Abstract
Background
Surgical resection is the most effective curative therapy for non-small cell lung cancer (NSCLC). However, many patients are unable to tolerate resection secondary to poor reserve or comorbid disease. Radiofrequency ablation (RFA) and microwave ablation (MWA) are methods of percutaneous thermal ablation that can be used to treat medically inoperable patients with NSCLC. We present long-term outcomes following thermal ablation of stage IA NSCLC from a single center.
Methods
Patients with stage IA NSCLC and factors precluding resection who underwent RFA or MWA from July 2005 to September 2009 were studied. CT and PET-CT scans were performed at 3 and 6 month intervals, respectively, for first 24 months of follow-up. Factors associated with local progression (LP) and overall survival (OS) were analyzed.
Results
Twenty-one patients underwent 21 RFA and 4 MWA for a total of 25 ablations. Fifteen patients had T1a and six patients had T1b tumors. Mean follow-up was 42 months, median survival was 39 months, and OS at three years was 52%. There was no significant difference in median survival between T1a nodules and T1b nodules (36 vs. 39 months, P=0.29) or for RFA and MWA (36 vs. 50 months, P=0.80). Ten patients had LP (47.6%), at a median time of 35 months. There was no significant difference in LP between T1a and T1b tumors (22 vs. 35 months, P=0.94) or RFA and MWA (35 vs. 17 months, P=0.18). Median OS with LP was 32 months compared to 39 months without LP (P=0.68). Three patients underwent repeat ablations. Mean time to LP following repeat ablation was 14.75 months. One patient had two repeat ablations and was disease free at 40-month follow-up.
Conclusions
Thermal ablation effectively treated or controlled stage IA NSCLC in medically inoperable patients. Three-year OS exceeded 50%, and LP did not affect OS. Therefore, thermal ablation is a viable option for medically inoperable patients with early stage NSCLC.
Keywords: Lung cancer, thermal ablation, radiofrequency ablation (RFA), microwave ablation (MWA), stage IA non-small cell lung cancer (NSCLC)
Introduction
Lung cancer is one of the most common types of cancer in the world, and in the United States is the leading cause of cancer-related death among men and women (1). Stage I non-small cell lung cancer (NSCLC) comprises 15% of all cases and has an overall 5-year survival of 54% (2). Lobectomy remains the treatment of choice for early-stage disease in standard-risk operable patients, and sub-lobar resection is an appropriate strategy for high-risk operable patients. In stage IA NSCLC, video assisted thoracic surgery (VATS) lobectomy carries a 5-year survival rate of over 90%, and median survival for lobectomy and segmentectomy are 100 and 74 months, respectively (3,4). Different options, however, are now available for the medically inoperable patient that may still provide an opportunity for cure. In many centers, stereotactic body radiation therapy (SBRT) has replaced three-dimensional conformal radiation therapy (3D-CRT) for medically inoperable patients with stage I NSCLC. In a study conducted by the Radiation Therapy Oncology Group (RTOG 0236), the median overall survival (OS) was 48.1 months and the 3-year disease-free survival and OS was 48.3% and 55.8%, respectively (5).
Percutaneous thermal ablation has been increasingly used for treatment of lung tumors, and can be performed using either radiofrequency ablation (RFA) or microwave ablation (MWA). The major advantages of thermal ablation pertain to its ability to destroy lung tumors by locally heating pathologic lung parenchyma to a lethal temperature, while incurring minimal damage to the surrounding normal lung tissue during a treatment session (6). The evolution of thermal ablation for the treatment of lung tumors has its origins in the initial use of RFA and MWA in managing hepatic tumors (7,8). RFA and MWA for the treatment of lung tumors were first reported in the early 2000s and the technique has been validated to be feasible and safe in highly selected patients (9-12).
Crabtree and colleagues previously compared pre-operative clinical factors used to define risk, among three co-operative group multicenter clinical trials involving treatment of stage I NSCLC (13). Patients considered medically inoperable were treated with SBRT (RTOG 0236) and RFA (ACOSOG Z4033), and patients considered high-risk but still operable were treated with sublobar resection (ACOSOG Z4032). Patients undergoing RFA were significantly older and had a worse mean predicted carbon monoxide diffusion capacity compared to patients treated with SBRT.
