Introduction
Fluorescence imaging is a real-time intraoperative navigation modality. Generally, fluorescence-imaging is obtained by administrating a substance, defined fluorophore, and illuminating the areas of interest with a near-infrared (NIR) light source. The NIR light will modify the energetic status of the fluorophore which will result into the emission of a fluorescent signal in the visible range. The signal is collected by dedicated camera systems and displayed in real-time in the operative field. These characteristics make of fluorescence imaging an ideal surgical navigation modality, since it does not disrupt the surgical workflow and does not require a bulky equipment.
Fluorescence-guided surgery (FGS) has the potential to improve the diagnostic ability, to provide a real-time support to the surgical strategy decision making and provide an assessment of the efficacy of the treatment. So far, FGS has been used in many clinical situations. Relative to the digestive system, the mostly studied indications are fluorescence cholangiography (1) and the fluorescence-based angiography to estimate the perfusion of the anastomotic site (2). Certainly, the most groundbreaking application of FGS, in the field of surgical oncology, is in the use of cancer-specific fluorescent probes, enabling real-time precision identification of cancer tissue (3).
An extensive intelligence and networking activity, including main opinion leaders in the field, has allowed to identify major directions for future researches to improve the current performances of FGS, including: (I) the integration of computer-assisted interpretation of the fluorescent signal through dedicated software; (II) the development of targeted fluorescent probes which recognize precisely biological targets or tumor cells and allow for image-guided removal of cancers by focused energy delivery or surgical ablation; (III) the development of miniature flexible endoscopic platforms able to diagnose and treat precisely gastrointestinal neoplasia. Considering these windows of opportunity, an effective program to develop and implement FGS would require strong partnerships including optical engineers (for hardware development), computer scientists (for software), chemists (for fluorophore engineering) and obviously physicians to allow a clinical translation.
At our Institute of Image-Guided Surgery, IHU-Strasbourg, France, we have created a dedicated research unit named IHU-SPECTRA (Integrated Hub for Shining Perioperative EndoscopiC TheRAnostics; http://www.ihu-strasbourg.eu/ihu/en/innovation/translational-rd/research-teams/), consisting in a multilevel platform that aims at the development and implementation of new technologies related to FGS, as well as at the optimization of the use of current devices and fluorophores. IHU-SPECTRA gathers a team of digestive surgeons, scientists, engineers and several industrial partners, in order to provide an up-to-date and comprehensive portfolio of skills, products, contacts and business intelligence on both fluorophores (including regulatory aspects) and devices enabling FGS applied to the digestive system.
FGS could potentially help to solve some of the major challenges pertinent to the surgery of the digestive system, as follows:
❖ Challenge 1: accurate intraoperative evaluation of anastomotic perfusion;
❖ Challenge 2: visualization of critical structures;
❖ Challenge 3: accurate intraoperative staging;
❖ Challenge 4: precise evaluation of radical cancer removal/response;
❖ Challenge 5: improved diagnostic yield of endoscopic work-up.
In this Editorial, we will discuss the potential role of FGS in those issues.
Challenge 1: accurate anastomotic perfusion evaluation is a key factor to prevent complications. The diffusion over the bowel surface of a systemically injected fluorophore can be considered a surrogate marker of preserved vascular supply. Encouraging results are being collected with the use of fluorescence angiography (FA) to evaluate anastomotic perfusion (2). However, so far there is a lack of well-designed controlled studies and the potential impact of FA on reducing anastomotic leak rate remains to be demonstrated. Additionally, in all of the published studies, perfusion has been evaluated qualitatively, without taking into account the over-time diffusion of the fluorophore. Such diffusion might create an overestimation of the vascularized areas, since the dye can slowly flow to even ischemic parts of the bowel.
