Abstract
Background
Anastomotic leak is one of most significant causes of mortality after esophagectomy. Therefore, it is clinically valuable to detect anastomotic leak early after esophagectomy in esophageal cancer. The purpose of this study is to investigate the associations between routine postoperative laboratory findings and anastomotic leak and to analyze the laboratory findings to find out an independent predictive marker for anastomotic leak. In addition, this study compares cases treated with neoadjuvant therapy (NT) and those without (non-NT).
Methods
We retrospectively assessed the medical records of 201 consecutive cases that met this study’s criteria from January 2009 to December 2016. All patients underwent curative and complete esophagectomy for intra-thoracic esophageal cancer. We compiled and analyzed routine laboratory findings from the day before surgery to the eighth postoperative day on a daily basis. Routine laboratory tests consisted of 26 separate tests, including complete blood cell counts, blood chemistries, as well as erythrocyte sedimentation rate and C-reactive protein (CRP). Barium esophagogram with chest computed tomography (CT) was performed on the seventh postoperative day to evaluate the presence of an anastomotic leak.
Results
A total of 45 of 201 patients underwent NT. Anastomotic leaks were found in 23 (11.4%) of 201 patients (8 patients in NT and 15 patients in non-NT). White blood cell (WBC) from the second postoperative day (P=0.031, P=0.006, P=0.007, P=0.007, P=0.041, and P=0.003, respectively) and CRP from the third postoperative day (P=0.012, P<0.001, P=0.014, P<0.001, P=0.001, and P=0.006, respectively) were associated with anastomotic leak in non-NT; however, only CRP on the third, fifth, sixth, and seventh postoperative days (P=0.041, P=0.037, P=0.002, and P=0.003, respectively) was associated with anastomotic leak in NT. The CRP level on the third postoperative day was a significant independent predictive marker of anastomotic leak (P=0.041, odd ratio (OR) 1.056, 95% confidential interval (CI): 1.002–1.113) and had a significant diagnostic cutoff value for the development of anastomotic leak (non-NT: cutoff value 17.12 mg/dL, sensitivity 69.2%, specificity 78.1%, P<0.001, area 0.822; NT: cutoff value 16.42 mg/dL, sensitivity 80.0%, specificity 70.0%, P=0.042, area 0.7104).
Conclusions
There were divergent laboratory findings reflective of anastomotic leak between patients who underwent NT and those who did not. The CRP level on the third postoperative day had a significant cutoff value for early detection of anastomotic leak after esophagectomy in both NT and non-NT groups.
Keywords: Esophageal cancer, anastomotic leak, prediction
Introduction
Anastomotic leak is one of most important causes of mortality after esophagectomy and can lead to pneumonia, sepsis, and conduit loss (1-4). The overall incidence of an anastomotic leak is about 10%, with significant higher incidence for cervical anastomosis compared to intrathoracic one (1-4). Therefore, the early detection of anastomosis leak after esophagectomy in esophageal cancer is clinically valuable (5,6). Performing an esophagogram using contrast dye (in the evaluation of anastomotic leak) is harmful when a leak is actually present. Therefore, evaluation and management should be different according to the existence of an anastomotic leak (1,5-8). Prior studies have evaluated the early prediction of anastomotic leak after esophagectomy using laboratory findings (9-12). However, there were few studies addressing the prediction of anastomotic leak after esophagectomy in patients who have received neoadjuvant therapy (NT). Furthermore, it is unclear whether the laboratory findings used to predict anastomotic leak differ between patients who had received NT for esophageal cancer and those who have not (non-NT). Therefore, the purpose of this study is to investigate the associations between routine postoperative laboratory findings and anastomotic leak and to analyze the laboratory findings to find out an independent predictive marker for anastomotic leak. Furthermore, it clarifies the different findings between NT and non-NT in order to predict the presence of anastomotic leak early after esophagectomy. In addition, we analyzed the significant serum predictor for anastomotic leak according to NT, anastomotic type, surgical technique, and cancer progression.
