Abstract
Background
Drug resistant Mycoplasma pneumoniae (MP) is a rising issue in the management of community-acquired pneumonia (CAP). Epidemiological monitoring is essential for identifying resistant patterns of MP isolates against various antibiotics in adult CAP patients.
Methods
This is a prospectively designed multicenter study conducted on adult patients with CAP visiting six teaching hospitals in the cities of Beijing, Shanghai and Guangzhou between September 2010 and June 2012.
Results
A total of 520 adult patients (mean age: 45.7±26.2 years) with CAP visiting teaching hospitals in the cities of Beijing, Shanghai and Guangzhou were included. Of the 520 patients, only 75 (14.42%) were confirmed MP positive by means of culture and real-time PCR methods. Quinolones were the most common initially prescribed antimicrobial, followed by β-lactams and β-lactams plus quinolones. Macrolide resistance was as high as 80% and 72% against erythromycin (ERY) and azithromycin (AZM) respectively, which were associated with the A2063G transition mutation in domain V of the 23S ribosomal RNA (rRNA) gene. Six strains with mild to moderate ERY-resistant level were still susceptible to AZM. Tetracycline (TET), minocycline (MIN) and quinolones [moxifloxacin (MOX) and fluoroquinolones] had no signs of resistance.
Conclusions
High resistance was observed with macrolides, whereas, none of the MP strains were resistant to fluoroquinolones and TET. Hence, macrolide resistant MP (MRMP)_infections could be well treated with fluoroquinolones. However, few isolated strains had minimal inhibitory concentration (MIC) values on the edge of resistance to quinolones, alarming a quinolone-resistant MP in the near future.
Keywords: Mycoplasma pneumoniae (MP), fluoroquinolones, antibiotic resistance, beta-lactams, point mutation
Introduction
Community-acquired pneumonia (CAP) is a common infectious disease affecting people of any age. Mycoplasma pneumoniae (MP) is a major cause of CAP worldwide and accounts for up to 40% of the cases in China (1). International guidelines have recommended macrolides or tetracyclines (TET) as the first-line drugs and fluoroquinolones as the second-line drugs in adults, whereas azithromycin (AZM) as the first-line drug, and clarithromycin, erythromycin (ERY), or doxycycline (for patients aged >8 years) along with fluoroquinolones [levofloxacin (LVX) or MOX for adolescent patients] as the second-line oral drugs, for mild pediatric cases (2). However, the emergence of macrolide-resistant MP (MRMP) has gradually increased worldwide in the last decade, especially in China and Japan the resistance rate has reached up to 90% (2-5). Macrolides are preferred over other antimicrobials due to the lack of cell wall in MP; however, macrolides are susceptible to resistance due to point mutations in few positions of domain V of the peptidyl transferase loop of 23S ribosomal RNA (rRNA) gene (such as A2063G, A2064G, A2063C, A2063T, A2067G, and C2617G) and at the location of macrolide binding to the 50S bacterial ribosome subunit reduces the affinity of the antibiotic towards the ribosome (1,2,6). Eventually fluoroquinolone therapy has emerged as an efficient treatment option for MP CAP (7). However, in vitro studies have reported resistance of MP to fluoroquinolones which indicates a high chance of fluoroquinolone-resistant MP infection in the near future (8). Though, bacterial resistance is evident for different antibiotics, the correlation between the resistance and clinical failure of therapies are still controversial. Hence, epidemiological monitoring is essential to acquire a better understanding of clinical characteristics of MP infection with resistant strains and emphasizing the need for judicious use of antimicrobial agents. For this purpose, we conducted a multicenter surveillance study on adult Chinese CAP patients in three major provincial cities Beijing, Shanghai and Guangzhou, to monitor the resistance patterns and characterize the mechanisms of resistance.
Methods
Study design and population
This is a prospectively designed multicenter study conducted on adult patients (>18 years) with CAP visiting six teaching hospitals in the cities of Beijing, Shanghai and Guangzhou between September 2010 and June 2012 who had initial clinical presentations and chest radiography findings that were consistent with CAP. A signed informed consent was obtained from all enrolled patients. Patients who were immunocompromised due to HIV infection; neutropenic; receiving immunosuppressive chemotherapy; pregnant or nursing; or had known or suspected active tuberculosis were excluded from the study.
