Abstract
Background
Anhedonia, the diminished ability to experience pleasure, is an important dimensional entity linked to depression, schizophrenia and other emotional disorders, but its origins and mechanisms are poorly understood. We have previously identified anhedonia, manifest as decreased sucrose preference and social play, in adolescent male rats that experienced chronic early-life adversity/stress (CES). Here we probed the molecular, cellular and circuit processes underlying CES-induced anhedonia and tested them mechanistically.
Methods
We examined functional brain circuits and neuronal populations activated by social play in adolescent CES and control rats. Structural connectivity between stress- and reward-related networks was probed using high-resolution diffusion tensor imaging (DTI), and cellular/regional activation was probed using cFos. We employed viral-genetic approaches to reduce corticotropin-releasing hormone (Crh) expression in amygdalar central nucleus (ACe) in anhedonic rats, and tested for anhedonia reversal in the same animals.
Results
Sucrose preference was reduced in adolescent CES rats. Social play, generally considered an independent measure of pleasure, activated brain regions involved in reward circuitry in both control and CES groups. In CES rats, social play activated Crh-expressing neurons in ACe, typically involved in anxiety/fear, indicating aberrant functional connectivity of pleasure/reward and fear circuits. DTI-tractography revealed increased structural connectivity of amygdala to medial prefrontal cortex in CES rats. Crh-shRNA, but not control shRNA, given into ACe reversed CES-induced anhedonia without influencing other emotional measures.
Conclusions
These findings robustly demonstrate aberrant interactions of stress and reward networks after early-life adversity and suggest mechanistic roles for Crh-expressing amygdala neurons in emotional deficits portending major neuropsychiatric disorders.
Keywords: anhedonia, brain circuits, CRF, CRH, shRNA, diffusion tensor imaging, early-life stress, gene silencing, amygdala, reward circuit
Introduction
Vulnerability to emotional disorders such as depression is governed in part by early-life experiences. Indeed, early-life adversity and stress are strongly associated with the development of adult depression (1–4), as well as alcohol use and addiction (5,6). In rodent models, this association may be causal, demonstrated by the provocation of adult depressive-like behaviors by the experimental imposition of chronic early-life stress (7–10).
Human depression and schizophrenia are often associated with or preceded by adolescent anhedonia, a loss of the ability to experience pleasure (11). Indeed, adolescent anhedonia is considered a harbinger of major depressive disorder and schizophrenia (12,13), and has recently been recognized as a crucial Research Domain Criterion (RDoC) by the National Institute of Mental Health (NIMH) (14). In line with these observations in humans, we have recently found that chronic early-life adversity, induced by an impoverished environment and aberrant maternal care, provokes anhedonia-like behaviors in adolescent male rats (15). Sucrose preference and social play, often considered manifestations of pleasure-like behaviors [(16–18) but see (19)], were diminished in rats experiencing early-life adversity (CES rats), indicating significant disruption of pleasure/reward networks. However, the mechanisms underlying these serious problems have remained unknown.
Early-life adversity, and stress throughout life, engage a complex brain circuitry that communicates- and overlaps with structures and circuits involved in pleasure/reward (20–23). Stress influences the expression and function of several hormones and mediators, including glucocorticoids and their brain receptors and the neuropeptide corticotropin-releasing factor (CRF; encoded by the Crh gene). Early-life stress induces potent epigenetic mechanisms to persistently alter the expression of several of these molecules, and thus change enduringly responses to subsequent stress, as well as vulnerability and resilience to stress-related emotional disorders (24–29). A significant body of work has analyzed the effects of early-life stress on glucocorticoid receptors in regions influencing pleasure/reward function such as the hippocampus (26,27), amygdala (28,29) and prefrontal cortex (28,30,31). In addition, early-life stress influences the expression of Crh in the central nucleus of the amygdala (ACe)(32,33). Such changes in expression and function of amygdala Crh are important, because amygdala CRF has been strongly implicated in anxiety (38–40) and fear (41–43). Augmented anxiety would interfere with the ability to experience joy during normally pleasurable activities. Furthermore, the amygdala communicates directly with brain regions sub-serving pleasure/reward functions and has been implicated in reward learning and addiction (19,40–43).
Here, we found several measures of anhedonia in adolescent rats that had experienced early-life adversity, associated with aberrant structural and functional interaction of the pleasure/reward and stress circuitries. Because aberrant activation of the ACe during pleasurable activities involved CRF-producing neurons, we reasoned that the peptide might interfere with normal pleasure/reward functions. Indeed, reducing expression of Crh in the ACe using shRNA reversed the anhedonic phenotype without affecting other emotional function, suggesting a mechanistic role for ACe CRF in early-life stress-related anhedonia.
Materials & Methods
Animals
Primiparous, time-pregnant Sprague Dawley rat dams were obtained from Harlan (now Envigo; Livermore, CA) on E15, and maintained in an uncrowded, quiet animal facility room on a 12 h light/dark cycle with ad libitum access to lab chow and water. Parturition was checked daily, and the day of birth was considered postnatal day (P)0. Four separate cohorts of rats were used, as described below. All experiments were performed in accordance with National Institutes of Health (NIH) guidelines and were approved by the UC Irvine animal care and use committee.
Chronic early-life stress (CES)
CES was induced P2-P9 using our laboratory’s standard protocol, as described previously (44,45)(see Supplement).
Assessment of anhedonia- and depressive-like behaviors
To examine for anhedonia, we assessed sucrose consumption (17), as well as social play behavior, an independent measure of behaviors considered to denote pleasure (18) that involves the activation of brain pleasure/reward pathways (16,50,51). We also examined traditional measures of depressive-like behaviors in rodents, including immobility time in the forced-swim test, considered a measure of “behavioral despair” (52). Sucrose consumption and the forced-swim test were conducted in the same rats both prior to lentiviral injections and following recovery (Cohort 1), enabling a within-subjects design to better assess rescue by Crh-shRNA (see Figure 1A for experimental timeline). The social play test was conducted only after lentiviral injections in Cohort 1. Cohort 2 was tested for social play only, and sacrificed 90 min later for assessment of circuit activation using c-Fos immunocytochemistry. Behavioral tests were conducted as previously described (15)(see Supplement) during the light cycle between 8 am and 12 pm (lights on at 6:30 am), without knowledge of treatment group.
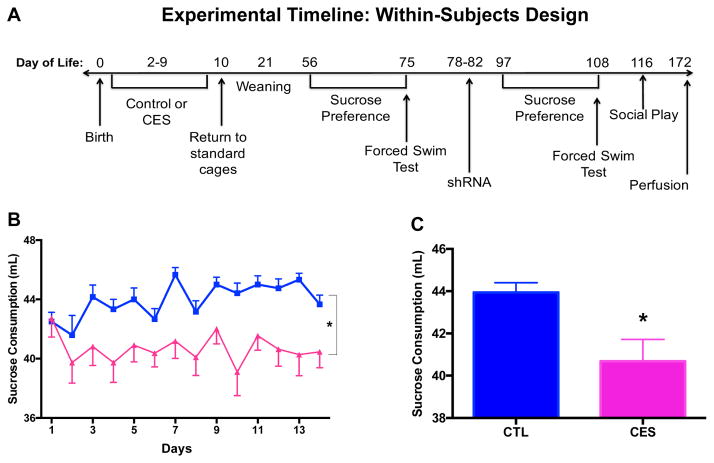
Figure 1.