Methods
We retrospectively assessed the medical records of 201 consecutive cases that met the study’s criteria from January 2009 to December 2016 (Figure 1). All patients underwent curative and complete esophagectomy for intra-thoracic esophageal cancer at the Department of Thoracic and Cardiovascular Surgery in a single tertiary hospital in Korea. The inclusion criteria were complete (R0) and curative surgery, intra-thoracic esophageal cancer, and preoperatively histology-proven squamous cell carcinoma. The exclusion criteria were palliation or salvage cases, other uncured prior or current primary cancers, and postoperative complications including pneumonia requiring ventilator care, chylothorax and bleeding requiring reoperation, acute renal failure requiring continuous renal replacement therapy, wound infection requiring exploration, and incomplete neoadjuvant treatment. Preoperative assessments included esophagogastroduodenoscopy, esophagography, chest computed tomography (CT), abdominal CT, positron emission tomography (PET)-CT, endoscopic ultrasound, and bone scan. Neoadjuvant therapies were conducted in accordance with the National Comprehensive Cancer Network guidelines and the suggestions of a multidisciplinary team with regard to each patient’s condition and cancer status including resectability. NT typically consisted of two cycles of cisplatin and 5-fluorouracil, as well as 25 fractions of radiation therapy with a total of 41–45 Gray (over 5 weeks). Esophagectomy was performed by 3 surgeons 5 or 6 weeks after completion of NT using the Ivor Lewis (28 mm-circular stapled anastomosis) or the McKeown procedures (sutured anastomosis) using a gastric conduit depending on the patient’s condition and cancer status (13). Two-field lymph node dissections were performed. Nasogastric tubes were routinely placed in all patients after surgery and oral feeding was started when esophagogram on the seventh postoperative day did not showed anastomotic leak. We retrospectively compiled and analyzed routine laboratory findings from the day before surgery to the eighth postoperative day on a daily basis. Routine laboratory tests consisted of 26 separate tests, including complete blood cell counts, blood chemistries, as well as erythrocyte sedimentation rate and C-reactive protein (CRP). All laboratory tests were measured by standard methods using an auto analyzer (Hitachi 7600-210; Hitachi, Tokyo, Japan) and available assay kits (Sekisui Medical Co., Ltd., Tokyo, Japan). We used barium esophagogram and chest CT on the seventh postoperative day to evaluate anastomotic leak. In this study, anastomotic leak was defined as the disruption of the anastomosis that leads to outflow of the intraluminal content, which is obvious leaks, as well as leaks without the presence of any clinical symptoms but with only occult leaks detected with esophagography followed by chest CT. We compared these routine laboratory findings between cases with (Leak) and without anastomotic leak (non-Leak). In addition, we evaluated the diagnosis accuracy of anastomotic leak using the significant serum markers. All analyses were performed separately for NT and non-NT. This study was approved by the Institutional Review Board of Seoul St. Mary’s Hospital (IRB approval number: KC17RESI0116).
Figure 1.
Consort flow diagram.
Statistical considerations
Due to non-normality distribution, comparison between the groups was evaluated using the Mann-Whitney U test. Chi-square or Fisher’s exact tests were used to compare categorical variables. The association between continuous variables was evaluated with the Spearman test. Receiver operating characteristic (ROC) analysis was used for diagnostic evaluations using serum laboratory marker. We used binary logistic regression analysis with backward selection of logarithmically transformed serum marker levels to analyze their interdependency for the diagnosis of anastomotic leak. Goodness of fit was assessed using the Hosmer-Lemeshow test. All analyses were performed after propensity score matching in order to overcome the data heterogeneity according to age, pathological stage, anastomosis type, surgical technique, and NT. Statistical Package of Social Sciences version 22.0 (SPSS Inc., Chicago, IL, USA) was used for all analyses. P value <0.05 (two-sided) was considered statistically significant.