The study was approved by the Ethics Committee of the Chinese People’s Liberation Army General Hospital (Approval number 20100727-003). A standardized data form was used to collect clinical information of individual patient. The severity of infection was assessed using CURB-65 scoring system.
Microbiological laboratory tests
Throat swab samples were obtained at the time of enrollment from all patients to identify MP cultures using colony morphology and polymerase chain reaction (9).
Clonogenic identification of MP
The throat swabs were re-suspended in 2 mL of transport medium (Oxoid, Basingstoke, UK) and then aliquoted (200 µL). A 200-µL aliquot was mixed with 1.8 mL of liquid broth medium (Oxoid) and incubated at 37 °C. Growth of MP produces acid by fermentation of glucose, which results in the color change of phenol red indicator to yellow. This change in color within one to six weeks was an indicator of positive MP growth.
PCR detection of MP
DNA samples were extracted from all isolated strains using the DNA Mini-kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol and the presence of MP DNA was diagnosed using PCR Fluorescence Probing kit (Zhi-Jiang Co., Ltd, Shanghai, China) with the following PCR amplification system: DNA template (2 µL), MP PCR reaction mix (40 µL) and Taq enzyme (3 µL). The PCR reaction was carried out using a quantitative PCR instrument (ABI Prism 7500, Applied Biosystems, Foster City, USA) under the following conditions: 93 °C for 2 min, followed by 10 cycles of 93 °C for 45 s 55 °C for 1 min, and another 30 cycles of 93 °C for 0.5 min and 55 °C for 10 min. Mutations associated with resistance to macrolides at sites 2063, 2064, and 2617 in the MP 23S rRNA domain V gene region were detected by a sequencing method described previously (10).
Determination of antimicrobial resistance
The isolates were cultured on a broth medium and the minimal inhibitory concentrations (MICs) of antimicrobial agents were determined by a broth microdilution method based on the document published by National Committee for Clinical Laboratory Standards (Clinical and Laboratory Standards Institute) (11).
The following antibiotics were tested: ERY, AZM, TET, minocycline (MIN), LVX, and MOX. Antimicrobial resistance was defined as: ERY: S ≤0.5 µg/mL; R ≥1 µg/mL; AZM: S ≤0.5 µg/mL; R ≥1 µg/mL; TET: S ≤2 µg/mL; MOX: S ≤0.5 µg/mL; LVX: S ≤1 µg/mL (12).
Sequence analysis
The total length of the 23S rRNA gene of each MP isolate was amplified and sequenced by the method described previously (11). The mutations of 23S rRNA have been deposited in the GenBank sequence database and were assigned the accession numbers HM043729, HM043730 and HM043731. Amplification of ribosomal protein L4 and L22 fragments was performed with the primers. Sequencing results were analysed with CLC Sequence Viewer (CLC Bio, Aarhus, Denmark) and were compared with the M129 complete genome sequence. PCR restriction fragment-length polymorphism typing of the P1 gene and pulsed-field gel electrophoresis typing were conducted for all MP isolates.
Statistical analysis
Comparisons of clinical characteristics were conducted using an unpaired Student’s t-test, the Mann-Whitney test, or the Chi-square test (SPSS for Windows 16.0, Chicago, IL, USA). A P value <0.05 was considered to be statistically significant. WHONET 5.6 software was used to set up a MICs database and perform resistance analysis.
Results
General characteristics
A total of 520 patients (mean age: 45.7±26.2 years; male, n=268) were enrolled in this study. Clinical characteristics of all patients were showed in Table 1. Of these patients, 342 (65.8%) were outpatients. The most common symptoms were cough (94.4%) and fever (86.5%). A total of 135 patients had co-morbid illness, majorly chronic obstructive pulmonary disease (COPD) and other pulmonary disease, followed by cardiac-cerebral vascular diseases and diabetes. According to the CRB-65 score system, scores of 0, 1–2 and ≥3 were observed in 380, 134, and 6 patients, respectively. Only 75 patients (14.42%) were confirmed to be MP positive by means of culture and real-time PCR methods.
