Abstract
Manganese (Mn) is an essential trace metal that is neurotoxic at high levels of exposure. Disruption of brain maturation processes during the prenatal period may have lasting consequences. During this critical period, the developing human brain is uniquely vulnerable to exposure to environmental toxicants such as Mn, and prenatal Mn exposure has been associated with changes in brain areas involved in emotion processing and regulation. The goal of the present pilot study was to examine whether prenatal Mn exposure is associated with changes in the intrinsic functional connectivity (iFC) of the brain in childhood, focusing on changes in emotional brain areas. We selected 15 subjects (age 6–7 years) from an ongoing longitudinal birth cohort study to participate in a resting state functional magnetic resonance imaging (fMRI) study. Prenatal Mn exposure was determined from maternal blood collected during the 2nd and 3rd trimesters of pregnancy. We used seed-based correlation analyses and independent component analyses to examine whether prenatal Mn exposure was associated with the iFC of the brain in children. We found that the right globus pallidus showed reduced iFC with the dorsal anterior cingulate cortex and lateral prefrontal cortex in children who were exposed to higher prenatal Mn levels, after controlling for sociodemographic confounders (SES, maternal education, child sex, home environment support) and environmental confounders (prenatal lead exposure and air pollution). These findings suggest that prenatal Mn exposure is associated with reduced iFC of brain areas involved in emotion processing and regulation in children. Future studies should investigate whether this reduced iFC mediates the association between prenatal Mn exposure and emotional dysfunction in childhood.
Keywords: Manganese, Prenatal exposure, Children, Functional Connectivity, Resting State fMRI
Manganese (Mn) is one of the most prevalent metals on earth 1. Widely used in industrial settings resulting in occupational exposure in adults, the general population is exposed to Mn through inhalation, dietary intake and drinking of contaminated water 2. As an essential trace metal, homeostatic levels of Mn are required for a variety of enzymatic and cellular processes within the human body, but levels outside of the homeostatic range can be neurotoxic 1. Neurotoxic effects of excess Mn include oxidative damage to neuronal cells, dopaminergic dysfunction and changes in the function of astrocytes 3. Extensive literature demonstrates the neurotoxic effects of excess Mn exposure in adults, particularly among exposed occupational workers 4,5. In adults, excess Mn characteristically accumulates in the basal ganglia and exposure is most commonly associated with clinical signs and symptoms resembling Parkinson’s disease (termed manganism) 6. While non-occupational exposure to Mn is often lower than occupational exposure, a rapidly growing body of literature reveals the complexity of associations between early life Mn exposure and adverse neurodevelopmental outcomes 7.
During prenatal and early postnatal periods, the developing human brain is uniquely vulnerable to exposure to environmental toxicants 8,9. Human brain development is a protracted process beginning early in pregnancy that relies on the temporal and regional emergence of critical developmental processes (i.e., proliferation, migration, differentiation, synaptogenesis, myelination and apoptosis) 8–10. The complexity and extent of human brain development throughout early life 11 result in a unique susceptibility to environmental chemicals, including metals such as Mn, which can override a normal growth trajectory towards a maladaptive phenotype 12,13. Pregnancy is a period of rapid growth and cell differentiation for both the mother and fetus and is associated with increased demand of many micronutrients including Mn 14. In humans, blood Mn levels increase markedly during pregnancy, peaking in the 3rd trimester 14. Mn is actively transported across the placental barrier 15, and accumulates in fetal and neonatal tissue 16. Infants and children absorb and retain a larger fraction of Mn than adults 16–18 and the fetal blood-brain barrier provides only partial protection against Mn 19–21. Brain areas implicated in emotion processing and regulation, including the prefrontal cortex (PFC), anterior cingulate cortex (ACC), insula, basal ganglia, and parietal cortex 22, are particularly vulnerable to prenatal and early-life exposure to Mn 23. Indeed, prenatal exposure to Mn has been linked to deficits in emotion processing and regulation in childhood, such as increased internalizing (e.g., anxiety and depression) and externalizing (e.g., aggression) symptoms 24,25, behavioral disinhibition 26 and hyperactivity 25.
While a growing number of researchers have examined associations between prenatal Mn exposure and behavioral outcomes including emotion regulation 24–26, the effects of prenatal Mn exposure on the functioning of brain areas subserving emotion processing and regulation in children remain poorly understood. Studying these neural mechanisms would improve our mechanistic insights into the effects of prenatal Mn exposure on emotional dysfunction in childhood, which is a growing public health problem 27. One recent functional magnetic resonance imaging (fMRI) pilot study explored the effects of Mn exposure on brain function in teens 28. Iannilli and colleagues (2016) found that teens raised in an Mn contaminated area showed reduced activity of brain areas involved in emotion processing and regulation during olfactory stimulation, compared to teens who were not exposed to Mn. Specifically, reduced activation of the dorsolateral PFC (DLPFC), parietal cortex and insula was observed in Mn-exposed teens.
The goal of the present study was to investigate whether prenatal Mn exposure is associated with intrinsic functional connectivity (iFC) of the brain 29,30 in children aged 6–7 years. These children were selected from the ongoing longitudinal birth cohort study PROGRESS 31,32 in Mexico City. We focused on prenatal Mn exposure specifically, because: 1) Mn is an essential nutrient and ubiquitous in the environment leading to widespread exposure 1; 2) while literature demonstrates associations between early life Mn exposure and adverse neurodevelopmental sequelae, the neural mechanisms underlying Mn neurotoxicity are poorly understood 33; 3) it is highly relevant to study the effects of prenatal Mn exposure, since Mn concentrations increase threefold during pregnancy and it is actively transported across the placenta 15,34; 4) Mn exposure in Mexico is higher than in the US and Canada 35, which makes the PROGRESS cohort uniquely poised to examine the effects of prenatal Mn exposure on iFC. We used resting state fMRI (rs-fMRI) to measure associations between spontaneous fluctuations in blood oxygen level dependent (BOLD) activity at rest, in the absence of a cognitive task 36,37. An rs-fMRI scan is used to measure correlations between distinct areas of the brain at rest, allowing one to focus on the iFC of distributed networks, instead of only focusing on activity of isolated brain areas 36,37. Moreover, rs-fMRI is a promising method to use in young children, as a scan lasting only a couple of minutes is administered, and participants are not required to perform a specific task 37. For the present study, we used both theory-driven (i.e., seed-based correlation analyses) and data-driven (i.e., independent component analysis) methods to assess iFC of the brain in children aged 6–7 years old. Given that Mn is both an essential nutrient (at low levels of exposure) and a neurotoxicant (at higher levels of exposure) 1, we examined both linear and quadratic associations between prenatal Mn exposure and iFC of the brain in childhood, since quadratic associations between prenatal and early postnatal Mn exposure and neurodevelopmental outcomes have been reported38,39.