Results
Study population
A total of 201 consecutive patients (male 184, female 17; mean age 63.9±9.0 years) who had undergone curative and complete surgery for intrathoracic esophageal cancers between January 2009 and December 2016 were included. Forty-five of these patients had received NT (initial clinical stage: IIa 9, IIb 23, IIIa 9, IIIb 1, and IIIc 3 cases). All of the cancers were squamous cell carcinomas on histology. Thirty-one tumors were located in the upper thoracic esophagus, 81 in the middle thoracic esophagus and 89 in the lower thoracic esophagus. Surgeries were performed using the Ivor Lewis (170 cases) and the McKeown procedures (31 cases); open (OE, 89 cases) and minimally invasive esophagectomy (MIE, 112 cases). The mean tumor length and size were 3.2 (±2.1) cm and 9.4 (±12.7) cm2, respectively. Anastomotic leaks were identified in 23 (11.4%) of 201 patients (8 patients in NT and 15 patients in non-NT). The overall clinicopathologic characteristics for the present study subjects are summarized in Table 1.
Table 1. Overall clinicopathologic characteristics for the study population.
| Characteristics | Number of patients (N=201) |
|---|---|
| Age (year) | Mean 63.9 (±9.0) |
| Sex | |
| Male | 184 |
| Female | 17 |
| Previous other primary cancers | |
| No | 181 |
| Yes | 20 |
| Current smoking | |
| No | 90 |
| Yes | 93 |
| Unknown | 18 |
| Preoperative stage after neoadjuvant therapy | |
| I a | 7 |
| I b | 54 |
| II a | 21 |
| II b | 85 |
| III a | 32 |
| III b | 2 |
| Pathologic stage after surgery | |
| Complete remission | 5 |
| I a | 18 |
| I b | 67 |
| II a | 13 |
| II b | 56 |
| III a | 19 |
| III b | 13 |
| III c | 10 |
| Location of cancer | |
| Upper thoracic | 31 |
| Middle thoracic | 81 |
| Lower thoracic | 89 |
| Method of surgery | |
| Ivor Lewis | 170 |
| McKeown | 31 |
| Leak | |
| Leak | 23 |
| No leak | 178 |
| Differentiation | |
| Well | 21 |
| Moderate | 152 |
| Poor | 28 |
| Neoadjuvant therapy | |
| No | 156 |
| Yes | 45 |
Laboratory findings between the leak and non-leak cases after esophagectomy
Non-NT cases
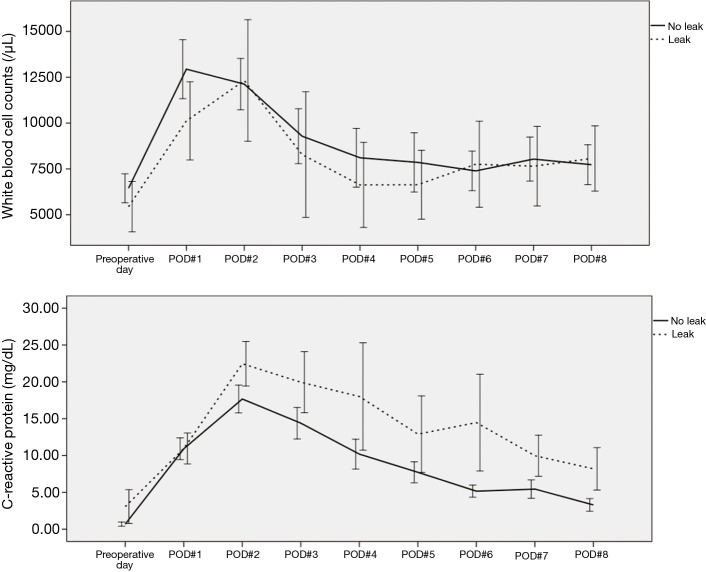
A total of 156 patients did not receive NT before esophagectomy. Their final pathology stages were as followed: 17 cases of Ia; 62 cases of Ib; 12 cases of IIa; 41 cases of IIb; 11 cases of IIIa; 11 cases of IIIb; and 2 cases of IIIc. Anastomotic leaks were identified in 15 of 156 patients. There was no difference in the routine preoperative laboratory findings between leak and non-leak. However, we found that white blood cell (WBC) and CRP out of 26 routine postoperative laboratory tests were significantly associated with anastomotic leak. The WBC counts from the second postoperative day were significantly associated with anastomotic leak (P=0.031, P=0.006, P=0.007, P=0.007, P=0.041, and P=0.003, respectively). In addition, the CRP level from the third postoperative day were also significantly associated with anastomotic leak (P=0.012, P<0.001, P=0.014, P<0.001, P=0.001, and P=0.006, respectively). After esophagogram, WBC and CRP was also significantly associated with anatomical leak (P<0.001, both) (Figure 2).
