Abstract
Background
Gene analysis could not be performed in all patients, especially in advanced non-small cell lung cancer (NSCLC). We aimed to find some clinical futures and CT or FDG-PET characteristics, which could be combined to help distinguish anaplastic lymphoma kinase (ALK) rearrangement form epidermal growth factor receptor (EGFR) mutations in treatment naïve advanced lung adenocarcinoma of Chinese patients.
Methods
We retrospectively reviewed clinical and radiological characteristics of 145 patients with treatment naïve advanced lung adenocarcinoma. The one-way ANOVA, the Mann-Whitney test, chi-square test and logistic regression were used for comparison between patients with ALK rearrangement and those with EGFR mutation.
Results
Among 145 patients with advanced lung adenocarcinoma, only six patients had both ALK rearrangement and EGFR mutation, the sample size was too small to analysis. Univariate analysis revealed that patients with ALK rearrangement were younger (P=0.001) and with lower serum carcinoembryonic antigen (CEA) level (P=0.008) than those with EGFR mutation. More of tumors with ALK rearrangement were well defined (P=0.023) and have bubble lucency (P=0.026) compared with those with EGFR mutation (P=0.026). Lymphadenopathy was seen more frequently in patients with ALK rearrangement (P=0.167). Twenty-six patients received FDG-PET/CT, among this population, lesion standardized uptake values (SUV) >6.95 and lymph nodes SUVmax >6.25 were more often seen in ALK rearrangement group (P=0.011, both). In multivariate analysis, patients younger than 50 years (RR =9.878, 95% CI: 2.318–42.090, P=0.002), with lower CEA level than 4.95 µg/L (RR =8.166, 95% CI: 1.085–31.983, P=0.003) and without brain metastasis (RR =7.304, 95% CI: 1.099–48.558, P=0.040) were more likely to be ALK rearrangement than EGFR mutation. Tumor diameter less than 36 mm were prone to be EGFR mutation (RR =0.078, 95% CI: 0.017–0.356, P=0.001).
Conclusions
Treatment naïve advanced lung adenocarcinomas with ALK rearrangement were more likely to have younger age, lower serum CEA level, larger tumor volume, well defined tumor border, and non-brain metastasis than those with EGFR mutation. Bubble lucency and higher FDG uptake of lesion and lymph nodes may help distinguish ALK rearrangement from EGFR mutation in the absence of genetic analysis.
Keywords: High-resolution computed tomography (HRCT); anaplastic lymphoma kinase (ALK); epidermal growth factor receptor (EGFR), advanced non-small cell lung cancer (NSCLC)
Introduction
Non-small cell lung cancer (NSCLC) accounts for a large proportion of lung carcinoma, which is one of the major causes of cancer death worldwide (1). During the past decade, adenocarcinoma has been the most common pathological type and the International Association for the Study of Lung Cancer (IASLC)/American Thoracic Society (ATS)/European Respiratory Society (ERS) published a new classification for lung adenocarcinoma in 2011 Feb (2,3). In this classification, guidance for small biopsies in advanced stage patients was also provided (2). It recommends gene test for epidermal growth factor receptor (EGFR) mutation in adenocarcinoma, because these mutations are related to high response to EGFR tyrosine kinase inhibitors (EGFR-TKI) (4,5). While Soda et al. identified anaplastic lymphoma kinase (ALK) gene rearrangement as another diagnostic marker and therapeutic target in 2007 (6). The fusion gene comprised the echinoderm microtubule-associated protein-like 4 (EML4) gene and ALK gene is occurring in 2–7% of NSCLC and 8–22% of light or never smokers suffered from lung cancer (7,8). ALK rearrangement targeting therapies have been investigated recent years. In a phase 3 study, crizotinib is found to be superior to standard chemotherapy in advanced NSCLC with ALK rearrangement (9). However, due to old age, serious diseases or other reasons, not every patient could undergo EGFR and ALK gene analysis. Although liquid biopsy has developed rapidly recent years, it is expensive and has not been popularized in clinic. It is important to find some specific clinical and radiologic characteristics to distinguish adenocarcinoma with ALK rearrangement from those with EGFR mutation.
Previous studies have attempted to investigate the clinicopathologic and radiogenomic features which could predict ALK rearrangement NSCLCs. Although a lot of studies found ALK rearrangement was highly occurred in younger, female and never smoked adenocarcinomas, it was overlapped with EGFR mutated patients on clinical profiles (10-12). Fortunately, some radiologic characteristics of ALK rearrangement were detected (13-16). Nakada et al. (14) reported that ALK rearrangement lesions were smaller and had a low tumor disappearance rate than EGFR mutated lesions on high-resolution computed tomography (HRCT). However, another study discovered ALK rearrangement positive lung adenocarcinomas were more likely to be large and with thoracic lymphadenopathy (15). A nearly research suggests that patents with ALK rearrangement is younger, and characterized by lobulated margin and solid lesion compared with EGFR mutant and wide type cohorts in surgically resected NSCLCs (16). These conclusions were mostly studied in early stage NSCLCs, and may remain some uncertainties.
