Abstract
Background
In recent years, the tumor-stroma ratio (TSR) has been considered to a new and independent predictive variable for the prognosis of some kinds of neoplasms. The objective of this study was to assess the prognostic significance of the TSR in non-small cell lung cancer (NSCLC).
Methods
A cohort of 261 NSCLC patients who underwent radical surgery of lung cancer were included in the present study. Two independent observers visually estimated the TSR on hematoxylin-eosin (H&E) stained tissue pathological slices. According to the proportion of stroma ≥50% or <50%, We separate the patients into two groups: those with stroma-poor and those with stroma-rich tumors.
Results
Both univariate and multivariate analyses disclosed that the TSR was associated with overall survival (OS) [hazard ratio (HR), 1.741; 95% confidence intervals (CI), 1.040–2.913 and HR, 1.904; 95% CI, 1.132–3.202, respectively]. The HR values for disease-free survival (DFS) were 1.795 (95% CI, 1.073–3.005) and 2.034 (95% CI, 1.210–3.420). The OS and DFS of patients with stroma-poor tumors were better than those with stroma-rich tumors.
Conclusions
These results demonstrated that the TSR is a new prognostic factor for NSCLC. Stroma-poor tumors were associated with longer disease-free period and better prognosis than were stroma-rich tumors in NSCLC patients. The TSR may contribute to the development of individualized treatment for NSCLC in the future.
Keywords: Non-small cell lung cancer (NSCLC), tumor-stroma ratio (TSR), prognosis, tumor microenvironment
Introduction
Based on the new data, among the incidence and mortality of cancer in China, lung cancer is the foremost (1). Non-small cell lung cancer (NSCLC) is the most prevalent histological types of lung cancer, accounting for approximately 80% of lung cancers worldwide. Although several new methods are applied to the diagnosis and treatment of lung cancer, the prognosis remains poor. According to the latest global cancer statistics, lung cancer is the most frequent cancer-related death among females in more developed countries and in males worldwide (2). Until recently, the five-year overall survival (OS) of carcinoma of the lungs is less than 15% (3).
In previous studies, it is confirmed that the TNM stage (4) was a crucial factor for the prognosis of lung cancer together with histology, age, patient sex (5), smoking history (6) etc. Although the current prognostic factors can predict the risk of recurrence to a certain degree, some early-stage cases have poor prognoses, reflecting a shortage to forebode the relative risk. Thus, new prognostic factors are needed to predict the recurrent risk.
Recently, the tumor-stroma ratio (TSR) has been regarded as a new prognostic factor for some kinds of cancers, such as the nasopharyngeal cancer (7), early cervical carcinoma (8), esophageal carcinoma (9) and epithelial ovarian cancer (10). These tumors comprise tumor cells and the corresponding stroma, including the pericytes leukocytes, extracellular matrix, fibroblasts and endothelial cells. Many studies have revealed that the tumor stroma may act as a pivotal part in malignant progression and metastasis. To the best of our knowledge, the role of the TSR in NSCLC has not been completed explored. Thus, the aim of the present study was to determine the prognostic significance of TSR in NSCLC and characterize the correlation of this value with other clinicopathologic variables.
