Abstract
No treatment is available for patients with spinal cord injury (SCI). Patients often arrive to the hospital hours after SCI suggesting the need of a therapy that can be used on a clinically relevant window. Previous studies showed that Tamoxifen (TAM) treatment 24 hours after SCI benefits locomotor recovery in female rats. Tamoxifen exerts beneficial effects in male and female rodents but a gap of knowledge exists on: the therapeutic window of TAM, the spatio-temporal mechanisms activated and if this response is sexually dimorphic. We hypothesized that TAM will favor locomotor recovery when administered up-to 24 hours after SCI in male Sprague-Dawley rats. Rats received a thoracic (T10) contusion using the MACSIS impactor followed by placebo or TAM (15mg/21 days) pellets in a therapeutic window of 0, 6, 12, or 24 hours. Animals were sacrificed at 2, 7, 14, 28 or 35 days post injury (DPI) to study the molecular and cellular changes in the acute and chronic stages. Immediate or delayed therapy (t=6 hours) improved locomotor function, increased white matter spared tissue, and neuronal survival. TAM reduced reactive gliosis during chronic stages and increased the expression of Olig-2. A significant difference was observed in estrogen receptor alpha between male and female rodents from 2–28 DPI suggesting a sexually dimorphic characteristic that could be related to the behavioral differences observed in the therapeutic window of TAM. This study supports the use of TAM in the SCI setting due to its neuroprotective effects but with a significant sexually dimorphic therapeutic window.
Keywords: Spinal cord injury, Tamoxifen, therapeutic window, sex differences, Estrogen receptor alpha, neuroprotection, astrogliosis, myelin spared tissue, Olig-2, selective estrogen receptor modulator
Introduction
Spinal cord injury (SCI) is a condition without a cure, with thousands of new cases reported each year. The major symptoms associated with SCI relate to the loss of voluntary muscle movement and somatosensory perception that, in some cases, trigger chronic pain (Huselbosch, 2002). The clinical symptoms associated with SCI are related to cellular events like: apoptosis, axonal degeneration, edema, vascular damage, demyelination, and the formation of a gliotic scar (Colón and Miranda, 2016; Fogaça Cristante et al., 2012). These cellular and molecular events complicate the search for a multi-active therapy that target most of them. However, any therapeutic intervention that restore even partial function would markedly increase the quality of life of these patients and reduce their high health care and living expenses.
Current interventions aim for patient stabilization and life preservation. Although some therapeutic strategies are under clinical investigation, a treatment to return locomotion after SCI in humans is yet to be determined (Ahuja et al., 2017). Human studies and models of rodents with SCI showed that locomotor recovery after SCI is sexually dimorphic; where female rodents recover locomotion better than male rats regardless of injury severity (Datto et al., 2015; Farooque et al., 2006; Hauben et al., 2002; Sipski et al., 2004). Several published articles pointed to the presence of estradiol (E2) as the neuroprotective hormone that contributed to the sex differences in response to SCI. This steroid hormone interacts with three estrogen receptors (ER): ER-α, ER-β, and GPR-30 to produce neuroprotective effects (Alexander et al., 2017; Levin, 2009; Nelson et al., 2013; Prossnitz and Arterburn, 2015). It promotes cell survival via activation of antiapoptotic, neurotrophic, and regeneration associated genes. The steroidal structure further confers anti-inflammatory and antioxidant properties, both reducing cellular toxicity and cell death (Chaovipoch et al., 2006; Cuzzocrea et al., 2008; Gupta and Hubscher, 2012; Jover-Mengual et al., 2010; Miller et al., 2005; Mosquera et al., 2014; Nilsen and Brinton, 2003; Ritz and Hausmann, 2008; Samantaray et al., 2011, 2016; Siriphorn et al., 2012; Sribnick et al., 2005, 2006, 2010; Yune et al., 2004, 2008; Zhao and Brinton, 2007). The GPER-1/GPR30 activation resulted in neuronal survival and better behavioral outcome after pathological conditions to the CNS, like SCI, traumatic brain injury and stroke (Cheng et al., 2016; Hu et al., 2012; Wang et al., 2017). The GPER-1 activation by G-1 (GPER-1 selective agonist) was shown to mediate a sexually dimorphic response after middle cerebral artery occlusion (MCAo) (Broughton et al., 2014). However, controversy exists regarding the effective dose, route of administration, and pleiotropic effects of continuous E2 use to activate its receptors. Among them, uncontrolled cell proliferation leading to cancer and feminizing effects in men. For that reason, Tamoxifen (TAM), a biochemical compound similar to E2, should be studied for the treatment of SCI but without the unfavorable side-effects.
Tamoxifen, is a selective estrogen receptor modulator that crosses the blood brain barrier and exerts neuroprotection through different mechanisms. Some of these mechanisms are: attenuating inflammatory damage, promoting sensorial cortex regeneration, anti-oxidant and anti-apoptotic effects in models of penetrating brain injury, MCAo, and SCI, among others (Colón and Miranda, 2016; De la Torre Valdovinos et al., 2016; Don Carlos et al., 2009; Franco Rodríguez et al., 2013; Guptarak et al., 2014; Ismailoğlu et al., 2010; Kimelberg et al., 2000; Tian et al., 2009; Wei and Ma, 2014; Zhang et al., 2007). In addition, TAM has been related to anti-inflammatory effects by acting on microglia through an ER-dependent mechanism in which it activates estrogen response elements to elicit E2-like effects (Ishihara et al., 2015). The favorable effects of TAM, like E2, suggest that this FDA approved drug may be translated into the SCI setting to provide a reduction of medical costs, and health benefits to patients.
Although the cellular-molecular effects and the therapeutic window of TAM after SCI were established in female rats (Colón et al., 2016), this has not been established in male animals. In addition, the molecular and cellular events affected by this drug at different time points after SCI are unknown. We hypothesized that TAM will improve locomotor activity in a defined therapeutic window by promoting neuronal survival, increase the extent of spared white matter, and reduce the gliotic response in male rodents. The objective of this study was to determine the therapeutic window of TAM after SCI in male rodents, and to establish the mechanisms activated (in the acute and chronic stages) by this drug. To study this, we used a SCI contusion model in male rats and provided the therapeutic intervention of TAM immediately, at 0 hours (hrs), or delayed in windows of 6, 12, or 24 hrs after SCI. Then, we performed anatomical (rostral, epicenter and caudal) and temporal studies (2, 7, 14, and 28 days post injury [DPI]) for neuronal and glial proteins. Here, we show that: 1) TAM improves behavioral activity when administered within 6 hrs after SCI in male rats, 2) TAM exerts neuroprotection by activating neuronal and oligodendroglial cellular machinery, and 3) TAM reduces secondary damage by reducing astrogliosis during chronic stages.
This study is novel because it provides a therapeutic window for TAM in male animals after SCI and gives an insight into the mechanism of action of this FDA approved drug. Also, these results present a possible mechanism to explain the difference in the therapeutic window of TAM between male and female rats with SCI. Therefore, since no treatment is available after SCI, the data of this work (and others published in several CNS pathologies) supported the beneficial use of TAM as a neuroprotective compound that benefits patients with this devastating condition.
Materials and Methods
Spinal cord injury surgery and post-operative care
The Institutional Animal Care and Use Committee of the University of Puerto Rico Medical Sciences Campus approved all surgical procedures and animal handling. Male Sprague Dawley rats (2 months old, ~300 g) were purchased from Hilltop Lab Animal (Scottsdale, PA) and maintained in a 12:12 hrs light-dark cycle. Also, we used total protein extract from female rats from our previous study (Colón et al., 2016) for some Western blot experiments. Rats received water and rat food chow (Harlan Teklad) at libitum. Upon arrival, animals were quarantined for 7 days and then, trained on the behavioral tests (BBB Open Field and grid-walk test). The animals were handled following the Guide for the Care and Use of Laboratory Animals from the National Institutes of Health (NIH). Efforts were made to collect and discuss all the minimum information required in the field of SCI, to replicate this study (Lemmon et al., 2014).
Surgical procedures were performed under aseptic conditions as previously described (Colón et al., 2016; Mosquera et al., 2014; Rosas et al., 2011; Santiago et al., 2009). Briefly, rats were sedated with Ketamine/Xylazine/Acepromazine, 87.5/4.2/0.8 mg/kg (Fort Dodge Animal Health, Fort Dodge, IO). The dorso-lateral surface was shaved and cleaned with iodine scrub, iodine solution, and 70% isopropyl alcohol. A midline incision in the dorsal thoracic area was performed, the muscles were spread and a laminectomy at T9-T10 level was performed. After laminectomy, the vertebral column was stabilized and the MACSIS impactor was used to exert a moderate contusion to the thoracic spinal cord. A 10 gram rod was dropped from 12.5 mm height, the compression was held for 5 seconds as previously reported (Colón et al., 2016). The sham groups did not receive any injury after the laminectomy. Afterward, placebo (PLB) or TAM pellets were implanted in the mid-scapular region at 0, 6, 12 or 24 hrs after SCI or laminectomy, as reported previously (Colón et al., 2016; Mosquera et al., 2014). Then, the animals were housed individually after the surgical procedures. TAM or PLB pellets were purchased from Innovative Research of America (Sarasota, FL, cat# E-361). TAM pellets were designed to release a constant dose of 0.71 mg/day for 21 days (15 mg pellet). PLB pellets were used as a control treatment for Sham-PLB and SCI-PLB animals (control groups). Rats were randomly selected and assigned to the following groups: Sham-PLB, Sham-TAM, SCI-PLB 0 hrs, SCI-PLB 6 hrs, SCI-PLB 12 hrs, SCI-PLB 24 hrs, SCI-TAM 0 hrs, SCI-TAM 6 hrs, SCI-TAM-12 hrs, and SCI-TAM 24 hrs. These 10 groups were established to investigate the therapeutic window of TAM when the treatment was delayed 0, 6, 12, or 24 hrs after SCI, including their respective controls. Post-operative care included 3 mL of isotonic saline, 0.05 mg/kg/day buprenorphine (Reckett & Colman Pharmaceuticals, Richmond, VA) for 3 days and 7 days of Cefazolin (25 mg/kg) as antibiotic treatment. Bladder manual voiding was conducted 3 times a day until voluntary micturition reflexes returned. All the animals received cereal and sterilized cardboards as enrichment throughout the duration of the experiments (2–35 days).
Functional locomotor recovery assessment
Functional locomotor recovery was evaluated at 2 DPI in order to establish the exclusion parameters for a successful compression injury (Colón et al., 2016). Once the appropriate injury was confirmed by the behavioral analysis at 2 DPI, locomotor activity was assessed weekly at 7, 14, 21, 28, and 35 DPI using the BBB Open field test (Basso et al., 1995). Two evaluators blinded to treatment scored each hindlimb movement, both scores were averaged and the total score was reported for each animal (Colón et al., 2016; Mosquera et al., 2014). The grid-walk test was used to evaluate recovery of coordination after SCI (Merkler et al., 2001). Briefly, animals were subjected to cross a horizontal ladder (3 feet long, bars separated by 1 or 2 inches) and scores were assigned according to the number of correct paw positions and the number of errors (missed footsteps) (Colón et al., 2016; Merkler et al., 2001). Every week, the experimenter randomized the bars to avoid memorization from the rats. The average score (both hindlimbs) was reported for each test.
