Abstract
Meningiomas frequently display activation of the PI3K/AKT/mTOR pathway, leading to elevated levels of phospho-4E binding proteins, which enhances protein synthesis; however, it is not known whether inhibition of protein translation is an effective treatment option for meningiomas. We found that human meningiomas expressed high levels of the three components of the eukaryotic initiation factor 4F (eIF4F) translation initiation complex, eIF4A, eIF4E, and eIF4G. The expression of eIF4A and eIF4E was important in sustaining the growth of NF2-deficient benign meningioma Ben-Men-1 cells, as shRNA-mediated knockdown of these proteins strongly reduced cell proliferation. Among a series of 23 natural compounds evaluated, silvestrol, which inhibits eIF4A, was identified as being the most growth inhibitory in both primary meningioma and Ben-Men-1 cells. Silvestrol treatment of meningioma cells prominently induced G2/M arrest. Consistently, silvestrol significantly decreased the amounts of cyclins D1, E1, A, and B, PCNA and Aurora A. In addition, total and phosphorylated AKT, ERK and FAK, which have been shown to be important drivers for meningioma cell proliferation, were markedly lower in silvestrol-treated Ben-Men-1 cells. Our findings suggest that inhibiting protein translation could be a potential treatment for meningiomas.
Keywords: Protein translation, eIF4F, eIF4A, eIF4E, eIF4G, meningioma, neurofibromatosis type 2 (NF2), merlin, silvestrol
1. Introduction
Meningiomas, accounting for over one third of all primary brain tumors, cause significant morbidity, including cranial nerve palsy, seizures, and brainstem compression, which may lead to paralysis, aspiration pneumonia, and death (Ostrom et al., 2015). Current treatment options for these tumors are limited to surgery and radiation. However, incomplete tumor resection is not uncommon and is one of the main causes of tumor recurrence (Goldbrunner et al., 2016). Even after total gross resection, the 5-year recurrence rate is about 7–23% (Rogers et al., 2015). Meningiomas can occur sporadically or in patients with neurofibromatosis 2 (NF2), which is caused by the inactivation of NF2/merlin tumor suppressor function (Rouleau et al., 1993; Trofatter et al., 1993). Importantly, patients with NF2 are at increased risk for multiple meningiomas, which often requires them to undergo several arduous treatment cycles. Development of an effective systemic medical therapy for meningiomas is therefore of urgent clinical need.
In addition to NF2-assocaited meningiomas, about 50% of sporadic tumors also harbor NF2 mutations. Loss of merlin abnormally activates several mitogenic signals, such as the PI3K/AKT/mTOR pathway (Ammoun et al., 2008; Jacob et al., 2008). Indeed, meningiomas often exhibit elevated levels of phospho-AKT (p-AKT), which can promote protein biosynthesis by stimulating p70 S6-kinase, leading to phosphorylation of the S6 ribosomal protein (Sorrells et al., 1999; De Benedetti and Graff, 2004; Holland et al., 2004; Anjum and Blenis, 2008; Menon and Manning, 2008; Kleiner et al., 2009; Ma and Blenis, 2009; Silvera et al., 2010; Li et al., 2012). Other important signaling proteins downstream of the PI3K/AKT/mTOR pathway include the 4E-binding protein (4E-BP) translational repressors; upon phosphorylation, these proteins are inactivated and protein biosynthesis is facilitated. Consistent with this notion, meningiomas display high p-4E-BP levels (Pachow et al., 2013), suggesting that protein translation initiation is likely enhanced in these tumors.
Initiation of protein translation is strictly-controlled and occurs when the eukaryotic initiation factor 4F (eIF4F) complex is recruited to the 5′ cap structure of mRNA, followed by the unwinding of the secondary structure of its 5′ untranslated region (Jackson et al., 2010; Silvera et al., 2010; Blagden and Willis, 2011). The eIF4F complex is composed of three components: eIF4E binds the 5′ cap; the eIF4A RNA helicase unwinds the secondary structure; and eIF4G is a scaffold for other eIFs and enhances the eIF4A helicase activity. Studies have shown that the 4E-BP proteins repress translation by binding to eIF4G and preventing its association with eIF4E. Phosphorylation of 4E-BPs causes these proteins to dissociate from eIF4G, thus promoting eIF4F assembly. The effective formation of the eIF4F complex may require sufficient levels of each eIF4F component.
As uncontrolled growth of tumor cells often requires a high degree of protein translation, increased expression of the eIF4F components has been reported in several cancer types (Sorrells et al., 1999; De Benedetti and Graff, 2004; Kleiner et al., 2009; Silvera et al., 2010; Li et al., 2012). Overexpression of eIF4E promotes cell transformation (Lazaris-Karatzas et al., 1990) and frequently correlates with high tumor grade and poor patient prognosis (Li et al., 1997; Berkel et al., 2001; Li et al., 2012). While the oncogenicity of eIF4A and eIF4G has not been as well characterized as for eIF4E, elevated eIF4A and eIF4G levels have been reported in hepatocellular and lung carcinomas, respectively (Shuda et al., 2000; Bauer et al., 2001; Comtesse et al., 2007). Also, forced overexpression of eIF4A enhances malignant progression in an acute lymphocytic leukemia mouse model (Wolfe et al., 2014). Furthermore, enhanced protein translation has been described in a spectrum of neurological disorders, including autism spectrum disorders and fragile X syndrome (Silvera et al., 2010; Gkogkas et al., 2013; Gkogkas et al., 2014), suggesting that targeting translation initiation may have therapeutic potential. Recently, we reported elevated expression of all three eIF4F components in NF2-deficient vestibular schwannomas, and depletion of these eIF4F components reduced schwannoma cell growth (Oblinger et al., 2016). However, the protein levels of eIF4A, eIF4E, and eIF4G have not been rigorously explored in meningiomas.
In this study, we used meningioma tumors, primary meningioma cell cultures, and the benign NF2-deficient meningioma Ben-Men-1 cell line to demonstrate that meningiomas overexpress all three eIF4F components. Overexpression of these components is an important driver of meningioma cell proliferation, as confirmed by shRNA-mediated knockdown and pharmacological inhibition. Our results suggest that inhibition of translation initiation factors should be further evaluated as a potential treatment for these tumors.
2. Materials and Methods
2.1. Tissue acquisition and cell cultures
The Ohio State University (OSU) Institutional Review Board (IRB) approved the human subjects protocols for the acquisition of meningioma specimens, the diagnosis of which was confirmed by a pathologist. Fresh meningioma tissues were finely minced and digested with collagenase/dispase at 37°C overnight as previously described (Chang and Welling, 2009). Primary meningioma cells, normal human meningeal cells (ScienCell), and NF2-deficient benign meningioma Ben-Men-1 cells were grown in Dulbecco’s modified Eagle medium (DMEM) and 10% fetal bovine serum (FBS) (Thermo Fisher) as described previously (Burns et al., 2013). All primary cell cultures were used at early passages (less than five passages).
2.2. Natural compound treatment, cell proliferation assays, and flow cytometry
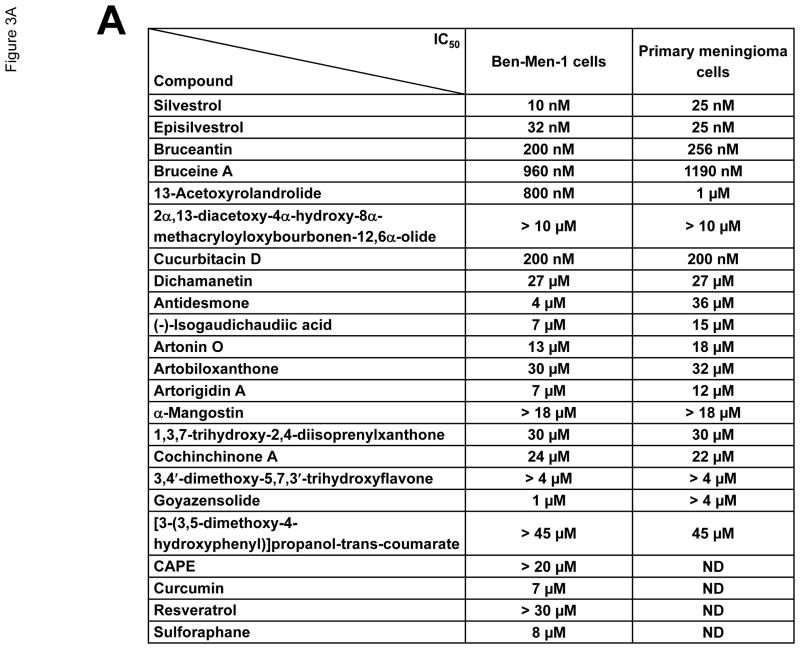
A series of 23 natural compounds, including silvestrol (Figure 3A), was isolated and their structures and absolute configurations were previously reported (Kinghorn et al., 2011; Oblinger et al., 2016). Purified compounds were dissolved in dimethyl sulfoxide (DMSO) to 1~10 mM and then diluted for cell proliferation assays. Briefly, Ben-Men-1 and primary meningioma cells were seeded in 96-well plates at 4,000 cells/well and treated the next day with various concentrations of each natural compound or DMSO as a control. After three days, cell proliferation was assessed using resazurin, and the IC50 values were determined (Burns et al., 2013).