Materials and Methods
Participants
A sample of 20 children were selected from the ongoing longitudinal birth cohort study PROGRESS (Programming Research in Obesity, Growth, Environment and Social Stressors) to participate in this pilot neuroimaging study. Original enrollment into the PROGRESS cohort is described at length elsewhere 31,32. Briefly, between July 2007 and February 2011, women attending a prenatal consult in 4 clinics belonging to the Mexican Social Security System (IMSS) in Mexico City were approached for enrollment. If women were in their first trimester, they completed a screening questionnaire and, if eligible, were invited to participate in the study. Inclusion criteria considered: being < 20 weeks pregnant, ≥ 18 years old (Mexican legal voting age), being heart or kidney disease free, having access to a telephone, planning to reside in Mexico City for the next 3 years, no use of steroids (including glucocorticoids) or anti-epilepsy drugs, and not consuming alcohol on a daily basis 31. At each visit the study protocol was explained to women, who provided informed consent before any procedure was carried out. From the 760 mother-infant pairs actively enrolled in PROGRESS we selected 20 subjects with the following criteria; 1) a 2nd and/or 3rd trimester maternal blood sample analyzed for metals and 2) a neurodevelopmental assessment completed at 5-years of age.
Socioeconomic status (SES) was calculated based on an index created by the Mexican Association of Market and Public opinion Research Agencies (Spanish acronym AMAI) using 13 variables derived from questionnaire results (education of the head of household, number of rooms, number of bathrooms with showers, type of floor, number of light bulbs, ownership of car/hot water/automatic washing machine/videocassette recorder/toaster/vacuum cleaner/microwave oven/personal computer) 40. Participants were classified as coming from a family of either low, middle or high SES 41,42. Maternal education was defined as low (<high school), medium (high school) or high (>high school). The Infant/Toddler version of the Home Observation for Measurement of the Environment (HOME) Inventory 43 was administered when subjects were 2 years old, to measure the quality and quantity of stimulation and support available to the child in the home environment.
All procedures of the pilot neuroimaging study were approved by the institutional review boards of the Icahn School of Medicine at Mount Sinai, Harvard T. H. Chan School of Public Health, the National Institute of Public Health Mexico, the Mexican Social Security System, and the National Institute of Perinatology, Mexico.
Blood manganese measurements
Venous whole blood samples were collected from the mothers of participants in trace metal-free tubes during the 2nd (between the 16th and 20th gestational weeks) and 3rd (between the 30th and 34th pregnancy weeks) trimesters of pregnancy 44. Manganese was measured with a dynamic reaction cell/inductively-coupled plasma mass spectrometer (ICP-MS) (Elan 6100; PerkinElmer, Norwalk, CT) using previously described methods and quality control measures 34.
For subjects with both 2nd and 3rd trimester blood Mn concentrations (n = 18), we averaged the two measures and used the mean level in our analyses. For subjects with only 2nd trimester blood manganese (n = 2), we used the available sample. 2nd and 3rd trimester blood Mn concentrations were highly correlated (rho =.55, p =.017). Blood Mn concentrations were skewed (Shapiro-Wilk statistic = .83, p =.012) and therefore log-transformed for analyses.
Environmental confounders
Lead (Pb) exposure is positively correlated with Mn exposure and Mn-Pb interactive effects on children’s neurodevelopment have been reported 45. We therefore assessed maternal blood Pb concentrations during the 2nd and 3rd trimesters of pregnancy. Data collection methods and quality control measures are described in detail elsewhere 31,42,44. For subjects with both 2nd and 3rd trimester blood Pb concentrations (n = 18), we averaged the two measures. For subjects with only 2nd trimester blood Pb (n = 2), we used the available sample. Blood Pb concentrations were skewed (Shapiro-Wilk statistic = .72, p <.001) and therefore log-transformed for analyses.
Air pollution is another important potential environmental confounder, as Mn is often a component of air pollution which could lead to inhalation exposure 2. We estimated ambient fine particulate matter (PM2.5 concentrations (μg/m3)) based on subjects’ residential address during enrollment (2nd trimester of pregnancy), using a hybrid satellite-land use regression model 46. Estimated daily PM2.5 concentrations were averaged for the 2nd and 3rd trimesters of pregnancy.
MRI data collection
MRI data were collected using a Philips Achieva 3T scanner equipped with an 8-channel head coil (Sense Head 8) at the Centro Nacional de Investigación en Imagenología e Instrumentación Médica (Ci3M) in Mexico City.
For registration purposes, an anatomical T1-weighted scan was collected using an MPRage sequence (301 volumes, TR = 7.45 ms, TE = 3.44 ms, FOV = 25 cm, Matrix =256×256, slice thickness = 1.2 mm, slice gap = 0.6 mm). Participants watched an age-appropriate cartoon video during the T1 acquisition.
In order to measure instrinsic functional connectivity, a 10 minute rs-fMRI scan was collected using a Field Echo-EPI gradient pulse (300 volumes, TR= 2000 ms, TE = 27 ms, slice thickness = 3 mm, 37 slices, ascending slice acquisition, FOV = 22 cm, Matrix = 80×80).
Resting state fMRI data analyses
Exclusion of participants
From 20 children invited to participate in the pilot imaging study, data from 15 was included in analyses. Two participants were excluded from the rs-fMRI analyses because the lateral PFC and superior parietal cortex were not covered in their collected images. Further, we excluded three additional participants because of excessive head motion during the rs-fMRI scan. Even small head movements have been shown to influence functional connectivity measures. Specifically, motion leads to overestimation of short-distance connections and underestimation of long-distance connections 47,48. Therefore, we used the fsl_motion_outliers tool (implemented in FSL version 6.00) to determine volumes that were corrupted by excessive motion based on the stringent threshold described in Power at al. 49: relative framewise displacement (FD) > 0.2 mm. Participants were excluded from the analyses if removal of these motion corrupted volumes resulted in having less than 4 minutes (120 volumes) of useable data 50.
Thus, 15 participants (7 girls) were included in all analyses described below.
Seed-based Correlation Analyses
Preprocessing was carried out using FEAT Version 6.00, part of FSL (FMRIB’s Software Library, www.fmrib.ox.ac.uk/fsl). The first two volumes were discarded to allow for T1-equilibration effects. We performed motion correction using MCFLIRT 51, slice-timing correction using Fourier-space time-series phase-shifting, non-brain removal using BET 52, spatial smoothing using a Gaussian kernel of 6 mm FWHM, and highpass temporal filtering (Gaussian-weighted least-squares straight line fitting, with sigma=100.0s). Registration of functional images to participants’ high resolution structural images was carried out using FLIRT 51,53. Given participants’ young age, we created a study-specific template by averaging participants’ high resolution structural images and registered this study-specific template to standard space (MNI-152) using FNIRT nonlinear registration 54,55. Next, we registered participants’ functional and high resolution structural images to this standard space, study-specific template using FNIRT.
