Abstract
Background
This study aimed to assess the efficiency of anterior fissureless uniport (AFU) thoracoscopic lobectomy for early stage right upper non-small cell lung cancer (NSCLC).
Methods
Between June 2014 and Dec 2016, 162 consecutive NSCLC patients who underwent thoracoscopic right upper lobectomy (RUL) by AFU approach (AFU group, n=65) or posterior intra-fissure triple-port dissection (PIFT group, n=97) were enrolled. A propensity-matched analysis was used to compare perioperative outcomes, safety and efficiency between the two groups.
Results
Propensity matching produced 40 pairs in this retrospective study. During the operation, lobectomy took less time in the AFU group compared with the PIFT group, while no statistical differences in mediastinal lymphadenectomy time, intraoperative blood loss, and total of lymph nodes harvested were found between the two groups. Postoperatively, length of hospital stay (LOS) and time of postoperative air leak were significantly reduced in AFU group than in PIFT group. However, the overall complication rate and volume of pleural effusion drainage within 48 h were similar. Compared with the PIFT group, visual analogue scale (VAS) of 3 postoperative days in AFU group was slighter.
Conclusions
In RUL, AFU thoracoscopic approach is safe, efficient and easily maneuverable, which would reduce the duration of lobectomy, LOS and time of postoperative air leak. Postoperative pain is also mild.
Keywords: Thoracoscopic lobectomy, fissureless, triple-port, uniport
Introduction
In conventional triple-port video-assisted thoracic surgery (VATS) right upper lobectomy (RUL), posterior approach is often selected, dissecting through the fissure in order to produce a tunnel above the pulmonary artery, which may increase the odds of air leak (1). Conversely, anterior fissureless lobectomy, which was first described in open thoracotomy in 1999 (2), decreases the chance of postoperative air leak. However, anterior fissureless lobectomy is rarely applied in triple-port VATS RUL, the mainly reason is that exposure of the superior border in hilum is limited, with the optical source generating a torsion angle not favorable with standard two-dimensional monitors (3).
Uniport VATS lobectomy has become popular in china since 2013 for reducing postoperative pain, length of hospital stay (LOS) and time to return to daily activities (4,5). Meanwhile, exposure of the hilum structure is clear (6) especially in upper lobectomy thanks to the camera in the upper location of the incision; the whole procedure is similar to open thoracotomy. However, performing uniport VATS lobectomy is also challenging, especially for patient with fused fissure because of the limited space.
In order to reduce postoperative pain, the chance of air leak and improve surgical maneuverability, we firstly combined anterior the fissureless technique and uniport VATS for RUL at the beginning of 2015, and obtained a favorable result. In this study, we performed a retrospective study comparing anterior fissureless uniport (AFU) VATS technique and posterior intra-fissure triple-port (PIFT) VATS dissection for RUL, evaluating the safety and efficacy of these procedures.
Methods
This retrospective propensity matched cohort study was approved by the institutional review board and the ethics committee of Affiliated Hospital of Nantong University, and the data were collected from the hospital’s database by trained surgical coordinators. During the period of study, two surgical procedures were conducted by two different senior consultant surgeons, one with 3 years of experience in uniport VATS lobectomy and the other with >4 years of experience in triple-port VATS lobectomy.
Study design
Between June 2014 and Dec 2016, 65 consecutive patients with early stage NSCLC who underwent uniport thoracoscopic RUL with anterior fissureless technique were included. During the same period, another 97 consecutive patients with early stage NSCLC who underwent triple-port thoracoscopic RUL with posterior intra-fissure dissection were also analyzed in order to avoid intention-to-treat selection bias. Preoperative examination, including lung function, electrocardiography, enhanced chest CT scan, head magnetic resonance imaging (MRI), abdominal ultrasound, and bone electroconvulsive therapy (ECT) were performed. Inclusion criteria were: (I) clinically diagnosed stage T1a–cN0M0 NSCLC (AJCC/UICC 8th edition) (7); (II) no previous history of thoracotomy, chemotherapy or radiotherapy; (III) no severe thoracic adhesion; (IV) American Society of Anesthesiologists (ASA) score of I–II.