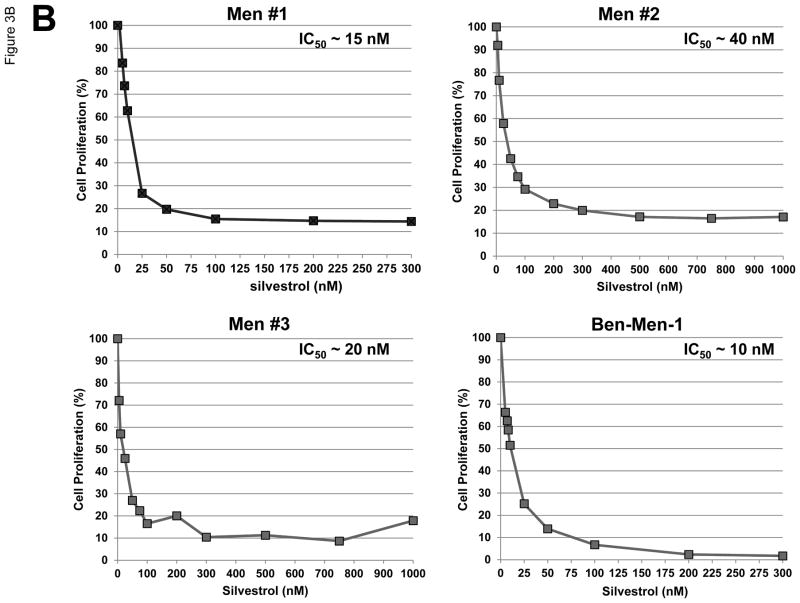
Figure 3.
Silvestrol potently inhibited proliferation of meningioma cells. (A) The natural compounds used in this study and their IC50 values in Ben-Men-1 and primary meningioma cells are summarized. Data shown are the mean IC50 values from three independent experiments. (B) Resazurin assays for cell proliferation were performed on primary meningioma cells and Ben-Men-1 cells treated with various concentrations of silvestrol for three days. Primary meningioma cells were derived from three separate tumors (Men #1~3) and assays were performed in six replicate wells; the IC50 values for each experiment are shown in the graph insets. The mean IC50 value for primary meningioma cells across the three experiments was ~25 nM. Experiments on Ben-Men-1 cells were performed in six replicates, and the experiments were independently repeated three times. Data shown are the mean of the six replicate wells from one representative experiment. The mean IC50 value for Ben-Men-1 cells across all three experiments was ~10 nM.
Cell cycle analysis of drug-treated Ben-Men-1 cells was performed as previously described (Oblinger et al., 2016). A FACSCalibur flow cytometer (Becton Dickinson) was used to analyze samples after gating around the diploid population (FL2-A/FL2-W). Histograms and cell cycle distribution were determined using ModFit LT software (Verity Software House).
2.3. Western blots
Subconfluent Ben-Men-1 cells were treated with the indicated concentrations of silvestrol or DMSO vehicle for 24 hours, followed by cell lysis and Western blotting according to Oblinger et al. (Oblinger et al., 2016). The antibodies used included anti-eIF4A1 (2490), AKT (9272), phospho-AKT [p-AKT(Ser473)] (4060), extracellular signal-regulated kinases 1 and 2 (ERK1/2; 4695), p-ERK (4370), proline-rich Akt substrate of 40 kDa (PRAS40; 2691), p-PRAS40(Thr246) (2997), focal adhesion kinase (FAK; 3285), cyclin B1 (4138), cyclin D1 (2978), cyclin E1 (4129), glyceraldehyde 3-phosphate dehydrogenase (GAPDH; 5174), merlin (12888) (all from Cell Signaling Technology), proliferating cell nuclear antigen (PCNA; sc-56), cyclin A (sc-751), eIF4A2 (sc-137148), and eIF4G (sc-133155) (all from Santa Cruz Biotechnology), p-FAK(Tyr397) (700255 from Thermo Fisher), and eIF4E (ab33768 from Abcam). Protein bands were detected using an HRP-conjugated secondary antibody and the ECL Plus Western Blotting Substrate (Thermo Fisher) or a fluorescently-labeled secondary antibody and the Odyssey CLx imaging system (LI-COR Biosciences).
2.4. Lentiviral-mediated short-hairpin RNA (shRNA) transduction
Ben-Men-1 cells were seeded in 6-well plates at 9,000 cells/well. The next day, cells were fed with fresh DMEM/10% FBS containing 8 μg/mL polybrene and then incubated overnight with MISSION shRNA lentiviruses targeting EIF4A1 (TRCN0000288729; target sequence 5′-GCCGTAAAGGTGTGGCTATTA-3′), EIF4A2 (TRCN0000051869; target sequence 5′-CGGGAGAGTGTTTGATATGTT-3′), EIF4E (TRCN0000062573; target sequence 5′-CCACTCTGTAATAGTTCAGTA-3′) or shNon-targeting lentivirus (#SHC002V, insert sequence 5′-CAACAAGATGAAGAGCACCAA-3′; all from Sigma) at a multiplicity of infection (MOI) of 10. Two days following transduction, puromycin (Corning) was added to the culture medium to a final concentration of 2.5 μg/mL. Cells were grown for another 8 days before counting on a hemocytometer. Subsequently, the cells were centrifuged, and the resulting pellets were lysed in 2% SDS with protease and phosphatase inhibitors (Sigma). Equal amounts of protein were analyzed by Western blot for eIF4A1, eIF4A2, eIF4E, and GAPDH (Oblinger et al., 2016). Fluorescently-labeled protein bands were captured on an Odyssey CLx Imager (LI-COR BioSciences), quantitated using Image Studio, and calculated as percent of the nontargeting control after normalization to GAPDH.
2.5. Immunohistochemistry (IHC)
Archived formalin-fixed, paraffin-embedded sections of human meningioma tissues were acquired, deparaffinized, and immunostained for eIF4AI-II (sc-50354) and eIF4G (sc-133155) (both from Santa Cruz) and eIF4E (ab33768 from Abcam) as previously described (Burns et al., 2013). Quantification of immunostaining signal was performed by a pathologist and an investigator using visual analysis based on the intensity and percentage of immunopositive cells.