Further defining the utility of RFA is important especially in the management of stage IA NSCLC in medically inoperable patients. MWA may offer some advantages over RFA as it is not dependent on current flow or thermal conduction, is not limited by charring or boiling of the tissue, and may destroy tumors at higher temperatures than RFA. It may also result in a larger zone of ablation created within a shorter timeframe (14). To that end, the purpose of this paper is to report the outcomes, safety, and effectiveness from a single institutional experience on the use of thermal ablation for the treatment of Stage IA NSCLC in medically inoperable patients.
Methods
We retrospectively reviewed our experience with thermal ablation for the treatment of Stage IA NSCLC in medically inoperable patients at Boston Medical Center over 50 months, from July 2005 to September 2009. Informed consent was obtained from all patients, and this study was approved by the Institutional Review Board of Boston University (H-31629).
Patient selection
Patients eligible for this study had lung tumors ≤3 cm in maximum diameter. Biopsies were performed in all patients. Patients with lesions suspicious for, or confirmed to be NSCLC were included for review. All patients had PET or PET-CT for staging and pulmonary function testing within 2 months prior to treatment. Patients with mediastinal lymph nodes greater than 1 cm in the short axis and/or a positive PET scan result underwent mediastinoscopy. The inclusion criteria for thermal ablation in the treatment of patients with stage IA NSCLC in this study were: (I) patients who were considered medically inoperable because of poor pulmonary function, high cardiac risk, and other comorbidities, and (II) presence of a target tumor of 3 cm or smaller. Exclusion criteria included central tumors. All patients were evaluated by a thoracic surgeon to determine inoperability and suitability for thermal ablation.
Technology
A percutaneous CT-guided approach was used in all patients, and all procedures were performed with a thoracic surgeon and radiologist present. For tumors treated with RFA, one of three systems were used: the Boston Scientific LeVeen system (Boston Scientific Co., Natick, MA), the Angiodynamics (formerly RITA Medical) system (Angiodynamics, Latham, NY), or the Cool-Tip (formerly ValleyLab) system (Covidien, Mansfield, MA). For tumors treated with MWA, the Evident (formerly ValleyLab) system (Covidien, Mansfield, MA) was used. In general, the thermal ablation equipment consists of a generator and an active probe. In the case of RFA, dispersive pads are also applied to the patient’s thighs and plugged into the return socket on the RFA generator. The MWA procedures were undertaken during a clinical evaluation of the system in our institution. Choice of ablation modality was not based on specific selection criteria.
The Boston Scientific RFA system utilizes uses a probe containing multiple tines that are expanded within the tumor being ablated. As ablation takes place, electrical conduction of the ablated tissue becomes progressively limited secondary to coagulation necrosis. The resultant significant rise in resistance to current serves as the endpoint of treatment. The Angiodynamics (RITA) RFA system also uses a probe containing multiple, expandable tines that are deployed within the tumor being ablated. Unlike the Boston Scientific RFA system, the Angiodynamics system ablates the tumor by heating to a target temperature of 90 °C which, when reached, serves as the endpoint of treatment. The Covidien Cool-Tip system uses a straight probe (or a series of three straight probes) that is advanced into a target tumor, and uses impedance to determine the endpoint of ablation. In contrast to these systems, the Covidien Evident MWA system utilizes a straight probe and a microwave generator that causes polar water molecules within the target tissue to vibrate with the alternating electromagnetic field, generating heat and eventually causing localized cell death and tissue necrosis.
For all systems, the generators and devices were set up and used in accordance with the system’s instructions for use. The ablation probes in each case were positioned with the goal of ablating the target tumor and a surrounding rim of 0.5 to 1 cm of normal lung parenchyma to ensure an adequate treatment margin.