Table 1. Clinical characteristics of enrolled patients.
| Variables | All patients | MP positive patients | MP negative patients |
|---|---|---|---|
| Patients (n) | 520 | 75 | 445 |
| Sex (male/female) | 268/252 | 30/45 | 238/207 |
| Age (years) | 45.7±26.2 | 36.20±17.54 | 43.49±19.79* |
| Inpatients/outpatients | 178/342 | 28/47 | 147/298 |
| Laboratory findings | |||
| White blood cell count (×109/L) | 8.83±3.77 | 7.80±4.27 | 8.81±3.69 |
| Clinical symptoms, n (%) | |||
| Cough | 491 (94.4) | 67 (89.3) | 424 (95.2) |
| Fever | 450 (86.5) | 57 (76.0) | 393 (88.3) |
| Breathlessness | 72 (13.8) | 12 (16) | 60 (13.4) |
| Chest pain | 49 (9.4) | 6 (8.0) | 43 (9.96) |
| Hemoptysis | 20 (3.8) | 3 (4.0) | 17 (3.8) |
| Previous disease history, n (%) | |||
| COPD | 22 (4.2) | 2 (2.6) | 20 (4.5) |
| Other pulmonary diseases | 37 (7.1) | 1 (1.3) | 36 (8.1) |
| Diabetes | 25 (4.8) | 3 (4) | 22 (4.9) |
| Cardiac-cerebral vascular diseases | 35 (6.7) | 3 (4) | 32 (7.2) |
| Cancer | 20 (3.8) | None | 20 (3.8) |
| Others | 14 (2.6) | None | 14 (2.6) |
*, comparison between MP positive patients and MP negative patients, P=0.005. MP, Mycoplasma pneumoniae; COPD, chronic obstructive pulmonary disease.
Patients medication
Of the 520 patients, 331 (63.65%) had received antibiotic therapy within 72 h prior to enrollment. β-lactams, quinolones and macrolides were the most commonly used antibiotics (Figure 1). Post-enrollment in the study, data for 426 patients receiving antibiotic treatment, quinolones were the most common initially prescribed (269/426), followed by β-lactams (78/426) and β-lactams plus quinolones (35/426). Only 28 patients were initially treated with combination of macrolides with β-lactams and 7 patients treated with macrolides alone, Figure 2.
Figure 1.
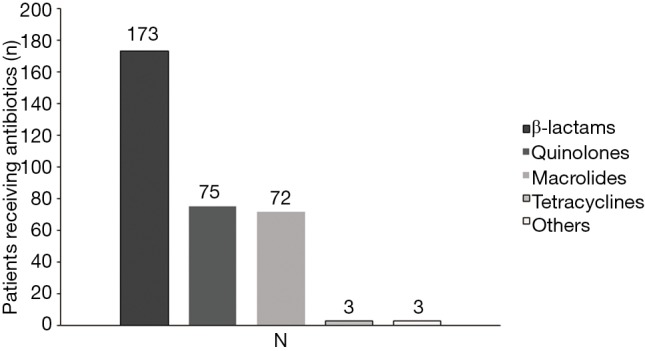
Prescription of antibiotics within 72-h after enrollment (n=520).
Figure 2.
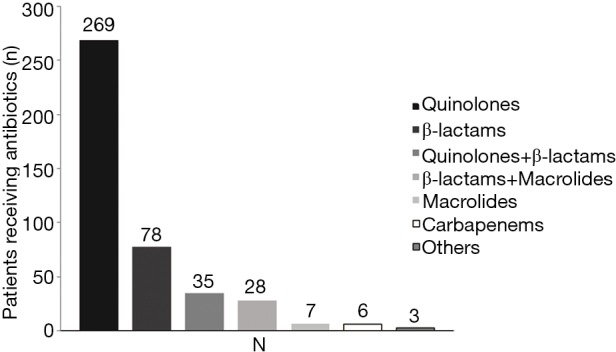
Initial prescriptions of antibiotics after enrollment (n=426).
