Abstract
Background
Epilepsy and ADHD are strongly associated, however, the underlying factors contributing to their co-occurrence remain unclear. A shared genetic liability has been proposed as one possible mechanism. Our goal was therefore to investigate the familial co-aggregation of epilepsy and ADHD, and to estimate the contribution of genetic and environmental risk factors to their co-occurrence.
Methods
We identified 1 899 654 individuals born 1987–2006 via national Swedish registers and linked each individual to their biological relatives. We used logistic regression to estimate the association between epilepsy and ADHD, within-individual and across relatives. Quantitative genetic modelling was used to decompose the cross-disorder covariance into genetic and environmental factors.
Results
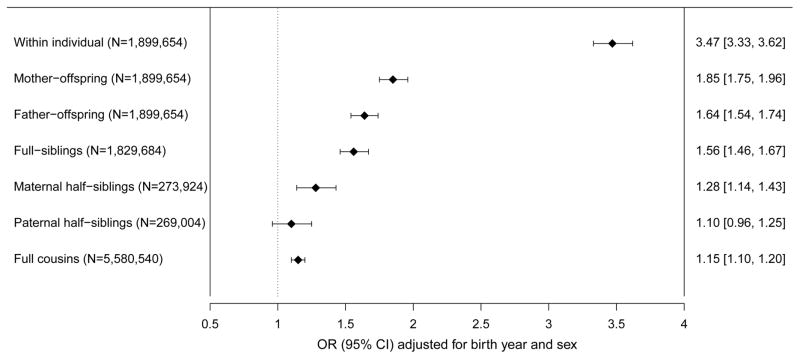
Individuals with epilepsy had a statistically significant increased risk of ADHD (OR=3·47, 95%CI=3·33-3·62). This risk increase extended to children whose mothers had epilepsy (OR=1·85, 95%CI=1·75-1·96), children whose fathers had epilepsy (OR=1·64, 95%CI=1·54-1·74), full-siblings (OR=1·56, 95%CI=1·46-1·67), maternal half-siblings (OR=1·28, 95%CI=1·14-1·43), paternal half-siblings (OR=1·10, 95%CI=0·96-1·25), and cousins (OR=1·15, 95%CI=1·10-1·20). The genetic correlation was 0·21 (95%CI=0·02-0·40) and explained 40% the phenotypic correlation between epilepsy and ADHD, with remaining variance largely explained by non-shared environmental factors (49%, rE 0·36, 95%CI=0·23-0·49). The contribution of shared environmental factors to the cross-disorder overlap was not statically significant (11%, rC 0·32, 95%CI=−0·16-0·79).
Conclusions
This study demonstrates a strong and etiologically complex association between epilepsy and ADHD, with shared familial factors and risk factors unique the individual contributing to co-occurrence between the disorders. Our findings suggest that epilepsy may share less genetic risk with ADHD, as compared to other neurodevelopmental disorders.
Keywords: ADHD, epilepsy, genetics, comorbidity, risk factors, neurodevelopment
Introduction
Epilepsy and ADHD are among the most common conditions in pediatric neurology and psychiatry (1, 2). Children with epilepsy have a 3 to 5-fold increased risk of ADHD (3, 4), and 10–30% meet diagnostic criteria for the disorder (1, 3, 5). This makes ADHD one of the most commonly co-occurring neurodevelopmental conditions in epilepsy (3, 6). Conversely, individuals with ADHD have a reported 4-fold increased risk of epilepsy (7). Despite the strong association, the underlying factors contributing to their co-occurrence are not well understood.
Several mechanisms have been proposed, including adverse effects of antiepileptic drugs and chronic seizures, lowered seizure threshold related to ADHD medications, and an underlying brain dysfunction increasing the risk for both conditions (5, 6). Although some antiepileptic drugs can increase inattention and hyperactivity problems, two studies have shown that ADHD symptoms are often present prior to first epileptic seizure, and in drug naïve patients. This suggests that epilepsy and ADHD are associated independently of the effect of seizures and medications (8, 9). There is evidence for common neurophysiological markers across the disorder. Frontal lobe dysfunction and adrenergic system dysregulation have been implicated in both epilepsy and ADHD (5). Further, subclinical epileptiform discharges are considerably more common in children with ADHD, compared to the general population (5, 10).
In the last years, specific genetic factors have been implicated in a range of clinically distinct neurodevelopmental disorders, including epilepsy and ADHD (11). Such findings, together with evidence of phenotypic and neurophysiological overlap, have led to the hypothesis that both disorders lie on a continuum of neurodevelopmental liability, that is underpinned by common genetic risk factors (11). However, there are few genetically-informative studies addressing the common etiology of epilepsy and ADHD. In a study of 308 epilepsy cases, considerable familial clustering was found for behavioural disorders, although the association with ADHD in first-degree relatives was not statistically significant (12). A pilot study examining the co-morbidity between ADHD and epilepsy found that the prevalence of ADHD in mothers of children with ADHD and epilepsy was elevated (13). Similarly, results from a population-based Norwegian study found that children of mothers with epilepsy had a nearly two-fold increased risk of ADHD as adults (14), suggesting that ADHD symptoms and epilepsy may cluster in families due to genetic or environmental causes passed from the mother to the child. However, familial transmission cannot be disentangled from pregnancy related risk factors using only mother-offspring pairs.
Further research is therefore needed to assess the contribution, and source, of familial liability to the co-occurrence of epilepsy and ADHD. To address this, we used nationwide Swedish data to assess the association between epilepsy and ADHD across multiple types of relatives, and quantitative genetic modelling to estimate the genetic and environmental contributions to their co-occurrence.