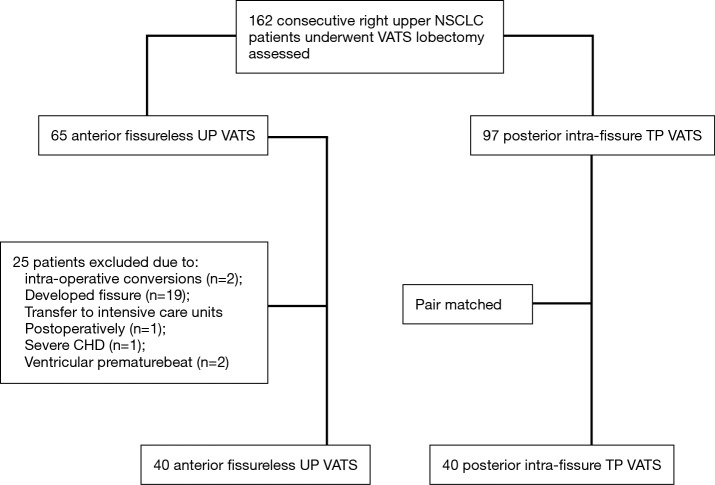
In this study, we modelled the likelihood of AFU group with logistic regression, and age, gender, body mass index (BMI), tumor size, histology, pulmonary function, fissural grade (8) and co-morbidities were used. Subsequently, PIFT group were matched with the nearest propensity score (within a range of 0.03 on a scale from 0 to 1) (Table 1). The flowchart summarizing the inclusion and matching procedure was found in Figure 1.
Table 1. Original data of preoperative variables.
| Parameter | AFU (n=65) | PIFT (n=97) | P value |
|---|---|---|---|
| Age | 61.5±10.4 | 63.2±12.1 | 0.539** |
| Gender | 0.417* | ||
| Male | 17 | 28 | |
| Female | 48 | 69 | |
| BMI | 21.5±2.7 | 23.1±2.2 | 0.782** |
| Histological type | 0.615* | ||
| SC | 5 | 12 | |
| AD | 60 | 85 | |
| Clinical TNM stage | 0.421* | ||
| T1aN0M0 | 16 | 29 | |
| T1bN0M0 | 34 | 41 | |
| T1cN0M0 | 15 | 27 | |
| Tumor size | 0.421* | ||
| <1 cm | 16 | 29 | |
| 1–2 cm | 34 | 41 | |
| 2–3 cm | 15 | 27 | |
| Pulmonary function | 0.643** | ||
| FEV1% | 77.8±12.1 | 75.6±10.8 | |
| DLCO% | 65.6±12.7 | 64.7±11.8 | |
| Fissure grade | |||
| I | 9 | 26 | 0.017* |
| II | 12 | 25 | 0.023* |
| III | 35 | 40 | 0.656* |
| IV | 9 | 6 | 0.778* |
| Comorbidity | |||
| Primary hypertension | 25 | 31 | 0.515* |
| grade I | 12 | 11 | |
| grade II | 13 | 20 | |
| II type diabetic mellitus | 12 | 18 | 0.871* |
Data are mean ± standard deviation or N (percentage). *, McNemar’s test; **, paired Student’s t-test. FEV1, forced expiratory volume in the first second; DLCO, diffusing capacity of carbon monoxide; SC, squamous cell cancer; AD, adenocarcinoma; AFU, anterior fissureless uniport; PIFT, posterior intra-fissure triple-port; BMI, body mass index.
Figure 1.
Study flowchart. UP, uniport; TP, triple port; CHD, coronary heart disease; VATS, video-assisted thoracoscopic surgery; NSCLC, non-small-cell lung cancer.