We used FSL’s Featquery to extract the mean timeseries of each seed region in participant’s native space focusing on 6 brain regions shown to be affected by prenatal and early-life Mn exposure in prior research 23,28,56. Specifically, we selected four seeds from the probabilistic Harvard-Oxford Cortical Structural Atlas 57: bilateral ACC, bilateral insula, bilateral middle frontal gyrus, and bilateral superior parietal lobule. We further selected two seeds from the probabilistic Harvard-Oxford Subcortical Structural Atlas 58: right and left globus pallidus. Using FEAT, the extracted timeseries of each seed was included as a predictor in a lower-level multiple regression analysis for each participant and seed separately, which produced Z-value correlation maps of all voxels that positively and/or negatively correlated with the seed timeseries. This analysis was carried out using FILM with local autocorrelation correction 59. In order to control for the confounding effects of head motion, we included 24 motion parameters 60 as nuisance regressors, and also regressed out volumes that were corrupted by excessive motion (relative FD > 0.2 mm; i.e., motion scrubbing).
Group-level analyses were carried out using a mixed-effects model implemented in FSL FLAME (stage 1). The general linear model included the mean-centered linear and quadratic effects of Mn exposure as predictors. Statistic images were thresholded using clusters determined by Z > 2.3 and cluster-corrected (using Gaussian Random Field theory) threshold of p < 0.05.
Independent Component Analysis (ICA)
We used ICA-AROMA 61 to first denoise the data by regressing out motion components. Denoised data were preprocessed for the ICA with FSL MELODIC ICA version 3.14, using the same preprocessing steps that were used for the seed-based correlation analyses. Multi-session temporal concatenation was performed to obtain group-level average spatial maps. We limited the output of this analysis to 20 components. Visual inspection of these components revealed 12 components that were core resting state networks: a default mode network, a fronto-temporal-occipital network, a cognitive control network (i.e., lateral PFC, insula), two subcortical reward networks (i.e., ventral striatum, thalamus), two motor networks (left and right pre- and postcentral gyrus), two visual networks (occipital cortex), two emotion/memory networks (OFC, inferior frontal gyrus, temporal cortex), and a posterior ACC network (dACC, PCC, postcentral gyrus)62–64. The remaining 8 components represented physiological noise (e.g., heart rate and respiration) or white matter.
The set of 12 spatial maps that were identified as core resting state networks from the group-average analysis was used to generate subject-specific versions of the spatial maps, and associated timeseries, using dual regression 65. First, for each subject, we regressed the group-average set of spatial maps (as spatial regressors in a multiple regression) into the subject’s 4D space-time dataset. This results in a set of subject-specific timeseries, one per group-level spatial map. Next, those timeseries are regressed (as temporal regressors, again in a multiple regression) into the same 4D dataset, resulting in a set of subject-specific spatial maps, one per group-level spatial map. We then tested for the linear and quadratic effects of prenatal Mn exposure (using mean-centered scores) with FSL’s randomise permutation-testing tool (5000 permutations) 66. Statistical maps were family-wise error (FWE) corrected with a threshold of p < .05, based on the threshold-free cluster enhancement (TFCE) statistical image 67.
Results
Participant Demographics and Mn exposure estimates
Demographic characteristics of the 15 subjects included in this rs-fMRI pilot study are presented in Table 1. The average age of the subjects was 6.8 years (SD: 0.4 years, range: 6.3–7.6 years). Participants were born full term (mean gestational age: 38 weeks, SD: 0.9 weeks) and birth weight ranged between 2000 and 4000 grams (mean: 2970 grams, SD: 314). Based on our indicator of SES, the majority of subjects were born to families of low- to middle SES.
Table 1.
Demographic and Mn exposure characteristics of the PROGRESS cohort and rs-fMRI pilot subjects
| Characteristic | PROGRESS (n = 760) Mean or % (SD) |
rs-fMRI pilot (n = 15) Mean or % (SD) |
|---|---|---|
| Child age (years) | 6.8 (0.4) | 6.9 (0.4) |
| Child sex (% female) | 50.1 | 46.7 |
| Birth weight (grams) | 3100 (400) | 2970 (314) |
| Gestational age (weeks) | 38.3 (1.8) | 38.0 (0.9) |
| Maternal SES (%) | ||
| Low | 53 | 53 |
| Middle | 37 | 47 |
| High | 10 | 0 |
| Maternal blood Mn at 2nd and/or 3rd trimester (ug/L)* | 17 (6) | 17 (6) |
Note. The demographic and exposure characteristics of the 5 subjects excluded from these analyses were not different from the 15 included in this study, except for child age (the 5 excluded subjects were younger than the 15 included subjects).
Mn blood manganese represented the average of 2nd and 3rd trimesters or of the 2nd or 3rd trimester, depending on sample availability.
Maternal blood Mn concentrations were detectable in all maternal blood samples and ranged from 2.7 to 41.1 μg/L (mean μg/L: 17, SD: 6). Mn concentrations were not related to sociodemographic variables, and the participants of the rs-fMRI pilot study did not differ significantly from the participants enrolled in the overall study (n = 948) or those still participating in the most recent follow-up (n = 760) in terms of sociodemographic variables. Maternal blood Mn concentrations were significantly correlated with maternal blood Pb concentrations (r = .59, p = .021). PM2.5 concentrations during the 2nd and 3rd trimesters of pregnancy were not correlated with maternal blood Mn concentrations (p’s > .76).
Seed-based Correlation Analyses
Bilateral ACC seed
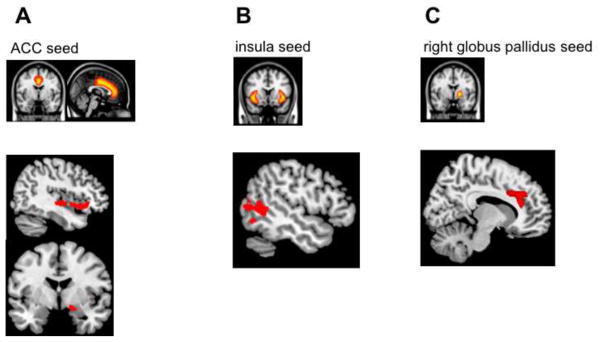
We found negative associations between prenatal Mn and functional connectivity between the ACC and orbitofrontal cortex (OFC), inferior frontal gyrus, insula and amygdala (see Table 2 and Figure 1A). Thus, children who were exposed to higher levels of Mn during pregnancy showed reduced functional connectivity between the ACC and these prefrontal and limbic regions.
Table 2.
Negative correlations between Mn (log) measured in maternal blood and functional connectivity with the bilateral ACC seed of 6–7-year-old children (corrected p <.05).
| Brain regions | MNI coordinates | Z value | ||
|---|---|---|---|---|
| X | Y | Z | ||
| Inferior frontal gyrus | 51 | 17 | −1 | 4.00 |
| Orbital frontal cortex | 38 | 26 | −11 | 3.72 |
| Orbital frontal cortex/Subgenual ACC/Amygdala | 27 | 21 | −15 | 3.64 |
| Orbital frontal cortex | 14 | 21 | −15 | 3.56 |
| Insula | 40 | 17 | −9 | 3.38 |
| Orbital frontal cortex | 40 | 20 | −9 | 3.35 |
Note. ACC = anterior cingulate cortex. All regions are part of a single, extended cluster (11263 voxels). MNI coordinates refer to the peak voxels, i.e., the locations of maximum activation.
Figure 1. Negative correlations between maternal blood Mn (log) and functional connectivity with the (A) bilateral ACC; (B) bilateral insula; (C) right globus pallidus of 6–7-year-old children.
