Abstract
Background
Diffuse alveolar haemorrhage (DAH) may accompany or complicate various diseases with different pathophysiology. The diagnosis of DAH, especially when presenting as diffuse non-resolving and predominantly upper lobe lung consolidations, is particularly difficult in patients with cardiac disease.
Methods
We retrospectively reviewed seven cases of DAH with predominant upper lobe involvement in the setting of acute or acute-on-chronic congestive heart failure (CHF).
Results
In general, time from onset till diagnostic confirmation of DAH was long. Chest CT scan mostly confirmed diffuse lung infiltrates without preferential side location but with typical cortical sparing in all patients. Echocardiography showed presence of some degree of mitral valve insufficiency in all subjects.
Conclusions
Diagnosis of DAH presenting as non-resolving upper lobe densities, accompanying CHF remains cumbersome but may be suggested indirectly by the presence of cortical sparing and mitral valve insufficiency. The latter may play a crucial role in the development of DAH.
Keywords: Congestive heart failure (CHF), diffuse alveolar haemorrhage (DAH), upper lung consolidations
Introduction
Diffuse alveolar haemorrhage (DAH) is a devastating condition that may present as microscopic or macroscopic haemoptysis often with a fall in haemoglobin, hypoxic respiratory failure, and diffuse pulmonary infiltrates on chest X-ray. However, the full “triad” occurs only in one third of cases (1). Distinct histological patterns of DAH comprise pulmonary capillaritis, diffuse alveolar damage, or bland pulmonary haemorrhage. DAH associated with pulmonary capillaritis accompanies a large variety of auto-immune diseases and is characterized by diffuse neutrophil infiltration in the alveolar septa with foci of necrosis, loss of capillary structural integrity, and extravascular red blood cell spilling (2). Cases of diffuse alveolar damage display the histopathology of the acute respiratory distress syndrome, including presence of capillary congestion, microthrombi, and intra-alveolar hyaline membrane formation (3). These two DAH types are potentially life-threatening and require prompt and adequate treatment (1). However, patients with DAH presenting as bland lung bleeding do not present predominant inflammation or alveolar damage (2). This particular DAH type may occur occasionally in the absence of underlying heart disease but mostly affects patients with congestive heart failure (CHF) due to systolic or diastolic left ventricular dysfunction (4). The pathophysiology of DAH associated with heart failure remains speculative. Increased pulmonary capillary pressure may induce hydrostatic venous pulmonary congestion leading to rupture of the microvasculature with intra-alveolar blood spilling. In case of chronically elevated pulmonary venous pressure, bleeding may occur at the anastomoses between submucosal bronchial and pulmonary veins as the latter become more resistant to a higher pressure (5,6).
An haemorrhagic broncho-alveolar lavage (BAL) fluid points to the diagnosis, especially if the amount of blood increases in sequentially retrieved aliquots. It also allows to discriminate between DAH and infection or drug-toxicity (1).
We present a case series of patients with non-resolving upper lung lobe densities related to DAH accompanying CHF and particularly focus on diagnostic pitfalls and potential diagnostic aids.
Methods
From June 2011 till December 2015, all cases of CHF-associated DAH (CHF-DAH) with predominant upper lung lobe involvement admitted to the intensive care unit (ICU) of a large university-affiliated hospital were reviewed. The study was presented to the Institutional Review Board of the Middelheim Hospital which waived the need for ethical approval due to its retrospective, observational, and non-interventional design. CHF was diagnosed according to the Framingham criteria (7) and confirmed by echocardiography, plasma NT pro-BNP measurement, or a combination of both. All non-resolving upper lung lobe(s) consolidation were reviewed by the same pneumologist. Focus was laid on excluding non-cardiac DAH cases, particularly those associated with auto-immune disorders or complicating the acute respiratory distress syndrome. Disease history and evolution, bronchoscopy, radiography, analysis of endobronchial aspirate or BAL fluid, and echocardiographic findings were retrieved from the patients’ files. Whenever possible, side and location of lung consolidations were documented on a chest CT scan.