In vitro antimicrobials susceptibility
A total of 75 MP strains were obtained by culture, of which 60 strains (80%) were resistant to ERY and only 54 strains (72%) were resistant to AZM. Six strains with mild to moderate ERY-resistant level were still susceptible to AZM. TET and MIN showed similar MICs distribution for MP and had no signs of resistance. MIC of ERY ranged from <0.004–256 µg/mL, Table 2. MIC value of M129 strain (µg/mL) was significant for ERY and AZM (P≤0.008). Of the 54 ERY&AZM double-resistant strains, 53 strains harbored an A2063G transition in domain V of the 23S rRNA gene and only 1 strain harbored an A2064G transition. In the rest 6 strains that were resistant to ERY but susceptible to AZM, only 2 strains harbored an A2063G transition (Table 3).
Table 2. MIC values of all isolated 75 MP strains.
| Antibiotics | MICs range (μg/mL) | MIC50 (μg/mL) | MIC90 (μg/mL) | MIC value of M129 strain (μg/mL) |
|---|---|---|---|---|
| ERY | <0.004–256 | 64 | 256 | ≤0.008 |
| AZM | <0.004–128 | 8 | 128 | ≤0.008 |
| TET | 0.008–1 | 0.25 | 0.25 | 0.125 |
| MIN | <0.016–0.5 | 0.064 | 0.125 | 0.016 |
| LVX | 0.032–0.5 | 0.25 | 0.5 | 0.064 |
| MOX | 0.016–0.5 | 0.125 | 0.125 | 0.064 |
ERY, erythromycin; AZM, azithromycin; TET, tetracycline; MIN, minocycline; LVX, levofloxacin; MOX, moxifloxacin; MIC, minimal inhibitory concentration.
Table 3. Characteristics of 6 ERY-resistant but AZM-susceptible MP strains.
| Strains | Clinical characteristics | Point transition | MICs value (μg/mL) | |||||
|---|---|---|---|---|---|---|---|---|
| ERY | AZM | TET | MIN | MOX | LVX | |||
| 1 | Female/21 years/outpatient | None | 1 | 0.5 | 0.064 | 0.064 | 0.032 | 0.25 |
| 2 | Female/56 years/inpatient | None | 4 | 0.008 | 0.125 | 0.125 | 0.032 | 0.064 |
| 3 | Female/20 years/inpatient | None | 2 | 0.5 | 0.25 | 0.25 | 0.125 | 0.064 |
| 4 | Male/80 years/inpatient | None | 2 | 0.008 | 0.125 | 0.125 | 0.064 | 0.125 |
| 5 | Female/29 years/outpatient | A2063G | 16 | 0.5 | 0.032 | 0.032 | 0.016 | 0.25 |
| 6 | Female/21 years/outpatient | A2063G | 32 | 0.5 | 0.25 | 0.125 | 0.032 | 0.032 |
Discussion
MP is a common pathogen generally associated with mild to moderate CAP (9,13). This cell wall deficient atypical microorganism shows insensitivity to antibiotics such as beta-lactams and aminoglycosides leading to the undisputed use of macrolide antibiotics as first line drug of choice in CAP. However, the use of macrolides has begun to subside due to a high rate of macrolide resistance exhibited by MP globally (6,13-17).
MP can develop macrolide resistance by point mutations in the 23S rRNA gene, which reduces the affinity of the antibiotic for the ribosome. Mutations in the sequence of 23S rRNA, A2063G was the most prevalent, followed by A2064G, A2063T and A1290G. A2063G and A2064G mutations are always responsible for the high-level macrolide resistance in MP (2). In the present study, we found that 80% of MP isolated strains were resistant to ERY, and 72% were resistant to AZM. Fifty-five ERY-resistant MP strains harbored an A2063G transition in the 23S rRNA genes and one harbored an A2064G transition, which was consistent with previous reports that the A2063G transition was the most frequent mutation related to macrolides resistance (2,10). Further, it is interesting to note that 6 strains with mild to moderate ERY-resistance level were still susceptible to AZM, among which only 2 strains (MIC value 16 and 32 µg/mL, respectively) harbored an A2063G transition.
