Abstract
Background
Research in adolescents and adults has suggested that altered neural processing of reward following early life adversity is a highly promising depressive intermediate phenotype. However, very little is known about how stress reactivity, neural processing of reward, and depression are related in very young children. Motivated by this knowledge gap, the present study examined the concurrent associations between cortisol response following a stressor, functional brain activity to reward, and depression severity in 4–6 year old children.
Methods
Fifty-two medication naïve 4–6 year olds participated in a study using functional magnetic resonance imaging (fMRI) to assess neural reactivity to reward, including gain, loss, and neutral outcomes. Parent-reported child depression severity and child cortisol response following stress were also measured.
Results
Greater caudate and medial prefrontal cortex reactivity to gain outcomes and increased amygdala reactivity to salient (i.e., both gain and loss) outcomes were observed. Higher total cortisol output following a stressor was associated with increased depression severity and reduced amygdala reactivity to salient outcomes. Amygdala reactivity was also inversely associated with depression severity and found to mediate the relationship between cortisol output and depression severity.
Conclusions
Results suggest that altered neural processing of reward is already related to increased cortisol output and depression severity in preschoolers. They also demonstrate an important role for amygdala function as a mediator of this relationship at a very early age. Our results further underscore early childhood as an important developmental period for understanding the neurobiological correlates of early stress and increased risk for depression.
Keywords: depression, development, stress, reward processing, fMRI, amygdala
INTRODUCTION
Major depressive disorder (MDD) is one of the most common psychiatric conditions and a leading cause of impairment, disability, and morbidity (1). Given a growing consensus that the origins of depression are likely neurodevelopmental in nature (2), remarkably little is known about its neurobiological roots. As a result, identifying early occurring neurobiological intermediate phenotypes associated with depression is critical for advancing efforts to establish predictive biomarkers of relative risk and resilience to this disorder. Research now clearly demonstrates that depression during the preschool period is a precursor of later school age and adolescent MDD (3, 4). As such, investigations of brain function in preschoolers with elevated symptoms of depression are likely to provide crucial information informing the next generation of intervention strategies aimed at reducing the considerable public health burden of this disorder.
Altered neural processing of reward has emerged as a highly promising depressive intermediate phenotype (5). Reward processing relies on an interconnected network of brain regions, including the midbrain, amygdala, striatum, anterior cingulate cortex, orbitofrontal cortex, and medial prefrontal cortex (6). Functional magnetic resonance imaging (fMRI) research has provided key data supporting altered reward-related brain function in adults and adolescents with depression, including associations with depression severity (7), diminished daily experience of positive emotion (8), response to depression treatment (9), and later depression in adolescents (10, 11). Given that neural processing of reward undergoes a prolonged period of development beginning in early childhood (12), early experiences influencing this developmental process have been proposed to underlie the future emergence of depression in at least some individuals (13).
The very early experience of stress has emerged as one of the most salient factors that may negatively influence reward-related brain function and contribute to the development of depression (14). Consistent with this notion, recent research has shown that variability in neural response to reward partially mediates the relationship between stressful childhood experiences and elevated depressive symptomatology during adolescence and adulthood (15–17). However, this research has primarily relied on retrospective measures of early life stress and assessed brain function during adolescence or adulthood. As a result, whether similar associations are present in young children is unknown and the putative mechanisms through which early life adversity is associated with neural processing of reward remains poorly understood.
Emerging independent lines of evidence raise the possibility that hypothalamic–pituitary–adrenal axis (HPA) function may play a mechanistic role in the expression of early life stress-related neural reward processing dysfunction (14). First, preclinical work indicates that the development of reward-related brain regions rich in glucocorticoid receptors is negatively affected by increased levels of glucocorticoids during prolonged periods of elevated stress (18). Second, previous research has reported altered HPA reactivity in groups of children exposed to early stressful life events (19, 20) and attenuated reward-related brain function in adolescents and adults with a history of early life stress (17, 21), including those who eventually develop depression (15). Lastly, recent fMRI data suggests that acute cortisol administration blunts reward-related neural activity (22, 23). Collectively, these data suggest that altered HPA stress reactivity following repeated exposure to stressors during early childhood may result in relatively blunted neural responses to reward, potentially conferring increased risk for depression. However, data directly informing the relationship between HPA function and neural response to reward during early childhood is not available. Such data would provide critical insight into our mechanistic understanding of how early life stress conveys increased risk for depression.
The present study investigates whether altered HPA functioning is associated with altered neural reactivity to reward and depression severity in preschoolers using fMRI. It also tests whether altered neural reactivity to reward mediates the relationship between cortisol output following stress and depression severity in preschoolers. Following previous research, it was predicted that greater depression severity in preschoolers would be linked to higher total cortisol output to an in-lab psychosocial stressor (24). Based on evidence that cortisol administration blunts reward-related activity in the amygdala and striatum, and data suggesting these regions as highly susceptible to the effects of early life stress and altered in pediatric depression (25) (26), we predicted that higher total cortisol output following stress would be associated with diminished reactivity to reward related outcomes in these regions. Lastly, we anticipated that altered neural reactivity to reward in these regions would mediate the relationship between cortisol output and depression severity.
METHODS AND MATERIALS
Participants
Eighty-eight preschoolers between 4–6 years of age were recruited from pediatrician’s offices, daycares, and other community resources throughout the greater St. Louis area. In order to increase sample variance in depressive symptoms, a validated screening checklist (Preschool Feelings Checklist (27); PFC) was used to identify preschoolers with and without elevated depressive symptoms. Caregivers indicating that their preschoolers had “low” (≤1 PFC items endorsed) or “high” (≥3 PFC items endorsed) levels of depressive symptoms were contacted and invited to complete additional phone screening steps assessing for the presence of neurological disorders (e.g., seizure disorder), autism spectrum disorders or developmental delays, premature birth (<36 weeks gestation), and psychotropic medication use. Endorsement of any of these conditions acted as exclusionary for all children. Children passing the exclusion criteria were invited to enroll in the full study. Following study enrollment, each family was asked to complete an age appropriate mental health and developmental assessment and an fMRI scan within 7–10 days of their assessment. Of the 88 children completing the study, complete fMRI data were not collected for 9 children due to equipment failure (N=3), falling asleep during scan (N=1), refusal to play fMRI task (N=1), or request to end scan (N=4). Of the 79 children completing the fMRI scan, 60 provided data passing quality control (QC) measures (76%; see Supplemental Information). Of the 60 children with usable fMRI data, 52 also had stress reactivity cortisol data passing QC (see Supplemental Information) and were included in the analyses addressing our a priori hypotheses. Parental written consent and child verbal assent were obtained for all subjects. The Institutional Review Board at Washington University in St. Louis approved all experimental procedures.