Methods and Materials
Study population
The study population was identified using data from nationwide Swedish registers linked via personal identification numbers (15). All individuals born in Sweden between 1987–2006 where identified via the Medical Birth Register (MBR) (16). We obtained demographic information from the Total Population Register (TPR), the Migration Register, the Cause of Death Register, and the Multi-Generation Register (MGR) (17). We excluded individuals with a recorded congenital malformation in the MBR based on the International Classification of Diseases, 9th (ICD-9, 1987–1996) and 10th revision (ICD-10, 1997 onwards) (ICD-9 740–759: ICD-10 Q00-Q99) (18). Individuals who died or migrated prior to age seven, were adopted, or whose biological parents were not identifiable were also excluded, resulting in a study population of 1 899 654 individuals. Each individual was then linked to their mother, father, full-siblings, maternal and paternal half-siblings, and cousins using the MGR, to create six relative sub-cohorts (Table 1) (17). All possible combinations of sibling and cousin pairs were identified, meaning each individual contributed at least once as exposing relative and once as outcome relative. The number of parent-offspring pairs corresponds to the number of individuals in the cohort as we only estimated the association between parental epilepsy and offspring ADHD, due to incomplete coverage for parental ADHD (Table 1) (19).
Table 1.
Number of relatives in each sub-cohort and a summary of the unique information provided by each relative sub-cohort to the familial aggregation analysis
| Type | N Individuals | N unique pairs | N pairs in analyses | Degree of genetic and environmental sharing |
|---|---|---|---|---|
| Mother-offspring | 1 899 654 | 1 899 654 | 1 899 654 |
|
| Father-offspring | 1 899 654 | 1 899 654 | 1 899 654 |
|
| Full-siblings | 1 312 567 | 914 824 | 1 829 684 |
|
| Maternal half-siblings | 193 186 | 136 269 | 273 924 |
|
| Paternal half-sibling | 183 938 | 134 502 | 269 004 |
|
| Full cousins | 1 413 024 | 2 790 270 | 5 580 540 |
|
N of unique pairs refers to the unique number of relative pairs in the cohort. 2N pairs in analyses refer to the number of possible pair combinations that contributed to the analyses.
Epilepsy and ADHD cases
We identified ADHD cases from the Swedish National Patient Register (NPR) (19) and the Swedish Prescribed Drug Register (PDR) (20) until December 31, 2013. Diagnoses in the NPR are coded according to the ICD (18). The PDR includes all medication dispensations in Sweden since mid-2005 (19, 20). ADHD cases were defined as ever having received a discharge diagnosis of hyperkinetic disorder (ICD-9 314, ICD-10 F90) in the NPR, and/or ever having received a prescription of ADHD medication after age three. Approved medications for the treatment of ADHD in Sweden during the study period were methylphenidate, amphetamine, dexamphetamine, lisdexamfetamine and atomoxetine. Epilepsy cases were identified via the NPR and defined as ever having received a discharge diagnosis for any epilepsy (ICD-9 345, ICD-10 G40, G41). Earlier ICD revisions were included to identify epilepsy cases in the parent generation (ICD-7 353, ICD-8 345).
The study was approved by the regional ethics review board in Stockholm, Sweden and all personal information from the registers was anonymized by Statistics Sweden. By Swedish law, there is no requirement for informed consent in register based research.
Statistical Analysis
Familial co-aggregation analyses
We used a cohort design to evaluate the association between epilepsy and ADHD, coded as lifetime diagnoses (0/1). Logistic regression was used to estimate the within-individual association, comparing the risk of ADHD in individuals with epilepsy to the risk in individuals without epilepsy. To estimate the familial co-aggregation, we fitted a logistic regression in each relative sub-cohort, comparing the risk of ADHD in individuals with a relative with epilepsy, to the risk in individuals without a relative with epilepsy. A summary of the unique information provided by each relative sub-cohort is provided in Table 1. Associations are presented as odds ratios (ORs) with 95% confidence intervals. Analyses were adjusted for birth-year and sex. A higher risk of ADHD in relatives of individuals with epilepsy indicates that etiological factors shared among relatives contribute to the co-occurrence of the disorders. Further, differences in the magnitude of the association across different types of relatives can point to the sources (i.e. genetic or environmental) of familial co-aggregation. Parents and their offspring share 50% of their segregating genes. Mothers also provide the in-utero environment, meaning a higher risk in mother-offspring may indicate the importance of pregnancy related factors. Full-siblings share on average 50% of their segregating genes, whereas half-sibling share 25%. In addition, full-siblings and maternal half-siblings tend to share environmental factors to a greater extent than paternal half-siblings, since children more often reside predominantly with mothers after parental separation (21). A higher risk in full-siblings, relative to maternal half-siblings, may therefore suggest that genetic factors are operative, whereas a higher risk in maternal versus paternal half-siblings suggest that shared environmental factors may be of importance.
Sensitivity analyses
Sensitivity analyses were only performed in the full cohort and in full-siblings to ensure sufficient power. First, we used a stricter definition of epilepsy and ADHD to explore whether case definition affected our estimates. We required ≥ two discharge diagnoses for ADHD and epilepsy from the NPR and excluded individuals who only ever had a diagnosis of status epilepticus (ICD-9: 345Q; ICD-10: G41). To assess the validity of using prescription data to define ADHD we also excluded information on ADHD from the PDR. Second, we used a restricted cohort born 1994–1999, excluding individuals who migrated or died during follow-up. This was done to assess potential bias due to differential follow-up time and incomplete coverage in the NPR, which only includes outpatient care from 2001(19). Third, we examined the hypothesis that a familial association between ADHD and epilepsy is better explained by direct effects of one disorder on the other, within an individual. This was done in the full-sibling cohort, firstly by adjusting for epilepsy in the outcome relative, and secondly by adjusting for ADHD in the exposing relative. If the OR’s remain statistically significant after adjustment, the contribution of common familial risk factors to epilepsy and ADHD is further supported. A detailed description of the rationale for these analyses (based on directed acyclic graphs, DAGs) has been provided elsewhere (22, 23). Finally, we stratified the analyses by sex.
Data management was done in SAS version 9.4 (SAS Institute, Inc) and analyses in Stata version 13.1 (StataCorp). A cluster robust sandwich estimator was used to remove distributional assumptions and correct confidence intervals for the non-independence of family data.
