Abstract
As extinctions continue across the globe, conservation biologists are turning to species reintroduction programs as one optimistic tool for addressing the biodiversity crisis. For repatriation to become a viable strategy, fundamental prerequisites include determining the causes of declines and assessing whether the causes persist in the environment. Invasive species—especially pathogens—are an increasingly significant factor contributing to biodiversity loss. We hypothesized that Batrachochytrium dendrobatidis (Bd), the causative agent of the deadly amphibian disease chytridiomycosis, was important in the rapid (<10 years) localized extirpation of a North American frog (Rana boylii) and that Bd remains widespread among extant amphibians in the region of extirpation. We used an interdisciplinary approach, combining interviews with herpetological experts, analysis of archived field notes and museum specimen collections, and field sampling of the extant amphibian assemblage to examine (1) historical relative abundance of R. boylii; (2) potential causes of R. boylii declines; and (3) historical and contemporary prevalence of Bd. We found that R. boylii were relatively abundant prior to their rapid extirpation, and an increase in Bd prevalence coincided with R. boylii declines during a time of rapid change in the region, wherein backcountry recreation, urban development, and the amphibian pet trade were all on the rise. In addition, extreme flooding during the winter of 1969 coincided with localized extirpations in R. boylii populations observed by interview respondents. We conclude that Bd likely played an important role in the rapid extirpation of R. boylii from southern California and that multiple natural and anthropogenic factors may have worked in concert to make this possible in a relatively short period of time. This study emphasizes the importance of recognizing historical ecological contexts in making future management and reintroduction decisions.
Keywords: amphibian decline, Batrachochytrium dendrobatidis, biodiversity, historical ecology, local ecological knowledge, oral history, reintroduction
1. INTRODUCTION
Conservation biology is gradually transitioning from a discipline of crisis to one of hope. In an era of “crisis fatigue” (Redford & Sanjayan, 2003), reintroduction programs provide much‐needed optimism for recovery (Redford, Aune, & Plumb, 2016). With approximately one‐third of species threatened with extinction, amphibians are the most vulnerable vertebrate group, and creative tools are urgently needed to stem their declines (Kissel, Palen, & Govindarajulu, 2017; Stuart et al., 2004). Amphibian ecologists are beginning to reverse catastrophic losses of some species through reintroductions (Griffiths & Pavajeau, 2008). The spread of non‐native and invasive species, including pathogens, remains a pressing problem, and a critical element in the viability of reintroduction as a conservation strategy is an understanding of the factors causing the geographic emergence and expansion of novel pathogens. Reconstructing historical pathogen distributions and emergence can inform predictions of future pathogen trajectories and the development of reintroduction programs where pathogens have driven localized species extinctions (Kauffman & Jules, 2006; Sainsbury & Vaughan‐Higgins, 2012).
For decades, many amphibian declines remained enigmatic. Batrachochytrium dendrobatidis (Bd), the fungal pathogen responsible for the amphibian disease chytridiomycosis, was not identified and described as a causative factor until the late 20th century, owing in part to its unique role, within the phylum Chytridiomycota, as a pathogen of vertebrates (Berger et al., 1998; Longcore, Pessier, & Nichols, 1999). Since then, chytridiomycosis has been implicated in declines or extinctions of hundreds of amphibian species globally (Berger et al., 1998; Fisher et al., 2012; Skerratt et al., 2007). In general, it is rare for disease to be the sole cause of species extinctions (Smith, Sax, & Lafferty, 2006), and until the discovery of chytridiomycosis, disease was largely overlooked as a primary conservation concern in amphibians, as the pathogens known to cause severe declines and extinctions were limited (Collins, Crump, & Lovejoy, 2009). Many theoretic models predict that host populations cannot be driven to extinction by disease alone (Anderson, May, & Anderson, 1992), although host extinction can occur if a reservoir for the pathogen exists, and pathogens can amplify the extinction risk of small host populations (Briggs, Knapp, & Vredenburg, 2010; De Castro & Bolker, 2005). As a result, initially, scientists were hesitant to attribute widespread amphibian declines to epidemic disease (Alford & Richards, 1997; Hero & Gillespie, 1997). Lack of historical information on pathogens may also explain why infectious disease has not traditionally been regarded as an important driver of extinctions (Smith et al., 2006).
Historical ecological knowledge and information, combined with interpretation of ecological change, can make important contributions to informed decision making and addressing contemporary conservation issues (Muths et al., 2016; Swetnam, Allen, & Betancourt, 1999). Diverse historical resources can be drawn upon to measure environmental change, from interviews (Golden, Naisilsisili, Ligairi, & Drew, 2014; Jennings & Hayes, 1994b) to specimens (Lips, 2011), and even works of art (Zerefos et al., 2013). Erroneous perceptions that contemporary conditions are the most accurate standard for ecological health, resulting from a lack of intergenerational communication, lapses in human memory, or the imperceptibly slow deterioration of ecosystems—a phenomenon referred to as shifting baseline syndrome—can lead to inaccurate assumptions about historical abundances and trends and result in poorly informed management decisions (Papworth, Rist, Coad, & Milner‐Gulland, 2009; Pauly, 1995). Bringing historical ecological information to the forefront of contemporary ecological analysis can help guard against this.
Here, we combined historical and contemporary approaches to determine the timing of extirpation of the foothill yellow‐legged frog, Rana boylii (Figure 1), from southern California, USA, and examine its potential causes. R. boylii exemplifies many amphibian species in western North America and around the world that experienced marked declines beginning in the 1970s (Berger et al., 2016; Blaustein & Wake, 1990; Corn, 2000; Green & Kagarise Sherman, 2001; Houlahan, Findlay, Schmidt, Meyer, & Kuzmin, 2000; Lips, Mendelson, Muñoz‐Alonso, Canseco‐Márquez, & Mulcahy, 2004; Whitfield et al., 2007). With R. boylii extirpated from much of its range, ecologists have begun planning for future reintroduction efforts (Lind, 2005; Lind, Spinks, Fellers, & Shaffer, 2011), yet no previous studies have specifically examined the potential role of disease in its decline. In evaluating the relevance and potential success of a reintroduction program, a comprehensive understanding of the original causes of the decline is essential to ensure that the threats have been adequately ameliorated, and to most efficiently and effectively allocate often‐scarce conservation resources (IUCN, 2013).
Figure 1.

Foothill yellow‐legged frog, Rana boylii, from an extant population in northern California. Photograph courtesy of Alessandro Catenazzi
Often, for amphibian species that have been extirpated from relatively pristine environments, the best‐supported evidence suggests that chytridiomycosis was a significant factor (Berger et al., 1998; Rachowicz et al., 2006; Skerratt et al., 2007). Because the southern California R. boylii habitats were relatively remote and unaltered at the time declines occurred, we hypothesized that chytridiomycosis was an important causative factor in R. boylii's rapid extirpation from the region. To test this hypothesis, we sampled for Bd in archived museum collections and in live amphibians in the field and combined these data with field notes and interviews with experts. We also aimed to determine how quickly the species was extirpated in order to compare trends in R. boylii occupancy with the timing of Bd emergence. Species are often at more risk of extinction from disease once they have been negatively affected by non‐disease‐related factors (Heard et al., 2013), so we also interviewed experts to examine other threats that may have contributed to declines. Combining these different data types, we expected to find rapid R. boylii declines coincident with Bd emergence and spread in the region. This study differs from the results of previous work in the R. boylii decline literature (Davidson, 2004; Davidson, Shaffer, & Jennings, 2002; Kupferberg et al., 2012; Lind, 2005; Lind, Welsh, & Wilson, 1996) by both specifically examining disease as a causative population‐level factor and focusing on southern California—the region from which the species was most rapidly extirpated and for which there is very limited information as a result.
2. MATERIALS AND METHODS
2.1. Study species
Rana boylii (Figure 1) is largely a stream‐dwelling and obligate stream‐breeding species that can inhabit a wide range of fluvial habitats, from first‐ to seventh‐order streams within a single watershed (Bury & Sisk, 1997). Historically they ranged from southern Oregon to Los Angeles County, California, USA, with an isolated population in the Sierra San Pedro Martir in Baja California, Mexico, and from near sea level to 1,219 m (Stewart, Jennings, & Robert, 2005). Listed as a candidate under the California Endangered Species Act and proposed for federal Endangered Species Act listing (U.S. Fish and Wildlife Service, 2015), R. boylii is absent from approximately half of its formerly occupied sites, with the most pronounced declines and local extirpations concentrated in northern Oregon, southern California, and Baja California, although the extent of disappearances from historical localities may be underestimated (Davidson, 2004; Hayes, Wheeler, Lind, Green, & Macfarlane, 2016; Lind, 2005). Most R. boylii populations in southern California were apparently stable until the mid‐1970s, when populations collapsed (Jennings, 2005; Jennings & Hayes, 1994a). R. boylii persistence at historical sites has been positively associated with latitude and precipitation and negatively associated with both upwind and surrounding agriculture (Davidson et al., 2002). Elsewhere in the range, dams and diversions that change flow and thermal regimes have been identified as primary causes of R. boylii declines (Catenazzi & Kupferberg, 2017; Hayes et al., 2016); however, in southern California, streams that supported the species either were largely undammed or, where dams existed, R. boylii had persisted for decades at high abundances despite flow regulation.
Until recently, little was known about the susceptibility of R. boylii to Bd. Experimental infections in the laboratory resulted in either mortality of Bd‐positive individuals not significantly different from those of controls (Davidson et al., 2003) or reduced growth in Bd‐positive juveniles but no mortality (Davidson et al., 2007). In a repeat experiment of Davidson et al. (2007), 16 R. boylii from the same source population exposed to the same Bd isolate experienced 100% mortality, while no individuals in the non‐Bd treatments (n = 16) died (C. Davidson, unpublished data). In 2013, a central California population of R. boylii experienced a chytridiomycosis‐induced mortality event (Adams et al., 2017), the first such record for this species in the wild.
2.2. Interviews and field notes
We used interviews and field notes to determine pre‐extirpation R. boylii abundance and to assess the rapidity with which the species disappeared from the southern California region. We conducted semi‐directive interviews, which enable the interviewee to guide the conversation beyond a few basic questions, and for which there is no time limit. Semi‐directive interviews are powerful tools for accessing and documenting ecological knowledge that is not available from any other source (Huntington, 1998).
To identify candidate interviewees, we used VertNet (National Science Foundation, 2016) records to identify the collectors of southern California R. boylii specimens. We identified additional interviewees through conversations with these and other experts in the herpetological and California natural history fields. Candidate interviewees were contacted via email, except for a few who were contacted through Field Herp Forum (Herp Nation Media, 2016) when no email address was readily available. If they consented to an interview, respondents were given an information sheet and oral history interview agreement. The information sheet formally invited the interviewee to participate in the study, briefly described the objective of the study, explained why they were selected as a participant, and explained that participation was entirely voluntary. The interview agreement provided for formal consent to the interview and gave options (via checkboxes) for whether interviewees assented to recording, transcription, and archiving. These instruments were reviewed and approved by the University of California Santa Barbara Human Subjects Committee (protocol # 1‐14‐0245). Interviews took place in person, over the telephone, or via email. We asked a series of general questions about the interviewees’ herpetological expertise, observations of amphibian mortality events and declines, observations of R. boylii natural history and abundance, and perceived causes of amphibian declines and R. boylii extirpation (Appendix S1).