Diagnostic Assessment
Diagnostic assessments were conducted using the Kiddie Schedule for Affective Disorders-Early Childhood version (K-SADS-EC; (28), a developmentally modified version of the Kiddie Schedule for Affective Disorders and Schizophrenia for School age Children-Present and Lifetime Version (K-SADS-PL)(29). See Supplemental Information for greater detail.
Depression Severity
Child
The Preschool Feelings Checklist – Scale Version (PFC-S; (30) is a 23 item measure that uses a Likert rating scale (0 = never, 4 = most of time; range of possible scores 0–92) designed to assess depression severity in preschool children and has established validity at this age (31). Example items include, ‘My child appears sad or says he/she feels sad’ and ‘Enjoys activities and play (reverse scored).’ See Supplemental Information for additional information.
Parent
Parents filled out the Beck Depression Inventory–II (BDI–II; (32), a validated 21-item measure of depression symptom presence and severity in adults.
Cortisol Collection and Analysis Procedures
Children completed a stress-inducing ‘frustration’ task that reliably induces a cortisol response in preschoolers (33). Briefly, children were instructed to match colored wooden chips with corresponding shapes to earn a prize before time ran out (~3 minutes). A toy traffic light indicated how much time they had remaining and experimental manipulation of timing ensured task failure. One saliva sample was collected prior to the frustration task as a baseline measurement of cortisol (preceded by a half-hour period of neutral activities) and six saliva samples were collected every ten minutes during the hour following the task while a neutral movie was watched. See Supplemental Information for detailed collection, assay, and data quality control methods.
Consistent with prior observations, cortisol data were skewed and subsequently log10 transformed prior to all analyses (34). Following previous research suggesting that total cortisol output following stress is associated with depression and depression risk (20, 35), total cortisol production during the stress task was calculated using standard area under the curve with respect to ground (AUCg) procedures (36), incorporating actual time between cortisol sample collection in these calculations.
Child fMRI Gambling Task
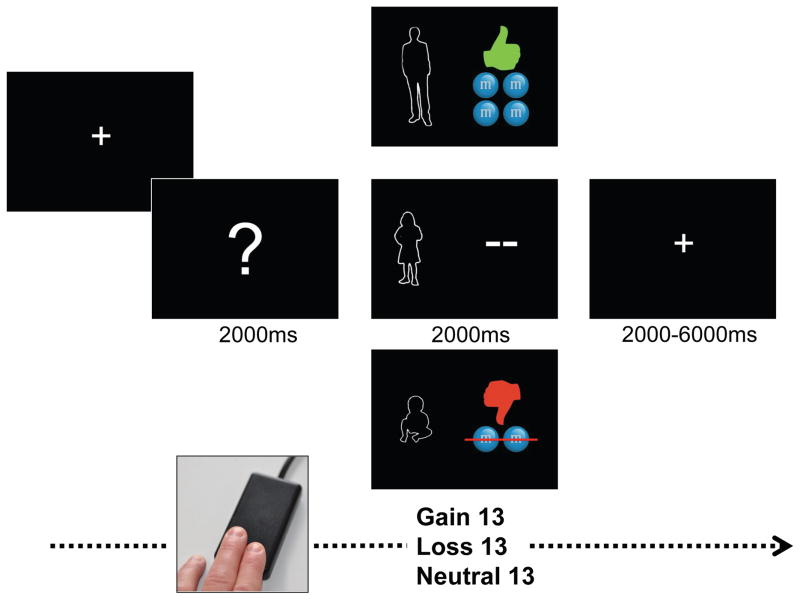
fMRI data were collected as children completed the Child Gambling Task (CGT) approximately 7–10 days following their in-person assessment. The CGT is a developmentally adapted form of a commonly used ‘gambling’ reward processing task (37) previously shown to elicit robust and reliable activation in reward related regions in older age groups (37–42). It has also been used in prior studies of reward and loss sensitivity in relation to depression (8, 15–17, 43–45). The CGT was presented with E-Prime (Psychology Software Tools, Inc.) using an event related design with 13 trials of each outcome (i.e., gain, loss, neutral) presented in a predetermined pseudo randomized order (no more than 3 of the same type in a row) per run (Figure 1). During the CGT, children are asked to guess whether the next person they see is going to be bigger or smaller than them to win or lose candy. To reduce the potential for movement, only one response (i.e., either ‘bigger’ or ‘smaller’) is assigned to a single button, with nonresponses (i.e., no button press) representing the alternate choice. The assignment of bigger or smaller as the active response was counterbalanced across children. The gain and loss amounts were chosen to give gains and losses of similar subjective values (46). Each child completed two ~6 minute runs and were given an amount of candy matching the maximum gained during the CGT following scan completion.
Figure 1.
Child Gambling Task (CGT). Each trial of the CGT begins with a white fixation cue presented in the center of a black screen for 2000ms. Next, a screen displays a question mark for 2000ms. Children are asked to guess whether the person hiding behind the question mark is bigger or smaller than them and to indicate their choice by pressing a button on an MRI compatible single button response box designed specifically for use with young children. Following their choice, feedback is generated as a function of whether the trial was scheduled to be a reward, loss or neutral outcome and presented for 2000ms. Feedback images included either a baby, adult, or similarly sized child paired with: 1) a green up thumbs up next to 4 candies for gain, 2) a red thumbs down next to an image of 2 candies with a line through them for loss; or 3) two dashes (“- -”) for neutral trials. A jittered inter-trial interval using a black screen with central fixation cross occurred between each trial (M=4000ms, Min.=2000ms, Max=6000ms).