Quantitative Genetic Modelling
Quantitative genetic modelling was used to estimate the contribution of genetic and environmental factors to the association between epilepsy and ADHD. Quantitative genetic models capitalizes on the different levels of genetic and environmental sharing between different types of relatives, in order to decompose variance and covariance into additive genetic (A), dominant genetic (D), shared environmental (C), and non-shared environmental factors (E). Bivariate models also provide an estimate of the genetic (rg) and environmental correlations (rC, rE) across disorders, which indicates the degree to which etiological factors are shared between disorders (24). As epilepsy and ADHD were binary, we used a liability threshold framework where diagnoses were assumed to represent an underlying normal distribution of liability (24). We used all possible full-sibling, maternal half-sibling, and paternal half-sibling pairs in our cohort, resulting in a sample of 1,186,306 sibling pairs (Table 3). We calculated phenotypic (i.e. the correlation between epilepsy and ADHD within individual), intra-class (ICC’s; the correlation between the same trait across siblings), and cross-sibling-cross-trait correlations (CSCT; the correlation between trait one in one sibling with trait two in the other sibling) to glean what type of model to fit (ADCE, ACE, or AE). We proceeded to fit a quantitative genetic sibling model based on the following assumptions (25, 26); Additive genetic factors correlate 0·5 for full-siblings and 0·25 for half-siblings. Dominant genetic factors correlate 0·25 for full-siblings and zero for half-siblings. Shared environmental factors correlate at 1 for full-siblings and maternal half-siblings and 0 for paternal half-siblings. Non-shared environmental factors do not correlate across siblings. The model was fitted using the Weighted Least Squares method implemented in OpenMx (27). To obtain valid standard errors when including multiple sibling pairs per family, we used non-parametric bootstrap sampling (28). A bootstrap sample was created by repeatedly drawing (with replacement) families, sampling as many families as in original dataset. For a given bootstrap sample, the estimates from the quantitative genetic model were computed. After repeating this process across 1000 bootstrap samples, standard errors were obtained from the bootstrap estimates and 95% confidence intervals were computed.
Table 3.
Phenotypic, intra-class and cross-trait-cross-sibling correlations between ADHD and epilepsy, presented with 95% confidence intervals
| N pairs | N Concordant Pairs | Phenotypic r | ICC ADHD | ICC EP | CTCS | |
|---|---|---|---|---|---|---|
| Full cohort | 1 186 306 | 946 | 0·24 (0·23-0·25) | na | na | na |
| Full-siblings | 914 842 | 598 | 0·25 (0·24-0·26) | 0·45 (0·44-0·45) | 0·24 (0·22-0·26) | 0·08 (0·06-0·09) |
| Maternal half-siblings | 136 962 | 198 | 0·22 (0·20-0·25) | 0·26 (0·24-0·27) | 0·17 (0·12-0·22) | 0·04 (0·01-0·07) |
| Paternal half-siblings | 134 502 | 150 | 0·22 (0·19-0·24) | 0·19 (0·17-0·20) | 0·07(0·02-0·13) | 0·01(−0·02-0·05) |
Abbreviation: ICC, intra-class correlation. CTCS, cross-trait-cross-sibling correlation. na, not applicable.
N concordant pairs refer to the number of sibling pairs where one sibling had a diagnosis of epilepsy and the other sibling a diagnosis of ADHD. 95% confidence intervals are presented in brackets.
Results
Descriptive statistics
Descriptive statistics for the full cohort are presented in Table 2. The prevalence at the end of follow-up was 1·1% for epilepsy and 4·3% for ADHD (Table 2). In comparison, 13·5% of those with epilepsy also had a diagnosis of ADHD, and 3·4% of those with ADHD had a diagnosis of epilepsy.
Table 2.
Prevalence of epilepsy and ADHD, age of first diagnosis and % female in full cohort (N= 1,899,654) born 1987–2006
| N Cases Min 1 Diagnosis | N Cases >= 2 Diagnoses | Mean age first diagnosis (SD) | % Female | |
|---|---|---|---|---|
| Epilepsy | 20 625 (1·09%) | 15 634 (0·82%) | 9·29 (6·23) | 47·24% |
| ADHD | 82 399 (4·34%) | 51 239 (2·70%) | 13·72 (4·85) | 33·54% |
| Comorbid cases | 2789 (0·15%) | 1463 (0·08%) | 8·88 (5·94)/12·10 (5·01) | 35·14% |
Abbreviation: SD, standard deviation.
Prevalence refers to the number of diagnosed individuals in the cohort at the end of follow-up (December 31st, 2013). 2N Cases >= 2 only includes cases identified via the National Patients Register. In this definition of epilepsy, codes ICD-9, 345Q and ICD-10, G41 (Status Epilepticus) were excluded. 3In comorbid cases, age at first diagnosis is presented first for epilepsy and second for ADHD.
Within-individual and familial co-aggregation analyses
Individuals with epilepsy had a statistically significant increased risk of ADHD (OR=3.47, 95%CI 3.33–3.62), compared to individuals without epilepsy (Figure 1). Further, individuals with a relative with epilepsy also had a statistically significant increased risk of ADHD (Figure 1). The risk of ADHD in children whose mothers had epilepsy was elevated (OR=1·85, 95%CI=1·75-1·96), compared to the risk of ADHD in children whose mothers did not have epilepsy. The risk was also statistically significantly higher (p=0·004) than the risk in children whose fathers had epilepsy (OR=1·64, 95%CI=1·54-1·74). Individuals with a full-sibling with epilepsy also had an increased risk of ADHD (OR=1·56, 95%CI=1·46-1·67), which was statistically significantly higher (p=0·002) than the risk in maternal half-siblings (OR=1·28, 95%CI=1·14-1·43). The risk was attenuated, but remained statistically significant in cousins (OR=1·15, 95%CI=1·10-1·20). We did not observe a statistically significant risk increase in paternal half-sibling (OR=1·10, 95%CI=0·96-1·25).
Figure 1.
Within-individual and family level co-aggregation of ADHD and epilepsy.
Odds ratios (OR) represent the association between ADHD and epilepsy, within-individual, and across different types of relatives. ORs are adjusted for birth year and sex and presented together with 95% confidence intervals.