Terminal analysis: Luxol fast blue histology
To determine the effects of chronic TAM treatment after SCI at the anatomical and cellular level, animals were sacrificed at 35 DPI for histology and immunofluorescence studies (Colón et al., 2016). Briefly, animals were sedated, the thoracic cavity was exposed and the animals were perfused intracardially with 250 mL of ice-cold phosphate buffered saline (PBS) followed by 250 mL of ice-cold 4% Paraformaldehyde (PFA) solution. The thoracic area of the spinal cord was removed and representative sections of the spinal cord containing the rostral, epicenter, and caudal areas were dissected (1.5 cm containing the lesion epicenter and rostral-caudal penumbra). The spinal cord segment was post-fixed in cold 4% PFA for 3 hrs followed by cryoprotection in 30% sucrose/PBS solution. Then, the tissue was embedded on freezing medium.
Representative transverse sections (20 μm) of sham or injured spinal cords (treated with PLB or TAM) were collected in gelatin coated slides using a cryostat at −20°C (Leica microsystems, model CM1850). Sections were stored at −20°C until additional processing. Luxol Fast Blue histology was performed to determine the amount of white matter spared tissue, as previously reported (Colón et al., 2016; Mosquera et al., 2014). Briefly, 3 to 5 slides containing representative sections from rostral, epicenter, and caudal areas (~200 μm apart) were dehydrated to 95% ethanol followed by immersion in Luxol Fast Blue solution (95% ethanol) at 37°C overnight. Slides were washed in series (95%–70% ethanol) and differentiated with 0.05% lithium carbonate solution followed by 95% ethanol. After staining, slides were washed in histoclear, mounted using Permount® (Fisher Scientific), dried overnight, and visualized with Z1 Zeiss observer microscope (Carl Zeiss MicroImaging Inc., Thornwood, NY) and photographed using AxioCam MRm at 21 × magnification (Rev3).
The Otsu method was used to threshold the amount of positive staining and relatively quantify the effects of TAM treatment on white matter preservation, as previously reported (Colón et al., 2016; Otsu, 1979). The external area of the epicenter was delineated, the cavity was outlined and the image was brought to threshold using ImageJ and the Fiji processing package (Colón et al., 2016; Schindelin et al., 2015). The selected area representing the epicenter penumbra was quantified. Then the cavity was demarcated, and quantified. The quantified epicenter penumbra was subtracted from the lesion cavity and selected as the amount of white matter spared tissue for this sample. For sham animals, intact grey matter was observed towards the center of the spinal cord. Grey matter was delineated and brought to threshold, and the amount of grey matter was subtracted from the total white matter. At least three sections per animal that correspond to the lesion epicenter were stained and quantified.
Terminal analysis: Immunofluorescence
For immunofluorescence studies, spinal cord tissue slides were dried at 60°C for 30 minutes and the freezing media was removed. The sections were probed using primary antibodies for neuronal and astrocytic markers (see Table 1 for details). Immunofluorescence experiments were performed as described previously by our group (Colón et al., 2016). Briefly, samples were permeabilized using 0.1% Triton X-100/PBS, post-fixed with 4% PFA, and blocked with 3% bovine serum albumin (BSA)/0.01%sodium azide solution in PBS for 2 hrs at room temperature. Sections were incubated with primary antibody overnight at 4°C (against NeuN or GFAP, Table 1). After the incubation, slides were washed with PBS and probed with Alexa Fluor® 488 donkey anti-mouse (1:500). Sections were incubated with isotype antibodies (IgG1 or IgG2b) at the same concentration of the primary antibody that were probed with the appropriate secondary antibodies to establish non-specific binding (negative controls). These negative control sections also served to establish the exposure for each experiment, and the imaging parameters were kept constant throughout the analyses. Sections were visualized with Axio Observer Z1 Zeiss microscope (Carl Zeiss MicroImaging Inc., Thornwood, NY) and photographed using AxioCam MRm (Rev3).
Table 1. Primary and secondary antibodies used for immunofluorescence and immunoblot experiments.
Primary and secondary antibodies were diluted to final concentration in 3% BSA for immunofluorescence (IMF) studies and 5% for western blot (WB) experiments.
| Antibody | Dilution by application | Company/Catalog number | Antibody Registry |
|---|---|---|---|
| Primary antibodies | |||
| Olig-2 | WB 1:3,000 | Phosphosolutions; cat# 1538-Olig2 | AB_2492193 |
| NeuN | IMF 1:1,000 WB 1:500 |
Millipore; cat# MAB377 | AB_2298772 |
| GFAP | IMF 1:2,000 WB 1:1,000 |
BD Biosciences; cat# 556327) | AB_396365 |
| ER-α | WB 1:500 | Millipore; cat# 06-935 | AB_310305 |
| GAPDH | WB 1:10,000 | Sigma; cat# G9545 | AB_796208 |
| Secondary antibodies | |||
| Alexa Fluor® 488 donkey anti-mouse | IMF 1:500 | Molecular Probes; cat#A21202 | AB_141607 |
| Goat Anti-Rabbit IgG, IRDye® 800CW Conjugated antibody | WB 1:25,000 | LI-COR; cat# 926-32211 | AB_621843 |
| IRDye 680RD Goat anti-Mouse IgG (H + L) | WB 1:25,000 | LI-COR; cat# 926-68070) | AB_10956588 |
| Isotype controls | |||
| IgG1 | Same concentration as primary | Cell Signaling Technologies; cat# 5415 | AB_10829607 |
| IgG2b | Same concentration as primary | Millipore; cat#MABC006 | AB_11213150 |
An antibody against NeuN (1:1,000) was used as a cellular marker to identify the neuronal populations on the dorsal and ventral horns in regions rostral and caudal to the lesion epicenter. Two sections (~200 μm apart) from each rostral and caudal areas were photographed. Neurons from the left and right horns (from the dorsal and ventral area) were counted by two evaluators, which were blinded to treatment groups. The averaged count was normalized to the total number of neurons counted for sham animals. Astrocytic marker GFAP (1:2,000) was used as a marker for reactive gliosis at the lesion epicenter. The dorsal, ventral, and lateral areas of the lesion epicenter or laminectomized spinal cord were photographed and presented.
Terminal analysis: spatio-temporal protein expression
Animals were sacrificed by decapitation at 2, 7, 14, and 28 DPI to study the mechanisms activated by TAM in the acute and chronic stages after the trauma. The spinal cord was quickly dissected, segments of 0.5 cm representing the rostral, epicenter, and caudal area were removed and placed in a cold 1.5 mL microcentrifuge tube containing 700 μL ice-cold lysis buffer (CelLytic MT Lysis/Extraction Buffer [cat# C3228], 1% Phosphatase Inhibitor Cocktail 3 [cat# P0044], and SigmaFast® Protease Inhibitor Cocktail Tablet EDTA free [cat# S8830], all from Sigma). Each segment was homogenized with a cold pellet pestle. Tubes with homogenized mixture were placed on a rocker/mixer for 45 min at 4°C. Samples were centrifuged for 10 minutes (20,000 rcf at 4°C). The supernatant was collected and stored at −80°C for long-term use. Protein concentration was determined using Bradford assay (Bio-Rad, cat# 500-0006) and quantified using a Microplate reader (Model 680, Bio-Rad Laboratories, Hercule, CA). Western blots were performed to evaluate the effect of TAM in proteins associated with neurons (NeuN), reactive astrocytes (GFAP), and oligodendrocytes (Olig-2). Uterine tissue (equal loading) was used to validate our antibodies. Uterus was used as positive control for ER-α (66 kDa) expression and as a negative control for Olig-2 expression.
Ten (10) μg of protein (amount needed to be within the linear range of detection) from the rostral, epicenter, and caudal region were separated individually in a 10% SDS-Polyacrylamide gel electrophoresis (PAGE) under constant voltage (50V 30 minutes followed by 150V 60 minutes). This was performed to study the effect of TAM in the temporal expression profile of NeuN and GFAP. To analyze Olig-2, an oligodendrocyte protein, 10 μg of homogenate from rostral, epicenter and caudal areas were individually separated on 15% SDS-PAGE under constant voltage parameters (50v 30 minutes followed by 100v 90 minutes). Proteins were transferred onto a nitrocellulose membrane with the Trans-Blot® Turbo Transfer System (Bio-Rad Laboratories, Hercules, CA). We used the 1.5 mm Bio-Rad protocol (25V; 1.3A; 10 minutes transfer). After transfer, the membranes for Olig-2 were washed in PBS and dried between two Whatman papers for 2 hrs. This allowed for better signal intensity after developing with secondary antibody. Membranes were blocked for 1 hr in 5% BSA/PBS/0.01% sodium azide followed by incubation with primary antibody (NeuN 1:500; GFAP 1:1,000; Olig-2 1:3,000) in a shaker at 4°C overnight.
The temporal pattern of ER-α was investigated using 25 μg of protein from the lesion epicenter and separated in a 10% SDS-PAGE (initially at 50V for 30 minutes and then at 150V for 60 minutes). To evaluate the temporal changes (2–28 DPI) in ER-α expression, we used protein extracts from the lesion epicenter of female rats from our previous study (Colón et al., 2016). Male and female total protein (25 μg) from the lesion epicenter or laminectomy (sham) at 2, 7, 14 and 28 DPI were separated by SDS PAGE. Experiments for ER-α temporal pattern of expression included control and experimental groups from box sex in each gel. After SDS-PAGE, nitrocellulose membranes (0.20 μm pore size, Bio-Rad, Hercules, CA) and blotting paper were soaked in Towbin transfer buffer (25 mM Tris, 192 mM glycine, 20% methanol, pH 8.3) for 10 minutes. Proteins were transferred overnight for 12–16 hrs at a constant 35V in Towbin transfer buffer at 4°C (Mosquera et al., 2014). After the transfer, nitrocellulose membranes were washed quickly in PBS and dried for 2 hrs between two Whatman papers. Membranes were blocked 1 hr in 5% BSA/PBS/0.01% Sodium Azide followed by incubation with ER-α (1:500) primary antibody overnight in shaker at 4°C. After primary antibody probing, the membranes were washed and incubated in IR Dye 800CW goat anti-rabbit secondary antibody (1:25,000) or IR Dye 680CW goat anti-mouse (1:25,000) secondary antibody for 1 hr at room temperature in a shaker. Secondary antibody was washed twice with PBS/0.1% Tween 20 and twice with PBS. Immunoblots were developed using the Odyssey CLx Quantitative Fluorescent Imaging System (LI-COR Biosciences, Lincoln, NE, USA). Results were quantified using Image Studio Lite Software (LI-COR Biosciences, version 5.2.5). Temporal expression (2–28 DPI) of neuronal (NeuN) and glial proteins (GFAP and Olig-2) was analyzed per area (rostral, epicenter, or caudal areas). The percentage fold change per area was combined to obtain the total representative change of neuronal and glial proteins at the epicenter, and rostral/caudal penumbra. Semi-quantitative expression of the target protein was calculated relative to loading control GAPDH (1:10,000) on each area, and results were presented as the percent of fold change relative to Sham-PLB from the combined data (rostral, epicenter, and caudal area).
