Abstract
Background
Anaplastic lymphoma kinase (ALK) and c-ros oncogene 1 (ROS1) rearrangements represent two most frequent fusion targets in lung adenocarcinoma. Our study was intended to explore the clinicopathological characteristics, coexistence and treatment of ALK/ROS1-rearranged patients of lung adenocarcinoma without epidermal growth factor receptor (EGFR) mutation.
Methods
Patients with wild-type EGFR mutation were screened for ALK/ROS1 at four domestic hospitals. ALK/ROS1 rearrangements were detected by reverse transcription-polymerase chain reaction (RT-PCR). Progression-free survival (PFS) curve was plotted with the Kaplan-Meier method.
Results
Among 732 eligible cases, ALK and ROS1 rearrangements were detected in 89 (12.2%) and 32 (4.4%) patients respectively. One patient harbored coexisting ALK/ROS1 fusion. Both ALK and ROS1-positive phenotypes were predominantly detected in younger non-smokers. More ALK/ROS1-rearranged patients were correlated with the expressions of TTF1, napsin A and solid predominant adenocarcinoma subtype. Thirty-three ALK and six ROS1 rearrangement patients received crizotinib treatment at an advanced stage. The median PFS was 9.5 months for ALK-positive patients and it was not attained in ROS1-rearranged counterparts.
Conclusions
The frequency of ALK and ROS1 rearrangements is elevated in EGFR-wild-type patients and the phenomenon of coexisting ALK/ROS1 has remained extremely rare. The rearrangements of ALK/ROS1 are correlated with age, smoking status, expressions of TTF1 & napsin A and solid predominant adenocarcinoma subtype.
Keywords: Lung adenocarcinoma; epidermal growth factor receptor (EGFR); anaplastic lymphoma kinase (ALK); c-ros oncogene 1 (ROS1); frequency, coexistence, efficacy
Introduction
Lung cancer has been the most common cause of cancer-related mortality in China (1). Most patients of non-small cell lung cancer (NSCLC) have already reached advanced stages during an initial diagnosis. Chemotherapy has been a mainstay treatment with limited efficacies over the last three decades. Treatment strategies for NSCLC based on molecular targets have been widely used with encouraging outcomes as compared to chemotherapy alone (2-5).
Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors are currently recommended as a first-line option for patients with EGFR-sensitizing mutations in metastatic setting. And EGFR mutation detection has been routinely recommended (6,7). Anaplastic lymphoma kinase (ALK) and c-ros oncogene 1 (ROS1) represent two most frequent rearrangement genes in NSCLC. Previous studies showed that the frequencies of ALK and ROS1 were approximately 3–7% and 1–2% in NSCLC respectively (8,9). Though as a common detecting modality, the clinicopathological characteristics are not widely recognized, especially for ROS1 rearrangement, due to the fact that merely 200 patients have been reported (10,11). However, most previous studies have focused largely on the relationship between clinical characteristics and gene frequency (12,13). However, immunohistochemical (IHC) markers and histological subtypes were not thoroughly examined. Furthermore, several recent studies have shown that driver genes could occur concurrently. However, most of them focused on EGFR concomitancy. And little has been known about the frequency of ALK/ROS1 coexistence.
It is well-known that patients with ALK/ROS1 rearrangement could benefit from crizotinib treatment (10,11). However, nearly all of them had ALK/ROS1 rearrangement based upon fluorescence in situ hybridization (FISH). Approved as a standard detection method in China, polymerase chain reaction (PCR) has been employed for examining the efficacy of crizotinib.
The ALK/ROS1 prevalence data of reverse transcription-polymerase chain reaction (RT-PCR) were used for examining the clinicopathological characteristics and gene coexistence in a cohort of 732 lung adenocarcinoma patients in present study. Also, the efficacy of ALK/ROS1 inhibitor was examined in rearrangement positive subjects.
