Abstract
Fibrosis can disrupt tissue structure and integrity and impair organ function. Fibrosis is characterized by abnormal collagen accumulation in the extracellular matrix. Pharmacological inhibition of collagen secretion therefore represents a promising strategy for the management of fibrotic disorders, such as liver and lung fibrosis. Hsp47 is an endoplasmic reticulum (ER)-resident collagen-specific molecular chaperone essential for correct folding of procollagen in the ER. Genetic deletion of Hsp47 or inhibition of its interaction with procollagen interferes with procollagen triple helix production, which vastly reduces procollagen secretion from fibroblasts. Thus, Hsp47 could be a potential and promising target for the management of fibrosis. In this study, we screened small-molecule compounds that inhibit the interaction of Hsp47 with collagen from chemical libraries using surface plasmon resonance (BIAcore), and we found a molecule AK778 and its cleavage product Col003 competitively inhibited the interaction and caused the inhibition of collagen secretion by destabilizing the collagen triple helix. Structural information obtained with NMR analysis revealed that Col003 competitively binds to the collagen-binding site on Hsp47. We propose that these structural insights could provide a basis for designing more effective therapeutic drugs for managing fibrosis.
Keywords: collagen, drug screening, endoplasmic reticulum (ER), fibrosis, heat-shock protein (HSP), molecular chaperone, protein-protein interaction, HSP47, serpinH1
Introduction
A number of fibrotic diseases, including liver cirrhosis, lung fibrosis, and idiopathic pulmonary fibrosis, are characterized by the abnormal deposition of collagens in the extracellular matrix (ECM).3 Long-term pathological accumulation of collagen in the ECM of an organ disrupts the normal structure and integrity and impairs the normal functions of the organ. Although a vast number of patients are suffering from fibrotic diseases, no specific treatment is currently available (1, 2). Compounds that regulate the biosynthesis and secretion of collagen might be promising targets for the treatment of these diseases (3–6).
Collagen is the most abundant protein in mammals. Twenty seven different types of collagen identified in mammalian cells have common primary structures and harbor a repeat of three amino acids (Gly-Xaa-Yaa), in which Pro is often located at the X and Y positions. When the α-chains of procollagen co-translationally enter the ER, the proline residue at the Y position is hydroxylated by prolyl 4-hydroxylase (P4H) and, in the case of type I collagen, two α1-chains and one α2-chain form a trimer at the C-propeptide region, where they undergo disulfide bond formation (7–10). Triple helix formation proceeds from the C to the N terminus in a zipper-like manner, and correctly folded procollagens in which the Gly-Xaa-Yaa repeats form a triple helix are transported from the ER to the cell surface via the Golgi apparatus (11, 12).
Hsp47 was initially identified as a collagen-binding heat-shock protein residing in the ER, and it was later reported to function as a collagen-specific molecular chaperone that is essential for the correct folding of procollagen in the ER (13–15). Hsp47 transiently associates with procollagen in the ER and dissociates before or during the stage at which procollagen reaches the cis-Golgi (16). This process is triggered by the change in pH because Hsp47 dissociates from procollagen completely at pH 6.3 (17, 18). We showed previously that Hsp47-knock-out mice (hsp47−/−) are embryonically lethal and display disrupted basement membranes and a reduced number of fibrillar structures composed of collagen in the ECM (19–21). In addition, type I collagen secreted from hsp47−/− fibroblasts is more susceptible to protease digestion due to malformation of its triple helix (19).
The synthesis of Hsp47 runs parallel to that of collagen in developing tissues and various cell lines, as well as in collagen-related pathological conditions. The expression of both collagen and Hsp47 increases dramatically with the onset of liver fibrosis, idiopathic pulmonary fibrosis, intestinal fibrosis, and glomerulonephritis (22–24). Furthermore, the progression of fibrosis is reduced markedly when Hsp47 expression is suppressed (23, 24), as confirmed by experiments using siRNA-mediated knockdown of Hsp47 (25, 26). Therefore, Hsp47 could be a potential therapeutic target for various fibrotic diseases; however, the strategy of siRNA-mediated down-regulation of Hsp47 has several critical issues that must be solved before the technique can be applied to humans in the clinical setting.