Figure 2.
WBC and CRP trends in non-NT. WBC counts from the second postoperative day were significantly associated with anastomotic leak (P=0.031, P=0.006, P=0.007, P=0.007, P=0.041, and P=0.003, respectively). In addition, CRP from the third postoperative day were also significantly associated with the anastomotic leak (P=0.012, P<0.001, P=0.014, P<0.001, P=0.001, and P=0.006, respectively). After esophagogram, WBC and CRP was also significantly associated with anatomical leak (P<0.001, both) (presented as 2 standard errors of the mean). WBC, white blood cell; CRP, C-reactive protein; NT, neoadjuvant therapy.
NT cases
Forty-five patients received NT prior to esophagectomy. Their final pathology stages included complete remission in 5 cases, stage Ia in 1 case, Ib in 5, IIa in 1, IIb in 15, IIIa in 8, IIIb in 2, and IIIc in 8 cases. Anastomotic leaks were identified in 8 of 45 patients. We found that only CRP out of 26 routine postoperative laboratory tests was associated with anastomotic leak. The CRP level were significantly associated with the anastomotic leak on the third, fifth, sixth, and seventh postoperative day (P=0.041, P=0.037, P=0.002, and P=0.003, respectively). After esophagogram, CRP was also significantly associated with anatomical leak (P<0.001) (Figure 3).
Figure 3.
WBC and CRP trends in NT. The CRP levels on the sixth and seventh postoperative days were significantly associated with anastomotic leak (P=0.028 and P=0.039, respectively). After esophagogram, the CRP level was also significantly associated with anatomical leak (P=0.008) (presented as 2 standard errors of the mean). WBC, white blood cell; CRP, C-reactive protein; NT, neoadjuvant therapy.
Independent predictive marker and diagnostic accuracy for anastomotic leak in non-NT and NT
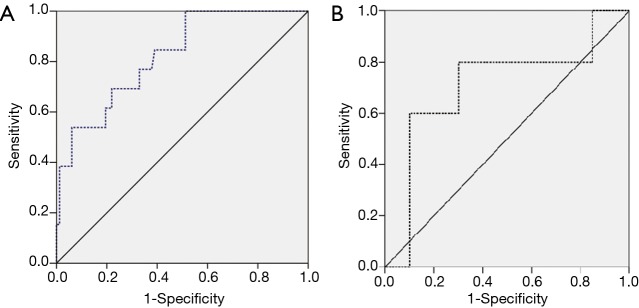
Propensity score matching analysis was used to find out an independent predictive marker and diagnostic accuracy for anastomotic leak in overall cases (Table 2). CRP was only associated with anastomotic leak on the third and sixth postoperative days out of 26 routine postoperative laboratory findings (P=0.028 and P=0.039, respectively). Logistic regression analysis was used to investigate the independent predictive factors using postoperative CRP, the significant serum marker. CRP on the third and sixth postoperative day were included as covariates, the logistic regression analysis revealed CRP on the third postoperative day as a significant independent predictive marker of anastomotic leak (P=0.041, OR 1.056, 95% CI: 1.002–1.113). CRP on the third postoperative day had a significant diagnostic cutoff value for the development of anastomotic leak (non-NT: cutoff value 17.12 mg/dL, sensitivity 69.2%, specificity 78.1%, P<0.001, area 0.822; NT: cutoff value 16.42 mg/dL, sensitivity 80.0%, specificity 70.0%, P=0.042, area 0.710) (Figure 4).
