Abstract
Background
Childhood maltreatment (CM) confers deleterious long-term consequences, and growing evidence suggests some of these effects may be transmitted across generations. We examined the intergenerational effect of maternal CM exposure on child brain structure and also addressed the hypothesis that this effect may start during the child’s intrauterine period of life.
Methods
A prospective longitudinal study was conducted in a clinical convenience sample of 80 mother-child dyads. Maternal CM exposure was assessed using the Childhood Trauma Questionnaire. Structural magnetic resonance imaging (MRI) was employed to characterize newborn global and regional brain (tissue) volumes near the time of birth.
Results
CM exposure was reported by 35% of the women. Maternal CM exposure was associated with lower child intracranial volume (F1,70=6.84, p=.011), which was primarily due to a global difference in cortical gray matter (F1,70=9.10, p=.004). The effect was independent of potential confounding variables, including maternal SES, obstetric complications, obesity, recent interpersonal violence, pre- and early postpartum stress, gestational age at birth, infant sex, and postnatal age at MRI scan. The observed group difference between offspring of CM-exposed versus non-exposed mothers was 6%.
Conclusions
These findings represent the first report to date associating maternal CM exposure with variation in newborn brain structure. These observations support our hypothesis of intergenerational transmission of the effects of maternal CM exposure on child brain development and suggest this effect may originate during the child’s intrauterine period of life, which may have downstream neurodevelopmental consequences.
Keywords: intergenerational transmission, childhood maltreatment, brain development, gray matter volume, pregnancy, newborn
Introduction
Exposure to childhood maltreatment (CM), such as child abuse or neglect, represents one of the most pervasive and pernicious stressors in society in terms of its widespread prevalence (1) and deleterious biological (neural, endocrine, immune, metabolic), psychological (depression, PTSD), biophysical (obesity) and behavioral (substance abuse, risky behavior) consequences (2). These sequelae can persist over the exposed individual’s life span, and growing evidence suggests that among women the adverse effects of CM exposure may be transmitted to the next generation. Children of mothers exposed to CM, even in the absence of maltreatment to themselves, have been shown to exhibit alterations in stress physiology systems and an increased risk for social-emotional and behavioral disorders (3–6).
To date, conceptual frameworks and empirical studies about the intergenerational effects of maternal CM have focused almost exclusively on the child’s postnatal period of life as the primary window of transmission (e.g., via CM-related maternal dysfunctional states underlying suboptimal parenting and care) (7, 8). We, however, hypothesize that the process of intergenerational transmission may start earlier during the period of intrauterine life, and that the developing fetal brain may represent a target of particular interest (9). Intergenerational transmission could potentially occur via maternal CM-related epigenetic alterations in her germ line (oocytes) that survive the re-establishment of postconceptional epigenetic marks. Findings from rodent models support the possibility of transgenerational epigenetic inheritance of adversity-related phenotypes via the paternal germ line (10, 11), however, evidence of such inheritance via the maternal germ line is still lacking. A more likely possibility is that the developing feto-placental unit senses and responds to biological cues in the maternal compartment that reflect the long-term sequelae that CM-exposed women may bring to their pregnancy and gestational state (e.g., alterations in glucocorticoid- and immune-related processes) (12). We suggest that stress-responsive biological systems, such as the hypothalamic-pituitary-adrenal (HPA) axis or the immune system, represent highly attractive candidate mechanisms as sensors, transducers, and effectors of CM-related states and conditions on the developing fetus.
The present study was conducted in a clinical convenience cohort of mother-child dyads assessed prospectively from early gestation through birth until infancy. To address our hypothesis that the effect of maternal CM exposure on her child’s brain may start as early as during intrauterine life, we selected the newborn period as the assessment time for characterization of child brain anatomy (because it precludes conflation with postnatal effects). We also sought to determine whether the hypothesized effect of maternal CM exposure (that occurred well before conception) persists over and beyond that of various potential CM-related psychological, biophysical and behavioral sequelae during pregnancy by controlling for maternal SES, obstetric complications, obesity, exposure to interpersonal violence, and pre- and early postpartum stress. Moreover, because gestational age at birth, infant sex, and postnatal age at MRI scan may relate to child brain anatomy, we also accounted for the effects of these variables in our analyses.
Methods and Materials
Study population
The study was conducted at the University of California, Irvine, Development, Health and Disease Research Program in a clinical convenience cohort of N=131 pregnant women receiving prenatal care at university and other affiliated institutions and clinics. Participants were recruited in the first trimester of pregnancy and followed-up with serial assessments at each trimester. All participants had singleton, intrauterine pregnancies, with no known cord, placental, or uterine anomalies, fetal congenital malformations, or presence of any conditions known to be associated with dysregulated neuroendocrine function or systemic corticosteroid medication use. Upon birth, the newborn children of those women who consented to an MRI scan of their child were included in the study. All newborns included were born full-term (> 37 weeks gestational age) or late preterm (n=7, range: 34.6 – 36.9 weeks; n=6 in the CM- group and n=1 in the CM+ group), and had no known congenital, genetic or neurological disorders. All study procedures were approved by the university’s IRB, and all participants (pregnant women, and parents on behalf of their infants) provided written informed consent.
Procedures
The study employed a prospective, longitudinal design with serial assessments of the pregnant women over the course of gestation (once in each trimester). Gestational age was confirmed by obstetric ultrasonographic biometry performed before 15 weeks gestation using standard clinical criteria (13). Study visit procedures included administration of structured socio-demographic and psychosocial interviews and questionnaires, and fetal ultrasonography. Magnetic resonance imaging (MRI) of the newborn brain was performed shortly after birth (mean: 26 ± 13 days; range: 5 – 64 days; 67.5% of children were scanned within 30 days of postnatal age).