However, a meta-analysis pooled 27 retrospective studies revealed that EML4-ALK was more common in adenocarcinoma lacking EGFR and KRAS mutation, never smoked and advanced NSCLC (17). Recently a retrospective study found that adenocarcinomas with ALK rearrangement were more likely to be solid masses and with lymph node metastasis or pleural metastasis compared with those with EGFR mutations (18). Nevertheless few investigations of ALK rearrangement and EGFR mutation have been conducted in advanced NSCLCs. Furthermore most of the patients who could not undergo EGFR and ALK gene analysis are first diagnosed as advanced lung cancer. We conducted this retrospective study attempted to investigate some clinical and radiologic differences between advanced NSCLCs with ALK rearrangement and those with EGFR mutations. The aim of our study was to find a few clinical futures and CT or FDG-PET characteristics, which could be combined to help distinguish ALK rearrangement form EGFR mutations in treatment naïve advanced lung adenocarcinoma of Chinese patients.
Methods
Patients
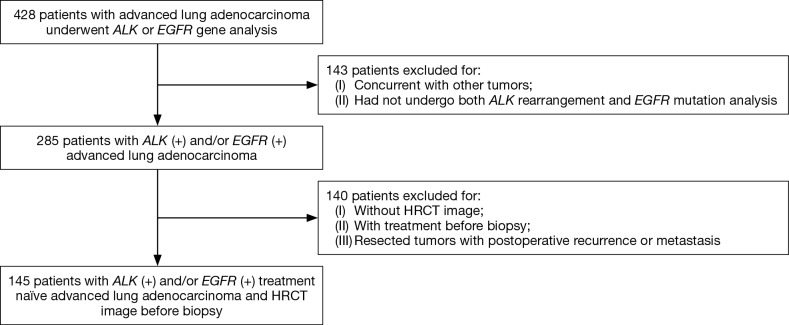
This retrospective study reviewed the clinical profiles, HRCT images, and FDG-PET features in patients with treatment naïve advanced lung adenocarcinoma (clinical stage IIIB or IV). These patients underwent small biopsies, both ALK rearrangement and EGFR mutation analysis in Jinling Hospital (Jiangsu, China) from January 2013 to December 2015. A total of 428 patients were identified. Eligible criteria included: 18 years or older; stage IIIB/IV NSCLC with percutaneous core needle and/or bronchoscopic biopsy diagnosed as adenocarcinoma; with ALK rearrangement and/or EGFR mutation, had not underwent any treatment (chemotherapy, radiation therapy, or immunologic therapy); performed chest HRCT before lung biopsy in our institution. Finally, 145 patients (91 female and 54 male; age range, 27–78 years; average age, 57.5 years) were included. In addition, patients concurrent with other tumors were excluded (Figure 1). This study design was approved by the Ethics Committee of Jinling Hospital. The requirement for informed consent was waived.
Figure 1.
Selection process for untreated advanced lung adenocarcinoma with ALK rearrangement and/or EGFR mutation and HRCT image. ALK, anaplastic lymphoma kinase; EGFR, epidermal growth factor receptor; HRVT, high-resolution computed tomography.
HRCT and FDG-PET evaluation
All studies were performed using a multi-slice CT scanner system (Somatom Sensation 64, Siemens, Erlangen, Germany). Scanning parameters were as following: tube voltage 120 kVp, tube current 150–200 mA, rotation time 0.5 s, and 2 mm reconstruction thickness with a 1 mm reconstruction interval. Two radiologists with more than 5 years of experience retrospectively interpreted the HRCT images independently. They were blinded to the pathological findings. When there were different opinions of the CT images, these two radiologists would discuss and reach a final consensus. All of the main HRCT characteristics were shown in Table 1. In Table 1, “well-defined borders” means the boundary between the nodule and the lung tissue is clearly defined and outlined as a pencil; “poorly-defined borders” means the boundary between the nodule and the lung tissue is not clear and cannot be completely outlined with a thin nodule contour; “bubble lucency” means bronchus encapsulated air sign and cavity.
Table 1. Univariate analysis of HRCT characteristics and driver mutations.