3. Results
3.1. Meningiomas expressed elevated levels of eIF4A, eIF4E, and eIF4G compared to normal meningeal cells
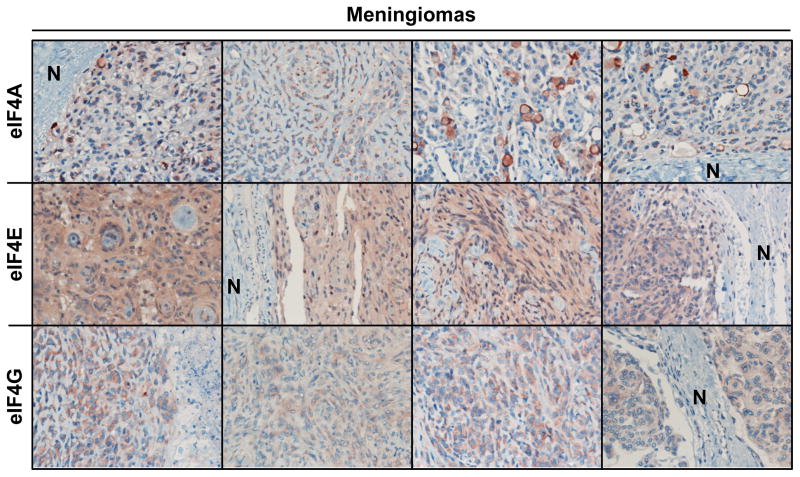
To determine the expression levels of the eIF4F components, we performed immunohistochemical staining for eIF4A, eIF4E and eIF4G on 16 meningiomas, including six grade I sporadic tumors, four grade I NF2-associated tumors, five grade II sporadic tumors, and one grade III sporadic tumor. All of these meningiomas showed more intense labeling of eIF4F components compared to adjacent normal meningeal tissues (Figure 1 and Table 1). Fifty percent of the meningioma specimens (8/16) were scored as having 1+ or greater labeling for eIF4A, 94% of the tumors (15/16) were scored as having 1+ or greater labeling for eIF4E, and 75% percent of the tumors (12/16) were scored as having 1+ or greater labeling for eIF4G. Notably, all four NF2-associated tumors had intense staining for eIF4A and eIF4E (Table 1).
Figure 1.
Meningioma tissues overexpressed all three components of the eIF4F complex. Shown are representative images of meningioma sections immunostained for eIF4A, eIF4E and eIF4G. “N” indicates adjacent normal meninges. The staining scores for all tumors are reported in Table 1.
Table 1. Clinicopathological information of meningiomas and their relative IHC staining signals for eIF4A, eIF4E, and eIF4G.
Meningioma tumor sections were processed for immunohistochemistry analysis as described in the Materials and Methods. Immunostaining signals were quantified using visual analysis based on the intensity and percentage of immunopositive cells (0 as negative, 0.5 = weak positive, 1 = moderate positive, 2 = strong positive, and 3 = very strong positive). Adjacent normal meningeal cells showed little to no staining and were scored as 0 (Figure 1).
| Tumor ID. | Gender | Tumor grade | Histological type | Clinical diagnosis | Tumor size (cm) | IHC staining signals
|
||
|---|---|---|---|---|---|---|---|---|
| eIF4A | eIF4E | eIF4G | ||||||
| Men01 | F | WHO grade I | Meningothelial | Sporadic | 3.3 × 2.7 × 1.6 | 0.5+ | 1+ | 0 ~ 0.5+ |
| Men02 | F | WHO grade I | Meningothelial | Sporadic | 2.0 × 1.0 × 1.0 | 1 ~ 2+ | 0.5+ | 1 ~ 2+ |
| Men03 | F | WHO grade I | Fibrous | Sporadic | 3.2 × 2.6 x1.3 | 0.5+ | 2+ | 0.5 ~ 1+ |
| Men04 | F | WHO grade I | Meningothelial | Sporadic | 2.5 × 2.0 × 1.8 | 0.5+ | 2+ | 1 ~ 1.5+ |
| Men05 | N/A* | WHO grade I | Meningothelial | Sporadic | 2.5 × 1.5 × 1.0 | 1 ~ 2+ | 1 ~ 2+ | 1 ~ 2+ |
| Men06 | F | WHO grade I | Meningothelial | Sporadic | 2.5 × 1.8 × 0.9 | 0.5+ | 2+ | 0.5 ~ 1+ |
| Men07 | M | WHO grade I | Meningothelial | NF2 | 2.0 × 1.2 × 0.8 | 1 ~ 3+ | 2 ~ 3+ | 2 ~3+ |
| Men08 | F | WHO grade I | Meningothelial | NF2 | 3.4 × 2.8 × 1.7 | 0.5 ~ 1+ | 1 ~ 2+ | 0.5+ |
| Men09 | M | WHO grade I | Meningothelial | NF2 | 3.2 × 2.9 × 3.4 | 1 ~ 3+ | 1 ~ 2+ | 0.5+ |
| Men10 | M | WHO grade I | Meningothelial | NF2 | 2.0 × 2.2 × 2.5 | 2 ~ 3+ | 1 ~ 2+ | 0.5+ |
| Men11 | F | WHO grade II | Atypical | Sporadic | 3.2 × 1.4 × 2.1 | 0.5 ~ 1+ | 1 ~ 3+ | 1 ~ 2+ |
| Men12 | F | WHO grade II | Atypical | Sporadic | 9.0 × 4.0 × 3.0 | 0.5+ | 1 ~ 1.5+ | 1 ~ 2+ |
| Men13 | F | WHO grade II | Atypical | Sporadic | 3.0 × 2.5 × 1.1 | 0.5+ | 2+ | 1+ |
| Men14 | M | WHO grade II | Atypical | Sporadic | 1.5 × 1.0 × 0.8 | 0.5+ | 0.5 ~ 1+ | 0.5 ~ 1+ |
| Men15 | F | WHO grade II | Chordoid | Sporadic | 6.5 × 6.0 × 3.0 | 0.5 ~ 1+ | 1+ | 1+ |
| Men16 | M | WHO grade III | Anaplastic | Sporadic | 5.8 × 4.0 × 3.1 | 0.5+ | 1+ | 1 ~ 1.5+ |
N/A, not available.
The RNA helicase eIF4A had prominent perinuclear staining, consistent with its role in translation initiation and localization to the rough endoplasmic reticulum. Staining for eIF4E occurred more diffusely throughout the cytoplasm, with faint nuclear labeling (Rosenwald et al., 1995; Culjkovic et al., 2008). The labeling pattern for eIF4G was diffusely cytoplasmic, with some conspicuous rimming around the nucleus that resembled the perinuclear concentrations observed with eIF4A. While the sample size was limited, the six high grade meningiomas (five grade II and one grade III) tended to show higher labeling in eIF4G, when compared to grade I tumors (Table 1).
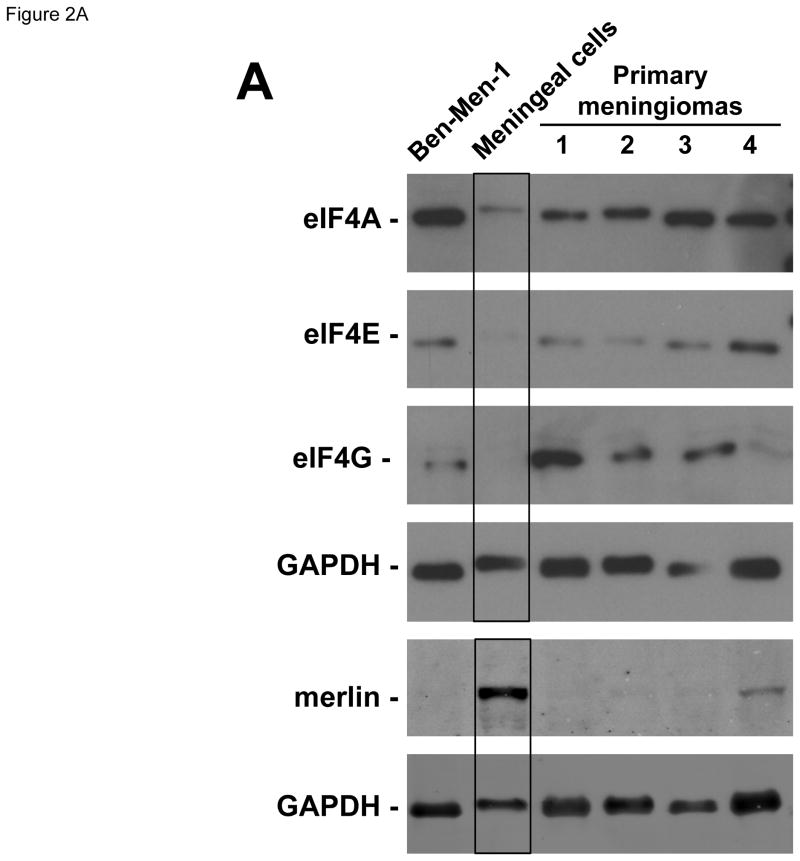
To confirm these findings, we compared eIF4A, eIF4E and eIF4G protein expression in four primary grade I meningioma cultures prepared from two sporadic (samples #1 and 2 in Figure 2A) and two NF2-associated tumors (samples #3 and 4) and the telomerase-immortalized Ben-Men-1 benign meningioma line with those in primary meningeal cells. All four primary cultures of meningioma cells and Ben-Men-1 cells uniformly exhibited higher levels of all three eIF4F components relative to normal meningeal cells (Figure 2A). As previously reported (Burns et al., 2013), Ben-Men-1 cells did not express merlin protein. In addition, merlin was not detected in three of the four primary meningioma cultures and was greatly reduced in the fourth meningioma culture. Collectively, these results indicate increased expression of eIF4A, eIF4E and eIF4G in meningiomas.