Maternal childhood maltreatment exposure
Maternal exposure to childhood maltreatment was ascertained at the mid-gestation visit using the Childhood Trauma Questionnaire (CTQ, 14), one of the most widely-used, reliable, and valid instruments for determination of abuse and neglect experiences in childhood and adolescence (15). This 28-item measure assesses five dimensions of childhood maltreatment: emotional abuse, physical abuse, sexual abuse, emotional neglect, and physical neglect. Cut-off values for moderate or greater exposure provided in the CTQ manual (14) were used to create dichotomous variables of exposure for each CTQ subscale (emotional abuse ≥13; physical abuse ≥10; sexual abuse ≥8; emotional neglect ≥15; and physical neglect ≥10). From these, for each subscale a binary variable was computed indicating at least moderate exposure to the respective type of abuse or neglect vs. no or low exposure to all five types of abuse and neglect, as well as an overall binary variable indicating exposure to at least one type of childhood maltreatment vs. no or low exposure (CM). CM was used as the principal predictor in statistical analysis. In addition, the overall CTQ score, a sum score over all items (range 25 – 125), was used as a measure of maltreatment severity.
Image acquisition
Magnetic resonance imaging (MRI) was performed in unsedated newborns during natural sleep using a Siemens 3T scanner (TIM Trio, Siemens Medical System Inc., Germany). T1-weighted images were obtained using a three-dimensional (3D) magnetization prepared rapid gradient echo (MP-RAGE) sequence (TR 2400 ms; TE 3.16 ms; TI 1200 ms; Flip Angle 8°; 6:18 minutes) and T2-weighted images were obtained with a turbo spin echo sequence (TR 3200 ms; TE1 13 ms; TE2 135 ms; Flip Angle 180°; 4:18 minutes). The spatial resolution was a 1 × 1 × 1 mm voxel for T1-weighted images and 1 × 1 × 1mm voxel with 0.5 mm interslice gap for T2-weighted images.
Covariates
At each pregnancy visit the Center for Epidemiological Studies Depression scale (CES-D, 16), the Perceived Stress Scale (PSS, 17), the state scale from the State-Trait Anxiety Inventory (STAI, 18), and the Prenatal Distress Questionnaire (PDQ, 19) were administered. The CES-D, PSS, and STAI scales were also administered at one month after birth. For individuals with < 3 missing items on any scale at any time point, the mean responses for that scale were calculated and then multiplied by the total number of items in the respective scale, in order to generate total scale scores that are comparable to those generated from participants without any missing data.
For the PDQ, a mean score was computed across the duration of pregnancy. For each of the CES-D, PSS and STAI scales mean scores were computed encompassing pregnancy and the first month of the postpartum period (pregnancy and early postpartum, PeP). Please see Supplemental Information for more details regarding these measures.
At each visit, participants were asked to report prescription medication use and to bring the packaging of any medication to the study visit. Based on this information, subjects on antidepressant/anxiety medication at any time during pregnancy were identified and excluded from the analyses (n=2).
Interpersonal violence (IPV) was assessed with the Abuse Assessment Screen (AAS, 20). A binary variable indicating exposure to physical and/or sexual violence within the last year was created from two questions assessing having been hit, slapped, kicked, or otherwise physically hurt by someone, and having been forced to have sexual activities by someone.
The presence of major obstetric risk complications in the index pregnancy (i.e., infection, hypertension, diabetes, anemia, vaginal bleeding, oligohydromnios, placental abruption), gestational age at birth, and infant sex were abstracted from the antepartum and delivery medical records, as previously described (21).
Maternal pre-pregnancy body-mass-index (BMI) was also abstracted from medical records. A binary variable indicating obesity (BMI ≥ 30) was created and used as a covariate.
Maternal socio-economic status (SES) was defined as a combination (mean) of maternal educational level (originally assessed in categories from less than high school to advanced degree (master’s/doctorate) and then recoded into values from 1 through 5) and household income (originally assessed in categories from ≤ 15,000$ to ≥ 100,000$ and then recoded into values from 1 through 5).
Image analysis
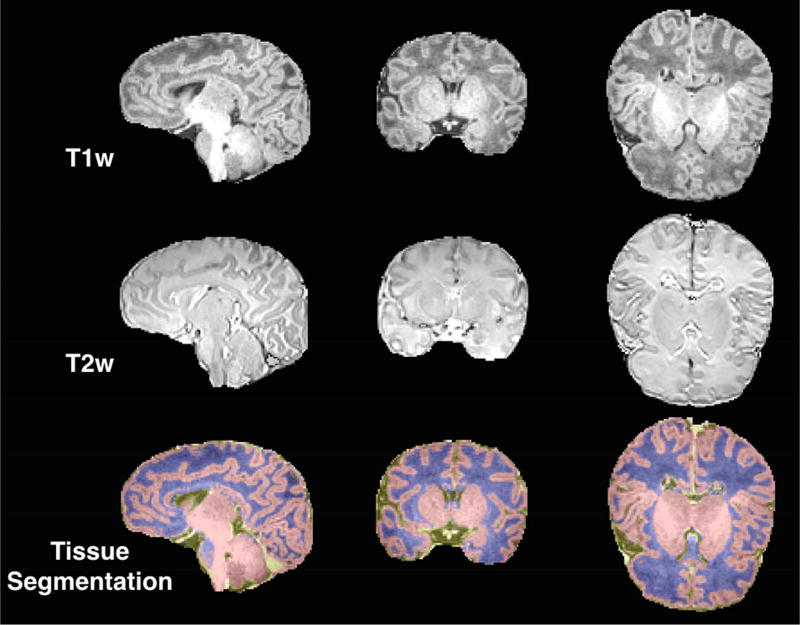
An MRI brain scan was attempted in 114 of the 131 infants included in the study. Scan acquisition was not attempted in n=17 infants because they did not fall asleep during the MRI study visit. The complete MRI sequence was obtained in 94 scans. These MRI scans were then independently screened for quality control and excluded if they had excessive motion (n=6) and/or significant abnormalities as reviewed by a clinical neuroradiologist (n=2). Tissue segmentation was performed using a multi-atlas based iterative expectation maximization segmentation algorithm as previously described (22, 23). The employed neonate multi-atlas is disseminated here: https://www.nitrc.org/projects/unc_brain_atlas/. Brain tissue was classified as gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF). A representative tissue segmentation is depicted in Figure 1. Taken together, these three tissue volumes comprise the intracranial volume (ICV). Regional anatomical subdivisions were defined by multi-modality (employing both T1 and T2 weighted images) non-linear warping of a newborn average brain atlas to native space via the Advanced Neuroimaging Tools (ANTs) toolkit (24, 25). The deformation field was then applied to the parcellation template (26) corresponding to the atlas, resulting in parcellated volumes in native space (see Supplementary Figure S1 and Supplemental Information for more details).