Statistics
Statistical analysis was performed using GraphPad Prism (version 4.0). All statistical tests were performed at an alpha<0.05 to be considered statistically significant. Two-way ANOVA followed by Bonferroni’s post hoc was used to demonstrate that SCI control subjects treated with PLB (0, 6, 12, and 24 hrs) in the behavioral assays (BBB Open field and Grid-walk tests) did not present any significant difference. Since no statistical difference was found between SCI-PLB 0 hrs, 6 hrs, 12 hrs and 24 hrs at 7, 14, 21, 28, and 35 DPI at the BBB Open field test (F(3,120)=0.7936; Treatment p=0.5071), these groups were pooled. For white matter quantification (Fig. 2), no statistical difference was observed between Sham-PLB and Sham-TAM (Unpaired t-test, p=0.774, n=3) or SCI-PLB 0 hrs and SCI-PLB 6 hrs (Unpaired t-test, p=0.515; n=3 for each group). For this reason, animals were pooled into a single group SCI-PLB (SCI-PLB 0 + 6 + 12 + 24 hrs) or Sham (PLB + TAM) for Fig. 1. In the Luxol histology experiment, the pooled groups were Sham (PLB + TAM) and SCI PLB (0 + 6 hrs).
Fig.2.
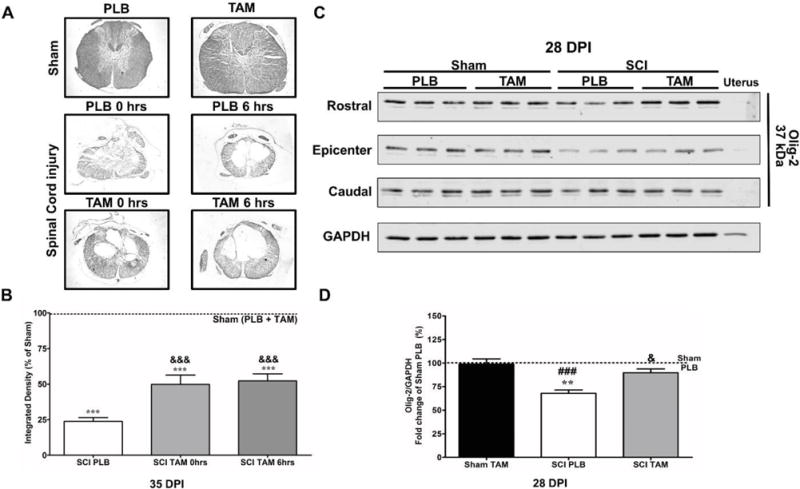
Tamoxifen favors white matter preservation at the lesion epicenter and stimulates Olig-2 expression in the chronic stages after SCI. (A) Luxol fast blue histology at the lesion epicenter show Sham (PLB or TAM) with intact myelin and injured animals with cavitation toward the epicenter at 35 DPI. (B) Immediate (t=0 hrs) and delayed (t=6 hrs) TAM treatment increased white matter preservation at the lesion epicenter after SCI. Data are mean±SEM, One-way ANOVA followed by Tukey’s multiple comparison test; Sham (PLB + TAM) n=6, SCI-PLB [0 hrs+6 hrs] n=6, SCI-TAM 0 hrs n=3, SCI-TAM 6 hrs n=3 (3 sections per animal). (C) Representative immunoblots for Olig-2 expression profile from the rostral, epicenter and caudal areas of sham or lesioned tissue at 28 DPI treated and untreated with TAM. (D) Semi-quantitative analysis revealed a significant increase in Olig-2 total expression (rostral, epicenter and caudal level) at 28 DPI (&p<0.05) relative to SCI-PLB group. Data are mean±SEM, One-way ANOVA followed by Tukey’s post hoc; Sham-PLB n=3, Sham-TAM n=5, SCI-PLB n=5; SCI-TAM n=5.
Fig.1.
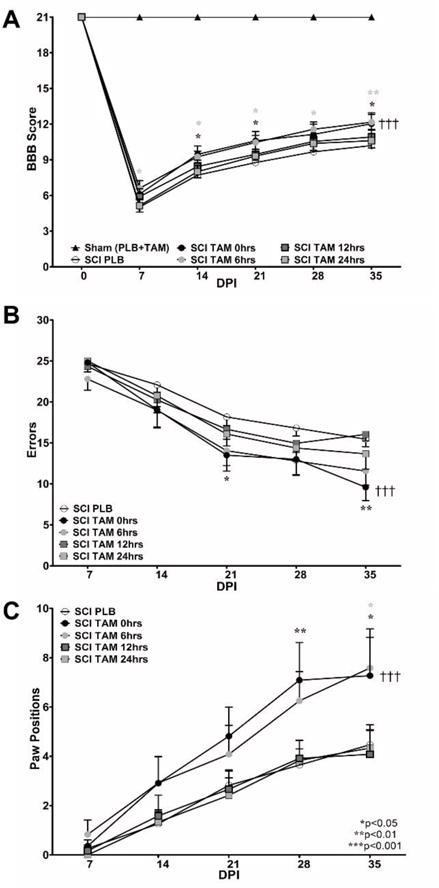
Immediate (0 hrs) or delayed (6 hrs) Tamoxifen treatment after SCI improves locomotor recovery and coordination in male rats. (A) The BBB Open Field test was performed weekly in all subjects. Sham animals received 21 points in the BBB Open Field Test at all test points. TAM favors locomotor recovery when the therapy was administered immediately or 6 hrs after SCI (&&&p<0.001 treatment, DPI and †††Interaction). However, if TAM was administered at 12 or 24 hrs after SCI no effect was observed. (B–C) Rats treated with TAM showed better coordination in the grid walking test during chronic stages (***Treatment, ***DPI and †††Interaction). TAM-treated animals showed reduced number of errors at 21 DPI and 35 DPI (SCI-PLB vs SCI-TAM 0 hrs) and increased number of paw positions when crossing the grid walk test at 28 DPI (SCI PLB vs SCI-TAM 0 hrs) and only at 35 DPI if TAM was applied 6 hrs after SCI (SCI-PLB vs SCI-TAM 6 hrs). Data are the mean±SEM (Sham [PLB+TAM] n=9, SCI-PLB (0 + 6 + 12 + 24 hrs) n=34, SCI-TAM 0 hrs n=11, SCI-TAM 6 hrs n=12, SCI-TAM 12 hrs n=12, SCI-TAM 24 hrs n=12); RM Two-way ANOVA followed by Bonferroni’s post hoc for both behavioral assays (statistical significance if p values were lower than 0.05).
For neuronal cell count at the Ventral horn (VH), no significant difference was found between Sham-PLB and Sham-TAM number of cells (average Sham-PLB=42.75± 2.4; n=6 versus Sham-TAM=43.25±0.87, n=6; un-paired t-test, p=0.8510). Also, neuronal count at the Dorsal Horn (DH) revealed no significant difference between cell number of sham animals (Sham-PLB=133.8± 7.22, n=6 versus Sham-TAM=135.9 ± 6.2, n=6; un-paired t-test, p=0.8243). Therefore, Sham-PLB and Sham-TAM animals were pooled into a single group (Fig. 3). For the temporal expression profile of ER-α, we evaluated the ER-α/GAPDH intensity from Sham-PLB male or Sham-PLB female rodents at 2, 7, 14, and 28 DPI (Fig. 5). No statistical difference was found in the ER-α/GAPDH intensity for the Sham-PLB male or Sham-PLB female among the selected time points according to One-way ANOVA followed by Tukey’s multiple comparison test (Sham-PLB male F(3,11)=1.685; Treatment MS=0.0001; p=0.247; and Sham-PLB female F(3,11)=2.932; Treatment MS=0.0003; p=0.010). Therefore, we grouped the Sham-PLB control rats (either male or female groups; separately) into a single group according to sex.
Fig.3.
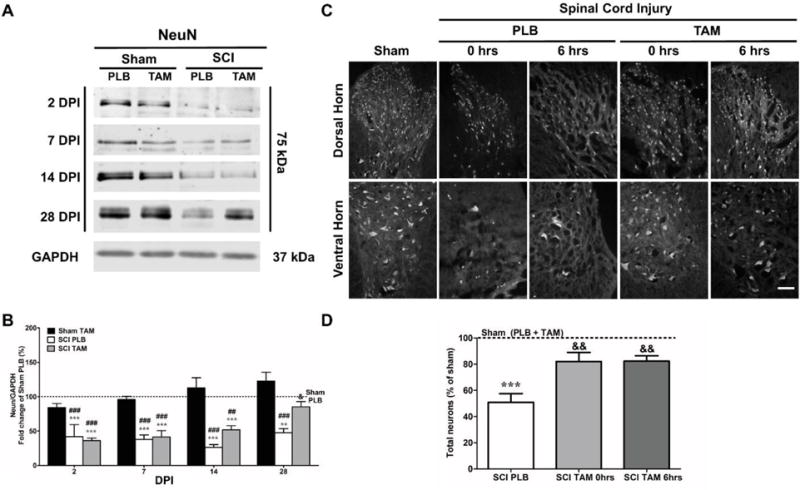
Tamoxifen promotes neuronal survival after spinal cord injury in male rats. (A) Representative immunoblots for NeuN protein from the injured or laminectomy spinal cord treated with TAM or PLB at 2, 7, 14 and 28 DPI. (B) Semi-quantitative analysis revealed that the group of SCI-PLB rats significantly decreased NeuN expression at all time points tested when compared to Sham-PLB (***p<0.001; **p<0.01) and Sham-TAM (###p<0.001; ##p<0.01). TAM treatment after SCI significantly increased NeuN expression at 28 DPI relative to SCI-PLB (&p<0.05). Data are mean±SEM, Two-way ANOVA followed by Bonferroni’s post hoc (Sham-PLB n=3,5,3,4; Sham-TAM n=3,5,3,3; SCI-PLB n=4,5,3,4; SCI-TAM n=4,4,4,3). C. Representative sections from DH and VH neurons at 35 DPI showed an increase in both horns of the gray matter when treated with TAM (magnification= 20×; scale bar= 100 μm). D. Dorsal (left and right side) and ventral (left and right side) areas were analyzed for NeuN immunoreactivity. Neurons from dorsal and ventral horn were counted, averaged, and compared to sham. Results are presented as the percentage of Sham (PLB + TAM). Neuronal count showed a significant reduction in neuronal cell number after SCI (***p<0.001). Immediate (t=0 hrs) and delayed (t=6 hrs) TAM administration significantly increased total number of neurons after SCI (&&p<0.01). Data are mean±SEM, One-way ANOVA followed by Tukey’s post hoc; Sham (PLB+TAM) n=6; SCI-PLB (0+6hrs) n=6; SCI-TAM 0 hrs n=3; SCI-TAM 6 hrs n=3.
Fig.5.