Methods
Study populations
Between December 2012 and July 2015, patients with a definite diagnosis of lung adenocarcinoma were selected from Zhejiang Cancer Hospital, Fuzhou General Hospital, Fujian Union Hospital and Zhangzhou Municipal Hospital. All diagnoses were confirmed as EGFR wild-type using ARMS kit (Amoy, Xiamen, China). The following 29 mutations were detected: 3 in exon 18 (G719A, G719C, and G719S), 19 deletions in exon 19, 2 mutations in exon 20 (T790M, S768I), 3 insertions in exon 20 and 2 mutations in exon 21 (L858R, L861Q). Lung cancer staging was performed according to the 7th TNM classification scheme. The study protocol was approved by Institutional Review Board of the above four institutions (2014-11-93).
Gene detection
Microscopy was used for ensuring that tumor tissues analyzed had >20% tumor contents. And ALK/ROS1 fusion mRNA was detected by PCR with fusion gene detection kit (Amoy, Xiamen, China). Briefly total RNA was extracted with QiagenRNeasy FFPE kit. And mRNA was reverse-transcribed into cDNA at 42 °C for 1 hour. Then β-actin was utilized as an internal control. The conditions of RT-PCR were specified as follows: initial denaturation at 95 °C for 5 min, followed by 95 °C for 25 s, 64 °C for 20 s and 72 °C for 20 s for ensuring specificity; and 31 cycles at 93 °C for 25 s, 60 °C for 35 s and 72 °C for 20 s. Data collection and sensitivity analysis were described previously (12).
Histological evaluations
Histological classification was based upon the IASLC/ATS/ERS classification of lung adenocarcinomas and 2015 WHO classification scheme. According to the IASLC/ATS/ERS criteria, tumors were classified as adenocarcinomas in situ (AIS), minimally invasive adenocarcinoma (MIA) and invasive adenocarcinomas. And the last type was further divided into lepidic predominant, acinar predominant, papillary predominant, micropapillary predominant, solid predominant and invasive mucinous adenocarcinoma, etc. IHC antibodies for lung adenocarcinoma included napsin A, CK5/6, P63 and TTF-1. All procedures were performed routinely. And all slides were independently reviewed by three experienced pathologists.
Statistical analyses
Wilcoxon’s rank-sum test was employed for assessing the baseline characteristics of different groups. Defined as the time from crizotinib treatment to documented progression or mortality from any cause, progression-free survival (PFS) of crizotinib was estimated by the Kaplan-Meier method. Data analysis was performed with Statistics 18.0 (SPSS Inc., Chicago, IL, USA). The last follow-up date was October 1, 2016.
Results
Patient characteristics
A total of 788 patients with lung adenocarcinoma with wild-type EGFR were enrolled and 732 of them were available for ALK/ROS1 detection. And 56 patients failed detection due to poor formalin-fixed paraffin-embedding (FFPE) quality. There were 391 males and 341 females with a median age of 59 [28–81] years. And 323 patients had a smoking history and 409 were never-smokers. None of them received any targeted treatment at an initial diagnosis. The clinical stages were advanced (n=219), I (n=205), II (n=112) and III (n=196). Their clinical characteristics were summarized in Tables 1,S1.
Table 1. Demographic characteristics of study population (n=732).
| Variables | Number |
|---|---|
| Gender | |
| Male | 391 |
| Female | 341 |
| Age (years) | |
| Range | 28–81 |
| Median | 59 |
| <60 | 415 |
| ≥60 | 317 |
| Smoking status | |
| Never | 409 |
| Former/current | 323 |
| Stage | |
| I | 205 |
| II | 112 |
| III | 196 |
| IV | 219 |
| TTF1 | |
| Positive | 509 |
| Negative | 95 |
| Unknown | 128 |
| Napsin A | |
| Positive | 546 |
| Negative | 58 |
| Unknown | 128 |
| P63 | |
| Positive | 25 |
| Negative | 520 |
| Unknown | 187 |
| CK5/6 | |
| Positive | 31 |
| Negative | 560 |
| Unknown | 141 |
| Histology | |
| Solid-predominant | 91 |
| Non-solid predominant | 530 |
| Unknown | 111 |
Histology
A total of 621 patients were evaluated for histological subtypes. None of the specimens fulfilled the criteria for AIS and 20 patients belonged to MIA. Among 601 cases of invasive adenocarcinoma, the distribution of dominant histological subtypes was 165 papillary (n=165, 27.5%), acinar (n=148, 24.6%), micropapillary (n=103, 17.1%), solid (n=91, 15.1%), epidic (14.0%) and 10 (1.7%) variants of invasive adenocarcinoma. And the detailed results of TTF1 and napsin A were provided for 604 patients; 509 and 546 patients were positive for TTF1 and napsin A respectively. The results of P63 were collected from 545 patients and 520 of them were negative; 560 patients were CK5/6 negative and 31 were positive.