Results
All non-resolving upper lung lobe(s) consolidation were reviewed by the same pneumologist. Focus was laid on excluding non-cardiac DAH cases, particularly those associated with auto-immune disorders or complicating the acute respiratory distress syndrome. Patients’ demographics and characteristics are depicted in Table 1. Most subjects were male (n=6) and suffered arterial hypertension (n=6) or chronic obstructive pulmonary disease (COPD) (n=4). Five patients had pre-existing chronic heart disease [ischaemic cardiopathy (n=4), non-compaction cardiomyopathy, and aortic valve stenosis]. Two patients presented with acute mitral valve endocarditis. All but one patient received some form of anticoagulant therapy.
Table 1. Patients' demographics and characteristics.
| Characteristics | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 |
|---|---|---|---|---|---|---|---|
| Age | 78 | 82 | 60 | 70 | 47 | 64 | 72 |
| Gender | Male | Female | Male | Male | Male | Male | Male |
| Comorbidities | COPD, AHT | SAD, AHT, arterial disease | COPD, AHT, CKD, arterial disease | COPD, AHT | AHT | None | COPD, AHT, CKD, arterial disease |
| Pre-existing heart condition | Ischaemic cardiopathy, normal EF | Ischaemic cardiopathy, normal EF | Ischaemic cardiopathy, reduced EF | N | N | Non-compaction cardiomyopathy, reduced EF | Ischemic cardiopathy, normal EF |
| Anticoagulantia | Y | Y | Y | U | N | Y | Y |
N, no; Y, yes, U, unknown; SAD, small airway disease; AHT, arterial hypertension; COPD, chronic obstructive pulmonary disease; CKD, chronic kidney disease; EF, ejection fraction.
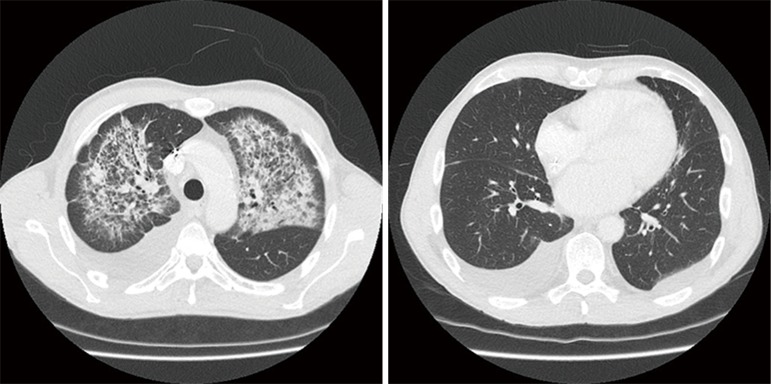
Clinical, bronchoscopic, CT scan, and echocardiographic findings are summarized in Table 2. Chest CT showed persistent cloudy or reticular “ground glass” infiltrates, preferentially located at the left side and sparing the lung cortex (as illustrated in Figure 1). Pleural effusion was present in 6 patients. Four patients had prominent diastolic heart failure. All patients had some degree of mitral valve insufficiency (MVI) with regurgitation fractions ranging from 20% to 60% (i.e., moderate to severe insufficiency) and atrial dilatation. DAH was confirmed bronchoscopically in five and clinically in two subjects who presented with massive haemoptysis on admission.
Table 2. Clinical, bronchoscopic, CT scan and echocardiographic findings.
| Findings | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 |
|---|---|---|---|---|---|---|---|
| Haemoptysis | N | N | Y | N | Y | Y | N |
| Bronchoscopy | |||||||
| Macroscopic blood | Y | Y | Y | Y | NA | NA | Y |
| Hemosiderin-laden macrophages | Y | U | Y | Y | NA | NA | U |
| Infiltrates on CT scan | |||||||
| Upper [1]/predomin. upper [2] | 2 | 1 | 1 | 1 | 2 | 1 | 2 |
| Central [1]/peripheral [2]/diffuse [3] | 3 | 1 | 3 | 2 | 1 | 1 | 3 |
| Right [1]/predomin. right [2]/diffuse [3]/predomin. left [4]/left [5] | 4 | 3 | 2 | 4 | 3 | 3 | 4 |
| Aspect | Ground glass/reticular | Ground glass/cloudy | Ground glass, central infiltrate | Dense infiltrate | Ground glass/cloudy | Ground glass/reticular | Ground glass/reticular |
| Cortical sparing | N | Y | N | Y | Y | Y | N |
| Pleural effusion | Y | Y | Y | Y | N | Y | Y |
| Echocardiography | |||||||
| Mitral insufficiency (gradient) | Y (2/4) | Y (1/4) | Y (1/4) | Y (3/4) | Y (3/4) | Y (2/4) | Y (2/4) |
| Elevated left ventricular end-diastolic pressure | U | U | Y | Y | U | Y | Y |
| Atrial dilatation | Y | Y | N | Y | Y | Y | Y |
| DAH-associated cardiac disease | Decompensated diastolic heart failure | Endocarditis with mitral valve insufficiency | Decompensated diastolic heart failure | Endocarditis with mitral valve insufficiency | Mitral valve insufficiency due to (idiopathic) chorda rupture | Decompensated diastolic heart failure | Decompensated diastolic heart failure |
Y, yes; N, no; U, unknown; NA, not applicable; DAH, diffuse alveolar haemorrhage.