Functional Imaging Data Acquisition and Preprocessing Procedures
To create familiarity and comfort with study procedures, each child was shown a child friendly video introducing the fMRI experience and introduced to the scanning environment using a mock scanner training protocol during their initial in-person assessment, allowed to watch a movie of their choice during structural scans, and rewarded with small prizes following scan completion. Imaging data were collected using a 3T TIM TRIO Siemens whole body system. See Supplemental Information for fMRI acquisition and preprocessing procedures.
Functional Imaging Data Analysis
A general linear model (GLM) approach incorporating regressors for outcome, linear trend, and baseline shift was used to estimate subject-specific voxel-wise task-related activity without assuming a hemodynamic response shape. Gain, loss, and neutral outcomes were modeled separately relative to fixation baseline for 10 frames following question mark onset (Figure 1). The estimates for the last 8 frames represent the different time points in 2-second increments following presentation of the reward outcome. The resulting beta estimates of the event-related response at each frame were entered into a second-level analysis treating subjects as a random factor. At the second level, we computed a voxel-wise repeated-measures analysis of variance (ANOVA) with time point (10 estimated frames) as a within-subject factor.
Both region-of-interest (ROI) and whole-brain approaches were used. The more conservative ROI approach was conducted using two a priori masks focused on 1) the left and right amygdala adopted from (47) and 2) an a priori network of regions implicated in reward processing including the dorsal and ventral striatum adopted from (42, 48). The choice of these two ROIs was based upon evidence indicating 1) that the amygdala plays an important and specific role in evaluating reward salience (49, 50), 2) amygdala reactivity is altered in depressed preschoolers (51), and 3) that developmental and depression related differences in striatal and cortical response to reward can be successfully identified in children using our a priori mask of reward-related regions (41, 52). To isolate task-evoked amygdala signals, we initially computed our ANOVA using the individually averaged beta values for each time point from our a priori amygdala ROI. Subsequent ANOVAs using our a priori reward processing mask or at the whole brain level were corrected for multiple comparisons (see Supplemental Information for additional details).
Following the identification of a significant main effect of time within a given brain region (e.g., amygdala), timecourses were subsequently inspected for time x outcome interactions using a 2-way repeated-measures ANOVA. When an outcome x time interaction was identified for a given brain region, follow-up paired t-tests were used to identify at which time point(s) conditions differed. Following previous event-related fMRI research (53–55), the two time points representing the period of peak difference between outcomes were identified, averaged within a given outcome (e.g., gain), and then subsequently subtracted between the differing outcomes (e.g., gain minus loss) to create a peak difference score. Peak difference scores were then examined in separate correlational and mediation analyses using PFC-S and AUCg cortisol scores and a 2-tailed approach to significance (IBM SPSS Statistics version 21; SPSS Inc., Chicago, IL, USA).
Brain Function, Stress, and Depression Severity
In order to test our a priori hypothesis that attenuated neural response to reward mediates the relationship between altered HPA function and depression severity in preschoolers, we used the PROCESS macro procedure for SPSS. Following Hayes (56), a significant effect of mediation would indicate that the association between AUCg and depression severity occurs indirectly through brain activity. Only difference scores generated from our a priori ROIs with a time x outcome effect were examined in the mediation analyses (see Figure 2A for complete model). A multivariate approach to identifying potential outliers using Mahalanobis D2 was conducted prior to carrying out our a priori correlational and mediation analyses. No outliers were identified.
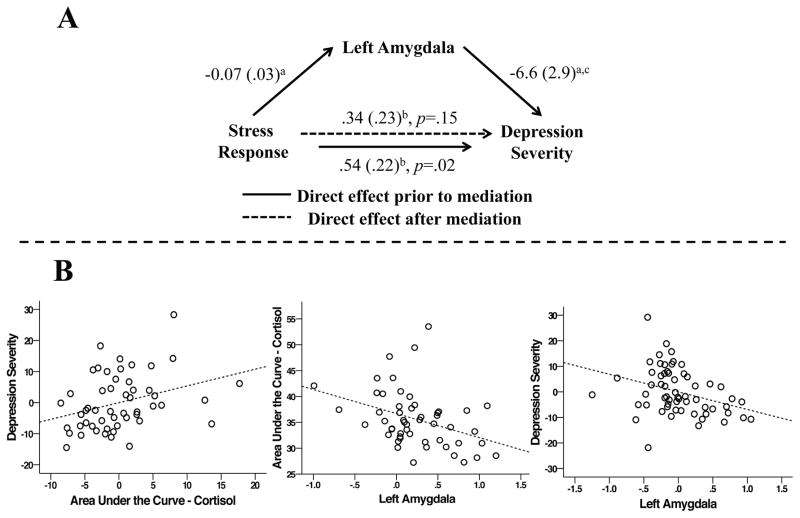
Figure 2.
A) Attenuated differential responding in the left amygdala to gain/loss versus neutral outcomes mediates the relationship between elevated stress reactivity and depression severity in preschool age children. Values represent beta coefficients generated by the SPSS PROCESS macro procedure for mediation model 4. a = p < .05; b = includes maternal depression as a covariate; c = includes maternal depression and stress reactivity as covariates B) Scatter plots illustrating the positive correlation between AUCg and child depression severity (r = .32, p = .021), the negative correlation between gain/loss minus neutral difference scores in the left amygdala and AUCg (r = −.37, p = .006), and the negative correlation between gain/loss minus neutral difference scores in the left amygdala and child depression severity (r = −.40, p = .003). Plots including depression severity scores represent the residualized values for each variable after controlling for maternal depression.
RESULTS
Demographic and Child Characteristics
See Table 1 for sample demographic and diagnostic characteristics. Averages scores were 16.1 (±6.3; range 1–47) for PFC-S, 8 (±9.2; range 0–34) for BDI-II, and 35.6 (±5.5; range 27.23–53.52) ng/ml for AUCg. Preschoolers with a diagnosis of MDD on the K-SADS-EC had higher PFC-S scores that those who did not (MDD = 28 (±10), No MDD (12.5 (±8.4); t50 = 5.4, p < .001) and those not providing usable fMRI data were younger (mean age 60 [11.5] months) than those who did (mean age 71 [9] months). Previous research suggests that maternal mood state likely inflates parent report of child psychopathology. In line with this, there was a significant positive correlation between PFC-S and BDI-II scores (r = .56, p < .001) in the current sample. Thus, all analyses including the PFC-S controlled for maternal BDI-II scores.
