Abstract
In infant tree shrews, exposure to narrow-band long-wavelength (red) light, that stimulates long-wavelength sensitive cones almost exclusively, slows axial elongation and produces hyperopia. We asked if red light produces hyperopia in juvenile and adolescent animals, ages when plus lenses are ineffective. Animals were raised in fluorescent colony lighting (100–300 lux) until they began 13 days of red-light treatment at 11 (n=5, “infant”), 35 (n=5, “juvenile”) or 95 (n=5, “adolescent”) days of visual experience (DVE). LEDs provided 527–749 lux on the cage floor. To control for the higher red illuminance, a fluorescent control group (n=5) of juvenile (35 DVE) animals was exposed to ~ 975 lux. Refractions were measured daily; ocular component dimensions at the start and end of treatment and end of recovery in colony lighting. These groups were compared with normals (n=7). In red light, the refractive state of both juvenile and adolescent animals became significantly (P<0.05) hyperopic: juvenile 3.9±1.0 diopters (D, mean±SEM) vs. normal 0.8±0.1 D; adolescent 1.6±0.2 D vs. normal 0.4±0.1 D. The fluorescent control group refractions (0.6±0.3 D) were normal. In red-treated juveniles the vitreous chamber was significantly smaller than normal (P<0.05): juvenile 2.67±0.03 mm vs. normal 2.75±0.02 mm. The choroid was also significantly thicker: juvenile 77±4 μm vs. normal 57±3 μm (P<0.05). Although plus lenses do not restrain eye growth in juvenile tree shrews, the red light-induced slowed growth and hyperopia in juvenile and adolescent tree shrews demonstrates that the emmetropization mechanism is still capable of restraining eye growth at these ages.
Keywords: Emmetropization, Myopia, Animal Models, Refraction, Wavelength, Longitudinal Chromatic Aberration
Graphical Abstract
1. Introduction
As noted in many recent articles, there is a myopia epidemic in the U.S. and around the globe. Myopia in U.S. adults has increased from around 25% in the 1970’s to over 40% in 2004 (Sperduto, Seigel et al. 1983, Lin, Chen et al. 1988, Quek, Chua et al. 2004, Vitale, Sperduto et al. 2009, Sun, Zhou et al. 2012, Dolgin 2015). Myopia, especially higher levels, produces an elevated risk for retinal tears and detachment, choroidal degeneration, glaucoma, and cataract, making myopia a significant cause of blindness in the U.S. (Burton 1989, Zadnik 2001, Vongphanit, Mitchell et al. 2002, Saw, Gazzard et al. 2005, Jones, Sinnott et al. 2007). Thus, the rising prevalence of myopia presents a serious public health issue now and in the years to come. As myopia prevalence rises and myopes age, myopia-related blindness will also rise (Holden, Jong et al. 2015) unless we can devise ways to block, or at least reduce, myopia development in children. Most studies in animal models have examined the emmetropization mechanism in the early postnatal stage (Zadnik and Mutti 1995). More information is needed on how this mechanism functions at older ages, similar to those when children develop myopia.
Most young animals and human infants are born with eyes that have large refractive errors – tree shrews (Norton and McBrien 1992), guinea pigs (Cook and Glasscock 1951), marmosets (Graham and Judge 1999), rhesus monkeys (Bradley, Fernandes et al. 1999), chicks (Pickett-Seltner, Sivak et al. 1988), and humans (Cook and Glasscock 1951, Chen, Xie et al. 2011), are generally hyperopic, although kestrels (Andison, Sivak et al. 1992) are initially myopic. With age, the axial length (primarily, vitreous chamber depth) is rapidly adjusted to match the power of the eye’s optics, achieving a state of emmetropia where the retina is located approximately at the focal plane (typically, a slight hyperopia remains that can be easily compensated for with accommodation, see Stenstrom 1948, Pickett-Seltner, Sivak et al. 1988, Norton and McBrien 1992, Bradley, Fernandes et al. 1999). The dimensional precision required to achieve emmetropia is very high: for an adult human eye a change in axial length of just 0.33 mm (out of a total axial length of about 24 mm) can result in a change in refraction of a diopter (Atchison, Jones et al. 2004).
It is now well established that an active emmetropization mechanism in the postnatal eye uses optical cues to dynamically adjust the eye’s elongation rate to match the retinal location to that eye’s focal plane (Schaeffel and Howland 1991, Wildsoet 1997, Norton 1999, Mutti, Mitchell et al. 2005, Smith, Hung et al. 2010). By fine-tuning the refractive state, the emmetropization mechanism achieves and maintains approximate emmetropia throughout the postnatal period (Wildsoet 1997, Norton 1999, Wallman and Winawer 2004, Norton, Amedo et al. 2010, Amedo and Norton 2012, Smith, Hung et al. 2014, Schaeffel and Feldkaemper 2015).
In early postnatal development, the emmetropization mechanism responds appropriately to the sign of the refractive error. Induced hyperopia (focal plane behind the retina), either naturally occurring or induced by placing a minus-power lens in front of the eye, produces an increase in the axial elongation rate which moves the retina outwards to the focal plane. This reduces the hyperopia so the eye becomes emmetropic while wearing the lens, (lens “compensation”). Induced myopia (focal plane in front of the retina), typically produced by placing a positive (plus-power) lens in front of the eye, produces slowed axial elongation if applied early in the postnatal period. The maturing optics at the front of the eye (which decrease in optical power with age) gradually move the focal plane back to the retina; the slowed axial elongation produces compensation for the plus lens so that the eye becomes emmetropic while wearing the lens and hyperopic with the lens removed (Schaeffel, Glasser et al. 1988, Schaeffel, Glasser et al. 1988, Irving, Sivak et al. 1992, Irving, Sivak et al. 1992, Hung, Crawford et al. 1995, Wildsoet 1997, Wildsoet 1997, Smith and Hung 1999, Wallman and Winawer 2004, Mutti, Mitchell et al. 2005, Shen and Sivak 2007, Metlapally and McBrien 2008, Howlett and McFadden 2009, Troilo, Totonelly et al. 2009, Norton, Amedo et al. 2010).
After achieving a near-emmetropic refractive state during infantile postnatal development, the emmetropization mechanism remains active through the juvenile and adolescent stages, maintaining emmetropia even though the axial length of the eyes continues to increase. During these older ages, many animals continue to show a robust response to the increased hyperopia produced by starting to wear a minus lens, or to the lack of images produced by wearing a translucent diffuser (“form deprivation,” which like minus lenses, also induces myopia) (Norton and Rada 1995, Papastergiou, Schmid et al. 1998, Smith, Bradley et al. 1999, Troilo, Nickla et al. 2000). However, in tree shrews, the ability of the emmetropization mechanism to compensate for a plus lens declines rapidly with age. Unlike infantile tree shrews, juvenile tree shrews wearing plus lenses do not slow the elongation rate of the eye. Normal elongation continues and the eyes remain myopic with the lens in place. (Siegwart and Norton 2010).
Although refractive myopia can be created in an emmetropic eye by wearing a plus lens, it can also be created by removing a negative lens or diffuser after the eyes have elongated and compensated for the minus lens. Upon lens removal, the elongated eyes are myopic (measured with the lens or diffuser removed). In response, the elongation rate is slowed and refractions return to normal levels, (“recovery from induced myopia”).
In contrast to the lack of compensation for plus lenses, juvenile and adolescent tree shrews recover robustly from an induced myopia (Norton, Amedo et al. 2010). The continued recovery response in juvenile and adolescent animals with induced myopia demonstrates that the emmetropization mechanism can detect and respond to the myopic refractive state over an extended age range with this species (Wallman and Adams 1987, Norton, Amedo et al. 2010). Refractively, the myopia that occurs when a minus lens is removed after compensation is very similar to the myopia that is measured when plus-lens wear begins in a normal eye. It is therefore a puzzle why plus lenses do not cause slowed axial elongation and refractive compensation at these older ages.
It has been suggested that an important difference between the lack of response to a plus lens in a normal eye vs. recovery from induced myopia is that eyes with induced myopia are elongated whereas a normal eye is not. Siegwart and Norton (2010) suggested that, in a normal-size eye, the emmetropization mechanism may not be able to slow axial elongation below a genetically pre-programmed minimum amount. In contrast, in an eye that has been elongated after minus lens wear, the sclera has undergone remodeling of the extracellular matrix and has altered protein and mRNA levels (Siegwart and Norton 1999, Frost, Guo et al. 2012, Guo, Frost et al. 2013, Grytz and Siegwart 2015). The altered sclera may respond to the signals from the emmetropization mechanism although normal sclera cannot.
Although optical blur is likely a powerful visual cue used by the emmetropization system to guide eyes to emmetropia and to maintain emmetropia, it need not be the only one. The full range of visual cues that the emmetropization mechanism uses to determine the amount and sign of refractive error are still poorly understood. Longitudinal chromatic aberration (LCA) could potentially be used as a cue to determine the sign of refractive error because long wavelength (“red”) light is focused farther from the cornea than is short wavelength (“blue”) light. Most mammals are dichromats, with their retinas containing only short-wavelength sensitive (SWS) cones and longer-wavelength sensitive (LWS) cones. Indeed, it has been argued that dichromacy is the baseline of mammalian color vision (Jacobs 1993). If a retinal image contains many wavelengths, and if the long (red) wavelengths are in focus but short (blue) wavelengths are out of focus, this could signal the emmetropization mechanism that the eye has become long enough, or too long, and the mechanism should slow the axial elongation rate. There is strong evidence that the emmetropization mechanism in chicks (which have four cone types) can make use of LCA cues (Rucker and Osorio 2008, Rucker and Wallman 2008, Rucker and Wallman 2009, Graef and Schaeffel 2012, Rucker and Wallman 2012, Britton, Hanowsky et al. 2014) (Rucker and Wallman 2008; Rucker and Osorio 2008; Rucker and Wallman 2009, 2012).
In infant tree shrews (dichromatic mammals closely related to primates) (Luckett 1980) we have recently found that narrow-band red light, that only stimulates the LWS cones, produces slowed axial elongation and prevents the normal decrease in refraction from hyperopia to emmetropia so that the eyes remain substantially hyperopic (Gawne, Siegwart et al. 2017). Similar results have been found in infant monkeys (Smith, Hung et al. 2015). The robust hyperopia and slowed axial elongation produced by red light in infant tree shrews led us to ask if red light can slow axial elongation in older, juvenile and adolescent, tree shrews at ages when plus lens wear is ineffective.