Sucrose consumption, a measure of anhedonia, is diminished in male adolescent rats that experienced chronic early-life adversity (CES) provoked by rearing in limited nesting and bedding cages during a sensitive early postnatal period. (A) Timeline of within-subjects experimental design for Cohort 1. Additional cohorts included Cohort 2, in which rats were sacrificed at P42 for Fos quantification following social play, and Cohorts 3 and 4, in which rats were sacrificed at P56 for ex vivo high-resolution MRI. (B) Consumption of sucrose (mL) was reduced over a 2-week period in Cohort 1 rats reared in a CES-promoting environment during P2-9 compared with those reared in routine cages (CTL). (C) Data are also presented as the daily average consumption of sucrose for the groups. Values are expressed as mean ± SEM (n = 11–12 rats per group; *p < 0.05).
Functional connectivity assessments using c-Fos immunocytochemistry
Ninety minutes following a 15-min social play experience, rats from Cohort 2 were euthanized with sodium pentobarbital and perfused with 0.9% saline followed by ice-cold, freshly prepared 4% paraformaldehyde in 0.1 M sodium phosphate buffer (PB; pH=7.4). Brains were cryoprotected and stored, then sectioned coronally into 30-μm thick slices using a cryostat (1:12 series). Fos immunocytochemistry (ICC) was performed on free-floating sections as previously described (53)(see Supplement).
c-Fos and CRF dual-label immunocytochemistry
For assessment of Fos, sections were processed as described above. Once this process was complete, sections were treated in 0.3% H2O2 in 0.01 M PBS-T for 20 min, followed by 4% paraformaldehyde for 15 minutes and then rinsed (3×5 min PBS-T). Sections were then processed for CRF immunohistochemistry as previously described (54)(see Supplement).
Structural circuitry assessments using Magnetic Resonance Imaging (MRI)
To avoid the brain artifacts resulting from viral injections, two separate cohorts (Cohorts 3 & 4) of 8-week-old male rats including both CTL and CES (n=8–9/group) were subjected to ex vivo high-resolution diffusion tensor imaging (DTI; see Supplement).
Stereotaxic injection of lentiviral vectors
Crh-shRNA-containing lentiviral vectors (Fig. S1), previously validated in vitro and in vivo to knock down Crh gene expression and CRF protein levels, were produced as described previously (38). To administer them into the ACe, rats were stereotaxically injected with 1 μl of lentiviral vectors using a Hamilton syringe connected to a motorized nanoinjector at a rate of 0.2 μl/min. Lentiviruses contained either Crh-shRNA or unrelated, non-specific small-interfering RNA (unrelated-shRNA) labeled with green fluorescent protein (GFP), and were lowered into the ACe (bregma coordinates= ML ±4.0, AP −2.0, DV −8.5) under isoflurane anesthesia, as previously described (38). To allow the solution time to diffuse into the brain tissue, the needle was left in the brains for an additional 5 min after injection and between sides. Following surgery, animals were single-housed and allowed to recover for 2 weeks before behavioral assessments.
Effect of Crh-shRNA on immunoreactive CRF
Two months following the end of behavioral testing, rats from Cohort 1 were euthanized with sodium pentobarbital and perfused as above. Brains were cryoprotected and sectioned, CRF immunocytochemistry was performed as described for Cohort 2 (see Supplement).
CRF-immunoreactivity was analyzed without knowledge of group on digitized images of sections at coronal levels corresponding to sections 18–21 of the Paxinos atlas (55) to examine the ACe, as well as the paraventricular nucleus of the hypothalamus (PVN) as a non-virally-manipulated region. ImageJ(v2) was used to determine the optical densities (ODs) of CRF-immunoreactive products. Background was corrected for by subtracting the OD of signal over the corpus callosum. Because the distribution of CRF is not homogenous throughout the amygdala, we selected a priori two anatomically matched regions rich in CRF-immunoreactive fibers and included these two matched sections per rat in the analysis.
Verification of injection site
Serial sections were employed to verify the injection site of the lentiviral vectors, which were engineered to express GFP. Briefly, sections were mounted onto gelatin-coated slides. DAPI was used to enhance visualization of landmarks to verify localization of the GFP-expressing lentivirus to the ACe. Two rats lacking evidence of successful injection (one CTL+Crh-shRNA and one CES+Crh-shRNA) were excluded from analysis. Every remaining rat had robust expression of the virus in the left hemisphere, whereas the right hemisphere did not consistently contain GFP in the ACe. Viral GFP expression in the right hemisphere was found in the caudate putamen or internal capsule (slightly dorsal or medial to the ACe) in the vast majority of brains (Table S1). However, these regions do not contain CRF-producing cell bodies, and thus could not have been impacted by the Crh-shRNA manipulation. Thus, virtually all brains had successful transfection of the shRNA in the left ACe, and lower expression in the right ACe, with effectively no meaningful non-specific transfection outside it.
Data analysis
All data throughout these experiments were analyzed without knowledge of treatment group. No data were eliminated arbitrarily, and the Grubb test was used to examine if a given value was an outlier. Data were analyzed by t-test or 2-way ANOVA as required (see Supplement), and significant interactions were followed up with Newman-Keuls multiple comparisons test to distinguish among groups. Significance levels were set at 0.05, and data are presented as mean ± SEM. All statistical analyses were performed using GraphPad Prism 6.0 software (San Diego, CA).
Results
Preference for sucrose is diminished in adolescent male rats that experienced early-life adversity
Sucrose consumption was significantly lower in 56-day-old rats that had experienced early-life stress (F[1,21]=8.85, p<0.01; Fig. 1B–C), in line with our prior report (15). This was the case for both absolute sucrose consumption as well as the proportion (%) of the dilute sucrose solution of overall fluid intake (Fig. S2A–B). Reduced sucrose preference is generally considered an indicator of anhedonia.
Aberrant structural connectivity of stress and pleasure/reward circuits in CES rats
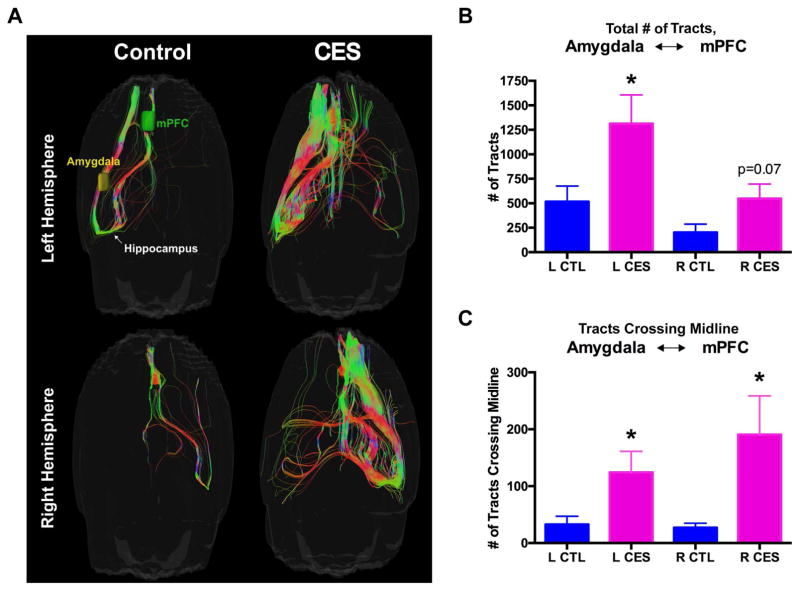
Complex behaviors including pleasure/reward and fear/anxiety are mediated via interactions of several underlying brain regions that are grouped into networks or circuits (19,53). The anhedonia in CES rats raised the possibility that pleasurable activities in these rats might be aborted or confounded by aberrant interactions of the pleasure/reward with the fear/anxiety circuits. We conducted high-resolution MRI followed by assessments of diffusion tensors and tractography (Fig. 2A). Analyses of tracts/streamlines connecting the amygdala [encompassing both the ACe and BLA subregions, which are extremely difficult to distinguish via MRI (57)] to the medial prefrontal cortex in control vs. CES rats was revealing: In the latter group, increased tract number was observed for both hemispheres of the brain (left hemisphere: t13=2.29, p<0.05; right hemisphere: t13=1.93, p=0.07; Fig. 2B) as well as an apparent increase in cross-midline connections originating from both hemispheres (left hemisphere origin: t13=2.30, p<0.05; right hemisphere origin; t13=2.23, p<0.05; Fig. 2C). Together, these data suggested that white-matter pathways among the components of the reward and stress networks were altered in CES rats, consistent with aberrant functional outputs, i.e., anhedonia.