Available data indicated that infection with MRMP will lead to a longer duration of therapy, persistent cough and increased time to resolution of fever compared with treatment-susceptible infection (2). Because of in vitro susceptibility, fluoroquinolones and TET are recommended as the effective alternatives for MRMP associated treatment failures (1,2,6). However, it is debatable how in vitro resistance translates into clinical outcomes. Large scale clinical trials are scarce on this subject. As macrolide resistance has clinical outcomes such as longer duration of fever, cough and hospital stay, alternative antibiotic treatment such as TET or fluoroquinolones are often being used (1,18). Whether macrolides should be removed as the first-line choice of MP pneumonia is still doubtful as in vitro study suggests that macrolides would still work in the treatment of mild to moderate MRMP infection due to their immunomodulatory and anti-inflammatory properties (19). Further, Fluoroquinolones might also show antimycoplasmal activities against macrolide- and tetracycline-resistant strains of MP due to the different mechanism of action and show excellent penetration into lung tissue, in particular bronchial secretions (20,21). Though fluoroquinolones are effective in the treatment of MP infection, these agents are not recommended in children due to their toxicity. The clinical importance of fluoroquinolones has not been demonstrated because they have been known to cause cartilage erosion in pre-clinical studies (7).
Furthermore, the prevalence of MRMP in adult CAP patients was lower than those reports in pediatric patients reported previously in China (1,7) and lower than adult CAP patients of Zhejiang province reported previously (1). This apparent inconsistency could be due to the varied nature of study population. Also, 65.7% patients of this study were outpatients, whereas, 60% of the patients from the pediatric studies were inpatients (7). A high proportion of inpatients might mean a greater chance of MRMP infected patients with severe illness.
Further, none of the strains showed resistance to quinolones or TET in this study. However, it should be noticed that few isolated strains with MIC values on the edge of resistance to quinolones (MIC value of 1.0 and 0.5 µg /mL for LVX and MOX, respectively) were observed, hence judicious use of quinolones should be considered in order to prevent development of quinolone resistant MP strains.
It is well known that the macrolide-lincosamide-streptogramin B (MLS) antibiotics inhibit protein synthesis by binding to domain II and/or domain V of 23S rRNA (22,23). The mutations of A-to-G transition at position 2063 induce a high-level of macrolide resistance, whereas, mutations of A-to-G transition at position 2064 and 2617 and A-to-T transition at position 2063 induce low-level resistance (10). Several studies have indicated that macrolide resistance in MP may have clinical significance in treatment failure of drugs (24-26). As an alternative to the time-consuming agar-and broth-based in vitro testing method, point mutations in the 23S rRNA region can be identified as a marker of macrolide resistance by PCR methods in respiratory samples. Results from clinical specimens revealed that the aforementioned 23S rRNA point mutations were not detected in some isolated strains with low to moderate level of macrolide resistance (27). Hence, whether those point mutations will be responsible for the all the MRMP strains should be elucidated in future prospective studies.
Our study has several limitations, which include: (I) low prevalence of MP CAP; (II) we performed in vitro resistance analysis in this study. Comparative resistance analysis of macrolides with other antimicrobials will be performed in future studies; (III) data from three large cities does not provide a picture of rural MRMP prevalence. Hence, the difference in prevalence of MRMP between large cities and rural areas need further investigations.
Conclusions
High resistance was observed with macrolides, whereas, none of the MP strains were resistant to fluoroquinolones and TET. Hence, MRMP infections could be well treated with fluoroquinolones. However, few isolated strains had MIC values on the edge of resistance to quinolones, alarming a quinolone-resistant MP in the near future.