Post-procedure follow-up and assessment of therapy
Patients were followed up with clinical examinations and chest CT scans at 3-month intervals, and with PET-CT imaging at 6-month intervals, for the first 24 months following intervention, and then at a reduced frequency. Response to treatment, determination of local progression at the ablation site, and distant recurrence were based on the modified Response Evaluation Criteria in Solid Tumors (RECIST) criteria as well as CT densitometry (15-17). The modified RECIST criteria allow for a determination of a treatment response as ‘complete’, ‘partial’, ‘stable lesion’, or ‘progression’ on the basis of the size and quality of the nodule on CT imaging, and the standardized uptake value (SUV) of fluorodeoxyglucose (18F) of the lesion on positron emission tomography (PET) imaging. CT densitometry involves acquiring a CT scan of the chest and measuring the Hounsfield units of the target lesion at 0, 45, 180, and 300 s following the administration of intervenous contrast. A change of 15 or more Hounsfield units over baseline in a lesion greater than 9 mm was considered suspicious for local progression. Patients were evaluated for initial response rate, time to local progression, and OS.
Data collection and statistical analysis
The objective of this study was to determine the outcomes of thermal ablation in the treatment of medically inoperable patients with stage IA NSCLC. Data were collected securely through a retrospective chart review using a data collection form that queried demographic information, preoperative clinical information, studies performed as part of each patient’s workup, and data from the perioperative and follow-up periods. Specific data and end points included patient demographics, tumor characteristics, treatment, comorbidities, complications, time to local progression, and OS. Local progression of the treated nodule was assessed as described above. Time to progression was calculated from the date of intervention. In some cases patients were lost to follow-up, therefore OS was determined by querying the Social Security Death Index. For OS, follow-up was censored on the date of the last documented visit, or on the date of death by the Social Security Death Index.
Statistical analysis was conducted using the SPSS (version 11 for Windows) statistical software package (SPSS, Inc., Chicago, IL). Kaplan-Meier plots were constructed to assess OS, and the log-rank test was used to determine differences between groups. Association between categorical variables was tested with the Fisher exact test or the χ2 test.
Results
Twenty-one patients underwent 25 thermal ablation procedures (21 RFA, 4 MWA) from July 2005 through September 2009; 18 patients had single ablations, 2 had repeat ablation once, and one had repeat ablation twice. Fifteen patients had T1a (≤2 cm) and 6 had T1b (>2–3 cm) tumors. Mean age was 69 [42–84] years. Ten patients were women. Mean BMI was 25. Sixteen patients were former smokers and 4 were active smokers, and the mean pack-years was 46.4 (range, 3–120). Seven patients were dependent on home oxygen, and 5 patients had prominent bullae on plain chest imaging. All patients had significant comorbidities, with a mean Charlson Comorbidity Index score of 5 (range, 2–8; median, 5.5). The median percent-predicted FEV1 was 39%, and DLCO was 47%.
The mean nodule diameter was 1.88 (0.8–3) cm. NSCLC was biopsy-confirmed in 17 patients (9 were adenocarcinoma, 3 were squamous cell carcinoma, 1 was large cell carcinoma, and 4 were unspecified), and 4 were suspicious on biopsy. Initial treatments involved RFA for 18 patients and MWA for 3 patients. The most common complication was pneumothorax requiring a chest tube in 12 (57.1%) patients. Prolonged air leak (>5 days) occurred in one patient, who required chemical pleurodesis and subsequently developed ventilator dependent respiratory failure necessitating tracheostomy placement. Additionally, this patient experienced a cerebrovascular accident. One patient required readmission with an exacerbation of chronic obstructive pulmonary disease (COPD), There was no procedure related mortality. The median hospital length of stay was 3 days.
Mean follow-up was 42 months. Two- and 3-year OS was 81% and 52%, respectively (Figure 1), and median survival was 39 months. This compares favorably with the often-quoted RTOG 0236 study, where SBRT was used in medically inoperable patients (5). In that study, median OS was 48.1 months and the 3-year OS was 55.8%. Median survival in our study was not significantly different between T1a nodules compared to T1b nodules (36 vs. 39 months, P=0.29), or nodules treated with RFA compared to MWA (36 vs. 50 months, P=0.80). Local progression occurred in 10 patients (47.6%). Median time to local progression was 35 months, and was not significantly different between T1a nodules compared to T1b nodules (22 vs. 35 months, P=0.94) or RFA compared to MWA (35 vs. 17 months, P=0.18). OS was not impacted by local progression (median 32 vs. 39 months with and without local progression, P=0.68).