2. Materials and methods
2.1 Subjects and Experimental Groups
The tree shrews used in this study were raised in the UAB Tree Shrew Core by their mothers until weaning. The colony is maintained on a 14-hour light on/10 hour light off cycle (McBrien and Norton 1992, Guo, Frost et al. 2013, He, Frost et al. 2014). Fluorescent lighting (F34CW RS WM ECO) provided illuminance of 100 to 300 lux on the floor of the cages. All procedures complied with the Association for Research in Vision and Ophthalmology (ARVO) statement for the use of animals in ophthalmic and visual research and were approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham (UAB).
Tree shrews are born with their eyes closed. The first day that both eyes are open, which typically occurs about three weeks after birth, is designated as the first day of visual experience (DVE). Eye opening status was checked daily, and all of our experimental manipulations were synchronized to this developmental time point.
One normal (n=7), three experimental (n = 5 per group, Fig. 1A) and one higher illuminance control (n=5) groups were included. These were balanced to include both males and females and excluded more than one pup from the same parents for all but one group. The three experimental groups had red light exposure for 14 hours a day (10 hours of darkness) starting at 11, 35, or 95 DVE and continuing for 13 days (Fig. 1A). The animals in the 11 DVE group were included in a previous study (Gawne, Siegwart et al. 2017). The 11 DVE group was at an age when tree shrews will respond to a plus lens; the 35 DVE group was past the age when tree shrews stop responding to plus lenses (Siegwart and Norton 2010, Guo, Frost et al. 2012); the 95 DVE group was nearing sexual maturity (comparable to human adolescents). In the 95 DVE group, there were six animals in total: two animals were siblings and the data from these two were averaged and treated as a single animal (for the same reason that we average data from the two eyes for a single animal: they are likely correlated and so should not be treated as separate data points. In addition, averaging them should reduce the effects of measurement variability).
Figure 1.
A. Schematic of the experimental paradigm. Animals in all three experimental groups were initially raised in colony fluorescent light, then placed in narrow-band red light for 13 days, starting at different ages. The fluorescent control group was placed in higher illuminance fluorescent light, also for 13 days. All groups then were returned to colony lighting. A normal group remained in colony fluorescent lighting throughout development. B. Example of a red LED array on the top of a cage. C. Representative red LED spectra superimposed on normalized tree shrew long wavelength sensitive (LWS) and short wavelength sensitive (SWS). The effect of the red LEDs on the SWS cones was minimal, calculated to be between 5 and 6 log units lower than the effect of the red LEDs on the LWS cones.
The illuminance of the red light (527 to 749 human lux) was higher than standard colony illuminance (100–300 lux). Because there is evidence that greatly elevated light levels have significant effects on refractive development (French, Ashby et al. 2013, Norton and Siegwart 2013), a fourth, higher illuminance fluorescent control group, started treatment at 35 DVE with white fluorescent light that was more intense (averaging, 975 lux) than the colony lighting. This higher illuminance was comparable to the illuminance of the red lights. We note that there is no evidence that tree shrews growing up outside under daylight become grossly hyperopic, and most studies that have found effects of elevated light levels on emmetropization have used 15,000 – 40,000 lux. These have shown that the elevated light levels primarily limit the effects of myopiagenic stimuli rather than creating hyperopia per se (Ashby, Ohlendorf et al. 2009, Smith, Hung et al. 2013). Therefore, this control condition is conservative.
After wavelength or higher illuminance fluorescent treatment, animals were returned to standard fluorescent colony lighting for a “recovery” period of 20 days (36 days for the infant group). The three experimental and the fluorescent control groups were compared with a normal group (n=7) that was exposed to only standard fluorescent colony lighting (100 to 300 lux) for 14 hours a day throughout development, and was derived from previous experiments (Ward, Siegwart et al. 2016, Gawne, Siegwart et al. 2017).
2.2 Visual Stimulators
The red light was produced either by 16 type 40200R narrow-band LED truck tail lights (peak wavelength 624 ± 10 nm; ASL American Superlite, San Fernando, CA) arranged in a 4×4 array (Fig. 1B) used in the initial studies, or, when these became unavailable, by six NFLS-X3 led light strips (peak wavelength 636 ± 10 nm; Superbrightleds, St. Louis Missouri) arranged in three parallel rows of two strips each. The spectrum of the emitted light was measured with a PhotoResearch LS670 spectrophotometer (Fig. 1C). The LED arrays were affixed to a white-painted 60 cm by 60 cm piece of plywood that was then placed on top of the cage, which was a cube, 60 cm on a side. The illuminance of the red light at the cage floor was measured with a LX1330B digital illuminance meter (Hisgadget, Inc.). A white shelf 28 cm above the cage floor was often used by the animals; the illuminance on the shelf was approximately 40 percent greater on the shelf than on the cage floor. An opaque gray PVC tube with one end open served as a nest tube that the animals could enter and leave at will. The amount of time that the animals spent in the tube was not recorded but did not appear to differ across groups.
The illuminance provided by the red LEDs (Fig. 1C) was very far removed from the SWS cone absorption curve for this dichromatic species (Petry and Harosi 1990); the effect on the SWS cones was between 5 and 6 log units less than on the LWS cones. Thus, the effect of the red LEDs (regardless of whether the peak wavelength was 624 or 636 nm) was to activate almost exclusively the LWS cones and the retinal neurons that receive red-cone inputs.
The light for the fluorescent control group was produced with an array of four “Ecosomart” model EDX0-14 compact fluorescent light bulbs atop the cage. They had the same wavelength profile as the colony light; the nominal color temperature of these lights was 5000K, 800 lumens per bulb. The illuminance at the cage floor was approximately 975 lux.
2.3 Pedestal Installation
In order to consistently align the animals for refractive and axial component measures, a dental acrylic pedestal was installed on the skull of all animals either one day before treatment began (11 DVE group), or 3 days before treatment for the juvenile (35 DVE) and adolescent (95 DVE) groups, following procedures described previously (Siegwart and Norton 1994). In brief, the animals were removed from their home cage and anesthetized i.m. with 100 mg/kg ketamine, 7 mg/kg xylazine. After initial anesthesia, but before the procedure began, they were given atropine i.p. 0.27 mg/kg, buprenorphine i.m. 0.02 mg/kg, and carprofen s.q. 5 mg/kg. Anesthesia was supplemented with 0.5–2.0% isoflurane as needed. After recovery from anesthesia, the animals were weaned and housed singly, or in pairs in colony lighting until the start of treatment.
2.4 Refractive and axial measures
The refractive measures were made in awake, gently restrained animals with a Nidek ARK-700A infrared autorefractor (Marco Ophthalmic, Jacksonville, FL) (Norton, Wu et al. 2003). As in previous studies, non-cycloplegic measures typically were made because atropine may interfere with emmetropization (McKanna and Casagrande 1981, McBrien, Moghaddam et al. 1993) and because non-cycloplegic measures have been shown to provide a valid estimate of a wide range of refractive states in tree shrews. Measured in the same animals, cycloplegic refractions for untreated and for myopic eyes were 0.8 D hyperopic in comparison to non-cycloplegic refractions (Norton, Siegwart et al. 2000, Norton, Wu et al. 2003) indicating the presence of a small tonic accommodation in treated and control eyes. Nonetheless, refractions under tropicamide cycloplegia were measured at the end of red treatment in four of the 95 DVE animals (technically on five animals, but two were siblings and data were averaged for them and treated as one animal). All refractive values were corrected for the “small eye artifact” (Glickstein and Millodot 1970) previously shown to be about +4 D in tree shrews (Norton, Wu et al. 2003).
For the animals in the red-light and fluorescent control groups, refractive state was measured daily during the 13 days of treatment shortly after 9 AM, approximately 30 minutes after the lights were turned on. Animals were kept in darkness while being transported between their treatment cage and the measurement room. Measurements were made in a room that was illuminated only with lights similar to the treatment cage (red LEDs or fluorescent light) so that the animals did not have a period of visual stimulation that was different from the treatment conditions. The internal white target light of the autorefractor was disabled to further avoid spurious non-red visual stimulation. During the post-treatment “recovery” period in colony lighting, frequent refractive measures were continued after the end of treatment to assess if animals that had developed refractive error returned to age-normal refractive states.
The axial component measures were made in awake hand-held animals with a LenStar LS-900 optical biometer (Haag-Streit, Mason, OH USA). This system uses optical low-coherence optical interferometry, and has been found to typically give similar dimensions as other techniques such as ultrasonography, but with greater repeatability (Penha, Burkhardt et al. 2012). The LenStar provides and stores waveforms with peaks that correspond to the front and back of the cornea, front and back of the lens, front and back of the retina, and the front and back of the choroid (Ward, Siegwart et al. 2016). Off-line, cursors were manually moved to each peak to provide measures of corneal thickness, anterior chamber depth, lens thickness, vitreous chamber depth (using the retinal cursors of the international software version), retinal, and choroidal thickness. These were summed to provide a measure of axial length.
Axial component dimensions were measured in all animals at the start and end of the red light or fluorescent control treatment. For all but the 11 DVE group, axial component dimensions were also taken at the end of the recovery period in colony lighting. Ocular component measures in the seven normal animals raised in colony lighting were taken at multiple time points between 11 and 140 DVE. There were two time points where the normal group did not have measures that matched the experimental groups. For the 48 DVE time-point we interpolated the data for the individual normal animals between 39 and 52 DVE, and for the 108 DVE point we interpolated between 95 and 120 DVE. Refractions were relatively stable across these time points, so these interpolations were small in magnitude (see Fig. 2).
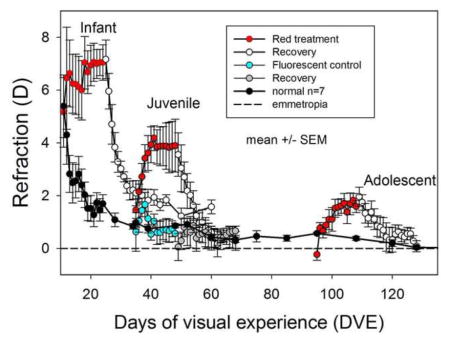
Fig. 2.