When field notes accompanied specimens or observations, we reviewed them for R. boylii abundance information. A collection of field notes was accessed online through the Museum of Vertebrate Zoology EcoReader (Museum of Vertebrate Zoology, 2016) and the California Academy of Sciences Herpetology Collection Database (California Academy of Sciences, 2016); others were reviewed at natural history museums where they were archived or were provided directly by interviewees. We transcribed responses to interview questions and relative abundance information from field notes and created a database for later analysis. We also used the number of R. boylii specimens in collections to estimate minimum abundance. To account for the differences between the reliability of primary sources (i.e., specimen collections and field notes) versus interviews that rely solely on memory (Alagona, Sandlos, & Wiersma, 2012), we coded interviews, field notes, and specimen collections as separate information categories.
2.3. Museum specimen sampling and analysis
Retrospective surveys of museum specimens can reveal signatures of chytridiomycosis effects on populations, even when observations of die‐offs and mass mortality are lacking (Burrowes, Joglar, & Green, 2004). We sampled postmetamorphic amphibian specimens and used quantitative PCR (qPCR) to detect Bd DNA in order to determine infection status. To identify desired specimens, we searched for species and county records in VertNet (National Science Foundation, 2016) and Specify (University of California Santa Barbara, 2016) and via interviews. In addition to R. boylii specimens from the southern California study area (defined as Ventura, Santa Barbara, San Luis Obispo, and Los Angeles counties), and because few R. boylii were collected relative to other species, we sampled all amphibian species that occupied the same streams as R. boylii in the region: Anaxyrus boreas halophilus (California toad); Anaxyrus californicus (arroyo toad); Rana catesbeiana (bullfrog—a non‐native species that now occupies some former R. boylii sites); Rana draytonii (California red‐legged frog); Hyliola cadaverina (California tree frog); Hyliola regilla (Pacific chorus frog); and Taricha torosa (California newt). We considered specimens desirable if they were one of the species listed above and occurred within the same stream, or the next stream order above or below the same stream as R. boylii, prior to extirpation.
Specimen sampling, DNA extraction using Macherey‐Nagel DNA FFPE, and qPCR protocols followed Adams et al. (2015), with the exception that 10 μl of extract was used per qPCR reaction. All samples were run in triplicate, with sample replicates run on separate qPCR plates. Specimens were considered positive when two or more samples exhibited qPCR amplification (i.e., as evidenced by an exponential amplification curve). In addition, 57 T. torosa specimens were tested for both Bd and Batrachochytrium salamandrivorans (Bsal; a fungal pathogen that has led to die‐offs of salamanders in Europe, but which has not yet been detected in the United States (Bsal Task Force, 2016, Martel et al., 2014, 2013)) using duplex PCR following Blooi et al. (2013). These specimens were sampled and DNA extracted as described above.
In addition to qPCR, we conducted histology on select specimens to examine chytridiomycosis status, following Reeder, Pessier, and Vredenburg (2012). This allowed us to confirm the Bd status of 13 specimens collected prior to 1970 that tested positive for Bd via qPCR (i.e., ≥2 amplified replicates in the sample). We also conducted histological examination on six R. boylii specimens collected from Clear Creek, San Benito County, California, in 1989 that died in captivity at a university laboratory within 6 weeks of their collection from the wild, and an A. californicus metamorph from Santa Barbara County, California, that also died in the same laboratory during that time.
2.4. Museum specimen statistical analysis
To test various hypotheses for Bd infection in museum specimens, we used an information‐theoretic approach and generalized linear models (GLM). The GLM included a binomial (Bd presence/absence) response and a logit‐link function. If any individuals collected from a site on a particular date were positive (≥2 amplified qPCR replicates), we declared the site positive on that date; if not, we then considered that site–date combination negative for the purposes of the GLM. When multiple species were collected on the same day at the same site, we treated individual species independently. We sampled 1,561 museum specimens for Bd and used 632 site–date–species combinations for the GLM analysis.
Based on other studies of historical Bd prevalence in California (Padgett‐Flohr & Hopkins, 2009; Sette, Vredenburg, & Zink, 2015) and our hypothesis that Bd was a causative factor in R. boylii's extirpation in the middle of the 20th century, we expected temporal and spatial variables to be significant predictors of Bd infection in museum specimens. Therefore, we included decade, year, month, 20‐year time interval, and county as categorical predictor variables in the GLM (Table 1). Because temporal variables were correlated, we included only one temporal variable in each model at one time. We also created a spatial variable—distance from earliest positive specimen detected—which enabled us to test the hypothesis that Bd could have spread spatiotemporally from an early Bd introduction (Table 1). Distance from first positive specimen was calculated, in kilometers, from latitude and longitude in decimal degrees using the Vincenty formula for ellipsoidal geodesic distances. As Bd susceptibility can be highly variable between taxa (Searle et al., 2011), we also included two biological variables—family and species—to determine whether there were taxonomic trends in historical Bd status.
Table 1.
Variables used in generalized linear models used to determine the best predictors of Bd presence/absence in museum specimens
| Covariate | Type | Range or levels | Description |
|---|---|---|---|
| Decade | Temporal | 1910; 1920; 1930; 1940; 1950; 1960; 1970; 1980; 1990; 2000 | Decade that specimen was collected from the wild |
| 20‐year interval | Temporal | 1910–1929; 1930–1949; 1950–1969; 1970–1989; 1990–2009 | 20‐year period that specimen was collected from the wild |
| Year collected | Temporal | 1911–2009 | Year that specimen was collected from the wild |
| Distance from first positive specimen | Spatiotemporal | 0–309.9 km | Vincenty distance from the earliest Bd positive detected in this study (z‐transformed) |
| Month collected | Temporal | 1–12 | Month of the year that specimen was collected from the wild |
| County | Spatial | Los Angeles; San Luis Obispo; Santa Barbara; Ventura | California County where specimen was collected from the wild |
| Species | Biological | Anaxyrus boreas; Anaxyrus californicus; Rana catesbeiana; Hyliola cadaverina, Hyliola regilla, Rana boylii, Rana draytonii, Taricha torosa | Species of specimen sampled |
| Family | Biological | Bufonidae, Hylidae, Ranidae, Salamandridae | Family to which specimen sampled belongs |
We conducted all analyses using R (R Core Team, 2016) and used a forward selection procedure to determine the predictor variables that best fit the data. To check for multicollinearity among predictors, we used “vif” function in package “car” to ensure that variance inflation factor values were <3 in candidate models that contained more than one predictor (Zuur, Ieno, & Elphick, 2010). Adding predictor variables sequentially in the order presented in Table 1, we used likelihood ratio tests to compare nested models during the forward selection procedure. After completing the forward selection process, we then ranked candidate models according to Akaike's information criterion (AIC) to determine the relative importance of predictor variables. The models with the lowest AIC were considered the best‐supported models by the data, and models with a ∆AIC >2 as compared to the model with the lowest AIC were considered not as well‐supported by the data (Burnham & Anderson, 2004). We used the “allEffects” function from the “effects” package to extract marginalized fixed effects from the model that was the best fit to the data as determined by AIC. We used the “glht” function in the “multcomp” package to conduct Tukey multiple‐comparisons tests to compare the effects differences among levels of the predictor variable in the best‐fit model.
2.5. Field sampling
To characterize the current status of Bd in extant stream‐dwelling amphibians within the former range of R. boylii, we conducted field sampling from 2011 to 2014 in formerly R. boylii‐occupied habitats in Ventura, Santa Barbara, and Los Angeles counties. All samples were collected in adherence to protocols approved by the University of California, Santa Barbara Institutional Animal Care and Use Committee (Protocol # 825). We sampled all species described above for specimen sampling except for T. torosa, because very few individuals of this species were encountered during the nocturnal surveys. Bd sampling and qPCR protocols for field‐collected swabs followed Hyatt et al. (2007) and Boyle, Boyle, Olsen, Morgan, and Hyatt (2004), respectively.
3. RESULTS
3.1. Interviews
We contacted 29 candidate interviewees and conducted interviews with 21 of them. Of the respondents, four communicated exclusively via email; the remainder were interviewed over the phone or in person. Average interviewee age was 69, and respondents collectively represented 873 years (mean 42 years) of herpetological field experience. Mean verbal interview duration was 1.2 hr.
3.1.1. Reasons for R. boylii decline
Most respondents declined to speculate what could have caused R. boylii extirpations from southern California. Responses centered around several common hypotheses of amphibian declines, including chytridiomycosis, pet trade, exotic species, and climate change (Catenazzi, 2015); however, two relatively unique threats emerged: increased recreational use of habitats and extreme flooding (Table 2). Three respondents reported observing the localized extirpation of once‐abundant R. boylii populations after extreme flood events in California: (1) Tulare County in the late 1960s (Interview 9); (2) Caliente Creek (Kern County) in the mid‐1970s (Interview 11); and (3) Evey Canyon in the San Gabriel Mountains (Los Angeles County) in the late 1960s (Interview 9). The Evey Canyon site shifted from having a very abundant population to no R. boylii found, according to the interviewee:
Table 2.
Interviewee responses to inquiry of potential causes of R. boylii extirpation from southern California
| Cause | Yearsa | Interview # | Explanation |
|---|---|---|---|
| Amphibian pet trade and exotic amphibians | 175 [58] | 10, 12, 20 | Amphibians wild‐caught; availability in local department stores; pet releases into the wild |
| Bd | 195 [49] | 3, 10, 12, 20 | Chytridiomycosis |
| Bd + climate change | 54 | 10 | Climate change exacerbates conditions when Bd is already present, making amphibians more susceptible to disease |
| Bd + fish stocking | 56 | 12 | Releases Bd zoospores into new waterways |
| Bd + flooding | 56 | 12 | Unusual frequency of rain events & overcast days reduces opportunities for frog basking (higher temperatures can mitigate chytridiomycosis) |
| Climate change | 54 | 10 | Declines trend from south to north; declines occurred earlier in drier climates |
| Drought | 60 | 4 | 1955–1960 and 1971–1978 were very dry years |
| Non‐native fish | 63 | 17 | Non‐native fish introduced, resulting in predation |
| Flooding (1968–1969) | 210 [53] | 9, 11, 12, 19, 20 | Extreme flood events scoured frogs to lower elevations/less suitable habitat; permanent change in habitat quality and suitability |
| Recreation | 234 [59] | 12, 17, 19, 20 | Increased and intensified recreational uses of streams |
Cumulative years of herpetological experience represented by respondents; number in brackets shows average number of years.
The frogs [R. boylii] were extremely abundant there…you'd walk along the creek for a couple hundred yards and collect a sample of 15 or 20…the frogs were just all over the place. But after the ‘69 floods…I went back in ‘70…there weren't frogs in that creek at all and there haven't been since. (Interview 9)
3.1.2. R. boylii occurrence and abundance in southern California
Despite the number of naturalists frequenting various portions of the study area, none were able to produce a record of R. boylii from later than 1977. One interviewee confirmed his sighting of one R. boylii individual in Piru Creek, Los Angeles County, on 6 July 1977, which is the last known record of the species in southern California (Hayes et al., 2016; Jennings & Hayes, 1994a). Extensive R. boylii resurveys were conducted in southern California by two independent research teams from 1981 to 1993 and from 1988 to 1991; however, none encountered the species. In addition, we did not encounter the species during our field surveys from 2011 to 2014. Several R. boylii collections were made in the 1940s and 1950s, along with field notes containing abundance information such as “very common” (R. Zweifel field notes, 20 March 1948) and “fairly abundant” (Interviewee 20 field notes, 3 May 1970); only one field note entry was found that reported a negative survey for R. boylii prior to the late 1970s (R. Zweifel field notes, 2 April 1950). Some interviewees reported abundance information such as “15‐20 individuals per mile of stream” (Interview 8).