Figure 2.
Aberrant structural connectivity of stress and reward circuits in CES rats. (A) Representative images of high-resolution MRI with assessments of diffusion tensors and tractography in 2 separate cohorts of CTL and CES rats (Cohorts 3 and 4), including analyses of tracts (i.e., streamlines) connecting the medial prefrontal cortex and the amygdala (encompassing the BLA and ACe, which are highly difficult to distinguish via MRI) (B) In the CES rats, an increased number of tracts connecting the amygdala and medial prefrontal cortex was observed in both hemispheres of the brain, as well as an apparent increase in cross-midline connections (C). Values are expressed as mean ± SEM (n = 8–9 rats per group; *p < 0.05).
ACe is aberrantly activated during social play in CES adolescents
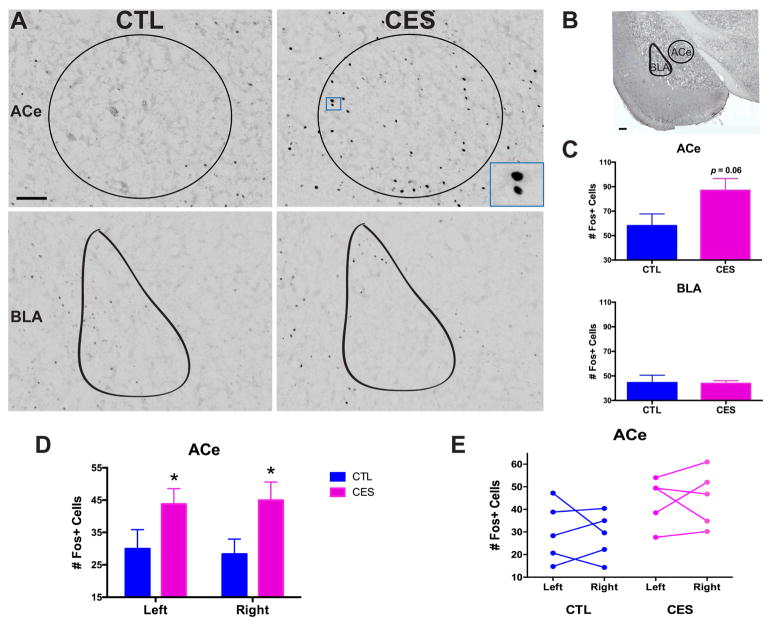
Anhedonia can arise from disruption of the function of several components of the pleasure/reward circuitry (54,55). To gain insight into the functional integrity and interconnections of the relevant brain structures after CES, we examined neuronal activation induced by social play, an acute measure for pleasure (as opposed to the chronic nature of sucrose consumption) in CES rats compared with controls. In our prior report, we demonstrated that CES resulted in decreased play with a younger, same-sex conspecific, without altering total interaction time (15). Accordingly, the pleasurable experience of social play induced c-Fos activation in salient brain regions including nucleus accumbens (NAcc), ventral tegmental area (VTA), medial prefrontal cortex (mPFC), habenula, and several amygdala nuclei, including medial and basolateral, in control rats (see Table S2). The same regions were activated in CES rats, but with several important distinctions: First, whereas the number of cells activated in the NAcc and VTA did not distinguish the groups, there was augmented c-Fos activation in the infralimbic mPFC (t8=2.55, p<0.05; Fig. S3B,C), a reward-related region functionally connected to the amygdala, and considered to constrain anxiety and fear (56,57). However, this activation by social play involved a larger percentage of the parvalbumin-producing interneurons in this region in CES rats than controls (t8=2.30, p<0.05; Fig. S3D,E; no change in total number of these interneurons, data not shown), predicting an overall decrease in functional output of the infralimbic cortex and thus more fear/anxiety in CES rats. Within amygdala, the medial nucleus (MeA), a region involved in several aspects of reward (60,62), expressed lower numbers of Fos+ neurons in CES rats than in controls (t8=2.81, p<0.05) following social play (Fig. S4). Remarkably, augmented activation of the ACe was noted in CES rats compared with controls, indicated by an increased number of Fos+ neurons in this region (Fig. 3A,C; t8= 2.12, p=0.06), regardless of hemisphere (Fig. 3D,E; nonsignificant repeated-measures ANOVA for left vs. right ACe, p>0.9). In contrast, there was no significant difference between groups in the number of Fos+ neurons in the neighboring basolateral amygdala (BLA; Fig. 3A,C). Together, these data suggest that a pleasure-like activity induces an aberrant pattern of activation in structures contributing to the regulation of pleasure/reward circuitry. Notably, ACe, an anxiety/fear hub that influences hedonic activity, was overactive, suggesting aberrant functional circuitry in CES rats.
Figure 3.
The central nucleus of the amygdala (ACe) is aberrantly activated during social play in CES adolescent rats. (A) Representative images of Fos+ cells in the counted regions, including ACe (top) and BLA (bottom), in CTL and CES adolescent rats sacrificed 90 min. following social play (Cohort 2). Scale bar = 100 μm. (B) Low-magnification image of the brain regions where Fos+ cells were counted, including the ACe and BLA. Scale bar = 200 μm. (C) Although the pleasurable experience of play induced c-Fos activation in multiple brain regions in both groups of rats, a key region that distinguished CES from CTL was the ACe: Social play activated a greater number of cells in the ACe of CES rats compared to controls (top), whereas Fos activation in the neighboring BLA did not distinguish between the groups (bottom). (D) When analyzed separately, both the right and left hemispheres of the ACe showed an increase of Fos+ cells in CES rats following social play. (E) Within individual rats, the number of Fos+ cells activated on the right vs. left hemisphere of the ACe did not significantly differ. Values are expressed as mean ± SEM (n = 5 rats per group; *p = 0.05).
ACe cell populations activated by social play secrete CRF
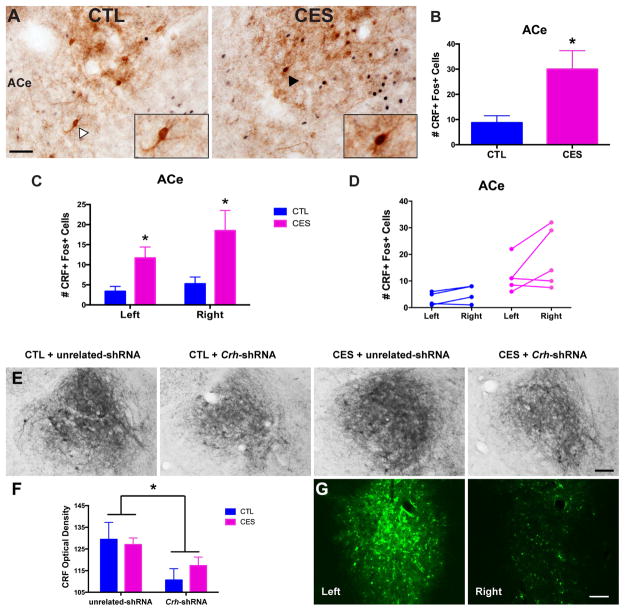
Because the ACe is a major hub of stress-related circuitry with a high density of CRF-producing neurons, we next queried whether the neurons activated by social play preferentially in CES rats secreted CRF, and if this activation of CRF neurons that are typically involved in fear and anxiety (28–31) differed between CES and control groups. Following social play, three times the number of CRF neurons were activated in CES rats than CTL rats (Fig. 4A,B; t7=2.45, p<0.05, no change in total number of CRF+ cells, data not shown), regardless of hemisphere (Fig. 4C,D; nonsignificant repeated-measures ANOVA for left vs. right ACe, p>0.05). Thus, a population of CRF-producing neurons in ACe was aberrantly activated by social play, suggesting a potential role for these stress- and anxiety-related neurons in the anhedonia in CES rats.