Figure 1.
Chest CT scan characteristic for diffuse alveolar haemorrhage in a patient with resolved acute heart failure showing diffuse bilateral ground glass opacities in upper lung parts sparing the cortex (left panel) and bilateral pleural effusion (right panel).
Discussion
The present case histories underscore the challenging diagnosis of DAH in patients with persisting upper lung lobe consolidations after initial treatment of an acute cardiac insult (8). Diagnosis was often not considered upfront and sometimes became apparent only after several hospitalisations.
de Prost et al. (1) described 33 CHF-DAH cases over a 30-year period in an 800-bed hospital. This corresponds with approximately one case per year which is substantially less than observed in our study (i.e., 3 DAH patients yearly in a 600-bed hospital). More recently, Tamai et al. reported 3.3 CHF-DAH cases per year in a 700-bed hospital (4) which more closely concurs with our observations. The difference in incidence can be explained by differences in defining heart failure but might as well highlight failure to establish a correct diagnosis. A greater awareness will obviously detect more cases of CHF-DAH.
Tamai et al. (4) documented predominantly right-sided DAH. Pulmonary vascular congestion and oedema involving the upper lobe of the right lung in patients with severe mitral regurgitation indeed occur more frequently than previously thought (9). Interestingly, we noticed more frequent and more voluminous left-sided consolidations. This is not readily explained but might be related to a regurgitating blood flow that is directed more towards the left pulmonary veins in our patient population.
Pulmonary ground glass infiltrates with central or peripheral location that persist despite resolving heart failure are cumbersome (10). Interestingly, cortical sparing was observed in four of our patients. Although far from proven and probably selection-biased, this might be a relevant sign pointing in the direction of CHF-DAH in case of unexplained persisting lung infiltrates.
The left atrium plays a crucial role in protecting lung vascular structures from bleeding by acting as a “compliant” reservoir. With progressive dilatation, however, atrial compliance recedes and its protective effect is lost (5). Atrial compliance can be indirectly assessed with echocardiography by evaluating ventricular filling velocities, venous pulmonary flow, degree of atrial dilatation, and presence of pulmonary hypertension (11). Six patients had dilated atria and one subject had an increased end-diastolic pressure and thus most likely an elevated pulmonary venous pressure. All patients presented MVI on previous or current echocardiographic exams. Differences in degree of regurgitation may be due to operator-dependent interpretation or difficult valve visualisation in patients suffering acute respiratory distress. Still, MVI might be considered as inherently associated or even as a possible culprit of DAH. In the presence of MVI, the systolic pressure generated by the left ventricle is not only transferred ahead but also backwards towards the left atrium. When the latter dilates excessively during compensation, pressure is transmitted further into the pulmonary veins and microcirculation. It should be underlined that ejection fraction is not a reliable substitute for elevated pulmonary venous pressure and reduced atrial compliance as it only reflects systolic and not diastolic ventricular function.