Figure 1.
Example Tissue Segmentation.
Note. An example of a typical three class (gray matter, white matter, cerebrospinal fluid) tissue segmentation (bottom row). Class definition used T1-weighted (top row) and T2-weighted (middle row) signal intensities as inputs.
Hippocampi and amygdalae were individually segmented initialized via a multi-modality, multi-template based automatic method combining T1- and T2-weighted high-resolution images (27), followed by manual correction in ITK-Snap (28) (see Supplemental Information for more details).
Statistical analysis
Global brain volumetric measures were available for n=86 newborns. Mother-child-dyads that could not be included in the analyses because no MRI scan was attempted or because of insufficient quality of the MRI scan (N=45) did not differ from the group with high quality structural MRI scans (N=86) with respect to maternal CM status, SES, maternal age, parity, obstetric complications, pre-pregnancy BMI, gestational age at birth, or birthweight percentile; drop-out infants were more likely to be of female sex (X2=6.1, p=.017). Of the 86 mother-child dyads with high quality structural MRI data, n=2 were excluded due to antidepressant/anxiety medication use in pregnancy and n=4 had missing information regarding CM, resulting in a final sample size of n=80.
Because of the high correlation between the different measures of maternal stress (r’s ranged between 0.60 and 0.86), we performed a factor analysis including the PeP-scores. Based on the eigenvalue > 1 criterion and visual inspection of the scree plot, the principal factor representing prenatal and early postnatal stress exposure was extracted. The factor explained 73.4% of the total variance. A composite PeP-stress variable score was computed for each subject using the weighted factor loadings (see Supplemental Information) and was used as a covariate in the analyses. Group-based differences in signal-to-noise ratio (SNR) and carrier-to-noise ratio (CNR) were evaluated, and there were no observed group differences between CM+ and CM− infants in GM SNR, WM SNR, or gray/white CNR for either the T1-weighted or T2-weighted images.
Analysis of variance (ANOVA) modeling was employed to statistically test the association of maternal CM with newborn global brain tissue volumes. All models controlled for maternal SES, obstetric complications, obesity, IPV, pre- and early postpartum stress, gestational age at birth, infant sex, and postnatal age at MRI scan. To determine the effect of maltreatment severity, the primary analyses were repeated using the overall CTQ score as the main predictor. As a second exploratory step, the association of maternal CM with offspring regional GM volumes was tested. To determine the relative strength of the effect of CM between the brain regions, the analyses were performed with and without controlling for overall brain size (ICV). To determine effect modification by infant sex, the main analyses were repeated including the interaction term between maternal CM and infant sex. Moderation by infant sex was investigated because many studies have reported sex-specific differences in the prevalence of neurodevelopmental disorders commonly observed in offspring of mothers with exposure to CM (29, 30), and because many of the intrauterine biological mechanisms that may underlie the intergenerational transmission of CM sequelae have been shown to exert sex-specific effects on developing offspring’s brain (31–33). All p-values were corrected for multiple comparisons using the False Discovery Rate method (34) implemented in R’s p.adjust function.
Results
Descriptives
Complete data were available in 80 mother-child dyads. 35% of these mothers (n=28) reported exposure to at least one type of moderate to severe CM. Key maternal and child characteristics, delineated by maternal CM status, are depicted in Table 1.
Table 1.
Characteristics of the Study Population.
| Characteristic | Complete sample N = 80 |
CM− group (no childhood maltreatment) n = 52 (65%) |
CM+ group (≥ 1 type of childhood maltreatment) n = 28 (35%) |
|---|---|---|---|
| Maternal age at baseline, yrs, mean ± SD | 28.06 ± 5.5 | 28.54 ± 5.7 | 27.18 ± 5.2 |
| SES, mean ± SD | 3.05 ± 0.9 | 3.14 ± 1.0 | 2.88 ± 0.8 |
| Race/ethnicity | |||
| Non-Hispanic White, n (%) | 31 (38.8) | 26 (50.0) | 5 (18.5)a |
| Hispanic White, n (%) | 26 (32.5) | 15 (21.2) | 11 (40.7) |
| Other, n (%) | 22 (27.5) | 11 (21.2) | 11 (40.7) |
| Presence of any major obstetric complication, n (%) | 20 (25) | 14 (26.9) | 6 (21.4) |
| Obesity, n (%) | 19 (23.8) | 14 (26.9) | 5 (17.9) |
| Interpersonal violence, n (%) | 11 (13.8) | 6 (11.5) | 5 (17.6) |
| Female infant sex, n (%) | 32 (40) | 23 (42.3) | 10 (35.7) |
| Gestational age at birth, wks, mean ± SD | 39.11 ± 1.5 | 39.04 ± 1.4 | 39.24 ± 1.6 |
| Infant birthweight, g, mean ± SD | 3313.90 ± 524.1 | 3355.78 ± 549.8 | 3236.11 ± 472.1 |
| Age at MRI scan, days, mean ± SD | 26.0 ± 13.1 | 24.77 ± 12.3 | 28.29 ± 14.6 |
| Psychosocial stress measures | |||
| PDQ in pregnancy, mean ± SD | 14.40 ± 6.8 | 13.32 ± 6.3 | 16.42 ± 7.1a |
| CES-D PeP, mean ± SD | 14.88 ± 8.7 | 13.43 ± 8.3 | 17.57 ± 9.0a |
| PSS PeP, mean ± SD | 15.86 ± 5.5 | 14.58 ± 5.5 | 18.25 ± 4.7a |
| STAI PeP, mean ± SD | 34.20 ± 8.6 | 32.49 ± 7.5 | 37.38 ± 9.7a |
| PeP-Stress, mean ± SD | 0.0 ± 1.0 | −0.20 ± 0.9 | 0.37± 1.0a |
Note. CM = childhood maltreatment; SES = Socio-economic status; PeP = pregnancy and early postpartum.