Here, we show that an Hsp47 mutant (Y365A) lacking the ability to bind to procollagen failed to recover collagen secretion in hsp47−/− fibroblasts, suggesting that inhibiting the interaction of Hsp47 with procollagen in the ER is another promising strategy for the treatment of fibrotic diseases. Thus, we performed a comprehensive screening of Hsp47 inhibitors in a library of natural and synthetic compounds. A small compound (Col003) that inhibited the interaction of Hsp47 with collagen was identified. When added to cultured cells, Col003 inhibited the secretion of collagen and induced the production of collagen with an unstable triple helix. We report that a novel small molecule may be a potential lead compound for the treatment of fibrosis and discuss the possible binding sites for this compound on Hsp47.
Results
Screening of Hsp47 inhibitors
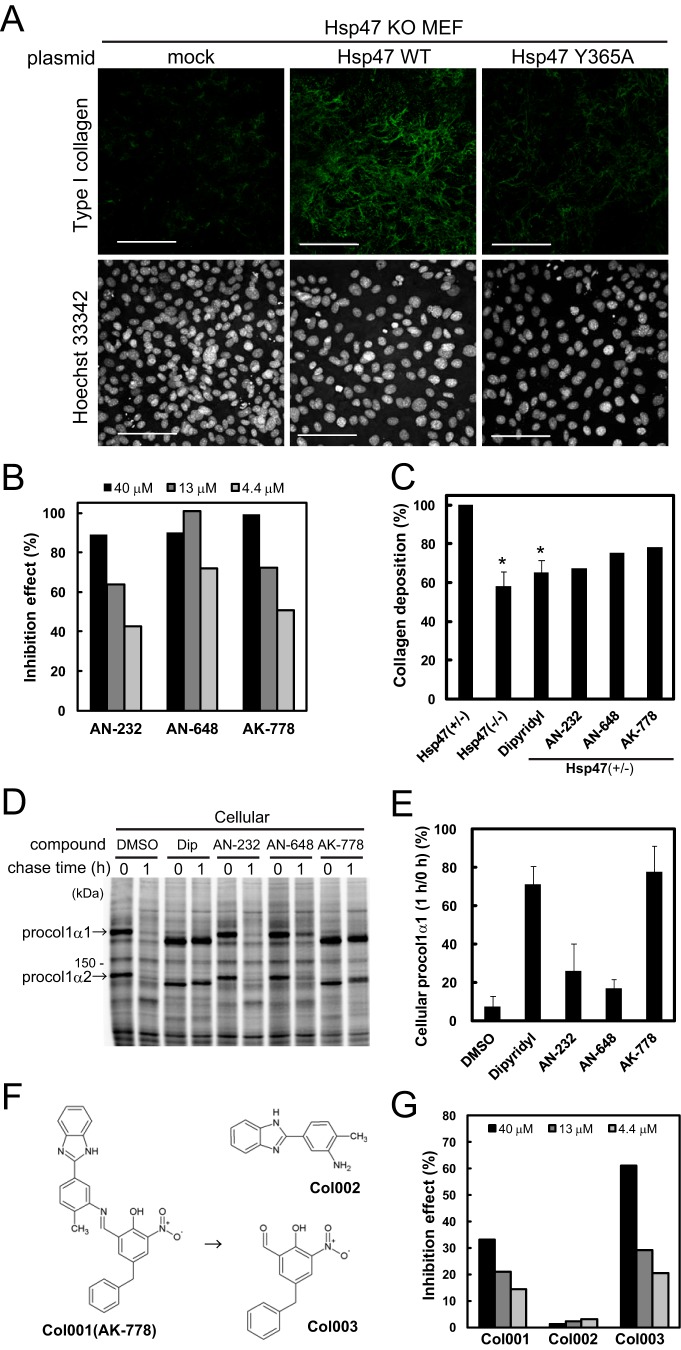
Previously, we showed that fibroblasts obtained from hsp47−/− mouse embryos secreted vastly reduced amounts of collagen, resulting in the loss of collagen fiber accumulation in the ECM, and we showed that transfection of Hsp47 into hsp47−/− mouse embryonic fibroblasts (MEF) restored collagen secretion (Fig. 1A) (20). However, if an Hsp47 mutant (Y365A) that could not bind to procollagen (27) was overexpressed instead, restoration of collagen secretion was not observed (Fig. 1A), suggesting that the interaction between procollagen and Hsp47 in the ER is indispensable for the correct folding of procollagen in the ER. These observations provided us with a rationale to screen for inhibitors of the interaction between Hsp47 and procollagen with the aim of developing a novel strategy for the treatment of fibrotic diseases.
Figure 1.