Surgical approach
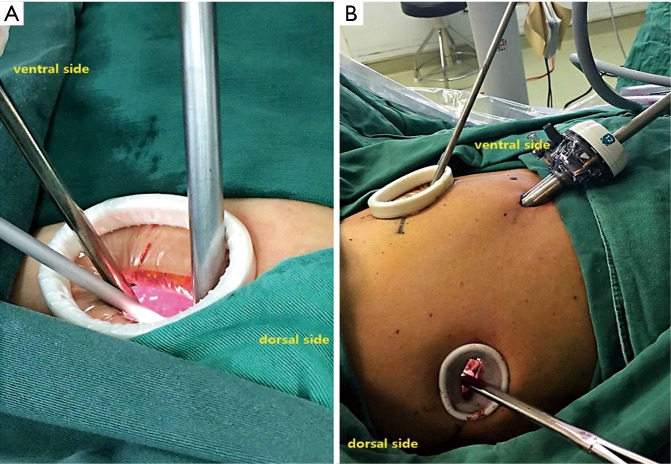
For general anesthesia, a double-lumen tracheal intubation plus intravenous anesthesia was applied in both groups. In AFU group, the surgical incision was performed at the fifth intercostal space along the anterior axillary line, which was approximately 4-cm. In PIFT group, a 1.0-cm camera incision was made at the 8th intercostal space along the mid-axillary line. The main operative incision (3-cm) was made at the fifth intercostal space along the anterior axillary line; a secondary incision was made at the 7th intercostal space along the triangle of auscultation
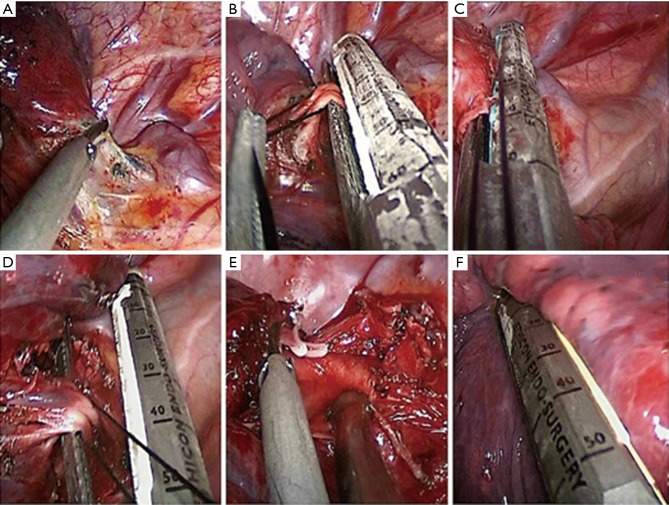
The surgical procedures in both groups were different. In the AFU group, the first step was involved the opening of the anterior mediastinal pleura with electrocautery to liberate the upper lobe pulmonary artery branch, anterior to the lung tip. This was followed by an approach that sequentially involved the upper lobe bronchus, posterior ascending branch of the pulmonary artery, upper pulmonary vein, and fissure last (Figure 2). This procedure is similar to single-direction (9). In PIFT group, the first step was to divide the oblique fissure with a harmonic scalpel (Ethicon Endo-Surgery, Inc, Cincinnati, Ohio, USA) or endoscopic staple if the fissure was fused, and then ascending pulmonary artery and upper lobe bronchus were exposed. After dividing them, the apical and anterior artery branched from the right pulmonary arterial trunk appeared separately and upper pulmonary vein can be dissected easily.
Figure 2.
The uniportal access incision (4-cm) was at the 5th intercostal space along the anterior axillary line (A). In triple-port access, camera incision was made at the eighth intercostal space along the mid-axillary line; the main operative incision (3-cm) was made at the fifth intercostal space along the anterior axillary line and the secondary (2-cm) at the 7th intercostal space along the triangle of auscultation (B).