Figure 1.
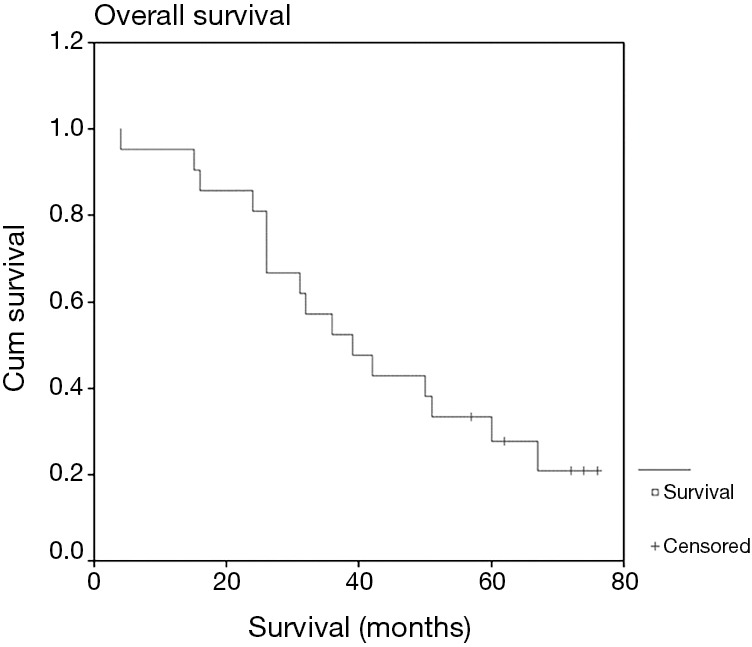
Overall survival.
Repeat ablation was performed once in 2 patients, and twice in 1 patient. One patient was treated with MWA for a 2 cm adenocarcinoma, and had local progression at 17 months. This lesion was retreated with RFA, and local progression recurred at 6 months. Another patient was treated with RFA for a 1.5 cm adenocarcinoma, and had local progression at 9 months; this lesion was retreated with RFA, and local progression also recurred at 6 months. The third patient was treated with RFA for a 1.5 cm non-specified NSCLC, and had local progression at 14 months. He was retreated with MWA, and local progression recurred at 6 months. Following this, he was retreated with RFA, and has been both local progression and recurrence free at 40 months. In these three patients, the mean local progression time following repeat ablation was 14.75 months.
Discussion
Thermal ablation for the treatment of lung tumors was first reported in 2000 for RFA, and in 2002 for MWA (9,10). This technique has been demonstrated to be feasible and safe in medically inoperable patients (11,12,18). However, many of these studies have involved heterogeneous patient populations that have included patients with various stages of NSCLC as well as lung metastases (18,19). As a result, there has not been widespread adoption of this technique, and many centers have adopted SBRT as their preferred approach for the medically inoperable patient. A better understanding of the long-term outcomes of thermal ablation for Stage IA NSCLC in medically inoperable patients is important to help physicians make treatment decisions for these compromised patients. Our report provides valuable information on thermal ablation in a cohort of medically inoperable patients with a mean follow-up of 42 months.
OS was 81% at 2 years and 52% at 3 years, with a median survival of 39 months in our study. Interestingly, the median survival was not impacted by the size of the nodule or the modality used for thermal ablation (RFA vs. MWA), although with respect to treatment modality there may have not been enough patients treated with MWA (n=3) to demonstrate a significant difference.
Our OS compares favorably to the RAPTURE trial of 106 patients, in which 33 Stage IA NSCLC patients were treated with RFA and the OS at 2 years was 48% (18). Pennathur and colleagues also reported a study of 19 patients with Stage I NSCLC (11 of which were stage IA) treated with RFA; OS at 2 years was estimated at 68% (20). Furthermore, our median survival compares favorably to the report by Simon and colleagues, in which 153 patients with lung tumors—56 of which had stage IA NSCLC—were treated with RFA, which demonstrated a median survival of 30 months among stage IA patients (19). More recently, Acksteiner and Steinke examined MWA in ten patients with early stage NSCLC, with one death occurring 19 months post-ablation and not secondary to cancer (21). Palussiere et al. had performed RFA in 82 patients and MWA in 5 patients in patients across all clinical stages. Stage I disease was present in 65 patients, and five-year overall and disease-free survival were 58.1% and 27.9%, respectively (22).