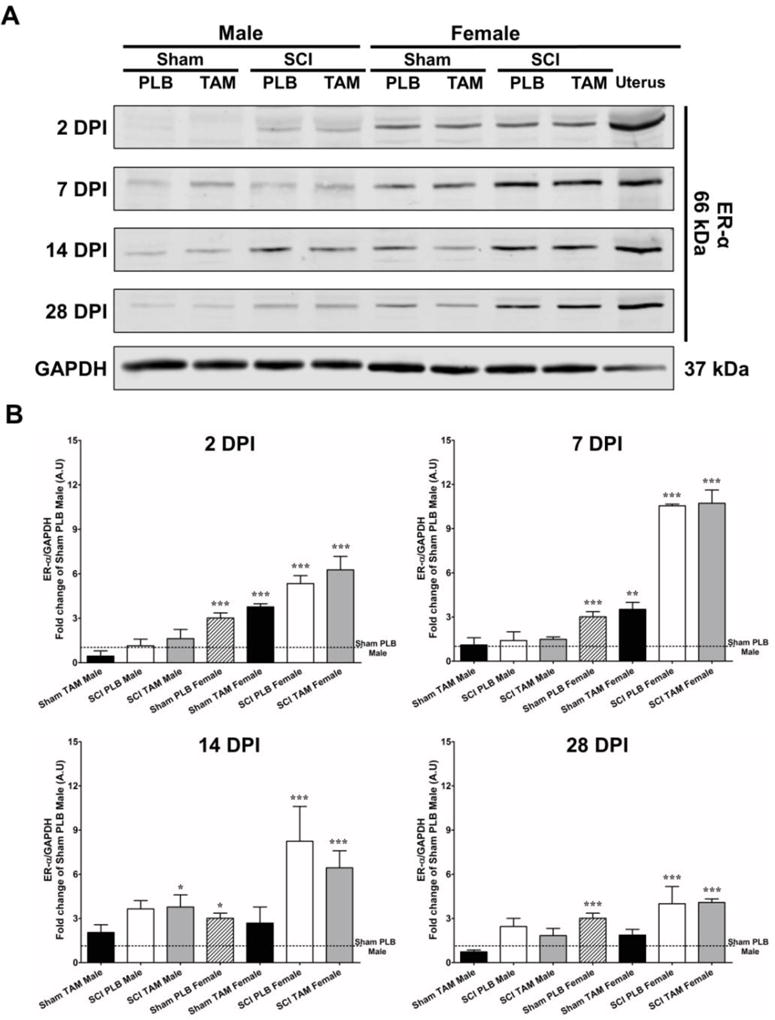
Differential expression of ER-α in male and female rats treated with Tamoxifen after SCI. (A) Representative immunoblots for ER-α expression at 2, 7, 14 and 28 DPI from the epicenter area in male and female rats. Immunoblots show a difference in the expression of ER-α (66 kDa) between male and female rats after SCI. (B) Semi-quantitative analysis showed a significant increase in ER-α expression SCI-PLB-female rats at 2,7,14 and 28 DPI and a significant increase in SCI-TAM-male at 14 DPI. Data are mean±SEM; One-way ANOVA followed by Dunnet’s post hoc was used to determine significant difference between groups and Sham-PLB-male at 2, 7, 14 and 28 DPI [*p<0.05; **p<0.01; ***p<0.001], (n=3 for each group, per time point).
Data are presented as mean ± standard error of the mean (SEM). A series of symbols are denoted to show statistical significance between groups. For behavioral assays, the ampersand symbol (&) denotes significance (p<0.05) between SCI-PLB (pooled data) and SCI-TAM (0 or 6 hrs) treatment groups while a dagger (†) symbol denotes statistical significance (p<0.05) for Interaction (DPI and Treatment) in Two-way ANOVA tests. For Luxol fast blue histology and NeuN cell count, an asterisk shows statistical significance between Sham (PLB + TAM) and treatment groups, while the ampersand (&) symbol shows significance between SCI-PLB (pooled 0 + 6 hrs groups) and SCI-TAM (0 or 6 hrs). For Western blot experiments, the asterisk (*) is used to denote significance for Sham-PLB male while the number sign (#) is used to denote significance between Sham-TAM and treatment groups. Sham-TAM rodents were not pooled for molecular experiments because we aimed to study the effects of TAM in target protein expression on intact animals. An increase in the number of symbols represent a higher statistical significance: 1 symbol= p<0.05; 2 symbols=p<0.01 and 3 symbols=p<0.001.
Results
TAM administration immediately and 6 hours after SCI favors locomotion and improves coordination
In this study, behavioral assays (BBB Open Field and Grid walk tests) were used to evaluate the effects of TAM in locomotor recovery when this drug was applied at different time points after SCI. Appropriate contusion injury to the spinal cord was verified by two evaluators blinded to treatment at 2 DPI (data not shown). Compression to the spinal cord was similar between all groups (SCI-PLB 0, 6, 12, 24 hrs or SCI-TAM 0, 6, 12, 24 hrs) at 2 DPI and no statistical difference was observed according to One-way ANOVA followed by Tukey’s multiple comparison post-hoc (F(7,73)=0.874; Treatment MS=0.062; p=0.5308). Immediate (t=0 hrs) TAM treatment favored locomotor activity at 14*,21*,35* DPI and delayed intervention (t=6 hrs) resulted in locomotor improvement at 7*,14*,21*,28** and 35** DPI. Delayed therapy for 12 or 24 hrs exerted no effect in locomotion after SCI according to Two-way ANOVA Bonferroni’s post hoc (F(5,84) =86.0; &&&Treatment p<0.0001; †††Interaction p<0.0001; &&&DPI p<0.001) [Fig. 1A].
The grid-walk test was used as a tool to evaluate the recovery of fine movements required for coordination (Colón et al., 2016; Merkler et al., 2001; Rosas et al., 2011; Santiago et al., 2009). Immediate (t=0 hrs) TAM administration resulted in a significant reduction in the number of errors at 21 and 35 DPI according to the Two-way ANOVA followed by Bonferroni’s post hoc (F(5,84)=35.7; Treatment p<0.0001) [Fig. 1B]. In addition, according to this analysis, both treatment and DPI significantly influenced the behavioral recovery after SCI when the animals were subjected to continuous TAM administration across the 35 days after the injury (F(4,84)=104.9, DPI p<0.0001; F(20,84)=3.99, Interaction p<0.0001). Delayed intervention at 12 or 24 hrs resulted in no effect in the number of errors. Immediate TAM delivery after SCI resulted in a significant increase in the number of paw positions during chronic stages (28 and 35 DPI). However, delayed intervention (6 hrs) resulted in an increase in the number of paw positions only at 35 DPI according to Two-way ANOVA followed by Bonferroni’s post hoc test (F(5,84)=12.00; Treatment p<0.0001) [Fig. 1C].
Tamoxifen increases white matter preservation and increases Olig-2 expression after SCI
Luxol fast blue histology was performed at the lesion epicenter of intact (sham) and injured rats. Positive staining (representative of intact myelin) was observed in the laminectomy area of Sham (PLB or TAM) animals and toward the periphery of the spinal cord of injured animals at the lesion epicenter (Fig. 2A). As expected, a meaningful reduction in white matter spared tissue was observed at 35 DPI after SCI and a significant increase in white matter spared tissue was observed in the SCI-TAM 0 hrs&&& and SCI-TAM 6 hrs&&& groups when compared to SCI-PLB (0 + 6 hrs) rats at 35 DPI (Fig. 2B) according to One-way ANOVA Tukey’s post hoc (F(3,17)= 109; Treatment MS=5867; ***p<0.0001).
We studied the effects of TAM in the expression of oligodendroglial transcription factor Olig-2 as a possible mechanism for the increase in myelin after TAM treatment. The protein migrated to the expected position of 37 kDa and no expression was observed in the negative control (uterus). SCI resulted in a significant reduction in Olig-2 expression at 28 DPI when compared to Sham-PLB* and Sham-TAM#. Immediate TAM treatment significantly increased Olig-2 expression when compared to SCI-PLB at 28&& DPI according to One-way ANOVA Tukey’s multiple comparison test (F(3,17)=11.62; MS=1041; p=0.0004). The up-regulation was mostly observed at the rostral area (Fig. 2C).
Tamoxifen favors neuronal survival after SCI
To determine the effects of TAM after SCI in neuronal survival, the expression of NeuN, a neuronal marker, was evaluated in the lesioned tissue at 2,7,14 and 28 DPI (Fig. 3A). Expression of NeuN was presented as a ratio relative to the loading control GAPDH and the total expression was normalized to Sham-PLB. The fold change (percentage) relative to Sham-PLB was presented in the acute and chronic stages after SCI (Fig. 3B). Immunoblot experiments showed a reduction in the expression of NeuN in SCI-PLB (0 hrs) at 2, 7, 14, and 28 DPI when compared to Sham-PLB* and Sham-TAM#. A similar pattern of expression was observed in SCI-TAM (0 hrs) animals at 2, 7, and 14 DPI. However, during chronic stages, a significant up-regulation of NeuN was observed in SCI-TAM animals (28& DPI) relative to SCI-PLB according to Two-way ANOVA, Bonferroni’s post hoc (F(3,54)=42.55; ***Treatment p<0.0001). NeuN expression changed with time and the effect was dependent solely on Treatment since no significant effect was observed for interaction (F(3,54)=5.456; **DPI p=0.0024; F(9,54)=1.051; Interaction p=0.4129).
To determine if the up-regulation of NeuN resulted in an increase in neuronal survival, we studied neuronal populations at the rostral and caudal penumbras at 35 DPI (Fig. 3C). Two evaluators blinded to treatment counted cells in the VH and DH (ventral and dorsal horn, respectively). Sham-PLB and TAM (pooled data; see statistics section) cell count was used as a point of reference to establish the percentage of neuronal loss after SCI as previously reported (Colón et al., 2016). Briefly, neurons from the DH and VH were identified and counted (Magnification= 20×, Fig. 3C). The total number of immuno-positive cells per area was compared to the total Sham (PLB + TAM) cell count per anatomical area (rostral and caudal). The data (Fig. 3D) for rostral and caudal area per animal was combined and presented as a total cell count percentage of Sham (PLB + TAM). Neuronal count revealed a significant decrease in the total number of immunopositive neurons in SCI-PLB (0 + 6 hrs) rats at 35*** DPI when compared to Sham (PLB + TAM). A significant increase in the total number of neurons at the VH and DH was found in the groups SCI-TAM (0 hrs)&& and SCI-TAM (6 hrs)&& relative to SCI-PLB, according to One-way ANOVA Tukey’s multiple comparison test (F(3,16)=22.59; Treatment MS=2227; p<0.0001).
Continuous TAM administration after SCI enhances gliosis in the acute stage and gradually decreases GFAP expression in the chronic stage
The effect of TAM in the temporal pattern of GFAP expression was established at the rostral, epicenter, and caudal areas (Fig. 4A). Target protein intensity from densitometry quantification was normalized to loading control GAPDH and standardized relative to Sham-PLB (percentage) expression at each area per time point (Fig. 4B). Following SCI, we observed a significant increase in total GFAP expression in SCI-PLB (2, 7, 14, and 28 DPI) and in SCI-TAM (2, 7 and 14 DPI) when compared to Sham-PLB* or Sham-TAM#. During the acute stages, TAM enhances GFAP expression at 2&&& DPI in SCI-TAM rats when compared to SCI-PLB. The enhancement in GFAP expression in the SCI-TAM group returned to SCI-PLB level at 7 DPI and gradually decreases until it is significantly down-regulated at 28& DPI according to Two-way ANOVA followed by Bonferroni’s post hoc test (F(3,47)=72.97; ***Treatment p<0.0001). The observed effect depends both, on DPI and Treatment (F(3,47)=17.78; ***DPI p<0.0001 and, F(9,47)=7.393; †††Interaction p<0.0001) [Fig. 4B]. We visualized GFAP immunoreactivity at the dorsal, ventral and lateral areas of Sham (PLB or TAM), and injured animals at the lesion epicenter 35 DPI. Imaging parameters were controlled using isotype primary antibody (IgG2b) as negative control as previously reported (Colón et al., 2016). We observed a reduction in GFAP immunoreactivity at the lesion epicenter at 35 DPI in SCI-TAM (0 hrs) and SCI-TAM (6 hrs) when compared to SCI-PLB animals (Fig. 4C).