Table 1.
Study Group Characteristics
| Characteristic | N = 52 |
|---|---|
| Age (months) | 71.9 (±8.9) |
| Gender | 28F/24M |
| Ethnicity | 35W/14AA/3O |
| PFC Screena | 34 low/18 high |
| Diagnosesb | |
| None | 37 |
| Internalizing | 9 |
| Externalizing | 2 |
| Int. and Ext. | 4 |
Note. F = female; M = male; W = white; AA = African American; O = other; PFC = Preschool Feelings Checklist
Number of children with caregiver reporting “low” (≤1 PFC items endorsed) or “high” (≥3 PFC items endorsed) levels of depressive symptoms during initial screen
Internalizing: Preschool Depression (N=8), Preschool Depression and Separation Anxiety Disorder (N=1), Generalized Anxiety Disorder (N=1)
Externalizing: Oppositional Defiant Disorder (N=1), Attention-Deficit Hyperactivity Disorder (N=1)
Internalizing and Externalizing: Preschool Depression and Oppositional Defiant Disorder (N=2), Oppositional Defiant Disorder and Attention- Deficit Hyperactivity Disorder (N=1)
Behavioral Results for Scanner Task
On average, children pressed the response button on 56% (44/78) of the CGT trials. Reaction time (RT) was missing for two children who did not push the response button during the CGT. Average win RT = 1001ms (±219), average loss RT = 972ms (±208), and average neutral RT = 963ms (±215). RT did not differ between outcome conditions (all t[50] ≤ 1.41, p ≥ .165).
Neuroimaging Findings
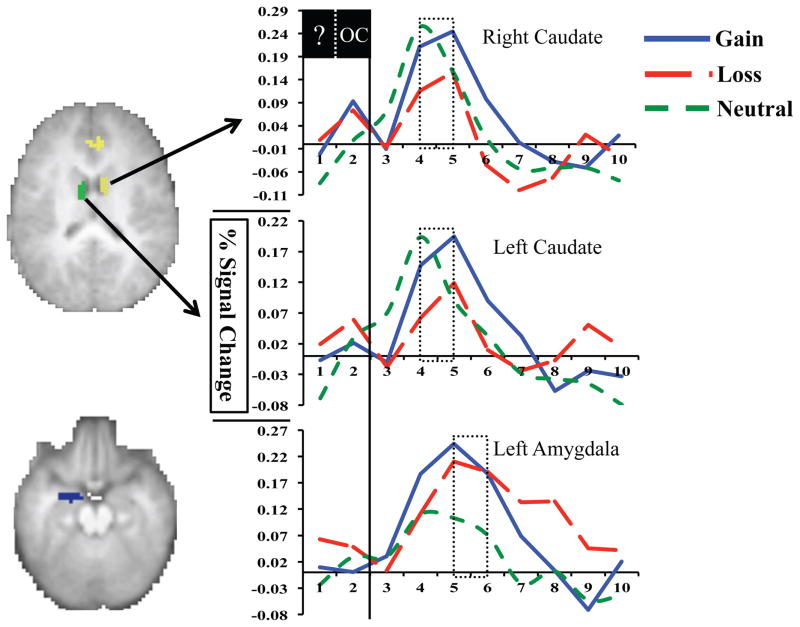
A main effect of time was found for the left and right amygdala ROIs as well as for multiple regions within our a priori reward processing mask, including the left anterior insula, anterior cingulate cortex (ACC), and bilateral caudate (Table 2). Time x outcome interactions were also noted, including greater left and right caudate reactivity for gain versus loss outcomes, greater ACC reactivity for gain versus loss and neutral outcomes, and increased left amygdala reactivity following gain and loss outcomes versus neutral ones (Figure 3). Consistent with previous research suggesting the amygdala is sensitive to stimulus salience rather than valence (49, 57), our paired t-tests revealed that gain and loss timecourses in the left amygdala did not differ from each other and were identical in their pattern of peak differences with neutral outcomes. Thus, we used an averaged timecourse for gain and loss outcomes (gain/loss) when creating left amygdala difference scores. Follow-up paired t-tests identified time points five and six as the period of peak difference between gain/loss and neutral outcomes in the left amygdala and between gain and loss and gain and neutral outcomes in the ACC. For the left and right caudate, follow-up paired t-tests indicated that peak differences between gain and loss outcomes were present at timepoints four and five. Individual peak difference scores were generated for the amygdala, caudate, and ACC (e.g., [average of gain timepoints 4 and 5] – [average of loss timepoints 4 and 5] for the left caudate) and used in all subsequent analyses.
Table 2.
Regions identified in a priori reward mask with main effect of time.
| Peak Voxel | Cluster (voxels) | Outcome X Time | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Region | Hemisphere | X | Y | Z | ||
| Globus Pallidus (includes amygdala) | R | 10 | 0 | 0 | 63 | NS |
| Caudate | L | −10 | 3 | 3 | 205 | NS |
| Caudate* | L | −10 | −4 | 18 | 34 | G > L |
| Putamen* | L | −26 | 6 | 4 | 32 | NS |
| Medial Globus Pallidus* (includes amygdala) | L | −12 | 0 | −5 | 35 | NS |
| Substantia Nigra | R | 10 | −21 | −9 | 42 | NS |
| Red Nucleus | L | −4 | −21 | −6 | 25 | NS |
| Insula | L | −34 | 9 | 3 | 144 | NS |
| Putamen | R | 20 | 6 | −3 | 17 | NS |
| Claustrum | R | 28 | 18 | 3 | 11 | NS |
| Caudate | R | 8 | 0 | 15 | 32 | G > L |
| Anterior Cingulate (BA 32) | L | 4 | 33 | 21 | 22 | G > L, N |
Following application of peak splitting algorithm to caudate cluster. BA = Brodmann Area; G = gain; L = loss; N = neutral; NS = not significant
Figure 3.