| HRCT characteristics | Number | Genetic alteration | ALK+ vs. EGFR+ | ||||
|---|---|---|---|---|---|---|---|
| ALK+ and EGFR+ | ALK+ | EGFR+ | χ2/F | P value | |||
| All patients [n (%)] | 145 | 6 [4] | 27 [19] | 112 [77] | – | – | |
| Diameter (mm)* | 7–120 | 12–75 | 7–120 | 10–102 | – | 0.549* | |
| Diameter (mm) | 145 | – | – | – | 2.129 | 0.198 | |
| ≤36 | 73 | 4 | 10 | 59 | – | – | |
| >36 | 72 | 2 | 17 | 53 | – | – | |
| Shape | 145 | – | – | – | 0.469 | 0.493 | |
| Round | 72 | 2 | 12 | 58 | – | – | |
| Irregular | 73 | 4 | 15 | 54 | – | – | |
| Border definition | 145 | – | – | – | 5.143 | 0.023 | |
| Poorly defined | 92 | 4 | 12 | 76 | – | – | |
| Well defined | 53 | 2 | 15 | 36 | – | – | |
| Margin | 145 | – | – | – | 1.250 | 0.262 | |
| Smooth | 5 | 0 | 0 | 5 | – | – | |
| Spiculation or lobulation | 140 | 6 | 27 | 107 | – | – | |
| Air bronchogram | 145 | – | – | – | 0.469 | 0.493 | |
| − | 72 | 2 | 12 | 58 | – | – | |
| + | 73 | 4 | 15 | 54 | – | – | |
| Bubble lucency | 145 | 4.959 | 0.026 | ||||
| − | 119 | 6 | 26 | 87 | – | – | |
| + | 26 | 0 | 1 | 25 | – | – | |
| Vessel convergence sign | 145 | – | – | – | 1.789 | 1.818 | |
| − | 47 | 1 | 6 | 40 | – | – | |
| + | 98 | 5 | 21 | 72 | – | – | |
| Pleural retraction | 145 | – | – | – | 2.734 | 0.098 | |
| − | 32 | 2 | 9 | 21 | – | – | |
| + | 113 | 4 | 18 | 91 | – | – | |
| Pleural thickening | 145 | – | – | – | 1.426 | 0.232 | |
| − | 89 | 3 | 14 | 72 | – | – | |
| + | 56 | 3 | 13 | 40 | – | – | |
| PLC | 145 | – | – | – | 0.150 | 0.699 | |
| − | 132 | 6 | 25 | 101 | – | – | |
| + | 13 | 0 | 2 | 11 | – | – | |
| Hydrothorax | 145 | – | – | – | 0.163 | 0.687 | |
| − | 91 | 3 | 18 | 70 | – | – | |
| + | 54 | 3 | 9 | 42 | – | – | |
| Lymphadenopathy | 145 | – | – | – | 1.911 | 0.167 | |
| − | 25 | 3 | 4 | 31 | – | – | |
| + | 120 | 3 | 23 | 81 | – | – | |
| Enhancement | 125 | – | – | – | 0.204 | 0.652 | |
| − | 2 | 1 | 0 | 1 | – | – | |
| + | 123 | 5 | 20 | 98 | – | – | |
*, diameter (mm) analysis used the Mann-Whitney test, others used chi-square test. +, positive; −, negative; HRVT, high-resolution computed tomography; PLC, pulmonary lymphangitic carcinomatosis; ALK, anaplastic lymphoma kinase; EGFR, epidermal growth factor receptor.
Only 26 of the included patients underwent integrated FDG-PET/CT before any clinical treatment. 18F-FDG was administered intravenously 1 hour prior to PET/CT scans. Fluorodeoxyglucose-positron emission tomography data was analyzed using standardized uptake values (SUV). We used trans-axial images for analysis. SUVs were calculated as decay-corrected activity (kBq) per milliliter of tissue volume divided by injected dose per weight (kBp/g). SUVmax of lymph nodes was measured after placing a region of interest over all masses.
ALK rearrangement and EGFR mutation analysis
DNA was extracted from five pieces of formalin-fixed, paraffin-embedded (FFPE) tumor tissue using the QIAamp FFPE Tissue Kit. Molecular analysis of mutation status of EGFR exons 18, 19, 20, and 21 was examined with Human EGFR Gene Mutations Detection Kit (AmoyDx, Xiamen, China), which is a polymerase chain reaction (PCR)—based amplification-refractory mutation system (ARMS). ALK-EML4 gene rearrangements were detected by VENTANA immunological histological chemistry (IHC) kits [ALK (D5F3), VENTANA, Roche].
Statistics
Continuous variables were compared using the one-way ANOVA or the Mann-Whitney test. Categorical variables were analyzed by chi-square test or Fisher’s exact test. Before compare the average diameter, serum carcinoembryonic antigen (CEA) and SUVmax values of tumors with ALK rearrangement and those with EGFR mutation, we calculated optimal cut-off values using a receiver operating characteristic (ROC)-based positive test with ALK rearrangement as categorical variables. Then the tumor diameters, serum CEA and SUVmax values of tumors were dichotomized as ≤ optimal cut-off value and > optimal cut-off value.
To investigate independent factors that may help distinguish tumors with ALK rearrangement from those with EGFR mutation, we performed multivariate logistic regression analysis. Variables were selected by a forward stepwise selection mode before multivariate analysis. Values of P<0.05 were considered statistically significant. We used Statistical Product and Service Solutions (SPSS) version 20.0 (SPSS Inc., Chicago, IL, USA) to conduct statistical analysis.
Results
Patient characteristics
A total of 145 patients were enrolled in this study. Their clinical characteristics are presented in Table 2. All of the patients were clinically diagnosed as advanced lung adenocarcinoma: IIIB stage in 7 (5%) patients and IV in 138 (95%) patients. Most patients were female (63%) and non-smoker (76%). A majority of the patients were more than 50 years old (79%) with a median age of 57.5 years (range, 27–78 years). Family tumor history was identified rarely (8%). More of the tumor located in right lung (55%). ALK rearrangement was positive in 33 (23%) patients and 118 (81%) patients were EGFR mutated. Among tumors with EGFR mutation, 57 (39%) had L858R mutation, 52 (36%) had 19-Del mutation, and 9 (6%) had other mutations (including Exon21 L861Q, Exon18 G719X, Exon20 S768I, L858R/T790M, L858R/19-Del, 19-Del/T790M and 19-Del/S768I). Tumors with both ALK rearrangement and EGFR mutation were happened in only six patients. No patient received chemotherapy, radiation therapy, or immunologic therapy.