Detection results of ALK and ROS1 genes
Eighty-nine samples were ALK positive and 32 ROS1 positive (Figure S1). The frequencies of ALK and ROS1 were 12.2% and 4.4% respectively. Among 89 ALK-positive patients, there were 42 males and 47 females with a median age of 49.5 years. And 19 patients had a smoking history and 70 belonged to never-smokers. For 32 ROS1-positive patients, the median age was 53.5 years and 46.8% of them were males. Comparison of ALK/ROS1 positive versus negative patients was summarized in Table 2.
Table 2. Comparison of clinicopathologic features among patients harboring different genes.
| Variables | ALK | ROS1 | Wild type | P (ALK vs. wild) | P (ROS1 vs. wild) |
|---|---|---|---|---|---|
| Gender | 0.19 | 0.39 | |||
| Male | 42 | 15 | 334 | ||
| Female | 47 | 17 | 277 | ||
| Age at diagnosis (years) | 0.08 | 0.65 | |||
| <60 | 58 | 19 | 338 | ||
| ≥60 | 31 | 13 | 273 | ||
| Smoking history | <0.001 | 0.0002 | |||
| Yes | 19 | 5 | 299 | ||
| No | 70 | 27 | 312 | ||
| Histology | 0.006 | 0.015 | |||
| Solid predominant | 18 | 8 | 66 | ||
| Non-solid predominant | 56 | 20 | 460 | ||
| Stage at diagnosis | 0.14 | 0.55 | |||
| I–IIIA | 67 | 20 | 413 | ||
| IIIB/IV | 22 | 12 | 198 | ||
| TTF1 | 0.01 | 0.035 | |||
| Positive | 70 | 26 | 413 | ||
| Negative | 5 | 0 | 90 | ||
| Napsin A | 0.04 | 0.25 | |||
| Positive | 76 | 28 | 443 | ||
| Negative | 2 | 0 | 52 | ||
| P63 | 0.32 | 0.57 | |||
| Positive | 1 | 0 | 24 | ||
| Negative | 64 | 21 | 435 | ||
| CK5/6 | 0.39 | 0.40 | |||
| Positive | 2 | 0 | 29 | ||
| Negative | 72 | 26 | 462 |
Correlation between ALK/ROS1 rearrangements and pathologic characteristics
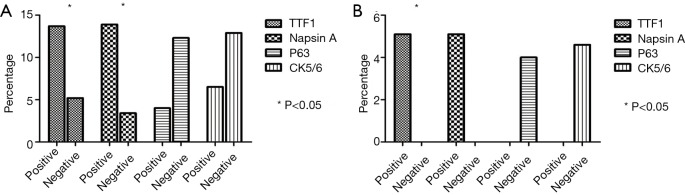
Both ALK and ROS1 rearrangements were significantly more frequent in solid predominant subtype than those in other subtypes (P=0.006 and 0.015). The frequencies of ALK and ROS1 in TTF1-positive patients were 13.8% and 5.1% respectively. And the frequencies of ALK and ROS1 in napsin A positive were 13.9% and 5.1% respectively. Significantly more frequent ALK and ROS1 rearrangements appeared in TTF1-positive than negative patients (P=0.01 and 0.035). A similar trend was found in napsin A positive and negative patients. Yet no such a relationship existed between expressions of CK5/6 and P63 and ALK/ROS1 frequencies (Table 2 and Figure 1).