Note. Seed-based correlation analyses were performed. Seed regions are displayed in the top row, while brain areas showing reduced functional connectivity with these seed regions (depicted in red) are displayed in the bottom row.
Bilateral insula seed
We found negative associations between prenatal Mn and functional connectivity between the insula and occipital cortex, middle temporal gyrus and angular gyrus (see Table 3 and Figure 1B). Thus, children who were exposed to higher levels of Mn during pregnancy showed reduced functional connectivity between the insula and these occipito-temporal regions.
Table 3.
Negative correlations between Mn (log) measured in maternal blood and functional connectivity with the bilateral insula seed of 6–7-year-old children (corrected p <.05).
| Brain regions | MNI coordinates | Z value | ||
|---|---|---|---|---|
| X | Y | Z | ||
| Occipital pole | −38 | −95 | 2 | 4.01 |
| Angular gyrus/Middle temporal gyrus | −61 | −56 | 13 | 3.86 |
| Occipital pole | −26 | −98 | 7 | 3.86 |
| Middle temporal gyrus/Lateral occipital cortex | −48 | −54 | 7 | 3.57 |
| Occipital pole | −29 | −100 | 1 | 3.56 |
| Middle temporal gyrus | −56 | −57 | 1 | 3.50 |
Note. All regions are part of a single, extended cluster (8537 voxels). MNI coordinates refer to the peak voxels, i.e., the locations of maximum activation. MNI coordinates refer to the peak voxels, i.e., the locations of maximum activation.
Right globus pallidus seed
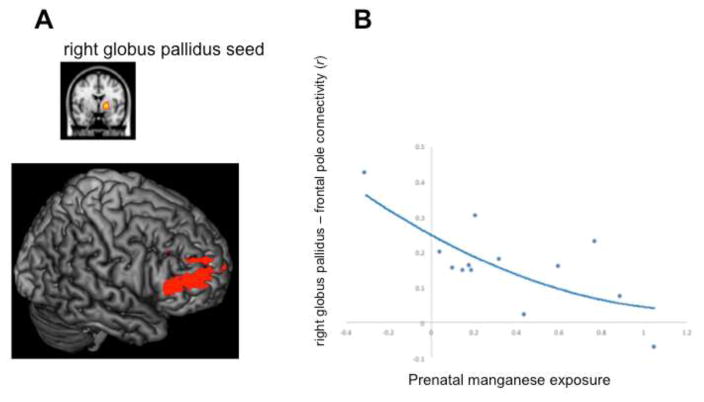
We found a negative correlation between Mn exposure and functional connectivity between the right globus pallidus and dorsal ACC (dACC; see Table 4 and Figure 1C). Thus, children with higher maternal blood Mn concentrations during pregnancy, showed reduced functional connectivity between the right globus pallidus and dACC. Additionally, we found a quadratic association between prenatal Mn and connectivity between the right globus pallidus and inferior frontal gyrus (see Table 4 and Figure 2).
Table 4.
Correlations between Mn (log) measured in maternal blood and functional connectivity with the right globus pallidus seed of 6–7-year-old children (corrected p <.05).
| Brain regions | MNI coordinates | Z value | ||
|---|---|---|---|---|
| X | Y | Z | ||
| Negative linear correlation | ||||
| Dorsal ACC | 8 | 31 | 21 | 4.02 |
| Dorsal ACC | 6 | 35 | 32 | 3.48 |
| Dorsal ACC | 7 | 29 | 31 | 3.35 |
| Rostral ACC | −11 | 30 | 20 | 3.34 |
| Dorsal ACC | −6 | 15 | 34 | 3.19 |
| Paracingulate gyrus | 1 | 29 | 33 | 3.10 |
| Quadratic correlation | ||||
| Frontal pole | 53 | 40 | 8 | 4.40 |
| Middle frontal gyrus | 41 | 53 | 8 | 4.18 |
| Inferior frontal gyrus | 52 | 10 | 16 | 4.14 |
| Inferior frontal gyrus | 54 | 37 | 10 | 4.05 |
| Inferior frontal gyrus | 57 | 34 | 9 | 4.01 |
| Middle frontal gyrus | 40 | 56 | 9 | 3.89 |
Note. All regions showing a negative linear correlation with prenatal Mn are part of a single, extended cluster (9774 voxels). All regions showing a quadratic correlation with prenatal Mn are part of a single, extended cluster (17242 voxels). ACC = anterior cingulate cortex. MNI coordinates refer to the peak voxels, i.e., the locations of maximum activation.
Figure 2. Quadratic correlation between prenatal Mn (log) and functional connectivity with the right globus pallidus of 6–7-year-old children.
Note. A seed-based correlation analysis was performed. (A) the right globus pallidus seed region is displayed in the top row, while the brain areas that showed reduced functional connectivity with this seed region (i.e., the frontal pole, inferior frontal gyrus and middle frontal gyrus; depicted in red) are displayed in the bottom row. (B) Quadratic association between prenatal manganese exposure (x-axis) and functional connectivity (r) of the right globus pallidus seed with the frontal pole (y-axis). In order to compute the functional connectivity with the seed for each subject, we masked the significant activation (displayed in red in figure 2A) with the corresponding region from the probablistic Harvard-Oxford cortical atlas (i.e., frontal pole). We calculated the mean r value for this region using matlab and python scripts.
There were no significant associations between prenatal Mn concentrations and functional connectivity with the middle frontal gyrus, superior parietal lobule and left globus pallidus seeds.
Controlling for multiple comparisons, sociodemographic variables and environmental confounders
In order to test whether these seed-based correlation analyses survived correction for the number of seeds (n = 6) that were analyzed, we repeated these analyses with a Z-score >2.3 and a corrected p<.0083 (p=.05/6). The findings for the bilateral ACC seed and right globus pallidus seed remained significant, even with this more conservative statistical threshold. The findings for the insula seed did not survive this more stringent threshold.
Moreover, adding either child sex, parental SES, maternal education or HOME environment as covariates in the seed-based correlation analyses did not change the findings for the bilateral ACC seed and right globus pallidus seed. However, the findings for the bilateral insula seed were no longer significant after controlling for either child sex or maternal education. Further, the findings for the right globus pallidus seed remained significant after controlling for prenatal Pb exposure and air pollution, separately, but the findings for the bilateral ACC seed were no longer significant when Pb exposure was included as a covariate.
Independent Component Analysis
There were no resting state networks derived from the independent component analysis that significantly correlated with prenatal Mn concentrations at a FWE- corrected threshold of significance.
However, when we used a more liberal, uncorrected threshold (uncorrected p <.005, with an extent threshold of 20 contiguous voxels 58), we observed several correlations between prenatal Mn concentrations and a fronto-temporal-occipital resting state network that mirrored the findings for the seed-based correlation analyses (see Table 5). Specifically, children who were exposed to higher prenatal Mn levels showed reduced functional connectivity between different parts of the fronto-temporal-occipital network, including the insula, lateral occipital cortex and medial and lateral PFC.
Table 5.