Local progression occurred in 10 patients (47.6%) and the median time to local progression was 35 months. Interestingly, local progression was not influenced by the size of the tumors or the modality that was used. This contrasts with the report by Simon et al.—where local recurrence rates were impacted by tumor size, and the study by Palussiere et al. who observed a 21.1% rate of local progression at 3 years post-ablation (19,22). Local progression in our study was comparable to the series by Pennathur and colleagues that demonstrated a local progression of 42% with a median time to local progression of 28 months (20). The high local recurrence may be attributable to the limitations of thermal ablation. In particular, a heat sink effect can occur in which blood vessels adjacent to the tumor can siphon thermal energy away during the ablation, limiting the therapeutic effect as well as the margin of therapy. Theoretically, MWA should not be limited by the same heat sink effects of RFA, but local progression still occurs. Liu and Steinke performed MWA on 16 patients with early stage NSCLC with a median follow-up of 1 year. The authors observed that 31.3% of these patients had local progression, with a median time to local progression of 9 months (23). Another factor that may account for higher rates of local progression in thermal ablation compared to that typically reported with SBRT may be related to the aggressiveness of follow-up. Many of the SBRT studies have only utilized CT scan follow-up. PET has been considered too sensitive after SBRT, and no SBRT studies have utilized densitometry (24,25). Our group has previously reported on the use of PET with SBRT and identified several cases of regional recurrence that would not have been identified with the more standard RTOG criteria using CT scan only (26). Interestingly, the presence of local progression did not impact OS as was also seen in a previous report (27).
In considering the treatment of the medically-inoperable patient with stage IA NSCLC, there are advantages to thermal ablation. Notably, there is no treatment ceiling unlike with SBRT. Retreatments are possible as demonstrated by our series in which two patients underwent repeat ablation once, and one patient twice (the latter patient was disease-free at 40 months). Although thermal ablation is associated with a short-hospital stay (the median length of stay in our series was 3 days), there is some evidence that thermal ablation is less expensive compared to treatment by SBRT and requires less total time, compared to the 3–5 sessions required by a SBRT treatment pathway over a 2-week period (28). Additionally, SBRT for stage IA NSCLC has an OS at 55.8% at 3 years, which is comparable to our experience with thermal ablation (5). The main disadvantage to thermal ablation is the risk of pneumothorax, which occurred in 57% of our series but was only associated with a prolonged air leak in one patient. In our series 13 out of 25 (52%) ablative procedures were performed with concurrent biopsies. The performance of biopsies at the time of ablation may have led to the increased the rate of pneumothorax in this study. It should be noted that in some approaches using SBRT, fiducial markers are placed, which also carries a high risk of pneumothorax often requiring chest tube insertion (29,30). Additionally, biopsy should be performed to confirm cancer prior to treating with SBRT.
In conclusion, thermal ablation—using RFA or MWA—is safe and effective in treating medically inoperable patients with stage IA NSCLC. OS exceeded 50% and was not impacted by local progression, the size of the tumors treated, or the treatment modality used. The rate of local progression was similar to other studies previously reported in the literature. Cases of local progression were successfully treated with repeat ablation. Further comparison of thermal ablation to SBRT and other treatment options, in the context of prospective clinical trials, can aid multidisciplinary care teams in selecting the appropriate management strategy for these challenging patients.
Acknowledgements
None.