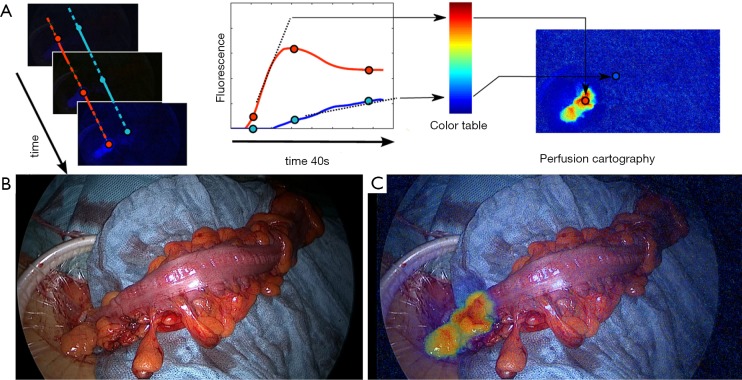
Considering this limitation, the real discriminant marker of perfusion is the “angiography effect” which is a rapid onset of the fluorescence signal within the first seconds following the injection. We have developed a quantitative, software-based analysis of the fluorescence signal, defined fluorescence-based enhanced reality (FLER), providing a real-time visualization of the perfusion values and the proposed resection lines directly on the bowel (4-7). The software computes the stiffness of the slope of the fluorescence signal to reach the peak of intensity, pixel-by-pixel. Based on those parameters, a virtual perfusion cartography is created, which stores into a single image the evolution of the signal over 40 seconds. This virtual image is subsequently superimposed onto real images during the surgical procedure in order to obtain an augmented reality effect.
This technology is currently being tested in a clinical trial at the IHU-Strasbourg (NCT02626091). The preliminary results demonstrate a good accuracy (Figure 1).
Figure 1.
Clinical application of the fluorescence-based enhanced reality to evaluate bowel perfusion. (A) The concept of FLER (fluorescence-based enhanced reality): after the i.v. administration of the Indocyanine Green, the fluorescence signal is analysed during 40 seconds by a specific software (VR-PERFUSION, IRCAD; France). The slope of the fluorescence time-to-peak is computed pixel-by-pixel and is converted into a color code to generate a virtual perfusion cartography. The resultant cartography is overlapped on real-time images [(B) white light images of the proximal resection during a sigmoidectomy] using a video-mixer [(C) augmented reality view of the proximal resection site].
Challenge 2: visualization of critical structures, such as the biliary tree, nerves, ureters etc. to prevent inadvertent lesions during the surgical procedure.
As an example, NIR fluorescence cholangiography (NIR-C) seems an accurate method to identify biliary structures and possibly to prevent bile duct injuries (1,8), with the advantages of being real-time, low-cost and radiation-free. NIR-C is based on the injection of a bile-excreted fluorophore [indocyanine green (ICG)], which becomes fluorescent upon excitation by a NIR light. A drawback with this technique is in the high background liver fluorescence. In our experience, NIR-C was considered effective to highlight the biliary anatomy. However, the high fluorescence background from the liver induced the surgeons to assign a lower score to the image quality obtained by NIR-C, when compared to standard intraoperative cholangiography (9). To reduce the fluorescence noise coming from the liver, a first strategy is to optimize the dosing and interval timing from the injection of the fluorophore to the visualization. The reported doses range from 2.5 mg in a single IV administration to 0.5 mg/kg (1). In a study, the best biliary ducts to liver fluorescence ratio was obtained with 0.25 mg/kg of ICG, administered at least 45 minutes before images were acquired (10). A prolonged time interval up to 24 h leads to a washout of the fluorophore with a clear view of the biliary tree and no background fluorescence from the liver (11). As an alternative strategy, we had proposed to inject the ICG directly into the gallbladder, with promising results in an experimental model (12). Subsequently, we moved from the bench to the clinical setting, and the results demonstrated that the fluorescence cholecystocholangiography provides clear delineation of the biliary tree and is particularly helpful in case of severe cholecystitis (13). Despite some limitations and the need for further refinements, it can be expected that fluorescence cholangiography will be one of the more largely adopted clinical applications of FGS.
Another important structure which is often at risk of iatrogenic lesions is the ureter, particularly in patients with previous abdominal surgeries.
