (4). The opposite is true for the mPFC: Netrin-1 expression is intense and DCC expression by mPFC dopamine axons is minimal (4). This complementary pattern of DCC to Netrin-1 expression is maintained before and after adolescence and appears to be determine the extent of the mPFC dopamine innervation (4; 20). We hypothesize that during normal adolescent development, DCC receptors in dopamine axons promote target recognition events in non-cortical regions. Dopamine axons with low levels of DCC expression in adolescence fail to recognize these targets, and instead continue to grow to the mPFC.
To test this idea, we applied axon-initiated recombination to Dcc floxed haploinsufficient mice (Dcclox/+). Thus, in addition to inducing the expression of eYFP in VTA neurons that have reached the NAcc by adolescence, we also reduced DCC expression selectively in these neurons (Figure 1D). We find that inducing Dcc haploinsufficiency in NAcc-projecting dopamine neurons at the start of adolescence leads to a dramatic increase in the number of eYFP-positive dopamine axons in the adult mPFC (Figure 1E). To compare the number of dopamine axons that continue to grow from the NAcc to the mPFC in adolescence between wild-type and Dcclox/+ mice, we quantified eYFP-positive dopamine varicosities in the cingulate and prelimbic subregions of the adult mPFC (Figure S1I). There is a 2-fold greater number of eYFP-positive dopamine varicosities in the mPFC of mice with both VTA eYFP expression and Dcc haploinsufficiency than in mice with VTA eYFP expression alone (Total mPFC eYFP-positive/TH-positive varicosities × 103, Mean±SEM: Wild-type = 36.84±5.24; Dcclox/+ = 76.86±10.36; Figure 1F).
DCC receptors promote target recognition events in the NAcc during adolescence
Importantly, we find that inducing Dcc haploinsufficiency dopamine neurons that project to the NAcc at the start of adolescence leads to a dramatic decrease in the number of eYFP-positive dopamine varicosities in the adult NAcc (Figure 1G). Remarkably, we found that the number of NAcc eYFP-positive dopamine varicosities is significantly and negatively correlated with the number of eYFP-positive dopamine varicosities in the prelimbic subregion of the mPFC (Figure 1H). This result is consistent with the idea that DCC receptors within dopamine neurons function to prevent the growth of mesolimbic axons to the mPFC during adolescence. Thus, reducing DCC signaling in these axons induces targeting errors and their ectopic growth into the mPFC (Figure 1L).
The increased number of dopamine axons that continue to grow from the NAcc to the mPFC in Dcclox/+ mice does not result from a greater number of dually-infected VTA neurons (Figure 1I, J), nor from differences in the population distribution of dually-infected neurons (Figure 1K). Furthermore, the distribution of VTA neurons expressing eYFP is consistent with previous reports that dopamine neurons in the medial VTA project to the medial NAcc or to the mPFC (24; 43; 45).
Wild-type mice that received dual viral injections in adulthood have a comparable (a) level of eYFP infection of VTA dopamine neurons (Figure S1D), and (b) distribution of eYFP-positive dopamine neurons (Figure S1E). However, eYFP-positive dopamine varicosities in the mPFC were rare and largely stereologically unreliable (Coefficient of error > 0.2, Figure S1B). Moreover, eYFP-positive/TH-negative fibers were found in the mPFC of all groups of mice, suggesting that other populations of midbrain neurons may send collaterals between the NAcc and mPFC (Figure S1C, G) (46).
Because we target only a subset of neurons with our injection technique (Figure 1I), these results are likely an underestimation of the total growth that takes place during normal adolescent development. Although we find that under the conditions of this experiment the effect appears to be more pronounced in the prelimbic subregion, we do not know whether this is the case during normal development.