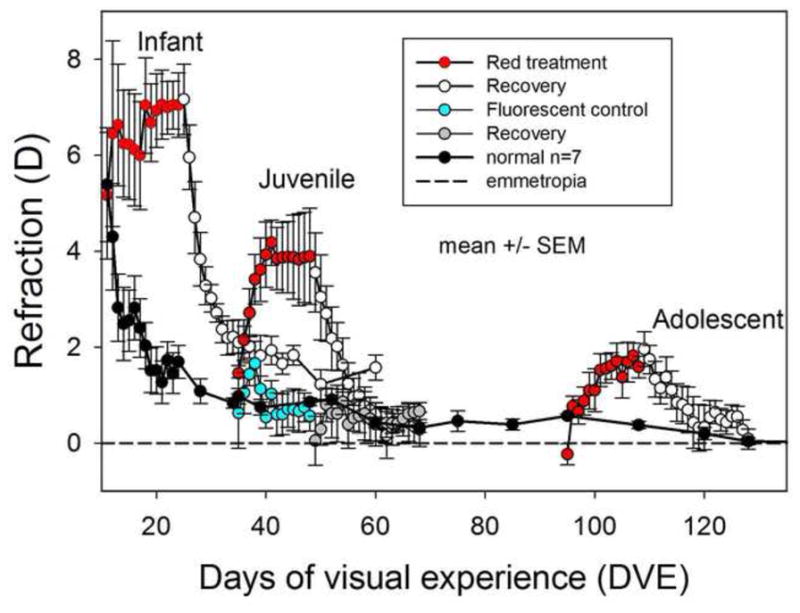
The effects of red light on refractive state (hyperopic refraction is positive) at different ages as a function of days of visual experience (DVE, horizontal axis). Error bars are mean ± standard error of the mean (SEM). The data for the infant group are from Gawne et al. (2017). The normal animals (black circles) show an initial hyperopia followed by a rapid decline and then a slower convergence towards emmetropia (dashed line). The animals exposed to the red light, however, developed a significant hyperopia (ANOVA, P<0.05) at all three age groups (red circles). Fluorescent control animals exposed to 975 lux (blue circles) did not become hyperopic. Upon return to standard colony lighting (open white circles), these animals all recovered back towards emmetropia.
2.5 Statistical Analysis
Refractive and axial component measures were summarized in Excel. For any single animal, both eyes (left and right) typically had very similar refractions: for all three red light groups the mean of the absolute value of the difference in refraction between the two eyes at the end of treatment was 0.6 ± 0.1 D (mean ± SEM), for the fluorescent control groups this was 0.4 ± 0.1 D, and for the normal animals at the same time as the end of treatment for the infant group was 0.4 ± 0.1 D. Therefore the data from the two eyes were averaged for each animal and treated as a single data point (Ederer 1973). Data on refractions were plotted as a function of time (days of visual experience). We calculated initial slopes of refractive development using the difference between the refraction on the fifth day of treatment, and the refraction at the first day of treatment (just before treatment began, therefore spanning four days in total). These were compared with slopes from previous studies (Norton, Amedo et al. 2010, Siegwart and Norton 2010, Guo, Frost et al. 2012).
We performed a two-way analysis of variance of the refractive and ocular component data using the ANOVAN function of Matlab (The math works, Natick, MA), using the factors of age (11/35/95 DVE) × light (red/colony). Significance was set at P < 0.05. If the 2-way ANOVA was significant, Tukey’s HSD (highly significant difference) test was used to learn if there were differences between the red-treated and normal groups at each age. Because there was only one fluorescent control group (35 DVE), intended as a control for illuminance, we compared the results of that group with the normal animals at 35 DVE with a t-test (P < 0.05 significance). We performed this test both at the end of treatment and at the end of the recovery period. However, as we did not have axial data at the end of recovery for the infant group, the ANOVA for axial data at the end of recovery was a 2X2 design.
3. Results
3.1 Refractive
Measures
The juvenile (treatment start at 35 DVE) and adolescent (95 DVE) animals had completed the initial emmetropization process, so their starting refractions were an age-appropriate low hyperopia for the juvenile group (1.4 ± 0.4 D) and near-emmetropia for the adolescent group (−0.2 ± 0.2 D). A two-way ANOVA for refraction at the start of red-light treatment on the factors of age and red light exposure showed significant results only for age, not treatment group. Therefore, the initial refractions of the treatment animals were well matched to those of the normal animals.
During red-light treatment (Fig. 2), as reported previously (Gawne, Siegwart et al. 2017), the refractions of the infant group remained hyperopic, whereas the refractive state of the normal colony-reared group decreased toward emmetropia. The refractive state of animals in the juvenile and adolescent groups moved rapidly away from normal and became significantly hyperopic compared to their pre-treatment refraction and to age-matched normal animals (see Table 1). At the end of red-light treatment the infant group was on average 5.36 D hyperopic compared to age-matched normals, the juvenile group 3.04 D, and the adolescent, 0.75D. A 2-way ANOVA showed that refraction at the end of treatment was significantly affected by both age and red light exposure, and a post-hoc test showed that all three red-light groups were hyperopic compared with normals (see supplementary table 2). The hyperopic shift was very rapid in the juvenile group. During the first four days of red-light treatment, the refraction rose rapidly, approximately 0.5 D/day. The effect of the red light was less, and less rapid, in the adolescent group, but the animals still became significantly hyperopic (over 1 D) compared with age-matched normals.
Table 1.
Refractions and axial length values at the end of the red light treatment period.
| 2-WAY ANOVA1 (P < 0.05, in bold) | |||||
|---|---|---|---|---|---|
| INFANT | JUVENILE | ADOLESCENT | AGE | LIGHT | |
| REFRACTION (D) | |||||
| RED | 7.05 ± 0.67 * | 3.89 ± 1.00 * | 1.59 ± 0.22 * | P = 0.0002 | P < 0.0001 |
| COLONY | 1.69 ± 0.41 | 0.85 ± 0.10 | 0.84 ± 0.09 | F = 11.38 | F = 38.88 |
| ANTERIOR CHAMBER DEPTH (μm) | |||||
| RED | 676.0 ± 4.3 | 712.4 ± 13.5 | 735.4 ± 13.3 | P = 0.0002 | P = 0.0345 |
| COLONY | 699.8 ± 12.4 | 740.1 ± 13.5 | 746.4 ± 15.2 | F = 11.17 | F = 4.88 |
| LENS THICKNESS (mm) | |||||
| RED | 3.22 ± 0.02 | 3.42 ± 0.02 | 3.73 ± 0.01 | P < 0.0001 | P = 0.0722 |
| COLONY | 3.18 ± 0.01 | 3.41 ± 0.01 | 3.71 ± 0.02 | F = 644.36 | F = 3.46 |
| VITREOUS CHAMBER DEPTH (mm) | |||||
| RED | 2.65 ± 0.02 * | 2.67 ± 0.03 * | 2.59 ± 0.03 * | P = 0.0005 | P = 0.0002 |
| COLONY | 2.78 ± 0.04 | 2.75 ± 0.03 | 2.65 ±0.02 | F = 9.62 | F = 17.3 |
| RETINAL THICKNESS (μm) | |||||
| RED | 206.6 ± 6.1 | 186.6 ± 4.6 | 187.6 ± 7.6 | P = 0.2658 | P = 0.1549 |
| COLONY | 184.4 ± 2.6 | 184.0 ± 4.8 | 193.3 ± 5.5 | F = 1.38 | F = 2.12 |
| CHOROIDAL THICKNESS (μm) | |||||
| RED | 75.2 ± 11.0 * | 77.4 ± 4.3 * | 72.2 ± 7.1 * | P = 0.4606 | P = 0.0003 |
| COLONY | 63.1 ± 4.2 | 56.1 ± 2.9 | 54.4 ± 1.9 | F = 0.79 | F = 16.8 |
| AXIAL LENGTH (mm) | |||||
| RED | 7.07 ± 0.01 | 7.28 ± 0.05 | 7.56 ± 0.05 | P < 0.0001 | P = 0.0138 |
| COLONY | 7.14 ± 0.03 | 7.38 ± 0.03 | 7.59 ± 0.03 | F = 116.17 | F = 6.79 |
| CORNEAL THICKNESS (μm) | |||||
| RED | 241.6 ± 5.9 | 218.8 ± 7.0 | 242.8 ± 5.9 | P = 0.0592 | P = 0.3553 |
| COLONY | 222.4 ± 6.8 | 229.0 ± 6.5 | 237.6 ± 5.3 | F = 3.09 | F = 0.88 |
A 2-way ANOVA was performed on each variable with the factors of age and red light. If the ANOVA showed significance P < 0.05, a single asterisk “*” indicates when the red group was different from the normal colony group at the same age using Tukey’s HSD test.
To verify that the non-cycloplegic refractions provided an accurate measure of the refractive state at the end of treatment, cycloplegic refractions were conducted in four of the five animals in the adolescent group. The non-cycloplegic refraction of these animals was 1.6 ± 0.3 D (mean ± SEM). With tropicamide cycloplegia, the refractions were 2.0 ± 0.5 D, a shift of only +0.4 D. This suggests that reduced tonic accommodation was not the cause of the red-light induced hyperopia.
The 35 DVE fluorescent control group, exposed to levels of fluorescent light at a comparable illuminance to the red showed, a brief hyperopic shift (Fig. 2) that peaked at three days of treatment and was not significantly different from the normal animals (t-test, P>0.05). At the end of treatment period the refraction of this group (0.6 ± 0.3 D) was not significantly different from normal (t-test, P > 0.05). The absence of an effect of the increased illuminance demonstrated that the effect of the red light was due to the wavelength, not the intensity.
Upon return to colony lighting, refractions of all three treated groups decreased toward normal levels, demonstrating that the red light had not damaged the emmetropization mechanism. After 36 days in colony lighting, the infant (11 DVE) group remained slightly hyperopic compared to age-matched (60 DVE) normals (1.6 ± 0.3 D vs 0.4 ± 0.1 D, mean ± SEM).