Figure 2.
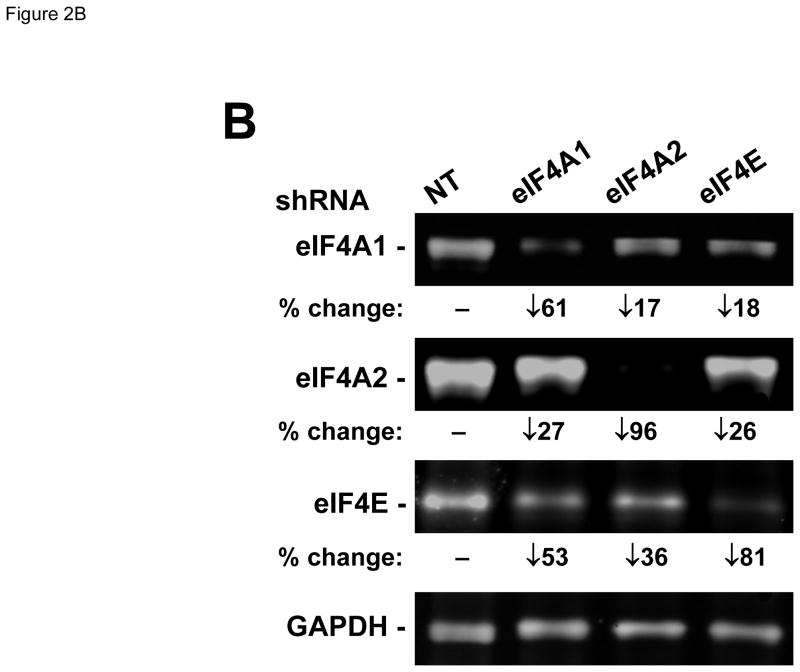
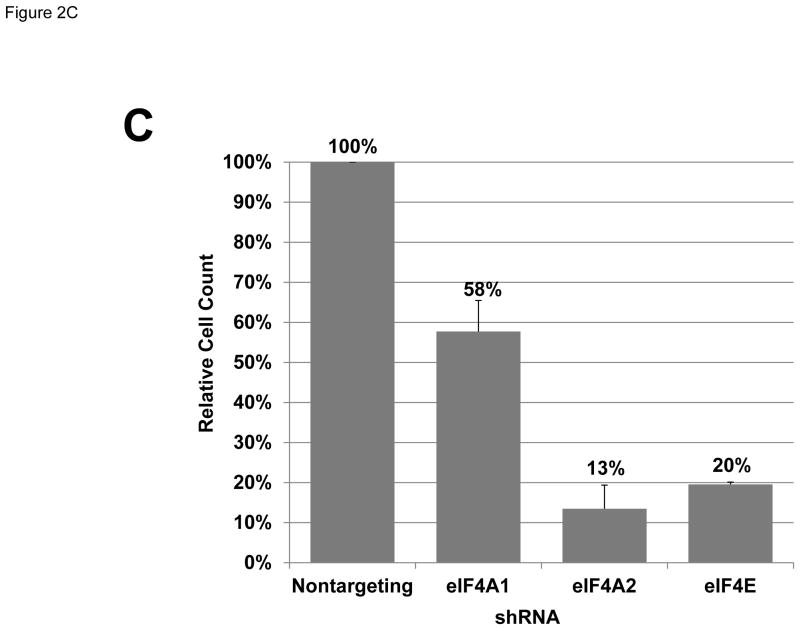
Meningioma cells expressed higher levels of eIF4F components. (A) Overexpression of eIF4F components in primary meningioma cells and the Ben-Men-1 benign meningioma cell line. Total cell lysates from four primary cultures of meningioma cells, normal human meningeal cells, and Ben-Men-1 cells were resolved by SDS-PAGE and probed for eIF4E, eIF4A, eIF4G, merlin, and GAPDH (loading control). The four meningiomas used to prepare primary cell cultures included two sporadic (#1 and #2) and two NF2-associated (#3 and #4) grade I tumors. (B) Silencing each indicated eIF4F component by shRNA. Ben-Men-1 cells were transduced with 10 MOI of lentiviruses expressing the indicated shRNAs (Materials and Methods). After 8 days, cells were lysed and analyzed by Western blotting for eIF4A1, eIF4A2, eIF4E, and GAPDH. Fluorescently-labeled protein bands were detected on the Odyssey CLx and quantitated in Image Studio. Shown below the blots is the % decrease in the eIF4A1, eIF4A2, or eIF4E protein level in shRNA-transduced cells relative to the nontargeting (NT) control. (C) Suppression of meningioma cell growth following depletion of eIF4A or eIF4E. Ben-Men-1 cells transduced as described in (B) were counted. Shown are the means and standard deviations (SDs) from two independent experiments run in technical duplicates.
3.2. Inhibition of eIF4A and eIF4E impaired meningioma cell proliferation
To verify the importance of eIF4F components in meningioma cell growth, we used shRNA-containing lentiviruses to silence eIF4A and eIF4E expression in Ben-Men-1 cells. Consistent with the knockdown efficiencies (Figure 2B), depletion of eIF4A2 or eIF4E dramatically reduced cell numbers by about 87% and 80%, respectively, relative to control cells that were transduced with a nontargeting shRNA construct (Figure 2C). Silencing eIF4A1 gave rise to partial growth suppression, which was commensurate with a moderate knockdown efficiency (Figures 2B and 2C). These results indicate that silencing of either eIF4A1 or eIF4A2 impairs meningioma cell proliferation. We also noted that knockdown of any individual eIF resulted in modest decreases in the other eIF components. In these conditions, the levels of eIF4E seemed to be particularly sensitive, decreasing by 53% and 36% after depletion of eIF4A1 or eIF4A2, respectively.
As an FDA-approved medical therapy is currently not available for the treatment of meningiomas, identification of new therapeutics for patients with meningiomas is of great clinical interest. The growth inhibition observed from the eIF4A and eIF4E RNA interference experiments (Figures 2B and 2C) suggested that meningioma cells may be susceptible to pharmacological inhibition of these eIF4F components. Interestingly, we found that among a series of botanical compounds evaluated, the eIF4A inhibitors silvestrol and episilvestrol, an isomer of silvestrol, were the most potent in inhibiting proliferation of Ben-Men-1 cells and primary meningioma cells based on the IC50 values and maximal killing effects (Figure 3A). Silvestrol consistently inhibited meningioma cell proliferation at very low IC50 values (~10 nM in Ben-Men-1 and ~25 nM in primary meningioma cultures) (Figures 3A and 3B). These results highlight the importance of eIF4F components in the growth of meningioma cells.