Ethical Statement: This study was approved by the Institutional Review Board of Boston University (H-31629) and written informed consent was obtained from all patients.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.American Cancer Society (2014). Cancer Facts and Figures 2014. Available online: http://www.cancer.org/acs/groups/content/@research/documents/webcontent/acspc-042151.pdf. Last accessed 08/02/2014
- 2.Cancer Stat Facts: Lung and Bronchus Cancer. Surveillance, Epidemiology, and End Results Program. National Cancer Institute. Available online: https://seer.cancer.gov/statfacts/html/lungb.html
- 3.Khullar OV, Liu Y, Gillespie T, et al. Survival after sublobar resection versus lobectomy for clinical stage IA lung cancer. An analysis from the National Cancer Data Base. J Thorac Oncol 2015;10:1625-33. 10.1097/JTO.0000000000000664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Higuchi M, Yaginuma H, Yonechi A, et al. Long-term outcomes after video-assisted thoracic surgery (VATS) lobectomy versus lobectomy via open thoracotomy for clinical stage IA non-small cell lung cancer. J Cardiothorac Surg 2014;9:88. 10.1186/1749-8090-9-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010;303:1070-6. 10.1001/jama.2010.261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Narsule CK, Daly BD, Fernando HC. Thermal Ablation of Lung Tumors and Pulmonary Metastases. In: Goodman MD. editor. Regional Therapeutics for Advanced Malignancies, 1st edition. New Delhi: Jaypee Brothers Medical Publishers Pvt. Ltd., 2012. [Google Scholar]
- 7.Curley SA, Izzo F, Delrio P, et al. Radiofrequency ablation of unresectable primary and metastatic hepatic malignancies: results in 123 patients. Ann Surg 1999;230:1-8. 10.1097/00000658-199907000-00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu MD, Chen JW, Xie XY, et al. Hepatocellular carcinoma: US-guided percutaneous microwave coagulation therapy. Radiology 2001;221:167-72. 10.1148/radiol.2211001783 [DOI] [PubMed] [Google Scholar]
- 9.Dupuy DE, Zagoria RJ, Akerley W, et al. Percutaneous radiofrequency ablation of malignancies in the lung. AJR Am J Roentgenol 2000;174:57-9. 10.2214/ajr.174.1.1740057 [DOI] [PubMed] [Google Scholar]
- 10.Feng W, Liu W, Li C, et al. Percutaneous microwave coagulation therapy for lung cancer. Zhonghua Zhong Liu Za Zhi 2002;24:388-90. [PubMed] [Google Scholar]
- 11.Zemlyak A, Moore WH, Bilfinger TV. Comparison of survival after sublobar resections and ablative therapies for state I non-small cell lung cancer. J Am Coll Surg 2010;211:68-72. 10.1016/j.jamcollsurg.2010.03.020 [DOI] [PubMed] [Google Scholar]
- 12.Ambrogi MC, Fanucchi O, Cioni R, et al. Long-term results of radiofrequency ablation treatment of stage I non-small cell lung cancer: a prospective intention-to-treat study. J Thorac Oncol 2011;6:2044-51. 10.1097/JTO.0b013e31822d538d [DOI] [PubMed] [Google Scholar]
- 13.Crabtree T, Puri V, Timmerman R, et al. Treatment of stage I lunc cancer in high-risk and inoperable patients: comparison of prospective clinical trials using stereotactic body radiotherapy (RTOG 0236), sublobar resection (ACOSOG Z4032), and radiofrequency ablation (ACOSOG Z4033). J Thorac Cardiovasc Surg 2013:145:692-9. 10.1016/j.jtcvs.2012.10.038 [DOI] [PubMed] [Google Scholar]
- 14.Dupuy DE. Image-guided thermal ablation of lung malignancies. Radiology 2011;260:633-55. 10.1148/radiol.11091126 [DOI] [PubMed] [Google Scholar]
- 15.Suh RD, Wallace AB, Sheehan RE, et al. Unresectable pulmonary malignancies: CT-guided percutaneous radiofrequency ablation – preliminary results. Radiology 2003;229:821-9. 10.1148/radiol.2293021756 [DOI] [PubMed] [Google Scholar]
- 16.Yasui K, Kanazawa S, Sano Y, et al. Thoracic tumors treated with CT-guided radiofrequency ablation: initial experience. Radiology 2004:231:850-7. 10.1148/radiol.2313030347 [DOI] [PubMed] [Google Scholar]