3.2 Axial Component Measures
Fig. 3 illustrates the axial component dimensions at the start and end of treatment, and the end of recovery. Table 1 shows end-of-treatment values only. Corneal thickness was unaffected by the red light treatment (Table 1). The depth of the anterior chamber at the end of red-light treatment (Fig. 3A, Table 1) did appear to be less in the red light group vs. the normals. A two-way ANOVA showed significant effects of both age and red light treatment on this parameter, but no post-hoc test was significant. Thus we cannot rule out that our red treatment has some effect on anterior chamber depth, but these would seem to be relatively small compared to normal variation.
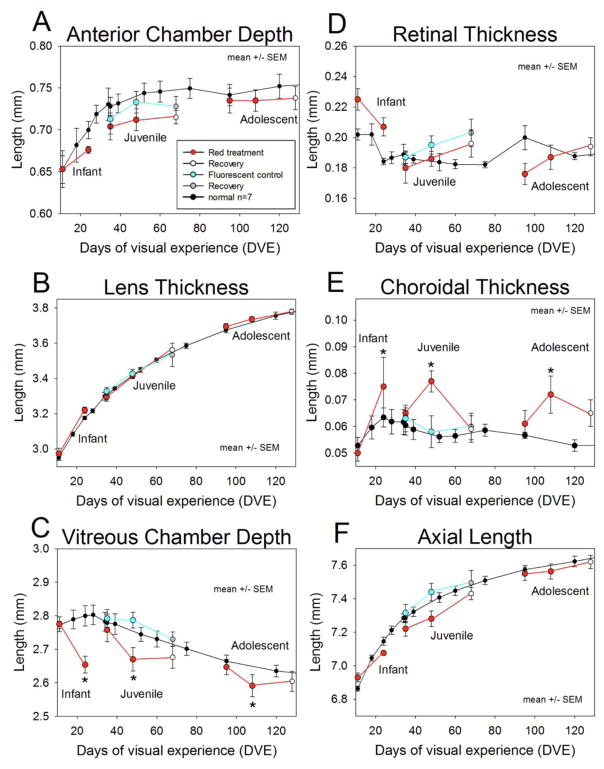
Fig. 3.
Axial component dimensions as measured with a LenStar biometer (mean ± SEM). Unlike the measures of refraction, which were typically made every day in the treatment groups (see Fig. 2), the axial component dimensions were only measured at the start of treatment, the end of treatment, and (for the 35 and 95 DVE groups) at the end of recovery. The normal group was measured frequently throughout development. A. Anterior chamber depth B. Lens thickness. C. Vitreous chamber depth D. Retinal thickness E. Choroidal thickness and F. Axial length as a function of days of visual experience (DVE). Asterisks represent where the red light group is statistically different from normals via Tukey’s HSD test.
Lens thickness in the red light group, as a function of age (Fig 3B), was very similar to the normal pattern of steady growth. In keeping with this, a 2-way ANOVA showed significant effects for age only and not red light treatment. The growth of the lens does not appear to be affected by our experimental conditions.
Fig. 3C shows the vitreous chamber depth as a function of time. The normal data at first appears somewhat paradoxical: initially the vitreous chamber depth increases (until about 24 DVE) but then becomes smaller even as the eye overall continues to grow as shown previously by Norton & McBrien (1992). This occurs because the lens is large in the tree shrew and grows continuously. As the axial elongation rate slows but the lens thickness continues to increase, the vitreous chamber depth decreases. Compared to age-matched normals, at the end of treatment the infant group vitreous chamber was on average 130 μm shorter than the age-matched control, the juvenile group 80μm shorter, and the adolescent group 60 μm shorter. A 2-way ANOVA showed that vitreous chamber depth was significantly affected by both age and red light exposure, and a post-hoc test showed that for each age the vitreous chamber depth in the red light groups was smaller than normal (see Table 1).
Retinal thickness (Fig. 3D) was highly variable and a 2-way ANOVA did not show significant effects of either age or red light treatment. Choroidal thickness (Fig. 3E) appeared to be strongly increased by the red light treatment. At the end of treatment, the infant group choroid was on average 12.1 μm thicker than age-matched normals, the juvenile group 21.3 μm thicker, and the adolescent group 17.8 μm. A 2-way ANOVA showed significant thickening only in the red light treatment groups, and post-hoc tests showed significant end-or red treatment differences between the red light groups and normal animals at all ages.
The axial length of the eye (sum of the components, Fig. 3F) in the normal animals increased monotonically. During red-light treatment, the increase in axial length slowed, so that the axial lengths were shorter at the end of treatment. A two-way ANOVA showed significant effects of both age and red light treatment on axial length at the end of treatment, but no single post-hoc comparison was significant. This was not surprising given that the axial length is the sum of all ocular components and that the vitreous chamber decrease was somewhat offset by the increased choroidal thickness.
At the end of recovery, we only had axial component measures for the juvenile and adolescent groups. Overall, the data showed a strong trend for axial length parameters to move back towards the age-matched controls. A two-way ANOVA with the factors of age and light using values at the end of recovery only showed a persisting effect of red light treatment on choroid thickness, although post-hoc Tukey HSD tests were not significant for either age group (see table 2 in the supplementary material).
3.3 Comparison with plus lens wear and recovery from minus lens wear
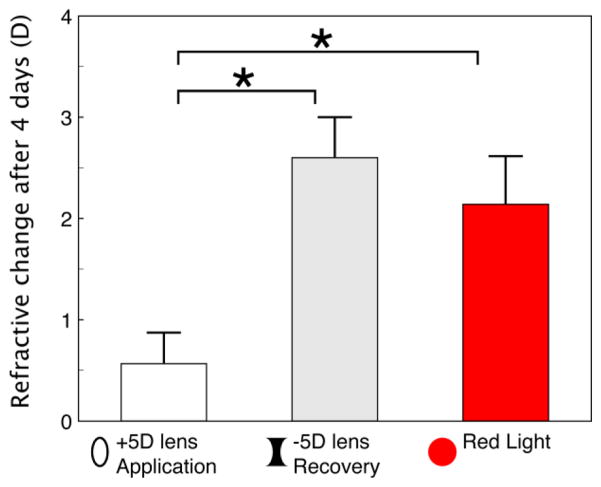
As noted in the Introduction, Siegwart and Norton (2010) found that infant tree shrews (11 DVE) responded to plus lens wear by slowing their axial elongation rate so that the eyes became hyperopic, measured with the lens removed. However, juvenile tree shrews (24 DVE) responded only slightly to plus lens wear. In contrast, tree shrews of the same age, that had been made myopic with a minus lens, showed robust recovery when the lens was removed, with slowed axial elongation and a hyperopic refractive shift back toward emmetropia. Guo et al. (2012) directly compared the response to four days of plus lens wear vs. recovery from minus lens wear in tree shrews starting at 35 DVE. As shown in Fig. 4, after four days, Guo et al. observed only a small (0.6 ± 0.3 D) hyperopic shift in the plus lens group. The refraction of the recovering myopic animals moved rapidly in a hyperopic direction (toward emmetropia) by 2.6 ± 0.4 D. As also shown in Fig. 4, the refractions of the juvenile red light group, that began treatment at the same age (35 DVE) became hyperopic by 2.2 ± 0.5 D during the same four-day time span. This hyperopic shift in animals with an initially normal axial length and, presumably, normal sclera was similar to the hyperopic change during recovery found in animals with elongated eyes and remodeled sclera, and was significantly larger than the response to plus-lens wear in normal animals.
Fig. 4.
Refractive change (final refraction - initial refraction) after four days of (left to right) application of a +5d lens (n=7), removal of a −5 D lens (n=7), and four days of red light treatment (n=5), all starting at 35 DVE. Error bars are +/− SEM. A one-way ANOVA showed significant effects (P < 0.05), starred bars indicate differences between groups via Tukey’s post-hoc HSD test. The +5 D application and −5 D removal data are from (Guo, Frost et al. 2012)
3.4 Initial Slope of Refractive Changes as a Function of Age
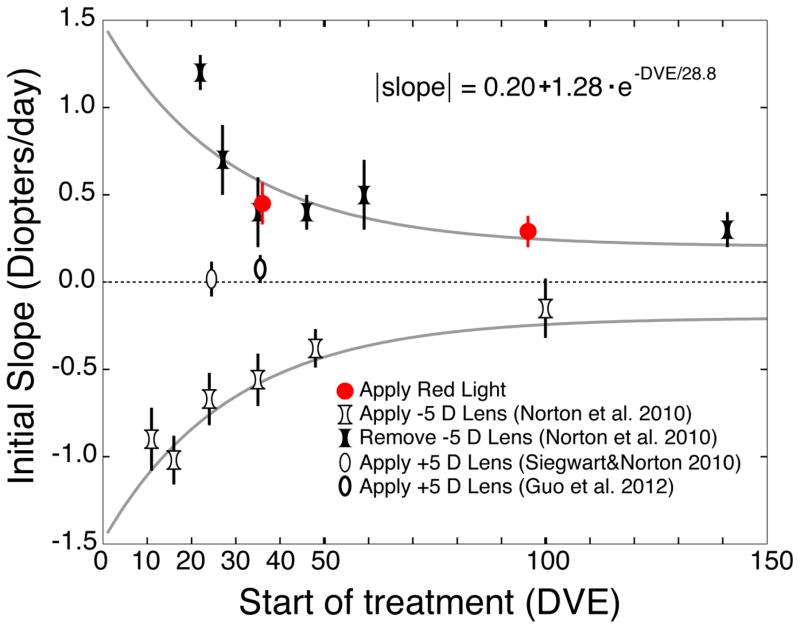
When lens-wear is either started or discontinued, the initial slope of the change in refraction as a function of time can be considered as a measure of ability of the emmetropization mechanism to detect and respond to this intervention. The white concave symbols in the bottom half of Fig. 5 plot the slope of myopia development (in diopters/day) during the first four days of wearing a −5 D lens at a variety of ages (Norton et al 2010). The response to a monocular −5 D lens is a myopic shift (negative slope) in the refractive state of the treated eye compared with its fellow control eye. At young ages, (leftmost data points) the initial change in refraction is quite rapid, on the order of −1 D/day. The slope of myopia development decreases with age, but even adolescent tree shrews (right-most data point) still respond to the −5 D lens.
Fig. 5.