Differential responses to reward outcomes were found in bilateral caudate and left amygdala. Specifically, greater reactivity to gain versus loss outcomes was found in the left and right caudate while great reactivity to both gain and loss outcomes versus neutral ones was found in the left amygdala. Dashed boxes highlight frames used to generate difference scores. ? = task guess period; OC = task outcome period
Whole brain results were significant for a main effect of time in multiple cortical and subcortical regions. Follow-up analyses found outcome x time effects in parahippocampla gyrus, fusiform gyrus and postcentral gyrus. See Supplemental Information for additional information.
Brain Function, Stress, and Depression Severity
Following our a priori hypotheses, AUCg was positively correlated with child depression severity (r =.32, p = .021) and negatively correlated with differences between gain/loss and neutral outcomes in the left amygdala (r = −.37, p = .006). In addition, differences between gain/loss and neutral outcomes in the left amygdala were negatively correlated with child depression severity (r = −.40, p = .003; Figure 2B). Further, reduced gain/loss versus neutral difference scores in the left amygdala were found to mediate the significant relationship between elevated AUCg and increased depression severity in preschoolers (PROCESS Indirect Effect [10,000 bootstrap samples]: .2 (.11), bias corrected 95% CI: .05/.5, Figure 2A). The relationships between AUCg and left and right caudate gain versus loss difference scores were not significant, though in the expected direction (right caudate r = −.19, p = .17; left caudate r = −.27, p = .052). AUCg was not related to either of the ACC difference scores (gain versus loss r = −.12, p = .39; gain versus neutral r = −.22, p = .13). The pattern and significance of observed results did not change when gender or age was included as a covariate. Please see Supplemental Information for additional analyses supporting the specificity of the mediation results to AUCg, neural response to highly salient (i.e., gain/loss) outcomes, and their robustness to additional covariates.
DISCUSSION
The current study used fMRI to examine whether neural reactivity to reward mediates the relationship between cortisol response following a stressor and depression severity in preschool age children. Our results extend prior reports in older age groups (14) by showing that both higher total cortisol output following a stressor and attenuated neural sensitivity to highly salient outcomes (i.e., gain and loss) are already related to increased depression severity in preschoolers. They also match prior findings suggesting a negative relationship between cortisol and reward-related brain activity (22, 23). Importantly, the current findings provide novel evidence further supporting attenuated neural sensitivity to reward-related information as a putative mechanism through which early life adversity is associated with increased risk for depression.
Attenuated neural processing of reward following early life stress has emerged as one of the most promising depressive intermediate phenotypes. More specifically, it has been suggested that under conditions of chronic stress and adversity, physiological responses to stress occur more frequently, tend to increase in magnitude and duration, and take longer to recover to baseline levels (58). Over time, the repeated, excessive activations and inefficient down-regulation of stress response systems - including the HPA – has a significant and negative affect on developing reward-related brain function, increasing risk for later MDD (59). However, data directly informing the relationship between individual HPA stress response and neural processing of reward during early childhood has remained largely unavailable, leaving the developmental trajectory of this intermediate phenotype uncharted. As a first step in filling this knowledge gap, the current findings indicate that higher total cortisol output following a mild stressor in preschoolers is associated with diminished amygdala reactivity to highly salient reward processing outcomes. The amygdala has been consistently shown to play an important role in evaluating the motivational significance of a given stimulus (57). Recent work has suggested that stress may dampen amygdala reactivity in this regard. More specifically, oral administration of cortisol has been reported to dampen amygdala reactivity to reward in older samples (22, 23). Preclinical work has also suggested that chronic stress induces significant dendritic spine loss in the medial amygdala (60), a major efferent nucleus of the amygdala sensitive to the motivational salience of events and strongly interconnected with the mesolimbic dopamine pathway (61). The current findings extend this work by providing unique insight into how developing stress and brain reward systems are related to each other very early in life. They also provide critical support for theoretical models suggesting that repeated activation of the HPA system may eventually facilitate the development of attenuated neural reactivity to reward as a more stable ‘trait’ like neurobiological endophenotype linking early adversity and MDD risk (14) (62). However, the current findings cannot address to what degree attenuated amygdala reactivity in our preschoolers is reflective of repeated exposure to prolonged HPA stress-related activity or establish a causal relationship. Nevertheless, they do provide important evidence suggesting that stress and brain reward systems are already tightly entwined as early as the preschool period.
Disrupted incentive-based learning has emerged as one potential mechanistic explanation of how altered reward processing mediates the relationship between early stress and increased risk for depression (14). Appropriate processing of reward outcomes plays a central role in incentive-based learning, with intact sensitivity to salient events (e.g., gains and/or losses) believed to be critical for learning reward-predicting cues that shape later self-regulation and goal directed behavior (63), both of which are disrupted in depression. Behavioral studies indicate that developmental changes in reward learning are already underway during the preschool period (64–66). Importantly, this work also suggests that developmental changes in early reward learning may lay a critical foundation for the ongoing development of self-regulation (64) and goal directed behavior (66). For example, recent behavioral data has illustrated that intact sensitivity to gain outcomes results in increased inhibitory control in preschoolers (66) and that diminished reward learning is associated with significant behavior regulation difficulties at this age (64). Previous work has suggested that the amygdala plays a critical role in reward learning; with disruptions affecting the ability to acquire as well as generalize learned responses (49, 50). Previous preclinical work also suggests that early disruptions in amygdala functioning may negatively influence the ongoing development of later maturing brain regions also important for reward processing and learning, including the medial prefrontal cortex (67). However, longitudinal studies beginning very early in development will be needed to more fully understand the complex relationships between brain development, reward learning, and emerging depression.
In contrast to previous work, higher total cortisol output following a stressor was not associated with caudate reactivity to gain versus loss. Previous research has suggested that attenuated reward-related activity in the striatum may be most evident during the experience of an acute stressor (23, 68, 69). Given the very young age of our children, cortisol response to stress was measured prior to their scan. As a result, the current study is unable to inform the relationship between cortisol and caudate reactivity when measured concurrently. Interestingly, recent functional connectivity work has suggested that the amygdala and striatum are positively connected in preschoolers, adolescents, and adults (70). As a result, it has been speculated that early alterations in amygdala reactivity to stimulus salience may negatively influence ongoing development of the striatum, with altered striatal response to reward following early stress emerging later in development as a result (25). However, longitudinal studies will be needed to answer this question. Alternatively, stress related attenuation of reward processing in the caudate might be most apparent during tasks involving reward anticipation and/or learning (71), two aspects of reward processing not directly tested in this study. Future work directly investigating these possibilities will be necessary to better understand the relationship between stress and caudate activity during early childhood.