Fig.4.
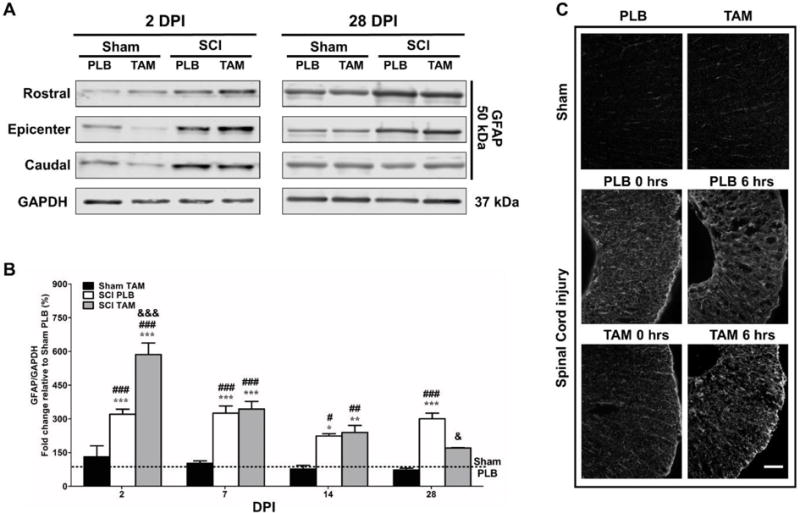
Tamoxifen enhances gliosis in the acute phase after SCI and gradually decreases GFAP total expression (rostral, epicenter and caudal levels) in the chronic stages. (A) Representative immunoblots for GFAP expression from rostral, epicenter and caudal areas at 2 and 28 DPI. (B) Semi-quantitative analysis showed a significant increase in GFAP total expression in SCI-PLB animals at 2, 7, 14 and 28 DPI when compared to Sham-PLB (***p<0.001) and a significant increase at 2, 7, 14 and 28 DPI when compared to Sham-TAM (###p<0.001). TAM significantly increased GFAP expression at 2 DPI in SCI-TAM rats (&&&p<0.001) and decreased GFAP expression gradually until a significant difference was observed between SCI-PLB and SCI-TAM at 28 DPI (&p<0.05). Data are mean±SEM Two-way ANOVA 2, 7, 14 and 28 DPI (Sham-PLB n=4,5,3,4; Sham-TAM n=4,4,3,3; SCI-PLB n=4,5,3,4; SCI-TAM n=4,5,5,3). (C) Representative sections of GFAP immunoreactivity at the lesion epicenter in the lateral thoracic spinal cord at 35 DPI (Magnification= 20×; scale bar= 100 μm). Sham animals (PLB or TAM) showed low levels of GFAP immunoreactivity. SCI increased GFAP immunoreactivity in PLB animals. Immediate (t= 0 hrs) or delayed (t= 6 hrs) TAM treatment reduced GFAP at the lesion epicenter. Sections from dorsal, lateral and ventral areas were visualized for each rat (Sham-PLB n=3; Sham-TAM n=3; SCI-PLB 0 hrs n=3; SCI-PLB 6 hrs n=3; SCI-TAM 0 hrs n=3; SCI-TAM 6 hrs n=3).
Estrogen receptor alpha differential expression influences locomotor recovery in male and female rodents after SCI
Since the TAM-mediated therapeutic window in male rats is 6 hrs (Fig. 1) but 24 hrs in female rats (Colón et al., 2016), the expression profile of ER-α during acute and chronic stages were studied to correlate if the behavioral observation could be attributed to a differential expression of this receptor. The temporal expression of ER-α was studied at the lesion epicenter in male and female rats treated with TAM (Fig. 5A). The immunoreactive band migrated to the expected position of 66 kDa and co-migrated with the ER-α receptors in the positive control (uterus homogenate). Sham-PLB male and Sham-PLB female rodents from all time points were pooled (by sex) and used to compare the expression of ER-α from the other groups since no significant difference was observed among each of the Sham-PLB groups across time. The temporal expression pattern of ER-α (66 kDa) revealed low levels of this receptor in the laminectomy area in Sham-PLB and Sham-TAM male groups at all time points (Fig. 5B). The expression of ER-α in SCI-PLB and SCI-TAM-male rodents remained relatively constant at all time-points except at 14 *DPI where SCI-TAM-male rodents exhibited a significant up-regulation when compared to Sham-PLB-male. Sham-PLB or TAM female rodents showed an overall increase in the expression of ER-α when compared to Sham-PLB-male at all time points (2–28 DPI). Sham-TAM-female rodents showed a significant up-regulation only at 2 and 7 DPI. After SCI, female rodents (SCI-PLB and SCI-TAM) exhibited a significant increase in ER-α at the lesion epicenter at all time points. Importantly, the female SCI-PLB and SCI-TAM groups exhibited similar up-regulation of ER-α expression at the lesion epicenter. A One-way ANOVA followed by Dunnett’s comparison test was performed to establish statistical significance between treatment groups using Sham-PLB-male (pooled) as control. Statistical analysis was performed per time-point as shown in Fig. 5B (2 DPI F(7,41)=18.26; Treatment MS=17.46; p<0.0001; 7 DPI F(7,41)=65.10; Treatment MS=60.31; p<0.0001; 14 DPI F(7,41)=10.74; Treatment MS=24.54; p<0.0001; 28 DPI F(7,41)=7.497; Treatment MS=7.566; p<0.0001).
Discussion
The results demonstrate that TAM provides behavioral recovery when administered 6 hrs after SCI. Moreover, for the first time, we also showed an increase in white matter spared tissue when this drug was used within this period in male rats and the difference in the therapeutic window with female animals could be attributed to the levels of ER-α. In addition, we found that TAM exerts neuroprotection in neurons and glia (oligodendrocytes and astrocytes) up-to 6 hrs after a contusion to the spinal cord in male rodents. Therefore, the results from this study validate the hypothesis about the use of TAM as a neuroprotective drug after SCI and show that sex differences may influence the therapeutic window of TAM (behavioral endpoint) without alterations in the mechanisms activated (neuronal and glial), which are similar in male or female rodents. In this study, together with published articles in other CNS pathologies, we provide mechanistic and therapeutic evidence for the translation of TAM into the clinical setting.
Our behavioral data is consistent with studies by others, showing that TAM favors locomotor recovery in male rodents when administered 30 minutes or 2 hrs after SCI using a weight drop method or the Infinite Horizon impactor, respectively (Guptarak et al., 2014; Tian et al., 2009). Moreover, our results agree with experiments performed in male cats that received a penetrating hemisection and were treated with TAM (de la Torre Valdovinos et al., 2016). Cats injected with TAM immediately and two more times (at 24 and 48 hrs after SCI) resulted in an improvement in gait locomotion that was correlated with a reduced cavity and axonal preservation. In contrast, our group showed that continuous delivery of TAM improves locomotor recovery in female rodents when therapy is delayed up-to 24 hrs after SCI (Colón et al., 2016). Here, we show that continuous TAM administration immediately or 6 hrs after SCI improves locomotion in male rodents (Fig. 1A). Delayed therapy 12 or 24 hrs after SCI exerted no effect in locomotion, which suggests the therapeutic window is shortened in male when compared to female subjects. The observed effects depend on Treatment and DPI, as evidenced by the rate of change in the acute stages that later plateau by 28 DPI. Importantly, male rodents with TAM exhibited a significant increase in locomotor recovery when compared to SCI-PLB (10.1 vs 12.0) but a marked sex difference was observed when SCI-TAM-male rodents are compared to SCI-TAM-female (12.0 vs 14.3) from our previous study (Colón et al., 2016). Also, this is the first study to include the grid walking test in injured male rats treated and untreated with TAM to analyze refined locomotor behavior. Tamoxifen treatment resulted in better coordination as evidenced by a reduction in the number or errors and increased paw positions when therapy is administered immediately (t=0 hrs) or delayed (t=6 hrs) and these effects were dependent on Treatment and DPI as evidenced by the rate of change for both tests until an eventual plateau for the paw positions (Fig. 1B–C). Since the grid walking test helps to discriminate between PLB and TAM treated rodents that exert a similar stepping frequency (from the BBB Open field test), the results in this task support the beneficial effects of TAM applied within the first 6 hrs after the SCI (Cummings et al., 2007; Patel et al., 2017). Based on the marked sex difference in the behavioral assays and the effects of Treatment and DPI, further studies must be conducted with a higher dose of TAM to consider if the maximum response obtained could be increased, if the therapeutic window obtained in this study is dose dependent or if the differential behavioral responses in male and female rats depend on a particular estrogen receptor.
The effects of TAM in white matter spared tissue has been evidenced in different models of SCI (NYU vs. Infinite Horizon impactors vs penetrating hemisection), animal species (rat vs. cat) and sex (male vs. female) (Colón et al., 2016; De la Torre Valdovinos et al., 2016; Guptarak et al., 2014; Mosquera et al., 2014; Osuna-Carrasco et al., 2016; Tian et al., 2009). The anatomical results in this project and others correlate with an increase in tissue sparing and enhanced locomotor activity. Here, we provide evidence of Olig-2 (oligodendroglial transcription factor) as a mechanism by which TAM promotes white matter preservation (Fig. 2A, 2D). The effect of Olig-2 increase (compared to SCI-PLB), in our model, was mostly restricted to the rostral and caudal areas in SCI-TAM group animals. This transcription factor has been associated to oligodendrocyte maturation in stem cell experiments, which suggests TAM may stimulate oligodendroglial progenitor cells (OPCs) to promote re-myelinating processes after SCI (Jadasz et al., 2013). Similarly, when a pellet of TAM was implanted 2 hrs after SCI in male rats, the investigators observed an increase in myelin associated proteins (MBP, MOG and CNPase) at 35 DPI (Guptarak et al., 2014). They correlated the increase in these proteins with an increase in myelin when the animals were treated with TAM. On the other hand, Tian and colleagues (2009) showed that a single bolus of TAM (5 mg/kg) 30 minutes after SCI reduced myelin associated axonal inhibitors at 7 DPI (Guptarak et al., 2014). However, this contradictory result could be related to the injury model, end-point studied, dose, duration, and delivery of TAM. Therefore, the maintenance of Olig-2 could lead to an increase in the amount of white matter spared tissue at the lesion epicenter 35 DPI (Fig. 2A–B). Nevertheless, this effect may also be attributed to the anti-oxidant, anti-apoptotic, and anti-inflammatory effects of TAM, as has been observed in several models of CNS pathologies (Kimelberg et al., 2000; Liu et al., 2010; Tian et al., 2009; Wei and Ma, 2014; Zhang et al., 2005, 2007).