Several important shortcomings and limitations of our study must be taken into account. Although rigorously scrutinized for a common pathway leading to DAH, our study population is small and heterogeneous with regard to underlying cardiac disease. This precluded to observe differences between systolic and diastolic heart failure. Disease staging in the COPD patients (e.g., by using Global Initiative for Chronic Obstructive Lung Disease criteria) was not performed. However, COPD stage is not related with DAH and could also have been overestimated by a CHF-associated restrictive lung function. A COPD “exacerbation” either combined with or inducing heart failure may have caused severe hypoxaemia and haemoptysis mimicking CHF-DAH. DAH may not be linked to endocarditis in se but rather occur as a consequence of its immunologic and embolic manifestations. All patients received some form of anticoagulation or antiplatelet therapy. This could have facilitated, yet may not have been the main cause of bleeding. The vascular barrier must indeed be altered to account for extravascular leakage. In our patients, leakage rather was triggered by heart failure-induced elevated pulmonary venous pressure ripping up the vascular wall. Finally, mortality rate at discharge in our patient population was low. However, the number of patients is too small and solid comparative data on CHF-DAH mortality are not available.
Conclusions
DAH should be suspected in ICU patients treated for heart failure who present non- or slow-resolving upper lung lobe densities. The real incidence of DAH in this particular patient population is probably highly underestimated. Diagnosis is difficult in the acute setting and often not considered because of the atypical distribution of lung consolidations. BAL can help in the differential diagnosis but is not always feasible. Cortical sparing with associated pleural effusion are typical radiological findings. Some degree of MVI was present in all patients. Detecting a MVI with elevated pulmonary venous pressure in patients with suggestive radiological abnormalities not only should trigger a search for CHF-DAH but might well play a key role in the pathogenesis of this disease.
Acknowledgements
None.
Ethical Statement: The study was presented to the Institutional Review Board of the Middelheim Hospital which waived the need for ethical approval due to its retrospective, observational, and non-interventional design.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.de Prost N, Parrot A, Cuquelmelle E, et al. Diffuse alveolar hemorrhage in immunocompetent patients: etiologies and prognosis revisited. Respir Med 2012;106:1021-32. 10.1016/j.rmed.2012.03.015 [DOI] [PubMed] [Google Scholar]
- 2.Colby T, Fukuoka J, Ewaskow SP, et al. Pathologic approach to pulmonary haemorrhage. Ann Diagn Pathol 2001;5:309-19. 10.1053/adpa.2001.27923 [DOI] [PubMed] [Google Scholar]
- 3.Katzenstein AL, Bloor CM, Leibow AA. Diffuse alveolar damage--the role of oxygen, shock, and related factors. A review. Am J Pathol 1976;85:209-28. [PMC free article] [PubMed] [Google Scholar]
- 4.Tamai K, Tomii K, Nakagawa A, et al. Diffuse alveolar hemorrhage with predominantly right-sided infiltration resulting from cardiac comorbidities. Intern Med 2015;54:319-24. 10.2169/internalmedicine.54.3057 [DOI] [PubMed] [Google Scholar]
- 5.Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. N Engl J Med 1995;1566-72. [DOI] [PubMed] [Google Scholar]
- 6.Jouve A, Gabriel B, Revelin JF, et al. Les hémoptysies graves de l’insufficiance mitrale pure ou prédominante. Arch Mal Coeur 1965;58:1213-32. [PubMed] [Google Scholar]
- 7.McKee PA, Castelli WP, McNamara PM, et al. The natural history of congestive heart failure: the Framingham study. N Engl J Med 1971;285:1441-6. 10.1056/NEJM197112232852601 [DOI] [PubMed] [Google Scholar]
- 8.Kuru T, Lynch JP., 3rd Nonresolving or slowly resolving pneumonia. Clin Chest Med 1999;20:623-51. 10.1016/S0272-5231(05)70241-0 [DOI] [PubMed] [Google Scholar]
- 9.Schnyder PA, Sarraj AM, Duvoisin BE, et al. Pulmonary edema associated with mitral regurgitation: prevalence of predominant involvement of the right upper lobe. AJR Am J Roentgenol 1993;161:33-36. 10.2214/ajr.161.1.8517316 [DOI] [PubMed] [Google Scholar]
- 10.Lichtenberger JP, 3rd, Digumarthy SR, Abbott GF, et al. Diffuse pulmonary hemorrhage: clues to the diagnosis. Curr Probl Diagn Radiol 2014;43:128-39. 10.1067/j.cpradiol.2014.01.002 [DOI] [PubMed] [Google Scholar]
- 11.Stefanadis C, Dernellis J, Toutouzas P. A clinical appraisal of left atrial function. Eur Heart J 2001;22:22-36. 10.1053/euhj.1999.2581 [DOI] [PubMed] [Google Scholar]