Dcc haploinsufficiency does not promote branching of dopamine axons in the mPFC
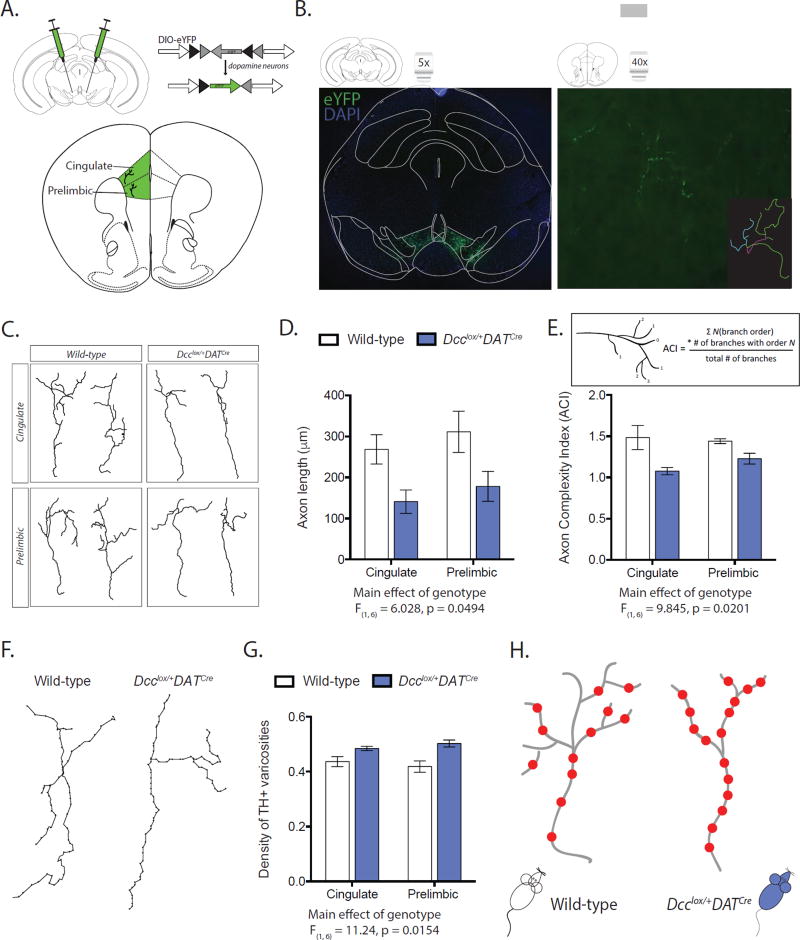
DCC-mediated Netrin-1 signaling participates in axonal arborization once axons reach their final targets (47; 48). We next determined whether compromised DCC expression within dopamine neurons also increases their branching within the mPFC. We quantified the fine architecture of individual eYFP axon arbors in the cingulate and prelimbic subregions of the mPFC of adult wild-type (DATCre) and conditional Dcc haploinsufficient (Dcclox/+DATCre) mice (Figure 2B, C). We found that dopamine axon arbors across the mPFC are shorter in conditional Dcc haploinsufficient mice than in wild-type controls (Figure 2D). We adapted the Axon Complexity Index measure (ACI) to assess the branching of dopamine axon arbors in the mPFC (Figure 2E, inset) (33). A higher ACI value indicates that axons are prominently arborized with many higher-order branches, while a lower ACI value indicates that axons are simpler, with arbors composed of mainly lower-order branches. We found that dopamine axons of conditional Dcc haploinsufficient mice also have a lower ACI value, indicating that their arbors are less complex (Figure 2E). Thus, Dcc haploinsufficiency within dopamine neurons triggers their growth to the mPFC, but reduces their branching. Importantly, we found that mPFC dopamine axons of conditional Dcc haploinsufficient mice have a greater density of dopamine varicosities (Figure 2F, G), which may compensate functionally for their reduced arborization (Figure 2H). Consistent with the fact that dopamine varicosities in the mPFC nearly always form synapses (49), Dcc haploinsufficiency leads to increased extracellular dopamine in this region (30). It is important to note that conditional Dcc haploinsufficiency in dopamine neurons does not lead to compensatory changes in Netrin-1 protein expression in target regions (Figure S2).
Figure 2. DCC receptors within dopamine neurons determine their fine axonal architecture in the mPFC.
(A) Infection strategy for labeling dopamine neurons with eYFP. (B) Right panel: Representative labeling of dopamine neurons in the ventral tegmental area. Left panel: single eYFP-positive mPFC dopamine axon. Inset: Neurolucida tracing of eYFP labeled axon. (C) Neurolucida tracings of representative dopamine axons from the cingulate (Cg1) and prelimbic (PrL) subregions of the mPFC. (D) Dcclox/+DATCre mice have shorter mPFC dopamine axons (Two-way mixed design ANOVA, main effect of genotype, F(1, 6) = 6.028, p = 0.0494; main effect of subregion, F(1, 6) = 10.11, p = 0.0191; no interaction, F(1, 6) = 0.04470, p = 0.8396; n = 4 per group). (E) Dcclox/+DATCre mice have less complex mPFC dopamine axons (Two-way mixed design ANOVA, main effect of genotype, F(1, 6) = 9.845, p = 0.0201; no effect of subregion, F(1, 6) = 0.6213, p = 0.4606; no interaction, F(1, 6) = 2.097, p = 0.1978. n = 4 per group. Inset: ACI calculation (33)). (F) Representative Neurolucida tracings of mPFC dopamine axons, circles represent varicosities. (G) mPFC dopamine axons of Dcclox/+DATCre mice have greater density of varicosities (Two-way mixed design ANOVA, main effect of genotype, F(1, 6) = 11.24, p = 0.0154; no effect of subregion, F(1, 6) = 0.0000004733, p = 0.9983; no interaction, F(1, 6) = 3.056, p = 0.1310. Dcclox/+DATCre n = 4; Wild-type n = 4). (H) Model of fine mPFC dopamine axonal architecture.