3.2. Chytridiomycosis in Rana boylii
Interviews revealed two previously unknown R. boylii mortality events attributable to chytridiomycosis, both outside of the immediate study area. In 1989, six live R. boylii individuals collected from Clear Creek, San Benito County (central California), were taken to a university laboratory where they were housed in a 60‐gallon tank with recirculating water. Within 6 weeks, all frogs succumbed to an unknown infection, which we confirmed to be chytridiomycosis (see the Section “ 3.1.2 ” below). Histological examination of these frogs revealed hyperkeratosis and hyperplasia in all six animals, consistent with chytridiomycosis, and Bd organisms in one (Table 3). Necropsy conducted on one of the individuals suggests that this animal died of chytridiomycosis. These animals appeared healthy when collected from the stream where they occurred, although they were taken from a stream that was actively being used by off‐road vehicles (Interview 19). It is unknown whether they contracted Bd from the field or in the laboratory, as this event occurred a decade before Bd was described (Longcore et al., 1999). The second mortality event was a R. boylii die‐off in a stream in Stanislaus County (central California) in 1986. Approximately 85 dead individuals were observed over a period of 2 weeks, until there were only a few animals left (Interview 12). Retrospective histology on these specimens confirmed that they were infected with Bd and showed symptoms of chytridiomycosis (Interviews 10 and 12). Although in a part of California where R. boylii is extant, the R. boylii population at this locality has not recovered to the level of abundance present prior to this chytridiomycosis outbreak (Interview 12).
Table 3.
Results of histological examination of formalin‐fixed, ethanol‐preserved museum specimens
| Institution | Collection # | Species | County | Year | qPCR replicates positive | Histology result |
|---|---|---|---|---|---|---|
| CAS | 39865 | Anaxyrus boreas | Los Angeles | 1915 | 2 | Lesions of hyperplasia and hyperkeratosis, no Bd organisms |
| CAS | 39867 | A. boreas | Los Angeles | 1915 | 3 | Lesions of hyperplasia and hyperkeratosis, no Bd organisms |
| CAS | 63049 | Hyliola cadaverina | Ventura | 1927 | 2 | Negative |
| MVZ | 27881 | Rana catesbeiana | Los Angeles | 1939 | 3 | Negative |
| MVZ | 33668 | Rana boylii | Ventura | 1940 | 2 | Negative |
| MVZ | 33672 | R. boylii | Ventura | 1940 | 2 | Negative |
| LACM | 13455 | Rana draytonii | Los Angeles | 1947 | 2 | Negative |
| LACM | 13703 | R. boylii | Ventura | 1954 | 2 | Negative |
| LACM | 13496 | R. draytonii | Ventura | 1954 | 2 | Negative |
| CAS | 181067 | A. boreas | Santa Barbara | 1961 | 2 | Negative |
| LACM | 13457 | R. draytonii | Los Angeles | 1963 | 2 | Negative |
| CAS | 190944 | Anaxyrus californicus | Santa Barbara | 1966 | 2 | Negative |
| LACM | 76274 | R. draytonii | Ventura | 1968 | 3 | Negative |
| Private | NA | A. californicus | Santa Barbara | 1989 | 1 | Hyperplasia and hyperkeratosis, no Bd organisms |
| Private | NA | R. boylii | San Benito | 1989 | 0 | Hyperplasia and hyperkeratosis with Bd organisms |
| Private | NA | R. boylii | San Benito | 1989 | 0 | Hyperplasia and hyperkeratosis, no Bd organisms |
| Private | NA | R. boylii | San Benito | 1989 | 0 | Hyperplasia and hyperkeratosis, no Bd organisms |
| Private | NA | R. boylii | San Benito | 1989 | 0 | Hyperplasia and hyperkeratosis, no Bd organisms |
| Private | NA | R. boylii | San Benito | 1989 | 0 | Hyperplasia and hyperkeratosis, no Bd organisms |
| Private | NA | R. boylii | San Benito | 1989 | 0 | Hyperplasia and hyperkeratosis, no Bd organisms |
3.3. Museum specimens
We sampled 1,561 museum specimens from eight institutional collections (Appendix S2). All species tested positive for Bd, and overall infection prevalence was 10.6% (149 of 1,561 samples). Bd‐positive individuals were present in all counties sampled. The earliest Bd positives detected with qPCR were two A. boreas specimens collected from Los Angeles County in 1915. Bd prevalence varied temporally and showed a steady increase beginning in the 1970s and continuing through the 1990s, followed by a decline in prevalence after the 1990s (Figure 2). California newts (T. torosa) and non‐native American bullfrogs (R. catesbeiana) had the highest prevalence of infection among species (Figure 3a). Bd prevalence in R. boylii was among the lowest (with higher prevalence in the 1970s); however, samples from this species were limited to the period prior to its extirpation, with prevalence in other species increasing during the 1990s, by which time R. boylii was extirpated from the study area (Figures 3b and 4).
Figure 2.
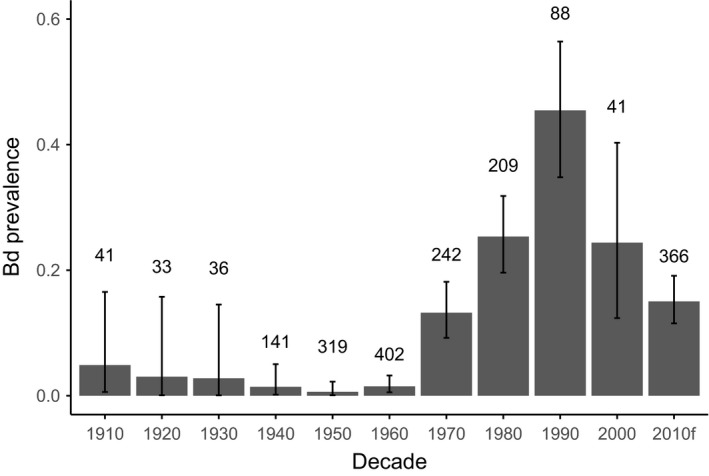
Bd prevalence through time for all museum specimens sampled. Each bar represents a decade that begins with the year indicated by the x‐axis label. Error bars represent Clopper–Pearson binomial confidence intervals. Numbers above the bars denote sample size. Data are from specimens collected from the study area, including Los Angeles, Ventura, and San Luis Obispo, and Santa Barbara counties. The “2010f” category represents Bd samples collected from live postmetamorphic amphibians sampled in the field from 2011 to 2014
Figure 3.
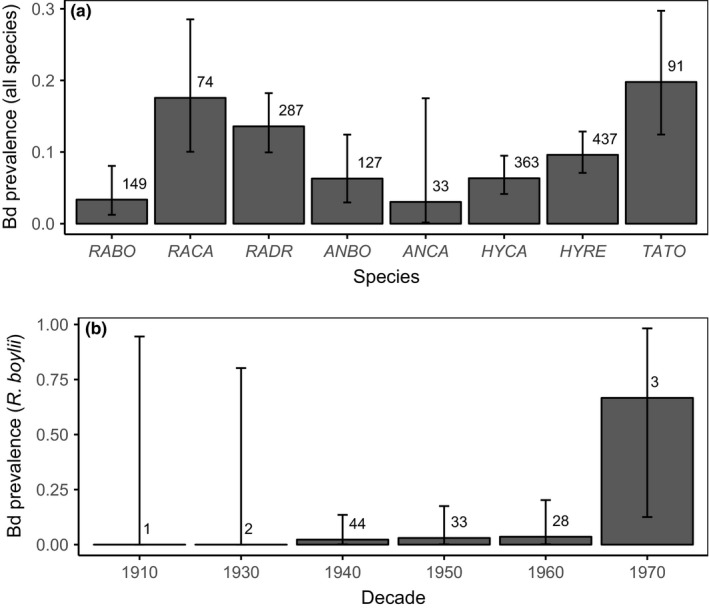
Bd prevalence of museum specimens, representing (a) all species; and (b) R. boylii only, by decade. Species codes: RABO = Rana boylii;RACA = Rana catesbeiana;RADR = Rana draytonii;ANBO = Anaxyrus boreas;ANCA = Anaxyrus californicus;HYCA = Hyliola cadaverina;HYRE = Hyliola regilla;TATO = Taricha torosa. Decades are indicated on the x‐axis in (b) by the first year in each decade
Figure 4.
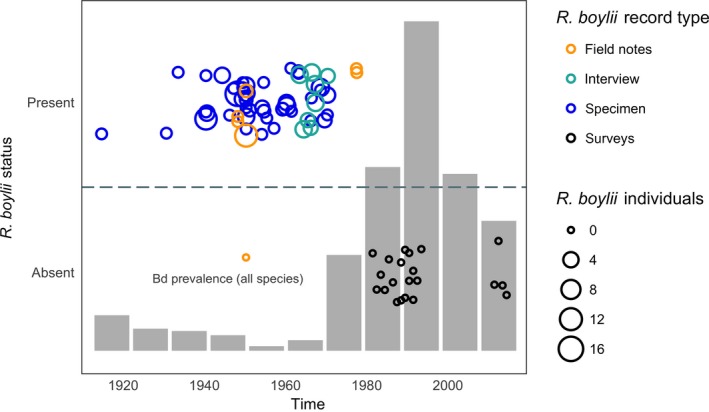
Rana boylii relative abundance (jittered circles) by record type through time. Specimen abundances are represented by the number of postmetamorphic individuals collected at a site on a particular date. Background plot (histogram in gray) shows relative Bd prevalence of all species detected from museum specimens through time (see Figure 2 for details). For Bd prevalence through time in R. boylii, refer to Figure 3b
The GLM analysis indicated that Bd presence/absence in museum specimens was best predicted by 20‐year time interval and distance from the first positive specimen (Table 4 and Figure 5), suggesting that Bd spread spatiotemporally from the area that is now the greater Los Angeles area in the early 1900s (Figure 6). Species, month, year, decade, family, and county were not important predictors in the GLM. As species was not an important predictor, the intrinsic confounding of species and time, due to historically uneven specimen collection, likely did not drive the pattern we observed. The importance of 20‐year time interval despite the relative unimportance of decade indicates that longer time intervals are required to observe a clear trend in the independent samples we collected.
Table 4.
Candidate generalized linear models used to determine the best predictors of Bd presence/absence in museum specimens
| Rank | Model | K | logLik | AICc | ∆AICc | AICw |
|---|---|---|---|---|---|---|
| 1 | 20‐year interval | 5 | −195.39 | 400.87 | 0 | 0.49 |
| 2 | 20‐year interval + Distance from first positive specimen | 6 | −195.11 | 402.36 | 1.49 | 0.23 |
| 3 | 20‐year interval + Family | 8 | −193.37 | 402.96 | 2.09 | 0.17 |
| 4 | 20‐year interval + County | 8 | −194.53 | 405.29 | 4.42 | 0.05 |
| 5 | Decade | 10 | −193.46 | 407.27 | 6.4 | 0.02 |
| 6 | 20‐year interval + Species | 12 | −191.49 | 407.48 | 6.61 | 0.02 |
| 7 | 20‐year interval + Month | 16 | −188.61 | 410.11 | 9.24 | 0 |
| 8 | Year | 80 | −153.14 | 489.8 | 88.93 | 0 |
| 9 | Null | 1 | −255.06 | 512.13 | 111.26 | 0 |
K = Number of parameters in the model.
Figure 5.