Slopes of refractive change across the first four days of treatment for four types of treatments. Error bars are mean ± SEM. The open concave symbols indicate the myopic shift in groups of animals that began monocular minus-lens wear or recovery from induced myopia at a variety of ages ranging from infantile to adolescent (Norton et al, 2010). The filled concave symbols show the four-day hyperopic shift (recovery) in groups after removal of a minus lens in the same study. The open convex symbols show the small four-day refractive shift in animals exposed to binocular plus-lens wear at 24 DVE (Siegwart & Norton 2010) or monocular plus lens wear at 35 DVE (Guo, Frost et al. 2012). The red symbols indicate the hyperopic shift after 4 days of binocular red-light treatment at 35 and 95 DVE. The solid gray curves are fits of an exponential function to the absolute value of the slopes of the response to −5 D lens application and removal: the two curves are thus the same curve reflected about the horizontal axis. Each data point is plotted at the age (DVE) at which the treatment began. The precise times in DVE have been slightly displaced for the 35 DVE Apply Red Light and the Remove −5D Lens data points to avoid overlapping error bars. This fit accounts for 76% of the variance of the slope. We added in a constant term to better fit the data at the older ages, but we do not claim that this constant will apply to animals with ages outside the range of this fit. The data for the infant red-light group is not shown because the refraction of that group remained at a stable hyperopia while the normal group’s refractions decreased (Fig. 2).
When an animal has compensated to −5 D lens wear, removing the lens produces a myopic refractive state, triggering refractive recovery: a hyperopic shift in refraction. In the top half of Fig. 5, the black concave figures plot the slope of the recovery (hyperopic shift) after the removal of a −5 D lens (Norton et al. 2010). In young animals, the hyperopic shift is relatively rapid, more than 1 D/day. The slope of the refractive recovery decreases with age, but recovery occurs even in young adult tree shrews.
The two curves (dashed lines) in Fig. 5 are exponential functions fit to the absolute value of the slope for both myopia development and recovery. Thus, they plot the same equation reflected about the x-axis, representing the magnitude of the slope to both the start and removal of a −5 D lens.
The slope of the hyperopic shift in response to the onset of wearing a +5 D lens (thin white ellipses in Fig. 5) at 24 DVE is very small (Siegwart and Norton 2010) or at 35 DVE (thick white ellipse) (Guo et al. (2012). In contrast, the slope of the hyperopic response to red-light treatment at both ages (red symbols) is similar to the slope of similar-aged recovery from induced myopia.
4.0 Discussion
4.1 Red light is effective at older ages
We previously found that ambient narrow-band red light produced a substantial reduction in vitreous chamber depth and refractive hyperopia in infant tree shrews (Gawne, Siegwart et al. 2017). The primary finding of this study is that exposure to ambient long-wavelength light also produces substantial and significant hyperopia in juvenile and adolescent tree shrews. During red-light treatment, the refractions moved away from emmetropia and became significantly hyperopic compared to age-matched normals. At all ages, the refractive shift was produced by a reduction in the depth of the vitreous chamber, coupled with an increase in the thickness of the choroid. Upon return to standard colony lighting, there was refractive and axial recovery from the induced hyperopia, suggesting that the emmetropization mechanism continued to function both during and after the red-light treatment.
Not only does the red light produce hyperopia and slowed axial (vitreous chamber) elongation when imposed on emmetropic eyes, the rate (slope, Fig. 5) at which the refraction responds to the red light is similar to the rate at which eyes recover from lens-induced myopia. As in the case of recovery, the juvenile red-light tree shrews responded more quickly to the long-wavelength stimulus condition than did the adolescent group. Even though the axial length (and presumably other aspects of the eye, such as the structure of the sclera) was initially normal, the long-wavelength light produced a hyperopic refractive shift that was nearly as strong as the refractive recovery in animals with an induced myopia caused and altered scleral remodeling (Guo, Frost et al. 2012, Guo, Frost et al. 2013).
The long wavelength light used in this study produced slowed axial elongation even though that increased the refractive error, producing hyperopia. In a normal colony environment, hyperopia is a cue used by the emmetropization mechanism to increase axial elongation, reducing the refractive error (Wildsoet 1997, Norton 1999, Wallman and Winawer 2004, Smith, Hung et al. 2010, Schaeffel and Feldkaemper 2015). The increasing hyperopia, sustained during red treatment, suggests that the long wavelength light interfered in some way with the production, or utilization, of hyperopia-generated retinal signals.
We hypothesize that if only long wavelengths are present in the image, as is the case with narrow-band red light that contains no short (blue) wavelengths, then this could be interpreted by the emmetropization mechanism as red image contrast being present, and blue image contrast being so low that it cannot even be detected. In the context of using LCA as a cue, this might signal that the eye is too long (red in better focus than blue) and should slow its axial elongation rate. This stimulus condition would not change, even if the refractive error changes, because there is no light that stimulates the short-wavelength sensitive cones. This could potentially explain why the red light produced slowed elongation even though that increased the hyperopic refractive error.
The effects of monochromatic/narrow band illumination on emmetropization have been inconsistent in previous studies of several species (For discussion see Smith, Hung et al. 2015). It appears that the emmetropization mechanism can sometimes use narrow-band wavelength information as a target where the eye attempts to elongate to match the focal plane of the specific wavelength, and sometimes use it as a cue, as in the present study. In some previous studies in red light, the eyes became longer, which is consistent with the long-wavelength stimulus serving as a target (Fish: Kröger and Fernald 1994, Chicks: Seidemann and Schaeffel 2002, Rucker and Wallman 2008, Guinea Pigs: Long, Chen et al. 2009, Liu, Qian et al. 2011, Qian, Dai et al. 2013, Jiang, Zhang et al. 2014). However, our data here, and a study in rhesus monkeys (Smith, Hung et al. 2015), are consistent with wavelength being used as a cue. Why sometimes the emmetropization system reacts one way and sometimes another is not currently known, although luminous intensity, age, species, the specific wavelength distributions and their interactions with cone-pigment absorption curves in different species, and the duration of exposure may be involved (Smith, Hung et al. 2015). In particular, most mammals are dichromats (Jacobs 1993). As trichromacy evolved relatively recently in primates, we speculate that that the human emmetropization system relies primarily on long-vs-short wavelength opponency. In support of this, human dichromats with red-green color blindness do not appear to develop significantly more refractive error than trichromats – indeed, some forms of red/green color blindness may actually be protective against myopia (Qian, Chu et al. 2009) suggesting that the red/green system can sometimes interfere with emmetropization. On the other hand, chicks come from a long evolutionary lineage of tetrachromacy, and use oil droplets to further sharpen the wavelength tuning of their cones (Wilby and Roberts 2017). We should not therefore be surprised if the emmetropization systems of mammals and birds both use wavelength cues, but differ in how they use them.
The results of the present study, taken together with the effect of minus-lenses in juvenile and adolescent tree shrews, provide strong evidence that the apparently stable refractions at these older ages are the result of continued functioning of the emmetropization mechanism. As shown in Fig. 3F, the axial length of normal eyes increases by about 0.5 mm between the juvenile period (24 DVE) and the end of the adolescent period (120 DVE). During this period of substantial growth, normal refractions are very stable near emmetropia (Fig. 2). To accomplish this requires continuous monitoring of refractive error and continued modulation of axial elongation; a mismatch of as little as 25 – 40 μm would produce one diopter of refractive error (Gawne, Siegwart et al. 2017). The results of the present study show the same relationship between the refractive and axial (vitreous chamber) changes and provide further evidence that the emmetropization mechanism is not only capable of increasing elongation during this period in response to a minus lens or from deprivation, but also of slowing axial elongation throughout the adolescent period.
4.2. Contrast between red light and plus-lens wear
The second main finding of this study is that normal juvenile and adolescent tree shrews can develop significant hyperopia in response to the red light at ages (35 and 95 DVE) when plus-lens wear has been found to be ineffective. One possible explanation for this difference might be that, with age, a myopic refractive state becomes a less effective signal to slow axial elongation. However, we cannot exclude the possibility that the plus-power lenses (a human PMMA lens, 7.5 mm radius, no peripheral curves), held in a goggle frame, used in previous studies in tree shrews, may produce other refractive stimuli (aberrations, peripheral refractive effects) that interfere with signals produced by the lens-induced myopic refractive state. However, when similar lenses, but with smaller plus power, were used in recovering animals, they guided eyes to nearly appropriate refractions (Amedo and Norton 2012). There is little information on the responses of other species to plus lenses or recovery at similar (juvenile and adolescent) ages. However, recently, Ashby and Karouta (2017) reported that plus lenses in young chicks do not produce the same changes in Egr-1 mRNA as does recovery from induced myopia, suggesting that plus lenses are different from recovery, even though the eyes respond to the plus lenses.
In the juvenile and adolescent tree shrews of this study, the eyes, and presumably the scleras, were normal at the start of treatment. So, too, were tree shrews that began to wear plus lenses at similar ages. In contrast, tree shrews at similar ages that recover from an induced myopia begin recovery with elongated eyes and scleras that have altered protein levels, mRNA levels and biomechanical properties. (Siegwart and Norton 1999, Frost and Norton 2012, Guo, Frost et al. 2012, Guo, Frost et al. 2013, Grytz and Siegwart 2015). The effectiveness of the red light in slowing axial elongation in eyes with normal scleras suggests that elongated eyes (an “eye-size” factor) does not explain the difference between the lack of response to plus lens wear and the recovery response when a minus lens is removed after compensation has occurred.
The most prominent anatomical change during red-light treatment was reduced vitreous chamber elongation, which moved the retina toward the cornea. There also were increases in choroidal thickness that also moved the retina toward the cornea and affected refraction to some degree (Wallman, Wildsoet et al. 1995). The decrease in vitreous chamber depth was greatest in the infant group and decreased with age, but the increase in choroid thickness remained relatively constant across age groups, so that the choroidal change was an increasing fraction of the two components. Based on the observed change in refraction vs. normal, the decrease in vitreous chamber, and the increase in choroid in the three groups, it appears that the choroid may have contributed 0.8 D of the induced hyperopia in the juvenile group and 0.4 D in the adolescent group. If the pattern of less vitreous change and constant choroid change continues beyond the ages studied here, at some age there might be only choroidal changes and a small, transient induced hyperopia from red-light treatment. While not yet demonstrated in tree shrews, it has been shown that in young adult human subjects, choroidal thickness can still respond significantly to changes in optical focus (Read, Collins et al. 2010).