We evaluated the effects of TAM in the expression of neuronal (NeuN) and astrocytic (GFAP) proteins after SCI. Because SCI is a multifactorial disease, we studied the expression of these proteins at the rostral, epicenter, and caudal areas during the acute (2, 7 DPI) and chronic (14, 28 DPI) stages. The temporal profile for NeuN shows that SCI significantly reduced the expression of this protein from 2–14 DPI in SCI-PLB and SCI-TAM rodents but the expression was significantly up-regulated at 28 DPI in SCI-TAM rats only. The observed effects suggest that TAM may initiate neuroprotective mechanisms such as anti-inflammatory, anti-oxidant, and anti-apoptotic effects during acute stages, which will reduce the detrimental environment promoting neuronal survival as shown by others during acute SCI (Tian et al., 2009; Wei and Ma, 2014) [Fig. 4]. Contrary to our expectations, a decrease in NeuN was observed during acute stages followed by an increase in NeuN expression and in the number of cells during chronic stages. This implies that TAM is initiating neuroprotective mechanisms that promote cell survival regardless of the negative input provided by the injury to the cord. Therefore, the beneficial effects of TAM are sufficient to promote survival regardless of the downregulation of this protein during the acute stages. Statistical analysis showed that the effects of TAM rely only on Treatment and not DPI (Interaction p=0.4129), which implicates that the TAM-mediated effect in neurons may relate to the presence of TAM metabolites during acute and chronic stages. For this study, we used a 15 mg TAM pellet that released a continuous dose for 21 days (0.71mg/day). However, we observe beneficial effects during chronic stages 28 and 35 DPI, which may be occurring either because TAM initiates cellular mechanisms during acute stages that extend towards the chronic stages or because the observed effect may be mediated by the active metabolites of TAM (4-hydroxy-Tamoxifen, half-life less than 7 days) (O’Neill et al., 2004).
As expected, we observed a significant decrease in GFAP expression at 28 DPI in SCI-TAM rats. Similar observations were reported by others, when selective estrogen receptor modulators reduced the gliotic response in the injured brain (Arevalo et al., 2012; Barreto et al., 2009). The observed effect in our experimental animals was dependent on Treatment and DPI (time), as evidenced by the dual effect observed in SCI-TAM rats; a significant increase compared to SCI-PLB at 2 DPI a gradual decline until a significant decrease at 28 DPI (Fig. 4A). The significant decrease was maintained as evidenced by GFAP immunoreactivity at 35 DPI in SCI-TAM 0 hrs. Although we observe a decrease in SCI-TAM 6 hrs rats, the effect of TAM in reactive gliosis was less in this group suggesting that the delayed therapy of TAM may exert cell specific effects (neuronal and oligodendroglial versus astrocytic; Fig. 2, 3, 4). Although unexpected, the 2 DPI increase in GFAP expression may be related to the activation of estrogen receptors (ER), similar to the effects shown by others when supra-physiological doses of E2 (4mg/kg) stimulate GFAP and vimentin expression during acute SCI (3 DPI) without affecting locomotor improvement (Ritz and Hausmann, 2008). Importantly, we interpret that the effect at rostral and caudal areas depend on the stimulating effects of TAM in GFAP expression during the acute stages. The up-regulation of GFAP at 2 DPI at the rostral, epicenter, and caudal areas may act as a neuroprotective mechanism that accelerates the gliotic process to create a stronger cavity that encapsulates the lesion area containing the damaged area from extending further (Fig. 4). This will provide a safer environment in areas close to the epicenter, favoring myelin preservation (Fig. 2), and neuronal survival (Fig. 3).
Our results show a marked sexually dimorphic difference when rodents are subjected to continuous TAM treatment. Studies by others suggested that female rats have an intrinsic capability to recover better from an injury to the central nervous system (CNS) when compared to their male counterparts (Farooque et al., 2006). Other studies showed that the sex-dependent effects were not related to estrogen since the administration of E2 to injured male rats exerted no effect in locomotor recovery (Swartz et al., 2007). However, this last result is controversial because of the noticeable neuroprotective effects of E2 during traumatic conditions to the CNS and some of these effects have been associated to the activation of ERs (Acaz-Fonseca et al., 2014; Bourque et al., 2013; Day et al., 2013; Jover-Mengual et al., 2010; Mosquera et al., 2014; Samantaray et al., 2016). Therefore, a possible explanation for the observed behavioral difference in male and female rodents could be attributed to the differential expression of ERs in male and female rodents. The basal expression of ER-α in female rats is higher than in male rodents and SCI upregulates the levels of this receptor only in female animals (Fig. 5). This differential pattern of ER-α expression support the sex difference in the therapeutic window of TAM after SCI, and helps to explain the response of female rats to this drug up-to 24 hrs after the trauma and only 6 hrs in male rats. However, we could not discard the possible involvement of other ERs, like the ER-β (cytoplasmic/nuclear receptor) or GPER-1/GPR-30 (G protein coupled transmembrane receptor).
The ER- β receptor has been poorly studied in a traumatic situation such as the SCI setting. Its role has being mainly attributed to aggression and anxiety-like behaviors (male mice), and neurogenesis in the dentate gyrus suggesting that this receptor plays a role mostly during cognitive and behavioral processes (Handa et al., 2012). On the other hand, several reports demonstrated that activation of GPER-1/GPR-30 mediates locomotor recovery after SCI. In addition, administration of E2 resulted in protection mediated by GPER-1/GPR-30 during traumatic brain injury, some of the protective effects were sexually dimorphic as shown by others (Broughton et al., 2014; Day et al., 2013). Moreover, G1 (GPER-1/GPR-30 agonist) or E2 treatment resulted in a temporal up-regulation of this receptor from 1–14 DPI, suggesting a possible role for this receptor after SCI (Hu et al., 2012). Therefore, the high levels of estradiol in female rats may activate these receptors resulting in a better neuroprotection of nervous tissue and behavioral outcomes than in male animals. Since previous studies have demonstrated the role of GPER-1/GPR-30 in locomotor recovery after SCI, future experiments must determine the role of GPER-1 in the TAM-mediated locomotor recovery.
Prior reports have demonstrated that sex differences resulted in rats recovery after SCI (Hauben et al., 2002) and brain injury (Bramlett & Dietrich, 2001). Therefore, we could not discard the possibility of high estradiol levels in female rats as responsible for their better behavioral outcomes than male animals. The phenol hydroxyl ring of estradiol may provide antioxidant and anti-inflammatory responses (Behl et al., 1997; Sugioka et al., 1987; Winterle et al., 2001). In clinical studies, lipid peroxidation is more prominent in males than females early after severe brain jury, suggesting an antioxidant activity conferred by female hormones (Bayir et al., 2004). In animal studies, endogenous levels of E2 also offer protection after brain injury (Bramlet and Dietrich, 2001), which could be another possibility to explain the marked sex difference between the intact female and male rats in this study. Spinal cord injury increases oxidative stress and the production of superoxide, peroxynitrite, hydroxyl radical and hydrogen peroxide, which induces damage to the surviving tissue (Xiong and Hall, 2009; Xu et al., 2005). The steroidal structure of estradiol could act as a radical scavenger in the early stages after injury. Since this neuroprotective hormone is in higher concentrations in the female rats, may be more beneficial than in male animals by reducing oxidative stress, independent of the ER-α (Mosquera et al., 2014). However, possible activation of superoxide dismutase or catalase enzymes in the acute and chronic stages after SCI should be evaluated for estradiol and TAM.
An important consideration during the TAM-mediated recovery in male and female rats is the anti-oxidant activity of TAM. Previous studies using the middle cerebral artery occlusion model showed that TAM produces antioxidant activity (Zhang et al., 2007), as well as studies from our laboratory with a SCI contusion model (Mosquera et al., 2014). Both studies demonstrated that TAM could act as an antioxidant drug, independent of estrogen receptors, which may contribute to its beneficial effects during CNS pathologies and therefore, should not be discarded as a non-genomic mechanism of action. Tamoxifen, as a selective estrogen receptor modulator, interacts with the genomic machinery to activate or inhibit gene transcription in estrogen responsive tissues (Robinson et al., 1991). Another alternative to TAM-mediated neuroprotective effects and sex response differences, is that the differential effect could be associated to the expression of co-activators and co-repressors in male and female rats, which facilitate or inhibit transcription depending on tissue type (Lonard and Smith, 2002; McDonnell and Wardell, 2010). We hypothesize that TAM administration will activate antioxidant mechanisms and gene transcription in CNS tissue, like the genomic effects of E2 promoting cell survival and better outcome after SCI.
The results provided in this work support the use of TAM in the SCI setting, providing a clinically relevant window of intervention for male subjects. If translated into the clinic, this window provides valuable time for TAM administration. The influence of sex differences on recovery of SCI should be considered upon therapy design. Here, we studied for the first time, the effects of TAM after a delayed acute therapeutic intervention and the involvement of ERs that could mediate this neuroprotection. Further studies must consider increasing the dose of TAM to maximize the effects, the involvement of ERs in locomotor recovery, and the effects of the TAM metabolites in the activation of cellular mechanisms after SCI.
Highlights.
Immediate and delayed Tamoxifen treatment improves locomotion in male rats.
TAM exerts acute and chronic effects in neurons, astrocytes and oligodendrocytes
Spinal cord injury changes the expression of the estrogen receptor alpha
Tamoxifen induces Olig-2 expression in male rats after injury.
Tamoxifen augment GFAP expression acutely and reduces it chronically.
Acknowledgments
This research project is in partial fulfillment of Jennifer M. Colón’s doctoral thesis dissertation. The authors acknowledge the work performed by Dr. Yaria Arroyo Torres from the Metropolitan University (UMET, Cupey Campus) for some of the molecular experiments. We acknowledge Mr. Luis Colón for his technical support during the immunofluorescence experiments. Images were captured with a Nikon inverted microscope (grant P031S130068) and a Zeiss fluorescent microscope (grant 1P30NS069258-01). We would like to recognize all personnel from the UPR-MSC Animal Resources for their assistance during surgical procedures and post-operative care (Dr. Idia Vanessa Rodriguez, Lucila Lopez-Lavoy, Frances Venegas, Migdaliz De Jesus and Yazmin Massa-Muñoz).
Funding
This work was supported by the National Institute of Health grants: COBRE (P20-GM103642), MBRS-RISE Program (R25 GM061838), NIH-MARC (5T34GM007821-35) and RCMI (5G12MD007600) programs. Experiments from this research project were partially funded by a Cooperative Title V (grant number P031S130068) and the University of Puerto Rico Carolina Campus Seed Funds. JMC was supported by RISE and AC by the MARC programs, respectively.
Abbreviations
- ANOVA
Analysis of Variance
- CNS
Central nervous system
- DPI
days post-injury
- E2
Estradiol
- ER
estrogen receptor
- ER-α
Estrogen receptor alpha
- GFAP
glial fibrillary acidic protein
- MCAo
Middle Cerebral Artery Occlusion
- OPC
Oligodendroglial progenitor cells
- PFA
paraformaldehyde
- PLB
Placebo
- PAGE
polyacrylamide gel electrophoresis
- SCI
spinal cord injury
- TAM
Tamoxifen
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
This study was supported by federal and Institutional funds and the authors declare no conflict of interest with commercial or private associations.