Figure 1.
Frequency of positive ALK/ROS1 rearrangements based upon IHC markers. (A) ALK; (B) ROS1. ALK, anaplastic lymphoma kinase; ROS1, c-ros oncogene 1.
Among 89 ALK-positive patients by RT-PCR, 34 were with FISH detection and 32 were positive. Among 643 ALK negative patients, 102 were with FISH detection and one was positive. Among 32 ROS1-positive patients, 7 were confirmed by FISH and all were positive.
Treatment
For 89 ALK-positive patients, 33 received crizotinib after recurrence or metastasis. For 32 ROS1-positive patients, 6 received crizotinib at an advanced stage. The disease control rates were 87.9% and 100% in ALK and ROS1 patients. And the objective response rates (ORRs) were 60.6% and 83.3% in ALK and ROS1-positive patients respectively. Median PFS was 9.5 months in ALK-positive patients and not attained in ROS1-positive patients (Figure 2).
Figure 2.
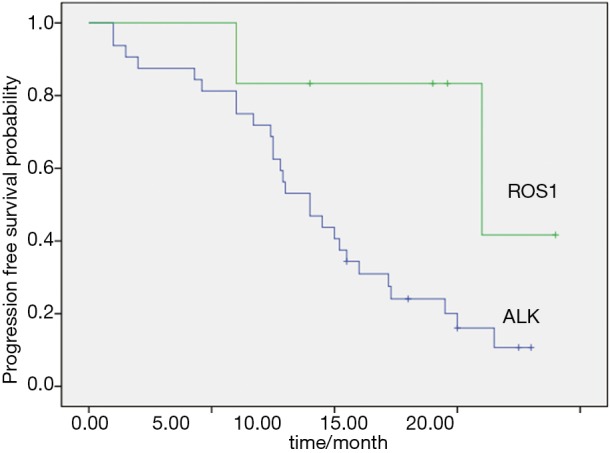
PFS of crizotinib treatment in ALK and ROS1 positive patients. PFS, progression-free survival; ALK, anaplastic lymphoma kinase; ROS1, c-ros oncogene 1.
One patient with concurrent ALK/ROS1 rearrangements
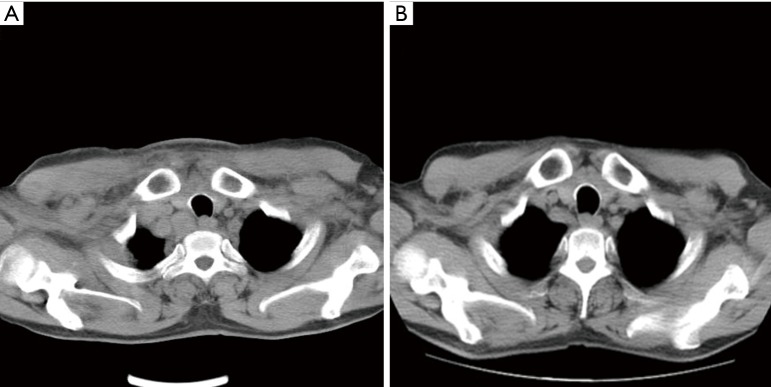
In May 2014, a 56-year-old male patient was referred for dyspnea to Zhejiang Cancer Hospital. Imaging study revealed a right upper lung lobe mass with adrenal metastases. A biopsy of lung mass hinted at the histology of lung adenocarcinoma (partially signet ring cell carcinoma). The status of EGFR mutation was wild-type. A first-line chemotherapeutic protocol of pemetrexed and cisplatin (PFS =5 months) was followed by second-line docetaxel (PFS =2 months). Unfortunately, imaging report demonstrated progressive disease with mediastinal, supraclavicular and axillar lymph node involvements. After enrollment, ALK/ROS1 detection was dually positive. FISH was used for confirming the presence of dual ALK/ROS1 rearrangements. Crizotinib (250 mg, bid) was initiated on February 24, 2015, and imaging examination indicated a remarkable response of lymph nodes after 2 months and crizotinib was extended until the last follow-up session with a PFS >1 year (Figure 3).