Conclusion
It has previously been shown that, while infant tree shrew eyes can adapt to a plus lens by restraining axial elongation, they lose this ability with age. We have previously shown that narrow-band red light strongly restrains eye elongation in infant tree shrews: here we show that narrow-band red light maintains this effect even in older juvenile and adolescent animals. Most children initially emmetropize successfully, it is only later in childhood and adolescence that myopia typically develops.
Perhaps the inability of plus-lens induced myopia to restrain axial elongation in older tree shrews reflects a similar inability of the human emmetropization mechanism to respond, with age, to the defocus associated with becoming myopic. The continued effectiveness of our narrow-band long-wavelength light in juvenile and adolescent tree shrews demonstrates that, given appropriate stimuli, the emmetropization mechanism can still slow eye the axial elongation rate. This raises the possibility that, while the human emmetropization mechanism may lose the ability to respond to one class of visual cues with age, perhaps there are other visual cues (red light, or something else) to which they may still be responsive. If so, this could open the door to developing new approaches to limit the progression of myopia.
Supplementary Material
Table 2.
Refractions and axial length values at the end of the recovery period.
| RECOVERY VALUES | 2-WAY ANOVA1 (P < 0.05, in bold) | ||||
|---|---|---|---|---|---|
| INFANT | JUVENILE | ADOLESCENT | AGE | LIGHT | |
| REFRACTION (D) | |||||
| RED | 1.57 ± 0.26 | 0.29 ± 0.35 | 0.08 ± 0.21 | P = 0.0066 | P = 0.1300 |
| COLONY | 0.43 ± 0.12 | 0.44 ± 0.13 | 0.20 ± 0.15 | F = 5.90 | F = 2.41 |
| ANTERIOR CHAMBER DEPTH (μm) | |||||
| RED | 715.2 ± 7.5 | 737.6 ± 14.4 | P = 0.4059 | P = 0.0872 | |
| COLONY | 748.6 ± 12.2 | 751.4 ± 14.7 | F = 0.72 | F = 3.22 | |
| LENS THICKNESS (mm) | |||||
| RED | 3.56 ± 0.01 | 3.78 ± 0.01 | P < 0.0001 | P = 0.0636 | |
| COLONY | 3.52 ± 0.02 | 3.75 ± 0.02 | F = 173.02 | F = 3.83 | |
| VITREOUS CHAMBER DEPTH (mm) | |||||
| RED | 2.68 ± 0.03 | 2.60 ± 0.03 | P = 0.0083 | P = 0.2429 | |
| COLONY | 2.70 ± 0.02 | 2.63 ±0.02 | F = 8.50 | F = 1.44 | |
| RETINAL THICKNESS (μm) | |||||
| RED | 196.0 ± 8.8 | 194.0 ± 6.0 | P = 0.7363 | P = 0.0564 | |
| COLONY | 182.0 ± 2.9 | 187.1 ± 2.9 | F = 0.12 | F = 4.08 | |
| CHOROIDAL THICKNESS (μm) | |||||
| RED | 59.4 ± 5.3 | 65.4 ± 4.6 | P = 0.8066 | P = 0.0286 | |
| COLONY | 55.7 ± 2.0 | 52.8 ± 1.8 | F = 0.06 | F = 5.67 | |
| AXIAL LENGTH (mm) | |||||
| RED | 7.43 ± 0.04 | 7.62 ± 0.04 | P < 0.0001 | P = 0.7873 | |
| COLONY | 7.45 ± 0.03 | 7.62 ± 0.03 | F = 31.2 | F = 0.07 | |
| CORNEAL THICKNESS (μm) | |||||
| RED | 222.4 ± 5.0 | 238.6 ± 8.6 | P = 0.0185 | P = 0.5929 | |
| COLONY | 227.1 ± 4.7 | 240.0 ± 4.9 | F = 6.52 | F = 0.29 | |
We did not have axial component data for the end of recovery for the infant group, so those values are left blank and the ANOVA is simply 2×2 with the factors of age and light.
Highlights.
Juvenile and adolescent tree shrews become hyperopic in narrow-band red light
The vitreous chamber is smaller than normal and the choroid is thicker
Tree shrews at these ages do not become hyperopic in response to a plus lens
The continuing response to red contrasts with the lack of response to a plus lens
The emmetropization mechanism can slow eye growth in juvenile and adolescent animals
Acknowledgments
Supported by the UAB Tree Shrew Core, the UAB Faculty Development Grant, the UAB Comprehensive Neuroscience Center (CNC), NEI P30 EY003909 (core), NEI R21 EY025254, and the Alabama Eyesight Foundation. The authors acknowledge the technical assistance of Regina Rab, Alex Zotov, and Eric Worthington.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amedo AO, Norton TT. Visual guidance of recovery from lens-induced myopia in tree shrews (Tupaia glis belangeri) Ophthalmic Physiol Opt. 2012;32(2):89–99. doi: 10.1111/j.1475-1313.2011.00875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andison ME, Sivak JG, Bird DM. The refractive development of the eye of the American kestrel (Falco sparverius): a new avian model. Journal of Comparative Physiology [A] 1992;170:565–574. doi: 10.1007/BF00199333. [DOI] [PubMed] [Google Scholar]
- Ashby R, Karouta C. Egr-1 mRNA Expression in Response to Myopic Defocus. 2017 ARVO E-Abstract 5640. [Google Scholar]
- Ashby R, Ohlendorf A, Schaeffel F. The effect of ambient illuminance on the development of deprivation myopia in chicks. Invest Ophthalmol Vis Sci. 2009;50(11):5348–5354. doi: 10.1167/iovs.09-3419. [DOI] [PubMed] [Google Scholar]
- Atchison DA, Jones CE, Schmid KL, Pritchard N, Pope JM, Strugnell WE, Riley RA. Eye shape in emmetropia and myopia. Invest Ophthalmol Vis Sci. 2004;45(10):3380–3386. doi: 10.1167/iovs.04-0292. [DOI] [PubMed] [Google Scholar]
- Bradley DV, Fernandes A, Lynn M, Tigges M, Boothe RG. Emmetropization in the rhesus monkey (Macaca mulatta): birth to young adulthood. Invest Ophthalmol Vis Sci. 1999;40(1):214–229. [PubMed] [Google Scholar]
- Bradley DV, Fernandes A, Lynn M, Tigges M, Boothe RG. Emmetropization in the rhesus monkey (Macaca mulatta): birth to young adulthood. Invest Ophthalmol Vis Sci. 1999;40(1):214–229. [PubMed] [Google Scholar]
- Britton S, Hanowsky S, Rucker FJ. Blue light protects against temporal frequency dependent refractive changes. ARVO Meeting Abstracts. 2014;55(5):3598. doi: 10.1167/iovs.15-17238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton TC. The influence of refractive error and lattice degeneration on the incidence of retinal detachment. Trans Am Ophthalmol Soc. 1989;87:143–155. [PMC free article] [PubMed] [Google Scholar]
- Chen J, Xie A, Hou L, Su Y, Lu F, Thorn F. Cycloplegic and noncycloplegic refractions of Chinese neonatal infants. Invest Ophthalmol Vis Sci. 2011;52(5):2456–2461. doi: 10.1167/iovs.10-5441. [DOI] [PubMed] [Google Scholar]
- Cook RC, Glasscock RE. Refractive and ocular findings in the newborn. American Journal of Ophthalmology. 1951;34:1407–1413. doi: 10.1016/0002-9394(51)90481-3. [DOI] [PubMed] [Google Scholar]
- Dolgin E. The myopia boom. Nature. 2015;519(7543):276–278. doi: 10.1038/519276a. [DOI] [PubMed] [Google Scholar]
- Ederer F. Shall we count numbers of eyes or numbers of subject? Archives of Ophthalmology. 1973;89:1–2. doi: 10.1001/archopht.1973.01000040003001. [DOI] [PubMed] [Google Scholar]
- French AN, Ashby RS, Morgan IG, Rose KA. Time outdoors and the prevention of myopia. Exp Eye Res. 2013;114:58–68. doi: 10.1016/j.exer.2013.04.018. [DOI] [PubMed] [Google Scholar]
- Frost MR, Guo L, Norton TT. Whole transcriptome analysis of tree shrew sclera during the development of lens-induced myopia. Investigative Ophthalmology and Visual Science. 2012;53 doi: 10.1167/iovs.11-8354. ARVO E-abstract 3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost MR, Norton TT. Alterations in protein expression in tree shrew sclera during development of lens-induced myopia and recovery. Invest Ophthalmol Vis Sci. 2012;53(1):322–336. doi: 10.1167/iovs.11-8354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawne TJ, Siegwart JT, Jr, Ward AH, Norton TT. The wavelength composition and temporal modulation of ambient lighting strongly affect refractive development in young tree shrews. Exp Eye Res. 2017;155:75–84. doi: 10.1016/j.exer.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickstein M, Millodot M. Retinoscopy and eye size. Science. 1970;168:605–606. doi: 10.1126/science.168.3931.605. [DOI] [PubMed] [Google Scholar]
- Graef K, Schaeffel F. Control of accommodation by longitudinal chromatic aberration and blue cones. J Vis. 2012;12(1):14. doi: 10.1167/12.1.14. [DOI] [PubMed] [Google Scholar]
- Graham B, Judge SJ. Normal development of refractive state and ocular component dimensions in the marmoset (Callithrix jacchus) Vision Research. 1999;39(2):177–187. doi: 10.1016/s0042-6989(98)00188-6. [DOI] [PubMed] [Google Scholar]
- Grytz R, Siegwart JT., Jr Changing material properties of the tree shrew sclera during minus lens compensation and recovery. Invest Ophthalmol Vis Sci. 2015;56(3):2065–2078. doi: 10.1167/iovs.14-15352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Frost MR, He L, Siegwart JT, Jr, Norton TT. Gene expression signatures in tree shrew sclera in response to three myopiagenic conditions. Invest Ophthalmol Vis Sci. 2013;54(10):6806–6819. doi: 10.1167/iovs.13-12551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Frost MR, Siegwart JT, Jr, Norton TT. Gene expression signatures in tree shrew sclera in response to two STOP visual conditions: recovery from minus-lens wear, and plus-lens wear. Invest Ophthalmol Vis Sci. 2012;53 ARVO E-Abstract 3455. [Google Scholar]