Screening of Hsp47 inhibitors. A, effects of transient expression of Hsp47 WT and Hsp47 Y365A in Hsp47 KO (−/−) MEFs on collagen accumulation in the ECM. Nuclei were stained with Hoechst 33342. Scale bar, 100 μm. B, effects of three representative compounds on the interaction of collagen with recombinant chicken Hsp47. A total of 52,560 natural and synthetic compounds were screened using SPR. Collagen was immobilized on the sensor chip. C, Sirius Red assay to detect collagen production by Hsp47+/− MEFs treated with the compounds shown in A. Hsp47−/− cells and α,α′-dipyridyl (50 μm) were used as positive controls. The control experiments were performed in triplicate, and data are represented as the mean ± S.D. *, p < 0.01 versus Hsp47+/− cells by a Student's t test. Initially, duplicate analyses of the test compounds were performed at 50 μm; the doses of highly toxic compounds were then reduced. The actual doses of AN-232, AK-648, and AK-778 were 10, 50, and 50 μm, respectively. D, pulse-chase experiments to evaluate the effects of the compounds shown in B on collagen secretion. The doses of AN-232, AK-648, and AK-778 were 10, 20, and 100 μm, respectively. Dip, α,α′-dipyridyl; procol1α1, procollagen type 1 α1. E, quantitative analysis of the data shown in D. Data are represented as the mean ± S.D. of n = 3 replicates. F, degradation of AK-778 (Col001) to Col002 and Col003. G, inhibitory effects of Col001, Col002, and Col003 on the interaction of collagen with Hsp47. The experimental conditions were identical to those described for B.
To identify compounds that inhibit the interaction of Hsp47 with collagen, we used a surface plasmon resonance (SPR) screening system in which a collagen was coupled to sensor tips, and recombinant chicken Hsp47 was used as an analyte. From the 52,560 chemical and natural compounds screened, 176 hit compounds that inhibited the interaction between collagen and Hsp47 in a dose-dependent manner were identified; the inhibitory effects of three of these compounds (AN-232, AN-648, and AK-778) are shown in Fig. 1B. The effects of these compounds on collagen deposition in the ECM were examined using a Sirius Red assay to evaluate the amount of collagen accumulated in the ECM (25). The hsp47+/− MEFs treated with α,α′-dipyridyl, an inhibitor of P4H, and hsp47−/− MEFs were used as positive controls. Of the 176 original hit compounds tested in the assay, 30 inhibited collagen depositions in the ECM (the effects of three representative compounds are shown in Fig. 1C).
Next, pulse-chase experiments were performed to determine whether the observed reductions in collagen deposition were caused by down-regulation of collagen synthesis or inhibition of collagen secretion. Treatment of the cells with α,α′-dipyridyl or AK-778 inhibited the secretion of collagen without affecting its synthesis (Fig. 1, D and E), whereas AK-232 and AN-648 did not inhibit collagen secretion. The other 27 compounds that were evaluated to have positive effects in Sirius Red assay failed to show inhibitory effects on the secretion of collagen. Therefore, AK-778 was identified as a hit compound after three successive qualitatively distinct screenings. Notably, AK-778 was found to be degraded into two fragments in the medium or buffer, which were tentatively named Col002 and Col003 (Fig. 1F). An SPR analysis showed that Col003 had an inhibitory effect on the interaction of Hsp47 with collagen, whereas Col002 did not (Fig. 1G).
Col003 inhibits the interactions of Hsp47 and P4H with collagen
In the pulse-chase experiment (Fig. 1D), treatment of the cells with AK-778 reduced the apparent molecular size of procollagen. This band shift was also observed for cells treated with α,α′-dipyridyl, suggesting that Col003 might also inhibit prolyl 4-hydroxylation of procollagen by P4H. To examine this possibility, we examined the prolyl 4-hydroxylation of (GPP)10, a collagen model peptide using purified P4H in the presence of the essential cofactors ferrous iron and α-ketoglutarate. α,α′-Dipyridyl, a ferrous iron chelator, inhibited the prolyl 4-hydroxylation of (GPP)10 almost completely, and Col003 also partially inhibited this process (supplemental Fig. S1).