Figure 3.
Computed tomography image in one patient before and after crizotinib treatment. (A) Thoracic computed tomography before crizotinib treatment; (B) 2 months after crizotinib treatment.
Discussion
As demonstrated here, 12.2% and 4.4% patients harbored ALK and ROS1 rearrangements in EGFR wild-type lung adenocarcinoma patients. Concurrent ALK/ROS1 rearrangement was extremely rare. And there was only one reported case of ALK/ROS1 dual positivity. Both ALK and ROS1 rearrangements were predominantly found in solid predominant adenocarcinoma subtype, TTF1 and napsin A positive samples. Detection of ALK/ROS1 rearrangements is preferably performed with RT-PCR.
The frequencies of ALK and ROS1 rearrangements were estimated at 3–7% and 1.0–4.5% among a non-selected NSCLC population (14-16). The detection of EGFR and ALK have been routinely recommended in clinical practice. And EGFR is preferred when there is a shortage of tumor tissue. For EGFR wild-types, both ALK and ROS1 genes are routinely detected. The frequencies of ALK and ROS1 spiked to 12.2% and 4.4% in our study. Here a higher prevalence of ALK/ROS1 rearrangements in younger and never-smokers accorded with previous studies of correlations between clinical characteristics and frequency (14-16). However, the association between histological markers and gene alternations has remained ill-elucidated. TTF1-positive samples had a higher frequency of ROS1 protein expression in one previous study (17). However, only nine patients had ROS1 rearrangement and no conclusion was reached about the correlation of TTF1 expression and ROS1 rearrangement. Thirty-two ROS1-rearranged patients were enrolled into the present study. The frequency was higher in TTF1 positive than negative samples. Furthermore, there was a similar trend for napsin A expression. However, no association existed between ALK/ROS1 frequencies and P63 & CK5/6 expression. It might contribute to a small number of P63 or CK5/6 positive patients. Thus, some IHC markers might become candidates for detecting ALK/ROS1. Lung adenocarcinoma subtypes have been found to be associated with genes and EGFR mutation tends to be more frequent in micropapillary predominant adenocarcinoma subtype (18). In our study, both ALK and ROS1 fusions were dominant in solid predominant subtype and the results agreed with those of previous studies.
Although gene rearrangements or mutations might be mutually exclusive, several studies have indicated that different genes appeared concurrently (19-21). Concomitant EGFR mutations and ALK rearrangements were previously reported (19,20). However, there has been no concurrency of fusion genes and ALK/ROS1. One patient of ALK/ROS1 concurrence responded favorably to crizotinib in our cohort. Of note, the fusions of ALK and ROS1 were confirmed by FISH. To our knowledge, it is the first ever case of ALK/ROS1 co-existence and crizotinib treatment offered marked benefits.
Currently, FISH has been a standard method of detecting ALK/ROS1. Both D5F3 antibody-based IHC and RT-PCR are recommended for ALK detection in China. A high concordance rate existed between FISH, IHC and RT-PCR for ALK detection (22). For ROS1 detection, no studies have compared the concordance rate between RT-PCR and FISH. In our study, all seven ROS1-positive samples were confirmed by FISH. Despite a limited number of samples, RT-PCR is an alternative method for ROS1 detection based on current data.
Our study examined the clinical efficacy of crizotinib for some ALK/ROS1-positive patients. The results hinted at a high response rate and disease control after crizotinib treatment. And ORR for ALK-positive patients was consistent with previous studies (23,24). For ROS1-positive patients, five of six achieved partial response. Therefore RT-PCR may be suitable for selecting the benefactors of crizotinib treatment.