Maternal socio-economic status was defined as a combination of maternal educational level (originally assessed in categories from less than high school to advanced degree and then recoded into values from 1 through 5) and household income (originally assessed in categories from ≤ 15,000$ to ≥ 100,000$ and then recoded into values from 1 through 5).
significantly different from CM− (p < .05)
Global brain and tissue volumes
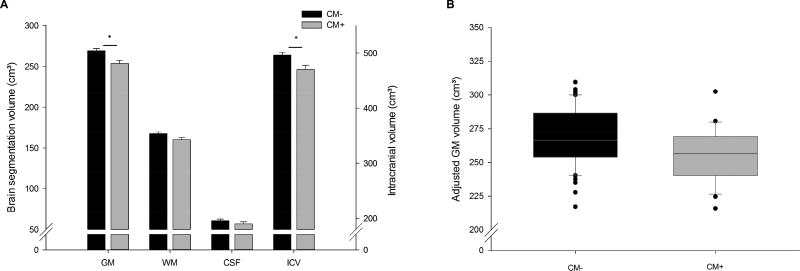
Compared to newborns of mothers without CM, newborns of CM+ mothers had a significantly smaller ICV (F1,70 = 6.84, p = .011, padj = .022, partial η2 = .089). Specifically, there was a significant difference in GM volume (F1,70 = 9.10, p = .004, padj = .016, partial η2 = .115). There also was a trend effect on newborn WM volume (F1,70 = 3.87, p = .053); however, CSF was not significantly different between the two groups (F1,70 = 1.30, p = .258, see Figure 2 and Supplementary Tables S2 and S3). These findings suggest that GM volume is the principal contributor to the observed overall difference in ICV. This observed difference of 14.8 cm³ in gray matter between newborns of CM+ and CM− mothers corresponds to approximately 6%, or half a standard deviation (SD=33.3), smaller volume in overall cortical GM.
Figure 2.
Mean intracranial volume and brain segmentation volumes in newborns of mothers with (CM+, n=28) and without childhood maltreatment (CM−, n=52).
Note. A) Depicted are adjusted means from the ANOVA models controlling for maternal SES, obstetric complications, maternal depression in pregnancy, infant sex, gestational age at birth and age at MRI scan. B) Boxplot illustrating the difference in GM volume adjusted for gestational age at birth and age at MRI scan between infants of mothers with vs. without CM. CM = childhood maltreatment; GM = gray matter; WM = white matter; CSF = cerebrospinal fluid; ICV = intracranial volume.
* p < .0125
To determine the influence of maltreatment severity, the main analyses were repeated using the CTQ total score. A higher overall CTQ score was associated with lower ICV and GM volume (B = −0.686, SE = 0.30, p = .025, padj = .050; and B = −0.363, SE = 0.15, p = .018, padj = .050, respectively). WM volume and CSF were not significantly related to maltreatment severity.
Regional gray matter volume
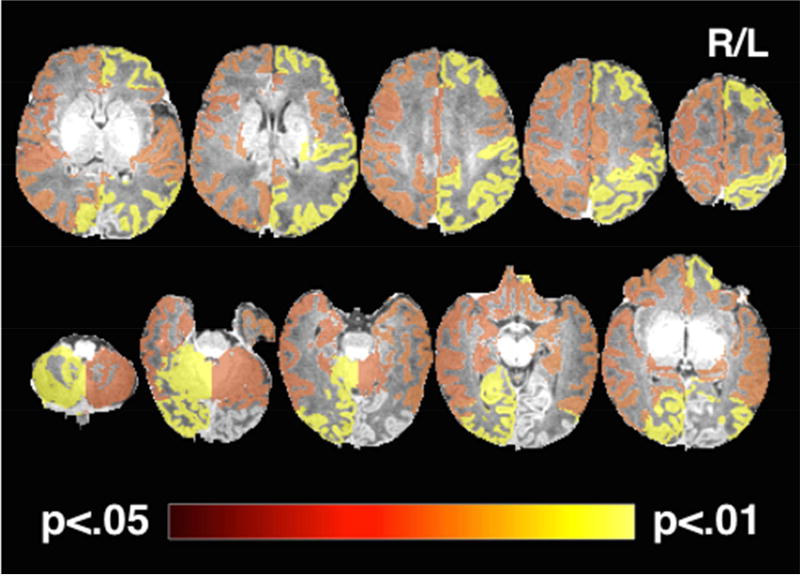
Next, we performed exploratory analyses to examine which specific regions of the newborn brain were most prominently associated with maternal CM exposure. Results suggest the effect was particularly pronounced in the right posterior parts of the brain, the left cingulate cortex, and the bilateral parietal, temporal, frontal and prefrontal areas, with a stronger effect in the left than in the right hemisphere (see Figure 2 and Supplementary Table S4). Regional GM volume analyses were repeated to determine the relative strength of GM volume reduction between these specific brain regions after additionally controlling for overall brain size (ICV). These analyses did not yield any significant results, suggesting that the observed effect of maternal CM on newborn GM maturation appears to be global in nature rather than region-specific, further underscoring its potential importance. Maternal CM status was not significantly associated with either hippocampus or amygdala volumes (Supplementary Table S4).