Fluorescence imaging can provide a non-invasive and effective enhanced visualization of the ureters to possibly or minimize the risks of iatrogenic lesions. Promising trials are ongoing with modified fluorophores that have, unlike the ICG, the ability to be excreted by the urinary system, such as the IRDye800CW-CA (14).
FGS has also the potential to visualize nerves, with the use of nerve-specific tracers, enabling to possibly reduce the risk of nerve injuries, particularly during cervical or pelvic surgery. However, fluorescence imaging of nerves is still at the preclinical stage.
Challenge 3: accurate intraoperative staging, the precise analysis of the lymph nodes status is crucial in the oncologic decision-making process. SLN navigation, irrespective of the method, is an oncologically validated approach for early stage Gastric cancer Eastern Countries (15), while it remains controversial in the management of colorectal cancer (16). Controversy can be explained by the widely variable and non-standardized approaches published in the literature, leading to highly variable results in terms of accuracy.
However, based on a systematic review and meta-analysis of 52 clinical trials, the authors could conclude that the sentinel node procedure should be considered in all patients diagnosed with colorectal cancer, without clinical evidence of lymph node involvement, since it may improve staging of patients (upstaging in 15% of cases) (16).
In the same meta-analysis, authors could show that a sensitivity up to 90% can be achieved by a refined patient selection and standardized techniques. Additionally, authors stated that further studies should focus on stage I and II, since in more advanced cases, Node Positive by definition, the sentinel node procedure will not affect the therapeutic strategy.
Additionally, micro-metastases can be missed by conventional histopathological examination. Accurate analysis of all the withdrawn lymph nodes with the surgical specimen to detect micro-metastases is time-consuming and expensive. SLN techniques can help focusing on a reduced number of lymph nodes on which it is worthy to perform more accurate pathological tests (ultrastaging) (16).
ICG fluorescence-guided navigation of the lymph node is a relatively novel and effective technique which has been successfully employed in various kinds of tumors, including GI cancers, showing high detection and sensitivity rates. However, ICG is not a good molecule for the purpose of identifying the sentinel lymph node (SLN), and ICG-based techniques of SLN navigation are limited. Actually, ICG has a low fluorescence brightness and has a low retention in the lymph nodes, being a too small molecule which diffuses too rapidly (17).
Recently, the Japan Clinical Oncology Group (18), in a trial on SLN in gastric cancer surgery, reported a high false-negative rate, leading to a premature suspension of the trial.
Improved performance of SLN navigation can be achieved by acting at various levels. Firstly, by engineering more adapted, smart fluorescent probes to counteract ICG deficiencies, including increased retention rate, increased quantum yield and selectivity to the target tumor tissue. As an example, the retention rate can be improved by engineered fluorescent molecules combined with large molecules, such as nanocolloids, which demonstrate less dispersion and longer retention in the nodes than ICG alone (19). Secondly, the performances can be improved by using a combined dual-mode 3D imaging lymphography (CT or MRI) and optical fluorescence-based navigation. Thirdly, using software image analysis, enabling quantification of the fluorescence signal through standardized digital imaging and optimal benchmark of fluorophores and hardware. In fact, there is currently a need of identifying unique reporting criteria and intraoperative objective fluorescence threshold that could be predictive of lymph node positivity, based on the fluorescence signal pattern.