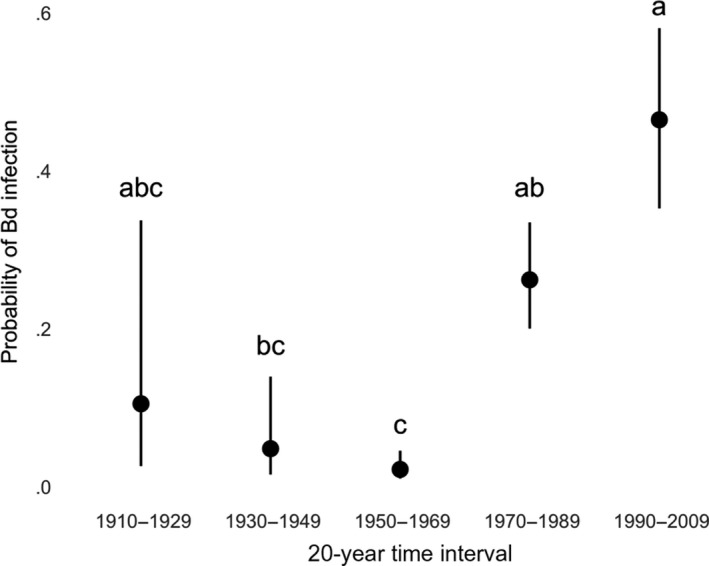
Probability of Bd infection for 20‐year time intervals as indicated by the best‐fit generalized linear model of museum specimen samples. Error bars indicate 95% confidence intervals. Letters above the bars indicate Tukey groups for 20‐year time intervals from the best‐fitting model of Bd presence/absence in all species: Time intervals that do not share a letter are significantly different from one another
Figure 6.
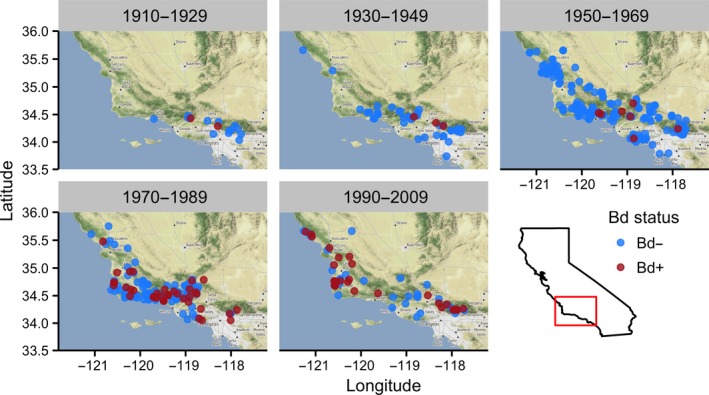
Map of localities sampled for Bd in museum specimens from 1915 to 2009 as used in the generalized linear model. Dates above each map represent the 20‐year time interval in which each specimen was collected. Data were arranged prior to mapping so that Bd‐positive samples would be superimposed on Bd‐negative samples when they overlapped. Data include sampled specimens collected from Ventura, Santa Barbara, Los Angeles, and San Luis Obispo counties
Histological examination revealed evidence of chytridiomycosis in two of the 13 specimens collected prior to 1970 that were qPCR‐positive for Bd (Table 3)—two A. boreas specimens collected from Los Angeles County in 1915 (CAS 39865, 39867). Both individuals had lesions of hyperplasia and hyperkeratosis (a thickened stratum corneum, consistent with chytridiomycosis infection (Pessier, Nichols, Longcore, & Fuller, 1999)). All of the R. boylii collected from Clear Creek in San Benito County in 1989 that later died in captivity exhibited hyperkeratosis and hyperplasia, as did the A. californicus metamorph that died in the same university laboratory at the same time as the R. boylii collected from Clear Creek. No visceral lesions of other infectious diseases known to cause mortality events of amphibians (e.g., Ranavirus) were observed in any of the animals histologically examined. None of the 59 specimens additionally tested for Bsal were positive for that pathogen, consistent with sampling efforts that have not yet found Bsal infection in North America (Bsal Task Force, 2016).
3.4. Field sampling
Between 12 March 2011 and 9 September 2014, we captured and sampled 366 postmetamorphic anurans. Only A. californicus and H. cadaverina did not test positive for Bd; infection prevalence (Figure 7a) for all species combined was 15% (55 of 366 samples). Among species, Bd prevalence and load were highest in the two ranid species sampled (R. draytonii and R. catesbeiana) and H. regilla (Figure 7b).
Figure 7.
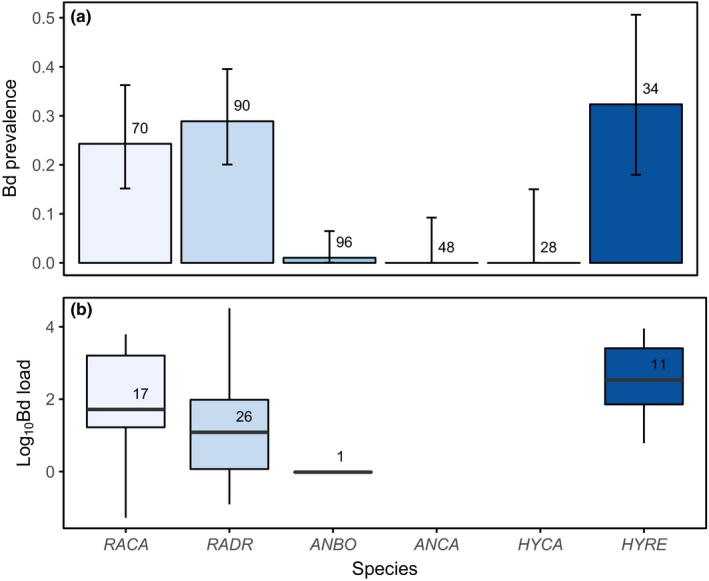
Bd infection among live anurans sampled for Bd in the wild during 2011–2014 within the former range of Rana boylii in southern California (Ventura, Santa Barbara, and Los Angeles counties), indicating (a) Bd prevalence; and (b) pathogen load of Bd‐positive individuals. Error bars in (a) represent the 95% Clopper–Pearson binomial confidence intervals. Bold horizontal lines within each boxplot in (b) indicate the median, boxes show the interquartile (IQ) range, and whiskers show the range within 1.5 times the IQ range. Numbers above the bars indicate (a) total sample size for each species or (b) the number of Bd‐positive individuals. RACA = Rana catesbeiana;RADR = Rana draytonii;ANBO = Anaxyrus boreas;ANCA = Anaxyrus californicus;HYCA = Hyliola cadaverina;HYRE = Hyliola regilla
4. DISCUSSION
4.1. Bd prevalence coincident with R. boylii extirpation
This study provides a clearer estimate of the timing of R. boylii extirpation from southern California, focuses the geographic scope of R. boylii decline analysis to this region, and is the first to examine disease as a causative factor in the historically precipitous declines of this species. One of the most important findings of the current study is that the proliferation of Bd in southern California coincided with the rapid extirpation of R. boylii from the region (Figures 2 and 4). Many declines ascribed to Bd have been unusually rapid as compared to those attributed to other causes, and in retrospective disease‐related analyses of museum specimens, high Bd prevalence is often observed during and after declines, but not before (Berger et al., 1998; Cheng, Rovito, Wake, & Vredenburg, 2011; Gillespie, Hunter, Berger, & Marantelli, 2015; Lips et al., 2006; Muths, Corn, Pessier, & Green, 2003; Vredenburg, Knapp, Tunstall, & Briggs, 2010). When Bd first arrives in a naïve population of susceptible hosts, infection prevalence rapidly increases, followed by either extirpation or transition to an eventual enzootic steady state of disease dynamics, wherein disease prevalence decreases (Briggs et al., 2010; Vredenburg et al., 2010).
The spatial pattern of R. boylii decline also mirrors patterns of Bd emergence. The gradual, progressive south‐to‐north trend of R. boylii declines and extirpations in California was mentioned by two R. boylii experts during their interviews (9 and 10) and is supported by the literature (Davidson et al., 2002; Lind, 2005). In addition, the wavelike pattern of Bd emergence and proliferation observed in this study (Figure 6) is consistent with observations elsewhere in California (Padgett‐Flohr & Hopkins, 2009; Vredenburg et al., 2010) and other parts of the world (Laurance, McDonald, & Speare, 1996; Lips, Diffendorfer, Mendelson, & Sears, 2008). Abundance information estimated from interviews, specimen collections, and field notes suggests that R. boylii populations in southern California prior to the proliferation of Bd were not small (Figure 4). While these are only relative abundance estimates and not the results of field surveys specifically targeted for quantifying R. boylii occupancy, they suggest that the species was relatively abundant shortly before it was extirpated (Figure 4).
4.2. Pathogen detection from museum specimens
Because of the large sample sizes in every decade from 1940 to 1980, we can be quite confident that Bd prevalence was initially low and then increased markedly. Because of the smaller sample sizes in the early decades and the failure of histology to detect Bd in early‐collected specimens, we are less confident of the prevalence prior to 1940. The probability of Bd detection from formalin‐fixed, ethanol‐preserved specimens increases as prefixation (live animal) infection load increases (Adams et al., 2015); therefore, it is likely that higher Bd prevalence observed between the 1970s and 1990s in this study also indicates higher pathogen burden, which is consistent with an epizootic disease phase.
It is important to note that due to the difficulty in recovering Bd DNA from formalin‐fixed museum specimens using qPCR (Adams et al., 2015), estimated Bd prevalence from museum specimens will appear lower than is actually the case; therefore, comparing contemporary, field‐based Bd data to specimen Bd data should be conducted with care. Although the prevalence estimates for field and specimen samples collected since the 2000s appear similar (Figures 3a and 7a), prevalences are likely much lower now than they were during the initial epizootic stage from the 1970s through the 1990s, due to better detection of Bd DNA from fresh field samples as compared to formalin‐fixed specimens (Figure 2). In addition, postfixation Bd load does not correlate with prefixation Bd load (Adams et al., 2015; Richards‐Hrdlicka, 2012); therefore, we are not able to infer prefixation (i.e., live animal) loads from the qPCR results we obtained from the specimens.
Despite not being able to detect chytrid organisms via histology in some qPCR‐positive specimens sampled from prior to 1970, we considered these positive for the purposes of this study, based on the following: (1) The two A. boreas specimens from 1915 (the earliest positives detected in this study) had lesions consistent with chytridiomycosis despite lacking Bd organisms; (2) histology can produce false negatives even in highly infected individuals due to the patchy or uneven distribution of infection on amphibian skin or low zoosporangia density (Boyle et al., 2004; Pessier et al., 1999; Reeder et al., 2012); (3) we were limited to sampling a single, small patch of skin on each specimen by some lending institutions, so the possibility that we missed sampling infected areas, due to the patchy distribution of chytridiomycosis on amphibian skin, is high; (4) earlier swabbing events may have removed some of the zoosporangia‐containing tissue that is important for histological diagnosis (Fong et al., 2015); (5) when the specimen sampling and qPCR protocols we followed are used, Bd contamination is unlikely (Adams et al., 2015); and (6) considering the pre‐1950s specimens positive based on two qPCR positives was conservative to our hypothesis that Bd prevalence increased in the middle of the 20th century. Items 1–5 above have implications extending beyond this study—we urge curators of natural history collections and the researchers that use them to communicate with one another regarding the specimens that have been sampled for Bd so that subsequent researchers can be aware of the increased risk of false histology negatives after specimens have been sampled, as the act of swabbing may remove zoosporangia‐containing tissue (Adams et al., 2015; Fong et al., 2015).