3.3. Silvestrol treatment induced G2/M arrest and reduced the levels of several cell-cycle and mitogenic proteins
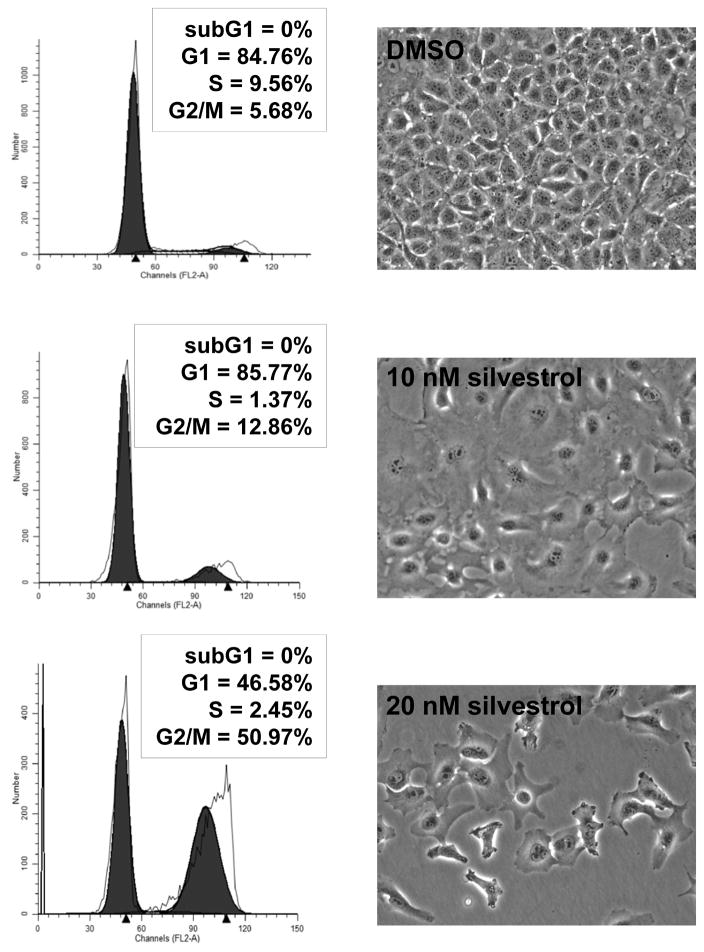
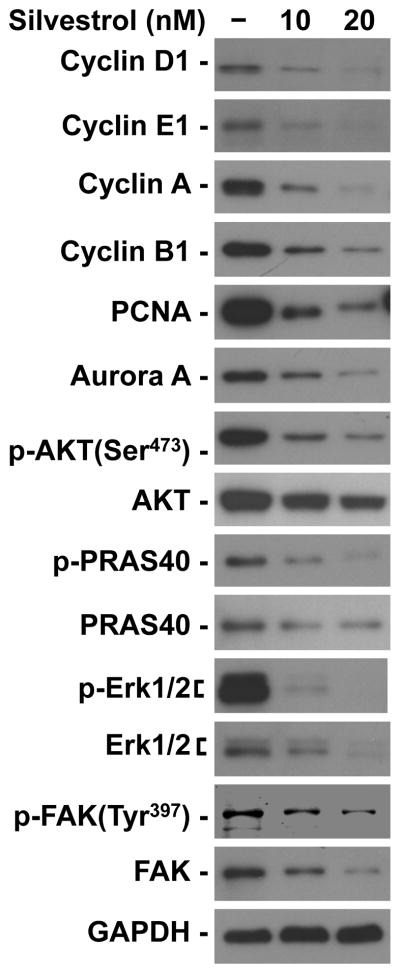
Consistent with previous reports (Mi et al., 2006; Oblinger et al., 2016), silvestrol induced cell cycle arrest at the G2/M phase. Ben-Men-1 cells treated with the IC50 dose of silvestrol for three days had an over two-fold increase in the proportion of cells in G2/M (untreated, 5.68% versus silvestrol-treated, 12.86%; Figure 4). This effect became even more pronounced at twice the IC50 dose, in which over half the cell population was in the G2/M fraction. To further examine the effects of silvestrol on the cell cycle, we profiled the expression of various cell cycle proteins. Ben-Men-1 cells treated with silvestrol exhibited sharp reductions in all examined cyclins (D1, E1, A, and B1) as well as the mitotic Aurora A kinase (Figure 5). PCNA, which acts during S phase to facilitate DNA synthesis, was also markedly reduced. This is consistent with the finding that silvestrol treatment of Ben-Men-1 cells reduced the S phase fraction (Figure 4).
Figure 4.
Silvestrol induced G2/M arrest in meningioma cells. Ben-Men-1 cells were treated with the indicated concentrations of silvestrol for three days, and phase contrast images of treated cells were taken, followed by cell harvesting for flow cytometry analysis as described in the Materials and Methods. Cell cycle histograms of propidium iodide-labeled cells revealed a prominent increase in the G2/M peak after silvestrol treatment.
Figure 5.
Silvestrol suppressed the expression of multiple cell cycle proteins and mitogenic kinases. Ben-Men-1 cells were treated with 1x and 2x the IC50 dose of silvestrol for 24 hours followed by cell lysis and Western blotting for the indicated proteins. GAPDH served as a loading control.
NF2-deficient tumors often exhibit increased phosphorylation of the AKT, ERK 1/2, and FAK kinases (Poulikakos et al., 2006; Jacob et al., 2008; Hilton et al., 2009; Endo et al., 2013; Ammoun et al., 2014), and activation of these kinases can lead to enhanced protein biosynthesis (Silvera et al., 2010). We found that silvestrol treatment decreased both the total and phosphorylated AKT as well as its downstream substrate PRAS40 in Ben-Men-1 cells (Figure 5). Likewise, both the total and phosphorylated ERK1/2 and FAK were suppressed in silvestrol-treated meningioma cells. Taken together, these results suggest that silvestrol exerts its antiproliferative action by simultaneously reducing multiple pro-growth signaling molecules.
4. Discussion
Despite being the most frequent brain tumors, the biology of meningiomas is not well understood and an FDA-approved medical therapy is currently not available for these tumors. While alterations in the NF2 gene frequently occur, recent molecular genetic studies have identified mutations in AKT1, SMO, KLF4, TRAF7, POLR2A in non-NF2 meningiomas (Brastianos et al., 2013; Clark et al., 2013; Clark et al., 2016). In addition, gene expression analyses have identified AKT as one of the key drivers for meningioma growth (Wang et al., 2012; Hilton et al., 2016). Activation of AKT promotes protein translation initiation, which is dependent upon expression and assembly of several eIF complexes (Jackson et al., 2010; Silvera et al., 2010). Intriguingly, high levels of eIF4E correlate with meningioma grade (Tejada et al., 2009). Meningiomas lacking NF2 frequently have more abundant eIF3C than NF2-expressing tumors (Scoles et al., 2006). We now report that all three components of the eIF4F complex are overexpressed in meningiomas. While the number of meningiomas that we analyzed is small, the NF2-associated grade I meningiomas that we analyzed exhibited high levels of these proteins, particularly eIF4A and eIF4E, when compared to sporadic grade I tumors (Table 1 and Figure 2A). Similarly, we recently reported that NF2-associated vestibular schwannomas also appeared to have more eIF4F components (Oblinger et al., 2016). In addition, we observed that the six high grade (II and III) tumors expressed higher amounts of eIF4E and eIF4G, compared to sporadic grade I tumors (Table 1). In line with this tumor study, our primary cultures of meningioma cells also showed elevated levels of eIF4F components compared with normal meningeal cells. It would be interesting to extend our study to a larger cohort of tumors to see if these findings can be confirmed and if they correlate with any clinical outcome parameters.
Both RNA silencing and pharmacological inhibition verified that these eIF4F components are critical for meningioma cell growth. Using shRNA-mediated knockdown in Ben-Men-1 cells, we found that silencing eIF4A1, eIF4A2, or eIF4E was sufficient to inhibit cell growth. Curiously, eIF4A2 appeared to be as important for proliferation as eIF4A1 since eIF4A2 knockdown profoundly impaired proliferation of meningioma cells (Figure 2C). Previously, we observed similar results in malignant peripheral nerve sheath tumor (MPNST) cells (Oblinger et al., 2016). However, the role of eIF4A2 on cell proliferation may depend upon the cell type (Galicia-Vázquez et al., 2012). In a similar context, it would be also interesting to investigate the role of eIF4G in meningioma cell growth. As isoforms exist in eIF4G (Silvera et al., 2010), the contribution of each isoform should be evaluated as we did with eIF4A1 and eIF4A2. Unexpectedly, we did not observe that depletion of eIF4A1, eIF4A2, or eIF4E resulted in a compensatory increase of the other two eIF4F components, but instead, we saw modest decreases in the levels of these components (Figure 2B). Our previous study in MPNST cells also showed reduced eIF4E levels following knockdown of eIF4A1 or eIF4A2 protein (Oblinger et al., 2016), suggesting coordinate regulation of these eIF4F components.