- Guo L, Frost MR, Siegwart JT, Jr, Norton TT. Gene expression signatures in tree shrew sclera in response to two STOP visual conditions: recovery from minus-lens wear, and plus-lens wear. Invest Ophthalmol Vis Sci. 2012;53:3455. [Google Scholar]
- He L, Frost MR, Siegwart JT, Jr, Norton TT. Gene expression signatures in tree shrew choroid during lens-induced myopia and recovery. Exp Eye Res. 2014;123:56–71. doi: 10.1016/j.exer.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden BA, Jong M, Davis S, Wilson D, Fricke T, Resnikoff S. Nearly 1 billion myopes at risk of myopia-related sight-threatening conditions by 2. Clin Exp Optom. 2015;98(6):491–493. doi: 10.1111/cxo.12339. [DOI] [PubMed] [Google Scholar]
- Howlett MH, McFadden SA. Spectacle lens compensation in the pigmented guinea pig. Vision Res. 2009;49(2):219–227. doi: 10.1016/j.visres.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Hung LF, Crawford ML, Smith EL. Spectacle lenses alter eye growth and the refractive status of young monkeys. Nature Medicine. 1995;1(8):761–765. doi: 10.1038/nm0895-761. [DOI] [PubMed] [Google Scholar]
- Irving EL, Sivak JG, Callender MG. Refractive plasticity of the developing chick eye. Ophthalmic Physiol Opt. 1992;12(4):448–456. [PubMed] [Google Scholar]
- Irving EL, Sivak JG, Callender MG. Refractive plasticity of the developing chick eye. Ophthalmic and Physiological Optics. 1992;12:448–456. [PubMed] [Google Scholar]
- Jacobs GH. The distribution and nature of colour vision among the mammals. Biol Rev Camb Philos Soc. 1993;68(3):413–471. doi: 10.1111/j.1469-185x.1993.tb00738.x. [DOI] [PubMed] [Google Scholar]
- Jiang L, Zhang S, Schaeffel F, Xiong S, Zheng Y, Zhou X, Lu F, Qu J. Interactions of chromatic and lens-induced defocus during visual control of eye growth in guinea pigs (Cavia porcellus) Vision Res. 2014;94:24–32. doi: 10.1016/j.visres.2013.10.020. [DOI] [PubMed] [Google Scholar]
- Jones LA, Sinnott LT, Mutti DO, Mitchell GL, Moeschberger ML, Zadnik K. Parental history of myopia, sports and outdoor activities, and future myopia. Invest Ophthalmol Vis Sci. 2007;48(8):3524–3532. doi: 10.1167/iovs.06-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kröger RHH, Fernald RD. Regulation of eye growth in the African cichlid fish Haplochromis burtoni. Vision Research. 1994;34:1807–1814. doi: 10.1016/0042-6989(94)90305-0. [DOI] [PubMed] [Google Scholar]
- Lin LLK, Chen CJ, Hung PT, Ko LS. Nation-wide survey of myopia among schoolchildren in Taiwan, 1986. Acta Ophthalmologica (Suppl) 1988;185:29–33. doi: 10.1111/j.1755-3768.1988.tb02657.x. [DOI] [PubMed] [Google Scholar]
- Liu R, Qian YF, He JC, Hu M, Zhou XT, Dai JH, Qu XM, Chu RY. Effects of different monochromatic lights on refractive development and eye growth in guinea pigs. Exp Eye Res. 2011;92(6):447–453. doi: 10.1016/j.exer.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Long Q, Chen D, Chu R. Illumination with monochromatic long-wavelength light promotes myopic shift and ocular elongation in newborn pigmented guinea pigs. Cutan Ocul Toxicol. 2009;28(4):176–180. doi: 10.3109/15569520903178364. [DOI] [PubMed] [Google Scholar]
- Luckett WP. Comparative Biology and Evolutionary Relationships of Tree Shrews. New York: Plenum Press; 1980. [Google Scholar]
- McBrien NA, Moghaddam HO, Reeder AP. Atropine reduces experimental myopia and eye enlargement via a nonaccommodative mechanism. Investigative Ophthalmology and Visual Science. 1993;34:205–215. [PubMed] [Google Scholar]
- McBrien NA, Norton TT. The development of experimental myopia and ocular component dimensions in monocularly lid-sutured tree shrews (Tupaia belangeri) Vision Res. 1992;32(5):843–852. doi: 10.1016/0042-6989(92)90027-g. [DOI] [PubMed] [Google Scholar]
- McKanna JA, Casagrande VA. Atropine affects lid-suture myopia development. Doc Ophthalmol Proc Ser. 1981;28:187–192. [Google Scholar]
- Metlapally S, McBrien NA. The effect of positive lens defocus on ocular growth and emmetropization in the tree shrew. J Vis. 2008;8(3):1–12. doi: 10.1167/8.3.1. [DOI] [PubMed] [Google Scholar]
- Mutti DO, Mitchell GL, Jones LA, Friedman NE, Frane SL, Lin WK, Moeschberger ML, Zadnik K. Axial growth and changes in lenticular and corneal power during emmetropization in infants. Invest Ophthalmol Vis Sci. 2005;46(9):3074–3080. doi: 10.1167/iovs.04-1040. [DOI] [PubMed] [Google Scholar]
- Norton TT. Animal models of myopia: Learning how vision controls the size of the eye. ILAR J. 1999;40(2):59–77. doi: 10.1093/ilar.40.2.59. [DOI] [PubMed] [Google Scholar]
- Norton TT, Amedo AO, Siegwart JT., Jr The effect of age on compensation for a negative lens and recovery from lens-induced myopia in tree shrews (Tupaia glis belangeri) Vision Res. 2010;50(6):564–576. doi: 10.1016/j.visres.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton TT, McBrien NA. Normal development of refractive state and ocular component dimensions in the tree shrew (Tupaia belangeri) Vision Research. 1992;32(5):833–842. doi: 10.1016/0042-6989(92)90026-f. [DOI] [PubMed] [Google Scholar]
- Norton TT, McBrien NA. Normal development of refractive state and ocular component dimensions in the tree shrew (Tupaia belangeri) Vision Res. 1992;32(5):833–842. doi: 10.1016/0042-6989(92)90026-f. [DOI] [PubMed] [Google Scholar]
- Norton TT, Rada JA. Reduced extracellular matrix accumulation in mammalian sclera with induced myopia. Vision Research. 1995;35(9):1271–1281. doi: 10.1016/0042-6989(94)00243-f. [DOI] [PubMed] [Google Scholar]
- Norton TT, Siegwart JT, German AJ, Robertson J, Wu WW. Comparison of cycloplegic streak retinoscopy with autorefractor measures in tree shrew eyes with, and without, induced myopia. Invest Ophthalmol Vis Sci. 2000;41 ARVO Abstract 563. [Google Scholar]
- Norton TT, Siegwart JT., Jr Light levels, refractive development, and myopia - A speculative review. Exp Eye Res. 2013;114:48–57. doi: 10.1016/j.exer.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton TT, Wu WW, Siegwart JT., Jr Refractive state of tree shrew eyes measured with cortical visual evoked potentials. Optom Vis Sci. 2003;80(9):623–631. doi: 10.1097/00006324-200309000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton TT, Wu WW, Siegwart JT., Jr Refractive state of tree shrew eyes measured with cortical visual evoked potentials. Optom Vis Sci. 2003;80(9):623–631. doi: 10.1097/00006324-200309000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papastergiou GI, Schmid GF, Laties AM, Pendrak K, Lin T, Stone RA. Induction of axial eye elongation and myopic refractive shift in one-year-old chickens. Vision Res. 1998;38(12):1883–1888. doi: 10.1016/s0042-6989(97)00347-7. [DOI] [PubMed] [Google Scholar]
- Penha AM, Burkhardt E, Schaeffel F, Feldkaemper MP. Ultrasonography and optical low-coherence interferometry compared in the chicken eye. Optom Vis Sci. 2012;89(6):916–921. doi: 10.1097/OPX.0b013e318257a255. [DOI] [PubMed] [Google Scholar]
- Petry HM, Harosi FI. Visual pigments of the tree shrew (Tupaia Belangeri) and greater galago (Galago Crassicaudatus): A microspectrophotometric investigation. Vision Research. 1990;30(6):839–851. doi: 10.1016/0042-6989(90)90053-n. [DOI] [PubMed] [Google Scholar]
- Pickett-Seltner RL, Sivak JG, Pasternak JJ. Experimentally induced myopia in chicks: morphometric and biochemical analysis during the first 14 days after hatching. Vision Res. 1988;28(2):323–328. doi: 10.1016/0042-6989(88)90160-5. [DOI] [PubMed] [Google Scholar]
- Pickett-Seltner RL, Sivak JG, Paternak JJ. Experimentally induced myopia in chicks: morphometric and biochemical analysis during the first 14 days after hatching. Vision Research. 1988;28:323–328. doi: 10.1016/0042-6989(88)90160-5. [DOI] [PubMed] [Google Scholar]
- Qian YF, Dai JH, Liu R, Chen MJ, Zhou XT, Chu RY. Effects of the Chromatic Defocus Caused by Interchange of Two Monochromatic Lights on Refraction and Ocular Dimension in Guinea Pigs. PLoS ONE. 2013;8(5):e63229. doi: 10.1371/journal.pone.0063229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian YS, Chu RY, He JC, Sun XH, Zhou XT, Zhao NQ, Hu DN, Hoffman MR, Dai JH, Qu XM, Pao KE. Incidence of myopia in high school students with and without red-green color vision deficiency. Invest Ophthalmol Vis Sci. 2009;50(4):1598–1605. doi: 10.1167/iovs.07-1362. [DOI] [PubMed] [Google Scholar]
- Quek TP, Chua CG, Chong CS, Chong JH, Hey HW, Lee J, Lim YF, Saw SM. Prevalence of refractive errors in teenage high school students in Singapore. Ophthalmic Physiol Opt. 2004;24(1):47–55. doi: 10.1046/j.1475-1313.2003.00166.x. [DOI] [PubMed] [Google Scholar]
- Read SA, Collins MJ, Sander BP. Human Optical Axial Length and Defocus. Investigative Ophthalmology & Visual Science. 2010;51(12):6262–6269. doi: 10.1167/iovs.10-5457. [DOI] [PubMed] [Google Scholar]