Figure 4.
ACe cell populations activated by social play secrete CRF, and administration of lentiviral Crh-shRNA reduces CRF levels in the ACe. (A) Representative images of CRF and Fos immunostaining in the ACe from CTL and CES adolescent rats sacrificed 90 min. following social play (Cohort 2). Insets display a CRF+ cell that lacks Fos in a CTL rat (left, marked by white arrowhead), and a CRF+ Fos+ (dual-labelled) cell in a CES rat (right, marked by black arrowhead). Scale bar= 50 μm. (B) Of the total number of neurons activated in ACe by social play, three times as many were identified to be CRF-producing cells (CRF+ Fos+/Fos+) in CES adolescent rats compared to CTL. (C) When analyzed separately, both the left and right hemispheres of the ACe showed an increase in the number of CRF+ cells activated by social play in CES rats. (D) Within individual rats, the number of CRF+ cells activated on the left vs. right hemisphere of the ACe did not significantly differ. (E) Representative images from each group (Cohort 1) of the ACe after immunohistochemistry using an antiserum directed against CRF. (F) Crh-shRNA significantly reduced CRF immunoreactivity in the ACe of both CTL and CES rats (data shown for left hemisphere). (G) GFP expression, indicating viral and shRNA presence, was more robust in the left hemisphere and specifically the left ACe. Values are expressed as mean ± SEM (n = 4–5 rats per group for A–D; n = 5–6 rats per group for E–G; *p < 0.05).
To test directly if ACe-CRF is causally involved in the observed anhedonia-like behaviors of CES rats, we employed viral-directed reduction of Crh expression in ACe of young-adult rats and tested for anhedonia in the same animals before and after intervention.
Administration of Crh-shRNA reduces CRF levels in ACe
To examine the efficacy of the viral-targeted approach in silencing Crh expression, we assessed peptide expression using CRF immunocytochemistry (Fig. 4C). Crh-shRNA lentiviral infusion decreased the optical density (OD) of CRF cells and fibers in the ACe of both control and CES rats (Fig. 4D; significant main effect of Crh-shRNA, F[1,17]=6.19, p<0.05); this effect was independent of early-life experience. GFP expression was examined as an indicator of viral and shRNA presence, and was robust in the left ACe (Fig. 4E). Surprisingly, GFP intensity was low in the right ACe, likely as a result of technical error during the stereotaxic surgical set-up (see Methods and Table S1 for more details). Therefore, the left amygdala was used for measuring the effects of intervention on CRF levels (Fig. 4D). As an additional specificity control, we analyzed peptide levels in the hypothalamic paraventricular nucleus (PVN; Fig. S5A), a CRF-rich area not targeted by viral infusions. CRF levels in the PVN did not distinguish among groups and were not affected by viral injections (no significant main effects or interactions, p>0.05). These data suggest that the shRNA intervention specifically attenuated CRF levels in the ACe, and not in other Crh-expressing regions, a conclusion supported by the (repeated-measures ANOVA for interaction of brain region X treatment group (F[3,17]=4.01, p<0.05).
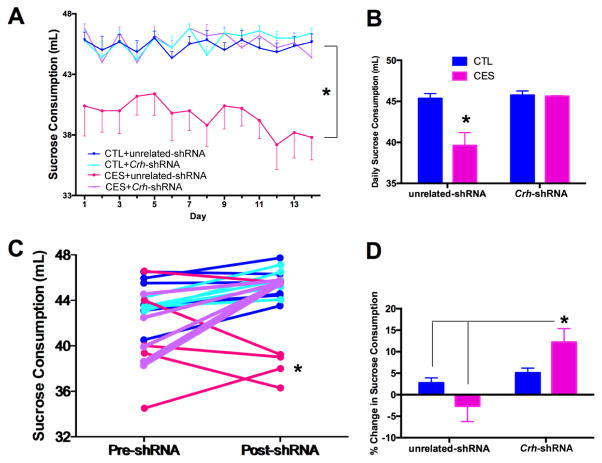
Partial silencing of ACe-Crh reverses CES-induced anhedonia in individual rats
To test if ACe-CRF influenced anhedonia-like behaviors in individual rats, we examined sucrose preference after the viral-genetic interventions in the same rats tested prior to interventions. Sucrose consumption remained lower in CES rats provided with a Crh-unrelated shRNA compared with the other groups (Fig. 5A–B; post hoc, p<0.05). In contrast, sucrose consumption of CES rats provided with Crh-targeting viruses were indistinguishable from that of controls (Fig. 5A–B; CES X Crh-shRNA interaction, F[1,17]=10.42, p<0.005). This indicated that intervention to knock-down Crh expression in the ACe during adulthood, after the emergence of poor sucrose preference, was effective in reversing this measure of CES-induced anhedonia. Notably, overall fluid consumption was not influenced by Crh-shRNA administration (Fig. S6A), and relative amount of sucrose consumed showed a similar trend to absolute sucrose consumption (Fig. S6B).
Figure 5.
Partial silencing of ACe-Crh reverses CES-induced anhedonia in individual rats. (A) Sucrose preference was reduced in CES rats provided with shRNA unrelated to Crh. In contrast, sucrose consumption of CES rats provided with Crh-targeting viruses were indistinguishable from that of controls. Data are also presented as daily average consumption of sucrose (mL) for each group after shRNA administration (B). (C) Within individual rats, the augmentation in sucrose preference provided by Crh-targeting was apparent in CES but not control rats. Sucrose consumption did not change with unrelated constructs (pre-shRNA data from Figure 1). Data are also presented as the relative change in daily sucrose consumption from pre-shRNA to post-shRNA for each group (D). Values are expressed as mean ± SEM (n = 5–6 rats per group; *p < 0.05).
We next examined directly the effects of shRNA on sucrose consumption in the same individual rat (pre- vs. post- intervention). Change in sucrose consumption varied significantly by treatment group (Fig. 5C; significant CES X Crh-shRNA within-subjects interaction, F[1,17]=7.31, p<0.05). Specifically, sucrose preference was increased by Crh-targeting only in CES rats and not in controls. Notably, no change in sucrose preference was observed when unrelated shRNA constructs were used. (Fig. 5D; significant CES x Crh-shRNA between-subjects interaction, F[1,17]=6.64, p<0.05; post hoc, p<0.05).