Table 2. Clinical characteristics of patients with advanced lung adenocarcinoma.
| Characteristics | Overall (n=145), [%] or [range] |
|---|---|
| Sex | |
| Female | 91 [63] |
| Male | 54 [37] |
| Age (years) | |
| <50 | 30 [21] |
| ≥50 | 115 [79] |
| Median | 57.50±10.04 [27–78] |
| Smoking history | |
| No | 110 [76] |
| Yes | 35 [24] |
| Family tumor history | |
| No | 133 [92] |
| Yes | 12 [8] |
| Tumor location | |
| RUL | 35 [24] |
| RML | 7 [5] |
| RLL | 37 [26] |
| LUL | 35 [24] |
| LLL | 31 [21] |
| Tumor stage | |
| T1 or T2 | 32 [22] |
| T3 or T4 | 113 [78] |
| Node stage | |
| N0 or N1 | 23 [16] |
| N2 or N3 | 122 [84] |
| Metastasis | |
| 0 | 7 [5] |
| 1a | 47 [32] |
| 1b | 91 [63] |
| Clinical stage | |
| IIIB | 7 [5] |
| IV | 138 [95] |
| ALK-EML4 | |
| + | 33 [23] |
| − | 112 [77] |
| EGFR status | |
| EGFR+ | 118 [81] |
| L858R | 57 [39] |
| 19-Del | 52 [36] |
| Others# | 9 [6] |
| EGFR− | 27 [19] |
#, other EGFR mutations including: Exon21 L861Q, Exon18 G719X, Exon20 S768I, L858R/T790M, L858R/19-Del, 19-Del/T790M, and 19-Del/S768I. RUL, right upper lobe; RML, right middle lobe; RLL, right lower lobe; LUL, left upper lobe; LLL, left lower lobe; ALK, anaplastic lymphoma kinase; EML4, echinderm microtubule-associated protein-like 4; EGFR, epidermal growth factor receptor.
Clinical features
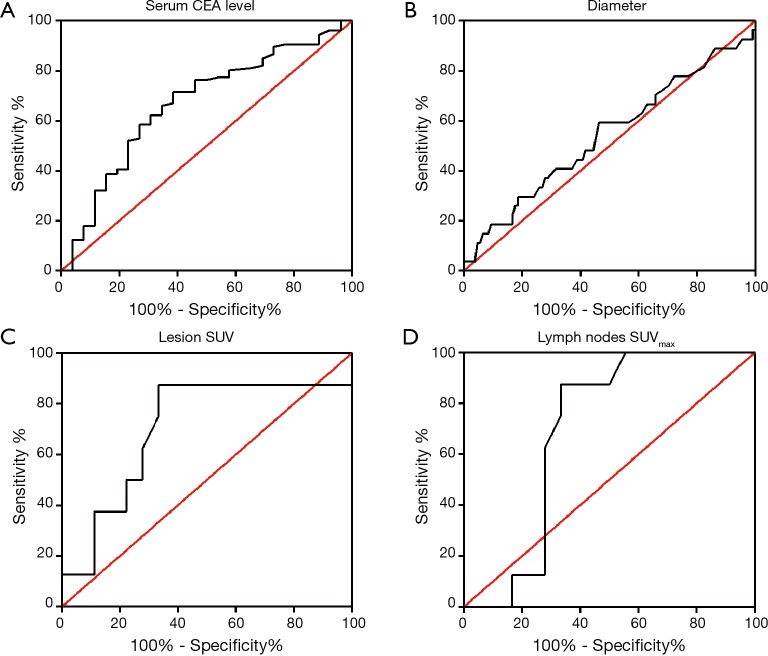
Although there were six patients with both ALK rearrangement and EGFR mutation, the sample size was too small to analysis. We just compared ALK rearrangement positive group and EGFR mutation positive group. Significant differences were only found in age and serum CEA level (Table 3). Patients with ALK rearrangement were younger (51.85±10.37) than those with EGFR mutation (58.98±9.42, P=0.001). EGFR mutation was more likely to be positive in patients older than 50 years old (P=0.015). ALK rearrangement had no preference in sex (female 48% vs. male 52%), while EGFR mutation was seen more frequently in female (65%), however there was no significant difference. Serum CEA level had significant difference in two driver mutations (P=0.005). We conducted ROC (ROC) analysis to determine the cut-off value for CEA level (Figure 2A). Patients were divided into two groups: CEA level less or equal than 4.95 µg/L and CEA level more than 4.95 µg/L. Patients with ALK rearrangement were more likely to have lower serum CEA level, while those with EGFR mutation were mostly with CEA level more than 4.95 µg/L (P=0.008). Although no statistically significant difference was found, tumors with ALK rearrangement had higher rate of pleura metastasis (52% vs. 35%) and pericardial metastasis (15% vs. 9.8%), lower rate of lung metastasis (52% vs. 67%), bone metastasis (44% vs. 55%) and brain metastasis (15% vs. 29%) compared with those with EGFR mutations. There was also no significant difference in smoking history, family tumor history, clinical stage, clinical symptom and liver metastasis between patients with ALK rearrangement and EGFR mutations (Table 3).