After the resection of the right upper lobe, the 2nd, 4th, 7th, 8th and 9th groups of lymphatic nodes were removed by Harmonic scalpel (Ethicon Endo-Surgery, Inc., Cincinnati, Ohio, USA). Intraoperative air leak were always tested by submerging the residual lung in sterile saline and inflating it to a pressure of 25–30 cmH2O. Sutures with 4-0 propylene or Fibrin Sealant (5 mL, Guangzhou Bioseal biotech Co, Ltd., China) are often used to minimize the air leak. At the end of both procedures, a 28-French intrathoracic tube was placed in the camera incision with a single stitch.
Criteria of postoperative parameters
We investigated the postoperative complications including pneumonia, atelectasis, chylothorax, arrhythmia, hypoxia, prolonged air leak and Cerebrovascular accident (CVA). Pneumonia and atelectasis were defined by purulent sputum and chest radiography, or computed tomography. If the pleural effusion appeared lacte and the chyle test was positive, chylothorax was confirmed. Arrhythmia was defined by the need for treatment, and hypoxia by a decline in oxygen saturation, as measured by pulse oximetry, to ≤90%. Air leak was assessed twice daily (during the morning and evening rounds) and the patients were instructed to perform standardised repeated forced expiratory manoeuvres (coughing and blowing). Prolonged air leak was diagnosed when a patient needed chest tube drainage for >5 days. CVA is defined when blood flow to a part of your brain is stopped either by a blockage or the rupture of a blood vessel. Angiogram, CT and MRI are often used for diagnosis in our hospital.
Chest tube was removed if no air leak was detectable, the pleural effusion was less than 100 mL in the last 24 h and chest X-ray showed satisfactory lung expansion. Subsequently, the patients were discharged when routine blood analysis showed no obvious abnormalities.
Pain management and visual analogue scale (VAS) scores
Pain management was the same in both groups, including a vein analgesic protocol and intercostal nerve block using Naropin (7.5 mg/mL, AstraZeneca) postoperatively. VAS from 0 to 10 was used to assess the maximum pain scores; pain scores were recorded at postoperative 1 h and days 1, 3, 7 and 30, respectively (Figure 3A-F). After surgery, the patients were followed up for at least 3 months at the outpatient department.
Figure 3.
Anterior fissureless uniport thoracoscopic approach for RUL. (A) Opening of the anterior mediastinal pleura; (B) dissection of the pulmonary artery branch anterior to the lung tip; (C) dissection of the bronchus; (D) dissection of the pulmonary vein; (E) dissection of the pulmonary artery ascending branch; (F) completing the fissure.
Statistical analysis
Propensity scores were calculated by multivariate logistic regression. Descriptive statistical analysis was performed. Continuous variables were expressed as mean ±standard deviation according to data distribution. Comparisons between the two groups were performed by paired Student’s test for continuous variables or McNemar’s test with continuity correction for categorical variables, to account for the matching design. Statistical analyses were performed with the SAS software package (SAS Institute, Inc., Cary, NC, USA). Statistical significance was set at P<0.05.
Results
Clinical features
From June 2014 and Dec 2016, after propensity scored analysis, 40 AFU VATS RUL were included. Meanwhile, 40 PIFT VATS RUL were matched in this retrospective study. Patients in the two groups were similar in age, gender, BMI, tumor size, clinical TNM stage, pulmonary function, fissural grade and comorbidities (Table 2). Postoperative pathological results revealed that the current patient cohort consisted of 71 adenocarcinoma and 9 squamous carcinoma, respectively. Furthermore, postoperative pathological TNM staging revealed that 29 cases were stage pT1aN0M0, 31 were stage pT1bN0M0, 17 were stage pT1cN0M0, 1were stage pT1bN1M0, and 2 were stage pT1cN1M0. Therefore, the current patient cohort was in principle suitable for assessing the performance of VATS RUL, being representative for clinical practice.