Negative correlations between Mn (log) measured in maternal blood and functional connectivity within the fronto-temporal-occipital network of 6–7-year-old children (uncorrected p <.005, k = 20 voxels).
| Fronto-temporal-occipital network | MNI coordinates | t value | ||
|---|---|---|---|---|
| X | Y | Z | ||
| Inferior temporal gyrus | −50 | −58 | −20 | 5.81 |
| Lateral occipital cortex | 18 | −86 | 32 | 8.57 |
| Temporal pole | 58 | 18 | −12 | 7.22 |
| Lateral occipital cortex | −30 | −78 | 24 | 6.00 |
| Precentral gyrus | 10 | −26 | 52 | 5.62 |
| Lateral occipital cortex | −18 | −70 | 52 | 5.64 |
| Middle temporal gyrus | 46 | 2 | −32 | 5.07 |
| Orbitofrontal cortex | 34 | 58 | −4 | 4.89 |
| Fusiform cortex | −26 | −46 | −20 | 5.08 |
| Precentral gyrus | 42 | −14 | 48 | 7.26 |
| Brain areas showing reduced functional connectivity with the fronto-temporal-occipital network in children with higher prenatal Mn exposure | ||||
| Insula | −34 | −6 | 12 | 6.63 |
| Superior temporal gyrus | −58 | −2 | −8 | 8.44 |
| Lateral occipital cortex | 38 | −74 | 36 | 5.32 |
| Frontal pole | 10 | 54 | 44 | 6.79 |
| Inferior temporal gyrus | −54 | −18 | −28 | 5.31 |
| Occipital pole | 2 | −98 | −4 | 5.62 |
| Supramarginal gyrus | 58 | −30 | 56 | 7.61 |
| Lateral occipital cortex | −22 | −82 | 32 | 4.94 |
| Middle temporal gyrus | 58 | −42 | 0 | 5.67 |
| Lateral occipital cortex | 46 | −82 | −8 | 6.05 |
| Fusiform cortex | 30 | −42 | −12 | 4.32 |
Note. An independent component analysis was performed. Brain areas that are consistent with the seed-based correlation analyses are printed in bold. MNI coordinates refer to the peak voxels, i.e., the locations of maximum activation.
Discussion
The goal of this pilot study was to examine whether prenatal Mn exposure is associated with the intrinsic functional connectivity of the brain in childhood. Fifteen subjects aged 6–7 years from the ongoing longitudinal birth cohort study PROGRESS participated in a resting state fMRI study, in order to measure the intrinsic functional connectivity of their brains. Among these 15 subjects, higher levels of maternal blood Mn were associated with reduced functional connectivity of brain areas implicated in emotion processing and regulation in children. Specifically, three brain regions of interest (i.e., seeds) showed reduced functional connectivity with other brain areas in children who were exposed to higher prenatal Mn: the globus pallidus, ACC, and insula. Other studies have demonstrated similar associations between early life Mn exposure and the changes in the globus pallidus and insula in childhood, suggesting these areas may be particularly sensitive to Mn exposure during brain development 23,28,56.
Our three main findings are as follows; First, the right globus pallidus showed reduced connectivity with the dACC and lateral PFC in children who were exposed to higher prenatal Mn levels. This finding remained significant after controlling for sociodemographic confounders (SES, maternal education, child sex, home environment support) and environmental confounders (prenatal Pb exposure and air pollution). The globus pallidus is part of the basal ganglia, and is involved in reward anticipation and processing 68, while the dorsal ACC plays a key role in performance monitoring, and may help track the extent to which rewarding behaviors are performed 69. The lateral PFC is involved in regulating emotions 70,71.
Second, the ACC showed reduced functional connectivity with the OFC, inferior frontal gyrus, amygdala and insula in children who were exposed to higher prenatal Mn. Third, the insula showed reduced functional connectivity with the occipital cortex and middle temporal gyrus in children who were exposed to higher prenatal Mn levels. However, the ACC and insula findings were no longer significant after controlling for prenatal lead exposure, suggesting that there may be complex interactive effects of manganese and lead exposure on brain development 45, which should be explored in more detail in future research.
Reduced connectivity between these brain areas, particularly between the globus pallidus and medial and lateral PFC, may partially explain why prenatal Mn exposure is linked to emotional dysfunction in childhood in other studies, such as such as increased internalizing (e.g., anxiety and depression) and externalizing (e.g., aggression) symptoms 24,25, behavioral disinhibition 26 and hyperactivity 25. In the future, we aim to extend the findings of this pilot study by collecting data on internalizing and externalizing symptoms and intrinsic functional connectivity in a larger sample of children. This would enable us to investigate whether the reduced functional connectivity between emotion processing and regulation areas we observed in this study mediates the association between prenatal Mn exposure and emotional dysfunction in childhood.
There are several potential mechanisms that might underpin the association between Mn exposure during the 2nd and 3rd trimesters of pregnancy and reduced functional connectivity of the brain in childhood. Structural connections underlie the brain’s functional connectivity, although the relationship between structure and function is not perfect 72. The structural foundation critical to the development of functional connectivity is established early in gestation 73. The structural framework and functional capacities of the major neurotransmitter systems, including dopamine, are established early in gestation, and exposure to environmental toxicants may significantly affect the development of neural circuits and neurotransmitter systems 73. Indeed, exposure to Mn is associated with dopaminergic dysfunction 3. Further, Mn has been shown to impair the function of astrocytes 3. Astrocytes and other glia cells play an important role in white matter development and myelination, which increases five-fold during the 3rd trimester of pregnancy 74. Disruption of myelination by environmental toxicants, such as Mn, during this time could predispose to poor neurodevelopmental outcomes 73. Moreover, given that optimal brain development requires extraordinarily complex processes to occur at the right time and in the right sequence, disruption of these processes during prenatal life might have lasting consequences that become apparent only later in life, such as in childhood 12,13.
In this study, maternal blood Mn concentrations during the 2nd and 3rd trimesters of pregnancy ranged from 2.7–41.1 μg/L, with a mean of 17 μg/L. While the range of blood Mn concentrations in our sample was comparable to prior studies that measured blood Mn concentrations in pregnant women in the US and Canada 75,76, mean blood Mn concentrations were higher in our sample than in the studies of Oulhote and colleagues 75 and Takser et al. 76, who both reported a mean of 13.1 μg/L. Several unique demographic and geographic aspects of Mexico, and Mexico City in particular, might explain the higher prenatal Mn concentrations in the present study. First, Mn concentrations in soil and water and consumption of Mn in foods are higher in Mexico than in the US and Canada 35. Second, Mexico City has among the highest levels of air pollution in the world, partly because the city sits in an elevated basin and is surrounded on three sides by mountain ridges 46. Importantly, however, our most robust finding of reduced functional connectivity between the right globus pallidus and dACC and lateral PFC in children with relatively high prenatal Mn exposure remained significant after controlling for air pollution.