4.3. Reservoir hosts and Bd introduction
The presence of a pathogen reservoir can allow a pathogen to lead to disease‐induced extinction of some host species (Briggs et al., 2010; De Castro & Bolker, 2005). Our 2011–2014 field sampling of extant species within the former range of R. boylii indicates that Bd prevalence and loads are highest in R. catesbeiana (American bullfrog) and H. regilla (Figure 7). Both R. catesbeiana and H. regilla are considered to be vectors of Bd in California (Adams et al., 2017; Padgett‐Flohr & Hopkins, 2009; Reeder et al., 2012), and as an often‐tolerant Bd reservoir host, bullfrogs have been implicated in declines of amphibians in places throughout the world where they are not native, including California (Daszak et al., 2004; Jennings & Hayes, 1994a; Miaud et al., 2016). H. regilla and R. catesbeiana may have provided sufficient reservoir hosts to allow for Bd persistence in the system, facilitating chytridiomycosis‐induced extinction of R. boylii. While some laboratory studies have suggested that bullfrogs may not always make suitable reservoir hosts for Bd (Eskew, Worth, Foley, & Todd, 2015; Gervasi et al., 2013), our observation that bullfrogs have the highest Bd prevalence and loads among species sampled in the field (Figure 7), and previous work showing that bullfrog sympatry is an important predictor of Bd infection in R. boylii (Adams et al., 2017), supports the hypothesis that bullfrogs are a vector of Bd in California, including where R. boylii are extant.
Bullfrog farming may have played a key role in introducing and spreading Bd in California. Bullfrogs were brought to California in the early 20th century from the eastern United States, where Bd may have occurred in an enzootic state for much longer than in the western United States (Talley, Muletz, Vredenburg, Fleischer, & Lips, 2015). Between 1900 and 1930, several farms that originally imported bullfrogs from the eastern United States were present throughout California, resulting in bullfrog introductions to the wild (Collins et al., 2009). Around 1912, bullfrogs were introduced to an artificial lake near Topanga Canyon (Los Angeles County) from New Orleans, Louisiana, which provided a source population for subsequent introductions throughout California (Jennings & Hayes, 1994b). The earliest Bd positives detected in our study, two A. boreas collected from Big Tujunga Canyon (Los Angeles County) in 1915, may have been the result of nearby bullfrog farming operations such as the one in Topanga Canyon. Although Bd appears to have arrived early in California—even predating introductions of the Bd‐tolerant African clawed frog, Xenopus laevis (Huss, Huntley, Vredenburg, Johns, & Green, 2013; Weldon, du Preez, Hyatt, Muller, & Speare, 2004)—our results suggest that Bd may have experienced multiple invasions with limited spread (Sette et al., 2015) prior to its proliferation beginning in the 1970s in southern California (Figure 2). In addition, Bd's ubiquity among sites sampled in the 2010s, its relatively low prevalence and pathogen loads among the anuran community, and a postepizootic pattern of decline in prevalence after the 1990s indicate that it may have reached an enzootic state in the study area (Figures 2 and 7).
4.4. Anthropogenic stressors
The 1970s extirpation of R. boylii from southern California is contemporaneous with some of the earliest reports of enigmatic amphibian declines in western North America (Blaustein & Wake, 1990; Drost & Fellers, 1996; Green & Kagarise Sherman, 2001; Sherman & Morton, 1993), Puerto Rico (Burrowes et al., 2004), and Australia (Berger, Speare, & Hyatt, 1999). After World War II, California's human population grew much faster than most other U.S. regions (Hope, 2011), sparking sprawling development and facilitating the spread of exotic pathogens that were once restricted to their native habitats or near ports of entry. Cities and intensive human activity increase opportunities for direct amphibian pathogen transport (Price, Garner, Cunningham, Langton, & Nichols, 2016) and spread via other hosts (Schloegel et al., 2009; Weldon et al., 2004). Roads are often associated with increased pathogen transmission (Jules, Kauffman, Ritts, & Carroll, 2002), and in a retrospective study in northern California, the earliest Bd positives occurred closest to a transportation corridor (De León, Vredenburg, & Piovia‐Scott, 2016), suggesting that roads are able to facilitate the spread of Bd.
Increased human use of natural areas within R. boylii habitat during the rapid development of southern California in the middle of the 20th century was likely exacerbated by the proliferation and wide availability of the pet trade. At this time, the pet trade was thriving—wild‐caught red‐eared sliders (Trachemys scripta elegans) and frogs were widely available at department stores such as Grant's in southern California (Interview 12) (Salisbury Post, 2014). A burgeoning pet trade and increased use of backcountry areas may have contributed to the spread of Bd, due to the increased likelihood of captive releases of pets back into the wild.
Several interviewees cited an increase in human use of habitats immediately prior to R. boylii extirpations (Table 2). In the two chytridiomycosis‐related R. boylii mortality events reported in this study, the habitats of both populations were actively being used by off‐road vehicles at the time (Interviews 12 and 19; although the source population for the captive animals that experienced mortality in the laboratory did not succumb to chytridiomycosis in the field). This coincidence suggests that increased recreational use by humans could facilitate the spread and proliferation of Bd in these habitats. In addition, environmental disturbances may stress amphibians enough to suppress their immune defenses against Bd (Rollins‐Smith et al., 2002) (but see Searle, Belden, Du, & Blaustein, 2014).
4.5. Extreme flooding
In January and February 1969, extreme flooding caused extensive damage in southern California, killing at least 115 people (U.S. Army Corps of Engineers, 1969). It has been suggested that the 1969 flood events led to the extirpation of R. boylii from southern California (Sweet, 1983), and five interviewees mentioned flooding as a proximate or ultimate cause of R. boylii extirpation (Table 2). Although only one of these observations of R. boylii extirpations following flood events occurred in the southern California study area (Evey Canyon, Los Angeles County), they suggest that the species could be vulnerable to extreme flood events. R. boylii is a stream obligate and rarely found more than a few meters from water (Kupferberg, 1996; Zweifel, 1955). Radio‐tracked R. boylii in northern California have been observed up to 40 m from the stream channel during relatively extreme precipitation events, suggesting that they will use uplands to avoid flows that would otherwise sweep them downstream, and will avoid peak flows in the main stream channel by overwintering in tributaries (Bourque, 2008). In another study, the magnitude of winter floods did not affect R. boylii clutch density (a measure of adult abundance), but dam releases timed asynchronously with environmental cues for flooding caused scouring of clutches and R. boylii recruitment losses (Kupferberg et al., 2012).
Evey Canyon is a steep site where frogs may not have been able to retreat to uplands during flooding. The 1969 flood event, however, was not the first of its kind in the region. The 1969 floods are considered the most damaging on record because they occurred after extensive development, but flooding in 1907, 1914, 1916, and 1938 may have equaled the 1969 floods in magnitude (U.S. Army Corps of Engineers, 1969). In addition, sediment deposition indicates that the 1969 floods were predated by much larger flood events (Hendy, Napier, & Schimmelmann, 2015; Ingram, 2013). Flooding has the potential to cause catastrophic mortality events, affecting population age structure, but is unlikely, on its own, to cause the rapid extirpation of a species from an entire region (Corn, 1994; Metter, 1968). Therefore, flooding was probably not the sole cause of R. boylii extirpation from southern California. Instead, we suggest that the flood event occurred at the same time as when Bd prevalence was on the rise (Figure 2), so chytridiomycosis and extreme flooding may have acted in concert to extirpate the species.
4.6. Why does R. boylii persist elsewhere in its range?
Regional contrasts in phylogeography, geomorphology, and climate may contribute to the absence of R. boylii in southern California relative to northern California and southern Oregon. R. boylii is more phylogenetically distinct at the edges of its range than at the core—the most distinct and divergent lineages occur at the southern portion of its range (Lind et al., 2011)—suggesting that the southern California populations may have differed in their susceptibility to Bd and subsequent extirpation. Differential outcomes in Bd susceptibility have been observed in the field (Briggs et al., 2010), and under identical conditions in the laboratory (Searle et al., 2011) (C. Davidson, unpublished data). Immunological and genetic responses to Bd infection may reduce frogs’ Bd susceptibility, resulting in persistent populations that are able to recover if there is a large enough population to sustain itself while this evolution occurs over generations (Knapp et al., 2016; Ramsey, Reinert, Harper, Woodhams, & Rollins‐Smith, 2010; Savage & Zamudio, 2011).
Although the interviews and histological examinations presented here, combined with a field study (Adams et al., 2017), indicate that R. boylii populations in central and northern California have also been affected by Bd and flooding, differences in environmental context may have contributed to a different outcome. The Mediterranean streams of southern California are qualitatively different from those in northern California. In southern California, highly variable hydrologic regimes are characterized by flashier flows, more ephemeral channels, and a higher degree of intermittency due to the lower total rainfall of the region (Cooper, Lake, Sabater, Melack, & Sabo, 2013). In R. boylii‐occupied regions of northern California, the greater density of dendritic river networks creates wetter habitats with more tributaries and wider floodplains, making R. boylii populations less isolated, facilitating dispersal and recolonization of unoccupied streams (Groom, Meffe, & Carroll, 2006; Larned, Datry, Arscott, & Tockner, 2010).
Differences in timing of pathogen emergence may also contribute to amphibian persistence. Bd may not have arrived in central and northern California until later in the 20th century than our observations for southern California: Retrospective surveys of museum specimens suggest that Bd arrived in the San Francisco Bay area of California in the late 1950s (Padgett‐Flohr & Hopkins, 2009) (but see Huss et al., 2013) and as late as the mid‐1970s in parts of northern California (De León et al., 2016). One interviewee (10) reported heavy flooding in northern California in 1956 and 1964, which may have occurred prior to Bd's arrival in that region.
Bd has a low tolerance for warm temperatures, leading many to hypothesize that latitude and altitude are positively correlated with Bd infection (Knapp, Briggs, Smith, & Maurer, 2011; Kriger & Hero, 2008; Kriger, Pereoglou, & Hero, 2007). Under this assumption, one might expect southern California R. boylii populations to be less susceptible to Bd as compared to those further north. Southern California's Mediterranean climate is characterized by cool, wet winters and warm, dry summers. While the mean temperature is warmer overall as compared to the rest of California, southern California's streams and microhabitats are often within the temperature threshold for Bd's optimal growth (Klose, Cooper, Leydecker, & Kreitler, 2012; Piotrowski, Annis, & Longcore, 2004). In an extant population of R. boylii in central California, water temperature was not predictive of either Bd prevalence or load in R. boylii, although low stream flows were predictive (Adams et al., 2017). Therefore, it may be other aspects of climate beyond ambient temperatures (i.e., precipitation, alternation of extreme droughts and floods) that distinguish the southern part of R. boylii's range.
Frogs vary in their responses to different strains and isolates of Bd (Piovia‐Scott et al., 2015) and novel strains can cause negative impacts to species at the same time that endemic strains do not (Gahl, Longcore, & Houlahan, 2012). Yet, separate Bd strains do not always result in different Bd outcomes (Knapp et al., 2016). There is no evidence to suggest that the Bd DNA detected in this study is from a strain endemic to the region. All of the wild Bd isolates genotyped to date from California and the Pacific coast of North America are part of the Bd‐GPL (Global Panzootic Lineage), belonging to the rapidly dispersed hypervirulent lineage of Bd, and there is no genetic evidence of any endemic lineages in North America (James et al., 2015). Where endemic and epizootic Bd lineages are sympatric, the endemic Bd shows a much more limited geographic distribution, and Bd‐GPL may be better at dispersing in a heterogeneous landscape as well as infecting a broader range of host species (Jenkinson et al., 2016).