Several limitations should be noted. First, future investigations into other constructs (e.g., threat processing) and disorders (e.g., anxiety) will be necessary to inform the specificity of our results to reward processing and depression. Given that all measures were taken concurrently, the current results cannot inform directions of causality (see Supplemental Information for discussion of alternative mediation models). As a result, longitudinal studies will likely be critical for identifying trajectories of risk for depression and related psychopathology and informing interventions that can successfully target them. Nevertheless, the current study supports stress attenuated neural sensitivity to salient, reward-related outcomes as one potential mechanism that increases depression risk and further underscores early childhood as an important developmental period for understanding its earliest roots (72).
Supplementary Material
Acknowledgments
We would like to thank the children and their families who participated in this study. The National Institute of Mental Health (NIMH; Grants K23 MH098176 and R01 MH110488 to MSG) and the McDonnell Center for Systems Neuroscience (MSG) supported this work. The NIMH and the McDonnell Center for Systems Neuroscience had no further \role in the design and conduct of the study; collection, management, analysis, and interpretation of data; or preparation, review, or approval of the manuscript.
Footnotes
FINANCIAL DISCLOSURE
The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mathers CD, Lopez AD, Murray CJL. The Burden of Disease and Mortality by Condition: Data, Methods, and Results for. 2006 [PubMed] [Google Scholar]
- 2.Insel TR. Mental disorders in childhood: shifting the focus from behavioral symptoms to neurodevelopmental trajectories. JAMA. 2014;311:1727–1728. doi: 10.1001/jama.2014.1193. [DOI] [PubMed] [Google Scholar]
- 3.Luby JL, Si X, Belden AC, Tandon M, Spitznagel E. Preschool depression: homotypic continuity and course over 24 months. Arch Gen Psychiatry. 2009;66:897–905. doi: 10.1001/archgenpsychiatry.2009.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luby JL, Gaffrey MS, Tillman R, April LM, Belden AC. Trajectories of Preschool Disorders to Full DSM Depression at School Age and Early Adolescence: Continuity of Preschool Depression. Am J Psychiatry. 2014 doi: 10.1176/appi.ajp.2014.13091198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luking KR, Pagliaccio D, Luby JL, Barch DM. Reward Processing and Risk for Depression Across Development. Trends in Cognitive Sciences. 2016;20:456–468. doi: 10.1016/j.tics.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forbes EE, Christopher May J, Siegle GJ, Ladouceur CD, Ryan ND, Carter CS, et al. Reward-related decision-making in pediatric major depressive disorder: an fMRI study. J Child Psychol Psychiatry. 2006;47:1031–1040. doi: 10.1111/j.1469-7610.2006.01673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forbes EE, Hariri AR, Martin SL, Silk JS, Moyles DL, Fisher PM, et al. Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. Am J Psychiatry. 2009;166:64–73. doi: 10.1176/appi.ajp.2008.07081336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forbes EE, Olino TM, Ryan ND, Birmaher B, Axelson D, Moyles DL, et al. Reward-related brain function as a predictor of treatment response in adolescents with major depressive disorder. Cogn Affect Behav Neurosci. 2010;10:107–118. doi: 10.3758/CABN.10.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forbes EE, Shaw DS, Dahl RE. Alterations in reward-related decision making in boys with recent and future depression. Biol Psychiatry. 2007;61:633–639. doi: 10.1016/j.biopsych.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 11.Stringaris A, Vidal-Ribas Belil P, Artiges E, Lemaitre H, Gollier-Briant F, Wolke S, et al. The Brain’s Response to Reward Anticipation and Depression in Adolescence: Dimensionality, Specificity, and Longitudinal Predictions in a Community-Based Sample. Am J Psychiatry. 2015;172:1215–1223. doi: 10.1176/appi.ajp.2015.14101298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galvan A. Neural systems underlying reward and approach behaviors in childhood and adolescence. Curr Top Behav Neurosci. 2014;16:167–188. doi: 10.1007/7854_2013_240. [DOI] [PubMed] [Google Scholar]
- 13.Davey CG, Yucel M, Allen NB. The emergence of depression in adolescence: development of the prefrontal cortex and the representation of reward. Neurosci Biobehav Rev. 2008;32:1–19. doi: 10.1016/j.neubiorev.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 14.Pizzagalli DA. Depression, stress, and anhedonia: toward a synthesis and integrated model. Annu Rev Clin Psychol. 2014;10:393–423. doi: 10.1146/annurev-clinpsy-050212-185606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanson JL, Hariri AR, Williamson DE. Blunted Ventral Striatum Development in Adolescence Reflects Emotional Neglect and Predicts Depressive Symptoms. Biol Psychiatry. 2015;78:598–605. doi: 10.1016/j.biopsych.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Romens SE, Casement MD, McAloon R, Keenan K, Hipwell AE, Guyer AE, et al. Adolescent girls’ neural response to reward mediates the relation between childhood financial disadvantage and depression. J Child Psychol Psychiatry. 2015;56:1177–1184. doi: 10.1111/jcpp.12410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanson JL, Albert D, Iselin AM, Carre JM, Dodge KA, Hariri AR. Cumulative stress in childhood is associated with blunted reward-related brain activity in adulthood. Soc Cogn Affect Neurosci. 2016;11:405–412. doi: 10.1093/scan/nsv124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qiao H, Li MX, Xu C, Chen HB, An SC, Ma XM. Dendritic Spines in Depression: What We Learned from Animal Models. Neural Plast. 2016;2016:8056370. doi: 10.1155/2016/8056370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Struber N, Struber D, Roth G. Impact of early adversity on glucocorticoid regulation and later mental disorders. Neurosci Biobehav Rev. 2014;38:17–37. doi: 10.1016/j.neubiorev.