Table 2. Propensity score matching description.
| Variable | Total population | Propensity-matched population | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Non-leak (n=178) | Leak (n=23) | P value | Standardized difference | Non-leak (n=23) | Leak (n=23) | P value | Standardized difference | ||
| Age | 64.5±7.5 | 65.1±8.5 | 0.840 | 0.152 | 64.5±7.5 | 65.1±8.5 | 0.991 | 0.071 | |
| p Stage | 3.24±1.7 | 3.8±1.7 | 0.038 | 0.200 | 3.5±1.6 | 3.6±1.7 | 0.708 | 0.053 | |
| NT | 37 (20.8%) | 8 (34.8%) | 0.180 | 0.287 | 9 (39.1%) | 8 (34.8%) | 1.000 | −0.089 | |
| MIE | 104 (58.4%) | 8 (34.8) | 0.044 | −0.486 | 8 (34.8%) | 8 (34.8%) | 1.000 | 0.000 | |
| Ivor Lewis | 156 (87.6%) | 14 (60.9%) | 0.003 | 0.536 | 9 (39.1%) | 9 (39.1%) | 1.000 | 0.000 | |
p Stage, pathological stage; NT, neoadjuvant therapy; MIE, minimally invasive esophagectomy.
Figure 4.
ROC analysis curve in the diagnostic evaluation of anastomotic leak. CRP on the third postoperative day was an independent predictive serum marker of anastomotic leak and had a significant diagnostic cutoff value for the development of anastomotic leak (non- NT: cutoff value 17.12, sensitivity 69.2%, specificity 78.1%, P<0.001, area 0.822; NT: cutoff value 16.42, sensitivity 80.0%, specificity 70.0%, P=0.042 area 0.710) (A, NT; B, non- NT). ROC, receiver operating characteristic; CRP, C-reactive protein; NT, neoadjuvant therapy.
Anastomotic leak and CRP according to NT, anastomosis type, surgical technique, and cancer progression
We analyzed the significant serum predictor, CRP for anastomotic leak according to NT, anastomotic type, surgical technique, and cancer progression (Table 3).
Table 3. Anastomotic leak and CRP according to neoadjuvant therapy, anastomosis type, surgical technique, and cancer progression.
| Variables | Neoadjuvant therapy | Anastomotic type | Surgical technique | Cancer progression |
|---|---|---|---|---|
| Anastomotic leakage | NA | NA | NA | NA |
| CRP | NA | NA | Associated | NA |
CRP, C-reactive protein; NA, not associated.
Anastomotic leak and CRP according to NT
Anastomotic leaks were found in 23 (11.4%) of 201 patients (8 patients in NT and 15 patients in non-NT). There was no significant difference in the prevalence of anastomotic leak between NT and non- NT. There was also no significant difference of CRP between the two groups.
Anastomotic leak and CRP according to anastomosis types (the Ivor Lewis and the McKeown procedure)
A total of 140 patients underwent the Ivor Lewis and 16 patients underwent the McKeown procedure in non-NT, and 30 patients underwent the Ivor Lewis and 15 patients underwent the McKeown procedure in NT. There was no significant difference in the prevalence of anastomotic leak between the Ivor Lewis and the McKeown procedure in either group. There was also no significant difference in CRP between anastomotic types in either group.
Anastomotic leak and CRP according to surgical technique (MIE and OE)
Sixty-seven patients underwent OE and 89 patients underwent MIE in non-NT, and in NT, 22 patients underwent OE and 23 patients underwent MIE. There was no difference in the prevalence of anastomotic leak between MIE and OE techniques in either group. However, there was significant difference in CRP on the third postoperative day between MIE and OE (11.8±5.0 vs. 16.4±7.3 mg/dL, P=0.001). We separately investigate the diagnostic accuracy of CRP level for anastomotic leak after MIE and OE. In non-NT, the CRP level on the third postoperative day had a significant diagnostic cutoff value for the development of anastomotic leak (MIE: cutoff value 12.86, sensitivity 83.3%, specificity 64.9%, P=0.016, area 0.800, 95% CI: 0.647–0.953; OE: cutoff value 17.94 mg/dL, sensitivity 71.4%, specificity 72.0%, P=0.008, area 0.834, 95% CI: 0.652–1.000). In NT, the CRP level on the third postoperative day also had a significant diagnostic cutoff values for the development of anastomotic leak (MIE: none; OE: cutoff value 21.35 mg/dL, sensitivity 66.7%, specificity 88.9%, P=0.046, area 0.778, 95% CI: 0.581–0.974).