Table 2. Propensity score matching of preoperative variables.
| Parameter | AFU (n=40) | PIFT (n=40) | P value |
|---|---|---|---|
| Age | 59.3±11.2 | 60.2±11.7 | 0.639** |
| Gender | 0.597* | ||
| Male | 7 | 8 | |
| Female | 23 | 22 | |
| BMI | 19.5±2.3 | 20.7±2.0 | 0.772** |
| Histological type | 0.655* | ||
| SC | 3 | 6 | |
| AD | 37 | 34 | |
| Clinical TNM Stage | 0.371* | ||
| T1aN0M0 | 16 | 13 | |
| T1bN0M0 | 12 | 20 | |
| T1cN0M0 | 12 | 17 | |
| Tumor size | 0.371* | ||
| <1 cm | 16 | 13 | |
| 1–2 cm | 12 | 20 | |
| 2–3 cm | 12 | 17 | |
| Pulmonary Function | 0.842** | ||
| FEV1% | 75.4±11.6 | 77.6±12.1 | |
| DLCO% | 65.6±12.7 | 64.7±11.8 | |
| Fissure grade | |||
| III | 34 | 35 | 0.883* |
| IV | 6 | 5 | 0.778* |
| Comorbidity | |||
| Primary hypertension | 15 | 12 | 0.685* |
| Grade I | 7 | 5 | 0.878* |
| Grade II | 8 | 7 | |
| II type diabetic mellitus | 8 | 7 |
Data are mean ± standard deviation or N (percentage). *, McNemar’s test; **, paired Student’s t-test. FEV1, forced expiratory volume in the first second; DLCO, diffusing capacity of carbon monoxide; SC, squamous cell cancer; AD, adenocarcinoma; AFU, anterior fissureless uniport: PIFT, posterior intra-fissure triple-port; BMI, body mass index.
Surgery characteristics
There were no perioperative deaths or conversions to open chest surgery in either groups. Duration of lobectomy (85.2±35.1 vs. 116.2±49.2 min; P=0.001), LOS (4.2±1.7 vs. 7.5±3.1 d; P=0.048) and postoperative air leak (2.0±1.3 vs. 4.4±2.6 d; P=0.014) were significantly reduced in the AFU group compared with the PIFT group. Duration of systemic mediastinal lymphadenectomy, intraoperative blood loss, total lymph nodes harvested and volume of pleural effusion drainage within 48 h were similar in both groups (Table 3).There was also no significant difference in complication occurrence (Table 4).
Table 3. Perioperative outcomes in the AFU and PIFT groups.
| Parameter | AFU (n=40) | PIFT (n=40) | P value |
|---|---|---|---|
| Lobectomy time (min) | 85.2±35.1 | 116.2±49.2 | 0.001 |
| Mediastinal lymphadenectomy time (min) | 23.2±11.5 | 20.4±13.7 | 0.336 |
| Total lymph nodes harvest (n) | 13±9 | 16±8 | 0.882 |
| Intraoperative blood loss (mL) | 77.8±33.6 | 82.5±42.7 | 0.656 |
| Time of postoperative drainage (d) | 2.0±1.3 | 4.4±2.6 | 0.014 |
| Volume of chest tube drainage within 48 h (mL) | 323.7±155.2 | 486.5±183.3 | 0.227 |
| Postoperative hospital stay (d) | 4.2±1.7 | 7.5±3.1 | 0.048 |
Table 4. Postoperative complications.
| Parameter | AFU (n=40) | PIFT (n=40) | P value |
|---|---|---|---|
| Total, n [%] | 4 [10] | 6 [15] | 0.337 |
| Prolonged air leakage (>5 d) | 0 | 3 | |
| Arrhythmia | 2 | 1 | |
| Chylothorax | 0 | 1 | |
| hypoxia | 0 | 0 | |
| Pneumonia | 1 | 1 | |
| Atelectasis | 0 | 0 | |
| Cerebrovascular accident | 1 | 0 |
Pain scores comparison between the two groups
There were no significant differences in the pain scores at postoperative 1 h and POD 1, 7, and 30. However, at POD 3, a significant difference was found (P=0.011) (Table 5). In both groups, only a small number of patients tended to receive normal doses of analgesics (e.g., nonsteroidal anti-inflammatory drugs, NSAIDs) after surgery. No side effects appeared due to these analgesics.