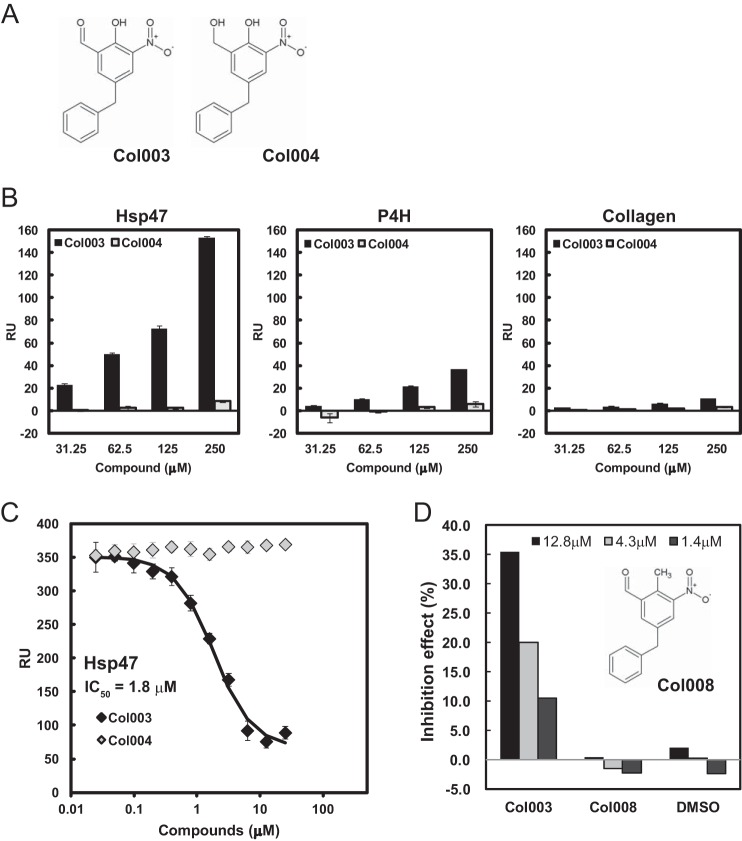
Next, we examined the direct binding of Col003 to Hsp47 by SPR analysis using Hsp47 immobilized to the sensor tip. As Col003 partially inhibited P4H activity, we also examined the binding of Col003 to P4H in parallel. An inactive analog of Col003, in which the aldehyde group was replaced by a hydroxyl group (Col004; Fig. 2A), was used as a negative control in this experiment. Col003 strongly bound to Hsp47 coupled to the sensor tip, although the binding of Col003 to P4H was much weaker (Fig. 2B and supplemental Fig. S2). It is worthwhile to note that Col003 did not bind to collagen. Next, the inhibitory effect of Col003 on the interaction between Hsp47 and collagen was also analyzed by SPR analysis. As shown in Fig. 2C, Col003, but not Col004, inhibited the interaction between Hsp47 and collagen with an IC50 of 1.8 μm (supplemental Fig. S2). Col003 bears a reactive aldehyde, which can potentially modify many proteins in a non-specific manner. Thus, we examined the inhibitory effect of one Col003 derivative, Col008, which has an aldehyde group at the same position as in Col003, with the interaction between Hsp47 and collagen. As shown in Fig. 2D, Col008 did not show any inhibitory effect, indicating that the aldehyde group of Col003 does not exert a non-specific side effect.
Figure 2.
Inhibitory effects of Col003 on the interactions between analytes and substrates in vitro. A, chemical structures of Col003 and Col004. B, SPR analyses of the direct binding of Col003 and Col004 to Hsp47, P4H, and collagen, which were immobilized to the sensor tip. C, SPR analyses of the dose-dependent effects of Col003 and Col004 on the interactions of Hsp47 with collagen. The IC50 value for Hsp47 is determined based on Ref. 33. Data are represented as the mean ± S.D. of n = 3 replicates. RU, response units. D, inhibitory effects of Col003 derivatives on the interaction between collagen and Hsp47. Data are represented as the mean values of duplicate analyses.