References
- Acaz-Fonseca E, Sanchez-Gonzalez R, Azcoitia I, Arevalo MA, Garcia-Segura LM. Role of astrocytes in the neuroprotective actions of 17β-estradiol and selective estrogen receptor modulators. Mol Cell Endocrinol. 2014;389:48–57. doi: 10.1016/j.mce.2014.01.009. [DOI] [PubMed] [Google Scholar]
- Ahuja CS, Nori S, Tetreault L, Wilson J, Kwon B, Harrop J, Choi D, Fehlings MG. Traumatic Spinal Cord Injury—Repair and Regeneration. Neurosurgery. 2017;80:S9–S22. doi: 10.1093/neuros/nyw080. [DOI] [PubMed] [Google Scholar]
- Alexander A, Irving AJ, Harvey J. Emerging roles for the novel estrogen-sensing receptor GPER1 in the CNS. Neuropharmacol. 2017;113:652–660. doi: 10.1016/j.neuropharm.2016.07.003. [DOI] [PubMed] [Google Scholar]
- Arevalo MA, Diz-Chaves Y, Santos-Galindo M, Bellini MJ, Garcia-Segura LM. Selective Ooestrogen receptor modulators decrease the inflammatory response of glial cells. J of Neuroendocrinol. 2012;24:183–190. doi: 10.1111/j.1365-2826.2011.02156.x. [DOI] [PubMed] [Google Scholar]
- Barreto G, Santos-Galindo M, Diz-Chaves Y, Pernia O, Carrero P, Azcoitia I, Garcia-Segura LM. Selective estrogen receptor modulators decrease reactive astrogliosis in the injured brain: effects of aging and prolonged depletion of ovarian hormones. Neuroendocrinol. 2009;150:5010–5015. doi: 10.1210/en.2009-0352. [DOI] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- Bayir H, Marion DW, Puccio AM, Wisniewski SR, Janesko KL, Clark RSB, Kochanek PM. Marked gender effect on lipid peroxidation after severe traumatic brain injury in adult patients. J Neurotrauma. 2004;21:1–8. doi: 10.1089/089771504772695896. [DOI] [PubMed] [Google Scholar]
- Behl C, Skutella T, Lezoualc’h F, Post A, Widmann M, Newton CJ, Holsboer F. Neuroprotection against oxidative stress by estrogens: structure-activity relationship. Mol Pharmacol. 1997;51:535–541. [PubMed] [Google Scholar]
- Bourque M, Morissette M, Côté M, Soulet D, Di Paolo T. Implication of GPER1 in neuroprotection in a mouse model of Parkinson’s disease. Neurobiol Aging. 2013;34:887–901. doi: 10.1016/j.neurobiolaging.2012.05.022. [DOI] [PubMed] [Google Scholar]
- Bramlett HM, Dietrich WD. Neuropathological protection after traumatic brain injury in intact female rats versus males or ovariectomized females. J Neurotrauma. 2001;18(9):891–900. doi: 10.1089/089771501750451811. [DOI] [PubMed] [Google Scholar]
- Broughton BRS, Brait VH, Kim HA, Lee S, Chu HX, Gardiner-Mann CV, Guida E, Evans MA, Miller AA, Arumugam TV, Drummond GR, Sobey CG. Sex-dependent effects of G protein-coupled estrogen receptor activity on outcome after ischemic stroke. Stroke. 2014;45:835–841. doi: 10.1161/STROKEAHA.113.001499. [DOI] [PubMed] [Google Scholar]
- Chaovipoch P, Bozak KA, Gernhold LM, West EJ, Chongthammakun S, Foyd CL. 17β-Estradiol is protective in spinal cord injury in post- and pre-menopausal rats. J Neurotrauma. 2006;23:830–852. doi: 10.1089/neu.2006.23.830. [DOI] [PubMed] [Google Scholar]
- Cheng Q, Meng J, Wang XS, Kang WB, Tian Z, Zhang K, Liu G, Zhao JN. G-1 Exerts Neuroprotective Effects Through G Protein-coupled Estrogen Receptor 1 Following Spinal Cord Injury In Mice. Biosci Rep. 2016;0:1–10. doi: 10.1042/BSR20160134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colón JM, Miranda JD. Tamoxifen: an FDA approved drug with neuroprotective effects for spinal cord injury recovery. Neural Regen Res. 2016;11:1208–1211. doi: 10.4103/1673-5374.189164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colón JM, Torrado AI, Cajigas Á, Santiago JM, Salgado IK, Arroyo Y, Miranda JD. Tamoxifen Administration Immediately or 24 Hours after Spinal Cord Injury Improves Locomotor Recovery and Reduces Secondary Damage in Female Rats. J Neurotrauma. 2016;33:1696–1708. doi: 10.1089/neu.2015.4111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings BJ, Engesser-Cesar C, Cadena G, Anderson AJ. Adaptation of a ladder beam walking task to assess locomotor recovery in mice following spinal cord injury. Behav Brain Res. 2007;177:232–241. doi: 10.1016/j.bbr.2006.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzzocrea S, Genovese T, Mazzon E, Esposito E, Di Paola R, Muia C, Crisafulli C, Peli A, Bramanti P, Chaudry IH. Effect of 17[beta]-estradiol on signal transduction pathways and secondary damage in experimental spinal cord trauma. Shock. 2008;29:362–371. doi: 10.1097/shk.0b013e31814545dc. [DOI] [PubMed] [Google Scholar]
- Datto JP, Bastidas JC, Miller NL, Shah AK, Arheart KL, Marcillo AE, Dietrich WD, Pearse DD. Female Rats Demonstrate Improved Locomotor Recovery and Greater Preservation of White and Gray Matter after Traumatic Spinal Cord Injury Compared to Males. J Neurotrauma. 2015;32:1146–1157. doi: 10.1089/neu.2014.3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day NL, Floyd CL, D’Alessandro TL, Hubbard WJ, Chaudry IH. 17B-Estradiol Confers Protection After Traumatic Brain Injury in the Rat and Involves Activation of G Protein-Coupled Estrogen Receptor 1. J Neurotrauma. 2013;30:1531–41. doi: 10.1089/neu.2013.2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De la Torre Valdovinos B, Dueñas Jiménez JM, Jimenez Estada I, Banuelos Pineda J, Franco Rodríguez NE, Lopez Ruiz J, Osuna Carrasco L, Candanedo Arellano A, Dueñas Jiménez SH. Tamoxifen promotes axonal preservation and gait locomotion recovery after spinal cord injury in cats. J Vet Med. 2016:1–16. doi: 10.1155/2016/9561968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Don Carlos LL, Azcoitia I, Garcia-Segura LM. Neuroprotective actions of selective estrogen receptor modulators. Psychoneuroendocrinology. 2009;34S1:113–122. doi: 10.1016/j.psyneuen.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooque M, Suo Z, Arnold PM, Wulser MJ, Chou CT, Vancura RW, Fowler S, Festoff BW. Gender-related differences in recovery of locomotor function after spinal cord injury in mice. Spinal Cord. 2006;44:182–7. doi: 10.1038/sj.sc.3101816. [DOI] [PubMed] [Google Scholar]
- Fogaça Cristante A, Pessoa de Barros Filho TE, Martus Marcon R, Biraghi Letaif O, Dias da Rocha I. Therapeutic approaches for spinal cord injury. Clinics. 2012;67:1219–1224. doi: 10.6061/clinics/2012(10)16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco Rodríguez NE, Dueñas Jiménez JM, De la Torre Valdovinos B, López Ruiz JR, Hernández Hernández L, Dueñas Jiménez SH. Tamoxifen favoured the rat sensorial cortex regeneration after a penetrating brain injury. Brain Res Bull. 2013;98:64–75. doi: 10.1016/j.brainresbull.2013.07.007. [DOI] [PubMed] [Google Scholar]
- Gupta DS, Hubscher CH. Estradiol treatment prevents injury induced enhancement in spinal cord dynorphin expression. Front Physiol. 2012;3:28. doi: 10.3389/fphys.2012.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guptarak J, Wiktorowicz JE, Sadygov RG, Zivadinovic D, Paulucci-Holthauzen AA, Vergara L, Nesic O. The cancer drug tamoxifen: a potential therapeutic treatment for spinal cord injury. J Neurotrauma. 2014;31:268–83. doi: 10.1089/neu.2013.3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauben E, Mizrahi T, Agranov E, Schwartz M. Sexual dimorphism in the spontaneous recovery from spinal cord injury: a gender gap in beneficial autoimmunity? Eur J Neurosci. 2002;16:1731–1740. doi: 10.1046/j.1460-9568.2002.02241.x. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Ogawa S, Wang JM, Herbison AE, Ogawat S, Wang JM, Herbison AE. Roles for Oestrogen Receptor β in Adult Brain Function. J Neuroendocrinol. 2012;24:160–173. doi: 10.1111/j.1365-2826.2011.02206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu R, Sun H, Zhang Q, Chen J, Wu N, Meng H, Cui G, Hu S, Li F, Lin J, Wan Q, Feng H. G-protein coupled estrogen receptor 1 mediated estrogenic neuroprotection against spinal cord injury. Crit Care Med. 2012;40:3230–7. doi: 10.1097/CCM.0b013e3182657560. [DOI] [PubMed] [Google Scholar]
- Hulsebosch CE. Recent advances in pathophysiology and treatment of spinal cord injury. Adv Physiol Edu. 2002;26:238–55. doi: 10.1152/advan.00039.2002. [DOI] [PubMed] [Google Scholar]
- Ishihara Y, Itoh K, Ishida A, Yamazaki T. Selective estrogen-receptor modulators suppress microglial activation and neuronal cell death via an estrogen receptor-dependent pathway. J Steroid Biochem Mol Biol. 2015;145:85–93. doi: 10.1016/j.jsbmb.2014.10.002. [DOI] [PubMed] [Google Scholar]
- Ismailoğlu O, Oral B, Görgülü A, Sütçü R, Demir N. Neuroprotective effects of tamoxifen on experimental spinal cord injury in rats. J Clin Neurosci. 2010;17:1306–10. doi: 10.1016/j.jocn.2010.01.049. [DOI] [PubMed] [Google Scholar]
- Jadasz JJ, Kremer D, Göttle P, Tzekova N, Domke J, Rivera FJ, Adjaye J, Hartung HP, Aigner L, Küry P. Mesenchymal Stem Cell Conditioning Promotes Rat Oligodendroglial Cell Maturation. PLoS One. 2013;8:1–11. doi: 10.1371/journal.pone.0071814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jover-Mengual T, Miyawaki T, Latuszek A, Alborch E, R Suzzanne Z, Etgen AM. Acute estradiol protects CA1 neurons from ischemia-induced apoptotic cell death via the PI3K/Akt pathway. Brain Res. 2010;1321:1–12. doi: 10.1038/jid.2014.371. C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelberg HK, Feustel PJ, Yiqiang J, Paquette J, Boulus A, Keller RW, Jr, Tranmer BI. Acute treatment with tamoxifen reduces ischemic damage following middle cerebral artery occlusion. Neuroreport. 2000;11:2675–2679. doi: 10.1097/00001756-200008210-00014. [DOI] [PubMed] [Google Scholar]
- Lemmon VP, Ferguson AR, Popovich PG, Xu XM, Snow DM, Igarashi M, Beattie CE, Bixby JL. Minimum Information about a Spinal Cord Injury Experiment: A Proposed Reporting Standard for Spinal Cord Injury Experiments. J Neurotrauma. 2014;31:1354–1361. doi: 10.1089/neu.2014.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ER. Plasma membrane estrogen receptors. Trends in Endrocrinology and Metabolism. 2009;20:477–482. doi: 10.1016/j.tem.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JL, Tian DS, Li ZW, Qu WS, Zhan Y, Xie MJ, Yu ZY, Wang W, Wu G. Tamoxifen alleviates irradiation-induced brain injury by attenuating microglial inflammatory response in vitro and in vivo. Brain Res. 2010;1316:101–111. doi: 10.1016/j.brainres.2009.12.055. [DOI] [PubMed] [Google Scholar]