Acknowledgements
We express our deepest gratitude to all physicians who collaborated with this surveillance study and provided valuable data. Participants in the Adult MRMP Study Group of Chinese Society of Respiratory Diseases: Ying-Mei Liu, Jiu-Xin Qu (Beijing Chao-Yang Hospital), Yan Gao (RenMin Hospital, Peking University), Yang Liu (Shanghai Hua-Shan Hospital), (Guangzhou Institute of Respiratory Medicine), Kang Liao (the First Affiliated Hospital of Zhongshan University). This work was supported by Bayer China. The authors also thank Dr. Amit Bhat (Indegene Private Limited, Bangalore, India) for providing medical writing assistance in the preparation of this manuscript.
Ethical Statement: The study was approved by the Ethics Committee of the Chinese People’s Liberation Army General Hospital (No. 20100727-003). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Zhou Z, Li X, Chen X, et al. Macrolide-resistant Mycoplasma pneumoniae in adults in Zhejiang, China. Antimicrob Agents Chemother 2015;59:1048-51. 10.1128/AAC.04308-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cao B, Qu JX, Yin YD, et al. Overview of antimicrobial options for Mycoplasma pneumoniae pneumonia: focus on macrolide resistance. Clin Respir J 2017;11:419-29. 10.1111/crj.12379 [DOI] [PubMed] [Google Scholar]
- 3.Liu Y, Ye X, Zhang H, et al. Characterization of macrolide resistance in Mycoplasma pneumoniae isolated from children in Shanghai, China. Diagn Microbiol Infect Dis 2010;67:355-8. 10.1016/j.diagmicrobio.2010.03.004 [DOI] [PubMed] [Google Scholar]
- 4.Qin Q, Xu B, Liu X, et al. Status of Mycoplasma pneumoniae Pneumonia in Chinese Children: A Systematic Review. Adv Microbiol 2014;4:704-11. 10.4236/aim.2014.411076 [DOI] [Google Scholar]
- 5.Yamazaki T, Kenri T. Epidemiology of Mycoplasma pneumoniae Infections in Japan and Therapeutic Strategies for Macrolide-Resistant M. pneumoniae. Front Microbiol 2016;7:693. 10.3389/fmicb.2016.00693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng X, Lee S, Selvarangan R, et al. Macrolide-Resistant Mycoplasma pneumoniae, United States. Emerg Infect Dis 2015;21:1470-2. 10.3201/eid2108.150273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miyashita N, Kawai Y, Akaike H, et al. Macrolide-resistant Mycoplasma pneumoniae in adolescents with community-acquired pneumonia. BMC Infect Dis 2012;12:126. 10.1186/1471-2334-12-126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dégrang S, Renaudin H, Charron A, et al. Reduced susceptibility to tetracyclines is associated in vitro with the presence of 16S rRNA mutations in Mycoplasma hominis and Mycoplasma pneumoniae. J Antimicrob Chemother 2008;61:1390-2. 10.1093/jac/dkn118 [DOI] [PubMed] [Google Scholar]
- 9.Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among US adults. N Engl J Med 2015;373:415-27. 10.1056/NEJMoa1500245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morozumi M, Hasegawa K, Kobayashi R, et al. Emergence of macrolide-resistant Mycoplasma pneumoniae with a 23S rRNA gene mutation. Antimicrob Agents Chemother 2005;49:2302-6. 10.1128/AAC.49.6.2302-2306.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okada T, Morozumi M, Tajima T, et al. Rapid effectiveness of minocycline or doxycycline against macrolide-resistant Mycoplasma pneumoniae infection in a 2011 outbreak among Japanese children. Clin Infect Dis 2012;55:1642-9. 10.1093/cid/cis784 [DOI] [PubMed] [Google Scholar]
- 12.Waites KB, Bade DJ, Bébéar C, et al. Methods for antimicrobial susceptibility testing for human mycoplasmas: Approved guideline. Wayne: Clinical and Laboratory Standards Institute, 2011. [PubMed] [Google Scholar]