Col003 inhibits collagen secretion, triple helix formation, and accumulation in the ECM
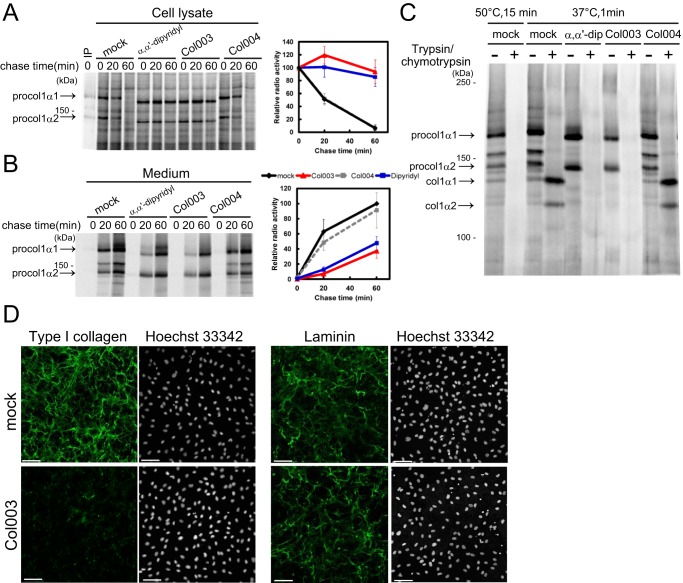
Next, the inhibitory effects of Col003 on collagen secretion and accumulation were determined in vivo. Col003 inhibited collagen secretion by wild-type MEFs (Fig. 3, A and B). Collagen secretion was also inhibited by α,α′-dipyridyl but was not affected by Col004. Because collagen secretion was not abolished completely by exposure of the cells to α,α′-dipyridyl or Col003, the triple helix formation of secreted collagen was assessed via trypsin digestion. Collagen secreted from the wild-type MEFs was degraded completely by incubation with trypsin at a high temperature (50 °C for 15 min; positive control), but it was resistant to trypsin digestion at 37 °C, indicating that the triple helix with α1- and α2-chains was retained after the propeptides of procollagen were cleaved off (Fig. 3C). By contrast, the collagen peptides secreted from Col003- and α,α′-dipyridyl-treated cells, but not those secreted from Col004-treated cells, exhibited sensitivity to trypsin digestion at 37 °C, suggesting that they did not retain correctly folded triple helix structures (Fig. 3C). The accumulation of collagen in the ECM was also examined by immunostaining MEFs with an anti-collagen antibody. Although the cell number examined by Hoechst staining was not affected by treatment with Col003, Col003 reduced collagen accumulation in the ECM drastically (Fig. 3D). Laminin accumulation in the ECM was not affected by treatment of the cells with Col003 (Fig. 3D), suggesting that the inhibitory effect of Col003 was specific for collagen secretion. Taken together, these results suggest that Col003 prevents the proper triple helix production of procollagen by inhibiting its interaction with Hsp47 in the ER, resulting in a reduction in collagen secretion. Because Col003 also inhibits P4H activity, at present we cannot exclude the possibility that Col003 prevents collagen secretion by inhibiting P4H, although the affinity of Col003 for P4H was much weaker than its affinity for Hsp47.
Figure 3.
Inhibitory effects of Col003 and α,α′-dipyridyl on collagen secretion by MEFs. A and B, pulse-chase analyses of collagen (procollagen type 1 α1) levels in the lysates and culture media of MEFs that were treated with 100 μm α,α′-dipyridyl, 100 μm Col003, or 100 μm Col004. At the 1st lane in A, the sample immunoprecipitated (IP) with type I procollagen antibody was loaded as the marker of type I procollagens. Data are represented as the mean ± S.D. of n = 4 replicates. C, susceptibilities to trypsin digestion of collagens secreted from wild-type MEFs that were treated with 100 μm α,α′-dipyridyl, 100 μm Col003, or 100 μm Col004. D, effects of Col003 treatment of wild-type MEFs on collagen and laminin accumulation in the ECM. The nuclei were stained with Hoechst 33342. Scale bar, 100 μm.