Sensitivity analyses
Results from the sensitivity analyses are presented in Supplementary Table S1. Estimates using a stricter definition of epilepsy and ADHD (Model 1; S1) were similar to results from the main analyses, suggesting that our definition (>1 or >2 diagnoses) and the use of prescription data from the PDR did not affect the results. Risk estimates obtained in the restricted cohort born 1994–1999, excluding all deaths and migrations, were somewhat attenuated for the within-individual association (OR=3·11 in restricted cohort vs OR=3·47 in full cohort), whereas CI’s for the OR in full-siblings were almost completely overlapping with those from the main analyses (Model 2; S1). This suggests that censoring and incomplete follow-up may have slightly biased the estimated within-individual association, but had limited impact on the estimated familial association. Adjusting for the exposing condition (epilepsy) in the outcome relative attenuated the OR in full-siblings, as did adjusting for the outcome condition (ADHD) in the exposing relative (Model 3&4; S1). However, the estimates remained positive and statistically significant, providing further support for the presence of common familial risk factors for epilepsy and ADHD (22, 23, 29). Estimates stratified by sex were not statistically significantly different (Supplementary Table S2).
Quantitative Genetic Modelling
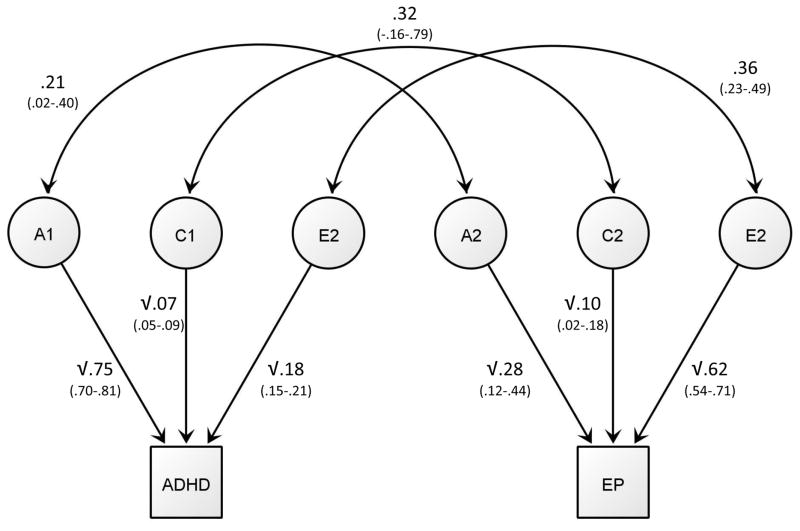
The phenotypic correlation between epilepsy and ADHD was 0·24 (95%CI=0·23-0·25). ICC’s and CSCT’s were higher in full-siblings than in maternal half-siblings, indicating the contribution of genetic factors to variance and covariance (Table 3). Further, all correlations were somewhat higher in maternal than paternal half-siblings, indicating a possible contribution of shared environmental factors. As there was no indication of dominance genetic effects, indexed by maternal half-sibling correlations being less than half of the full-sibling correlations, we fitted a bivariate ACE model (Figure 2). Genetic and shared environmental factors together explained 51% of the phenotypic correlation between epilepsy and ADHD (additive genetic contribution 40%, 95%CI=9–70%; shared environmental contribution 11%, 95%CI=−3–25%), with the remaining overlap (49%, 95%CI=32–67%) attributable to non-shared environmental factors. Although the genetic correlation was statistically significant (p = 0.03), confidence intervals were wide (rG=0·21, 95%CI=0·02-0·40). The shared environmental correlation was not statistically significant (p=0.20) as confidence intervals crossed zero (rC=0·32, 95%CI=−0·16-0·79). The non-shared environmental correlation (rE) was estimated to 0·36 (95%CI=0·23-0·49). Parameter estimates are presented in Figure 2, and full results from the ACE model are presented in Supplementary Table S3.
Figure 2.
Path estimates for the bivariate ACE model presented for one sibling only
Rectangles represent the liability for ADHD and epilepsy and are fixed at a variance of 1. Circles represent latent additive genetic (A), shared environmental (C) and non-shared environmental (E) factors. Each latent variable has a variance of 1. Values within the square root sign are the squared path estimates and represent the percentage of variance accounted for by the A, C and E parameter for each trait. Curved double-headed arrows refer to the correlations between A (rG), C (rC) and E (rE) across ADHD and epilepsy. 95% confidence intervals are presented in parentheses.
Discussion
In this nationwide family-study, individuals with epilepsy had a 3.5-fold increased risk of ADHD, compared to individuals without epilepsy, which is in line with previous studies (3, 4, 7). We also show that relatives of individuals with epilepsy have a statistically significant increased risk of ADHD, suggesting that a shared familial liability contributes to the co-occurrence between epilepsy and ADHD.
Based on the pattern of risk across relatives, we found support for both environmental and genetic factors contributing to a shared familial liability. Maternal half-siblings had a somewhat higher risk of ADHD than paternal half-siblings, indicating that shared environmental factors may be operative. This is because both types of half-siblings share 25% of additive genetic factors, but maternal half-siblings tend to share more environmental factors (21). We also found that the risk of ADHD was higher in full-siblings compared to maternal half-siblings, suggesting that at least part of the familial liability is genetic in origin.
When estimating the genetic overlap across epilepsy and ADHD using quantitative genetic modeling, our results revealed a modest genetic correlation between the disorders. Shared genetic factors explained about 40% of the phenotypic cross-disorder correlation. A similar effect remains to be identified in molecular genetic data. In the largest study to date, including 7,779 epilepsy and 12,645 ADHD cases, result showed no statistically significant genetic cross-disorder correlation based on common genetic variants (30). Nevertheless, rare variants, including de novo mutations which are not inherited, may play a role for the within-individual association between epilepsy and ADHD (11, 31). The genetic correlation across epilepsy and ADHD in the current study was also lower than correlations previously reported in twin studies of ADHD and other neurodevelopment conditions, including autism spectrum disorder (ASD) and developmental coordination disorder, which range from 0·50-0·90 (32–34). Further, our results also differ from estimates in two studies investigating the familial aggregation between ASD, intellectual disabilities and ADHD using similar cohorts and analytic approaches as the current study (26, 35). Both studies showed strong evidence for a genetic contribution to the cross-disorder familial aggregation; quantitative genetic analysis of the overlap between ADHD and intellectual disabilities indicated that 91% of phenotypic cross-disorder correlation could be attributed to genetic factors (26). This suggests that the modest genetic correlation between epilepsy and ADHD observed in our study is not merely due to methodological differences across studies. Rather, our results suggest that epilepsy may have a weaker genetic sharing with ADHD and the broader neurodevelopmental continuum, as compared to traditional neurodevelopment disorder such as ASD and ID (11).