The importance of eIF4A activity for meningioma cell proliferation was also validated by the identification of the eIF4A inhibitor silvestrol as being profoundly growth inhibitory in both primary meningioma and Ben-Men-1 cells. Consistent with previous reports (Mi et al., 2006; Oblinger et al., 2016), silvestrol treatment resulted in a prominent G2/M arrest in meningioma cells (Figure 4). Cyclin levels are induced during specific phases of the cell cycle and are well-known for having a fast protein turnover rate (Pines, 1996). While tumor cells frequently exhibit abnormal G1 checkpoint, sharp declines in multiple cyclins following silvestrol treatment render them liable to G2/M arrest. Additionally, the pro-growth signaling molecules AKT, ERK1/2 and FAK are frequently phosphorylated and activated in NF2-related tumor cells (Poulikakos et al., 2006; Jacob et al., 2008). Reducing the levels of these mitogenic signals would be expected to reinforce silvestrol’s growth inhibitory properties.
With its high potency in several tumor types, silvestrol has been rigorously investigated as a potential cancer therapeutic (Pan et al., 2014). While it is well tolerated in mice, studies are ongoing to determine dosing schedules and toxicity in large animals. We have demonstrated that silvestrol exhibits consistent strong suppression of the growth of meningioma cells in culture (Figure 3). Previous pharmacokinetic analysis showed that silvestrol requires intraperitoneal or intravenous delivery for maximal bioavailability (Saradhi et al., 2011). However, even with these delivery methods, the distribution to the brain is relatively low, suggesting that silvestrol may not readily cross the blood-brain barrier. Silvestrol has a bulky sugar-like dioxanyl ring, which has been shown to confer susceptibility to the multi-drug resistance protein 1 (MDR1) transporter (Gupta et al., 2011). This characteristic may limit the distribution of silvestrol to the brain. Intriguingly, rocaglaol, a silvestrol-related compound lacking this ring, possesses antitumor activity and a synthetic analog of rocaglaol displays activity in drug-resistant breast carcinoma cells that overexpress MDR1 (Mi et al., 2006; Pan et al., 2013). The finding that the growth-suppressive effects of rocaglaol are also mediated by inhibiting protein translation indicates that the dioxanyl ring of silvestrol is dispensable for eIF4A inhibition (Ohse et al., 1996). Moreover, rocaglaol analogs may exhibit cardioprotective and/or neuroprotective effects (Bernard et al., 2011; Thuaud et al., 2011). A halogenated rocaglaol derivative has been shown to be capable of crossing the blood-brain barrier (Fahrig et al., 2005; Thuaud et al., 2011). In addition, our preliminary study has identified several silvestrol-related rocaglates that lack the dioxanyl ring but exhibit potent growth-inhibitory activity in meningioma cells (data not shown). Nevertheless, it should be noted that meningiomas may disrupt the blood-brain barrier. Experiments are in progress to evaluate the anti-tumor effects of silvestrol and a series of silvestrol-related rocaglates lacking this dioxanyl ring in an orthotopic mouse model for meningioma (Burns et al., 2013). Identification of an effective treatment for meningioma would significantly advance our efforts to improve clinical care and long-term treatment outcomes for these patients.
Acknowledgments
We sincerely thank Drs. Aaron Moberly and Oliver Adunka for meningioma specimens and the OSU Cooperative Human Tissue Network and Tissue Archives for tissue sections. This study was supported by grants from the US Department of Defense, Advocure NF2, the Galloway family, Meningioma Mommas, and CancerFree Kids to LSC and from the National Cancer Institute to ADK (P01 CA125066) and to the OSU Comprehensive Cancer Center (P30 CA16058).
Footnotes
Conflict of interest statement
The authors declare no conflict of interest.
Authorship
Conception and design of the study: JLO, SSB, and LSC.
Performance of experiments: JLO, SSB, JH, LP, YR, ADK, and LSC
Analysis and interpretation of the data: JLO, SSB, RS, DBW, and LSC.
Manuscript writing: JLO and LSC.
References
- Ammoun S, Flaiz C, Ristic N, Schuldt J, Hanemann CO. Dissecting and targeting the growth factor-dependent and growth factor-independent extracellular signal-regulated kinase pathway in human schwannoma. Cancer Res. 2008;68:5236–5245. doi: 10.1158/0008-5472.CAN-07-5849. [DOI] [PubMed] [Google Scholar]
- Ammoun S, Provenzano L, Zhou L, Barczyk M, Evans K, Hilton DA, Hafizi S, Hanemann CO. Axl/Gas6/NFκB signalling in schwannoma pathological proliferation, adhesion and survival. Oncogene. 2014;33:336–346. doi: 10.1038/onc.2012.587. [DOI] [PubMed] [Google Scholar]
- Anjum R, Blenis J. The RSK family of kinases: emerging roles in cellular signalling. Nat Rev Mol Cell Biol. 2008;9:747–758. doi: 10.1038/nrm2509. [DOI] [PubMed] [Google Scholar]
- Bauer C, Diesinger I, Brass N, Steinhart H, Iro H, Meese EU. Translation initiation factor eIF-4G is immunogenic, overexpressed, and amplified in patients with squamous cell lung carcinoma. Cancer. 2001;92:822–829. doi: 10.1002/1097-0142(20010815)92:4<822::aid-cncr1388>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Berkel HJ, Turbat-Herrera EA, Shi R, de Benedetti A. Expression of the translation initiation factor eIF4E in the polyp-cancer sequence in the colon. Cancer Epidemiol Biomarkers Prev. 2001;10:663–666. [PubMed] [Google Scholar]
- Bernard Y, Ribeiro N, Thuaud F, Türkeri G, Dirr R, Boulberdaa M, Nebigil CG, Désaubry L. Flavaglines alleviate doxorubicin cardiotoxicity: implication of Hsp27. PLoS One. 2011;6:e25302. doi: 10.1371/journal.pone.0025302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagden SP, Willis AE. The biological and therapeutic relevance of mRNA translation in cancer. Nat Rev Clin Oncol. 2011;8:280–291. doi: 10.1038/nrclinonc.2011.16. [DOI] [PubMed] [Google Scholar]
- Brastianos PK, Horowitz PM, Santagata S, Jones RT, McKenna A, Getz G, Ligon KL, Palescandolo E, Van Hummelen P, Ducar MD, Raza A, Sunkavalli A, Macconaill LE, Stemmer-Rachamimov AO, Louis DN, Hahn WC, Dunn IF, Beroukhim R. Genomic sequencing of meningiomas identifies oncogenic SMO and AKT1 mutations. Nat Genet. 2013;45:285–289. doi: 10.1038/ng.2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns SS, Akhmametyeva EM, Oblinger JL, Bush ML, Huang J, Senner V, Chen CS, Jacob A, Welling DB, Chang LS. Histone deacetylase inhibitor AR-42 differentially affects cell-cycle transit in meningeal and meningioma cells, potently inhibiting NF2-deficient meningioma growth. Cancer Res. 2013;73:792–803. doi: 10.1158/0008-5472.CAN-12-1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang LS, Welling DB. Molecular biology of vestibular schwannomas. Methods Mol Biol. 2009;493:163–177. doi: 10.1007/978-1-59745-523-7_10. [DOI] [PubMed] [Google Scholar]