Col003 interacts with Hsp47 at its collagen-binding region
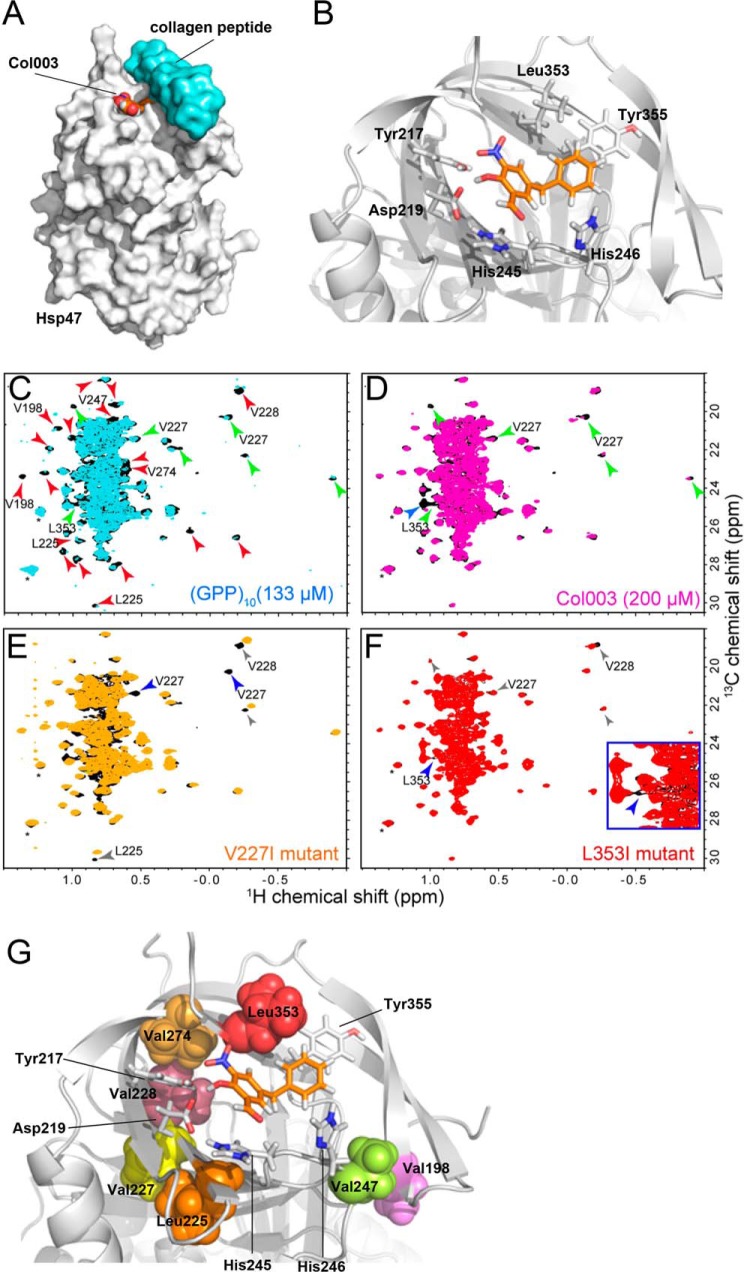
Because the inhibitory effect of Col003 on the interaction of Hsp47 with collagen was relatively weak (IC50 = 1.8 μm), modification of the compound may be required to improve its efficiency. For this purpose, the identification of binding sites of Col003 on Hsp47 is required as the necessary information. Therefore, we examined the Col003-binding site of chicken Hsp47 using the crystal structure of canine Hsp47 (Protein Data Bank code 4AU2) (28), which is so far available as a reference. A potential binding site for Col003 was identified using a druggable pocket analysis (Fig. 4, A and B). Based on this model, the nitro group and the hydroxyl group of Col003 form three hydrogen bonds with the hydroxyl group of Tyr-217, the carboxyl group of Asp-219, and the backbone amide group of Leu-353 in Hsp47. In addition, two aromatic rings of Col003 interact with the ring system of Tyr-355 and His-245 in Hsp47. It should be noted that Leu-353 and Tyr-355 were previously shown to be essential for the interaction of collagen with canine Hsp47 (28).
Figure 4.
Proposed model of the binding of Col003 and NMR analyses of Hsp47. A, overview of the structure of Hsp47 (gray surface model) in complex with Col003 (space-filling model with orange carbon atoms) and collagen peptide (light blue surface model). X-ray structure of Hsp47: Protein Data Bank code 4AU2. B, detailed view of the key residues of Hsp47 (stick model with gray carbon atoms) that interact with and surround Col003 (stick model with orange carbon atoms). C, comparison of the Leu/Val methyl regions of the 1H-13C HMQC spectra of Hsp47 in the presence (cyan) and absence (black) of 133 μm (GPP)10 as a trimer. D, comparison of the Leu/Val methyl regions of the 1H-13C HMQC spectra of Hsp47 in the presence (magenta) and absence (black) of 200 μm Col003. The red and blue arrowheads indicate resonances that were substantially perturbed by binding of Hsp47 to (GPP)10 and Col003, respectively. The green arrowheads indicate resonances that were commonly perturbed by the binding of Hsp47 to either substrate. The solvent peaks are indicated by asterisks. E and F, comparisons of the Leu/Val methyl regions of the 1H-13C HMQC spectra of wild-type Hsp47 (black) and Hsp47 containing a V227I (E; orange) or L353I (F; red) mutation. The resonances that disappeared upon introduction of the mutations are indicated by blue arrowheads. The signals that were affected by the mutation and by binding of (GPP)10 or Col003 are indicated by gray arrowheads. Resonances that were mutated in subsequent analyses (see supplemental Fig. S3) are annotated. G, positions of the mutated residues in the theoretical model of the Hsp47–Col003 complex. The mutated residues are indicated by surface representations. The residues that are located in close proximity to Col003 in the model are shown as stick representations. All of the residues are located within the collagen-binding interface.