Half of the phenotypic association between epilepsy and ADHD was attributed to factors unique to the individual. Pregnancy-related complications and pre-term birth have been associated with both epilepsy and ADHD in a potentially causal way (14, 36, 37). In the extent that such pre- and perinatal risk factors are not due to maternal effects consistent across pregnancies, they could potentially contribute to the strong non-shared environmental correlation we observed between epilepsy and ADHD. Another possible explanation is that part of the within-individual association is driven by one disorder increasing the risk of the other disorder. Nearly 75% of comorbid cases in our cohort were diagnosed with epilepsy prior to ADHD. This could indicate that epilepsy related factors may exacerbate ADHD symptoms, increasing the risk of a subsequent ADHD diagnosis. Indeed, previous research suggests that adverse effects of antiepileptic drugs and chronic seizures can lead to attention and hyperactivity problems (6). However, based on register data alone we cannot determine to what extent receiving an epilepsy diagnosis prior to an ADHD diagnosis reflects the actual temporal order of diagnoses and to what extent it reflects clinical practices in Sweden. Further, the mean age for first ADHD diagnosis (14 years) was relatively late in our cohort and is therefore unlikely to represent the onset of ADHD symptoms.
Finally, we found that children of mothers with epilepsy had a nearly two-fold risk increase of ADHD, compared to children of mothers who did not have epilepsy. This is consistent with previous research (14). Further, the risk of ADHD in children of mothers with epilepsy was somewhat higher than the risk in children of fathers with epilepsy. We also found a higher risk of ADHD in full-siblings and maternal half-siblings, relative to the risk in paternal half-siblings. These findings converge with evidence from familial aggregation studies of epilepsy, which have consistently reported a higher risk in children of mothers with epilepsy relative to children of fathers with epilepsy (38). Our results indicate that this maternal effect may not be specific to epilepsy, as re-running the analysis in the mother-child and father-child sub-cohorts, adjusting for epilepsy in the child, did not change the patter of higher risk in mother-offspring pairs (OR=1·77, 95%CI=1·67-1·88) compared to father-offspring pairs (OR=1·60, 95%CI=1·50-1·70). Maternal specific transmission could have several, not mutually exclusive, interpretations. Exposure to epilepsy related factors during pregnancy may further increase the risk of ADHD, over and above familial transmission. Intrauterine exposure to certain antiepileptic drugs has been associated with an increased risk of adverse neurodevelopmental outcomes, whereas evidence for a similar risk linked to seizures during pregnancy is less clear (39). Results have also been interpreted as suggestive of maternal-specific genetic transmission, however there is currently only limited evidence for such a parent-of-origin effect (40).
Limitations
Our results must be interpreted in the context of the study limitations. We relied on register-based diagnoses of epilepsy and ADHD and did not have access to structured interviews to diagnose ADHD or neurological assessments to confirm epilepsy. Nonetheless, prior research supports the validity of register based epilepsy and ADHD diagnoses. Two previous studies have shown that majority of individuals with epilepsy in Sweden are included in the NPR (41, 42). Further, the validity of epilepsy diagnosis in the Swedish Inpatient Register (a large part of the NPR) is fairly high, with positive predictive validity estimated at 79% (42). ADHD diagnosis in the Swedish NPR have been validated against childhood psychiatric symptoms assessed in 19,150 twins, showing that 70% of children with a NPR ADHD diagnosis meet screening-cut off for ADHD based on parent ratings (43). Chart validation studies in the Danish Psychiatric Central Register, a country with similar national health-care registers to Sweden, have shown strong agreement between ADHD register diagnosis and medical chart records (44, 45). Further, research using information on ADHD from the Swedish NPR and PDR show similar heritability (46), psychiatry comorbidity (35, 43, 44, 46–52) and adverse functional outcomes (49) as research using structured interviews to diagnose ADHD (50). Together, this suggests that our definitions ADHD and epilepsy are reasonably accurate and valid. Nevertheless, the use of register-based diagnoses has limitations. Firstly, we were not able to study the association between different ADHD subtypes (inattentive, hyperactive/impulsive and combined) and different types of epilepsy; partly because the ICD definition of hyperkinetic disorder only recognizes ADHD combined subtype, and partly due to insufficient power and discriminative validity to study different types of epilepsy (53). We can therefore not rule out that the strength of association, and the underlying etiology, may differ across disorder subtypes. Second, we were not able to study the bidirectional relationship between parental ADHD and offspring epilepsy due to poor coverage of ADHD diagnosis in the NPR prior to 2001, when the outpatient register where most ADHD cases are recorded was included (19). Third, we did not account for differential follow-up time, censoring due to death or migration after age seven, or possible misclassification due to incomplete cover of ADHD diagnoses in the NPR prior to 2001. Nonetheless, sensitivity analyses suggested that the risk estimates did not differ substantially in a restricted birth cohort with a minimum of 14 years of follow-up time and no censoring (S1). Some ADHD cases may also have been misclassified when defining ADHD from prescription data as certain ADHD medications are also approved for the treatment of narcolepsy. Nonetheless, narcolepsy is a very rare disorder and results from sensitivity analyses excluding cases identified only in the PDR did not differ markedly from those including information from the PDR. Fourth, we cannot exclude that detection bias may have artificially inflated the risk estimates. Patients with epilepsy, and their family members, are more likely to be in contact with clinical services than the general public, thus increasing the opportunity to detect and get help for ADHD, if present. However, the increased risk among cousins, who do not share parents and rarely live together, suggests that the observed familial risk is unlikely to be entirely explained by detection bias. Finally, despite the large sample size and the indication of shared environmental effects based on the observed ICC’s and CSCT’s (see Table 3), the estimated shared environmental correlation was not statistically significant. Quantitative genetic models using binary variables require very large samples sizes, particularly when modelling disorders with low prevalence and associations of small effect (24). It is therefore possible that shared environmental risk factors do contribute to the familial aggregation of epilepsy and ADHD, however we did not have the power to detect such an effect.