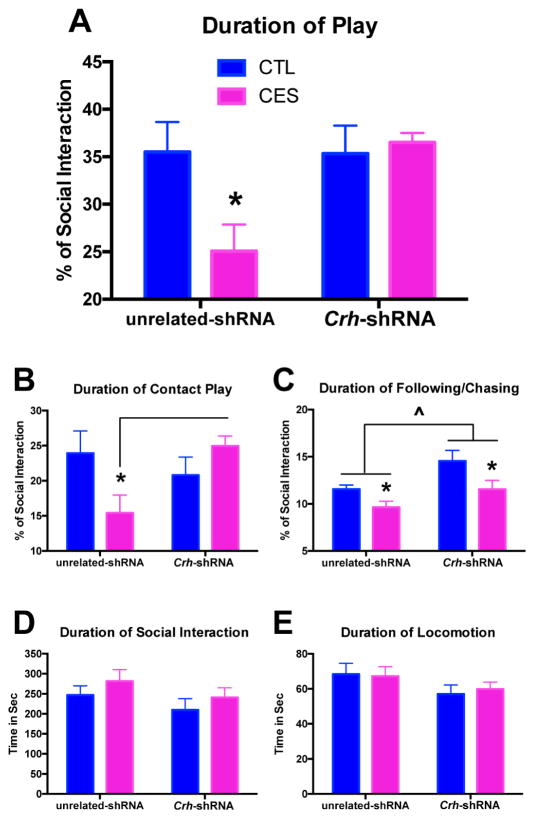
CRF reduction in the ACe rescues CES rats from deficits in social play
As a second, independent measure of anhedonia in rodents (15,47), we assessed peer-play behaviors. CES rats provided with an unrelated-shRNA engaged in play behaviors for shorter durations compared with controls (Fig. 6A; significant CES X Crh-shRNA interaction, F[1,16]=4.96, p<0.05; post hoc, p<0.05), in agreement with our previous findings (15). In this measure of anhedonia as well, partial silencing of Crh in the ACe of young-adult rats was sufficient to rescue social play behavior in CES animals. Interestingly, CRF reduction seemed to specifically rescue contact play behavior, such as pinning, pouncing, kicking, boxing and wrestling (Fig. 6B; significant CES X Crh-shRNA interaction, F[1,16]=6.40, p<0.05; post hoc, p<0.05), with little effect on deficits in playful following/chasing (Fig. 6C; significant main effect of CES, F[1,16]=9.02, p<0.01), though these were somewhat increased overall (significant main effect of Crh-shRNA, F[1,16]=8.92, p<0.01). Notably, neither CES nor Crh-shRNA altered total social interaction or locomotion, indicating specific effects on behaviors considered to denote pleasure, rather than a change in general activity or social anxiety (Fig. 6D,E).
Figure 6.
CRF reduction in the ACe rescues CES rats from deficits in social play. (A) As a second, independent measure of anhedonia in rodents, we assessed the duration of play behavior during a social interaction test. CES rats provided with an unrelated-shRNA engaged in lower durations of play behaviors. In this measure of pleasure as well, Crh-targeting in the ACe was sufficient to rescue play behavior in CES rats. Of the multiple play behaviors measured, CRF reduction specifically rescued contact play such as pinning, pouncing, kicking, boxing and wrestling in CES rats (B), with little effect on deficits in playful following/chasing (C). Overall, Crh-shRNA did slightly increase the duration of following/chasing behavior, regardless of early-life experience. (D) Neither CES nor Crh-shRNA altered the total duration of social interaction, which includes play behavior as well as non-play activities such as anogenital investigation. (E) Neither CES nor Crh-shRNA altered the total duration of locomotion, or walking around the apparatus. Values are expressed as mean ± SEM (n = 5–6 rats per group; *p < 0.05).
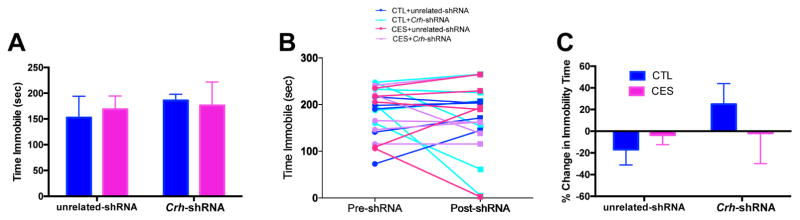
CES and Crh-shRNA do not influence depressive-like behavior in young-adult male rats
Anhedonia often precedes or accompanies depression in humans, though it can be found alone or within the context of other neuropsychiatric disorders such as schizophrenia. Here, to examine the spectrum of depressive-like behaviors influenced by amygdala CRF levels, we tested CES rats provided with Crh-specific or unrelated constructs in the Porsolt forced-swim test. In line with previous results (15), CES did not influence immobility time in adolescent/young-adult male rats in comparison to controls (Fig. 7A–B). Lentivirus administration did not affect this result (Fig. 7B), and Crh-shRNA had no effect on immobility time (Fig. 7A) or the percent change in despair-like behavior from pre- to post-shRNA (Fig. 7C).
Figure 7.
Neither early-life experience nor CRF reduction affect depressive-like behavior in young-adult male rats. (A) CES did not influence immobility time in the forced-swim test, and lentivirus administration did not influence this result. (B) The change in immobility time following Crh-targeting did not differ significantly by treatment group. Data are also presented as the relative change in immobility time in the forced-swim test from pre-shRNA to post-shRNA for each group (C). Values are expressed as mean ± SEM (n = 5–6 rats per group; *p < 0.05).
Discussion
Here we demonstrate that early-life adversity provokes severe anhedonia by altering interactions of pleasure/reward and stress-related networks involving CRF-producing neurons in the amygdala. Specifically, we found that CES caused anhedonia, measured as decreased sucrose preference and reduced social play. During the normally pleasurable experience of play, early-life stress resulted in augmented, abnormal activation of CRF-producing neurons in the ACe of adolescent rats compared with controls, and diffusion tensor imaging delineated heightened connection of both amygdalae and components of the pleasure/reward circuit. Accordingly, targeted unilateral reduction of CRF levels in the ACe sufficed to reverse the CES-induced anhedonia in individual rats. Together, these findings provide the first mechanistic evidence for aberrant structural and functional circuitry encompassing reward and anxiety/stress networks after CES, and for a role of CRF-producing amygdala neurons in emotional deficits that portend major neuropsychiatric disorders.
Anhedonia during adolescence had originally been implicated as a harbinger or accompanying feature of later depression (61) or schizophrenia (12,13). Notably, the importance of anhedonia as a biological entity is being increasingly recognized (14). Anhedonic behavior can arise as a result of a number of problems at different ‘nodes’ of the mesocorticolimbic pleasure/reward circuitry (16,19). This system encompasses the nucleus accumbens, ventral pallidum and prefrontal cortex, with critical input from amygdala, hippocampus, habenula and other regions and pathways (59,60). Recently, the concept that anhedonia might arise from dysfunctional interactions between the stress and reward/pleasure systems has been proposed (61), and has significant support from clinical studies. Here, we employed mechanistic interventions in rodent models to provide plausible, biologically-relevant potential mechanisms for the relationship of stress, fear/anxiety and anhedonia.
Indeed, in the current set of experiments, anhedonia in late-adolescent and adult rats was a result of experimentally imposed early-life adversity. Therefore, we focused on the interaction of stress and pleasure/reward networks. We have previously demonstrated abnormal development of brain circuits following CES (61,62), as well as augmented CRF levels in a number of brain regions. These included the hippocampus and amygdala (32,61), and there is a robust literature implicating amygdala CRF in emotional functions including anxiety, fear and depression-like behaviors (34,35,63,64). These converging observations provided the impetus for testing the hypotheses that aberrant development of the amygdala and brain circuitry involved in reward/pleasure might underlie CES-induced anhedonia, and that amygdala CRF contributes to abnormal responses to pleasurable cues, including sucrose and social partners.