Sexual dimorphism
In concordance with previous studies, we observed significant sexual dimorphism in the newborn brain (23). Males had a larger global brain volume as well as larger GM and WM volumes, but not larger volume of CSF compared to female newborns (ICV: F1,70 = 12.86, p = .001, padj = .004; GM: F1,70 = 10.65, p = .002, padj = .004; WM: F1,70 = 14.30, p < .001, padj < .004; and CSF: F1,70 = 2.70, p = .105 respectively). Infant sex did not moderate the effect of maternal CM on newborn ICV, GM volume or WM volume. There was an interaction effect of CM and infant sex on CSF volume, however, this effect was not significant after adjusting for multiple comparisons (F1,69 = 4.55, p = .036, padj = .144).
Discussion
To the best of our knowledge, this is the first study to establish an association between maternal CM exposure and her child’s brain anatomy. Newborns of mothers who had experienced CM had a significantly smaller overall brain size and less GM volume than those born to mothers who had not experienced CM. The observation that this effect of maternal CM on child brain anatomy is already evident at birth supports our premise that the intergenerational, mother-to-child transmission of the adverse sequelae of CM exposure may begin as early as during the child’s intrauterine period of life. Our results showed a global, rather than regionally-specific, difference in GM volume, corresponding to approximately 6% less volume in cortical GM in the CM+ group compared to the CM− group. Global GM volume changes of similar magnitude have been reported in children with neurodevelopmental disorders (35), as well as in 1-year old rhesus monkeys whose mothers were exposed to influenza infection during pregnancy (36).
During normal brain development, the trajectory of cortical GM growth exhibits a particularly pronounced increase during the prenatal and early postnatal period (23, 37). Because analyses of the newborn brain metrics were adjusted for gestational age at birth as well as postnatal age at MRI scan, our finding suggests children of CM+ mothers may exhibit a delay in the rate of intrauterine maturation of cortical gray matter. Alterations in the maturational trajectory of cortical GM have previously been associated with various forms of psychopathology (38, 39), and this may, thus, represent a pathway underlying previously-observed associations between maternal CM exposure and increased risk in their children of neurodevelopmental and neuropsychiatric disorders.
We did not observe a region-specific effect of maternal CM exposure on newborn hippocampus and amygdala volumes. Previous studies that have focused on maternal stress during pregnancy (as opposed to preconceptional stress) have reported an association with hippocampus and amygdala volumes (32, 40). To examine whether maternal preconceptional conditions (in this case, maternal CM exposure) may affect fetal development independent of maternal psychological state and other typical CM sequelae during pregnancy, we controlled for these states in our statistical analyses. However, it is possible that these CM sequelae may moderate the association between CM and fetal brain development (12), which our study was not powered to assess. It will, therefore, be important to investigate the potential moderating role of CM-related sequelae in future studies, and to specifically examine whether maternal stress during pregnancy moderates the association between maternal CM and her offspring’s hippocampus and amygdala volumes.
We suggest that future studies should replicate the present findings in larger, independent cohorts and also elucidate underlying biological transmission pathways, with a focus on the role of maternal, placental and fetal endocrine and immune/inflammatory processes and epigenetic characteristics. During gestation, endocrine and immune processes play an obligatory role in the development of the fetal brain, and perturbations are likely to adversely affect its developmental trajectory (9, 41), with long-term consequences in terms of increased susceptibility for neurodevelopmental and psychiatric disorders. Alterations in endocrine and immune mediators are well-established consequences of CM exposure (2). Importantly, empirical evidence suggests that such CM and other preconceptional trauma-related alterations in endocrine and immune biology may also carry forward into the gestational biological state of a woman when she becomes pregnant (42–45). For instance, we recently determined that the long reach of maternal CM exposure extends during gestation to the stress physiology of the developing placental-fetal unit. Women with a higher number of abuse and neglect exposures in childhood exhibited higher placental CRH production and a steeper trajectory of placental CRH increase across gestation (46). Higher concentrations of placental CRH during pregnancy have been associated in the offspring with lower physical and neurological maturity and a more difficult temperament (47–49). Furthermore, placental CRH is known to exert a stimulatory effect on cortisol production from the maternal and fetal adrenals (50), and increased maternal and fetal cortisol concentrations in pregnancy have been shown in offspring to predict difficult temperament and behavior (51, 52), delayed cognitive development (53), and alterations in HPA axis regulation (54, 55).
Our study has some limitations. The sample size was relatively small, possibly limiting the ability to detect small or more nuanced effects. In addition, the study was conducted in a clinical convenience sample of healthy pregnant women and their children; the study population was not enriched for CM exposure. The prevalence of moderate to severe CM in our study is consistent with estimates from larger epidemiological studies in the general population (56–58), however, as can be expected, the prevalence of maltreatment at the more severe end of the spectrum was limited. We did find a dose-dependent effect of CM severity (using the overall CTQ score as a predictor), so perhaps an even larger effect might be expected when including mothers with a more pronounced CM severity. Thus, future studies should include a more detailed and extensive CM characterization, possibly in a high risk cohort enriched for CM exposure. Another limitation relates to the retrospective method of CM assessment, which may be subject to problems such as non-awareness (e.g. when CM occurred before the age of 3), non-disclosure, and reporting biases due to mood state or personality factors (59). While we controlled for current depressive symptoms and anxiety and tried to limit the rate of false-positives by choosing a moderate cut-off score to classify exposure to CM (14), this does not preclude the possibility of false negatives. Finally, we cannot completely rule out that other unmeasured factors during the prenatal (e.g. maternal diet quality) or the early postnatal period may have influenced our results. We limited the influence of the postnatal environment by performing the MRI scan shortly after birth, however, a third of the MRI scans were obtained after the newborn period (between 31–64 days postnatal age). Theoretically, the size of any postnatal effect should increase as a function of the length of the postnatal exposure, i.e. the older the child at the assessment, which is why controlling for age at scan should at least partly account for postnatal influences. In addition, we have tried to limit the possibility of postnatal environmental influences by controlling for the possible effects of maternal stress during the early postnatal period.