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 20.Lopez-Duran NL, Kovacs M, George CJ. Hypothalamic-pituitary-adrenal axis dysregulation in depressed children and adolescents: a meta-analysis. Psychoneuroendocrinology. 2009;34:1272–1283. doi: 10.1016/j.psyneuen.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dillon DG, Holmes AJ, Birk JL, Brooks N, Lyons-Ruth K, Pizzagalli DA. Childhood adversity is associated with left basal ganglia dysfunction during reward anticipation in adulthood. Biol Psychiatry. 2009;66:206–213. doi: 10.1016/j.biopsych.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kinner VL, Wolf OT, Merz CJ. Cortisol alters reward processing in the human brain. Horm Behav. 2016;84:75–83. doi: 10.1016/j.yhbeh.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Montoya ER, Bos PA, Terburg D, Rosenberger LA, van Honk J. Cortisol administration induces global down-regulation of the brain’s reward circuitry. Psychoneuroendocrinology. 2014;47:31–42. doi: 10.1016/j.psyneuen.2014.04.022. [DOI] [PubMed] [Google Scholar]
- 24.Luby JL, Heffelfinger A, Mrakotsky C, Brown K, Hessler M, Spitznagel E. Alterations in stress cortisol reactivity in depressed preschoolers relative to psychiatric and no-disorder comparison groups. Arch Gen Psychiatry. 2003;60:1248–1255. doi: 10.1001/archpsyc.60.12.1248. [DOI] [PubMed] [Google Scholar]
- 25.Fareri DS, Tottenham N. Effects of early life stress on amygdala and striatal development. Dev Cogn Neurosci. 2016;19:233–247. doi: 10.1016/j.dcn.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller CH, Hamilton JP, Sacchet MD, Gotlib IH. Meta-analysis of Functional Neuroimaging of Major Depressive Disorder in Youth. JAMA Psychiatry. 2015;72:1045–1053. doi: 10.1001/jamapsychiatry.2015.1376. [DOI] [PubMed] [Google Scholar]
- 27.Luby J, Heffelfinger A, Koenig-McNaught A, Brown K, Spitznagel E. The preschool feelings checklist: A brief and sensitive screening measure for depression in young children. J Am Acad Child Adolesc Psychiatry. 2004;43:708–717. doi: 10.1097/01.chi.0000121066.29744.08. [DOI] [PubMed] [Google Scholar]
- 28.Gaffrey MS, Luby JL. Kiddie Schedule for Affective Disorders and Schizophrenia - Early Childhood Version, 2012 Working Draft (K-SAD-EC) St. Louis, MO: Washington University School of Medicine; 2012. [Google Scholar]
- 29.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 30.Luby J, Heffelfinger A, Mrakeotsky C, Hildebrand T. Preschool Feelings Checklist. St. Louis, Missouri: Washington University; 1999. [Google Scholar]
- 31.Luby J, Lenze S, Tillman R. A novel early intervention for preschool depression: findings from a pilot randomized controlled trial. J Child Psychol Psychiatry. 2012;53:313–322. doi: 10.1111/j.1469-7610.2011.02483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beck A, Steer R, Brown G. Manual for Beck Depression Inventory-II. San Antonio, Texas: Psychological Corporation; 1996. [Google Scholar]
- 33.Kryski KR, Smith HJ, Sheikh HI, Singh SM, Hayden EP. Assessing stress reactivity indexed via salivary cortisol in preschool-aged children. Psychoneuroendocrinology. 2011;36:1127–1136. doi: 10.1016/j.psyneuen.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 34.Gunnar MR, Talge NM. Neuroendrocrine Measures in Developmental Research. In: Schmidt LA, Segalowitz SJ, editors. Developmental Psychophysiology: Theory, Systems, and Methods. Cambridge: Cambridge Univesity Press; 2008. [Google Scholar]
- 35.LeMoult J, Ordaz SJ, Kircanski K, Singh MK, Gotlib IH. Predicting first onset of depression in young girls: Interaction of diurnal cortisol and negative life events. J Abnorm Psychol. 2015;124:850–859. doi: 10.1037/abn0000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- 37.Delgado MR, Nystrom LE, Fissell C, Noll DC, Fiez JA. Tracking the hemodynamic responses to reward and punishment in the striatum. Journal of Neurophysiology. 2000;84:3072–3077. doi: 10.1152/jn.2000.84.6.3072. [DOI] [PubMed] [Google Scholar]
- 38.Tricomi EM, Delgado MR, Fiez JA. Modulation of caudate activity by action contingency. Neuron. 2004;41:281–292. doi: 10.1016/s0896-6273(03)00848-1. [DOI] [PubMed] [Google Scholar]
- 39.May JC, Delgado MR, Dahl RE, Stenger VA, Ryan ND, Fiez JA, et al. Event-related functional magnetic resonance imaging of reward-related brain circuitry in children and adolescents. Biological psychiatry. 2004;55:359–366. doi: 10.1016/j.biopsych.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 40.Forbes EE, Hariri AR, Martin SL, Silk JS, Moyles DL, Fisher PM, et al. Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. The American journal of psychiatry. 2009;166:64–73. doi: 10.1176/appi.ajp.2008.07081336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luking KR, Luby JL, Barch DM. Kids, candy, brain and behavior: age differences in responses to candy gains and losses. Dev Cogn Neurosci. 2014;9:82–92. doi: 10.1016/j.dcn.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luking KR, Barch DM. Candy and the brain: neural response to candy gains and losses. Cogn Affect Behav Neurosci. 2013;13:437–451. doi: 10.3758/s13415-013-0156-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gotlib IH, Hamilton JP, Cooney RE, Singh MK, Henry ML, Joormann J. Neural processing of reward and loss in girls at risk for major depression. Arch Gen Psychiatry. 2010;67:380–387. doi: 10.1001/archgenpsychiatry.2010.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang WN, Chang SH, Guo LY, Zhang KL, Wang J. The neural correlates of reward-related processing in major depressive disorder: a meta-analysis of functional magnetic resonance imaging studies. J Affect Disord. 2013;151:531–539. doi: 10.1016/j.jad.2013.06.039. [DOI] [PubMed] [Google Scholar]
- 45.Belden AC, Irvin K, Hajcak G, Kappenman ES, Kelly D, Karlow S, et al. Neural Correlates of Reward Processing in Depressed and Healthy Preschool-Age Children. J Am Acad Child Adolesc Psychiatry. 2016;55:1081–1089. doi: 10.1016/j.jaac.2016.09.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tversky A, Kahneman D. The framing of decisions and the psychology of choice. Science. 1981;211:453–458. doi: 10.1126/science.7455683. [DOI] [PubMed] [Google Scholar]