Table 5. Pain scores comparison between the two groups.
| Groups | Postoperative 1 h | POD 1 d | POD 3 d | POD 7 d | POD 30 d |
|---|---|---|---|---|---|
| AFU (n=40) | 2.3±0.8 | 2.4±0.5 | 2.4±0.3 | 2.8±0.7 | 1.3±0.2 |
| PIFT (n=40) | 2.5±1.2 | 2.7±0.6 | 3.1±0.4 | 3.5±1.2 | 1.2±0.7 |
| P value | 0.898 | 0.724 | 0.011 | 0.365 | 0.866 |
Discussion
Uniport VATS lobectomy (10,11) is widely accepted, especially in East Asia since 2011. There are multiple advantages, such as minimal trauma, beauty of the incision, and mild postoperative pain. In addition, compared with conventional TP VATS, a specific advantage of uniport VATS is exposure especially in upper lobectomy (12). For example, the high camera provides the shortest observation distance to the hilar structure, without overt reversal of the lobe. The sagittal view of the surgical field may be more conductive to anterior operation (13). However, though uniportal VATS has a good visibility, a narrow space often restricts the operative procedure. Many beginners feel uncomfortable in the initial learning curve, and the lobectomy time is not increased (14).
The fissureless technique was applied firstly in open thoracotomy in 1999, and reports related to VATS lobectomy appeared recently (15). In terms of technology, the process is simple and easy to learn, especially for RUL (16). The advantages of this approach are reduced operating time and decreased odds of postoperative air leak. Therefore, in early 2015, we combined anterior the fissureless technique and uniportal VATS in RUL; after a preliminary exploration of 20 cases, our learning curve became stable gradually. In this study, we found AFU VATS RUL was associated with shortened lobectomy time, LOS and postoperative drainage.
In 2013, Gonzalez (17) found that uniport VATS lobectomy has multiple advantages, including shortened operative time, corroborating our findings. Skillful manipulation and good visualization may be crucial factors. Nevertheless, Shen et al. (18) performed a propensity-matched study, and showed that the total operation duration, volume of intraoperative blood loss, total of lymph nodes and length of postoperative hospital stay are similar between the single incision and multi-incision VATS groups. The discrepant conclusions may be associated with the proficiency of surgeons. Furthermore, statistical errors may be produced if taking into account all lobes for analysis. Indeed, resection of different lobes has varying complexity because of anatomical structure differences. It would be more accurate if each lobe was assessed separately. In this study, only RUL were enrolled; more cases with shortened lobectomy time were obtained, which may contribute to the novel surgical process, although we are beginners.
In addition, there were no significant differences between the two groups in terms of mediastinal lymphadenectomy time, intraoperative blood loss, total of lymph nodes harvested, overall complications, and volume of pleural effusion drainage within 48 h, in agreement with other studies (19,20). However, duration of postoperative drainage and hospital stay in the UP group were shorter than the of TP group. This may contribute to the anterior fissureless technique. Ng et al. (1) and Refai et al. (21) have been verifying its safety and efficacy for many years, although only in open thoracotomy or muscle-sparing lateral thoracotomy. Davor et al. (15) recently reported that the fissureless technique applied in VATS lobectomy appears to be superior to conventional VATS lobectomy in terms of preventing postoperative drainage and reducing LOS, corroborating our findings. Nevertheless, the numbers of patients in subgroups were too small for detailed analysis. Therefore, a larger, randomized study is required to confirm our findings.