In conclusion, the findings of this study support a concerted focus on the intrauterine period of development as one of the primary windows for the intergenerational transmission of the effects of exposure to CM. This has obvious and important implications for the development and timing of intervention strategies to ultimately break the vicious cycle of the enduring consequences of abuse and neglect passed down from a vulnerable population of abused women to the even more vulnerable population of their unborn children.
Supplementary Material
Figure 3.
Regions with reduced GM volume in newborns of mothers exposed to CM.
Note. The statistical map is overlaid on a typical neonatal T1-weighted image in radiologic convention and thresholded at padj < 0.05 with hot colors representing a more significant finding. The most significant reduction of GM volume was observed in the right occipital cortex and cerebellum, as well as the left parietal and prefrontal cortex.
Acknowledgments
This research was supported by US Public Health Service (National Institutes of Health) grants R01 MH-091351, R01 MH-105538, R01 HD-060628, and UG3 OD-O23349 (all to PDW and CB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
FINANCIAL DISCLOSURES
All authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.U.S. Department of Health & Human Services Administration for Children and Families, Administration on Children, Youth and Families, Children’s Bureau. Child maltreatment. 2014 Available from http://www.acf.hhs.gov/programs/cb/research-data-technology/statistics-research/child-maltreatment.
- 2.Heim C, Shugart M, Craighead WE, Nemeroff CB. Neurobiological and psychiatric consequences of child abuse and neglect. Dev Psychobiol. 2010;52:671–690. doi: 10.1002/dev.20494. [DOI] [PubMed] [Google Scholar]
- 3.Lehrner A, Bierer LM, Passarelli V, Pratchett LC, Flory JD, Bader HN, et al. Maternal PTSD associates with greater glucocorticoid sensitivity in offspring of Holocaust survivors. Psychoneuroendocrinology. 2014;40:213–220. doi: 10.1016/j.psyneuen.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collishaw S, Dunn J, O’Connor TG, Golding J. Maternal childhood abuse and offspring adjustment over time. Dev Psychopathol. 2007;19:367–383. doi: 10.1017/S0954579407070186. [DOI] [PubMed] [Google Scholar]
- 5.Thompson R. Mothers’ violence victimization and child behavior problems: examining the link. Am J Orthopsychiatry. 2007;77:306–315. doi: 10.1037/0002-9432.77.2.306. [DOI] [PubMed] [Google Scholar]
- 6.Roberts AL, Lyall K, Rich-Edwards JW, Ascherio A, Weisskopf MG. Association of maternal exposure to childhood abuse with elevated risk for autism in offspring. JAMA Psychiatry. 2013;70:508–515. doi: 10.1001/jamapsychiatry.2013.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plant DT, Barker ED, Waters CS, Pawlby S, Pariante CM. Intergenerational transmission of maltreatment and psychopathology: the role of antenatal depression. Psychol Med. 2013;43:519–528. doi: 10.1017/S0033291712001298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berlin LJ, Appleyard K, Dodge KA. Intergenerational continuity in child maltreatment: mediating mechanisms and implications for prevention. Child Dev. 2011;82:162–176. doi: 10.1111/j.1467-8624.2010.01547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buss C, Entringer S, Wadhwa PD. Fetal Programming of Brain Development: Role of Intrauterine Stress in Susceptibility to Psychopathology. Science Signaling. 2012;5(pt7) doi: 10.1126/scisignal.2003406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dias BG, Ressler KJ. Parental olfactory experience influences behavior and neural structure in subsequent generations. Nat Neurosci. 2014;17:89–96. doi: 10.1038/nn.3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodgers AB, Morgan CP, Bronson SL, Revello S, Bale TL. Paternal stress exposure alters sperm microRNA content and reprograms offspring HPA stress axis regulation. J Neurosci. 2013;33:9003–9012. doi: 10.1523/JNEUROSCI.0914-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buss C, Entringer S, Moog NK, Toepfer P, Fair DA, Simhan HN, et al. Intergenerational Transmission of Maternal Childhood Maltreatment Exposure: Implications for Fetal Brain Development. J Am Acad Child Adolesc Psychiatry. 2017;56:373–382. doi: 10.1016/j.jaac.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Brien GD, Queenan JT, Campbell S. Assessment of gestational age in the second trimester by real-time ultrasound measurement of the femur length. Am J Obstet Gynecol. 1981;139:540–545. doi: 10.1016/0002-9378(81)90514-7. [DOI] [PubMed] [Google Scholar]
- 14.Bernstein DP, Fink L. Childhood Trauma Questionnaire: a retrospective self-report manual. San Antonio, TX: The Psychological Corporation; 1998. [Google Scholar]
- 15.Baker AJL. Adult recall of childhood psychological maltreatment: Definitional strategies and challenges. Child Youth Serv Rev. 2009;31:703–714. [Google Scholar]
- 16.Radloff LS. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 17.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 18.Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 19.Yali AM, Lobel M. Coping and distress in pregnancy: an investigation of medically high risk women. J Psychosom Obstet Gynaecol. 1999;20:39–52. doi: 10.3109/01674829909075575. [DOI] [PubMed] [Google Scholar]