Inconsistent with previous studies38,39, we did not observe inverted u-shaped associations between Mn exposure and functional connectivity in the present study. In prior research, inverted u-shaped associations were observed between prenatal and early postnatal Mn exposure and infant neurodevelopment38,39. Specifically, infants with lower levels (<20μg/L) and higher levels (>30 μg/L) of Mn exposure had lower neurodevelopmental scores than infants with moderate exposure (20–30μg/L). These discrepant findings between the current study and prior studies might be explained by differences across studies in the distribution of Mn exposure (i.e., only 2 subjects in the present study had Mn levels >30 μg/L), the age of participants (6–12 months in prior studies vs. 6–7 years in this study), and the neurodevelopmental outcome measure that was used (mental and psychomotor development as assessed by the Bayley Scales of Infant Development in prior studies vs. resting state functional connectivity in the present study).
To our knowledge, this study is the first to explore the association between prenatal Mn exposure and functional brain connectivity in children. The use of neuroimaging tools in studies of environmental exposure in children is an emerging science, with the potential to provide mechanistic insights into the effects of environmental toxicants on neurodevelopment, cognition and behavior 77. However, several limitations of the current study need to be mentioned as well. The modest sample size may have precluded us from finding additional associations between Mn exposure and intrinsic functional connectivity. Nevertheless, we did observe robust findings that were consistent with prior studies, and the observed correlations between Mn exposure and functional connectivity survived a stringent Bonferroni correction for the number of brain areas (i.e., seeds) that we focused on. In addition, the right globus pallidus findings remained significant after controlling for potential sociodemographic confounders, (i.e., child sex, SES, home environment support and maternal education) and environmental confounders (i.e., prenatal lead exposure and air pollution). We aim to replicate and extend these promising pilot findings, by adding measures of emotional dysfunction in a larger sample, in order to examine whether reduced functional connectivity mediates the association between Mn exposure and emotional dysfunction in children and adolescents.
We did not collect information on the exact sources of Mn exposure in our subjects, which is a limitation. Mn exposure might have occurred through inhalation of polluted air, dietary intake and drinking water 2,56.
We measured Mn exposure in maternal blood at two timepoints during pregnancy; 2nd and 3rd trimesters and concentrations were highly correlated. While blood Mn is considered an appropriate indicator of environmental exposure 78, measuring Mn exposure in blood at two timepoints does not allow one to determine windows of susceptibility, during which exposure may be particularly detrimental. A newly developed biomarker of metal exposure in deciduous teeth does allow for the identification of potential windows of susceptibility during prenatal life and early childhood 79–81. Future studies should determine temporally resolved Mn exposure from teeth in order to examine whether associations between Mn exposure and functional connectivity differ as a function of the timing of the exposure. Future researchers may also include children and adolescents from a wide age range, to test whether the reduced functional connectivity in Mn exposed children simply indicates a developmental delay, or that it remains stable or even increases with age. Further, measures of structural connectivity (e.g., Diffusion Tensor Imaging; DTI) could be included in future investigations, to provide more insight into the potential underlying mechanisms of reduced functional connectivity in Mn exposed children. Finally, we focused specifically on prenatal Mn exposure, since Mn concentrations increase during pregnancy 14; Mn is an understudied metal 33; and environmental exposure to Mn is relatively high in Mexico, where this study was performed. However, several other environmental toxicants and environmental factors may influence intrinsic functional connectivity of the developing brain. We included several of these variables as covariates, such as prenatal lead exposure, air pollution, and home environment support (HOME). However, the effects of prenatal exposure to other environmental toxicants (e.g., other metals, phtalates, PBDEs, pesticides) on the developing brain deserve to be explored in future investigations.
To conclude, we found that the right globus pallidus showed reduced intrinsic functional connectivity with other brain areas involved in emotion processing and regulation in children who were exposed to higher prenatal Mn levels, even when controlling for sociodemographic and environmental confounders. Future studies need to examine whether this reduced functional connectivity underpins the association between prenatal Mn exposure and emotional dysfunction (e.g., internalizing and externalizing problems) in childhood.
Highlights.
Prenatal manganese exposure is associated with the intrinsic functional connectivity of children’s brains
Children with higher prenatal exposure show reduced connectivity between the right globus pallidus and medial and lateral prefrontal cortex
These findings remain significant after controlling for sociodemographic (e.g., SES) and environmental (lead, air pollution) confounders
Acknowledgments
We are indebted to the American British Cowdray (ABC) hospital for providing cohort support, the National Center for Medical Instrumentation and Imaging (Ci3M) for providing the imaging facilities and to Edmund Wong for extracting the functional connectivity values depicted in Figure 4. This research was supported by the National Institute of Environmental Health Sciences (NIEH) grant P30ES023515.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Roth JA. Homeostatic and toxic mechanisms regulating manganese uptake, retention, and elimination. Biological Research. 2006;39:45–57. doi: 10.4067/s0716-97602006000100006. [DOI] [PubMed] [Google Scholar]
- 2.Wright RO, Baccarelli A. Metals and neurotoxicology. The Journal of nutrition. 2007;137:2809–13. doi: 10.1093/jn/137.12.2809. [DOI] [PubMed] [Google Scholar]
- 3.Normandin L, Hazell AS. Manganese neurotoxicity: an update of pathophysiologic mechanisms. Metabolic brain disease. 2002;17:375–87. doi: 10.1023/a:1021970120965. [DOI] [PubMed] [Google Scholar]
- 4.Mergler D, Baldwin M. Early manifestations of manganese neurotoxicity in humans: an update. Environmental research. 1997;73:92–100. doi: 10.1006/enrs.1997.3710. [DOI] [PubMed] [Google Scholar]
- 5.Registry AfTSaD. Toxicological profile for manganese. Atlanta, GA: 2000. [Google Scholar]
- 6.Bowler RM, Koller W, Schulz PE. Parkinsonism due to manganism in a welder: neurological and neuropsychological sequelae. Neurotoxicology. 2006;27:327–32. doi: 10.1016/j.neuro.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 7.Zoni S, Lucchini RG. Manganese exposure: cognitive, motor and behavioral effects on children: a review of recent findings. Current opinion in pediatrics. 2013;25:255–60. doi: 10.1097/MOP.0b013e32835e906b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rice D, Barone S. Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environmental Health Perspectives. 2000;108:511–33. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodier PM. Developing brain as a target of toxicity. Environ Health Perspect. 1995;103(Suppl 6):73–6. doi: 10.1289/ehp.95103s673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Osmond C, Barker DJ. Fetal, infant, and childhood growth are predictors of coronary heart disease, diabetes, and hypertension in adult men and women. Environ Health Perspect. 2000;108(Suppl 3):545–53. doi: 10.1289/ehp.00108s3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faustman EM, Silbernagel SM, Fenske RA, Burbacher TM, Ponce RA. Mechanisms underlying Children’s susceptibility to environmental toxicants. Environ Health Perspect. 2000;108(Suppl 1):13–21. doi: 10.1289/ehp.00108s113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grandjean P, Landrigan PJ. Developmental neurotoxicity of industrial chemicals. Lancet (London, England) 2006;368:2167–78. doi: 10.1016/S0140-6736(06)69665-7. [DOI] [PubMed] [Google Scholar]