Methods
Patient population
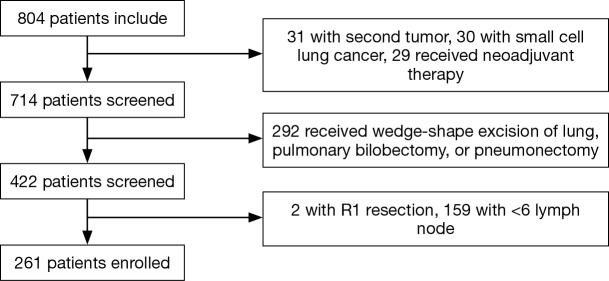
In the present retrospective study, we picked out 261 patients who underwent radical surgery and systematic lymphadenectomy at the Department of Thoracic Surgery of Sun Yat-sen University Cancer Center from January 2007 to December 2009. The information for each patient was retrieved from the Medical Record Room, and we collected hematoxylin-eosin (H&E) stained slides and the tumor characteristics from the Department of Pathology. We obtained written informed consent of all patients before the use of their tumor tissue when necessary, and the study was approved by Institute Research Medical Ethics Committee of Sun Yat-sen University Cancer Center (No. B2017-060-01). Patients were enrolled in this study according to the following eligibility criteria: (I) all patients underwent radical surgery and systematic lymphadenectomy, and confirmed R0 resection; (II) all patients had the pathologically confirmed NSCLC; (III) evidence of distant metastasis was not detected by the preoperative examination, like the sonography, computed tomography (CT) and so on; (IV) not less than 6 lymph nodes were dissected during the lung resection. The exclusion criteria were as shown below: (I) patients had a second primary tumor; (II) patients received wedge-shape excision of lung, pulmonary bilobectomy, or pneumonectomy; (III) patients received neoadjuvant therapy, including radiotherapy, chemotherapy, or chemoradiotherapy; (IV) patients without complete follow-up information; (V) patients died within 30 days after surgery. Finally, 261 patients were enrolled in this study. The flow was shown in Figure 1.
Figure 1.
Screening chart for 804 patients received lung resection at Sun Yat-sen University Cancer Center.
Histopathological protocol
We selected tissue samples from the pathology archives, and 5 µm H&E stained histologic sections were microscopically analyzed. The most invasive tumor areas were selected using a 4× objective. Subsequently, a 10× objective was chosen to pick out the part of the sample which contained both tumor and stroma tissue. The fields where tumor cells were visualized on all sides were scored per ten-fold-percentage (10%, 20%, 30%, etc.). The evaluation was based on the analyses of more than one microscopic field. Areas with the highest TSR were considered crucial. Two independent investigators (Huang J and Jing J) assessed the TSR. A third pathologist made the final decision when the two investigators disagreed. As previously reported for malignant hepatoma (11), esophageal squamous cell carcinoma (9) and breast carcinoma (12), the cutoff value for the TSR was 50%. The TSR was used to divide the tumors into stroma-poor (percentage of tumor stroma <50%) and stroma-rich (percentage of tumor stroma ≥50%) groups. The TNM stage was determined based on the TNM classification of malignant tumors according to the International Association for the Study of Lung Cancer (7th ed, 2009).
Follow-up
The deadline of collecting follow-up data were March 2016 or death. Every patient was regularly followed, within the first 2 years of surgery, every 3 or 4 months, the patients were followed by physical examination and the complete history, also with the medical imaging examinations, including thoracoabdominal CT, brain MRI, even positron emission tomography (PET) when it needed. And by then extend to every 6 months. We defined the time interval between the date of surgery and the end of follow-up or the date of death as OS. And defined the time period between the date of surgery and the date of death or the appearance of tumor progression or recurrence as disease-free survival (DFS).
Statistical analysis
Chi-square test was applied to measure the correlation between the TSR and other clinicopathological factors along with Fisher’s exact test. OS and DFS curves were draw by Kaplan-Meier survival analysis, the survival curves was compared using log-rank tests. Inter-observer agreement was analyzed according to Cohen’s kappa. We assessed the hazard ratio (HR) and 95% confidence interval (CI) for OS and DFS by univariate and multivariate Cox regression models. Software version 21 (SPSS Inc., Chicago, IL, USA) were applied to perform data analysis. The results were considered statistically significant at P values less than 0.05.