Conclusions
We demonstrate a strong association between epilepsy and ADHD and extend previous research by showing that the association is partly explained by a shared familial liability. Our results indicate a complex relationship between epilepsy and ADHD, where both shared familial factors and risk factors unique the individual contribute to the co-occurrence between the disorders. We found only a modest genetic correlation between the disorders, indicating that shared genetics is not the primary factor underpinning the co-occurrence of epilepsy and ADHD. Future research aimed at identifying genetic risk variants shared across epilepsy and ADHD will likely require very large sample sizes. Further, the strong contribution of unique environmental factors to the cross-disorder association suggest that research focused on environmental risk factors, including direct effects of one disorder on the other, may prove more effective. For clinicians, evidence of familial co-aggregation suggest that ADHD should not merely be regarded an as epiphenomenon of epilepsy and may aid early identification of children at risk of both disorders.
Supplementary Material
Supplementary Table S1. Sensitivity analyses of the within-individual and full-sibling association between epilepsy and ADHD
Supplementary Table S2. Odds ratios (ORs) and 95% confidence interval (CI) from sensitivity analyses stratified by sex
Supplementary Table S3. Genetic and environmental parameter estimates with Bootstrap standard errors and 95% confidence interval
Acknowledgments
We acknowledge financial support from the Swedish Research Council (2013-2280; 2014-3831) and through the Swedish Initiative for Research on Microdata in the Social And Medical Sciences (SIMSAM) framework Grant no. 340-2013-5867, and through the National Institute of Mental Health (NIMH) (Grant No. 1R01MH102221). The funding sources had no involvement in any part of study, or in the decision to submit the paper for publication. Preliminary findings from this work were presented at the 2016 EUNETHYDIS International Conference, in Berlin, Germany.
Footnotes
Financial disclosures
Dr Larsson has served as a speaker for Eli-Lilly and Shire and has received research grants from Shire; all outside the submitted work. Dr. Dunn reports grants from Eli Lilly and personal fees from US Department of Defense; all outside the submitted work. Dr. D’Onofrio reports grants from National Institute of Mental Health, grants from Swedish Initiative for Research on Microdata in the Social and Medical Sciences, during the conduct of the study; grants from National Science Foundation, grants from Indiana Clinical and Translational Sciences Institute: Pediatric Project Development Team, grants from National Institute on Drug Abuse, grants from American Foundation for Suicide Prevention; all outside the submitted work. All other authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Russ SA, Larson K, Halfon N. A national profile of childhood epilepsy and seizure disorder. Pediatrics. 2012;129:256–264. doi: 10.1542/peds.2010-1371. [DOI] [PubMed] [Google Scholar]
- 2.Polanczyk GV, Salum GA, Sugaya LS, Caye A, Rohde LA. Annual research review: A meta-analysis of the worldwide prevalence of mental disorders in children and adolescents. J Child Psychol Psychiatry. 2015;56:345–365. doi: 10.1111/jcpp.12381. [DOI] [PubMed] [Google Scholar]
- 3.Aaberg KM, Bakken IJ, Lossius MI, Lund Soraas C, Haberg SE, Stoltenberg C, et al. Comorbidity and Childhood Epilepsy: A Nationwide Registry Study. Pediatrics. 2016:138. doi: 10.1542/peds.2016-0921. [DOI] [PubMed] [Google Scholar]
- 4.Bertelsen EN, Larsen JT, Petersen L, Christensen J, Dalsgaard S. Childhood Epilepsy, Febrile Seizures, and Subsequent Risk of ADHD. Pediatrics. 2016:138. doi: 10.1542/peds.2015-4654. [DOI] [PubMed] [Google Scholar]
- 5.Hamoda HM, Guild DJ, Gumlak S, Travers BH, Gonzalez-Heydrich J. Association between attention-deficit/hyperactivity disorder and epilepsy in pediatric populations. Expert Rev Neurother. 2009;9:1747–1754. doi: 10.1586/ern.09.128. [DOI] [PubMed] [Google Scholar]
- 6.Williams AE, Giust JM, Kronenberger WG, Dunn DW. Epilepsy and attention-deficit hyperactivity disorder: links, risks, and challenges. Neuropsychiatr Dis Treat. 2016;12:287–296. doi: 10.2147/NDT.S81549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chou IC, Chang YT, Chin ZN, Muo CH, Sung FC, Kuo HT, et al. Correlation between epilepsy and attention deficit hyperactivity disorder: a population-based cohort study. PLoS One. 2013;8:e57926. doi: 10.1371/journal.pone.0057926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hesdorffer DC, Ludvigsson P, Olafsson E, Gudmundsson G, Kjartansson O, Hauser WA. ADHD as a risk factor for incident unprovoked seizures and epilepsy in children. Arch Gen Psychiatry. 2004;61:731–736. doi: 10.1001/archpsyc.61.7.731. [DOI] [PubMed] [Google Scholar]
- 9.Hermann B, Jones J, Dabbs K, Allen CA, Sheth R, Fine J, et al. The frequency, complications and aetiology of ADHD in new onset paediatric epilepsy. Brain. 2007;130:3135–3148. doi: 10.1093/brain/awm227. [DOI] [PubMed] [Google Scholar]
- 10.Kanazawa O. Reappraisal of abnormal EEG findings in children with ADHD: On the relationship between ADHD and epileptiform discharges. Epilepsy Behav. 2014;41:251–256. doi: 10.1016/j.yebeh.2014.09.078. [DOI] [PubMed] [Google Scholar]