Anastomotic leak and CRP according to cancer progression
There was no significant difference in the prevalence of anastomotic leak according to pathological stage in either the non-NT or NT. Preoperative CRP level was positively correlated with pathological stage (P=0.017) in non-NT. However, CRP level was not associated with pathological stage in NT. In addition, postoperative CRP level was not associated with pathological stage in either non-NT or NT.
Discussion
Despite progress with regard to esophagectomy techniques in esophageal cancer, anastomotic leak remains a fatal consequence of the procedure (1-4). Usually, anastomotic leak is evaluated after esophagectomy using contrast esophagogram on the seventh postoperative day (8,14,15). Its early detection is critical, as it prevents delay in therapeutic intervention and hopefully reduces its sequelae (5,6). Unfortunately, esophagogram using contrast dye, which is the procedure used to diagnose anastomotic leak, can cause severe complications in case of the existence of anastomotic leak (including aspiration pneumonia and mediastinitis) (6). Therefore, the evaluation and management must be tailored according to the existence of anastomotic leak (1,5-7).
There are many prior studies evaluating anastomotic leak after esophagectomy (4,9,10,14,16). The literature suggests that elevated CRP after esophagectomy is a marker that can be used for early detection of postoperative complications after esophagectomy (11,17,18). CRP is an acute-phase reactant that is produced in the liver and its levels start to increase approximately 2 hours after the onset of inflammation and peak at 48 hours (11). It has a half-life of 18 hours (11). Therefore, CRP is widely used as a non-specific indicator of inflammation after surgery (11). Other studies have also evaluated laboratory values in the early prediction of anastomotic leak after esophagectomy (10,12,17). However, there has not been extensive investigation of the prediction of anastomotic leak after esophagectomy using routine laboratory findings in patients who have undergone NT. In addition, it is unclear whether laboratory markers in the prediction of anastomotic leak differ between NT and non-NT.
In this study, WBC count and CRP (out of 26 routine laboratory tests) were associated with anastomotic leak in non-NT. However, only CRP was associated with anastomotic leak after esophagectomy in NT. In other words, while elevated WBC was marker in only non- NT, the implication could be, that WBC cannot be relied on suspecting leak in NT presumably due to NT ameliorating WBC response. In addition, serial changes in postoperative WBC counts were significantly different from the second postoperative day between Leak and non-Leak in non-NT. However, WBC count on the seventh postoperative day had a significant diagnostic cutoff value for predicting postoperative anastomotic leak. The diagnostic accuracy was low. The cutoff value was within normal range. Most postoperative complications due to anastomotic leak have obvious clinical symptoms by the seventh postoperative day. These findings indicate that postoperative WBC count does not contribute to the early prediction of anastomotic leak after esophagectomy with regard to diagnostic accuracy and timeliness. Instead, the postoperative CRP value is the only useful marker in the prediction of postoperative anastomotic leak, which is applicable in both NT and non-NT. Procalcitonin and interleukin-6 have been previously reported to be potential predictors of surgical complications after surgery (18). However, given that these markers are not included in routine laboratory tests, are not well-establish, and are expensive, we did not analyze these results. Unlike previous studies, we investigated the independent CRP level on a specific postoperative day in the early prediction of anastomotic leak in both non-NT and NT. The present study shows that CRP on the third postoperative day could make it possible to early detect anastomotic leak before the onset of overt clinical manifestations. This may be not enough to confirm anastomotic leak, however, this suggest that other modality such as endoscopy are required to evaluate anastomotic leak. Early detection would allow early therapeutic intervention and better prognosis.