Table 3. Univariate analysis of clinical characteristics and driver mutations.
| Clinical characteristics | Number | Genetic alteration | ALK+ vs. EGFR+ | ||||
|---|---|---|---|---|---|---|---|
| ALK+ and EGFR+ | ALK+ | EGFR+ | χ2/F | P value | |||
| All patients [n (%)] | 145 | 6 [4] | 27 [19] | 112 [77] | – | – | |
| Median age* | 57.50±10.04 [27–78] | 55.33±12.09 | 51.85±10.37 | 58.98±9.42 | 11.976 | 0.001* | |
| Age (years) | 145 | – | – | – | 5.945 | 0.015 | |
| <50 | 30 | 2 | 10 | 18 | – | – | |
| ≥50 | 115 | 4 | 17 | 94 | – | – | |
| Sex | 145 | – | – | – | 2.675 | 0.102 | |
| Female | 91 | 5 | 13 | 73 | – | – | |
| Male | 54 | 1 | 14 | 39 | – | – | |
| Smoking history | 145 | – | – | – | 0.789 | 0.374 | |
| Yes | 35 | 0 | 5 | 30 | – | – | |
| No | 110 | 6 | 22 | 82 | – | – | |
| Family tumor history | 145 | – | – | – | 0.470 | 0.493 | |
| Yes | 12 | 1 | 3 | 8 | – | – | |
| No | 133 | 5 | 24 | 104 | – | – | |
| Clinical stage | 145 | – | – | 2.586 | 0.108 | ||
| IIIB | 7 | 0 | 3 | 4 | – | – | |
| IV | 138 | 6 | 24 | 108 | – | – | |
| Clinical symptom | 145 | – | – | – | 0.665 | 0.717 | |
| No | 20 | 1 | 3 | 16 | – | – | |
| Pulmonary symptom | 101 | 5 | 18 | 78 | – | – | |
| Extrapulmonary symptom | 24 | 0 | 6 | 18 | – | – | |
| Serum CEA level (μg/L) | 0.7–1,123 | 1.1–156.6 | 0.7–1,123 | 0.8–1,010 | – | 0.005* | |
| Serum CEA (μg/L) | 141 | – | – | – | 7.094 | 0.008 | |
| ≤4.95 | 48 | 3 | 14 | 31 | – | – | |
| >4.95 | 93 | 3 | 11 | 79 | – | – | |
| Pleura metastasis | 145 | – | – | – | 2.675 | 0.102 | |
| − | 89 | 3 | 13 | 73 | – | – | |
| + | 56 | 3 | 14 | 39 | – | – | |
| Pericardial metastasis | 145 | – | – | – | 0.563 | 0.453 | |
| − | 129 | 5 | 23 | 101 | – | – | |
| + | 16 | 1 | 4 | 11 | – | – | |
| Lung metastasis | 145 | – | – | – | 2.157 | 0.142 | |
| − | 53 | 3 | 13 | 37 | – | – | |
| + | 92 | 3 | 14 | 75 | – | – | |
| Bone metastasis | 145 | – | – | – | 1.041 | 0.308 | |
| − | 71 | 6 | 15 | 50 | – | – | |
| + | 74 | 0 | 12 | 62 | – | – | |
| Brain metastasis | 145 | – | – | – | 2.145 | 0.143 | |
| − | 107 | 4 | 23 | 80 | – | – | |
| + | 38 | 2 | 4 | 32 | – | – | |
| Liver metastasis | 145 | – | – | – | 0.037 | 0.847 | |
| − | 126 | 6 | 23 | 97 | – | – | |
| + | 19 | 0 | 4 | 15 | – | – | |
*, median age analysis used the one-way ANOVA analysis, serum CEA level analysis used the Mann-Whitney test, and others used chi-square test. +, positive; −, negative; CEA, carcinoembryonic antigen; ALK, anaplastic lymphoma kinase; EGFR, epidermal growth factor receptor.
Figure 2.
ROC curve for clinical and radiological features. (A) Serum CEA level; (B) tumor diameter; (C) lesion SUV; (D) lymph nodes SUVmax. ROC, receiver operating characteristic; CEA, carcinoembryonic antigen. SUV, standard uptake value.
HRCT and FDG-PET features
We conducted the Mann-Whitney test or chi-square test to find some differences among ALK rearrangement and EGFR mutation groups (Table 1). Tumor diameter had no significant difference in these two groups (P=0.549). Then we used ROC analysis to determine the cut-off value for tumor diameter and the calculated cut-off value was 36 mm (Figure 2B). There was still no significant difference (P=0.198) when divided into less or equal than 36 mm group and more than 36 mm group, but tumors with ALK rearrangement had higher rate in diameter more than 36 mm than those with EGFR mutation. Most of the tumor borders with EGFR mutation were poorly defined, while more of those with ALK rearrangement were well defined (P=0.023). In addition, tumors with ALK rearrangement were more likely to have bubble lucency than those with EGFR mutation (P=0.026). Lymphadenopathy was seen more frequently in patients with ALK rearrangement (P=0.167). We did not find statistically significant difference in tumor shape, margin, air bronchogram, vessel convergence sign, pleural retraction, pleural thickening, pulmonary lymphangitic carcinomatosis (PLC), hydrothorax, and enhancement (Table 1).