- 11.Moreno-De-Luca A, Myers SM, Challman TD, Moreno-De-Luca D, Evans DW, Ledbetter DH. Developmental brain dysfunction: revival and expansion of old concepts based on new genetic evidence. The Lancet Neurology. 2013;12:406–414. doi: 10.1016/S1474-4422(13)70011-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hesdorffer DC, Caplan R, Berg AT. Familial clustering of epilepsy and behavioral disorders: evidence for a shared genetic basis. Epilepsia. 2012;53:301–307. doi: 10.1111/j.1528-1167.2011.03351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonzalez-Heydrich J, Hamoda HM, Luna L, Rao S, McClendon J, Rotella P, et al. Elevated rates of ADHD in mothers of children with comorbid ADHD and epilepsy. Neuropsychiatry (London) 2012;2:385–391. doi: 10.2217/NPY.12.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halmoy A, Klungsoyr K, Skjaerven R, Haavik J. Pre- and perinatal risk factors in adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2012;71:474–481. doi: 10.1016/j.biopsych.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 15.Ludvigsson JF, Otterblad-Olausson P, Pettersson BU, Ekbom A. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol. 2009;24:659–667. doi: 10.1007/s10654-009-9350-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Board of Health and Welfare CfE. The Swedish Medical Birth Registry. A summary of content and quality. Report 2003-112-3. Stockholm, Sweden: 2003. [Google Scholar]
- 17.Ekbom A. The Swedish Multi-generation Register. Methods Mol Biol. 2011;675:215–220. doi: 10.1007/978-1-59745-423-0_10. [DOI] [PubMed] [Google Scholar]
- 18.The ICD-10 classification of mental and behavioural disorders: Clinical descriptions and diagnostic guidelines. Geneva: World Health Organization; 1992. [Google Scholar]
- 19.Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450. doi: 10.1186/1471-2458-11-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wettermark B, Hammar N, Fored CM, Leimanis A, Otterblad Olausson P, Bergman U, et al. The new Swedish Prescribed Drug Register--opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf. 2007;16:726–735. doi: 10.1002/pds.1294. [DOI] [PubMed] [Google Scholar]
- 21.Statistics Sweden. 2014. Different families live in different ways – a survey on residence and support of children after a separation., 2014-02-21 ed. [Google Scholar]
- 22.Yao S, Kuja-Halkola R, Thornton LM, et al. Familial liability for eating disorders and suicide attempts: Evidence from a population registry in sweden. JAMA Psychiatry. 2016;73:284–291. doi: 10.1001/jamapsychiatry.2015.2737. [DOI] [PubMed] [Google Scholar]
- 23.Chen Q, Kuja-Halkola R, Sjolander A, Serlachius E, Cortese S, Faraone SV, et al. Shared familial risk factors between attention-deficit/hyperactivity disorder and overweight/obesity - a population-based familial coaggregation study in Sweden. J Child Psychol Psychiatry. 2017 doi: 10.1111/jcpp.12686. [DOI] [PubMed] [Google Scholar]
- 24.Neale M, Cardon L. Methodology for genetic studies of twins and families. Springer Science & Business Media; 1992. [Google Scholar]
- 25.Frisell T, Pawitan Y, Langstrom N, Lichtenstein P. Heritability, Assortative Mating and Gender Differences in Violent Crime: Results from a Total Population Sample Using Twin, Adoption, and Sibling Models. Behav Genet. 2012;42:3–18. doi: 10.1007/s10519-011-9483-0. [DOI] [PubMed] [Google Scholar]
- 26.Faraone SV, Ghirardi L, Kuja-Halkola R, Lichtenstein P, Larsson H. The Familial Co-Aggregation of Attention-Deficit/Hyperactivity Disorder and Intellectual Disability: A Register-Based Family Study. J Am Acad Child Adolesc Psychiatry. 2017;56:167–174.e161. doi: 10.1016/j.jaac.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 27.Neale MC, Hunter MD, Pritikin JN, Zahery M, Brick TR, Kirkpatrick RM, et al. OpenMx 2.0: Extended Structural Equation and Statistical Modeling. Psychometrika. 2015:1–15. doi: 10.1007/s11336-014-9435-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Efron B, Tibshirani R. Bootstrap methods for standard errors, confidence intervals, and other measures of statistical accuracy. Statistical science. 1986:54–75. [Google Scholar]
- 29.Hudson JI, Javaras KN, Laird NM, VanderWeele TJ, Pope HG, Jr, Hernan MA. A structural approach to the familial coaggregation of disorders. Epidemiology. 2008;19:431–439. doi: 10.1097/EDE.0b013e31816a9de7. [DOI] [PubMed] [Google Scholar]
- 30.Anttila V, Bulik-Sullivan B, Finucane HK, Bras J, Duncan L, Escott-Price V, et al. Analysis of shared heritability in common disorders of the brain. bioRxiv. 2016 doi: 10.1126/science.aap8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lo-Castro A, Curatolo P. Epilepsy associated with autism and attention deficit hyperactivity disorder: Is there a genetic link? Brain Dev. 2014;36:185–193. doi: 10.1016/j.braindev.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 32.Lichtenstein P, Carlstrom E, Rastam M, Gillberg C, Anckarsater H. The genetics of autism spectrum disorders and related neuropsychiatric disorders in childhood. Am J Psychiatry. 2010;167:1357–1363. doi: 10.1176/appi.ajp.2010.10020223. [DOI] [PubMed] [Google Scholar]
- 33.Reiersen AM, Constantino JN, Grimmer M, Martin NG, Todd RD. Evidence for shared genetic influences on self-reported ADHD and autistic symptoms in young adult Australian twins. Twin Res Hum Genet. 2008;11:579–585. doi: 10.1375/twin.11.6.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ronald A, Simonoff E, Kuntsi J, Asherson P, Plomin R. Evidence for overlapping genetic influences on autistic and ADHD behaviours in a community twin sample. Journal of child psychology and psychiatry, and allied disciplines. 2008;49:535– 542. doi: 10.1111/j.1469-7610.2007.01857.x. [DOI] [PubMed] [Google Scholar]