mPFC pyramidal neuron structure is altered in mice with increased dopamine input to the mPFC
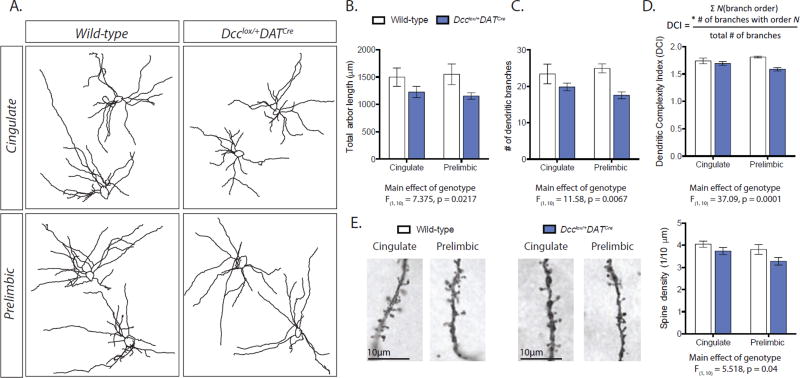
Dopamine input to pyramidal neurons, but not to local interneurons, increases across adolescence (13). In parallel to dopamine growth into the mPFC, the structure of pyramidal neurons changes across adolescence, including morphological modifications to their dendritic arbors and alterations to their dendritic spine density (13; 50). We analyzed mPFC layer V pyramidal neuron morphology in adult mice with conditional Dcc haploinsufficiency in dopamine neurons and in their wild-type littermates (Figure 3A). We found that in adult conditional Dcc haploinsufficient mice, which have augmented mPFC dopamine input (20), pyramidal neurons of the cingulate and prelimbic subregions have shorter basilar dendritic arbors and fewer basilar dendritic branches (Figure 3B, C). We quantified arbor complexity using the Dendritic Complexity Index (Figure 3D, inset) (34; 51), and found that pyramidal neurons in the mPFC of conditional Dcc haploinsufficient mice have less complex basilar dendritic arbors than those of their wild-type counterparts (Figure 3D). Pyramidal neurons of conditional Dcc haploinsufficient mice also have a significant reduction in dendritic spine density compared to wild-type controls across the cingulate and prelimbic subregions (Figure 3E) (20). These subregion-specific results replicate and expand our previous findings of structural alterations in layer V pyramidal neurons across the entire mPFC in our models of conditional and global Dcc haploinsufficiency (4; 20). PFC pyramidal neuron structure in adolescence can be shaped in response to environmental factors (52). Thus, our results raise the possibility that environmental stimuli may exert their effects by altering the DCC-dependent adolescent growth of dopamine axons to the mPFC, influencing in turn cognitive processing in adulthood.
Figure 3. Dcc drives the structural maturation of mPFC pyramidal neurons by limiting the growth of dopamine axons in adolescence.
(A) Representative Neurolucida tracings of the basilar dendritic arbors of layer V pyramidal neurons. (B) The dendritic arbors of layer V pyramidal neurons are shorter and less complex in Dcclox/+DATCre mice (Arbor Length: Two-way mixed design ANOVA, main effect of genotype, F(1, 10) = 7.375, p = 0.0217; no effect of subregion, F(1, 10) = 0.006162, p = 0.9390; no interaction, F(1,10) = 0.2289, p = 0.6427. Number of Branches: Two-way mixed design ANOVA, main effect of genotype, F(1, 10) = 11.58, p = 0.0067; no effect of subregion, F(1, 10) = 0.08324, p = 0.7788; no interaction, F(1,10) = 1.969, p = 0.1908. Dendritic Complexity: Two-way mixed design ANOVA, main effect of genotype, F(1, 10) = 37.09, p = 0.0001; no effect of subregion, F(1, 10) = 0.1742, p = 0.6852; no interaction, F(1,10) = 4.011, p = 0.0731. Dcclox/+DATCre n = 7; Wild-type n = 5). Inset: DCI calculation. (C) The dendritic spine density of layer V pyramidal neurons is reduced across cingulate and prelimbic mPFC subregions in Dcclox/+DATCre mice. (Two-way mixed design ANOVA, main effect of genotype, F(1, 10) = 5.518, p = 0.0407; no effect of subregion, F(1, 10) = 3.957, p = 0.0747; no interaction, F(1,10) = 0.4078, p = 0.5374. Dcclox/+DATCre n = 7; Wild-type n = 5).