Results
TSR in NSCLC
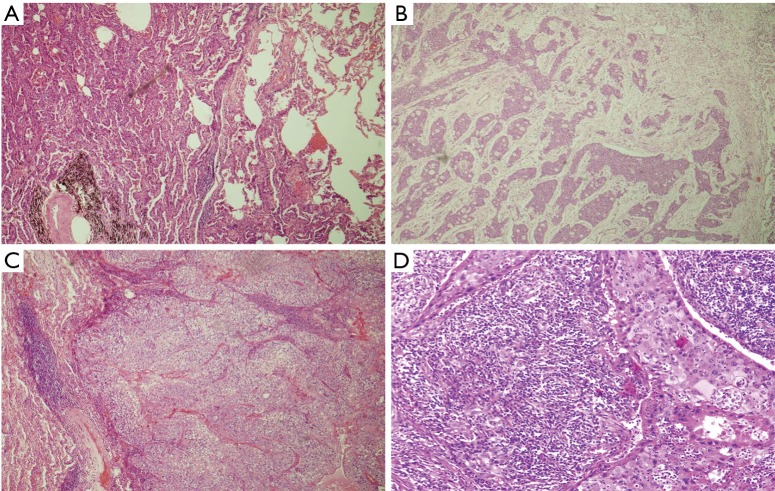
We affirmed the fact of stromal participation in routine H&E-stained tissue slice from the lung cancer samples. The TSR was assessed on sections from the most invasive tumors (representative images are shown in Figure 2). By any possibility heterogeneity, the highest TSR value was considered crucial. Two independent pathologists successfully estimated the TSR. The concordance rate of the two pathologists was 86.6%. Thirty-five cases required a third review. Cohen’s kappa indicated moderate agreement (Kappa =0.51). Among the 261 samples, 223 tumors were stroma poor, and all others were stroma rich.
Figure 2.
Hematoxylin and eosin-stained sections (5 µm) of lung squamous cell carcinoma and adenocarcinoma (original magnification, ×100). (A) The stroma-poor lung adenocarcinoma group; (B) the stroma-rich group lung adenocarcinoma; (C) the stroma-poor lung squamous cell carcinoma group; (D) the stroma-rich lung squamous cell carcinoma group.
Patient characteristics
In the present study, altogether 261 patients with NSCLC were selected, including 165 men and 96 women. Age of the selected patients ranged from 30–84 years (mean, 60 years) at the date of surgery. The follow-up time ranged from 4.50–111.87 months (mean, 83.07 months).
Relationships between TSR and clinicopathological features
The clinical and pathological characteristic of the patients are demonstrated in Table 1. Division of all 261 patients into two group: a stroma-poor tumor group (TSR <50%, n=223) and a stroma-rich tumor group (TSR ≥50%, n=38). Comparing the differences between the two groups was by Chi-square test along with Fisher’s exact test. According to the results of Table 1, gender, smoking history, age, differentiation grade, histology, pT status, pN status, adjuvant therapy and pTNM stage were associated with the TSR. Without a significant difference between the two groups.
Table 1. Tumor and patient characteristics with NSCLC (grouped by tumor-stroma ratio).
| Characteristics | Total (N=261), n (%) | Stroma poor (N=223), n (%) | Stroma rich (N=38), n (%) | P |
|---|---|---|---|---|
| Gender | 0.462 | |||
| Women | 96 (36.8) | 80 (35.9) | 16 (42.1) | |
| Men | 165 (63.2) | 143 (64.1) | 22 (57.9) | |
| Smoking history (P.Y) | 0.715 | |||
| <20 | 144 (55.2) | 122 (54.7) | 22 (57.9) | |
| ≥20 | 117 (44.8) | 101 (45.3) | 16 (42.1) | |
| Age (year) | 0.763 | |||
| ≤65 | 173 (66.3) | 147 (65.9) | 26 (68.4) | |
| >65 | 88 (33.7) | 76 (34.1) | 12 (31.6) | |
| Differentiation grade | 0.443 | |||
| Well | 27 (10.3) | 24 (10.8) | 3 (7.9) | |
| Moderate | 132 (50.6) | 109 (48.9) | 23 (60.5) | |
| Poor | 102 (39.1) | 90 (40.4) | 12 (31.6) | |
| Histology | 0.181 | |||
| ADC | 182 (69.7) | 152 (68.2) | 30 (78.9) | |
| SCC | 79 (30.3) | 71 (31.8) | 8 (21.1) | |
| pT status | 0.753 | |||
| pT1 | 119 (45.6) | 101 (45.3) | 18 (47.4) | |
| pT2 | 125 (47.9) | 106 (47.5) | 19 (50.0) | |
| pT3/4 | 17 (6.5) | 16 (7.2) | 1 (2.6) | |
| pN status | 0.202 | |||
| pN0 | 178 (68.2) | 151 (67.7) | 27 (71.1) | |
| pN1 | 36 (13.8) | 34 (15.2) | 2 (5.3) | |
| pN2 | 47 (18.0) | 38 (17.0) | 9 (23.7) | |
| Adjuvant therapy | 0.058 | |||
| No | 121 (46.4) | 98 (43.9) | 23 (60.5) | |
| Yes | 140 (53.6) | 125 (56.1) | 15 (39.5) | |
| pTNM stage | 0.188 | |||
| I | 157 (60.2) | 131 (58.7) | 26 (68.4) | |
| II | 57 (21.8) | 53 (23.8) | 4 (10.5) | |
| III | 47 (18.0) | 39 (17.5) | 8 (21.1) |
P<0.05 was considered significant. NSCLC, non-small cell lung cancer; ADC, adenocarcinoma; SCC, squamous carcinoma; pT, pathological tumor stage; pN, pathological node stage; pTNM, tumor node metastasis; P.Y, package year.