As the first large-scale study for detecting ALK/ROS1 rearrangements in EGFR wild-type patients, there were some inherent limitations. Firstly, this study was conducted for select patients so that it had a selection bias. Secondly, not all ALK/ROS1-positive samples were confirmed by another method. Thus, the sensitivity and specificity of RT-PCR were unsatisfactory. Thirdly, only a small number of patients received crizotinib, especially for ROS1-positive patients. A larger prospective study is warranted for validation.
Conclusions
In summary, 12.2% and 4.4% patients harbored ALK/ROS1 rearrangements in Chinese lung adenocarcinoma patients with EGFR wild-type. Concurrent ALK/ROS1 fusion is rare. The efficacy of crizotinib treatment is decent for ALK/ROS1-rearranged patients.
Acknowledgements
The authors thank all patients and investigators for participation. This study was supported by Amoy Diagnostics Company Ltd., Xiamen, China.
Funding: Our study was funded by Medical Scientific Research Foundation of Zhejiang Province (No. 2015KYA040).
Real time RT-PCR
The EML4-ALK and ROS1 fusion mRNA was readily detected by PCR using AmoyDx EML4-ALK and ROS1 Fusion Gene Detection Kit (Amoy Diagnostics, Xiamen, China), according to manufacturer’s instruction. In brief, total RNA was extracted with AmoyDx FFPE RNA Kit (Spin Column) from 5–10 µm thick FFPE sections with at least 20% tumor cells. For each sample, 100–500 ng of extracted RNA was used for reverse transcription into cDNA at 42 °C for 1 h. Real-time PCR was then carried out in each of the four reactions of the Fusion Gene Detection Kit according to the manufacturer’s protocol. All assays were performed on an Agilent Mx3000P QPCR instrument (Agilent Technologies, Santa Clara, CA). The following PCR procedure was used: an initial denaturation at 95 °C for 5 min, followed by 95 °C for 25 s, 64 °C for 20 s, 72 °C for 20 s to ensure the specificity; and 31 cycles of 93 °C for 25 s, 60 °C for 35 s, 72 °C for 20 s to perform the data collection. The qualitative judgment is according to the fusion fluorescence signal. Assay reactions achieving Ct values of ≤30 cycles were considered positive for one of the variants detected by that reaction mixture. Housekeeping gene (beta-actin) was used to control the integrity of RNA.
Table S1. Clinical characteristics in patients with ROS1-positive.
| Case | Gender/age (years) | Histology subtype | Fusion subtype | Crizotinib treatment | PFS/months |
|---|---|---|---|---|---|
| 1 | M/62 | Papillary pred | EZR | – | – |
| 2 | F/47 | Lepidic pred | CD74 | – | – |
| 3 | M/45 | Papillary pred | GOPC | Yes | 6.0 |
| 4 | F/45 | NA | CD74 | Yes | 15.5 |
| 5 | F/43 | Papillary pred | EZR | – | – |
| 6 | M/46 | Solid pred | CD74 | – | – |
| 7 | M/59 | Papillary pred | SDC4 | – | – |
| 8 | F/60 | Micropapillary pred | SLC34A2 | Yes | 18.0+ |
| 9 | M/49 | Micropapillary pred | SDC4 | – | – |
| 10 | F/72 | Minimally invasive | SLC34A2 | – | – |
| 11 | M/65 | Papillary pred | CD74 | – | – |
| 12 | F/47 | NA | SDC4 | – | – |
| 13 | M/56 | Micropapillary pred | EZR | Yes | 14.2+ |
| 14 | F/66 | Acinar pred | CD74 | – | – |
| 15 | F/44 | Micropapillary pred | CD74 | Yes | 15.0+ |
| 16 | F/67 | Acinar pred | CD74 | – | – |
| 17 | M/69 | Papillary pred | TPM3 | – | – |
| 18 | F/70 | Micropapillary pred | EZR | Yes | 9.5+ |
| 19 | F/45 | Solid pred | CD74 | – | – |
| 20 | F/69 | Solid pred | GOPC | – | – |
| 21 | F/61 | Solid pred | GOPC | – | – |
| 22 | M/61 | NA | SDC4 | – | – |
| 23 | M/49 | Lepidic pred | CD74 | – | – |
| 24 | M/48 | Papillary pred | SDC4 | – | – |
| 25 | F/47 | Solid pred | GOPC | – | – |
| 26 | F/51 | Papillary pred | CD74 | – | – |
| 27 | F/51 | Solid pred | CD74 | – | – |
| 28 | M/47 | NA | CD74 | – | – |
| 29 | M/57 | Acinar pred | LRIG3 | – | – |
| 30 | M/65 | Solid pred | TPM3 | – | – |
| 31 | M/60 | Lepidic pred | GOPC | – | – |
| 32 | F/39 | Solid pred | LRIG3 | – | – |
Pred, predominant; NA, not applicable; PFS, progression-free survival; ROS1, c-ros oncogene 1.