Improved cognitive outcome in mice with increased dopamine input to the mPFC
Multiple psychiatric disease states share common cognitive symptomology (1; 2; 53), suggesting that alterations in PFC circuitry may serve as a common neurobiological mechanism (21; 54). Performance in tasks that require cognitive control are emerging as a useful biomarker of disorders that involve PFC dysfunction (22). First, we tested cognitive flexibility in conditional Dcc haploinsufficient mice using the Attentional Set Shifting Task, as we have done previously in mice with global Dcc haploinsufficiency (20). Remarkably, conditional Dcc haploinsufficient mice performed significantly better than their wild-type counterparts in the extradimensional shift part of the task, fully recapitulating our findings in mice with global Dcc haploinsufficiency (Figure 4A)(20). Performance in the extradimensional shift depends on PFC function (55), and an improvement in this part of the task indicates greater cognitive flexibility. We next extended this finding by assessing behavioral inhibition, an aspect of cognitive control, in adult conditional Dcc haploinsufficient and wild-type mice using a Go/No-Go task (31). While both groups improved in their ability to inhibit disadvantageous responses over the course of the 10-day task, conditional Dcc haploinsufficient mice made fewer commission errors throughout the entire task, indicating superior behavioral inhibition (Figure 4C). There were no differences between genotypes in the correct responses to the ‘Go’ cue (Hits, Figure 4D), or in learning the components of the task (Figure S3A). Conditional Dcc haploinsufficient mice do not differ from wild-type mice in spatial learning and memory (Figure 4E, F), or in locomotor and anxiety-like phenotypes (Figure S3B, C). Together, we find that conditional Dcc haploinsufficiency leads to improvements in adult cognitive functioning, likely by altering the adolescent development of the mPFC. Factors regulating DCC in adolescence may be useful in early intervention strategies to improve cognitive control.
Discussion
The protracted increase in PFC dopamine innervation across adolescence has been described for some time in both rodents and primates (5–7; 10; 13). Here we show, for the first time, that the delayed increase in mPFC dopamine innervation results from the fact that axons are still growing to this region over the course of adolescence. We discover that DCC receptors within dopamine neurons control this process by inducing target recognition events in axons destined to innervate the NAcc, preventing their ectopic growth to the cortex. By determining mesocorticolimbic dopamine axon targeting in adolescence, DCC receptors in turn drive the maturation of the PFC. DCC-dependent rerouting of mesolimbic dopamine axons to the cortex alters the structure of mPFC layer V pyramidal neurons and improves behavioral inhibition in adulthood. Our results indicate that subtle alterations to the number of dopamine axons growing to the mPFC in adolescence have significant consequences on the structural and functional development of the mPFC. To the best of our knowledge, axon growth during adolescence has not previously been described. We estimate that growing mesocortical dopamine axons travel over an approximate distance of ~2–4 mm to reach their cortical targets during adolescence. Along their route they need to make frequent and cumulative decisions at intermediate targets, and thus remain very vulnerable for an extended spatiotemporal window.
Studies on molecular determinants of dopamine pathway development have largely focused on embryonic life, when various guidance cue systems have been shown to influence the growth of dopamine axons toward their targets (56–59). Target recognition regulates axonal growth to ensure that axons do not stop prematurely, or proceed past their target (16). How and where dopamine axons undergo target recognition events and the molecular cues regulating their target selection has been, until now, completely unknown (59). Here we show that the netrin-1 receptor, DCC, limits the adolescent growth of dopamine axons to the mPFC by promoting target recognition events in the NAcc. This role is in line with previous reports showing that DCC receptors mediate target recognition events at intermediate or final targets of axons of retinal ganglion cells in mice (60), and of axons of motor neurons and of photoreceptors in drosophila (61; 62).
Why dopamine axons destined to innervate the mPFC remain in the NAcc for a period of time before continuing to their target remains unknown. Waiting periods, where axons pause before continuing along their path, have been demonstrated in early dopamine development (5; 35; 63), and may also rely on the function of guidance cues (64). We have shown that DCC receptors are not required for dopamine axons to grow to the NAcc (4; 20), but are necessary for axons to recognize this area as their target during adolescence. VTA dopamine neurons express DCC receptors from embryonic life to adulthood, but levels decrease significantly in adolescence (17). In fact, VTA dopamine neurons begin to express another netrin-1 receptor, UNC5C, during adolescence, and the ratio of DCC:UNC5C expression inverts (17). This developmental switch may contribute to mesolimbic dopamine axon targeting, and to determining which axons pause in the NAcc before continuing to the mPFC (27; 65).
Cognitive control relies on PFC function and matures in tandem with its circuitry (66–69). Performance on cognitive tasks improves throughout adolescence, including measures of inhibitory control, behavioral flexibility, and working memory (70–73). Furthermore, impaired cognitive control is a symptom that spans multiple diagnoses based on the Diagnostic and Statistical Manual of Mental Disorders (DSM), including those with an adolescent onset, suggesting that disrupted PFC development is a common link between psychiatric diseases (54; 74). Indeed, cognitive control has been proposed to serve as a biomarker for disease states resulting from altered PFC circuitry (22). Our findings support the idea that work in rodents can begin to identify the molecular events underlying the development of circuitry involved in specific behaviors altered in psychiatric disease, such as behavioral inhibition, and inform research-based intervention strategies (21).
Taken together, our results indicate that the pathfinding trajectory of mesocortical dopamine axons is malleable in adolescence and could be easily modified by factors that target DCC receptors during this vulnerable age. These modifications would have profound consequences for adult cortical function. Indeed, we have shown that environmental factors in adolescence, such as exposure to drugs of abuse (75), alter DCC receptor expression in dopamine neurons and, in turn, disrupt the extent and the organization of mPFC dopamine input (28). We propose that DCC receptor signaling in dopamine neurons may be a molecular link where genetic and environmental factors interact in adolescence to influence mPFC dopamine axon growth and, thereby, vulnerability to psychopathology.