To verify that collagen and Col003 share a common binding site in Hsp47, we performed nuclear magnetic resonance (NMR) analyses of the interactions between Hsp47 and (GPP)10 (collagen model peptide with triple helix conformation) or Col003. The HMQC spectra of Hsp47 in which Leu/Val were labeled with methyl-1H13C were obtained before and after the binding of (GPP)10 or Col003. Upon binding to (GPP)10, the intensities of a substantial number of Hsp47 resonances were reduced due to binding-induced chemical shifts in the slow-to-intermediate time scale (Fig. 4C). By contrast, a smaller number of resonances were affected by the binding of Hsp47 to Col003 (Fig. 4D), which most likely reflects the smaller binding interface of Col003 with Hsp47. Nevertheless, some of the Hsp47 resonances showed substantial reduction in intensity and/or changes in chemical shift upon binding to Col003. Among the 24 and 7 resonances of Hsp47 that were perturbed upon binding to (GPP)10 and Col003, respectively, six resonances were affected by both (GPP)10 and Col003. These findings suggest that the Col003-binding site is located mainly within the collagen-binding interface and that Col003 should be a competitive inhibitor of the interaction between Hsp47 and collagen.
A further examination of the Hsp47 NMR signals affected by binding to Col003 was performed by mutating several Leu and Val residues located close to the collagen-binding interface and proposed Col003-binding site to Ile (Fig. 4, E and F, and supplemental Fig. S3). In the X-ray structure of the Hsp47–collagen complex and our model of the Hsp47–Col003 complex, Val-198 and Val-247 are located in the collagen-binding site but not the Col003-binding site (Fig. 4G). The NMR analyses revealed that the signals from these residues were affected by the binding of (GPP)10 but not the binding of Col003 (Fig. 4, C and D). By contrast, Val-227 and Leu-353, which are located close to the binding site of Col003, were affected by the binding of both (GPP)10 and Col003. These results provide further evidence that the Col003-binding site is located mostly within the collagen-binding interface. Notably, the signals from Leu-225, Val-228, and Val-274 were not affected by Col003 binding, but the Ile mutations introduced to these residues affected the same resonances that were affected by the Col003 titration, suggesting that these residues are located in close proximity to the residues involved in Col003 binding, although they are not directly involved in the Col003 binding. The distribution of these residues in the binding model supported the validity of our Col003–Hsp47 complex model.
Discussion
Fibrosis is characterized by the abnormal accumulation of ECM components such as collagen and fibronectin, followed by the onset of chronic inflammatory events (1, 29). An imbalance between collagen synthesis and degradation caused by chronic inflammation results in abnormal accumulation of collagen. Pirfenidone, a small molecule with anti-inflammatory activity that inhibits transforming growth factor β signaling, is approved as a therapeutic drug for fibrosis in Europe and Japan (30). Pirfenidone reportedly suppresses the expression of the mRNA-encoding collagen (31). Although the suppression of collagen expression could be a therapeutic strategy for fibrosis, prevention of the folding of the collagen protein should also be another effective strategy (3, 4).
The collagen-specific molecular chaperone Hsp47 is essential for the correct folding of procollagen in the ER, and Hsp47 expression is up-regulated markedly during the progression of fibrosis in various organs (22). Thus, Hsp47 could be a promising target for the treatment of fibrosis, and an Hsp47-specific siRNA is currently under evaluation in a phase 1b/2 study.