- 13.Grandjean P, Landrigan PJ. Neurobehavioural effects of developmental toxicity. The Lancet Neurology. 2014;13:330–8. doi: 10.1016/S1474-4422(13)70278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Jameil N, Tabassum H, Al-Mayouf H, et al. Analysis of serum trace elements-copper, manganese and zinc in preeclamptic pregnant women by inductively coupled plasma optical emission spectrometry: a prospective case controlled study in Riyadh, Saudi Arabia. International Journal of Clinical and Experimental Pathology. 2014;7:1900–10. [PMC free article] [PubMed] [Google Scholar]
- 15.Adinolfi M. The development of the human blood-CSF-brain barrier. Developmental medicine and child neurology. 1985;27:532–7. doi: 10.1111/j.1469-8749.1985.tb04581.x. [DOI] [PubMed] [Google Scholar]
- 16.Fechter LD. Distribution of manganese in development. Neurotoxicology. 1999;20:197–201. [PubMed] [Google Scholar]
- 17.Aschner JL, Aschner M. Nutritional aspects of manganese homeostasis. Molecular aspects of medicine. 2005;26:353–62. doi: 10.1016/j.mam.2005.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoon M, Schroeter JD, Nong A, et al. Physiologically based pharmacokinetic modeling of fetal and neonatal manganese exposure in humans: describing manganese homeostasis during development. Toxicological sciences: an official journal of the Society of Toxicology. 2011;122:297–316. doi: 10.1093/toxsci/kfr141. [DOI] [PubMed] [Google Scholar]
- 19.Aschner M, Aschner JL. Manganese neurotoxicity: cellular effects and blood-brain barrier transport. Neuroscience and biobehavioral reviews. 1991;15:333–40. doi: 10.1016/s0149-7634(05)80026-0. [DOI] [PubMed] [Google Scholar]
- 20.Aschner M. Manganese: brain transport and emerging research needs. Environ Health Perspect. 2000;108(Suppl 3):429–32. doi: 10.1289/ehp.00108s3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ljung K, Vahter M. Time to Re-evaluate the Guideline Value for Manganese in Drinking Water? Environ Health Perspect. 2007;115:1533–8. doi: 10.1289/ehp.10316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ochsner KN, Silvers JA, Buhle JT. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Sciences. 2012;1251:E1–24. doi: 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aschner JL, Anderson A, Slaughter JC, et al. Neuroimaging identifies increased manganese deposition in infants receiving parenteral nutrition. The American journal of clinical nutrition. 2015;102:1482–9. doi: 10.3945/ajcn.115.116285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khan K, Factor-Litvak P, Wasserman GA, et al. Manganese exposure from drinking water and children’s classroom behavior in Bangladesh. Environ Health Perspect. 2011;119:1501–6. doi: 10.1289/ehp.1003397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mora AM, Arora M, Harley KG, et al. Prenatal and postnatal manganese teeth levels and neurodevelopment at 7, 9, and 10. 5 years in the CHAMACOS cohort. Environment international. 2015;84:39–54. doi: 10.1016/j.envint.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ericson JE, Crinella FM, Clarke-Stewart KA, Allhusen VD, Chan T, Robertson RT. Prenatal manganese levels linked to childhood behavioral disinhibition. Neurotoxicology and teratology. 2007;29:181–7. doi: 10.1016/j.ntt.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 27.Belfer ML. Child and adolescent mental disorders: the magnitude of the problem across the globe. Journal of child psychology and psychiatry, and allied disciplines. 2008;49:226–36. doi: 10.1111/j.1469-7610.2007.01855.x. [DOI] [PubMed] [Google Scholar]
- 28.Iannilli E, Gasparotti R, Hummel T, et al. Effects of Manganese Exposure on Olfactory Functions in Teenagers: A Pilot Study. PloS one. 2016;11:e0144783. doi: 10.1371/journal.pone.0144783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic resonance in medicine. 1995;34:537–41. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 30.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature reviews Neuroscience. 2007;8:700–11. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 31.Braun JM, Wright RJ, Just AC, et al. Relationships between lead biomarkers and diurnal salivary cortisol indices in pregnant women from Mexico City: a cross-sectional study. Environmental health: a global access science source. 2014;13:50. doi: 10.1186/1476-069X-13-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burris HH, Braun JM, Byun HM, et al. Association between birth weight and DNA methylation of IGF2, glucocorticoid receptor and repetitive elements LINE-1 and Alu. Epigenomics. 2013;5:271–81. doi: 10.2217/epi.13.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanders AP, Claus Henn B, Wright RO. Perinatal and Childhood Exposure to Cadmium, Manganese, and Metal Mixtures and Effects on Cognition and Behavior: A Review of Recent Literature. Current environmental health reports. 2015;2:284–94. doi: 10.1007/s40572-015-0058-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zota AR, Ettinger AS, Bouchard M, et al. Maternal Blood Manganese Levels and Infant Birth Weight. Epidemiology (Cambridge, Mass) 2009;20:367–73. doi: 10.1097/EDE.0b013e31819b93c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Santos-Burgoa C, Rios C, Mercado LA, et al. Exposure to manganese: health effects on the general population, a pilot study in central Mexico. Environmental research. 2001;85:90–104. doi: 10.1006/enrs.2000.4108. [DOI] [PubMed] [Google Scholar]
- 36.Kelly C, Castellanos FX. Strengthening connections: functional connectivity and brain plasticity. Neuropsychology review. 2014;24:63–76. doi: 10.1007/s11065-014-9252-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smyser CD, Snyder AZ, Neil JJ. Functional Connectivity MRI in Infants: Exploration of the Functional Organization of the Developing Brain. NeuroImage. 2011;56:1437–52. doi: 10.1016/j.neuroimage.2011.02.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chung SE, Cheong HK, Ha EH, et al. Maternal Blood Manganese and Early Neurodevelopment: The Mothers and Children’s Environmental Health (MOCEH) Study. Environ Health Perspect. 2015;123:717–22. doi: 10.1289/ehp.1307865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Claus Henn B, Ettinger AS, Schwartz J, et al. Early postnatal blood manganese levels and children’s neurodevelopment. Epidemiology. 2010;21:433–9. doi: 10.1097/ede.0b013e3181df8e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carrasco AV. The Amai System of Classifying Households by Socio-economic Level: The Experience of Mexico and its Comparison with Brazil and Argentina. ESOMAR. 2002 [Google Scholar]
- 41.Rodosthenous RS, Burris HH, Svensson K, et al. Prenatal lead exposure and fetal growth: Smaller infants have heightened susceptibility. Environment international. 2016 doi: 10.1016/j.envint.2016.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stroustrup A, Hsu HH, Svensson K, et al. Toddler temperament and prenatal exposure to lead and maternal depression. Environmental health: a global access science source. 2016;15:71. doi: 10.1186/s12940-016-0147-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bradley RH, Caldwell BM, Corwyn RF. The Child Care HOME Inventories: assessing the quality of family child care homes. Early Childhood Research Quarterly. 2003;18:294–309. [Google Scholar]