Supplementary Material
Acknowledgments
This work was supported by the National Institute on Drug Abuse (R01DA037911 to C.F.; F31DA041188 to L.M.R.), the Canadian Institutes of Health Research (MOP-74709 to C.F.), and the Natural Science and Engineering Research Council of Canada (2982226 to C.F.). C.F. is a research scholar of the Fonds de Recherche du Québec - Santé. L.M.R. was supported by predoctoral fellowships from The Djavad Mowafaghian Foundation and Fulbright Canada. The authors would like to thank Dr. Roy Wise and Dr. Andreas Arvanitogiannis for their critical reading of the manuscript, and Dr. Joseph Rochford for MEDPC-IV programming assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions:
L.M.R., C.M. and C.F. conceived and designed the experiments. L.M.R., M.P., A.T.B, S.C., L.C.L., E.D.C.P., M.W., and B.K. performed the experiments. L.M.R., A.T.B., S.C., L.C.L., and C.F. analyzed the results. P.K. provided Dcc+/− mice. L.M.R. and C.F. wrote the manuscript with input from the other authors.
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Lee FS, Heimer H, Giedd JN, Lein ES, Šestan N, Weinberger DR, Casey BJ. Mental health. Adolescent mental health--opportunity and obligation. Science. 2014;346:547–549. doi: 10.1126/science.1260497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. 2008;9:947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manitt C, Mimee A, Eng C, Pokinko M, Stroh T, Cooper HM, et al. The netrin receptor DCC is required in the pubertal organization of mesocortical dopamine circuitry. J Neurosci. 2011;31:8381–8394. doi: 10.1523/JNEUROSCI.0606-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalsbeek A, Voorn P, Buijs RM, Pool CW, Uylings HB. Development of the dopaminergic innervation in the prefrontal cortex of the rat. J Comp Neurol. 1988;269:58–72. doi: 10.1002/cne.902690105. [DOI] [PubMed] [Google Scholar]
- 6.Naneix F, Marchand AR, Di Scala G, Pape J-R, Coutureau E. Parallel Maturation of Goal-Directed Behavior and Dopaminergic Systems during Adolescence. J Neurosci. 2012;32:16223–16232. doi: 10.1523/JNEUROSCI.3080-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benes FM, Taylor JB, Cunningham MC. Convergence and plasticity of monoaminergic systems in the medial prefrontal cortex during the postnatal period: implications for the development of psychopathology. Cereb Cortex. 2000;10:1014–1027. doi: 10.1093/cercor/10.10.1014. [DOI] [PubMed] [Google Scholar]
- 8.Caballero A, Granberg R, Tseng KY. Mechanisms contributing to prefrontal cortex maturation during adolescence. Neurosci Biobehav Rev. 2016 doi: 10.1016/j.neubiorev.2016.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Donnell P. Adolescent onset of cortical disinhibition in schizophrenia: insights from animal models. Schizophr Bull. 2011;37:484–492. doi: 10.1093/schbul/sbr028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenberg DR, Lewis DA. Postnatal maturation of the dopaminergic innervation of monkey prefrontal and motor cortices: a tyrosine hydroxylase immunohistochemical analysis. J Comp Neurol. 1995;358:383–400. doi: 10.1002/cne.903580306. [DOI] [PubMed] [Google Scholar]
- 11.Weickert CS, Webster MJ, Gondipalli P, Rothmond D, Fatula RJ, Herman MM, et al. Postnatal alterations in dopaminergic markers in the human prefrontal cortex. Neuroscience. 2007;144:1109–1119. doi: 10.1016/j.neuroscience.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 12.Rothmond DA, Weickert CS, Webster MJ. Developmental changes in human dopamine neurotransmission: cortical receptors and terminators. BMC Neurosci. 2012;13:18. doi: 10.1186/1471-2202-13-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lambe EK, Krimer LS, Goldman-Rakic PS. Differential postnatal development of catecholamine and serotonin inputs to identified neurons in prefrontal cortex of rhesus monkey. J Neurosci. 2000;20:8780–8787. doi: 10.1523/JNEUROSCI.20-23-08780.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Money KM, Stanwood GD. Developmental origins of brain disorders: roles for dopamine. Front Cell Neurosci. 2013;7:260. doi: 10.3389/fncel.2013.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schubert D, Martens GJM, Kolk SM. Molecular underpinnings of prefrontal cortex development in rodents provide insights into the etiology of neurodevelopmental disorders. Mol Psychiatry. 2014 doi: 10.1038/mp.2014.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chao DL, Ma L, Shen K. Transient cell-cell interactions in neural circuit formation. Nat Rev Neurosci. 