- 20.McFarlane J, Parker B, Soeken K, Bullock L. Assessing for abuse during pregnancy. Severity and frequency of injuries and associated entry into prenatal care. JAMA. 1992;267:3176–3178. doi: 10.1001/jama.267.23.3176. [DOI] [PubMed] [Google Scholar]
- 21.Wadhwa PD, Garite TJ, Porto M, Glynn L, Chicz-DeMet A, Dunkel-Schetter C, et al. Placental corticotropin-releasing hormone (CRH), spontaneous preterm birth, and fetal growth restriction: a prospective investigation. Am J Obstet Gynecol. 2004;191:1063–1069. doi: 10.1016/j.ajog.2004.06.070. [DOI] [PubMed] [Google Scholar]
- 22.Cherel M, Budin F, Prastawa M, Gerig G, Lee K, Buss C, et al. Automatic tissue segmentation of neonate brain MR Images with subject-specific atlases. Proc SPIE Int Soc Opt Eng. 2015:9413. doi: 10.1117/12.2082209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gilmore JH, Lin W, Prastawa MW, Looney CB, Vetsa YS, Knickmeyer RC, et al. Regional gray matter growth, sexual dimorphism, and cerebral asymmetry in the neonatal brain. J Neurosci. 2007;27:1255–1260. doi: 10.1523/JNEUROSCI.3339-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage. 2011;54:2033–2044. doi: 10.1016/j.neuroimage.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prastawa M, Gilmore JH, Lin W, Gerig G. Automatic segmentation of MR images of the developing newborn brain. Med Image Anal. 2005;9:457–466. doi: 10.1016/j.media.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 26.Gousias IS, Rueckert D, Heckemann RA, Dyet LE, Boardman JP, Edwards AD, et al. Automatic segmentation of brain MRIs of 2-year-olds into 83 regions of interest. Neuroimage. 2008;40:672–684. doi: 10.1016/j.neuroimage.2007.11.034. [DOI] [PubMed] [Google Scholar]
- 27.Wang J, Vachet C, Rumple A, Gouttard S, Ouziel C, Perrot E, et al. Multi-atlas segmentation of subcortical brain structures via the AutoSeg software pipeline. Front Neuroinform. 2014;8:7. doi: 10.3389/fninf.2014.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. 2006;31:1116–1128. doi: 10.1016/j.neuroimage.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 29.Carter AS, Wagmiller RJ, Gray SA, McCarthy KJ, Horwitz SM, Briggs-Gowan MJ. Prevalence of DSM-IV disorder in a representative, healthy birth cohort at school entry: sociodemographic risks and social adaptation. J Am Acad Child Adolesc Psychiatry. 2010;49:686–698. doi: 10.1016/j.jaac.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lavigne JV, Gibbons RD, Christoffel KK, Arend R, Rosenbaum D, Binns H, et al. Prevalence rates and correlates of psychiatric disorders among preschool children. J Am Acad Child Adolesc Psychiatry. 1996;35:204–214. doi: 10.1097/00004583-199602000-00014. [DOI] [PubMed] [Google Scholar]
- 31.Bergeron JDL, Deslauriers J, Grignon S, Fortier LC, Lepage M, Stroh T, et al. White Matter Injury and Autistic-Like Behavior Predominantly Affecting Male Rat Offspring Exposed to Group B Streptococcal Maternal Inflammation. Dev Neurosci. 2013;35:504–515. doi: 10.1159/000355656. [DOI] [PubMed] [Google Scholar]
- 32.Buss C, Davis EP, Shahbaba B, Pruessner JC, Head K, Sandman CA. Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems. Proc Natl Acad Sci U S A. 2012;109:E1312–1319. doi: 10.1073/pnas.1201295109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu L, Li A, Matthews SG. Maternal glucocorticoid treatment programs HPA regulation in adult offspring: sex-specific effects. Am J Physiol Endocrinol Metab. 2001;280:E729–E739. doi: 10.1152/ajpendo.2001.280.5.E729. [DOI] [PubMed] [Google Scholar]
- 34.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995:289–300. [Google Scholar]
- 35.Carmona S, Vilarroya O, Bielsa A, Tremols V, Soliva JC, Rovira M, et al. Global and regional gray matter reductions in ADHD: a voxel-based morphometric study. Neurosci Lett. 2005;389:88–93. doi: 10.1016/j.neulet.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 36.Short SJ, Lubach GR, Karasin AI, Olsen CW, Styner M, Knickmeyer RC, et al. Maternal influenza infection during pregnancy impacts postnatal brain development in the rhesus monkey. Biol Psychiatry. 2010;67:965–973. doi: 10.1016/j.biopsych.2009.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andescavage NN, du Plessis A, McCarter R, Serag A, Evangelou I, Vezina G, et al. Complex Trajectories of Brain Development in the Healthy Human Fetus. Cereb Cortex. 2016:1–10. doi: 10.1093/cercor/bhw306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gogtay N, Thompson PM. Mapping gray matter development: implications for typical development and vulnerability to psychopathology. Brain Cogn. 2010;72:6–15. doi: 10.1016/j.bandc.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Greimel E, Nehrkorn B, Schulte-Ruther M, Fink GR, Nickl-Jockschat T, Herpertz-Dahlmann B, et al. Changes in grey matter development in autism spectrum disorder. Brain Struct Funct. 2013;218:929–942. doi: 10.1007/s00429-012-0439-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wen DJ, Poh JS, Ni SN, Chong YS, Chen H, Kwek K, et al. Influences of prenatal and postnatal maternal depression on amygdala volume and microstructure in young children. Transl Psychiat. 