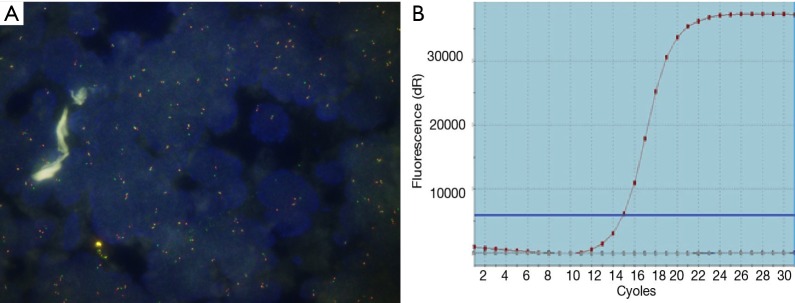
Figure S1.
ROS1 image with FISH and RT-PCR detection. (A) ROS1 FISH (+); (B) ROS1 RT-PCR (+). ROS1, c-ros oncogene 1; FISH, fluorescence in situ hybridization; RT-PCR, reverse transcription-polymerase chain reaction.
Ethical Statement: The study protocol was approved by Institutional Review Board (2014-11-93) of Zhejiang Cancer Hospital, Fuzhou General Hospital, Fujian Union Hospital and Zhangzhou Municipal Hospital and written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. 10.3322/caac.21338 [DOI] [PubMed] [Google Scholar]
- 2.Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42. 10.1016/S1470-2045(11)70184-X [DOI] [PubMed] [Google Scholar]
- 3.Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121-8. 10.1016/S1470-2045(09)70364-X [DOI] [PubMed] [Google Scholar]
- 4.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. 10.1056/NEJMoa0810699 [DOI] [PubMed] [Google Scholar]
- 5.Sequist LV, Soria JC, Goldman JW, et al. Rociletinib in EGFR-mutated non-small-cell lung cancer. N Engl J Med 2015;372:1700-9. 10.1056/NEJMoa1413654 [DOI] [PubMed] [Google Scholar]
- 6.Leighl NB, Rekhtman N, Biermann WA, et al. Molecular testing for selection of patients with lung cancer for epidermal growth factor receptor and anaplastic lymphoma kinase tyrosine kinase inhibitors: American Society of Clinical Oncology endorsement of the College of American Pathologists/International Association for the study of lung cancer/association for molecular pathology guideline. J Clin Oncol 2014;32:3673-9. 10.1200/JCO.2014.57.3055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindeman NI, Cagle PT, Beasley MB, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. J Thorac Oncol 2013;8:823-59. 10.1097/JTO.0b013e318290868f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang X, Zhang S, Yang X, et al. Fusion of EML4 and ALK is associated with development of lung adenocarcinomas lacking EGFR and KRAS mutations and is correlated with ALK expression. Mol Cancer 2010;9:188. 10.1186/1476-4598-9-188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kohno T, Nakaoku T, Tsuta K, et al. Beyond ALK-RET, ROS1 and other oncogene fusions in lung cancer. Transl Lung Cancer Res 2015;4:156-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaw AT, Ou SH, Bang YJ, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med 2014;371:1963-71. 10.1056/NEJMoa1406766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mazières J, Zalcman G, Crinò L, et al. Crizotinib therapy for advanced lung adenocarcinoma and a ROS1 rearrangement: results from the EUROS1 cohort. J Clin Oncol 2015;33:992-9. 10.1200/JCO.2014.58.3302 [DOI] [PubMed] [Google Scholar]
- 12.Wu C, Zhao C, Yang Y, et al. High Discrepancy of Driver Mutations in Patients with NSCLC and Synchronous Multiple Lung Ground-Glass Nodules. J Thorac Oncol 2015;10:778-83. 10.1097/JTO.0000000000000487 [DOI] [PubMed] [Google Scholar]