- 17.Swensen SJ, Viggiano RW, Midthun DE, et al. Lung nodule enhancement at CT: a multicenter study. Radiology 2000;214:73-80. 10.1148/radiology.214.1.r00ja1473 [DOI] [PubMed] [Google Scholar]
- 18.Lencioni R, Crocetti L, Cioni R, et al. Response to radiofrequency ablation of pulmonary tumors: a prospective, intention-to-treat multicenter clinical trial (the RAPTURE study). Lancet Oncol 2008;9:621-8. 10.1016/S1470-2045(08)70155-4 [DOI] [PubMed] [Google Scholar]
- 19.Simon CJ, Dupuy DE, DiPetrillo TP, et al. Pulmonary radiofrequency ablation: long-term safety and efficacy. Radiology 2007;243:268-75. 10.1148/radiol.2431060088 [DOI] [PubMed] [Google Scholar]
- 20.Pennathur A, Luketich JD, Abbas G, et al. Radiofrequency ablation for the treatment of stage I non-small cell lung cancer in high-risk patients. J Thorac Cardiovasc Surg 2007;134:857-64. 10.1016/j.jtcvs.2007.04.060 [DOI] [PubMed] [Google Scholar]
- 21.Acksteiner C, Steinke K. Percutaneous microwave ablation for early-stage non-small cell lung cancer (NSCLC) in the elderly: a promising outlook. J Med Imaging Radiat Oncol 2015;59:82-90. 10.1111/1754-9485.12251 [DOI] [PubMed] [Google Scholar]
- 22.Palussiere J, Lagarde P, Aupérin A, et al. Percutaneous lung thermal ablation of non-surgical clinical N0 non-small cell lung cancer: results of eight years of experience in 87 patients from two centers. Cardiovasc Intervent Radiol 2015;38:160-6. 10.1007/s00270-014-0999-6 [DOI] [PubMed] [Google Scholar]
- 23.Liu H, Steinke K. High-powered percutaneous microwave ablation of stage I medically inoperable non-small cell lung cancer: a preliminary study. J Med Imaging Radiat Oncol 2013;57:466-74. 10.1111/1754-9485.12068 [DOI] [PubMed] [Google Scholar]
- 24.Hoopes DJ, Tann M, Fletcher JW, et al. FDG-PET and stereotactic body radiotherapy (SBRT) for stage I non-small cell lung cancer. Lung Cancer 2007;56:229-34. 10.1016/j.lungcan.2006.12.009 [DOI] [PubMed] [Google Scholar]
- 25.Henderson MA, Hoopes DJ, Fletcher JW, et al. A pilot trial of serial 18F-fluorodeoxyglucose positron emission tomography in patients with medically inoperable stage I non-small cell lung cancer reated with hypofractionated stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys 2010;76:789-95. 10.1016/j.ijrobp.2009.02.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ebright MI, Russo GA, Gupta A, et al. Positron emission tomography combined with diagnostic chest computed tomography enhances detection of regional recurrence after stereotactic body radiation therapy for early stage non-small cell lung cancer. J Thorac Cardiovasc Surg 2013;145:709-15. 10.1016/j.jtcvs.2012.12.024 [DOI] [PubMed] [Google Scholar]
- 27.Lanuti M, Sharma A, Digumarthy SR, et al. Radiofrequency ablation for treatment of medically inoperable stage I non-small cell lung cancer. J Thorac Cardiovasc Surg 2009;137:160-6. 10.1016/j.jtcvs.2008.08.034 [DOI] [PubMed] [Google Scholar]
- 28.Dupuy DE. Treatment of medically inoperable non-small cell lung cancer with stereotactic body radiation therapy versus image-guided tumor ablation: can interventional radiology compete? J Vasc Interv Radiol 2013:24:1139-45. 10.1016/j.jvir.2013.04.021 [DOI] [PubMed] [Google Scholar]
- 29.Bhagat N, Fidelman N, Durack JC, et al. Complications associated with the percutaneous insertion of fiducial markers in the thorax. Cardiovasc Intervent Radiol 2010;33:1186-91. 10.1007/s00270-010-9949-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yousefi S, Collins BT, Reichner CA, et al. Complications of thoracic computed tomography-guided fiducial placement for the purpose of stereotactic body radiation therapy. Clin Lung Cancer 2007;8:252-6. 10.3816/CLC.2007.n.002 [DOI] [PubMed] [Google Scholar]