- 44.Tamayo Y, Ortiz M, Tellez-Rojo MM, Wright RJ, Coull BA, Wright RO. Longitudinal associations of age and prenatal lead exposure on cortisol secretion of 12–24 month-old infants from Mexico City. Environmental health: a global access science source. 2016;15:41. doi: 10.1186/s12940-016-0124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Claus Henn B, Schnaas L, Ettinger AS, Schwartz J, et al. Associations of early childhood manganese and lead coexposure with neurodevelopment. Environ Health Perspect. 2012 doi: 10.1289/ehp.1003300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Just AC, Wright RO, Schwartz J, et al. Using High-Resolution Satellite Aerosol Optical Depth To Estimate Daily PM2. 5 Geographical Distribution in Mexico City. Environmental science & technology. 2015;49:8576–84. doi: 10.1021/acs.est.5b00859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–54. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van Dijk KRA, Sabuncu MR, Buckner RL. The Influence of Head Motion on Intrinsic Functional Connectivity MRI. NeuroImage. 2012;59:431–8. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Steps toward optimizing motion artifact removal in functional connectivity MRI; a reply to Carp. NeuroImage. 2013;76 doi: 10.1016/j.neuroimage.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Satterthwaite TD, Elliott MA, Gerraty RT, et al. An Improved Framework for Confound Regression and Filtering for Control of Motion Artifact in the Preprocessing of Resting-State Functional Connectivity Data. NeuroImage. 2013;64 doi: 10.1016/j.neuroimage.2012.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–41. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 52.Smith SM. Fast robust automated brain extraction. Human brain mapping. 2002;17:143–55. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Medical image analysis. 2001;5:143–56. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- 54.Andersson JLR, Jenkinson M, Smith SM. Non-linear optimisation. FMRIB technical report TR07JA1. 2007 [Google Scholar]
- 55.Andersson JLR, Jenkinson M, Smith SM. Non-linear registration, aka Spatial normalisation. FMRIB technical report TR07JA2. 2007 [Google Scholar]
- 56.Dion LA, Bouchard MF, Sauve S, et al. MRI pallidal signal in children exposed to manganese in drinking water. Neurotoxicology. 2016;53:124–31. doi: 10.1016/j.neuro.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 57.Kennedy DN, Lange N, Makris N, Bates J, Meyer J, Caviness VS., Jr Gyri of the human neocortex: an MRI-based analysis of volume and variance. Cerebral cortex (New York, NY: 1991) 1998;8:372–84. doi: 10.1093/cercor/8.4.372. [DOI] [PubMed] [Google Scholar]
- 58.Cabezas M, Oliver A, Lladó X, Freixenet J, Bach Cuadra M. A review of atlas-based segmentation for magnetic resonance brain images. Computer Methods and Programs in Biomedicine. 2011;104:e158–e77. doi: 10.1016/j.cmpb.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 59.Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage. 2001;14:1370–86. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- 60.Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R. Movement-related effects in fMRI time-series. Magnetic resonance in medicine. 1996;35:346–55. doi: 10.1002/mrm.1910350312. [DOI] [PubMed] [Google Scholar]
- 61.Pruim RH, Mennes M, van Rooij D, Llera A, Buitelaar JK, Beckmann CF. ICA-AROMA: A robust ICA-based strategy for removing motion artifacts from fMRI data. Neuroimage. 2015;112:267–77. doi: 10.1016/j.neuroimage.2015.02.064. [DOI] [PubMed] [Google Scholar]
- 62.Damoiseaux JS, Rombouts S, Barkhof F, et al. Consistent resting-state networks across healthy subjects. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:13848–53. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jolles DD, van Buchem MA, Crone EA, Rombouts SARB. A Comprehensive Study of Whole-Brain Functional Connectivity in Children and Young Adults. Cerebral Cortex. 2011;21:385–91. doi: 10.1093/cercor/bhq104. [DOI] [PubMed] [Google Scholar]
- 64.van Duijvenvoorde AC, Achterberg M, Braams BR, Peters S, Crone EA. Testing a dual-systems model of adolescent brain development using resting-state connectivity analyses. Neuroimage. 2016;124:409–20. doi: 10.1016/j.neuroimage.2015.04.069. [DOI] [PubMed] [Google Scholar]
- 65.Filippini N, MacIntosh BJ, Hough MG, et al. Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:7209–14. doi: 10.1073/pnas.0811879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE. Permutation inference for the general linear model. Neuroimage. 2014;92:381–97. doi: 10.1016/j.neuroimage.2014.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 68.O’Doherty JP. Reward representations and reward-related learning in the human brain: insights from neuroimaging. 2004 doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 69.Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 70.Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends in cognitive sciences. 2004;8:170–7. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 71.Rolls ET. The functions of the orbitofrontal cortex. Brain and cognition. 2004;55:11–29. doi: 10.1016/S0278-2626(03)00277-X. [DOI] [PubMed] [Google Scholar]
- 72.Damoiseaux JS, Greicius MD. Greater than the sum of its parts: a review of studies combining structural connectivity and resting-state functional connectivity. Brain structure & function. 2009;213:525–33. doi: 10.1007/s00429-009-0208-6. [DOI] [PubMed] [Google Scholar]
- 73.Tau GZ, Peterson BS. Normal Development of Brain Circuits. Neuropsychopharmacology. 2010;35:147–68. doi: 10.1038/npp.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huppi PS, Maier SE, Peled S, et al. Microstructural development of human newborn cerebral white matter assessed in vivo by diffusion tensor magnetic resonance imaging. Pediatric research. 1998;44:584–90. doi: 10.1203/00006450-199810000-00019. [DOI] [PubMed] [Google Scholar]
- 75.Oulhote Y, Mergler D, Bouchard MF. Sex- and age-differences in blood manganese levels in the U.S. general population: national health and nutrition examination survey 2011–2012. Environmental health: a global access science source. 2014;13:87. doi: 10.1186/1476-069X-13-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Takser L, Lafond J, Bouchard M, St-Amour G, Mergler D. Manganese levels during pregnancy and at birth: relation to environmental factors and smoking in a Southwest Quebec population. Environmental research. 2004;95:119–25. doi: 10.1016/j.envres.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 77.Horton MK, Margolis AE, Tang C, Wright R. Neuroimaging is a novel tool to understand the impact of environmental chemicals on neurodevelopment. Current opinion in pediatrics. 2014;26:230–6. doi: 10.1097/MOP.0000000000000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Draft Toxicological Profile for Manganese. 2008 at http://www.atsdr.cdc.gov/toxprofiles/tp151.pdf.
- 79.Arora M, Austin C. Teeth as a biomarker of past chemical exposure. Current opinion in pediatrics. 2013;25:261–7. doi: 10.1097/MOP.0b013e32835e9084. [DOI] [PubMed] [Google Scholar]
- 80.Arora M, Austin C, Sarrafpour B, et al. Determining prenatal, early childhood and cumulative long-term lead exposure using micro-spatial deciduous dentine levels. PloS one. 2014;9:e97805. doi: 10.1371/journal.pone.0097805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Arora M, Bradman A, Austin C, et al. Determining Fetal Manganese Exposure from Mantle Dentine of Deciduous Teeth. Environmental science & technology. 2012;46:5118–25. doi: 10.1021/es203569f. [DOI] [PMC free article] [PubMed] [Google Scholar]