- 13.Pan Y, Zhang Y, Li Y, et al. ALK, ROS1 and RET fusions in 1139 lung adenocarcinomas: a comprehensive study of common and fusion pattern-specific clinicopathologic, histologic and cytologic features. Lung Cancer 2014;84:121-6. 10.1016/j.lungcan.2014.02.007 [DOI] [PubMed] [Google Scholar]
- 14.Li H, Pan Y, Li Y, et al. Frequency of well-identified oncogenic driver mutations in lung adenocarcinoma of smokers varies with histological subtypes and graduated smoking dose. Lung Cancer 2013;79:8-13. 10.1016/j.lungcan.2012.09.018 [DOI] [PubMed] [Google Scholar]
- 15.Sun Y, Ren Y, Fang Z, et al. Lung adenocarcinoma from East Asian never-smokers is a disease largely defined by targetable oncogenic mutant kinases. J Clin Oncol 2010;28:4616-20. 10.1200/JCO.2010.29.6038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, Sun Y, Pan Y, et al. Frequency of driver mutations in lung adenocarcinoma from female never-smokers varies with histologic subtypes and age at diagnosis. Clin Cancer Res 2012;18:1947-53. 10.1158/1078-0432.CCR-11-2511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Warth A, Muley T, Dienemann H, et al. ROS1 expression and translocations in non-small-cell lung cancer: clinicopathological analysis of 1478 cases. Histopathology 2014;65:187-94. 10.1111/his.12379 [DOI] [PubMed] [Google Scholar]
- 18.Song Z, Zhu H, Guo Z, et al. Correlation of EGFR mutation and predominant histologic subtype according to the new lung adenocarcinoma classification in Chinese patients. Med Oncol 2013;30:645. 10.1007/s12032-013-0645-1 [DOI] [PubMed] [Google Scholar]
- 19.Yang JJ, Zhang XC, Su J, et al. Lung cancers with concomitant EGFR mutations and ALK rearrangements: diverse responses to EGFR-TKI and crizotinib in relation to diverse receptors phosphorylation. Clin Cancer Res 2014;20:1383-92. 10.1158/1078-0432.CCR-13-0699 [DOI] [PubMed] [Google Scholar]
- 20.Eng J, Woo KM, Sima CS, et al. Impact of Concurrent PIK3CA Mutations on Response to EGFR Tyrosine Kinase Inhibition in EGFR-Mutant Lung Cancers and on Prognosis in Oncogene-Driven Lung Adenocarcinomas. J Thorac Oncol 2015;10:1713-9. 10.1097/JTO.0000000000000671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y, Sun Y, Pan Y, et al. Frequency of driver mutations in lung adenocarcinoma from female never-smokers varies with histologic subtypes and age at diagnosis. Clin Cancer Res 2012;18:1947-53. 10.1158/1078-0432.CCR-11-2511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ying J, Guo L, Qiu T, et al. Diagnostic value of a novel fully automated immunochemistry assay for detection of ALK rearrangement in primary lung adenocarcinoma. Ann Oncol 2013;24:2589-93. 10.1093/annonc/mdt295 [DOI] [PubMed] [Google Scholar]
- 23.Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013;368:2385-94. 10.1056/NEJMoa1214886 [DOI] [PubMed] [Google Scholar]
- 24.Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371:2167-77. 10.1056/NEJMoa1408440 [DOI] [PubMed] [Google Scholar]