- 13.Arnold FW, Summersgill JT, LaJoie AS, et al. A worldwide perspective of atypical pathogens in community-acquired pneumonia. Am J Respir Crit Care Med 2007;175:1086-93. 10.1164/rccm.200603-350OC [DOI] [PubMed] [Google Scholar]
- 14.Averbuch D, Hidalgo-Grass C, Moses AE, et al. Macrolide resistance in Mycoplasma pneumoniae, Israel, 2010. Emerg Infect Dis 2011;17:1079-82. 10.3201/eid/1706.101558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferguson GD, Gadsby NJ, Henderson SS, et al. Clinical outcomes and macrolide resistance in Mycoplasma pneumoniae infection in Scotland, UK. J Med Microbiol 2013;62:1876-82. 10.1099/jmm.0.066191-0 [DOI] [PubMed] [Google Scholar]
- 16.Peuchant O, Menard A, Renaudin H, et al. Increased macrolide resistance of Mycoplasma pneumoniae in France directly detected in clinical specimens by real-time PCR and melting curve analysis. J Antimicrob Chemother 2009;64:52-8. 10.1093/jac/dkp160 [DOI] [PubMed] [Google Scholar]
- 17.Wolff BJ, Thacker WL, Schwartz SB, et al. Detection of macrolide resistance in Mycoplasma pneumoniae by real-time PCR and high-resolution melt analysis. Antimicrob Agents Chemother 2008;52:3542-9. 10.1128/AAC.00582-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pereyre S, Goret J, Bébéar C. Mycoplasma pneumoniae: current knowledge on macrolide resistance and treatment. Front Microbiol 2016;7:974. 10.3389/fmicb.2016.00974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arai S, Gohara Y, Akashi A, et al. Effects of new quinolones on Mycoplasma pneumoniae-infected hamsters. Antimicrob Agents Chemother 1993;37:287-92. 10.1128/AAC.37.2.287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gohara Y, Arai S, Akashi A, et al. In vitro and in vivo activities of Q-35, a new fluoroquinolone, against Mycoplasma pneumoniae. Antimicrob Agents Chemother 1993;37:1826-30. 10.1128/AAC.37.9.1826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurata S, Taguchi H, Sasaki T, et al. Antimicrobial and immunomodulatory effect of clarithromycin on macrolide-resistant Mycoplasma pneumoniae. J Med Microbiol 2010;59:693-701. 10.1099/jmm.0.014191-0 [DOI] [PubMed] [Google Scholar]
- 22.Douthwaite S, Hansen LH, Mauvais P. Macrolide–ketolide inhibition of MLS‐resistant ribosomes is improved by alternative drug interaction with domain II of 23S rRNA. Mol Microbiol 2000;36:183-93. 10.1046/j.1365-2958.2000.01841.x [DOI] [PubMed] [Google Scholar]
- 23.Weisblum B. Erythromycin resistance by ribosome modification. Antimicrob Agents Chemother 1995;39:577. 10.1128/AAC.39.3.577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawai Y, Miyashita N, Yamaguchi T, et al. Clinical efficacy of macrolide antibiotics against genetically determined macrolide‐resistant Mycoplasma pneumoniae pneumonia in paediatric patients. Respirology 2012;17:354-62. 10.1111/j.1440-1843.2011.02102.x [DOI] [PubMed] [Google Scholar]
- 25.Matsubara K, Okada T, Matsushima T, et al. A comparative clinical study of macrolide-sensitive and macrolide-resistant Mycoplasma pneumoniae infections in pediatric patients. J Infect Chemother 2009;15:380-3. 10.1007/s10156-009-0715-7 [DOI] [PubMed] [Google Scholar]
- 26.Suzuki S, Yamazaki T, Narita M, et al. Clinical evaluation of macrolide-resistant Mycoplasma pneumoniae. Antimicrob Agents Chemother 2006;50:709-12. 10.1128/AAC.50.2.709-712.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yin YD, Cao B, Wang H, et al. Survey of macrolide resistance in Mycoplasma pneumoniae in adult patients with community-acquired pneumonia in Beijing, China. Zhonghua Jie He He Hu Xi Za Zhi 2013;36:954-8. [PubMed] [Google Scholar]