With respect to postoperative pain, most contrastive studies (22,23) showed either no difference or significant difference in the early postoperative stage, while assessing simple thoracic surgery, e.g., for spontaneous pneumothorax correction. In the current study, the overt alleviation of postoperative pain at POD 3 differed from the above reports. This difference may mainly result from the shortened duration of chest tubes, and likely independent of the number of incision. Before uniport can be recommended as a less painful option than multiport thoracoscopic surgery, higher quality prospective randomized studies, validated pain assessment tools, and longer follow-up are needed.
There were two limitations in this study. Firstly, the number of patients was relatively small, with the lobe type limited. However, after matching for confounding factors, the characteristic results of the comparison were reliable. Secondly, the results were from a single medical center, which limits the generalization of the findings. Multicenter randomized controlled trials are therefore required to confirm the role of the AFU thoracoscopic approach for RUL or the resection of another lobe.
Conclusions
This initial study suggests that the AFU thoracoscopic approach for RUL appears to be safe, efficient and easy to manipulate; therefore, it merits further studies for suitability for the other lobe, or widespread clinical implementation.
Acknowledgements
Funding: The present research was supported by a grant provided by the Science Foundation of Nantong City (MS22015123).
Ethical Statement: Written informed consent was obtained from patients with approval from the Institutional Review Board in Affiliated Hospital of Nantong University, China (2016-K143).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Ng T, Ryder BA, Machan JT, et al. Decreasing the incidence of prolonged air leak after right upper lobectomy with the anterior fissureless technique. J Thorac Cardiovasc Surg 2010;139:1007-11. 10.1016/j.jtcvs.2009.07.023 [DOI] [PubMed] [Google Scholar]
- 2.Temes RT, Willms CD, Endara SA, et al. Fissureless lobectomy. Ann Thorac Surg 1998;65:282-4. 10.1016/S0003-4975(97)01268-X [DOI] [PubMed] [Google Scholar]
- 3.Bertolaccini L, Viti A, Terzi A, et al. Geometric and ergonomic characteristics of the uniportal video-assisted thoracoscopic surgery (VATS) approach. Ann Cardiothorac Surg 2016;5:118-22. 10.21037/acs.2015.12.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gonzalez D, de la Torre M, Paradela M, et al. Video-assisted thoracic surgery lobectomy: 3-year initial experience with 200 cases. Eur J Cardiothorac Surg 2011;40:e21-8. 10.1016/j.ejcts.2011.02.051 [DOI] [PubMed] [Google Scholar]
- 5.Harris C, Croce B, Harris R. Uniportal video-assisted thoracoscopic surgery (VATS). Ann Cardiothorac Surg 2016;5:154. 10.21037/acs.2016.03.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bertolaccini L, Rocco G, Viti A, et al. Geometrical characteristics of uniportal VATS. J Thorac Dis 2013;5 Suppl 3:S214-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Detterbeck FC, Boffa DJ, Kim AW, et al. The Eighth Edition Lung Cancer Stage Classification. Chest 2017;151:193-203. [DOI] [PubMed] [Google Scholar]
- 8.Craig SR, Walker WS. A proposed anatomical classification of the pulmonary fissures. J R Coll Surg Edinb 1997;42:233-4. [PubMed] [Google Scholar]
- 9.Liu L, Che G, Pu Q, et al. A new concept of endoscopic lung cancer resection: Single-direction thoracoscopic lobectomy. Surg Oncol 2010;19:e71-7. 10.1016/j.suronc.2009.04.005 [DOI] [PubMed] [Google Scholar]