Twenty-six patients also received FDG-PET/CT in our institution. Among these patients, eight were ALK rearrangement positive and 18 were EGFR mutation positive. We pooled their lesion FDG uptake and lymph nodes FDG uptake in Table 4. The cut-off values of lesion SUV and lymph nodes SUVmax were calculated using ROC analysis (Figure 2C,D). The median SUV of lesions with ALK rearrangement and EGFR mutation was 10.79±6.57 and 7.78±5.61, respectively, however there was no significant difference (P=0.243). The median SUVmax of lymph nodes with ALK rearrangement and EGFR mutation was 7.56±1.92 and 5.56±5.00, respectively, and there was also no significant difference (P=0.309). Then we dichotomized the patients with cut-off value of lesion SUV 6.95 and lymph nodes SUVmax 6.25. Patients with lesion SUV >6.95 and lymph nodes SUVmax >6.25 were more frequently seen in ALK rearrangement group than EGFR mutation group (P=0.011, both).
Table 4. Univariate analysis of PET/CT characteristics and driver mutations.
| PET/CT characteristics | Number | Driver mutations | |||
|---|---|---|---|---|---|
| ALK+ | EGFR+ | χ2/F | P value | ||
| All patients [n (%)] | 26 | 8 [31] | 18 [69] | – | – |
| Lesion FDG uptake | – | – | – | – | – |
| − | 0 | 0 | 0 | – | – |
| + | 26 | 8 | 18 | – | – |
| Lesion SUV | 8.71±5.95* | 10.79±6.57* | 7.78±5.61* | 1.434 | 0.243 |
| ≤6.95 | 13 | 1 | 12 | 6.500 | 0.011 |
| >6.95 | 13 | 7 | 6 | – | – |
| Lymph nodes FDG uptake | – | – | – | 2.101 | 0.147 |
| − | 4 | 0 | 4 | – | – |
| + | 22 | 8 | 14 | – | – |
| Lymph nodes SUVmax | 6.24±4.34* | 7.56±1.92* | 5.65±5.00* | 1.079 | 0.309 |
| Lymph nodes SUVmax | – | – | – | 6.500 | 0.011 |
| ≤6.25 | 13 | 1 | 12 | – | – |
| >6.25 | 13 | 7 | 6 | – | – |
*, the mean and standard deviation of SUV. +, positive; −, negative. FDG, 18F-FDG; SUV, standard uptake value; ALK, anaplastic lymphoma kinase; EGFR, epidermal growth factor receptor.
Multivariable analysis
We conducted the multivariate analysis referenced to EGFR mutation group to pool influence of both clinical variables and HRCT features. Five features were found to have significant difference in this logistic regression model (Table 5). These independent prognostic factors to distinguish ALK rearrangement from EGFR mutation were age, serum CEA, tumor diameter, border definition, and brain metastasis. Patients younger than 50 years (RR=9.878, 95% CI: 2.318–42.090, P=0.002), with lower CEA level than 4.95 µg/L (RR=8.166, 95CI%: 1.085–31.983, P=0.003) and without brain metastasis (RR=7.304, 95% CI: 1.099‒48.558, P=0.040) were more likely to be ALK rearrangement than EGFR mutation. Tumor diameter less than 36 mm were prone to be EGFR mutation (RR=0.078, 95% CI: 0.017–0.356, P=0.001). The border definition of tumors with ALK rearrangement was preferred to be well defined (RR=4.902, 95% CI: 1.222–19.662, P=0.025).
Table 5. Multivariate analysis of differences in clinical/HRCT characteristics between patients with ALK rearrangement and those with EGFR mutation.
| Clinical/HRCT characteristics | P value | RR | 95% CI |
|---|---|---|---|
| Age (years) | |||
| <50 | 0.002 | 9.878 | 2.318–42.090 |
| ≥50 | Reference* | – | – |
| CEA (μg/L) | |||
| ≤4.95 | 0.003 | 8.166 | 1.085–31.983 |
| >4.95 | Reference* | – | – |
| Diameter (mm) | |||
| ≤36 | 0.001 | 0.078 | 0.017–0.356 |
| >36 | Reference* | – | – |
| Border definition | |||
| Well defined | 0.025 | 4.902 | 1.222–19.662 |
| Poorly defined | Reference* | – | – |
| Brain metastasis | |||
| − | 0.040 | 7.304 | 1.099–48.558 |
| + | Reference* | – | – |
*, the reference category is: EGFR+ group. +, positive; −, negative; RR, relative risk; CI, confidence interval; HRVT, high-resolution computed tomography; ALK, anaplastic lymphoma kinase; EGFR, epidermal growth factor receptor.
Discussion
Our study retrospectively reviewed 145 patients with untreated advanced adenocarcinoma in East Asian Chinese population. We investigated the clinical, HRCT, and FDG-PET features between tumors with ALK rearrangement and EGFR mutations.