We also analyzed anastomotic leak and CRP according to NT, anastomotic type, surgical technique, and cancer progression (16,19,20). In this study, NT did not increase the risk of anastomotic leak. In addition, the prevalence of anastomotic leak did not differ between intrathoracic and cervical anastomoses (2,20,21). Furthermore, CRP did not differ regardless of NT or anastomosis type. The present study also showed that surgical technique (MIE vs. OE) did not influence the prevalence of anastomotic leak (19). However, CRP was significantly lower after MIE than it was after OE. We attribute these results to the fact that MIE causes less trauma and therefore less inflammation than does OE. Future studies should separately investigate the diagnostic accuracy of CRP for anastomotic leak after MIE and OE. With exception of the MIE group in the NT (likely due to the small sample size), the CRP level on the third postoperative day had a significant diagnostic cutoff value for the development of anastomotic leak in both MIE and OE cases. The diagnostic cutoff values for anastomotic leak were also lower in those who underwent MIE than in those in had OE. We investigated the association of anastomotic leak, CRP level, and cancer progression (19,20). Anastomotic leak was not associated with cancer progression. Preoperative CRP was significantly correlated with pathological stage in non-NT, and CRP was not associated with pathological stage in NT. In order words, CRP was not associated with pathological stage after surgery or NT. However, CRP may have been significantly correlated with cancer progression prior to treatment. These findings also suggest the CRP level after esophagectomy is useful in the prediction of anastomotic leak regardless of cancer progression. To the best of our knowledge, our study is the first to assess the prediction of anastomotic leak using laboratory findings in both NT and non-NT after esophagectomy. Further prospective large-scale studies are needed to improve early diagnosis of anastomotic leak.
This study has several limitations. For instance, its retrospective nature as well as inclusions and exclusions criteria introduce some inherent bias in the evaluation of anastomotic leak. In addition, it was performed at a single-center, with a small sample of patients from a homogenous ethic group, which increases the likelihood of Type II error. Therefore, our findings must be generalized with caution. Furthermore, this study was not randomized, introducing the possibility of selection bias. Finally, we used propensity score matching analysis to overcome the data heterogeneity.
Conclusions
There were divergent laboratory findings reflective of anastomotic leak between patients who underwent NT and those who did not. The CRP level on the third postoperative day had a significant cutoff value for early detection of anastomotic leak after esophagectomy in both NT and non-NT groups. Therefore, CRP may contribute to early clinical decision-making with regard anastomotic leak after esophagectomy.
Acknowledgements
None.
Ethical Statement: This study was approved by the Institutional Review Board of Seoul St. Mary’s Hospital (IRB Approval number: KC17RESI0116).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Schlottmann F, Molena D. Anastomotic leak: an early complication with potentially long-term consequences. J Thorac Dis 2016;8:E1219-E20. 10.21037/jtd.2016.09.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ott K, Bader FG, Lordick F, et al. Surgical factors influence the outcome after Ivor-Lewis esophagectomy with intrathoracic anastomosis for adenocarcinoma of the esophagogastric junction: a consecutive series of 240 patients at an experienced center. Ann Surg Oncol 2009;16:1017-25. 10.1245/s10434-009-0336-5 [DOI] [PubMed] [Google Scholar]
- 3.Wright CD, Kucharczuk JC, O’Brien SM, et al. Predictors of major morbidity and mortality after esophagectomy for esophageal cancer: a Society of Thoracic Surgeons General Thoracic Surgery Database risk adjustment model. J Thorac Cardiovasc Surg 2009;137:587-95; discussion 596. 10.1016/j.jtcvs.2008.11.042 [DOI] [PubMed] [Google Scholar]
- 4.Booka E, Takeuchi H, Nishi T, et al. The Impact of Postoperative Complications on Survivals After Esophagectomy for Esophageal Cancer. Medicine (Baltimore) 2015;94:e1369. 10.1097/MD.0000000000001369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Low DE. Diagnosis and management of anastomotic leaks after esophagectomy. J Gastrointest Surg 2011;15:1319-22. 10.1007/s11605-011-1511-0 [DOI] [PubMed] [Google Scholar]