- 10.Hsu PK, Lin WC, Chang YC, et al. Multiinstitutional analysis of single-port video-assisted thoracoscopic anatomical resection for primary lung cancer. Ann Thorac Surg 2015;99:1739-44. 10.1016/j.athoracsur.2015.01.041 [DOI] [PubMed] [Google Scholar]
- 11.Ng CS, Kim HK, Wong RH, et al. Single-port video assisted thoracoscopic major lung resections: experience with 150 consecutive cases. Thorac Cardiovasc Surg 2016;64:348-53. 10.1055/s-0034-1396789 [DOI] [PubMed] [Google Scholar]
- 12.Bertolaccini L, Viti A, Terzi A, et al. Geometric and ergonomic characteristics of the uniportalvideo-assisted thoracoscopic surgery (VATS) approach. Ann Cardiothorac Surg 2016;5:118-22. 10.21037/acs.2015.12.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansen HJ, Petersen RH, Christensen M. Video-assisted thoracoscopic surgery (VATS) lobectomy using a standardized anterior approach. Surg Endosc 2011;25:1263-9. 10.1007/s00464-010-1355-9 [DOI] [PubMed] [Google Scholar]
- 14.Liu CC, Shih CS, Pennarun N, et al. Transition from a multiport technique to a single-port technique for lung cancer surgery: is lymph node dissection inferior using the single-port technique?†. Eur J Cardiothorac Surg 2016;49 Suppl 1:i64-72. [DOI] [PubMed] [Google Scholar]
- 15.Davor S, Korkut B, Antje M, et al. Fissureless fissure-last video-assisted thoracoscopic lobectomy for all lung lobes: a better alternative to decrease the incidence of prolonged air leak? Eur J Cardiothorac Surg 2016;50:118-23. 10.1093/ejcts/ezv455 [DOI] [PubMed] [Google Scholar]
- 16.Majed R, Alessandro B, Michele S, et al. Efficacy of anterior fissureless technique for right upper lobectomies:a case-matched analysis. Eur J Cardiothorac Surg 2011;39:1043-6. 10.1016/j.ejcts.2010.09.039 [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez-Rivas D, Paradela M, Fernandez R, et al. Uniportal video-assisted thoracoscopic lobectomy: two years of experience. Ann Thorac Surg 2013;95:426-32. 10.1016/j.athoracsur.2012.10.070 [DOI] [PubMed] [Google Scholar]
- 18.Shen Y, Wang H, Feng M, et al. Single- versus multiple-port thoracoscopic lobectomy for lung cancer: a propensity-matched study. Eur J Cardiothorac Surg 2016;49 Suppl 1:i48-53. [DOI] [PubMed] [Google Scholar]
- 19.French DG, Thompson C, Gilbert S. Transition from multiple port to single port video-assisted thoracoscopic anatomic pulmonary resection: early experience and comparison of perioperative outcomes. Ann Cardiothorac Surg 2016;5:92-9. 10.21037/acs.2016.03.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shih CS, Liu CC, Liu ZY, et al. Comparing the postoperative outcomes of video-assisted thoracoscopic surgery (VATS) segmentectomy using a multi-port technique versus a single-port technique for primary lung cancer. J Thorac Dis 2016;8:S287-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Refai M, Brunelli A, Salati M, et al. Efficacy of anterior fissureless technique for right upper lobectomies: a case-matched analysis. Eur J Cardiothorac Surg 2011;39:1043-6. 10.1016/j.ejcts.2010.09.039 [DOI] [PubMed] [Google Scholar]
- 22.Young R, McElnay P, Leslie R, et al. Is uniport thoracoscopic surgery less painful than multiple port approaches? Interact Cardiovasc Thorac Surg 2015;20:409-14. 10.1093/icvts/ivu391 [DOI] [PubMed] [Google Scholar]
- 23.Tamura M, Shimizu Y, Hashizume Y. Pain following thoracoscopic surgery: retrospective analysis between single-incision and three-port video-assisted thoracoscopic surgery. J Cardiothorac Surg 2013;8:153. 10.1186/1749-8090-8-153 [DOI] [PMC free article] [PubMed] [Google Scholar]