Patients with ALK rearrangement were younger than those with EGFR mutation. EGFR mutations were more likely to be positive in patients older than 50 years old. These results were consistent with previous studies in NSCLC (10,15,18). CEA is an important serum tumor marker; recent studies have found that patients with ALK rearrangement were seen more frequently to have normal serum CEA level in lung adenocarcinoma (19,20). However the study populations of these retrospective investigations were most with early clinical stage. In our study, we found that advanced lung adenocarcinomas with ALK rearrangement were more likely to have lower serum CEA level, while those with EGFR mutation were mostly with CEA level more than 4.95 µg/L. These clinical features may be helpful in distinguishing ALK rearrangement from EGFR mutation in the absence of genetic analysis.
Unfortunately, there was no significant difference in sex, smoking history, family tumor history, clinical stage, clinical symptom and liver metastasis between patients with ALK rearrangement and EGFR mutations. Both of the groups were prone to be non-smokers, which was consistent with previous studies (20-23). No statistically significant difference was found in tumor metastasis, but the tendencies were agree with Choi et al. (18). Tumors with ALK rearrangement had higher rate of pleura metastasis and pericardial metastasis, lower rate of lung metastasis, bone metastasis and brain metastasis compared with those with EGFR mutations (Table 3).
Our study identified several HRCT characteristics, among which only border definition and bubble lucency had significant difference. Tumors with ALK rearrangement were more likely to have well defined border and bubble lucency. Wang et al. demonstrated five CT characteristics had significant difference between ALK rearrangement and EGFR mutation, including tumor size, ground-glass opacity (GGO), bubble-like lucency, lymphadenopathy, and tumor shadow disappearance rate (24). However, in this research, we did not find significant difference in tumor diameter and lymphadenopathy, and GGO was not seen in all of the ALK rearrangement population. When we dichotomized the patients with cut-off value of diameter, there was still no significant difference. Whereas the tendency was in agreement, tumors with ALK rearrangement had higher rate in diameter more than 36 mm than those with EGFR mutation. Lymphadenopathy was also seen more frequently in patients with ALK rearrangement. And among the 26 patients received FDG-PET/CT in our institution, lesion FDG uptake and lymph nodes FDG uptake were higher in those with ALK rearrangement than with EGFR mutations. Patients with lesion SUV >6.95 and lymph nodes SUVmax >6.25 were more frequently seen in ALK rearrangement group than EGFR mutation group. These FDG-PET evaluations were keeping with the results of previous study (25).
In the logistic regression model, we found five independent prognostic factors to distinguish tumors with ALK rearrangement from those with EGFR mutation. Patients younger than 50 years and with lower CEA level than 4.95 µg/L were more prone to be ALK rearrangement than EGFR mutation. Tumor diameters less than 36 mm were likely to be EGFR mutation. The border definition of tumors with ALK rearrangement was preferred to be well defined. Tumors with ALK rearrangement were less seen to have brain metastasis. Our results were consistent with several previous studies (18,20,24,25).
There were also some limitations in our study. The present study was a retrospective study and was conducted in a single large institution. Patient number was relatively small for our strict inclusion criteria and low rate of ALK rearrangement. Some cases were also excluded for not available HRCT image. There should be a large sample prospective study to validate these results. It was not impossible to analyze clinical and HRCT features according to histopathologic subtypes of invasive adenocarcinoma for limited small biopsy. Most of the patients did not undergo KRAS, ROS1, or c-MET gene analysis; this may have some influence of the results. We could not get complete prognostic information now, because our study reviewed patients from January 2013 to December 2015. We look forward to confirm these results in advanced lung adenocarcinoma prognosis by follow-up research.
Conclusions
In summary, we demonstrated that patients with younger age, lower serum CEA level, large tumor volume, well defined tumor border, and non-brain metastasis are more likely to be ALK rearrangement positive than EGFR mutation positive in treatment naïve advanced lung adenocarcinoma. Other features such as bubble lucency and higher FDG uptake of lesion and lymph nodes may help distinguish advanced lung adenocarcinoma with ALK rearrangement from those with EGFR mutation in the absence of genetic analysis.
Acknowledgements
Funding: This work was supported by the Clinical Science and Technology Project of Jiangsu Province (No. BL2013026) and the National Natural Science Foundation of China (No. 81572273), Natural Science Foundation of Jiangsu Province (No. BK20161386).