Relationships between TSR and patients after lung resection
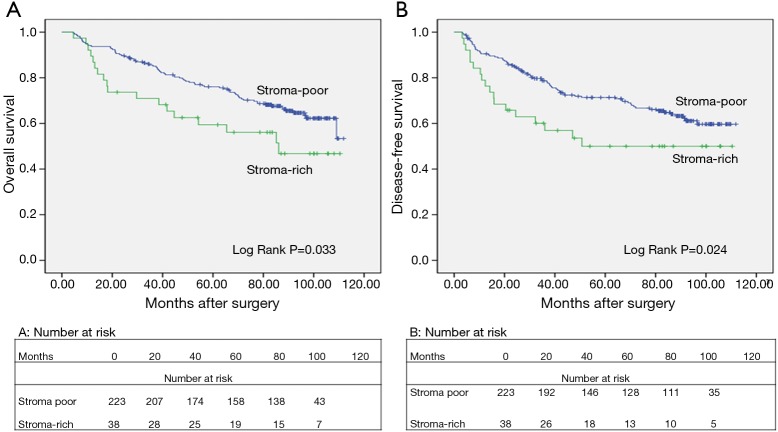
We analyzed the association between TSR, OS (log-rank P=0.033), and DFS (log rank P=0.024) using Kaplan-Meier curves (Figure 3). In all patients examined in the present study, the median OS was 86.07 months. The 5-year OS rate was 67.82%. In the stroma-rich and stroma-poor tumor groups, the 5-year OS was 50.00% and 70.85%, respectively. As shown in Tables 2 and 3, Cox univariate and multivariate models were applied to explore the association between clinicopathological characteristics and DFS and OS. The results of the Cox univariate analysis revealed the TSR, age, pT status, pN status and pTNM stage to be vital factors of DFS and OS. The HR of the TSR was 1.741 (95% CI, 1.040–2.913; P=0.035) for OS and 1.795 (95% CI, 1.073–3.005; P=0.026) for DFS. The results of the Cox multivariate model revealed the TSR, age, and pTNM stage to be significantly associated with OS and DFS. The HR of the TSR was 1.904 (95% CI, 1.132–3.202; P=0.015) for OS and 2.034 (95% CI, 1.210–3.420; P=0.007) for DFS. Thus, both Cox univariate and multivariate analyses revealed the TSR to be a crucial indicator of DFS and OS. In addition, we selected the cases of adenocarcinoma and used Kaplan-Meier curves to analyze the association between TSR, OS (log-rank P=0.001), and DFS (log rank P=0.001) in this study.
Figure 3.
Kaplan-Meier curves for the survival of 261 patients with NSCLC. (A) Kaplan-Meier curve for the OS of NSCLC; (B) Kaplan-Meier curve for the DFS of NSCLC. Patients with stroma-poor tumors showed significantly increased OS, and DFS compared to patients with stroma-rich tumors. NSCLC, non-small cell lung cancer; OS, overall survival; DFS, disease-free survival.