- Clark VE, Erson-Omay EZ, Serin A, Yin J, Cotney J, Ozduman K, Avşar T, Li J, Murray PB, Henegariu O, Yilmaz S, Günel JM, Carrión-Grant G, Yilmaz B, Grady C, Tanrikulu B, Bakircioğlu M, Kaymakçalan H, Caglayan AO, Sencar L, Ceyhun E, Atik AF, Bayri Y, Bai H, Kolb LE, Hebert RM, Omay SB, Mishra-Gorur K, Choi M, Overton JD, Holland EC, Mane S, State MW, Bilgüvar K, Baehring JM, Gutin PH, Piepmeier JM, Vortmeyer A, Brennan CW, Pamir MN, Kiliç T, Lifton RP, Noonan JP, Yasuno K, Günel M. Genomic analysis of non-NF2 meningiomas reveals mutations in TRAF7, KLF4, AKT1, and SMO. Science. 2013;339:1077–1080. doi: 10.1126/science.1233009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark VE, Harmancı AS, Bai H, Youngblood MW, Lee TI, Baranoski JF, Ercan-Sencicek AG, Abraham BJ, Weintraub AS, Hnisz D, Simon M, Krischek B, Erson-Omay EZ, Henegariu O, Carrión-Grant G, Mishra-Gorur K, Durán D, Goldmann JE, Schramm J, Goldbrunner R, Piepmeier JM, Vortmeyer AO, Günel JM, Bilgüvar K, Yasuno K, Young RA, Günel M. Recurrent somatic mutations in POLR2A define a distinct subset of meningiomas. Nat Genet. 2016;48:1253–1259. doi: 10.1038/ng.3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comtesse N, Keller A, Diesinger I, Bauer C, Kayser K, Huwer H, Lenhof HP, Meese E. Frequent overexpression of the genes FXR1, CLAPM1 and EIF4G located on amplicon 3q26-27 in squamous cell carcinoma of the lung. Int J Cancer. 2007;120:2538–2544. doi: 10.1002/ijc.22585. [DOI] [PubMed] [Google Scholar]
- Culjkovic B, Tan K, Orolicki S, Amri A, Meloche S, Borden KL. The eIF4E RNA regulon promotes the Akt signaling pathway. J Cell Biol. 2008;181:51–63. doi: 10.1083/jcb.200707018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Benedetti A, Graff JR. eIF-4E expression and its role in malignancies and metastases. Oncogene. 2004;23:3189–3199. doi: 10.1038/sj.onc.1207545. [DOI] [PubMed] [Google Scholar]
- Endo M, Yamamoto H, Setsu N, Kohashi K, Takahashi Y, Ishii T, Iida K, Matsumoto Y, Hakozaki M, Aoki M, Iwasaki H, Dobashi Y, Nishiyama K, Iwamoto Y, Oda Y. Prognostic significance of AKT/mTOR and MAPK pathways and antitumor effect of mTOR inhibitor in NF1-related and sporadic malignant peripheral nerve sheath tumors. Clin Cancer Res. 2013;19:450–461. doi: 10.1158/1078-0432.CCR-12-1067. [DOI] [PubMed] [Google Scholar]
- Fahrig T, Gerlach I, Horváth E. A synthetic derivative of the natural product rocaglaol is a potent inhibitor of cytokine-mediated signaling and shows neuroprotective activity in vitro and in animal models of Parkinson’s disease and traumatic brain injury. Mol Pharmacol. 2005;67:1544–1555. doi: 10.1124/mol.104.008177. [DOI] [PubMed] [Google Scholar]
- Galicia-Vázquez G, Cencic R, Robert F, Agenor AQ, Pelletier J. A cellular response linking eIF4AI activity to eIF4AII transcription. RNA. 2012;18:1373–1384. doi: 10.1261/rna.033209.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gkogkas CG, Khoutorsky A, Cao R, Jafarnejad SM, Prager-Khoutorsky M, Giannakas N, Kaminari A, Fragkouli A, Nader K, Price TJ, Konicek BW, Graff JR, Tzinia AK, Lacaille JC, Sonenberg N. Pharmacogenetic inhibition of eIF4E-dependent Mmp9 mRNA translation reverses fragile X syndrome-like phenotypes. Cell Rep. 2014;9:1742–1755. doi: 10.1016/j.celrep.2014.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gkogkas CG, Khoutorsky A, Ran I, Rampakakis E, Nevarko T, Weatherill DB, Vasuta C, Yee S, Truitt M, Dallaire P, Major F, Lasko P, Ruggero D, Nader K, Lacaille JC, Sonenberg N. Autism-related deficits via dysregulated eIF4E-dependent translational control. Nature. 2013;493:371–377. doi: 10.1038/nature11628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldbrunner R, Minniti G, Preusser M, Jenkinson MD, Sallabanda K, Houdart E, von Deimling A, Stavrinou P, Lefranc F, Lund-Johansen M, Moyal EC, Brandsma D, Henriksson R, Soffietti R, Weller M. EANO guidelines for the diagnosis and treatment of meningiomas. Lancet Oncol. 2016;17:e383–391. doi: 10.1016/S1470-2045(16)30321-7. [DOI] [PubMed] [Google Scholar]
- Gupta SV, Sass EJ, Davis ME, Edwards RB, Lozanski G, Heerema NA, Lehman A, Zhang X, Jarjoura D, Byrd JC, Pan L, Chan KK, Kinghorn AD, Phelps MA, Grever MR, Lucas DM. Resistance to the translation initiation inhibitor silvestrol is mediated by ABCB1/P-glycoprotein overexpression in acute lymphoblastic leukemia cells. AAPS J. 2011;13:357–364. doi: 10.1208/s12248-011-9276-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton DA, Ristic N, Hanemann CO. Activation of ERK, AKT and JNK signalling pathways in human schwannomas in situ. Histopathology. 2009;55:744–749. doi: 10.1111/j.1365-2559.2009.03440.x. [DOI] [PubMed] [Google Scholar]
- Hilton DA, Shivane A, Kirk L, Bassiri K, Enki DG, Hanemann CO. Activation of multiple growth factor signalling pathways is frequent in meningiomas. Neuropathology. 2016;36:250–261. doi: 10.1111/neup.12266. [DOI] [PubMed] [Google Scholar]
- Holland EC, Sonenberg N, Pandolfi PP, Thomas G. Signaling control of mRNA translation in cancer pathogenesis. Oncogene. 2004;23:3138–3144. doi: 10.1038/sj.onc.1207590. [DOI] [PubMed] [Google Scholar]
- Jackson RJ, Hellen CU, Pestova TV. The mechanism of eukaryotic translation initiation and principles of its regulation. Nat Rev Mol Cell Biol. 2010;11:113–127. doi: 10.1038/nrm2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob A, Lee TX, Neff BA, Miller S, Welling B, Chang LS. Phosphatidylinositol 3-kinase/AKT pathway activation in human vestibular schwannoma. Otol Neurotol. 2008;29:58–68. doi: 10.1097/mao.0b013e31816021f7. [DOI] [PubMed] [Google Scholar]
- Kinghorn AD, Pan L, Fletcher JN, Chai H. The relevance of higher plants in lead compound discovery programs. J Nat Prod. 2011;74:1539–1555. doi: 10.1021/np200391c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiner HE, Krishnan P, Tubbs J, Smith M, Meschonat C, Shi R, Lowery-Nordberg M, Adegboyega P, Unger M, Cardelli J, Chu Q, Mathis JM, Clifford J, De Benedetti A, Li BD. Tissue microarray analysis of eIF4E and its downstream effector proteins in human breast cancer. J Exp Clin Cancer Res. 2009;28:5. doi: 10.1186/1756-9966-28-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazaris-Karatzas A, Montine KS, Sonenberg N. Malignant transformation by a eukaryotic initiation factor subunit that binds to mRNA 5′ cap. Nature. 1990;345:544–547. doi: 10.1038/345544a0. [DOI] [PubMed] [Google Scholar]
- Li BD, Liu L, Dawson M, De Benedetti A. Overexpression of eukaryotic initiation factor 4E (eIF4E) in breast carcinoma. Cancer. 1997;79:2385–2390. [PubMed] [Google Scholar]