Challenge 4: precise evaluation of radical removal of cancer tissue, evaluation of tumor margins and evaluation of response to treatment—the Holy Grail in surgical oncology is the radical ablation of cancer tissue, which is the key to increase the disease free survival and to reduce the recurrence rate. Tumor-specific monoclonal antibodies coupled with fluorescent molecules targeting the tumor at a cellular level, could provide a real-time and precise estimation of radical tumor removal. In 2011, the first in human use of tumor-specific molecular FGS was reported (20). Thanks to the fluorescence enhancement, the surgeons could remove 34 intra-peritoneal implants of ovarian cancer metastasis, which were invisible at the naked eye. This was a groundbreaking proof of the concept of the surgical precision that tumor-specific fluorescence imaging could achieve. There is an increasing number of targeted probes which are being developed with the purpose of identifying cancer cells in a specific way. Despite we lack of scientific data, it is easy to foresee the potential of molecular fluorescence imaging to allow early stage cancer detection and precise tumor resection (21). One of the strategy, which is currently being tested in human trials, is based on the use of a fluorescent dye (IRDye800CW) coupled with humanized monoclonal antibodies, currently used in anticancer therapy [e.g., bevacizumab, which targets the vascular endothelial growth factor (VEGF) or the cetuximab, targeting the epithelial growth factor receptor (EGFR)].
Challenge 5: improve diagnostic yield, the rate of esophageal cancers missed at standard endoscopic work-up is as high as 7.8% (22) and can go up to 25% in patients with Barrett’s esophagus (BE) (23). The state of the art follow-up endoscopic protocol (Seattle protocol) includes direct biopsy sampling from suspicious spots integrated by systematic random 4 quadrants biopsies every 1–2 cm along the long axis of the Barrett’s segment. This approach, despite considered the gold standard today, is time consuming and inaccurate. Additionally, it does not enable inter-operative re-localization of the biopsy site (the depth localization of the biopsy site is recorded based on the markings on the endoscope shaft and is expressed as distance from the dental arcade).
Similarly, the missing rate of colorectal adenomas during standard colonoscopy has been reported to be particularly high, up to 31% (24). Numerous endoscopic innovations, including mechanical and image-processing improvements, have been developed to augment the diagnostic yield but still a significant 10% missing rate occurs with the most performing available technologies (25).
NIR imaging coupled with targeted molecular fluorophores is a promising method to improve detection of GI neoplasia and improve the diagnostic yields of the endoscopic procedures. There is a pilot clinical trial NCT02129933 ongoing at Groningen by the group of Professor Gooitzen Van Dam (The Netherlands), assessing the ability to improve detection of esophageal pre-malignant or malignant lesions during surveillance endoscopy of patients at risk of esophageal cancer or presenting BE, and a second pilot trial (run by the same group) in colon cancer enhanced detection (NCT01972373). They are using VEGF-targeting humanized monoclonal antibody Bevacizumab, coupled with the fluorophore IRDye800CW. At the IHU-Strasbourg we are also planning to start several clinical trials using those molecules.
Dissemination and data sharing around FGS
The IHU-Strasbourg in partnership with the European Association of Endoscopic Surgeons (EAES) and sponsored by the French Foundation ARC (Association for Cancer Research), has recently launched a registry to collect safety and efficacy data of FGS. The aim is also to share experiences and setting-up collaborations at the European level on clinical applications of FGS. The EURO-FIGS registry (www.euro-figs.eu) is a web-based, portable platform which is currently collecting data on three main and most common indications for FGS: fluorescence cholangiography, anastomotic perfusion and sentinel node navigation. The registry is modular, enabling to add new clinical applications at any time, upon participating surgeons’ request. Currently, the registry connects 53 centers across Europe performing FGS on a regular basis and is helping promoting networking and possibly to overcome some barriers to the widespread adoption of FGS. The mid-term plan is to extend the registry also to non-European centers and to create a core of experts to provide consensus statements and guidelines for FGS.
Additionally, with the same aim of promoting and developing the concept of FGS, in 2014 the International Society of Fluorescence Guided Surgery (ISFGS, https://www.isfgs.org/), was founded by renowned experts in the field, coming from all the surgical specialties.
Conclusions
FGS is experiencing a vibrant excitement from the surgical community, since it increasingly seems to be the next cutting-edge towards precision therapies. Extensive research is ongoing at various levels, including hardware, software and custom fluorophores to improve the current performances and provide the proofs of efficacy and the impact on surgical outcomes.
Acknowledgements
M Diana is the recipient of a research grant (project ELIOS) from the Foundation ARC (Association for Cancer Research) to develop and implement molecular fluorescence-guided surgery.