Table 2. Cox univariate and multivariate analysis for overall survival in 261 patients of NSCLC.
| Variables | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||
| TSR | 0.035 | 0.015 | |||
| Stroma-poor | Ref. | Ref. | |||
| Stroma-rich | 1.741 (1.040–2.913) | 1.904 (1.132–3.202) | |||
| Gender | 0.129 | ||||
| Women | Ref. | ||||
| Men | 1.408 (0.905–2.192) | ||||
| Smoking history (P.Y) | 0.152 | ||||
| <20 | Ref. | ||||
| ≥20 | 1.344 (0.897–2.014) | ||||
| Age (year) | 0.002 | 0.000 | |||
| ≤65 | Ref. | Ref. | |||
| >65 | 1.925 (1.283–2.890) | 2.570 (1.669–3.956) | |||
| Differentiation grade | 0.194 | ||||
| Well | Ref. | ||||
| Moderate | 1.342 (0.604–2.979) | 0.470 | |||
| Poor | 1.821 (0.818–4.056) | 0.142 | |||
| Histology | 0.259 | ||||
| ADC | Ref. | ||||
| SCC | 1.279 (0.834–1.961) | ||||
| pT status | 0.000 | ||||
| pT1 | Ref. | ||||
| pT2 | 1.554 (0.999–2.416) | 0.051 | |||
| pT3/4 | 4.057 (2.085–7.893) | 0.000 | |||
| pN status | 0.000 | ||||
| pN0 | Ref. | ||||
| pN1 | 1.565 (0.845–2.898) | 0.154 | |||
| pN2 | 4.423 (2.824–6.926) | 0.000 | |||
| Adjuvant therapy | 0.150 | ||||
| No | Ref. | ||||
| Yes | 1.354 (0.896–2.044) | ||||
| pTNM stage | 0.000 | 0.000 | |||
| I | Ref. | Ref. | |||
| II | 1.841 (1.091–3.107) | 0.022 | 1.729 (1.020–2.931) | 0.042 | |
| III | 4.484 (2.803–7.172) | 0.000 | 5.669 (3.479–9.236) | 0.000 | |
| Number of resected LNs | 0.992 | ||||
| <12 | Ref. | ||||
| ≥12 | 1.002 (0.626–1.605) | ||||
P<0.05 was considered significant. NSCLC, non-small cell lung cancer; TSR, tumor-stroma ratio; CI, confidence interval; HR, hazard ratio; ADC, adenocarcinoma; SCC, squamous carcinoma; pT, pathological tumor stage; pN, pathological node stage; pTNM, tumor node metastasis; P.Y, package year; LNs: lymph nodes.
Table 3. Cox univariate and multivariate analysis for disease-free survival in 261 patients of NSCLC.
| Variables | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||
| TSR | 0.026 | 0.007 | |||
| Stroma-poor | Ref. | Ref. | |||
| Stroma-rich | 1.795 (1.073–3.005) | 2.034 (1.210–3.420) | |||
| Gender | 0.110 | ||||
| Women | Ref. | ||||
| Men | 1.435 (0.922–2.233) | ||||
| Age (year) | 0.002 | 0.000 | |||
| ≤65 | Ref. | Ref. | |||
| >65 | 1.894 (1.262–2.844) | 2.309 (1.518–3.514) | |||
| Smoking history (P.Y) | 0.113 | ||||
| <20 | Ref. | ||||
| ≥20 | 1.387 (0.925–2.078) | ||||
| Differentiation grade | 0.211 | ||||
| Well | Ref. | ||||
| Moderate | 1.363 (0.614–3.023) | ||||
| Poor | 1.818 (0.817–4.048) | ||||
| Histology | 0.395 | ||||
| ADC | Ref. | ||||
| SCC | 1.204 (0.785–1.844) | ||||
| pT status | 0.000 | ||||
| pT1 | Ref. | ||||
| pT2 | 1.512 (0.973–2.352) | 0.066 | |||
| pT3/4 | 4.909 (2.516–9.578) | 0.000 | |||
| pN status | 0.000 | ||||
| pN0 | Ref. | ||||
| pN1 | 1.626 (0.879–3.008) | 0.122 | |||
| pN2 | 5.051 (3.210–7.947) | 0.000 | |||
| Adjuvant therapy | 0.129 | ||||
| No | Ref. | ||||
| Yes | 1.375 (0.911–2.073) | ||||
| pTNM stage | 0.000 | 0.000 | |||
| I | Ref. | Ref. | |||
| II | 1.927 (1.142–3.253) | 0.014 | 1.841 (1.088–3.115) | 0.023 | |
| III | 5.187 (3.231–8.327) | 0.000 | 6.150 (3.787–9.987) | 0.000 | |
| Number of resected LNs | 0.958 | ||||
| <12 | Ref. | ||||
| ≥12 | 1.013 (0.633–1.621) | ||||
P<0.05 was considered significant. NSCLC, non-small cell lung cancer; TSR, tumor-stroma ratio; CI, confidence interval; HR, hazard ratio; ADC, adenocarcinoma; SCC, squamous carcinoma; pT, pathological tumor stage; pN, pathological node stage; pTNM, tumor node metastasis; P.Y, package year; LNs, lymph nodes.