2017;7:e1103. doi: 10.1038/tp.2017.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Entringer S, Buss C, Wadhwa PD. Prenatal stress, development, health and disease risk: A psychobiological perspective-2015 Curt Richter Award Paper. Psychoneuroendocrinology. 2015;62:366–375. doi: 10.1016/j.psyneuen.2015.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cammack AL, Buss C, Entringer S, Hogue CJ, Hobel CJ, Wadhwa PD. The association between early life adversity and bacterial vaginosis during pregnancy. Am J Obstet Gynecol. 2011;204:431, e431–438. doi: 10.1016/j.ajog.2011.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bublitz MH, Stroud LR. Childhood sexual abuse is associated with cortisol awakening response over pregnancy: Preliminary findings. Psychoneuroendocrinology. 2012;37:1425–1430. doi: 10.1016/j.psyneuen.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blackmore ER, Moynihan JA, Rubinow DR, Pressman EK, Gilchrist M, O’Connor TG. Psychiatric Symptoms and Proinflammatory Cytokines in Pregnancy. Psychosom Med. 2011;73:656–663. doi: 10.1097/PSY.0b013e31822fc277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller GE, Culhane J, Grobman W, Simhan H, Williamson DE, Adam EK, et al. Mothers’ childhood hardship forecasts adverse pregnancy outcomes: Role of inflammatory, lifestyle, and psychosocial pathways. Brain Behav Immun. 2017 doi: 10.1016/j.bbi.2017.04.018. https://doi.org/10.1016/j.bbi.2017.04.018. [DOI] [PMC free article] [PubMed]
- 46.Moog NK, Buss C, Entringer S, Shahbaba B, Gillen DL, Hobel CJ, et al. Maternal Exposure to Childhood Trauma Is Associated During Pregnancy With Placental-Fetal Stress Physiology. Biol Psychiatry. 2016;79:831–839. doi: 10.1016/j.biopsych.2015.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sandman CA, Wadhwa PD, Chicz-DeMet A, Porto M, Garite TJ. Maternal corticotropin-releasing hormone and habituation in the human fetus. Dev Psychobiol. 1999;34:163–173. doi: 10.1002/(sici)1098-2302(199904)34:3<163::aid-dev1>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 48.Ellman LM, Schetter CD, Hobel CJ, Chicz-Demet A, Glynn LM, Sandman CA. Timing of fetal exposure to stress hormones: effects on newborn physical and neuromuscular maturation. Dev Psychobiol. 2008;50:232–241. doi: 10.1002/dev.20293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Davis EP, Glynn LM, Dunkel Schetter C, Hobel C, Chicz-Demet A, Sandman CA. Corticotropin-releasing hormone during pregnancy is associated with infant temperament. Dev Neurosci. 2005;27:299–305. doi: 10.1159/000086709. [DOI] [PubMed] [Google Scholar]
- 50.McLean M, Bisits A, Davies J, Woods R, Lowry P, Smith R. A placental clock controlling the length of human pregnancy. Nat Med. 1995;1:460–463. doi: 10.1038/nm0595-460. [DOI] [PubMed] [Google Scholar]
- 51.Davis EP, Glynn LM, Schetter CD, Hobel C, Chicz-Demet A, Sandman CA. Prenatal Exposure to Maternal Depression and Cortisol Influences Infant Temperament. J Am Acad Child Adolesc Psychiatry. 2007;46:737–746. doi: 10.1097/chi.0b013e318047b775. [DOI] [PubMed] [Google Scholar]
- 52.de Weerth C, van Hees Y, Buitelaar JK. Prenatal maternal cortisol levels and infant behavior during the first 5 months. Early Hum Dev. 2003;74:139–151. doi: 10.1016/s0378-3782(03)00088-4. [DOI] [PubMed] [Google Scholar]
- 53.Davis EP, Sandman CA. The Timing of Prenatal Exposure to Maternal Cortisol and Psychosocial Stress is Associated with Human Infant Cognitive Development. Child Dev. 2010;81:131–148. doi: 10.1111/j.1467-8624.2009.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O’Connor TG, Bergman K, Sarkar P, Glover V. Prenatal cortisol exposure predicts infant cortisol response to acute stress. Dev Psychobiol. 2013;55:145–155. doi: 10.1002/dev.21007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gutteling BM, de Weerth C, Buitelaar JK. Maternal prenatal stress and 4–6 year old children’s salivary cortisol concentrations pre- and post-vaccination. Stress. 2004;7:257–260. doi: 10.1080/10253890500044521. [DOI] [PubMed] [Google Scholar]
- 56.Finkelhor D, Turner HA, Shattuck A, Hamby SL. Prevalence of Childhood Exposure to Violence, Crime, and Abuse: Results From the National Survey of Children’s Exposure to Violence. JAMA Pediatr. 2015;169:746–754. doi: 10.1001/jamapediatrics.2015.0676. [DOI] [PubMed] [Google Scholar]
- 57.Hussey JM, Chang JJ, Kotch JB. Child maltreatment in the United States: prevalence, risk factors, and adolescent health consequences. Pediatrics. 2006;118:933–942. doi: 10.1542/peds.2005-2452. [DOI] [PubMed] [Google Scholar]
- 58.Walker EA, Unutzer J, Rutter C, Gelfand A, Saunders K, VonKorff M, et al. Costs of health care use by women HMO members with a history of childhood abuse and neglect. Arch Gen Psychiatry. 1999;56:609–613. doi: 10.1001/archpsyc.56.7.609. [DOI] [PubMed] [Google Scholar]
- 59.Reuben A, Moffitt TE, Caspi A, Belsky DW, Harrington H, Schroeder F, et al. Lest we forget: comparing retrospective and prospective assessments of adverse childhood experiences in the prediction of adult health. J Child Psychol Psyc. 2016;57:1103–1112. doi: 10.1111/jcpp.12621. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.