- 47.Roy AK, Shehzad Z, Margulies DS, Kelly AM, Uddin LQ, Gotimer K, et al. Functional connectivity of the human amygdala using resting state fMRI. Neuroimage. 2009;45:614–626. doi: 10.1016/j.neuroimage.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beck SM, Locke HS, Savine AC, Jimura K, Braver TS. Primary and secondary rewards differentially modulate neural activity dynamics during working memory. PLoS One. 2010;5:e9251. doi: 10.1371/journal.pone.0009251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baxter MG, Murray EA. The amygdala and reward. Nat Rev Neurosci. 2002;3:563–573. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- 50.Murray EA. The amygdala, reward and emotion. Trends Cogn Sci. 2007;11:489–497. doi: 10.1016/j.tics.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 51.Gaffrey MS, Barch DM, Singer J, Shenoy R, Luby JL. Disrupted amygdala reactivity in depressed 4- to 6-year-old children. J Am Acad Child Adolesc Psychiatry. 2013;52:737–746. doi: 10.1016/j.jaac.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Luking KR, Pagliaccio D, Luby JL, Barch DM. Depression Risk Predicts Blunted Neural Responses to Gains and Enhanced Responses to Losses in Healthy Children. J Am Acad Child Adolesc Psychiatry. 2016;55:328–337. doi: 10.1016/j.jaac.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Siegle GJ, Steinhauer SR, Thase ME, Stenger VA, Carter CS. Can’t shake that feeling: event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biol Psychiatry. 2002;51:693–707. doi: 10.1016/s0006-3223(02)01314-8. [DOI] [PubMed] [Google Scholar]
- 54.McClernon FJ, Hiott FB, Huettel SA, Rose JE. Abstinence-induced changes in self-report craving correlate with event-related FMRI responses to smoking cues. Neuropsychopharmacology. 2005;30:1940–1947. doi: 10.1038/sj.npp.1300780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Anticevic A, Repovs G, Corlett PR, Barch DM. Negative and nonemotional interference with visual working memory in schizophrenia. Biol Psychiatry. 2011;70:1159–1168. doi: 10.1016/j.biopsych.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 56.Hayes AF. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. New York: The Guilford Press; 2013. [Google Scholar]
- 57.Cunningham WA, Borsch T. Motivational Salience: Amygdala tuning from traits, needs, values, and goals. Current Directions in Psychological Science. 2012;21:54–59. [Google Scholar]
- 58.Gunnar M, Quevedo K. The neurobiology of stress and development. Annu Rev Psychol. 2007;58:145–173. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- 59.McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- 60.Bennur S, Shankaranarayana Rao BS, Pawlak R, Strickland S, McEwen BS, Chattarji S. Stress-induced spine loss in the medial amygdala is mediated by tissue-plasminogen activator. Neuroscience. 2007;144:8–16. doi: 10.1016/j.neuroscience.2006.08.075. [DOI] [PubMed] [Google Scholar]
- 61.Bromberg-Martin ES, Matsumoto M, Hikosaka O. Dopamine in motivational control: rewarding, aversive, and alerting. Neuron. 2010;68:815–834. doi: 10.1016/j.neuron.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ellis BJ, Del Giudice M. Beyond allostatic load: rethinking the role of stress in regulating human development. Dev Psychopathol. 2014;26:1–20. doi: 10.1017/S0954579413000849. [DOI] [PubMed] [Google Scholar]
- 63.Haber SN, Behrens TE. The neural network underlying incentive-based learning: implications for interpreting circuit disruptions in psychiatric disorders. Neuron. 2014;83:1019–1039. doi: 10.1016/j.neuron.2014.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Briggs-Gowan MJ, Nichols SR, Voss J, Zobel E, Carter AS, McCarthy KJ, et al. Punishment insensitivity and impaired reinforcement learning in preschoolers. J Child Psychol Psychiatry. 2014;55:154–161. doi: 10.1111/jcpp.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chelonis JJ, Gravelin CR, Paule MG. Assessing motivation in children using a progressive ratio task. Behav Processes. 2011;87:203–209. doi: 10.1016/j.beproc.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 66.Winter W, Sheridan M. Previous reward decreases errors of commission on later ‘No-Go’ trials in children 4 to 12 years of age: evidence for a context monitoring account. Dev Sci. 2014;17:797–807. doi: 10.1111/desc.12168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bouwmeester H, Gerrits MA, Roozemond JG, Snapper J, Ronken E, Kruse CG, et al. Neonatal basolateral amygdala lesions affect monoamine and cannabinoid brain systems in adult rats. Int J Neuropsychopharmacol. 2007;10:727–739. doi: 10.1017/S1461145706007346. [DOI] [PubMed] [Google Scholar]
- 68.Porcelli AJ, Lewis AH, Delgado MR. Acute stress influences neural circuits of reward processing. Front Neurosci. 2012;6:157. doi: 10.3389/fnins.2012.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kumar P, Berghorst LH, Nickerson LD, Dutra SJ, Goer FK, Greve DN, et al. Differential effects of acute stress on anticipatory and consummatory phases of reward processing. Neuroscience. 2014;266:1–12. doi: 10.1016/j.neuroscience.2014.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fareri DS, Gabard-Durnam L, Goff B, Flannery J, Gee DG, Lumian DS, et al. Normative development of ventral striatal resting state connectivity in humans. Neuroimage. 2015;118:422–437. doi: 10.1016/j.neuroimage.2015.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Delgado MR. Reward-related responses in the human striatum. Ann N Y Acad Sci. 2007;1104:70–88. doi: 10.1196/annals.1390.002. [DOI] [PubMed] [Google Scholar]
- 72.Neville H, Stevens C, Pakulak E, Bell TA. Commentary: neurocognitive consequences of socioeconomic disparities. Dev Sci. 2013;16:708–712. doi: 10.1111/desc.12081. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.