Discussion
In recent years, an increasing number of studies have emphasized the significance of the tumor microenvironment in cancer recurrence and progression, and the TSR has been reported as a predictive variable in previous studies and confirmed in the present study. Patients in the stroma-rich group had worse prognosis than those in the stroma-poor tumor group.
As a recently identified predictive variable, the TSR was associated with the prognosis of many solid tumors. Wang et al. (9) demonstrated that stroma-rich esophageal squamous cell carcinoma was associated with a poor prognosis. Zhang et al. (7) analyzed 93 patients with nasopharyngeal cancer and showed that a high TSR results in adverse survival. An analogous outcome could be verified in other tumors, such as carcinoma of colon (13-16), and early breast cancer (12). The outcomes of the present study pointed that the TSR, age, and pTNM stage were significantly associated with OS and DFS in univariate and multivariate survival analyses. However, the TSR was not correlated with gender, smoking history, age, differentiation grade, histology, pT status, pN status, adjuvant therapy or pTNM stage. Increased stromal proportion in NSCLC was also associated with poor prognosis and increased recurrence risk.
Carcinoma comprises tumor cells and the surrounding stroma, which originates from normal tissue. The stroma could serve as an obstacle in tumor formation by restraining the growth of tumor cell in normal tissue. However, in tumor tissue, the stroma portion can promote the course of tumor progression (17). Over the last decades, the role of the tumor stroma in tumor occurrence and progression had been emphasized. Tumor cells change the characteristics of stromal tissues by exchanging cytokines and enzymes with the surrounding stroma (18-20). This exchange could contribute to a microenvironment beneficial for the initiation, growth, and invasion of tumor (18). The interplay between tumor cells and stroma is significant in tumor progression. The stroma component primarily comprises endothelial cells, the basement membrane, the extracellular matrix, macrophages, and fibroblasts. Tumor cells encroaching on the mesenchyme by destroying the basement membrane, could ultimately induce the normal stroma-activated stroma transition. This process facilitates fibroblast proliferation and extracellular matrix deposition, thereby extending the tumor stroma (21).
In recent years, cancer-associated fibroblasts (CAFs) have drawn the attention of scholars as the main cellular components of the carcinoma microenvironment. Studies have shown that CAFs are associated with tumor proliferation, invasion, and progression via interactions with surrounding cancer cells through cytokine-mediated signal transmission and extracellular matrix remodeling (22). CAFs secrete various cytokines/growth factors, like proteases, transforming growth factor β, stroma cell-derived factor 1, etc. (23). Transforming growth factor-β plays a vital part in the evolution of the tumor microenvironment (24). Activated CAFs could secrete extracellular matrix metalloproteinase, which affects the progression of epithelial tumor by changing the components of the extracellular matrix. CAFs also regulate the recruitment of immune cells and their antitumor activity (25). The presence of a number of CAFs has been associated with the progression of malignant tumor and poor survival. In addition, macrophages are another key component of the tumor stroma. These cells secret angiogenic factors and produce matrix metalloproteinases, which facilitate matrix remodeling (26). Therefore, the inter-reactions between tumor cells and stroma tissue are complicated, and the stroma tissue should be regarded as an indispensable participant in the growth, invasion and progression of NSCLC.