- Rucker FJ, Osorio D. The effects of longitudinal chromatic aberration and a shift in the peak of the middle-wavelength sensitive cone fundamental on cone contrast. Vision Res. 2008;48(19):1929–1939. doi: 10.1016/j.visres.2008.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rucker FJ, Wallman J. Cone signals for spectacle-lens compensation: differential responses to short and long wavelengths. Vision Res. 2008;48(19):1980–1991. doi: 10.1016/j.visres.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rucker FJ, Wallman J. Cone signals for spectacle-lens compensation: differential responses to short and long wavelengths. Vision Res. 2008;48(19):1980–1991. doi: 10.1016/j.visres.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rucker FJ, Wallman J. Chick eyes compensate for chromatic simulations of hyperopic and myopic defocus: evidence that the eye uses longitudinal chromatic aberration to guide eye-growth. Vision Res. 2009;49(14):1775–1783. doi: 10.1016/j.visres.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rucker FJ, Wallman J. Chicks use changes in luminance and chromatic contrast as indicators of the sign of defocus. J Vis. 2012;12(6) doi: 10.1167/12.6.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saw SM, Gazzard G, Shih-Yen EC, Chua WH. Myopia and associated pathological complications. Ophthalmic Physiol Opt. 2005;25(5):381–391. doi: 10.1111/j.1475-1313.2005.00298.x. [DOI] [PubMed] [Google Scholar]
- Schaeffel F, Feldkaemper M. Animal models in myopia research. Clin Exp Optom. 2015;98(6):507–517. doi: 10.1111/cxo.12312. [DOI] [PubMed] [Google Scholar]
- Schaeffel F, Glasser A, Howland HC. Accommodation, refractive error and eye growth in chickens. Vision Res. 1988;28(5):639–657. doi: 10.1016/0042-6989(88)90113-7. [DOI] [PubMed] [Google Scholar]
- Schaeffel F, Glasser A, Howland HC. Accommodation, refractive error and eye growth in chickens. Vision Research. 1988;28(5):639–657. doi: 10.1016/0042-6989(88)90113-7. [DOI] [PubMed] [Google Scholar]
- Schaeffel F, Howland HC. Properties of the feedback loops controlling eye growth and refractive state in the chicken. Vision Res. 1991;31(4):717–734. doi: 10.1016/0042-6989(91)90011-s. [DOI] [PubMed] [Google Scholar]
- Seidemann A, Schaeffel F. Effects of longitudinal chromatic aberration on accommodation and emmetropization. Vision Res. 2002;42(21):2409–2417. doi: 10.1016/s0042-6989(02)00262-6. [DOI] [PubMed] [Google Scholar]
- Shen W, Sivak JG. Eyes of a lower vertebrate are susceptible to the visual environment. Invest Ophthalmol Vis Sci. 2007;48(10):4829–4837. doi: 10.1167/iovs.06-1273. [DOI] [PubMed] [Google Scholar]
- Siegwart JT, Jr, Norton TT. Regulation of the mechanical properties of tree shrew sclera by the visual environment. Vision Res. 1999;39(2):387–407. doi: 10.1016/s0042-6989(98)00150-3. [DOI] [PubMed] [Google Scholar]
- Siegwart JT, Jr, Norton TT. Binocular lens treatment in tree shrews: Effect of age and comparison of plus lens wear with recovery from minus lens-induced myopia. Exp Eye Res. 2010;91:660–669. doi: 10.1016/j.exer.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegwart JT, Jr, Norton TT. Binocular lens treatment in tree shrews: Effect of age and comparison of plus lens wear with recovery from minus lens-induced myopia. Exp Eye Res. 2010;91(5):660–669. doi: 10.1016/j.exer.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegwart JT, Norton TT. Goggles for controlling the visual environment of small animals. Laboratory Animal Science. 1994;44:292–294. [PubMed] [Google Scholar]
- Smith EL, 3rd, Hung LF, Arumugam B, Holden BA, Neitz M, Neitz J. Effects of Long-Wavelength Lighting on Refractive Development in Infant Rhesus Monkeys. Invest Ophthalmol Vis Sci. 2015;56(11):6490–6500. doi: 10.1167/iovs.15-17025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EL, Hung LF, Arumugam B, Huang J. Negative Lens-Induced Myopia in Infant Monkeys: Effects of High Ambient Lighting. Investigative Ophthalmology & Visual Science. 2013;54(4):2959–2969. doi: 10.1167/iovs.13-11713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EL, Hung LF, Huang J, Blasdel TL, Humbird TL, Bockhorst KH. Optical Defocus Influences Refractive Development in Monkeys via Local, Regionally Selective Mechanisms. Invest Ophthalmol Vis Sci. 2010 doi: 10.1167/iovs.09-4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EL, III, Bradley DV, Fernandes A, Boothe RG. Form deprivation myopia in adolescent monkeys. Optom Vis Sci. 1999;76(6):428–432. doi: 10.1097/00006324-199906000-00023. [DOI] [PubMed] [Google Scholar]
- Smith EL, III, Hung LF. The role of optical defocus in regulating refractive development in infant monkeys. Vision Research. 1999;39(8):1415–1435. doi: 10.1016/s0042-6989(98)00229-6. [DOI] [PubMed] [Google Scholar]
- Smith EL, III, Hung LF, Arumugam B. Visual regulation of refractive development: insights from animal studies. Eye (Lond) 2014;28(2):180–188. doi: 10.1038/eye.2013.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EL, III, Hung LF, Arumugam B, Holden BA, Neitz M, Neitz J. Effects of Long-Wavelength Lighting on Refractive Development in Infant Rhesus Monkeys. Invest Ophthalmol Vis Sci. 2015;56(11):6490–6500. doi: 10.1167/iovs.15-17025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EL, III, Hung LF, Huang J, Blasdel TL, Humbird TL, Bockhorst KH. Effects of optical defocus on refractive development in monkeys: evidence for local, regionally selective mechanisms. Invest Ophthalmol Vis Sci. 2010;51(8):3864–3873. doi: 10.1167/iovs.09-4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperduto RD, Seigel D, Roberts J, Rowland M. Prevalence of myopia in the United States. Arch Ophthalmol. 1983;101(3):405–407. doi: 10.1001/archopht.1983.01040010405011. [DOI] [PubMed] [Google Scholar]
- Stenstrom S. Investigation of the variation and the correlation of the optical elements of human eyes. Am J Optom Arch Am Acad Optom. 1948;25(10):496–504. doi: 10.1097/00006324-194810000-00006. [DOI] [PubMed] [Google Scholar]
- Sun J, Zhou J, Zhao P, Lian J, Zhu H, Zhou Y, SUN Y, Wang Y, Zhao L, Wei Y, Wang L, Cun B, Ge S, Fan X. High prevalence of myopia and high myopia in 5060 Chinese university students in Shanghai. Investigative Ophthalmology & Visual Science. 2012;53(12):7504–7509. doi: 10.1167/iovs.11-8343. [DOI] [PubMed] [Google Scholar]
- Troilo D, Nickla DL, Wildsoet CF. Form deprivation myopia in mature common marmosets (Callithrix jacchus) Invest Ophthalmol Vis Sci. 2000;41(8):2043–2049. [PubMed] [Google Scholar]
- Troilo D, Totonelly K, Harb E. Imposed anisometropia, accommodation, and regulation of refractive state. Optom Vis Sci. 2009;86(1):E31–E39. doi: 10.1097/OPX.0b013e318194072e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale S, Sperduto RD, Ferris FL., III Increased prevalence of myopia in the United States between 1971–1972 and 1999–2004. Arch Ophthalmol. 2009;127(12):1632–1639. doi: 10.1001/archophthalmol.2009.303. [DOI] [PubMed] [Google Scholar]
- Vongphanit J, Mitchell P, Wang JJ. Prevalence and progression of myopic retinopathy in an older population. Ophthalmology. 2002;109(4):704–711. doi: 10.1016/s0161-6420(01)01024-7. [DOI] [PubMed] [Google Scholar]
- Wallman J, Adams JI. Developmental aspects of experimental myopia in chicks: Susceptibility, recovery and relation to emmetropization. Vision Research. 1987;27(7):1139–1163. doi: 10.1016/0042-6989(87)90027-7. [DOI] [PubMed] [Google Scholar]
- Wallman J, Wildsoet C, Xu A, Gottlieb MD, Nickla DL, Marran L, Krebs W, Christensen AM. Moving the retina: choroidal modulation of refractive state. Vision Research. 1995;35(1):37–50. doi: 10.1016/0042-6989(94)e0049-q. [DOI] [PubMed] [Google Scholar]
- Wallman J, Winawer J. Homeostasis of eye growth and the question of myopia. Neuron. 2004;43(4):447–468. doi: 10.1016/j.neuron.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Ward AH, Siegwart JT, Jr, Frost MR, Norton TT. The effect of intravitreal injection of vehicle solutions on form deprivation myopia in tree shrews. Exp Eye Res. 2016;145:289–296. doi: 10.1016/j.exer.2016.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilby D, Roberts NW. Optical influence of oil droplets on cone photoreceptor sensitivity. J Exp Biol. 2017 doi: 10.1242/jeb.152918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildsoet CF. Active emmetropization - evidence for its existence and ramifications for clinical practice. Ophthalmic & Physiological Optics. 1997;17(4):279–290. [PubMed] [Google Scholar]
- Wildsoet CF. Active emmetropization--evidence for its existence and ramifications for clinical practice. Ophthalmic Physiol Opt. 1997;17(4):279–290. [PubMed] [Google Scholar]
- Zadnik K. It’s the retina, stupid. Optom Vis Sci. 2001;78(4):179–180. doi: 10.1097/00006324-200104000-00001. [DOI] [PubMed] [Google Scholar]
- Zadnik K, Mutti DO. How applicable are animal myopia models to human juvenile onset myopia? Vision Res. 1995;35(9):1283–1288. doi: 10.1016/0042-6989(94)00234-d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.