In this young-adult cohort we did not identify upregulation of CRF levels in the ACe. However, we identified a tripling of the activation of ACe-CRF cells by social play. The notion that the augmented activation of CRF-producing neurons in the ACe during social play was a result of hyper-connectivity of amygdala with reward circuitry (via the mPFC) was supported by high-resolution DTI. Notably, the mPFC and amygdala are classically part of both the reward and stress-related networks, which places them in an ideal position to mediate the interactions of these two circuits (23). The reversal of anhedonia in individual rats upon partial silencing of ACe-Crh supports a causal role for amygdala CRF in the disruption of stress- and reward-related circuitry that results in anhedonia. Notably, the current experimental design could not definitively ascertain unilateral and regional specificity of ACe-CRF in CES-induced anhedonia.
Surprisingly, we found complete reversal of CES-induced anhedonia upon reduction of CRF levels unilaterally. For technical reasons, the reduction took place in left amygdala. Although unilateral side-specific regulation of CRF production in the amygdala (69), ipsilateral effects of manipulating amygdala activity (70), and side-specific actions of several amygdala nuclei (71) have been demonstrated, we do not propose a functional distinction of left vs right amygdala. Indeed, Fos expression was indistinguishable in the left vs. right ACe following social play (Fig. 3D,E), and the number of CRF cells activated by play was also similar in both hemispheres (Fig. 4C,D). These data support the idea that the left amygdala is not functionally distinct from the right amygdala in the context of our experiments, and unilateral Crh shRNA knockdown in either ACe would likely have similar effects. To probe the potential mechanisms for bilateral effects of unilateral silencing of ACe-Crh, we carried out magnetic resonance imaging, focusing on detection of connections and circuits using high-resolution DTI. Unexpectedly, DTI revealed enriched bilateral connections of the amygdala to the mPFC in CES rats, which may have enabled unilateral Crh silencing to reverse CES-induced anhedonia. Together, these findings support the notion that early-life stress promotes anhedonia via aberrant maturation of reward and fear circuits and hyper-connectivity between them.
Notably, we found increased c-Fos activation in the portion of the mPFC that has been implicated in constraining fear and anxiety. Indeed, connections of PFC and amygdala have been well-studied (43,72). In rodents, the infralimbic PFC inhibits amygdala activity and fear responses, and enhanced fronto-amygdala connectivity is associated with decreased anxiety-like behaviors (73). Therefore, our finding of increased c-Fos+ cell number in the infralimbic mPFC (IL-mPFC) was surprising. To further probe this observation, we tested if the activated cells were glutamatergic or inhibitory interneurons. We found that whereas there was no difference in the overall numbers of parvalbumin (PV)-producing interneurons in IL-mPFC of CES and control rats, there was a significant increase in c-Fos+ PV+ neurons (Fig. S3). Thus, social play preferentially activated inhibitory interneurons in IL-mPFC of CES rats, likely inhibiting the fear-constraining function of the IL-mPFC projection onto the amygdala.
In summary, the findings described here suggest that anhedonia provoked by early-life stress involves aberrant interaction of stress-related and reward networks, mediated, at least in part, by augmented activation of CRF-producing amygdala neurons. CRF is also found in pleasure/reward-related brain regions, such as the nucleus accumbens (74–76), and future studies will investigate the potential disruption in expression or function of the peptide by CES. The current studies uncover plausible mechanisms for the major emotional consequences of CES; importantly, they point out novel, potentially effective interventional strategies in the adult, with strong translational potential.
Supplementary Material
Acknowledgments
We thank Gissell A. Sanchez, Jennifer Daglian and Pamela A. See for technical assistance. Funding was provided by NIH grants RO1s MH73136, NS28912, and P50 MH096889; J.L.B. is the recipient of a George E. Hewitt Foundation for Biomedical Research postdoctoral fellowship.
Footnotes
Financial Disclosures
Dr. Bolton reported no biomedical financial interests or potential conflicts of interest.
Dr. Molet reported no biomedical financial interests or potential conflicts of interest.
Dr. Regev reported no biomedical financial interests or potential conflicts of interest.
Dr. Chen reported no biomedical financial interests or potential conflicts of interest.
Dr. Rismanchi reported no biomedical financial interests or potential conflicts of interest.
Ms. Haddad reported no biomedical financial interests or potential conflicts of interest.
Mr. Yang reported no biomedical financial interests or potential conflicts of interest.
Dr. Obenaus reported no biomedical financial interests or potential conflicts of interest.
Dr. Baram reported no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: Insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33:693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 2.Klengel T, Binder EB. Epigenetics of Stress-Related Psychiatric Disorders and Gene × Environment Interactions. Neuron. 2015;86:1343–1357. doi: 10.1016/j.neuron.2015.05.036. [DOI] [PubMed] [Google Scholar]
- 3.Pratchett LC, Yehuda R. Foundations of posttraumatic stress disorder: does early life trauma lead to adult posttraumatic stress disorder? Dev Psychopathol. 2011;23:477–491. doi: 10.1017/S0954579411000186. [DOI] [PubMed] [Google Scholar]
- 4.Sharma S, Powers A, Bradley B, Ressler KJ. Gene × Environment Determinants of Stress- and Anxiety-Related Disorders. Annu Rev Psychol. 2016;67:239–261. doi: 10.1146/annurev-psych-122414-033408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lovallo WR. Early life adversity reduces stress reactivity and enhances impulsive behavior: Implications for health behaviors. Int J Psychophysiol. 2013;90:8–16. doi: 10.1016/j.ijpsycho.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Connor RM, Moloney RD, Glennon J, Vlachou S, Cryan JF. Enhancing glutamatergic transmission during adolescence reverses early-life stress-induced deficits in the rewarding effects of cocaine in rats. Neuropharmacology. 2015;99:168–176. doi: 10.1016/j.neuropharm.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 7.Raineki C, Cortés MR, Belnoue L, Sullivan RM. Effects of early-life abuse differ across development: infant social behavior deficits are followed by adolescent depressive-like behaviors mediated by the amygdala. J Neurosci. 2012;32:7758–65. doi: 10.1523/JNEUROSCI.5843-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kundakovic M, Gudsnuk K, Franks B, Madrid J, Miller RL, Perera FP, Champagne FA. Sex-specific epigenetic disruption and behavioral changes following low-dose in utero bisphenol A exposure. 2013;110:9956–9961. doi: 10.1073/pnas.1214056110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bolton JL, Molet J, Ivy A, Baram TZ. New insights into early-life stress and behavioral outcomes. Curr Opin Behav Sci. 2017;14:133–139. doi: 10.1016/j.cobeha.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rincón-Cortés M, Sullivan RM. Emergence of social behavior deficit, blunted corticolimbic activity and adult depression-like behavior in a rodent model of maternal maltreatment. Transl Psychiatry. 2016;6:e930. doi: 10.1038/tp.2016.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 12.Whitton AE, Treadway MT, Pizzagalli DA. Reward processing dysfunction in major depression, bipolar disorder and schizophrenia. Curr Opin Psychiatry. 2015;28:7–12. doi: 10.1097/YCO.0000000000000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Romer Thomsen K, Whybrow PC, Kringelbach ML. Reconceptualizing anhedonia: novel perspectives on balancing the pleasure networks in the human brain. Front Behav Neurosci. 2015;9:49. doi: 10.3389/fnbeh.2015.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.NIMH Research Domain Criteria (RDoc) Unit of Analysis: Behaviors. n.d Retrieved December 22, 2016, from https://www.nimh.nih.gov/research-priorities/rdoc/units/behaviors/index.shtml.