5. CONCLUSION
Emerging infectious diseases are a significant threat to global biodiversity, and information on the introduction, spread, and effects of novel pathogens is essential for enacting appropriate responses to this threat (Roy et al., 2016). Anthropogenic forces have probably played an important role in the spread of hypervirulent lineages of Bd globally (Farrer et al., 2011), and the rapid growth of mid‐20th‐century southern California coinciding with increased Bd prevalence and the extirpation of a once‐common anuran is consistent with this hypothesis. This study emphasizes the importance of the human context within which most host–pathogen systems occur, including perceptions of biodiversity loss. Many interviewees were reluctant to hypothesize why R. boylii disappeared in such a short period of time, in part because amphibian populations naturally fluctuate; many did not know that anything unusual was happening at the time. As one interviewee stated:
…I would go to places I had seen them [R. boylii] and they wouldn't be there anymore… I didn't think anything of it, of course—you see those patterns—but I saw 2 or 3 frogs at a spot one year and then went back 5 years later and didn't see anything. It didn't mean anything [at the time], but when you don't see them any more times that you're out there…then you realize that they're gone. (Interview 2)
Historical information can help guard against inaccurate assumptions about abundance and trends that can lead to poorly informed management decisions. Considering the extent of R. boylii's extirpation from many areas of California, biologists are examining the practicalities of reintroducing the species to areas where it has been extirpated (Lind, 2005). Gaining a better understanding of pattern and process of extinctions and extirpations is only the beginning of successfully predicting best management practices for repatriation programs. An important next phase is to describe the immunogenetic and ecological attributes of R. boylii populations that are persisting despite ongoing Bd infection, and use insights gained from these studies to design R. boylii reintroduction programs that maximize the probability of success. In combination with our findings about the current prevalence of Bd among the amphibians dwelling in the streams that could provide suitable habitat for R. boylii, our historical analysis provides a solid foundation for assessing the feasibility of repatriation of this species to southern California where it has long been extirpated.
CONFLICT OF INTEREST
None declared.
AUTHORSHIP
AJA designed the study, acquired the data, analyzed and interpreted the data, and wrote the manuscript; APP contributed to acquisition and interpretation of data and revised the manuscript; CJB contributed to conception and design and analysis and interpretation of data and revised the manuscript.
Supporting information
ACKNOWLEDGMENTS
We thank all of the respondents and interviewees for their generosity with their time, records, and insights; Greg Pauly, Neftali Camacho, Mireia Beas‐Moix, Jens Vindum, Carol Spencer, Brad Hollingsworth, Emily Taylor, A. Kristopher Lappin, and Paul Collins for accommodating access to their respective institutions’ collections; Sam Sweet for countless insights, information, and recommendations; John LaBonte for sharing ideas; Theora Tiffney, Paul Malone, and Megan Swanson for assistance in collecting and processing samples; Finn Christie for assistance with data entry; Peter Alagona for guidance with historical methods; and Sarah Kupferberg, Roland Knapp, and two anonymous reviewers for their perceptive and helpful comments on the manuscript. This work was financially supported by grants from the Robert and Patricia Switzer Foundation (awarded to AJA) and the National Science Foundation (DEB‐1557190; awarded to CJB).
Adams AJ, Pessier AP, Briggs CJ Rapid extirpation of a North American frog coincides with an increase in fungal pathogen prevalence: Historical analysis and implications for reintroduction. Ecol Evol. 2017;7:10216–10232. https://doi.org/10.1002/ece3.3468
REFERENCES
- Adams, A. J. , Kupferberg, S. J. , Wilber, M. Q. , Pessier, A. P. , Grefsrud, M. , Bobzien, S. , … Briggs, C. J. (2017). Extreme drought, host density, sex, and bullfrogs influence fungal pathogen infection in a declining lotic amphibian. Ecosphere, 8, e01740. [Google Scholar]
- Adams, A. J. , Labonte, J. P. , Ball, M. L. , Richards‐Hrdlicka, K. L. , Toothman, M. H. , & Briggs, C. J. (2015). DNA extraction method affects the detection of a fungal pathogen in formalin‐fixed specimens using qPCR. PLoS One, 10, e0135389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alagona, P. S. , Sandlos, J. , & Wiersma, Y. F. (2012). Past imperfect. Environmental Philosophy, 9, 49–70. [Google Scholar]
- Alford, R. A. , & Richards, S. J. (1997). Lack of evidence for epidemic disease as an agent in the catastrophic decline of Australian rain forest frogs. Conservation Biology, 11, 1026–1029. [Google Scholar]
- Anderson, R. M. , May, R. M. , & Anderson, B. (1992). Infectious diseases of humans: Dynamics and control. Oxford, UK: Oxford University Press. [Google Scholar]
- Berger, L. , Roberts, A. A. , Voyles, J. , Longcore, J. E. , Murray, K. A. , & Skerratt, L. F. (2016). History and recent progress on chytridiomycosis in amphibians. Fungal Ecology, 19, 89–99. [Google Scholar]
- Berger, L. , Speare, R. , Daszak, P. , Green, D. E. , Cunningham, A. A. , Goggin, C. L. , … Parkes, H. (1998). Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. Proceedings of the National Academy of Sciences of the United States of America, 95, 9031–9036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger, L. , Speare, R. , & Hyatt, A. (1999). Chytrid fungi and amphibian declines: Overview, implications and future directions In Campbell A. (Ed.), Declines and disappearances of Australian frogs (pp. 23–33). Canberra: Environment Australia. [Google Scholar]
- Blaustein, A. R. , & Wake, D. B. (1990). Declining amphibian populations: A global phenomenon? Trends in Ecology and Evolution, 5, 203–204. [Google Scholar]
- Blooi, M. , Pasmans, F. , Longcore, J. E. , Spitzen‐van der Sluijs, A. , Vercammen, F. , & Martel, A. (2013). Duplex real‐time PCR for rapid simultaneous detection of Batrachochytrium dendrobatidis and Batrachochytrium salamandrivorans in amphibian samples. Journal of Clinical Microbiology, 51, 4173–4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque, R. (2008). Spatial ecology of an inland population of the foothill yellow‐legged frog (Rana boylii) in Tehama County, California. Master's thesis, California State University, Humboldt. [Google Scholar]
- Boyle, D. G. , Boyle, D. B. , Olsen, V. , Morgan, J. A. T. , & Hyatt, A. D. (2004). Rapid quantitative detection of chytridiomycosis (Batrachochytrium dendrobatidis) in amphibian samples using real‐time Taqman PCR assay. Diseases of Aquatic Organisms, 60, 141–148. [DOI] [PubMed] [Google Scholar]
- Briggs, C. J. , Knapp, R. A. , & Vredenburg, V. T. (2010). Enzootic and epizootic dynamics of the chytrid fungal pathogen of amphibians. Proceedings of the National Academy of Sciences of the United States of America, 107, 9695–9700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bsal Task Force (2016). Bsal Task Force 2016 Annual Report. Retrieved from http://www.salamanderfungus.org/wp-content/uploads/2016/11/Bsal-Report_1610_4-0v.pdf.
- Burnham, K. P. , & Anderson, D. R. (2004). Multimodel inference: Understanding AIC and BIC in model selection. Sociological Methods & Research, 33, 261–304. [Google Scholar]
- Burrowes, P. A. , Joglar, R. L. , & Green, D. E. (2004). Potential causes for amphibian declines in Puerto Rico. Herpetologica, 60, 141–154. [Google Scholar]
- Bury, R. , & Sisk, N. (1997). Amphibians and reptiles of the Cow Creek Watershed in the BLM‐Roseburg District. Report submitted to BLM‐Roseburg District and Oregon Dept. Fish and Wildl., Roseburg. Biological Resources Division, USGS, Corvallis, OR. [Google Scholar]
- California Academy of Sciences (2016). Herpetology collection database. Retrieved from http://researcharchive.calacademy.org/research/herpetology/catalog/Index.asp
- Catenazzi, A. (2015). State of the world's amphibians. Annual Review of Environment and Resources, 40, 91–119. [Google Scholar]
- Catenazzi, A. , & Kupferberg, S. J. (2017). Variation in thermal niche of a declining river‐breeding frog: From counter‐gradient responses to population distribution patterns. Freshwater Biology, 62, 1255–1265. [Google Scholar]
- Cheng, T. L. , Rovito, S. M. , Wake, D. B. , & Vredenburg, V. T. (2011). Coincident mass extirpation of neotropical amphibians with the emergence of the infectious fungal pathogen Batrachochytrium dendrobatidis . Proceedings of the National Academy of Sciences of the United States of America, 108, 9502–9507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins, J. P. , Crump, M. L. , & Lovejoy, T. E. III (2009). Extinction in our times: Global amphibian decline. Oxford, UK: Oxford University Press. [Google Scholar]
- Cooper, S. D. , Lake, P. S. , Sabater, S. , Melack, J. M. , & Sabo, J. L. (2013). The effects of land use changes on streams and rivers in mediterranean climates. Hydrobiologia, 719, 383–425. [Google Scholar]
- Corn, P. S. (1994). What we know and don't know about amphibian declines in the West. USDA Forest Service, General Technical Report RM‐247 (May 1994).
- Corn, P. S. (2000). Amphibian declines: Review of some current hypotheses In Sparling D., Linder G. & Bishop C. A. (Eds.), Ecotoxicology of amphibians and reptiles (pp. 633–696). Pensacola, FL: SETAC Press. [Google Scholar]
- Daszak, P. , Strieby, A. , Cunningham, A. A. , Longcore, J. E. , Brown, C. C. , & Porter, D. (2004). Experimental evidence that the bullfrog (Rana catesbeiana) is a potential carrier of chytridiomycosis, an emerging fungal disease of amphibians. Herpetological Journal, 14, 201–207. [Google Scholar]
- Davidson, C. (2004). Declining downwind: Amphibian population declines in California and historical pesticide use. Ecological Applications, 14, 1892–1902. [Google Scholar]
- Davidson, C. , Benard, M. F. , Shaffer, H. B. , Parker, J. M. , O'Leary, C. , Conlon, J. M. , & Rollins‐Smith, L. A. (2007). Effects of chytrid and carbaryl exposure on survival, growth and skin peptide defenses in foothill yellow‐legged frogs. Environmental Science & Technology, 41, 1771–1776. [DOI] [PubMed] [Google Scholar]
- Davidson, E. W. , Parris, M. , Collins, J. P. , Longcore, J. E. , Pessier, A. P. , Brunner, J. , & Beaupre, S. J. (2003). Pathogenicity and transmission of chytridiomycosis in tiger salamanders (Ambystoma tigrinum). Copeia, 2003, 601–607. [Google Scholar]
- Davidson, C. , Shaffer, H. B. , & Jennings, M. R. (2002). Spatial tests of the pesticide drift, habitat destruction, UV‐B, and climate‐change hypotheses for California amphibian declines. Conservation Biology, 16, 1588–1601. [Google Scholar]
- De Castro, F. , & Bolker, B. (2005). Mechanisms of disease‐induced extinction. Ecology Letters, 8, 117–126. [Google Scholar]
- De León, M. E. , Vredenburg, V. T. , & Piovia‐Scott, J. (2016). Recent emergence of a chytrid fungal pathogen in California Cascades frogs (Rana cascadae). EcoHealth, 14, 1–7. [DOI] [PubMed] [Google Scholar]
- Drost, C. A. , & Fellers, G. M. (1996). Collapse of a regional frog fauna in the Yosemite area of the California Sierra Nevada, USA. Conservation Biology, 10, 414–425. [Google Scholar]
- Eskew, E. A. , Worth, S. J. , Foley, J. E. , & Todd, B. D. (2015). American Bullfrogs (Lithobates catesbeianus) resist infection by multiple isolates of Batrachochytrium dendrobatidis, including one implicated in wild mass mortality. EcoHealth, 12, 513–518. [DOI] [PubMed] [Google Scholar]
- Farrer, R. A. , Weinert, L. A. , Bielby, J. , Garner, T. W. , Balloux, F. , Clare, F. , … Fisher, M. C. (2011). Multiple emergences of genetically diverse amphibian‐infecting chytrids include a globalized hypervirulent recombinant lineage. Proceedings of the National Academy of Sciences of the United States of America, 108, 18732–18736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher, M. C. , Henk, D. A. , Briggs, C. J. , Brownstein, J. S. , Madoff, L. C. , McCraw, S. L. , & Gurr, S. J. (2012). Emerging fungal threats to animal, plant and ecosystem health. Nature, 484, 186–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong, J. J. , Cheng, T. L. , Bataille, A. , Pessier, A. P. , Waldman, B. , & Vredenburg, V. T. (2015). Early 1900s detection of Batrachochytrium dendrobatidis in Korean Amphibians. PLoS One, 10, e0115656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahl, M. K. , Longcore, J. E. , & Houlahan, J. E. (2012). Varying responses of northeastern North American amphibians to the chytrid pathogen Batrachochytrium dendrobatidis . Conservation Biology, 26, 135–141. [DOI] [PubMed] [Google Scholar]
- Gervasi, S. S. , Urbina, J. , Hua, J. , Chestnut, T. , Relyea, R. , & Blaustein, A. (2013). Experimental evidence for American bullfrog (Lithobates catesbeianus) susceptibility to chytrid fungus (Batrachochytrium dendrobatidis). EcoHealth, 10, 166–171. [DOI] [PubMed] [Google Scholar]
- Gillespie, G. R. , Hunter, D. , Berger, L. , & Marantelli, G. (2015). Rapid decline and extinction of a montane frog population in southern Australia follows detection of the amphibian pathogen Batrachochytrium dendrobatidis . Animal Conservation, 18, 295–302. [Google Scholar]
- Golden, A. S. , Naisilsisili, W. , Ligairi, I. , & Drew, J. A. (2014). Combining natural history collections with fisher knowledge for community‐based conservation in Fiji. PLoS One, 9, e98036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green, D. E. , & Kagarise Sherman, C. (2001). Diagnostic histological findings in Yosemite toads (Bufo canorus) from a die‐off in the 1970s. Journal of Herpetology, 35, 92–103. [Google Scholar]
- Griffiths, R. A. , & Pavajeau, L. (2008). Captive breeding, reintroduction, and the conservation of amphibians. Conservation Biology, 22, 852–861. [DOI] [PubMed] [Google Scholar]
- Groom, M. J. , Meffe, G. K. , & Carroll, C. R. (2006). Principles of conservation biology. Sunderland: Sinauer Associates. [Google Scholar]
- Hayes, M. P. , Wheeler, C. A. , Lind, A. J. , Green, G. A. , & Macfarlane, D. C. (2016). Foothill yellow‐legged frog conservation assessment in California. USDA General Technical Report PSW‐GTR‐248.