2009;10:262–271. doi: 10.1038/nrn2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manitt C, Labelle-Dumais C, Eng C, Grant A, Mimee A, Stroh T, Flores C. Peri-pubertal emergence of UNC-5 homologue expression by dopamine neurons in rodents. PLoS ONE. 2010;5:e11463. doi: 10.1371/journal.pone.0011463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reyes S, Fu Y, Double KL, Cottam V, Thompson LH, Kirik D, et al. Trophic factors differentiate dopamine neurons vulnerable to Parkinson's disease. Neurobiol Aging. 2013;34:873–886. doi: 10.1016/j.neurobiolaging.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 19.Osborne PB, Halliday GM, Cooper HM, Keast JR. Localization of immunoreactivity for deleted in colorectal cancer (DCC), the receptor for the guidance factor netrin-1, in ventral tier dopamine projection pathways in adult rodents. Neuroscience. 2005;131:671–681. doi: 10.1016/j.neuroscience.2004.11.043. [DOI] [PubMed] [Google Scholar]
- 20.Manitt C, Eng C, Pokinko M, Ryan RT, Torres-Berrío A, Lopez JP, et al. dcc orchestrates the development of the prefrontal cortex during adolescence and is altered in psychiatric patients. Transl Psychiatry. 2013;3:e338. doi: 10.1038/tp.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Young JW, Winstanley CA, Brady AM, Hall FS. Research Domain Criteria versus DSM V: How does this debate affect attempts to model corticostriatal dysfunction in animals? Neurosci Biobehav Rev. 2016:1–63. doi: 10.1016/j.neubiorev.2016.10.029. [DOI] [PubMed] [Google Scholar]
- 22.Clementz BA, Sweeney JA, Hamm JP, Ivleva EI, Ethridge LE, Pearlson GD, et al. Identification of Distinct Psychosis Biotypes Using Brain-Based Biomarkers. Am J Psychiatry. 2016;173:373–384. doi: 10.1176/appi.ajp.2015.14091200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krimpenfort P, Song J-Y, Proost N, Zevenhoven J, Jonkers J, Berns A. Deleted in colorectal carcinoma suppresses metastasis in p53-deficient mammary tumours. Nature. 2012;482:538–541. doi: 10.1038/nature10790. [DOI] [PubMed] [Google Scholar]
- 24.Beier KT, Steinberg EE, DeLoach KE, Xie S, Miyamichi K, Schwarz L, et al. Circuit Architecture of VTA Dopamine Neurons Revealed by Systematic Input-Output Mapping. Cell. 2015;162:622–634. doi: 10.1016/j.cell.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bru T, Salinas S, Kremer EJ. An update on canine adenovirus type 2 and its vectors. Viruses. 2010;2:2134–2153. doi: 10.3390/v2092134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Junyent F, Kremer EJ. CAV-2-why a canine virus is a neurobiologist's best friend. Curr Opin Pharmacol. 2015;24:86–93. doi: 10.1016/j.coph.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 27.Daubaras M, Dal Bo G, Flores C. Target-dependent expression of the netrin-1 receptor, UNC5C, in projection neurons of the ventral tegmental area. Neuroscience. 2013 doi: 10.1016/j.neuroscience.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 28.Reynolds LM, Makowski CS, Yogendran SV, Kiessling S, Cermakian N, Flores C. Amphetamine in Adolescence Disrupts the Development of Medial Prefrontal Cortex Dopamine Connectivity in a dcc-Dependent Manner. Neuropsychopharmacology. 2015;40:1101–1112. doi: 10.1038/npp.2014.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. Academic Press; 2008. [Google Scholar]
- 30.Grant A, Hoops D, Labelle-Dumais C, Prévost M, Rajabi H, Kolb B, et al. Netrin-1 receptor-deficient mice show enhanced mesocortical dopamine transmission and blunted behavioural responses to amphetamine. Eur J Neurosci. 2007;26:3215–3228. doi: 10.1111/j.1460-9568.2007.05888.x. [DOI] [PubMed] [Google Scholar]
- 31.Loos M, Staal J, Schoffelmeer ANM, Smit AB, Spijker S, Pattij T. Inhibitory control and response latency differences between C57BL/6J and DBA/2J mice in a Go/No-Go and 5-choice serial reaction time task and strain-specific responsivity to amphetamine. Behav Brain Res. 2010;214:216–224. doi: 10.1016/j.bbr.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 32.Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marshak S, Nikolakopoulou AM, Dirks R, Martens GJ, Cohen-Cory S. Cell-autonomous TrkB signaling in presynaptic retinal ganglion cells mediates axon arbor growth and synapse maturation during the establishment of retinotectal synaptic connectivity. J Neurosci. 2007;27:2444–2456. doi: 10.1523/JNEUROSCI.4434-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lom B, Cohen-Cory S. Brain-derived neurotrophic factor differentially regulates retinal ganglion cell dendritic and axonal arborization in vivo. J Neurosci. 1999;19:9928–9938. doi: 10.1523/JNEUROSCI.19-22-09928.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kolk SM, Gunput R-AF, Tran TS, Van den Heuvel DMA, Prasad AA, Hellemons AJCGM, et al. Semaphorin 3F is a bifunctional guidance cue for dopaminergic axons and controls their fasciculation, channeling, rostral growth, and intracortical targeting. J Neurosci. 2009;29:12542–12557. doi: 10.1523/JNEUROSCI.2521-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Voorn P, Kalsbeek A, Jorritsma-Byham B, Groenewegen HJ. The pre- and postnatal development of the dopaminergic cell groups in the ventral mesencephalon and the dopaminergic innervation of the striatum of the rat. Neuroscience. 1988;25:857–887. doi: 10.1016/0306-4522(88)90041-3. [DOI] [PubMed] [Google Scholar]