- 15.Molet J, Heins K, Zhuo X, Mei YT, Regev L, Baram TZ, Stern H. Fragmentation and high entropy of neonatal experience predict adolescent emotional outcome. Transl Psychiatry. 2016;6:e702. doi: 10.1038/tp.2015.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Der-Avakian A, Markou A. The neurobiology of anhedonia and other reward-related deficits. Trends Neurosci. 2012;35:68–77. doi: 10.1016/j.tins.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Papp M, Willner P, Muscat R. An animal model of anhedonia: attenuation of sucrose consumption and place preference conditioning by chronic unpredictable mild stress. Psychopharmacology (Berl) 1991;104:255–259. doi: 10.1007/BF02244188. [DOI] [PubMed] [Google Scholar]
- 18.Trezza V, Baarendse PJJ, Vanderschuren LJMJ. The pleasures of play: pharmacological insights into social reward mechanisms. Trends Pharmacol Sci. 2010;31:463–469. doi: 10.1016/j.tips.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kringelbach ML, Berridge KC. Towards a functional neuroanatomy of pleasure and happiness. Trends Cogn Sci. 2009;13:479–487. doi: 10.1016/j.tics.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joëls M, Baram TZ. The neuro-symphony of stress. Nat Rev Neurosci. 2009;10:459. doi: 10.1038/nrn2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 22.Nestler EJ. Role of the Brain’s Reward Circuitry in Depression: Transcriptional Mechanisms. Int Rev Neurobiol. 2015;124:151–70. doi: 10.1016/bs.irn.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nat Rev Neurosci. 2013;14:609–625. doi: 10.1038/nrn3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Russo SJ, Murrough JW, Han M-HH, Charney DS, Nestler EJ. Neurobiology of resilience. 2012;15:1475–1484. doi: 10.1038/nn.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Y, Baram TZ. Toward Understanding How {Early-Life} Stress Reprograms Cognitive and Emotional Brain Networks. 2015 doi: 10.1038/npp.2015.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bale TL. Sex differences in prenatal epigenetic programming of stress pathways. 2011;14:348–356. doi: 10.3109/10253890.2011.586447. [DOI] [PubMed] [Google Scholar]
- 27.Bagot RC, Labonté B, Peña CJ, Nestler EJ. Epigenetic signaling in psychiatric disorders: stress and depression. Dialogues Clin Neurosci. 2014;16:281–95. doi: 10.31887/DCNS.2014.16.3/rbagot. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lupien SJ, Ouellet-Morin I, Herba CM, Juster R, McEwen BS. Epigenetics Neuroendocrinol. Springer International Publishing; 2016. From Vulnerability to Neurotoxicity: A Developmental Approach to the Effects of Stress on the Brain and Behavior; pp. 3–48. [Google Scholar]
- 29.Bohacek J, Mansuy IM. Epigenetics Neuroendocrinol. Springer International Publishing; 2016. Epigenetic Risk Factors for Diseases: A Transgenerational Perspective; pp. 79–119. [Google Scholar]
- 30.McGowan PO, Sasaki A, D’Alessio AC, Dymov S, Labonté B, Szyf M, et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci. 2009;12:342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, et al. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science (80-) 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- 32.Meaney MJ, Diorio J, Francis D, Widdowson J, LaPlante P, Caldji C, et al. Early Environmental Regulation of Forebrain Glucocorticoid Receptor Gene Expression: Implications for Adrenocortical Responses to Stress; pp. 61–72. Dev Neurosci. 2010;18:61–72. doi: 10.1159/000111395. [DOI] [PubMed] [Google Scholar]
- 33.McEwen BS, Nasca C, Gray JD. Stress Effects on Neuronal Structure: Hippocampus, Amygdala, and Prefrontal Cortex. Neuropsychopharmacology. 2016;41:3–23. doi: 10.1038/npp.2015.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andersen SL, Teicher MH. Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci. 2008;31:183–191. doi: 10.1016/j.tins.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 35.McEwen BS, Morrison JH. The Brain on Stress: Vulnerability and Plasticity of the Prefrontal Cortex over the Life Course. Neuron. 2013;79:16–29. doi: 10.1016/j.neuron.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dubé CM, Molet J, Akanksha S-T, Ivy A, Maras PM, Baram TZ. Hyper-excitability and epilepsy generated by chronic early-life stress. 2015;2 doi: 10.1016/j.ynstr.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fenoglio KA, Brunson KL, Avishai-Eliner S, Chen Y, Baram TZ. Region-Specific Onset of Handling-Induced Changes in Corticotropin-Releasing Factor and Glucocorticoid Receptor Expression. Endocrinology. 2004;145:2702–2706. doi: 10.1210/en.2004-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Regev L, Tsoory M, Gil S, Chen A. Site-Specific Genetic Manipulation of Amygdala Corticotropin-Releasing Factor Reveals Its Imperative Role in Mediating Behavioral Response to Challenge. Biol Psychiatry. 2012;71:317–326. doi: 10.1016/j.biopsych.2011.05.036. [DOI] [PubMed] [Google Scholar]
- 39.Regev L, Neufeld-Cohen A, Tsoory M, Kuperman Y, Getselter D, Gil S, Chen A. Prolonged and site-specific over-expression of corticotropin-releasing factor reveals differential roles for extended amygdala nuclei in emotional regulation. Mol Psychiatry. 2011;16:714–728. doi: 10.1038/mp.2010.64. [DOI] [PubMed] [Google Scholar]
- 40.Gray TS, Bingaman EW. The Amygdala: Corticotropin-Releasing Factor, Steroids, and Stress. Crit Rev Neurobiol. 1996;10:155–168. doi: 10.1615/critrevneurobiol.v10.i2.10. [DOI] [PubMed] [Google Scholar]
- 41.Gafford GM, Guo J-D, Flandreau EI, Hazra R, Rainnie DG, Ressler KJ. Cell-type specific deletion of GABA(A)α1 in corticotropin-releasing factor-containing neurons enhances anxiety and disrupts fear extinction. Proc Natl Acad Sci U S A. 2012;109:16330–5. doi: 10.1073/pnas.1119261109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walker DL, Miles LA, Davis M. Selective participation of the bed nucleus of the stria terminalis and CRF in sustained anxiety-like versus phasic fear-like responses. Prog Neuro-Psychopharmacology Biol Psychiatry. 2009;33:1291–1308. doi: 10.1016/j.pnpbp.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Keen-Rhinehart E, Michopoulos V, Toufexis DJ, Martin EI, Nair H, Ressler KJ, et al. Continuous expression of corticotropin-releasing factor in the central nucleus of the amygdala emulates the dysregulation of the stress and reproductive axes. Mol Psychiatry. 2009;14:37–50. doi: 10.1038/mp.2008.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kalivas PW, Volkow ND. The Neural Basis of Addiction: A Pathology of Motivation and Choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- 45.Baxter MG, Murray EA. The amygdala and reward. Nat Rev Neurosci. 2002;3:563–573. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- 46.Maren S, Quirk GJ. Neuronal signalling of fear memory. Nat Rev Neurosci. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- 47.Klavir O, Prigge M, Sarel A, Paz R, Yizhar O. Manipulating fear associations via optogenetic modulation of amygdala inputs to prefrontal cortex. Nat Neurosci. 2017;20:836–844. doi: 10.1038/nn.4523. [DOI] [PubMed] [Google Scholar]