- Heard, M. J. , Smith, K. F. , Ripp, K. J. , Berger, M. , Chen, J. , Dittmeier, J. , … Ryan, E. (2013). The threat of disease increases as species move toward extinction. Conservation Biology, 27, 1378–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendy, I. L. , Napier, T. J. , & Schimmelmann, A. (2015). From extreme rainfall to drought: 250 years of annually resolved sediment deposition in Santa Barbara Basin, California. Quaternary International, 387, 3–12. [Google Scholar]
- Hero, J.‐M. , & Gillespie, G. R. (1997). Epidemic disease and Amphibian declines in Australia. Conservation Biology, 11, 1023–1025. [Google Scholar]
- Herp Nation Media (2016). Field Herp Forum: The Worldwide Community of Field Herpers [Online]. Retrieved from http://www.fieldherpforum.com/.
- Hope, A. (2011). Tract housing in California, 1945‐1973: A context for National Register evaluation. Sacramento: California Department of Transportation. [Google Scholar]
- Houlahan, J. E. , Findlay, C. S. , Schmidt, B. R. , Meyer, A. H. , & Kuzmin, S. L. (2000). Quantitative evidence for global amphibian population declines. Nature, 404, 752–755. [DOI] [PubMed] [Google Scholar]
- Huntington, H. P. (1998). Observations on the utility of the semi‐directive interview for documenting traditional ecological knowledge. Arctic, 51, 237–242. [Google Scholar]
- Huss, M. , Huntley, L. , Vredenburg, V. , Johns, J. , & Green, S. (2013). Prevalence of Batrachochytrium dendrobatidis in 120 Archived Specimens of Lithobates catesbeianus (American Bullfrog) Collected in California, 1924–2007. EcoHealth, 10, 339–343. [DOI] [PubMed] [Google Scholar]
- Hyatt, A. D. , Boyle, D. G. , Olsen, V. , Boyle, D. B. , Berger, L. , Obendorf, D. , … Colling, A. (2007). Diagnostic assays and sampling protocols for the detection of Batrachochytrium dendrobatidis . Diseases of Aquatic Organisms, 73, 175–192. [DOI] [PubMed] [Google Scholar]
- Ingram, B. L. (2013). California megaflood: Lessons from a forgotten catastrophe. Scientific American. [Google Scholar]
- IUCN (2013). Guidelines for reintroductions and other conservation translocations. Gland, Switzerland: IUCN Species Survival Commission. [Google Scholar]
- James, T. Y. , Toledo, L. F. , Rödder, D. , da Silva Leite, D. , Belasen, A. M. , Betancourt‐Román, C. M. , … Longcore, J. E. (2015). Disentangling host, pathogen, and environmental determinants of a recently emerged wildlife disease: Lessons from the first 15 years of amphibian chytridiomycosis research. Ecology and Evolution, 5, 4079–4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson, T. S. , Betancourt Román, C. M. , Lambertini, C. , Valencia‐Aguilar, A. , Rodriguez, D. , Nunes‐de‐Almeida, C. H. L. , … James, T. Y. (2016). Amphibian‐killing chytrid in Brazil comprises both locally endemic and globally expanding populations. Molecular Ecology, 25, 2978–2996. [DOI] [PubMed] [Google Scholar]
- Jennings, M. R. (2005). Native ranid frogs in California. [Online]. U.S. Geological Survey. Retrieved from http://biology.usgs.gov/s+t/noframe/d054.htm.
- Jennings, M. , & Hayes, M. P. (1994a). Amphibian and reptile species of special concern in California. Rancho Cordova, CA: California Department of Fish and Game. [Google Scholar]
- Jennings, M. R. , & Hayes, M. P. (1994b). Decline of native ranid frogs in the desert southwest. Herpetology of the North American deserts . Southwestern Herpetologists Society, Special Publication, 5, 183–211. [Google Scholar]
- Jules, E. S. , Kauffman, M. J. , Ritts, W. D. , & Carroll, A. L. (2002). Spread of an invasive pathogen over a variable landscape: A nonnative root rot on Port Orford cedar. Ecology, 83, 3167–3181. [Google Scholar]
- Kauffman, M. J. , & Jules, E. S. (2006). Heterogeneity shapes invasion: Host size and environment influence susceptibility to a nonnative pathogen. Ecological Applications, 16, 166–175. [DOI] [PubMed] [Google Scholar]
- Kissel, A. M. , Palen, W. J. , & Govindarajulu, P. (2017). A decision‐theory approach to cost‐effective population supplementation for imperiled species. Ecological Economics, 142, 194–202. [Google Scholar]
- Klose, K. , Cooper, S. D. , Leydecker, A. D. , & Kreitler, J. (2012). Relationships among catchment land use and concentrations of nutrients, algae, and dissolved oxygen in a southern California river. Freshwater Science, 31, 908–927. [Google Scholar]
- Knapp, R. A. , Briggs, C. J. , Smith, T. C. , & Maurer, J. R. (2011). Nowhere to hide: Impact of a temperature‐sensitive amphibian pathogen along an elevation gradient in the temperate zone. Ecosphere, 2, 1–26. [Google Scholar]
- Knapp, R. A. , Fellers, G. M. , Kleeman, P. M. , Miller, D. A. W. , Vredenburg, V. T. , Rosenblum, E. B. , & Briggs, C. J. (2016). Large‐scale recovery of an endangered amphibian despite ongoing exposure to multiple stressors. Proceedings of the National Academy of Sciences of the United States of America, 113, 11889–11894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriger, K. M. , & Hero, J.‐M. (2008). Altitudinal distribution of chytrid (Batrachochytrium dendrobatidis) infection in subtropical Australian frogs. Austral Ecology, 33, 1022–1032. [Google Scholar]
- Kriger, K. M. , Pereoglou, F. , & Hero, J. M. (2007). Latitudinal variation in the prevalence and intensity of chytrid (Batrachochytrium dendrobatidis) infection in eastern Australia. Conservation Biology, 21, 1280–1290. [DOI] [PubMed] [Google Scholar]
- Kupferberg, S. J. (1996). Hydrologic and geomorphic factors affecting conservation of a river‐breeding frog (Rana boylii). Ecological Applications, 6, 1332–1344. [Google Scholar]
- Kupferberg, S. J. , Palen, W. J. , Lind, A. J. , Bobzien, S. , Catenazzi, A. , Drennan, J. O. E. , & Power, M. E. (2012). Effects of flow regimes altered by dams on survival, population declines, and range‐wide losses of California river‐breeding frogs. Conservation Biology, 26, 513–524. [DOI] [PubMed] [Google Scholar]
- Larned, S. T. , Datry, T. , Arscott, D. B. , & Tockner, K. (2010). Emerging concepts in temporary‐river ecology. Freshwater Biology, 55, 717–738. [Google Scholar]
- Laurance, W. F. , McDonald, K. R. , & Speare, R. (1996). Epidemic disease and the catastrophic decline of Australian rain forest frogs. Conservation Biology, 10, 406–413. [Google Scholar]
- Lind, A. J. (2005). Reintroduction of a declining amphibian: Determining an ecologically feasible approach for the foothill yellow‐legged frog (Rana boylii) through analysis of decline factors, genetic structure, and habitat associations. Ph.D. dissertation, University of California, Davis, CA. [Google Scholar]
- Lind, A. J. , Spinks, P. Q. , Fellers, G. M. , & Shaffer, H. B. (2011). Rangewide phylogeography and landscape genetics of the Western U.S. endemic frog Rana boylii (Ranidae): Implications for the conservation of frogs and rivers. Conservation Genetics, 12, 269–284. [Google Scholar]
- Lind, A. J. , Welsh, H. H. , & Wilson, R. A. (1996). The effects of a dam on breeding habitat and egg survival of the foothill yellow‐legged frog (Rana boylii) in northwestern California. Herpetological Review, 27, 62–67. [Google Scholar]
- Lips, K. R. (2011). Museum collections: Mining the past to manage the future. Proceedings of the National Academy of Sciences of the United States of America, 108, 9323–9324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lips, K. R. , Brem, F. , Brenes, R. , Reeve, J. D. , Alford, R. A. , Voyles, J. , … Collins, J. P. (2006). Emerging infectious disease and the loss of biodiversity in a Neotropical amphibian community. Proceedings of the National Academy of Sciences of the United States of America, 103, 3165–3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lips, K. R. , Diffendorfer, J. , Mendelson, J. R. , & Sears, M. W. (2008). Riding the wave: Reconciling the roles of disease and climate change in amphibian declines. PLoS Biology, 6, e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lips, K. R. , Mendelson, J. R. III , Muñoz‐Alonso, A. , Canseco‐Márquez, L. , & Mulcahy, D. G. (2004). Amphibian population declines in montane southern Mexico: Resurveys of historical localities. Biological Conservation, 119, 555–564. [Google Scholar]
- Longcore, J. E. , Pessier, A. P. , & Nichols, D. K. (1999). Batrachochytrium dendrobatidis gen. et sp. nov., a chytrid pathogenic to amphibians. Mycologia, 91, 219–227. [Google Scholar]
- Martel, A. , Blooi, M. , Adriaensen, C. , van Rooij, P. , Beukema, W. , Fisher, M. C. , … Pasmans, F. (2014). Recent introduction of a chytrid fungus endangers Western Palearctic salamanders. Science, 346, 630–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel, A. , Spitzen‐Van der Sluijs, A. , Blooi, M. , Bert, W. , Ducatelle, R. , Fisher, M. C. , … Pasmans, F. (2013). Batrachochytrium salamandrivorans sp. nov. causes lethal chytridiomycosis in amphibians. Proceedings of the National Academy of Sciences of the United States of America, 110, 15325–15329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metter, E. (1968). The influence of floods on population structure of Ascaphus truei Stejneger. Journal of Herpetology, 1, 105–106. [Google Scholar]
- Miaud, C. , Dejean, T. , Savard, K. , Millery‐Vigues, A. , Valentini, A. , Curt Grand Gaudin, N. , & Garner, T. W. J. (2016). Invasive North American bullfrogs transmit lethal fungus Batrachochytrium dendrobatidis infections to native amphibian host species. Biological Invasions, 18, 2299–2308. [Google Scholar]
- Museum of Vertebrate Zoology (2016). EcoReader: Museum of Vertebrate Zoology archives. Berkeley, California: Museum of Vertebrate Zoology Archives. [Google Scholar]
- Muths, E. , Corn, P. S. , Pessier, A. P. , & Green, D. E. (2003). Evidence for disease‐related amphibian decline in Colorado. Biological Conservation, 110, 357–365. [Google Scholar]
- Muths, E. , Scherer, R. D. , Amburgey, S. M. , Matthews, T. , Spencer, A. W. , & Corn, P. S. (2016). First estimates of the probability of survival in a small‐bodied, high elevation frog or, how historical data can be useful. Canadian Journal of Zoology, 94, 599–606. [Google Scholar]
- National Science Foundation (2016). VertNet biodiversity database [Online]. Retrieved from http://portal.vertnet.org/search.