The present study has some disadvantages. First, the research was retrospective and used a relatively small sample capacity. Second, the proportion of pulmonary squamous cell carcinoma patients was relatively low. It would be valuable to conduct a prospective study with a large sample in the future.
In general, the findings of the present study confirmed the TSR to be a new prognostic factor for NSCLC. Estimating the TSR is relatively easy during routine pathological examination, and reflecting its availability and low expense, the TSR is a candidate factor that can facilitate the evaluation of prognosis, enabling better identification of high-risk patients who undergo radical resection of pulmonary carcinoma. We frequently use therapeutic measures for cancer according to the clinical stage, differentiation grade, histology, etc. However, understanding the tumor stroma has the potential to provide new methods for cancer therapy, and together with the development of other therapeutic agents, this factor treatments may contribute to the development of individualized treatments for NSCLC in the future.
Acknowledgements
The authors would like to thank to the three pathologists Huang J, Jing J and Zhu C, for expert suggestions and for the careful assessment of the TSR in the present study.
Ethical Statement: The study was approved by Institute Research Medical Ethics Committee of Sun Yat-sen University Cancer Center (No. B2017-060-01) and written informed consent was obtained from all patients.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. 10.3322/caac.21338 [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- 3.Wu YL, Zhou Q. Clinical trials and biomarker research on lung cancer in China. Expert Opin Ther Targets 2012;16:S45-50. 10.1517/14728222.2011.630663 [DOI] [PubMed] [Google Scholar]
- 4.Woodard GA, Jones KD, Jablons DM. Lung Cancer Staging and Prognosis. Cancer Treat Res 2016;170:47-75. 10.1007/978-3-319-40389-2_3 [DOI] [PubMed] [Google Scholar]
- 5.Salmerón D, Chirlaque MD, Isabel IM, et al. Lung cancer prognosis in Spain: the role of histology, age and sex. Respir Med 2012;106:1301-8. 10.1016/j.rmed.2012.06.006 [DOI] [PubMed] [Google Scholar]
- 6.Kogure Y, Ando M, Saka H, et al. Histology and smoking status predict survival of patients with advanced non-small-cell lung cancer. Results of West Japan Oncology Group (WJOG) Study 3906L. J Thorac Oncol 2013;8:753-8. 10.1097/JTO.0b013e31828b51f5 [DOI] [PubMed] [Google Scholar]
- 7.Zhang XL, Jiang C, Zhang ZX, et al. The tumor-stroma ratio is an independent predictor for survival in nasopharyngeal cancer. Oncol Res Treat 2014;37:480-4. 10.1159/000365165 [DOI] [PubMed] [Google Scholar]
- 8.Liu J, Liu J, Li J, et al. Tumor-stroma ratio is an independent predictor for survival in early cervical carcinoma. Gynecol Oncol 2014;132:81-6. 10.1016/j.ygyno.2013.11.003 [DOI] [PubMed] [Google Scholar]
- 9.Wang K, Ma W, Wang J, et al. Tumor-stroma ratio is an independent predictor for survival in esophageal squamous cell carcinoma. J Thorac Oncol 2012;7:1457-61. 10.1097/JTO.0b013e318260dfe8 [DOI] [PubMed] [Google Scholar]
- 10.Chen Y, Zhang L, Liu W, et al. Prognostic Significance of the Tumor-Stroma Ratio in Epithelial Ovarian Cancer. Biomed Res Int 2015;2015:589301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lv Z, Cai X, Weng X, et al. Tumor-stroma ratio is a prognostic factor for survival in hepatocellular carcinoma patients after liver resection or transplantation. Surgery 2015;158:142-50. 10.1016/j.surg.2015.02.013 [DOI] [PubMed] [Google Scholar]