Ethical Statement: The study was approved by the Institutional Ethical Committee (approval number: 2017NZHX-002). The requirement for informed consent was waived.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- 2.Travis WD, Brambilla E, Noguchi M, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society: international multidisciplinary classification of lung adenocarcinoma: executive summary. Proc Am Thorac Soc 2011;8:381-5. 10.1513/pats.201107-042ST [DOI] [PubMed] [Google Scholar]
- 3.Chen F, Cole P, Bina WF. Time trend and geographic patterns of lung adenocarcinoma in the United States, 1973-2002. Cancer Epidemiol Biomarkers Prev 2007;16:2724-9. 10.1158/1055-9965.EPI-07-0455 [DOI] [PubMed] [Google Scholar]
- 4.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. 10.1056/NEJMoa0810699 [DOI] [PubMed] [Google Scholar]
- 5.Fan TW, Lane AN, Higashi RM, et al. Metabolic profiling identifies lung tumor responsiveness to erlotinib. Exp Mol Pathol 2009;87:83-6. 10.1016/j.yexmp.2009.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007;448:561-6. 10.1038/nature05945 [DOI] [PubMed] [Google Scholar]
- 7.Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med 2010;363:1693-703. 10.1056/NEJMoa1006448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim HR, Shim HS, Chung JH, et al. Distinct clinical features and outcomes in never-smokers with nonsmall cell lung cancer who harbor EGFR or KRAS mutations or ALK rearrangement. Cancer 2012;118:729-39. 10.1002/cncr.26311 [DOI] [PubMed] [Google Scholar]
- 9.Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013;368:2385-94. 10.1056/NEJMoa1214886 [DOI] [PubMed] [Google Scholar]
- 10.Paik JH, Choi CM, Kim H, et al. Clinicopathologic implication of ALK rearrangement in surgically resected lung cancer: a proposal of diagnostic algorithm for ALK-rearranged adenocarcinoma. Lung Cancer 2012;76:403-9. 10.1016/j.lungcan.2011.11.008 [DOI] [PubMed] [Google Scholar]
- 11.Zheng D, Wang R, Zhang Y, et al. Prevalence and clinicopathological characteristics of ALK fusion subtypes in lung adenocarcinomas from Chinese populations. J Cancer Res Clin Oncol 2016;142:833-43. 10.1007/s00432-015-2081-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tian G, Zhao X, Nie J, et al. Clinical characteristics associated with non-small-cell lung cancer harboring ALK rearrangements in Chinese patients. Future Oncol 2016;12:1243-9. 10.2217/fon.15.361 [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto S, Korn RL, Oklu R, et al. ALK molecular phenotype in non-small cell lung cancer: CT radiogenomic characterization. Radiology 2014;272:568-76. 10.1148/radiol.14140789 [DOI] [PubMed] [Google Scholar]
- 14.Nakada T, Okumura S, Kuroda H, et al. Imaging Characteristics in ALK Fusion-Positive Lung Adenocarcinomas by Using HRCT. Ann Thorac Cardiovasc Surg 2015;21:102-8. 10.5761/atcs.oa.14-00093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halpenny DF, Riely GJ, Hayes S, et al. Are there imaging characteristics associated with lung adenocarcinomas harboring ALK rearrangements? Lung Cancer 2014;86:190-4. 10.1016/j.lungcan.2014.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim TJ, Lee CT, Jheon SH, et al. Radiologic Characteristics of Surgically Resected Non-Small Cell Lung Cancer With ALK Rearrangement or EGFR Mutations. Ann Thorac Surg 2016;101:473-80. 10.1016/j.athoracsur.2015.07.062 [DOI] [PubMed] [Google Scholar]
- 17.Zhao F, Xu M, Lei H, et al. Clinicopathological characteristics of patients with non-small-cell lung cancer who harbor EML4-ALK fusion gene: a meta-analysis. PLoS One 2015;10:e0117333. 10.1371/journal.pone.0117333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi CM, Kim MY, Hwang HJ, et al. Advanced adenocarcinoma of the lung: comparison of CT characteristics of patients with anaplastic lymphoma kinase gene rearrangement and those with epidermal growth factor receptor mutation. Radiology 2015;275:272-9. 10.1148/radiol.14140848 [DOI] [PubMed] [Google Scholar]
- 19.Wang WT, Li Y, Ma J, et al. Serum carcinoembryonic antigen levels before initial treatment are associated with EGFR mutations and EML4- ALK fusion gene in lung adenocarcinoma patients. Asian Pac J Cancer Prev 2014;15:3927-32. 10.7314/APJCP.2014.15.9.3927 [DOI] [PubMed] [Google Scholar]
- 20.Fukui T, Yatabe Y, Kobayashi Y, et al. Clinicoradiologic characteristics of patients with lung adenocarcinoma harboring EML4-ALK fusion oncogene. Lung Cancer 2012;77:319-25. 10.1016/j.lungcan.2012.03.013 [DOI] [PubMed] [Google Scholar]
- 21.Wong DW, Leung EL, So KK, et al. The EML4-ALK fusion gene is involved in various histologic types of lung cancers from nonsmokers with wild-type EGFR and KRAS. Cancer 2009;115:1723-33. 10.1002/cncr.24181 [DOI] [PubMed] [Google Scholar]
- 22.Shigematsu H, Lin L, Takahashi T, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst 2005;97:339-46. 10.1093/jnci/dji055 [DOI] [PubMed] [Google Scholar]
- 23.Tokumo M, Toyooka S, Kiura K, et al. The relationship between epidermal growth factor receptor mutations and clinicopathologic features in non-small cell lung cancers. Clin Cancer Res 2005;11:1167-73. [PubMed] [Google Scholar]
- 24.Wang H, Schabath MB, Liu Y, et al. Clinical and CT characteristics of surgically resected lung adenocarcinomas harboring ALK rearrangements or EGFR mutations. Eur J Radiol 2016;85:1934-40. 10.1016/j.ejrad.2016.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi H, Paeng JC, Kim DW, et al. Metabolic and metastatic characteristics of ALK-rearranged lung adenocarcinoma on FDG PET/CT. Lung Cancer 2013;79:242-7. 10.1016/j.lungcan.2012.11.021 [DOI] [PubMed] [Google Scholar]