- 6.Perry Y, Towe CW, Kwong J, et al. Serial Drain Amylase Can Accurately Detect Anastomotic Leak After Esophagectomy and May Facilitate Early Discharge. Ann Thorac Surg 2015;100:2041-6; discussion 2046-7. 10.1016/j.athoracsur.2015.05.092 [DOI] [PubMed] [Google Scholar]
- 7.Guo J, Chu X, Liu Y, et al. Choice of therapeutic strategies in intrathoracic anastomotic leak following esophagectomy. World J Surg Oncol 2014;12:402. 10.1186/1477-7819-12-402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roh S, Iannettoni MD, Keech JC, et al. Role of Barium Swallow in Diagnosing Clinically Significant Anastomotic Leak following Esophagectomy. Korean J Thorac Cardiovasc Surg 2016;49:99-106. 10.5090/kjtcs.2016.49.2.99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aminian A, Panahi N, Mirsharifi R, et al. Predictors and outcome of cervical anastomotic leakage after esophageal cancer surgery. J Cancer Res Ther 2011;7:448-53. 10.4103/0973-1482.92016 [DOI] [PubMed] [Google Scholar]
- 10.Noble F, Curtis N, Harris S, et al. Risk assessment using a novel score to predict anastomotic leak and major complications after oesophageal resection. J Gastrointest Surg 2012;16:1083-95. 10.1007/s11605-012-1867-9 [DOI] [PubMed] [Google Scholar]
- 11.Gordon AC, Cross AJ, Foo EW, et al. C-reactive protein is a useful negative predictor of anastomotic leak in oesophago-gastric resection. ANZ J Surg 2016. [Epub ahead of print]. 10.1111/ans.13681 [DOI] [PubMed] [Google Scholar]
- 12.Okamura A, Watanabe M, Imamura Y, et al. Preoperative Glycosylated Hemoglobin Levels Predict Anastomotic Leak After Esophagectomy with Cervical Esophagogastric Anastomosis. World J Surg 2017;41:200-7. 10.1007/s00268-016-3763-z [DOI] [PubMed] [Google Scholar]
- 13.Walther B, Johansson J, Johnsson F, et al. Cervical or thoracic anastomosis after esophageal resection and gastric tube reconstruction: a prospective randomized trial comparing sutured neck anastomosis with stapled intrathoracic anastomosis. Ann Surg 2003;238:803-12; discussion 812-4. 10.1097/01.sla.0000098624.04100.b1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooke DT, Lin GC, Lau CL, et al. Analysis of cervical esophagogastric anastomotic leaks after transhiatal esophagectomy: risk factors, presentation, and detection. Ann Thorac Surg 2009;88:177-84; discussion 184-5. 10.1016/j.athoracsur.2009.03.035 [DOI] [PubMed] [Google Scholar]
- 15.Schaheen L, Blackmon SH, Nason KS. Optimal approach to the management of intrathoracic esophageal leak following esophagectomy: a systematic review. Am J Surg 2014;208:536-43. 10.1016/j.amjsurg.2014.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kassis ES, Kosinski AS, Ross P, Jr, et al. Predictors of anastomotic leak after esophagectomy: an analysis of the society of thoracic surgeons general thoracic database. Ann Thorac Surg 2013;96:1919-26. 10.1016/j.athoracsur.2013.07.119 [DOI] [PubMed] [Google Scholar]
- 17.Goh SL, De Silva RP, Dhital K, et al. Is low serum albumin associated with postoperative complications in patients undergoing oesophagectomy for oesophageal malignancies? Interact Cardiovasc Thorac Surg 2015;20:107-13. 10.1093/icvts/ivu324 [DOI] [PubMed] [Google Scholar]
- 18.Hoeboer SH, Groeneveld AB, Engels N, et al. Rising C-reactive protein and procalcitonin levels precede early complications after esophagectomy. J Gastrointest Surg 2015;19:613-24. 10.1007/s11605-015-2745-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Markar SR, Arya S, Karthikesalingam A, et al. Technical factors that affect anastomotic integrity following esophagectomy: systematic review and meta-analysis. Ann Surg Oncol 2013;20:4274-81. 10.1245/s10434-013-3189-x [DOI] [PubMed] [Google Scholar]
- 20.Messager M, Warlaumont M, Renaud F, et al. Recent improvements in the management of esophageal anastomotic leak after surgery for cancer. Eur J Surg Oncol 2017;43:258-69. 10.1016/j.ejso.2016.06.394 [DOI] [PubMed] [Google Scholar]
- 21.Klink CD, Binnebösel M, Otto J, et al. Intrathoracic versus cervical anastomosis after resection of esophageal cancer: a matched pair analysis of 72 patients in a single center study. World J Surg Oncol 2012;10:159. 10.1186/1477-7819-10-159 [DOI] [PMC free article] [PubMed] [Google Scholar]