- Lonard DM, Smith CL. Molecular perspectives on selective estrogen receptor modulators (SERMs): progress in understanding their tissue-specific agonist and antagonist actions. Steroids. 2002;67:15–24. doi: 10.1016/s0039-128x(01)00133-7. [DOI] [PubMed] [Google Scholar]
- McDonnell DP, Wardell SE. The molecular mechanisms underlying the pharmacological actions of ER modulators: implications for new drug discovery in breast cancer. Curr Opin Pharmacol. 2010;10:620–8. doi: 10.1016/j.coph.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkler D, Metz GAS, Raineteau O, Dietz V, Schwab ME, Fouad K. Locomotor Recovery in Spinal Cord-Injured Rats Treated with an Antibody Neutralizing the Myelin-Associated Neurite Growth Inhibitor Nogo-A. J Neurosci. 2001;21:3665–3673. doi: 10.1523/JNEUROSCI.21-10-03665.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller NR, Jover T, Cohen HW, Zukin RS, Etgen AM. Estrogen can act via estrogen receptor alpha and beta to protect hippocampal neurons against global ischemia-induced cell death. Endocrinology. 2005;146:3070–3079. doi: 10.1210/en.2004-1515. [DOI] [PubMed] [Google Scholar]
- Mosquera L, Colón JM, Santiago JM, Torrado AI, Meléndez M, Segarra AC, Rodríguez-Orengo JF, Miranda JD. Tamoxifen and estradiol improved locomotor function and increased spared tissue in rats after spinal cord injury: their antioxidant effect and role of estrogen receptor alpha. Brain Res. 2014;1561:11–22. doi: 10.1016/j.brainres.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson ER, Wardell SE, McDonnell DP. The molecular mechanisms underlying the pharmacological actions of estrogens, SERMs and oxysterols: implications for the treatment and prevention of osteoporosis. Bone. 2013;53:42–50. doi: 10.1016/j.bone.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen J, Brinton RD. Mechanism of estrogen-mediated neuroprotection: Regulation of mitochondrial calcium and Bcl-2 expression. Proc Natl Acad Sci. 2003;100:2842–2847. doi: 10.1073/pnas.0438041100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill K, Chen S, Diaz Brinton R. Impact of the selective estrogen receptor modulator, tamoxifen, on neuronal outgrowth and survival following toxic insults associated with aging and Alzheimer’s disease. Exp Neurol. 2004;188:268–78. doi: 10.1016/j.expneurol.2004.01.014. [DOI] [PubMed] [Google Scholar]
- Otsu N. A Threshold Selection Method from Gray-Level Histograms. IEEE Trans Syst Man Cybern. 1979;9:62–66. doi: 10.1109/TSMC.1979.4310076. [DOI] [Google Scholar]
- Patel SP, Cox DH, Gollihue JL, Bailey WM, Geldenhuys WJ, Gensel JC, Sullivan PG, Rabchevsky AG. Pioglitazone treatment following spinal cord injury maintains acute mitochondrial integrity and increases chronic tissue sparing and functional recovery. Experimental Neurol. 2017;293:74–82. doi: 10.1016/j.expneurol.2017.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prossnitz ER, Arterburn JB. International Union of Basic and Clinical Pharmacology. XCVII G Protein-Coupled Estrogen Receptor and Its Pharmacologic Modulators. Pharmacol Rev. 2015;67:505–540. doi: 10.1124/pr.114.009712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz MF, Hausmann ON. Effect of 17beta-estradiol on functional outcome, release of cytokines, astrocyte reactivity and inflammatory spreading after spinal cord injury in male rats. Brain Res. 2008;1203:177–88. doi: 10.1016/j.brainres.2008.01.091. [DOI] [PubMed] [Google Scholar]
- Robinson S, Langa-fahey Susan M, Johnson Delinda A, Jordan VC. Metabolites, pharmacodynamics and pharmacokinetics of tamoxifen in rats and mice compared to the breast cancer patient. Drug Metab Dispos. 1991;19:36–43. [PubMed] [Google Scholar]
- Rosas OR, Figueroa JD, Torrado AI, Rivera M, Santiago JM, Konig-Toro F, Miranda JD. Expression and activation of ephexin is altered after spinal cord injury. Dev Neurobiol. 2011;71:595–607. doi: 10.1002/dneu.20848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samantaray S, Das A, Matzelle DC, Yu SP, Wei L, Varma A, Ray SK, Banik NL. Administration of low dose-estrogen attenuates persistent inflammation, promotes angiogenesis and improves locomotor function following chronic spinal cord injury in rats. J Neurochem. 2016;137:604–617. doi: 10.1111/jnc.13610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samantaray S, Smith JA, Das A, Matzelle DD, Varma AK, Ray SK, Banik NL. Low dose estrogen prevents neuronal degeneration and microglial reactivity in an acute model of spinal cord injury: effect of dosing, route of administration, and therapy delay. Neurochem Res. 2011;36:1809–1816. doi: 10.1007/s11064-011-0498-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago M, Rosas O, Torrado AI, Kalyan-masih PO, Miranda JD. Molecular, Anatomical, Physiological and Behavioral Studies of Rats Treated with Buprenorphine after Spinal Cord Injury. J Neurotrauma. 2009;29:1783–1793. doi: 10.1089/neu.2007.0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J, Rueden CT, Hiner MC, Eliceiri KW. The ImageJ ecosystem: An open platform for biomedical image analysis. Mol Reprod Dev. 2015 doi: 10.1002/mrd.22489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipski ML, Jackson AB, Gomez-Marin O, Estores I, Stein A. Effects of gender on neurologic and functional recovery after spinal cord injury. Arch Phys Med Rehabil. 2004;85:1826–1836. doi: 10.1016/j.apmr.2004.04.031. [DOI] [PubMed] [Google Scholar]
- Siriphorn A, Dunham KA, Chompoopong S, Floyd CL. Postinjury administration of 17β-estradiol induces protection in the gray and white matter with associated functional recovery after cervical spinal cord injury in male rats. J Comp Neurol. 2012;520:2630–46. doi: 10.1002/cne.23056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sribnick EA, Matzelle DD, Ray SK, Banik NL. Estrogen treatment of spinal cord injury attenuates calpain activation and apoptosis. J Neurosc Res. 2006a;84:1064–1075. doi: 10.1002/jnr.21016. [DOI] [PubMed] [Google Scholar]
- Sribnick EA, Ray SK, Banik NL. Estrogen prevents glutamate-induced apoptosis in C6 glioma cells by a receptor-mediated mechanism. Neuroscience. 2006;137:197–209. doi: 10.1016/j.neuroscience.2005.08.074. [DOI] [PubMed] [Google Scholar]
- Sribnick EA, Samantaray S, Das A, Smith J, Matzelle DD, Ray SK, Banik NL. Post-injury Estrogen Treatment of Chronic Spinal Cord Injury Improves Locomotor Function in Rats. J Neurosci Res. 2010;88:1738–1750. doi: 10.1002/jnr.22337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sribnick EA, Wingrave JM, Matzelle DD, Wilford GG, Ray SK, Banik NL. Estrogen Attenuated Markers of Inflammation and Decreased Lesion Volume in Acute Spinal Cord Injury in Rats. J Neurosc Res. 2005;82:283–293. doi: 10.1002/jnr.20622. [DOI] [PubMed] [Google Scholar]
- Sugioka K, Shimosegawa Y, Nakano M. Estrogens as natural antioxidants of membrane phospholipid peroxidation. FEBS Lett. 1987;210:37–39. doi: 10.1016/0014-5793(87)81293-0. [DOI] [PubMed] [Google Scholar]
- Swartz KR, Fee DB, Joy KM, Roberts KN, Sun S, Scheff NN, Wilson ME, Scheff SW. Gender differences in spinal cord injury are not estrogen-dependent. J Neurotrauma. 2007;24:473–80. doi: 10.1089/neu.2006.0167. [DOI] [PubMed] [Google Scholar]
- Tian DS, Liu JL, Xie MJ, Zhan Y, Qu WS, Yu ZY, Tang ZP, Pan DJ, Wang W. Tamoxifen attenuates inflammatory-mediated damage and improves functional outcome after spinal cord injury in rats. J Neurochem. 2009;109:1658–67. doi: 10.1111/j.1471-4159.2009.06077.x. [DOI] [PubMed] [Google Scholar]
- Wang ZF, Pan ZY, Xu CS, Li ZQ. Activation of G-protein coupled estrogen receptor 1 improves early-onset cognitive impairment via PI3K/Akt pathway in rats with traumatic brain injury. Biochem Biophys Res Commun. 2017;482:948–953. doi: 10.1016/j.bbrc.2016.11.138. [DOI] [PubMed] [Google Scholar]
- Wei HY, Ma X. Tamoxifen reduces infiltration of inflammatory cells, apoptosis and inhibits IKK/NF-kB pathway after spinal cord injury in rats. Neurol Sci. 2014;35:1763–8. doi: 10.1007/s10072-014-1828-z. [DOI] [PubMed] [Google Scholar]
- Winterle JS, Mill T, Harris T, Goldbeck RA. Absolute kinetic characterization of 17β-estradiol as a radical-scavenging, Antioxidant synergist. Arch Biochem Biophis. 2001;392:233–244. doi: 10.1006/abbi.2001.2431. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Hall ED. Pharmacological evidence for a role of peroxynitrite in the pathophysiology of spinal cord injury. Exp Neurol. 2009;216:105–114. doi: 10.1016/j.expneurol.2008.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Chi L, Xu R, Ke Y, Luo C, Cai J, Qiu M, Gozal D, Liu R. Increased production of reactive oxygen species contributes to motor neuron death in a compression mouse model of spinal cord injury. Spinal Cord. 2005;43:204–213. doi: 10.1038/sj.sc.3101674. [DOI] [PubMed] [Google Scholar]
- Yune TY, Kim SJ, Lee SM, Lee YK, Oh YJ, Kim YC, Markelonis GJ, Oh TH. Systemic administration of 17beta-estradiol reduces apoptotic cell death and improves functional recovery following traumatic spinal cord injury in rats. J Neurotrauma. 2004;21:293–306. doi: 10.1089/089771504322972086. [DOI] [PubMed] [Google Scholar]
- Yune TY, Park HG, Lee JY, Oh TH. Estrogen-Induced Bcl-2 Expression after Spinal Cord Injury Is Mediated through Phosphoinositide-3-Kinase/Akt-Dependent CREB Activation. J Neurotrauma. 2008;25:1121–1131. doi: 10.1089/neu.2008.0544. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Jin Y, Behr MJ, Feustel PJ, Morrison JP, Kimelberg HK. Behavioral and histological neuroprotection by tamoxifen after reversible focal cerebral ischemia. Exp Neurol. 2005;196:41–6. doi: 10.1016/j.expneurol.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Milatovic D, Aschner M, Feustel PJ, Kimelberg HK. Neuroprotection by tamoxifen in focal cerebral ischemia is not mediated by an agonist action at estrogen receptors but is associated with antioxidant activity. Exp Neurol. 2007;204:819–27. doi: 10.1016/j.expneurol.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Brinton RD. Estrogen receptor alpha and beta differentially regulate intracellular Ca2+ dynamics leading to ERK phosphorylation and estrogen neuroprotection in hippocampal neurons. Brain Res. 2007;1172:48–59. doi: 10.1016/j.brainres.2007.06.092. [DOI] [PubMed] [Google Scholar]