- 48.Gilles EE, Schultz L, Baram TZ. Abnormal corticosterone regulation in an immature rat model of continuous chronic stress. Pediatr Neurol. 1996;15:114–9. doi: 10.1016/0887-8994(96)00153-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Avishai-Eliner S, Eghbal-Ahmadi M, Tabachnik E, Brunson KL, Baram TZ. Down-regulation of hypothalamic corticotropin-releasing hormone messenger ribonucleic acid (mRNA) precedes early-life experience-induced changes in hippocampal glucocorticoid receptor mRNA. Endocrinology. 2001;142:89–97. doi: 10.1210/endo.142.1.7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Panksepp J, Siviy S, Normansell L. The psychobiology of play: Theoretical and methodological perspectives. Neurosci Biobehav Rev. 1984;8:465–492. doi: 10.1016/0149-7634(84)90005-8. [DOI] [PubMed] [Google Scholar]
- 51.Vanderschuren LJMJ, Niesink RJM, Van Pee JM. The neurobiology of social play behavior in rats. Neurosci Biobehav Rev. 1997;21:309–326. doi: 10.1016/s0149-7634(96)00020-6. [DOI] [PubMed] [Google Scholar]
- 52.Slattery DA, Cryan JF. Using the rat forced swim test to assess antidepressant-like activity in rodents. Nat Protoc. 2012;7:1009–1014. doi: 10.1038/nprot.2012.044. [DOI] [PubMed] [Google Scholar]
- 53.Chen Y, Fenoglio KA, Dubé CM, Grigoriadis DE, Baram TZ. Cellular and molecular mechanisms of hippocampal activation by acute stress are age-dependent. Mol Psychiatry. 2006;11:992–1002. doi: 10.1038/sj.mp.4001863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen Y, Bender RA, Frotscher M, Baram TZ. Novel and Transient Populations of Corticotropin-Releasing Hormone-Expressing Neurons in Developing Hippocampus Suggest Unique Functional Roles: A Quantitative Spatiotemporal Analysis. J Neurosci. 2001:21. doi: 10.1523/JNEUROSCI.21-18-07171.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paxinos G, Watson C. The rat brain atlas. San Diego, CA: Academic Press; 1986. [Google Scholar]
- 56.Redish AD, Gordon JA. 2. Breakdowns and Failure Modes An Engineer’s View The Computational Perspective n.d [Google Scholar]
- 57.Obenaus A, Kinney-Lang E, Shereen D, Solodkin ABT. A Novel Diffusion Tensor Imaging Strategy for Delineating the Neuroanatomical Boundaries of the Amygdala. Proc Intl Soc Mag Reson Med. 2016;24 Abstract 3310. [Google Scholar]
- 58.Vidal-Gonzalez I, Vidal-Gonzalez B, Rauch SL, Quirk GJ. Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learn Mem. 2006;13:728–33. doi: 10.1101/lm.306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Quirk GJ, Garcia R, González-Lima F. Prefrontal Mechanisms in Extinction of Conditioned Fear. Biol Psychiatry. 2006;60:337–343. doi: 10.1016/j.biopsych.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 60.Holder MK, Hadjimarkou MM, Zup SL, Blutstein T, Benham RS, McCarthy MM, Mong JA. Methamphetamine facilitates female sexual behavior and enhances neuronal activation in the medial amygdala and ventromedial nucleus of the hypothalamus. Psychoneuroendocrinology. 2010;35:197–208. doi: 10.1016/j.psyneuen.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pizzagalli DA. Depression, stress, and anhedonia: toward a synthesis and integrated model. Annu Rev Clin Psychol. 2014;10:393–423. doi: 10.1146/annurev-clinpsy-050212-185606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Georgiadis JR, Kringelbach ML, Pfaus JG. Sex for fun: a synthesis of human and animal neurobiology. Nat Rev Urol. 2012;9:486–498. doi: 10.1038/nrurol.2012.151. [DOI] [PubMed] [Google Scholar]
- 63.Berridge KC, Kringelbach ML. Pleasure Systems in the Brain. Neuron. 2015;86:646–664. doi: 10.1016/j.neuron.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Romer Thomsen K, Whybrow PC, Kringelbach ML. Reconceptualizing anhedonia: novel perspectives on balancing the pleasure networks in the human brain. Front Behav Neurosci. 2015;9:49. doi: 10.3389/fnbeh.2015.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ivy AS, Rex CS, Chen Y, Dubé C, Maras PM, Grigoriadis DE, et al. Hippocampal dysfunction and cognitive impairments provoked by chronic early-life stress involve excessive activation of CRH receptors. J Neurosci. 2010;30:13005–15. doi: 10.1523/JNEUROSCI.1784-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Molet J, Maras PM, Kinney-Lang E, Harris NG, Rashid F, Ivy AS, et al. MRI uncovers disrupted hippocampal microstructure that underlies memory impairments after early-life adversity. Hippocampus. 2016 doi: 10.1002/hipo.22661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Refojo D, Schweizer M, Kuehne C, Ehrenberg S, Thoeringer C, Vogl AM, et al. Glutamatergic and Dopaminergic Neurons Mediate Anxiogenic and Anxiolytic Effects of CRHR1. Science (80-) 2011:333. doi: 10.1126/science.1202107. [DOI] [PubMed] [Google Scholar]
- 68.Müller MB, Zimmermann S, Sillaber I, Hagemeyer TP, Deussing JM, Timpl P, et al. Limbic corticotropin-releasing hormone receptor 1 mediates anxiety-related behavior and hormonal adaptation to stress. Nat Neurosci. 2003;6:1100–1107. doi: 10.1038/nn1123. [DOI] [PubMed] [Google Scholar]
- 69.Day HE, Vittoz NM, Oates MM, Badiani A, Watson SJ, Robinson TE, Akil H. A 6-Hydroxydopamine lesion of the mesostriatal dopamine system decreases the expression of corticotropin releasing hormone and neurotensin mRNAs in the amygdala and bed nucleus of the stria terminalis. Brain Res. 2002;945:151–159. doi: 10.1016/s0006-8993(02)02747-6. [DOI] [PubMed] [Google Scholar]
- 70.Silveira DC, Klein P, Ransil BJ, Liu Z, Hori A, Holmes GL, et al. Lateral Asymmetry in Activation of Hypothalamic Neurons with Unilateral Amygdaloid Seizures. Epilepsia. 2000;41:34–41. doi: 10.1111/j.1528-1157.2000.tb01502.x. [DOI] [PubMed] [Google Scholar]
- 71.Sanchez MA, Dominguez R. Differential effects of unilateral lesions in the medial amygdala on spontaneous and induced ovulation. Brain Res Bull. 1995;38:313–317. doi: 10.1016/0361-9230(95)00094-u. [DOI] [PubMed] [Google Scholar]
- 72.Burgos-Robles A, Kimchi EY, Izadmehr EM, Porzenheim MJ, Ramos-Guasp WA, Nieh EH, et al. Amygdala inputs to prefrontal cortex guide behavior amid conflicting cues of reward and punishment. Nat Neurosci. 2017;20:824–835. doi: 10.1038/nn.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dincheva I, Drysdale AT, Hartley CA, Johnson DC, Jing D, King EC, et al. FAAH genetic variation enhances fronto-amygdala function in mouse and human. Nat Commun. 2015;6:6395. doi: 10.1038/ncomms7395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. The Lancet Psychiatry. 2016;3:760–773. doi: 10.1016/S2215-0366(16)00104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Walsh JJ, Friedman AK, Sun H, Heller EA, Ku SM, Juarez B, et al. Stress and CRF gate neural activation of BDNF in the mesolimbic reward pathway. Nat Neurosci. 2013;17:27–29. doi: 10.1038/nn.3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lemos JC, Wanat MJ, Smith JS, Reyes BAS, Hollon NG, Van Bockstaele EJ, et al. Severe stress switches CRF action in the nucleus accumbens from appetitive to aversive. Nature. 2012;490:402–406. doi: 10.1038/nature11436. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.