Provenance: This is a Guest Editorial commissioned by Editor-in-Chief Jiafu Ji, MD, FACS (Department of Gastrointestinal Surgery, Peking University School of Oncology & Beijing Cancer Hospital, Beijing, China).
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- 1.Pesce A, Piccolo G, La Greca G, et al. Utility of fluorescent cholangiography during laparoscopic cholecystectomy: A systematic review. World J Gastroenterol 2015;21:7877-83. 10.3748/wjg.v21.i25.7877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Degett TH, Andersen HS, Gögenur I. Indocyanine green fluorescence angiography for intraoperative assessment of gastrointestinal anastomotic perfusion: a systematic review of clinical trials. Langenbecks Arch Surg 2016;401:767-75. 10.1007/s00423-016-1400-9 [DOI] [PubMed] [Google Scholar]
- 3.Singhal S. The Future of Surgical Oncology: Image-Guided Cancer Surgery. JAMA Surg 2016;151:184-5. 10.1001/jamasurg.2015.3604 [DOI] [PubMed] [Google Scholar]
- 4.Diana M, Noll E, Diemunsch P, et al. Enhanced-reality video fluorescence: a real-time assessment of intestinal viability. Ann Surg 2014;259:700-7. 10.1097/SLA.0b013e31828d4ab3 [DOI] [PubMed] [Google Scholar]
- 5.Diana M, Agnus V, Halvax P, et al. Intraoperative fluorescence-based enhanced reality laparoscopic real-time imaging to assess bowel perfusion at the anastomotic site in an experimental model. Br J Surg 2015;102:e169-76. 10.1002/bjs.9725 [DOI] [PubMed] [Google Scholar]
- 6.Diana M, Dallemagne B, Chung H, et al. Probe-based confocal laser endomicroscopy and fluorescence-based enhanced reality for real-time assessment of intestinal microcirculation in a porcine model of sigmoid ischemia. Surg Endosc 2014;28:3224-33. 10.1007/s00464-014-3595-6 [DOI] [PubMed] [Google Scholar]
- 7.Diana M, Halvax P, Dallemagne B, et al. Real-time navigation by fluorescence-based enhanced reality for precise estimation of future anastomotic site in digestive surgery. Surg Endosc 2014;28:3108-18. 10.1007/s00464-014-3592-9 [DOI] [PubMed] [Google Scholar]
- 8.Conrad C, Wakabayashi G, Asbun HJ, et al. IRCAD recommendation on safe laparoscopic cholecystectomy. J Hepatobiliary Pancreat Sci 2017;24:603-15. 10.1002/jhbp.491 [DOI] [PubMed] [Google Scholar]
- 9.Diana M, Soler L, Agnus V, et al. Prospective Evaluation of Precision Multimodal Gallbladder Surgery Navigation: Virtual Reality, Near-infrared Fluorescence, and X-ray-based Intraoperative Cholangiography. Ann Surg 2017;266:890-7. 10.1097/SLA.0000000000002400 [DOI] [PubMed] [Google Scholar]
- 10.Zarrinpar A, Dutson EP, Mobley C, et al. Intraoperative Laparoscopic Near-Infrared Fluorescence Cholangiography to Facilitate Anatomical Identification: When to Give Indocyanine Green and How Much. Surg Innov 2016;23:360-5. 10.1177/1553350616637671 [DOI] [PubMed] [Google Scholar]
- 11.Verbeek FP, Schaafsma BE, Tummers QR, et al. Optimization of near-infrared fluorescence cholangiography for open and laparoscopic surgery. Surg Endosc 2014;28:1076-82. 10.1007/s00464-013-3305-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu YY, Kong SH, Diana M, et al. Near-infrared cholecysto-cholangiography with indocyanine green may secure cholecystectomy in difficult clinical situations: proof of the concept in a porcine model. Surg Endosc 2016;30:4115-23. 10.1007/s00464-015-4608-9 [DOI] [PubMed] [Google Scholar]