- 35.Ghirardi L, Brikell I, Kuja-Halkola R, Freitag CM, Franke B, Asherson P, et al. The familial co-aggregation of ASD and ADHD: a register-based cohort study. Mol Psychiatry. 2017 doi: 10.1038/mp.2017.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun Y, Vestergaard M, Pedersen CB, Christensen J, Basso O, Olsen J. Gestational age, birth weight, intrauterine growth, and the risk of epilepsy. Am J Epidemiol. 2008;167:262–270. doi: 10.1093/aje/kwm316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.D’Onofrio BM, Class QA, Rickert ME, Larsson H, Langstrom N, Lichtenstein P. Preterm birth and mortality and morbidity: A population-based quasi-experimental study. JAMA Psychiatry. 2013;70:1231–1240. doi: 10.1001/jamapsychiatry.2013.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peljto AL, Barker-Cummings C, Vasoli VM, Leibson CL, Hauser WA, Buchhalter JR, et al. Familial risk of epilepsy: a population-based study. Brain. 2014;137:795–805. doi: 10.1093/brain/awt368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bromley R. The treatment of epilepsy in pregnancy: The neurodevelopmental risks associated with exposure to antiepileptic drugs. Reprod Toxicol. 2016;64:203–210. doi: 10.1016/j.reprotox.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 40.Pal DK, Durner M, Klotz I, Dicker E, Shinnar S, Resor S, et al. Complex inheritance and parent-of-origin effect in juvenile myoclonic epilepsy. Brain Dev. 2006;28:92–98. doi: 10.1016/j.braindev.2005.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mattsson P, Tomson T, Eriksson O, Brannstrom L, Weitoft GR. Sociodemographic differences in antiepileptic drug prescriptions to adult epilepsy patients. Neurology. 2010;74:295–301. doi: 10.1212/WNL.0b013e3181cbcd5c. [DOI] [PubMed] [Google Scholar]
- 42.Nilsson L, Tomson T, Farahmand BY, Diwan V, Persson PG. Cause-specific mortality in epilepsy: a cohort study of more than 9,000 patients once hospitalized for epilepsy. Epilepsia. 1997;38:1062–1068. doi: 10.1111/j.1528-1157.1997.tb01194.x. [DOI] [PubMed] [Google Scholar]
- 43.Skoglund C, Chen Q, D′Onofrio BM, Lichtenstein P, Larsson H. Familial confounding of the association between maternal smoking during pregnancy and ADHD in offspring. Journal of Child Psychology and Psychiatry. 2014;55:61–68. doi: 10.1111/jcpp.12124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Linnet KM, Wisborg K, Secher NJ, Thomsen PH, Obel C, Dalsgaard S, et al. Coffee consumption during pregnancy and the risk of hyperkinetic disorder and ADHD: a prospective cohort study. Acta Paediatr. 2009;98:173–179. doi: 10.1111/j.1651-2227.2008.00980.x. [DOI] [PubMed] [Google Scholar]
- 45.Mohr-Jensen C, Vinkel Koch S, Briciet Lauritsen M, Steinhausen HC. The validity and reliability of the diagnosis of hyperkinetic disorders in the Danish Psychiatric Central Research Registry. Eur Psychiatry. 2016;35:16–24. doi: 10.1016/j.eurpsy.2016.01.2427. [DOI] [PubMed] [Google Scholar]
- 46.Larsson H, Chang Z, D’Onofrio BM, Lichtenstein P. The heritability of clinically diagnosed attention deficit hyperactivity disorder across the lifespan. Psychol Med. 2014;44:2223–2229. doi: 10.1017/S0033291713002493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Skoglund C, Chen Q, Franck J, Lichtenstein P, Larsson H. Attention-Deficit/Hyperactivity Disorder and Risk for Substance Use Disorders in Relatives. Biol Psychiatry. 2015;77:880–886. doi: 10.1016/j.biopsych.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 48.Larsson H, Ryden E, Boman M, Langstrom N, Lichtenstein P, Landen M. Risk of bipolar disorder and schizophrenia in relatives of people with attention-deficit hyperactivity disorder. Br J Psychiatry. 2013;203:103– 106. doi: 10.1192/bjp.bp.112.120808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chang Z, Lichtenstein P, D’Onofrio BM, Sjolander A, Larsson H. Serious transport accidents in adults with attention-deficit/hyperactivity disorder and the effect of medication: a population-based study. JAMA psychiatry. 2014;71:319–325. doi: 10.1001/jamapsychiatry.2013.4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Faraone SV, Asherson P, Banaschewski T, Biederman J, Buitelaar JK, Ramos-Quiroga JA, et al. Attention-deficit/hyperactivity disorder. Nature Reviews Disease Primers. 2015:15020. doi: 10.1038/nrdp.2015.20. [DOI] [PubMed] [Google Scholar]
- 51.Faraone SV, Biederman J, Wozniak J. Examining the comorbidity between attention deficit hyperactivity disorder and bipolar I disorder: a meta-analysis of family genetic studies. Am J Psychiatry. 2012;169:1256–1266. doi: 10.1176/appi.ajp.2012.12010087. [DOI] [PubMed] [Google Scholar]
- 52.Leitner Y. The Co-Occurrence of Autism and Attention Deficit Hyperactivity Disorder in Children – What Do We Know? Front Hum Neurosci. 2014:8. doi: 10.3389/fnhum.2014.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Christensen J, Vestergaard M, Olsen J, Sidenius P. Validation of epilepsy diagnoses in the Danish National Hospital Register. Epilepsy Res. 2007;75:162–170. doi: 10.1016/j.eplepsyres.2007.05.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1. Sensitivity analyses of the within-individual and full-sibling association between epilepsy and ADHD
Supplementary Table S2. Odds ratios (ORs) and 95% confidence interval (CI) from sensitivity analyses stratified by sex
Supplementary Table S3. Genetic and environmental parameter estimates with Bootstrap standard errors and 95% confidence interval