- Li Y, Fan S, Koo J, Yue P, Chen ZG, Owonikoko TK, Ramalingam SS, Khuri FR, Sun SY. Elevated expression of eukaryotic translation initiation factor 4E is associated with proliferation, invasion and acquired resistance to erlotinib in lung cancer. Cancer Biol Ther. 2012;13:272–280. doi: 10.4161/cbt.18923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol. 2009;10:307–318. doi: 10.1038/nrm2672. [DOI] [PubMed] [Google Scholar]
- Menon S, Manning BD. Common corruption of the mTOR signaling network in human tumors. Oncogene. 2008;27(Suppl 2):S43–51. doi: 10.1038/onc.2009.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi Q, Kim S, Hwang BY, Su BN, Chai H, Arbieva ZH, Kinghorn AD, Swanson SM. Silvestrol regulates G2/M checkpoint genes independent of p53 activity. Anticancer Res. 2006;26:3349–3356. [PubMed] [Google Scholar]
- Mi Q, Su BN, Chai H, Cordell GA, Farnsworth NR, Kinghorn AD, Swanson SM. Rocaglaol induces apoptosis and cell cycle arrest in LNCaP cells. Anticancer Res. 2006;26:947–952. [Google Scholar]
- Oblinger JL, Burns SS, Akhmametyeva EM, Huang J, Pan L, Ren Y, Shen R, Miles-Markley B, Moberly AC, Kinghorn AD, Welling DB, Chang LS. Components of the eIF4F complex are potential therapeutic targets for malignant peripheral nerve sheath tumors and vestibular schwannomas. Neuro Oncol. 2016;18:1265–1277. doi: 10.1093/neuonc/now032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohse T, Ohba S, Yamamoto T, Koyano T, Umezawa K. Cyclopentabenzofuran lignan protein synthesis inhibitors from Aglaia odorata. J Nat Prod. 1996;59:650–652. doi: 10.1021/np960346g. [DOI] [PubMed] [Google Scholar]
- Ostrom QT, Gittleman H, Fulop J, Liu M, Blanda R, Kromer C, Wolinsky Y, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro Oncol. 2015;17(Suppl 4):iv1–iv62. doi: 10.1093/neuonc/nov189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachow D, Andrae N, Kliese N, Angenstein F, Stork O, Wilisch-Neumann A, Kirches E, Mawrin C. mTORC1 inhibitors suppress meningioma growth in mouse models. Clin Cancer Res. 2013;19:1180–1189. doi: 10.1158/1078-0432.CCR-12-1904. [DOI] [PubMed] [Google Scholar]
- Pan L, Acuña UM, Li J, Jena N, Ninh TN, Pannell CM, Chai H, Fuchs JR, Carcache de Blanco EJ, Soejarto DD, Kinghorn AD. Bioactive flavaglines and other constituents isolated from Aglaia perviridis. J Nat Prod. 2013;76:394–404. doi: 10.1021/np3007588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan L, Woodard JL, Lucas DM, Fuchs JR, Kinghorn AD. Rocaglamide, silvestrol and structurally related bioactive compounds from Aglaia species. Nat Prod Rep. 2014;31:924–939. doi: 10.1039/c4np00006d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pines J. Cyclin from sea urchins to HeLas: making the human cell cycle. Biochem Soc Trans. 1996;24:15–33. doi: 10.1042/bst0240015. [DOI] [PubMed] [Google Scholar]
- Poulikakos PI, Xiao GH, Gallagher R, Jablonski S, Jhanwar SC, Testa JR. Re-expression of the tumor suppressor NF2/merlin inhibits invasiveness in mesothelioma cells and negatively regulates FAK. Oncogene. 2006;25:5960–5968. doi: 10.1038/sj.onc.1209587. [DOI] [PubMed] [Google Scholar]
- Rogers L, Barani I, Chamberlain M, Kaley TJ, McDermott M, Raizer J, Schiff D, Weber DC, Wen PY, Vogelbaum MA. Meningiomas: knowledge base, treatment outcomes, and uncertainties. A RANO review. J Neurosurg. 2015;122:4–23. doi: 10.3171/2014.7.JNS131644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenwald IB, Kaspar R, Rousseau D, Gehrke L, Leboulch P, Chen JJ, Schmidt EV, Sonenberg N, London IM. Eukaryotic translation initiation factor 4E regulates expression of cyclin D1 at transcriptional and post-transcriptional levels. J Biol Chem. 1995;270:21176–21180. doi: 10.1074/jbc.270.36.21176. [DOI] [PubMed] [Google Scholar]
- Rouleau GA, Merel P, Lutchman M, Sanson M, Zucman J, Marineau C, Hoang-Xuan K, Demczuk S, Desmaze C, Plougastel B. Alteration in a new gene encoding a putative membrane-organizing protein causes neuro-fibromatosis type 2. Nature. 1993;363:515–521. doi: 10.1038/363515a0. [DOI] [PubMed] [Google Scholar]
- Saradhi UV, Gupta SV, Chiu M, Wang J, Ling Y, Liu Z, Newman DJ, Covey JM, Kinghorn AD, Marcucci G, Lucas DM, Grever MR, Phelps MA, Chan KK. Characterization of silvestrol pharmacokinetics in mice using liquid chromatography-tandem mass spectrometry. AAPS J. 2011;13:347–356. doi: 10.1208/s12248-011-9273-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoles DR, Yong WH, Qin Y, Wawrowsky K, Pulst SM. Schwannomin inhibits tumorigenesis through direct interaction with the eukaryotic initiation factor subunit c (eIF3c) Hum Mol Genet. 2006;15:1059–1070. doi: 10.1093/hmg/ddl021. [DOI] [PubMed] [Google Scholar]
- Shuda M, Kondoh N, Tanaka K, Ryo A, Wakatsuki T, Hada A, Goseki N, Igari T, Hatsuse K, Aihara T, Horiuchi S, Shichita M, Yamamoto N, Yamamoto M. Enhanced expression of translation factor mRNAs in hepatocellular carcinoma. Anticancer Res. 2000;20:2489–2494. [PubMed] [Google Scholar]
- Silvera D, Formenti SC, Schneider RJ. Translational control in cancer. Nat Rev Cancer. 2010;10:254–266. doi: 10.1038/nrc2824. [DOI] [PubMed] [Google Scholar]
- Sorrells DL, Meschonat C, Black D, Li BD. Pattern of amplification and overexpression of the eukaryotic initiation factor 4E gene in solid tumor. J Surg Res. 1999;85:37–42. doi: 10.1006/jsre.1999.5653. [DOI] [PubMed] [Google Scholar]
- Tejada S, Lobo MV, García-Villanueva M, Sacristán S, Pérez-Morgado MI, Salinas M, Martín ME. Eukaryotic initiation factors (eIF) 2alpha and 4E expression, localization, and phosphorylation in brain tumors. J Histochem Cytochem. 2009;57:503–512. doi: 10.1369/jhc.2009.952929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuaud F, Ribeiro N, Gaiddon C, Cresteil T, Désaubry L. Novel flavaglines displaying improved cytotoxicity. J Med Chem. 2011;54:411–415. doi: 10.1021/jm101318b. [DOI] [PubMed] [Google Scholar]
- Trofatter JA, MacCollin MM, Rutter JL, Murrell JR, Duyao MP, Parry DM, Eldridge R, Kley N, Menon AG, Pulaski K. A novel moesin-, ezrin-, radixin-like gene is a candidate for the neurofibromatosis 2 tumor suppressor. Cell. 1993;72:791–800. doi: 10.1016/0092-8674(93)90406-g. [DOI] [PubMed] [Google Scholar]
- Wang X, Gong Y, Wang D, Xie Q, Zheng M, Zhou Y, Li Q, Yang Z, Tang H, Li Y, Hu R, Chen X, Mao Y. Analysis of gene expression profiling in meningioma: deregulated signaling pathways associated with meningioma and EGFL6 overexpression in benign meningioma tissue and serum. PLoS One. 2012;7:e52707. doi: 10.1371/journal.pone.0052707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe AL, Singh K, Zhong Y, Drewe P, Rajasekhar VK, Sanghvi VR, Mavrakis KJ, Jiang M, Roderick JE, Van der Meulen J, Schatz JH, Rodrigo CM, Zhao C, Rondou P, de Stanchina E, Teruya-Feldstein J, Kelliher MA, Speleman F, Porco JA, Pelletier J, Rätsch G, Wendel HG. RNA G-quadruplexes cause eIF4A-dependent oncogene translation in cancer. Nature. 2014;513:65–70. doi: 10.1038/nature13485. [DOI] [PMC free article] [PubMed] [Google Scholar]