- 13.Liu YY, Liao CH, Diana M, et al. Near-infrared cholecystocholangiography with direct intragallbladder indocyanine green injection: preliminary clinical results. Surg Endosc 2017. [Epub ahead of print]. 10.1007/s00464-017-5838-9 [DOI] [PubMed] [Google Scholar]
- 14.Korb ML, Huh WK, Boone JD, et al. Laparoscopic Fluorescent Visualization of the Ureter With Intravenous IRDye800CW. J Minim Invasive Gynecol 2015;22:799-806. 10.1016/j.jmig.2015.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitsumori N, Nimura H, Takahashi N, et al. Sentinel lymph node navigation surgery for early stage gastric cancer. World J Gastroenterol 2014;20:5685-93. 10.3748/wjg.v20.i19.5685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van der Pas MH, Meijer S, Hoekstra OS, et al. Sentinel-lymph-node procedure in colon and rectal cancer: a systematic review and meta-analysis. Lancet Oncol 2011;12:540-50. 10.1016/S1470-2045(11)70075-4 [DOI] [PubMed] [Google Scholar]
- 17.Cahill RA, Anderson M, Wang LM, et al. Near-infrared (NIR) laparoscopy for intraoperative lymphatic road-mapping and sentinel node identification during definitive surgical resection of early-stage colorectal neoplasia. Surg Endosc 2012;26:197-204. 10.1007/s00464-011-1854-3 [DOI] [PubMed] [Google Scholar]
- 18.Miyashiro I, Hiratsuka M, Sasako M, et al. High false-negative proportion of intraoperative histological examination as a serious problem for clinical application of sentinel node biopsy for early gastric cancer: final results of the Japan Clinical Oncology Group multicenter trial JCOG0302. Gastric Cancer 2014;17:316-23. 10.1007/s10120-013-0285-3 [DOI] [PubMed] [Google Scholar]
- 19.Tummers QR, Boogerd LS, de Steur WO, et al. Near-infrared fluorescence sentinel lymph node detection in gastric cancer: A pilot study. World J Gastroenterol 2016;22:3644-51. 10.3748/wjg.v22.i13.3644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Dam GM, Themelis G, Crane LM, et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-α targeting: first in-human results. Nat Med 2011;17:1315-9. 10.1038/nm.2472 [DOI] [PubMed] [Google Scholar]
- 21.Rosenthal EL, Warram JM, de Boer E, et al. Successful Translation of Fluorescence Navigation During Oncologic Surgery: A Consensus Report. J Nucl Med 2016;57:144-50. 10.2967/jnumed.115.158915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chadwick G, Groene O, Hoare J, et al. A population-based, retrospective, cohort study of esophageal cancer missed at endoscopy. Endoscopy 2014;46:553-60. 10.1055/s-0034-1365646 [DOI] [PubMed] [Google Scholar]
- 23.Visrodia K, Singh S, Krishnamoorthi R, et al. Magnitude of Missed Esophageal Adenocarcinoma After Barrett's Esophagus Diagnosis: A Systematic Review and Meta-analysis. Gastroenterology 2016;150:599-607.e7; quiz e14-5. [DOI] [PMC free article] [PubMed]
- 24.Gralnek IM. Emerging technological advancements in colonoscopy: Third Eye® Retroscope® and Third Eye® Panoramic(TM), Fuse® Full Spectrum Endoscopy® colonoscopy platform, Extra-Wide-Angle-View colonoscope, and NaviAid(TM) G-EYE(TM) balloon colonoscope. Dig Endosc 2015;27:223-31. 10.1111/den.12382 [DOI] [PubMed] [Google Scholar]
- 25.Dik VK, Moons LM, Siersema PD. Endoscopic innovations to increase the adenoma detection rate during colonoscopy. World J Gastroenterol 2014;20:2200-11. 10.3748/wjg.v20.i9.2200 [DOI] [PMC free article] [PubMed] [Google Scholar]