- 37.Antonopoulos J, Dori I, Dinopoulos A, Chiotelli M, Parnavelas JG. Postnatal development of the dopaminergic system of the striatum in the rat. Neuroscience. 2002;110:245–256. doi: 10.1016/s0306-4522(01)00575-9. [DOI] [PubMed] [Google Scholar]
- 38.Tepper JM, Sharpe NA, Koós TZ, Trent F. Postnatal development of the rat neostriatum: electrophysiological, light- and electron-microscopic studies. Dev Neurosci. 1998;20:125–145. doi: 10.1159/000017308. [DOI] [PubMed] [Google Scholar]
- 39.Brummelte S, Teuchert-Noodt G. Postnatal development of dopamine innervation in the amygdala and the entorhinal cortex of the gerbil (Meriones unguiculatus) Brain Res. 2006;1125:9–16. doi: 10.1016/j.brainres.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 40.Antonopoulos J, Dinopoulos A, Dori I, Parnavelas JG. Distribution and synaptology of dopaminergic fibers in the mature and developing lateral septum of the rat. Brain Res Dev Brain Res. 1997;102:135–141. doi: 10.1016/s0165-3806(97)00088-6. [DOI] [PubMed] [Google Scholar]
- 41.Loughlin SE, Fallon JH. Substantia nigra and ventral tegmental area projections to cortex: Topography and collateralization. Neuroscience. 1984;11:425–435. doi: 10.1016/0306-4522(84)90034-4. [DOI] [PubMed] [Google Scholar]
- 42.Aransay A, Rodríguez-López C, García-Amado M, Clascá F, Prensa L. Long-range projection neurons of the mouse ventral tegmental area: a single-cell axon tracing analysis. Front Neuroanat. 2015;9:59. doi: 10.3389/fnana.2015.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Björklund A, Dunnett SB. Dopamine neuron systems in the brain: an update. Trends Neurosci. 2007;30:194–202. doi: 10.1016/j.tins.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 44.Yetnikoff L, Lavezzi HN, Reichard RA, Zahm DS. An update on the connections of the ventral mesencephalic dopaminergic complex. Neuroscience. 2014;282:23–48. doi: 10.1016/j.neuroscience.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lammel S, Hetzel A, Häckel O, Jones I, Liss B, Roeper J. Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron. 2008;57:760–773. doi: 10.1016/j.neuron.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 46.Taylor SR, Badurek S, Dileone RJ, Nashmi R, Minichiello L, Picciotto MR. GABAergic and glutamatergic efferents of the mouse ventral tegmental area. J Comp Neurol. 2014;522:3308–3334. doi: 10.1002/cne.23603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dent EW, Barnes AM, Tang F, Kalil K. Netrin-1 and semaphorin 3A promote or inhibit cortical axon branching, respectively, by reorganization of the cytoskeleton. J Neurosci. 2004;24:3002–3012. doi: 10.1523/JNEUROSCI.4963-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Manitt C, Nikolakopoulou AM, Almario DR, Nguyen SA, Cohen-Cory S. Netrin participates in the development of retinotectal synaptic connectivity by modulating axon arborization and synapse formation in the developing brain. J Neurosci. 2009;29:11065–11077. doi: 10.1523/JNEUROSCI.0947-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Séguéla P, Watkins KC, Descarries L. Ultrastructural features of dopamine axon terminals in the anteromedial and the suprarhinal cortex of adult rat. Brain Res. 1988;442:11–22. doi: 10.1016/0006-8993(88)91427-8. [DOI] [PubMed] [Google Scholar]
- 50.Koss WA, Belden CE, Hristov AD, Juraska JM. Dendritic remodeling in the adolescent medial prefrontal cortex and the basolateral amygdala of male and female rats. Synapse. 2014;68:61–72. doi: 10.1002/syn.21716. [DOI] [PubMed] [Google Scholar]
- 51.Bagot RC, van Hasselt FN, Champagne DL, Meaney MJ, Krugers HJ, Joëls M. Maternal care determines rapid effects of stress mediators on synaptic plasticity in adult rat hippocampal dentate gyrus. Neurobiol Learn Mem. 2009;92:292–300. doi: 10.1016/j.nlm.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 52.Kolb B, Mychasiuk R, Muhammad A, Li Y, Frost DO, Gibb R. Experience and the developing prefrontal cortex. Proc Natl Acad Sci USA. 2012;109(Suppl 2):17186–17193. doi: 10.1073/pnas.1121251109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McTeague LM, Goodkind MS, Etkin A. Transdiagnostic impairment of cognitive control in mental illness. J Psychiatr Res. 2016;83:37–46. doi: 10.1016/j.jpsychires.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Van den Heuvel DMA, Pasterkamp RJ. Getting connected in the dopamine system. Prog Neurobiol. 2008;85:75–93. doi: 10.1016/j.pneurobio.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 57.Smidt MP, Burbach JPH. How to make a mesodiencephalic dopaminergic neuron. Nat Rev Neurosci. 2007;8:21–32. doi: 10.1038/nrn2039. [DOI] [PubMed] [Google Scholar]