- 12.de Kruijf EM, van Nes JG, van de Velde CJ, et al. Tumor-stroma ratio in the primary tumor is a prognostic factor in early breast cancer patients, especially in triple-negative carcinoma patients. Breast Cancer Res Treat 2011;125:687-96. 10.1007/s10549-010-0855-6 [DOI] [PubMed] [Google Scholar]
- 13.Mesker WE, Junggeburt JM, Szuhai K, et al. The carcinoma-stromal ratio of colon carcinoma is an independent factor for survival compared to lymph node status and tumor stage. Cell Oncol 2007;29:387-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.West NP, Dattani M, McShane P, et al. The proportion of tumour cells is an independent predictor for survival in colorectal cancer patients. Br J Cancer 2010;102:1519-23. 10.1038/sj.bjc.6605674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huijbers A, Tollenaar RA, v Pelt GW, et al. The proportion of tumor-stroma as a strong prognosticator for stage II and III colon cancer patients: validation in the VICTOR trial. Ann Oncol 2013;24:179-85. 10.1093/annonc/mds246 [DOI] [PubMed] [Google Scholar]
- 16.Park JH, Richards CH, McMillan DC, et al. The relationship between tumour stroma percentage, the tumour microenvironment and survival in patients with primary operable colorectal cancer. Ann Oncol 2014;25:644-51. 10.1093/annonc/mdt593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lorusso G, Rüegg C. The tumor microenvironment and its contribution to tumor evolution toward metastasis. Histochem Cell Biol 2008;130:1091-103. 10.1007/s00418-008-0530-8 [DOI] [PubMed] [Google Scholar]
- 18.Hemmings C. Is carcinoma a mesenchymal disease? The role of the stromal microenvironment in carcinogenesis. Pathology 2013;45:371-81. 10.1097/PAT.0b013e328360b600 [DOI] [PubMed] [Google Scholar]
- 19.Curry JM, Sprandio J, Cognetti D, et al. Tumor microenvironment in head and neck squamous cell carcinoma. Semin Oncol 2014;41:217-34. 10.1053/j.seminoncol.2014.03.003 [DOI] [PubMed] [Google Scholar]
- 20.Chung HW, Lim JB. Role of the tumor microenvironment in the pathogenesis of gastric carcinoma. World J Gastroenterol 2014;20:1667-80. 10.3748/wjg.v20.i7.1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shekhar MP, Pauley R, Heppner G. Host microenvironment in breast cancer development: extracellular matrix-stromal cell contribution to neoplastic phenotype of epithelial cells in the breast. Breast Cancer Res 2003;5:130-5. 10.1186/bcr580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Udagawa T, Wood M. Tumor-stromal cell interactions and opportunities for therapeutic intervention. Curr Opin Pharmacol 2010;10:369-74. 10.1016/j.coph.2010.06.010 [DOI] [PubMed] [Google Scholar]
- 23.Erez N, Truitt M, Olson P, et al. Cancer-Associated Fibroblasts Are Activated in Incipient Neoplasia to Orchestrate Tumor-Promoting Inflammation in an NF-kappaB-Dependent Manner. Cancer Cell 2010;17:135-47. 10.1016/j.ccr.2009.12.041 [DOI] [PubMed] [Google Scholar]
- 24.Gupta DK, Singh N, Sahu DK. TGF-β Mediated Crosstalk Between Malignant Hepatocyte and Tumor Microenvironment in Hepatocellular Carcinoma. Cancer Growth Metastasis 2014;7:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balsamo M, Scordamaglia F, Pietra G, et al. Melanoma-associated fibroblasts modulate NK cell phenotype and antitumor cytotoxicity. Proc Natl Acad Sci U S A 2009;106:20847-52. 10.1073/pnas.0906481106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gong Y, Chippada-Venkata UD, Oh WK. Roles of matrix metalloproteinases and their natural inhibitors in prostate cancer progression. Cancers (Basel) 2014;6:1298-327. 10.3390/cancers6031298 [DOI] [PMC free article] [PubMed] [Google Scholar]