- Padgett‐Flohr, G. E. , & Hopkins, R. L. 2nd (2009). Batrachochytrium dendrobatidis, a novel pathogen approaching endemism in central California. Diseases of Aquatic Organisms, 83, 1–9. [DOI] [PubMed] [Google Scholar]
- Papworth, S. K. , Rist, J. , Coad, L. , & Milner‐Gulland, E. J. (2009). Evidence for shifting baseline syndrome in conservation. Conservation Letters, 2, 93–100. [Google Scholar]
- Pauly, D. (1995). Anecdotes and shifting baseline syndrome of fisheries. Trends in Ecology and Evolution, 10, 430. [DOI] [PubMed] [Google Scholar]
- Pessier, A. P. , Nichols, D. K. , Longcore, J. E. , & Fuller, M. S. (1999). Cutaneous chytridiomycosis in poison dart frogs (Dendrobates spp.) and White's tree frogs (Litoria caerulea). Journal of Veterinary Diagnostic Investigation, 11, 194–199. [DOI] [PubMed] [Google Scholar]
- Piotrowski, J. S. , Annis, S. L. , & Longcore, J. E. (2004). Physiology of Batrachochytrium dendrobatidis, a chytrid pathogen of amphibians. Mycologia, 96, 9–15. [PubMed] [Google Scholar]
- Piovia‐Scott, J. , Pope, K. , Joy Worth, S. , Rosenblum, E. B. , Poorten, T. , Refsnider, J. , … Foley, J. (2015). Correlates of virulence in a frog‐killing fungal pathogen: Evidence from a California amphibian decline. The ISME Journal, 9, 1570–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price, S. J. , Garner, T. W. J. , Cunningham, A. A. , Langton, T. E. S. , & Nichols, R. A. (2016). Reconstructing the emergence of a lethal infectious disease of wildlife supports a key role for spread through translocations by humans. Proceedings of the Royal Society B: Biological Sciences, 283, 20160952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2016). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Rachowicz, L. J. , Knapp, R. A. , Morgan, J. A. T. , Stice, M. J. , Vredenburg, V. T. , Parker, J. M. , & Briggs, C. J. (2006). Emerging infectious disease as a proximate cause of amphibian mass mortality. Ecology, 87, 1671–1683. [DOI] [PubMed] [Google Scholar]
- Ramsey, J. P. , Reinert, L. K. , Harper, L. K. , Woodhams, D. C. , & Rollins‐Smith, L. A. (2010). Immune defenses against Batrachochytrium dendrobatidis, a fungus linked to global amphibian declines, in the South African clawed frog, Xenopus laevis . Infection and Immunity, 78, 3981–3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redford, K. H. , Aune, K. , & Plumb, G. (2016). Hope is a bison. Conservation Biology, 30, 689–691. [DOI] [PubMed] [Google Scholar]
- Redford, K. , & Sanjayan, M. A. (2003). Retiring Cassandra. Conservation Biology, 17, 1473–1474. [Google Scholar]
- Reeder, N. M. M. , Pessier, A. P. , & Vredenburg, V. T. (2012). A reservoir species for the emerging amphibian pathogen Batrachochytrium dendrobatidis thrives in a landscape decimated by disease. PLoS One, 7, e33567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards‐Hrdlicka, K. L. (2012). Extracting the amphibian chytrid fungus from formalin‐fixed specimens. Methods in Ecology and Evolution, 3, 842–849. [Google Scholar]
- Rollins‐Smith, L. A. , Carey, C. , Longcore, J. , Doersam, J. K. , Boutte, A. , Bruzgal, J. E. , & Conlon, J. M. (2002). Activity of antimicrobial skin peptides from ranid frogs against Batrachochytrium dendrobatidis, the chytrid fungus associated with global amphibian declines. Developmental and Comparative Immunology, 26, 471–479. [DOI] [PubMed] [Google Scholar]
- Roy, H. E. , Hesketh, H. , Purse, B. V. , Eilenberg, J. , Santini, A. , Scalera, R. , … Dunn, A. M. (2016). Alien pathogens on the horizon: Opportunities for predicting their threat to wildlife. Conservation Letters, 10, 477–484. [Google Scholar]
- Sainsbury, A. W. , & Vaughan‐Higgins, R. J. (2012). Analyzing disease risks associated with translocations. Conservation Biology, 26, 442–452. [DOI] [PubMed] [Google Scholar]
- Salisbury Post (2014). A few ‘five and dimes’: Not pocket change. Staff Report.
- Savage, A. E. , & Zamudio, K. R. (2011). MHC genotypes associate with resistance to a frog‐killing fungus. Proceedings of the National Academy of Sciences of the United States of America, 108, 16705–16710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloegel, L. M. , Picco, A. M. , Kilpatrick, A. M. , Davies, A. J. , Hyatt, A. D. , & Daszak, P. (2009). Magnitude of the US trade in amphibians and presence of Batrachochytrium dendrobatidis and ranavirus infection in imported North American bullfrogs (Rana catesbeiana). Biological Conservation, 142, 1420–1426. [Google Scholar]
- Searle, C. L. , Belden, L. K. , Du, P. , & Blaustein, A. R. (2014). Stress and chytridiomycosis: Exogenous exposure to corticosterone does not alter amphibian susceptibility to a fungal pathogen. Journal of Experimental Zoology Part A: Ecological Genetics and Physiology, 321, 243–253. [DOI] [PubMed] [Google Scholar]
- Searle, C. L. , Gervasi, S. S. , Hua, J. , Hammond, J. I. , Relyea, R. A. , Olson, D. H. , & Blaustein, A. R. (2011). Differential host susceptibility to Batrachochytrium dendrobatidis, an emerging amphibian pathogen. Conservation Biology, 25, 965–974. [DOI] [PubMed] [Google Scholar]
- Sette, C. M. , Vredenburg, V. T. , & Zink, A. G. (2015). Reconstructing historical and contemporary disease dynamics: A case study using the California slender salamander. Biological Conservation, 192, 20–29. [Google Scholar]
- Sherman, C. K. , & Morton, M. L. (1993). Population declines of Yosemite toads in the eastern Sierra Nevada of California. Journal of Herpetology, 27, 186–198. [Google Scholar]
- Skerratt, L. F. , Berger, L. , Speare, R. , Cashins, S. , McDonald, K. R. , Phillott, A. D. , … Kenyon, N. (2007). Spread of chytridiomycosis has caused the rapid global decline and extinction of frogs. EcoHealth, 4, 125–134. [Google Scholar]
- Smith, K. F. , Sax, D. F. , & Lafferty, K. D. (2006). Evidence for the role of infectious disease in species Extinction and endangerment. Conservation Biology, 20, 1349–1357. [DOI] [PubMed] [Google Scholar]
- Stewart, G. R. , Jennings, M. R. , & Robert, H. Jr (2005). Sensitive species of snakes, frogs, and salamanders in southern California conifer forest areas: status and management. USDA Forest Service General Technical Report PSW‐GTR‐195.
- Stuart, S. N. , Chanson, J. S. , Cox, N. A. , Young, B. E. , Rodrigues, A. S. , Fischman, D. L. , & Waller, R. W. (2004). Status and trends of amphibian declines and extinctions worldwide. Science, 306, 1783–1786. [DOI] [PubMed] [Google Scholar]
- Sweet, S. S. (1983). Mechanics of a natural extinction event: Rana boylii in southern California. 26th Annual Meeting of the Society for the Study of Amphibians and Reptiles, 31st Annual Meeting of the Herpetologist's League, University of Utah.
- Swetnam, T. W. , Allen, C. D. , & Betancourt, J. L. (1999). Applied historical ecology: Using the past to manage for the future. Ecological Applications, 9, 1189–1206. [Google Scholar]
- Talley, B. L. , Muletz, C. R. , Vredenburg, V. T. , Fleischer, R. C. , & Lips, K. R. (2015). A century of Batrachochytrium dendrobatidis in Illinois amphibians (1888–1989). Biological Conservation, 182, 254–261. [Google Scholar]
- University of California Santa Barbara (2016). Specify herpetology database web portal [Online]. Retrieved from http://biobase.eri.ucsb.edu:8080/specify-solr/herpetology/.
- U.S. Army Corps of Engineers (1969). Report on the floods of January and February 1969 in Southern California. Los Angeles, U.S. Army Corps of Engineers. [Google Scholar]
- U.S. Fish and Wildlife Service (2015). Endangered and Threatened Wildlife and Plants; 90‐Day Findings on 31 Petitions. Washington, DC: U.S. Fish and Wildlife Service. [Google Scholar]
- Vredenburg, V. T. , Knapp, R. A. , Tunstall, T. S. , & Briggs, C. J. (2010). Dynamics of an emerging disease drive large‐scale amphibian population extinctions. Proceedings of the National Academy of Sciences of the United States of America, 107, 9689–9694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weldon, C. , du Preez, L. , Hyatt, A. D. , Muller, R. , & Speare, R. (2004). Origin of the amphibian chytrid fungus. Emerging Infectious Diseases, 10, 2100–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield, S. M. , Bell, K. E. , Philippi, T. , Sasa, M. , Bolanos, F. , Chaves, G. , … Donnelly, M. A. (2007). Amphibian and reptile declines over 35 years at La Selva, Costa Rica. Proceedings of the National Academy of Sciences of the United States of America, 104, 8352–8356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerefos, C. S. , Tetsis, P. , Kazantzidis, A. , Amiridis, V. , Zerefos, S. C. , Luterbacher, J. , … Papayannis, A. (2013). Further evidence of important environmental information content in red‐to‐green ratios as depicted in paintings by great masters. Atmospheric Chemistry and Physics Discussions, 13, 33145–33176. [Google Scholar]
- Zuur, A. F. , Ieno, E. N. , & Elphick, C. S. (2010). A protocol for data exploration to avoid common statistical problems. Methods in Ecology and Evolution, 1, 3–14. [Google Scholar]
- Zweifel, R. (1955). The ecology and systematics of the Rana boylii species group. University of California Publications in Zoology, 54, 207–292. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