- 58.Prestoz L, Jaber M, Gaillard A. Dopaminergic axon guidance: which makes what? Front Cell Neurosci. 2012;6:32. doi: 10.3389/fncel.2012.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Riddle R, Pollock JD. Making connections: the development of mesencephalic dopaminergic neurons. Brain Res Dev Brain Res. 2003;147:3–21. doi: 10.1016/j.devbrainres.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 60.Deiner MS, Kennedy TE, Fazeli A, Serafini T, Tessier-Lavigne M, Sretavan DW. Netrin-1 and DCC mediate axon guidance locally at the optic disc: loss of function leads to optic nerve hypoplasia. Neuron. 1997;19:575–589. doi: 10.1016/s0896-6273(00)80373-6. [DOI] [PubMed] [Google Scholar]
- 61.Timofeev K, Joly W, Hadjieconomou D, Salecker I. Localized netrins act as positional cues to control layer-specific targeting of photoreceptor axons in Drosophila. Neuron. 2012;75:80–93. doi: 10.1016/j.neuron.2012.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Winberg ML, Mitchell KJ, Goodman CS. Genetic analysis of the mechanisms controlling target selection: complementary and combinatorial functions of netrins, semaphorins, and IgCAMs. Cell. 1998;93:581–591. doi: 10.1016/s0092-8674(00)81187-3. [DOI] [PubMed] [Google Scholar]
- 63.Verney C. Distribution of the catecholaminergic neurons in the central nervous system of human embryos and fetuses. Microsc Res Tech. 1999;46:24–47. doi: 10.1002/(SICI)1097-0029(19990701)46:1<24::AID-JEMT3>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 64.Deck M, Lokmane L, Chauvet S, Mailhes C, Keita M, Niquille M, et al. Pathfinding of corticothalamic axons relies on a rendezvous with thalamic projections. Neuron. 2013;77:472–484. doi: 10.1016/j.neuron.2012.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pokinko M, Moquin L, Torres-Berrío A, Gratton A, Flores C. Resilience to amphetamine in mouse models of netrin-1 haploinsufficiency: role of mesocortical dopamine. Psychopharmacology (Berl) 2015 doi: 10.1007/s00213-015-4032-9. [DOI] [PubMed] [Google Scholar]
- 66.Catts VS, Fung SJ, Long LE, Joshi D, Vercammen A, Allen KM, et al. Rethinking schizophrenia in the context of normal neurodevelopment. Front Cell Neurosci. 2013;7:60. doi: 10.3389/fncel.2013.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 68.Paus T. Mapping brain maturation and cognitive development during adolescence. Trends Cogn Sci (Regul Ed) 2005;9:60–68. doi: 10.1016/j.tics.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 69.Casey BJ, Jones RM, Hare TA. The adolescent brain. Ann N Y Acad Sci. 2008;1124:111–126. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ordaz SJ, Foran W, Velanova K, Luna B. Longitudinal Growth Curves of Brain Function Underlying Inhibitory Control through Adolescence. J Neurosci. 2013;33:18109–18124. doi: 10.1523/JNEUROSCI.1741-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Casey BJ, Trainor RJ, Orendi JL, Schubert AB, Nystrom LE, Giedd JN, et al. A Developmental Functional MRI Study of Prefrontal Activation during Performance of a Go-No-Go Task. J Cogn Neurosci. 1997;9:835–847. doi: 10.1162/jocn.1997.9.6.835. [DOI] [PubMed] [Google Scholar]
- 72.Luna B. Developmental changes in cognitive control through adolescence. Adv Child Dev Behav. 2009;37:233–278. doi: 10.1016/s0065-2407(09)03706-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Luna B, Padmanabhan A, O'Hearn K. What has fMRI told us about the development of cognitive control through adolescence? Brain Cogn. 2010;72:101–113. doi: 10.1016/j.bandc.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Millan MJ, Agid Y, Brüne M, Bullmore ET, Carter CS, Clayton NS, et al. Cognitive dysfunction in psychiatric disorders: characteristics, causes and the quest for improved therapy. Nat Rev Drug Discov. 2012;11:141–168. doi: 10.1038/nrd3628. [DOI] [PubMed] [Google Scholar]
- 75.Yetnikoff L, Almey A, Arvanitogiannis A, Flores C. Abolition of the behavioral phenotype of adult netrin-1 receptor deficient mice by exposure to amphetamine during the juvenile period. Psychopharmacology (Berl) 2011;217:505–514. doi: 10.1007/s00213-011-2312-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.