Because a small molecule that inhibits Hsp47 function would be a promising lead compound for the treatment of fibrosis, we performed a comprehensive screen of natural and chemical compound libraries containing more than 50,000 compounds. Three successive screening techniques were used as follows: (i) SPR analyses to examine the inhibition of the interaction of Hsp47 with collagen peptides; (ii) Sirius Red analyses of cultured cells to evaluate the inhibition of collagen accumulation in the ECM; and (iii) pulse-chase analyses to confirm the inhibition of collagen secretion. Using these methods, we identified a small molecule compound (Col003) that bound to Hsp47 directly in vitro and competitively inhibited the interaction between Hsp47 and collagen. We used recombinant chicken Hsp47 in in vitro assay because recombinant chicken Hsp47 is more stable than recombinant human and mouse Hsp47. We confirmed that Col003 inhibited the interaction of collagen with mouse Hsp47, the sequence of which is 96% identical with that of human Hsp47. Thus, it may be valid to conclude that the Hsp47 inhibitor identified here (Col003) could inhibit the interaction of human Hsp47 with human procollagen.
In a previous study using fibrotic model rats, down-regulation of Hsp47 using a specific siRNA targeted to hepatic stellate cells (HSCs) caused apoptosis of these collagen-producing cells, leading to a dramatic reduction in liver fibrosis (25). Our group found that HSC-specific depletion of the Hsp47 gene caused the apoptosis in HSCs due to the ER stress exerted by the accumulation of procollagen within the ER under the suppression of autophagy (32). When the interaction of Hsp47 with procollagen was inhibited, procollagen would accumulate in the ER, causing the apoptosis of collagen-producing HSCs. Hence, a small compound that inhibits Hsp47 would be a promising candidate for the treatment of fibrosis; however, the IC50 value of Col003 is too high for the compound to be tested in mouse models. An NMR analysis using the crystal structure of canine Hsp47 as a reference identified and validated the Col003-binding site of Hsp47 and confirmed that Col003 is a competitive inhibitor of the interaction between Hsp47 and collagen. The structural information provided in this study would be useful for screening more efficient small molecules that inhibit the interaction of collagen with Hsp47 in the future, and the crystal structure of the Hsp47–Col003 complex would provide useful information for further screening of efficient fibrosis inhibitors.
Experimental procedures
Screening library
The library used for inhibitor screening contained 52,560 diverse natural products and synthetic compounds. The synthetic library contained 10,240 compounds, and the natural product library contained crude metabolites of actinomycetes (33,440 samples), fungi (8,240 samples), and compounds isolated from microbial metabolites (640 samples). All samples were dissolved in dimethyl sulfoxide (DMSO). We found two compounds (Col002 and Col003) when AK778 was analyzed by mass spectrometry. Both Col002 and Col003 were synthesized and the inhibitory activities of which were measured.
SPR assay
SPR (Biacore A100; GE Healthcare) was used for high-throughput screening of inhibitors of the interaction between Hsp47 and collagen. Collagen (pig's tendon type I-A (Nitta Gelatin Inc.)) was covalently immobilized on spots 1 and 5 of each flow cell in a Biacore CM5 sensor chip via standard amine coupling chemistry for a contact time of 10 min. The final response was ∼6,000 response units (RUs), where 1 RU corresponds to 1 pg of protein per mm2, and the flow system was maintained in HBS-P running buffer (10 mm HEPES, 0.15 m NaCl, and 0.005% surfactant P20 (pH 7.4)). Spots 2 and 4 of each flow cell were coated with ethanolamine and used as reference spots. Chemical compounds in the Japan and Natural Product Library6 of Namiki Shoji Co., Ltd., were used for inhibitor screening. Purified chicken Hsp47 was pre-incubated with the chemical compounds (38.5 μm) in HBS-P at 25 °C for at least 30 min. The interactions between Hsp47 and collagen in the reaction mixtures were determined using a Biacore A100 instrument. The running buffer was HBS-P, and the temperature in the flow cells was maintained at 25 °C. The 384-well plates containing the reaction mixtures were maintained at 4 °C in the plate stacker of the Biacore A100 instrument until the detection procedure was performed. The reaction mixtures were injected separately onto spot 1 or spot 5 of each flow cell for 150 s at a flow rate of 10 μl/min. To regenerate the collagen immobilized on the sensor chips, 10 mm HCl was injected for 60 s to remove bound Hsp47. Data collection and analysis were performed using the Biacore A100 evaluation software. The level of inhibition (%) of the interaction between Hsp47 and collagen by each chemical compound was calculated from the sensorgram “baseline” and “binding late” values of reaction mixtures with and without the compound. Positive hits had inhibition rates of at least 60%. Finally, (GPP)10 (100 μg/ml) was used as a competitor to validate the inhibitor screening system. The IC50 values for Hsp47 and P4H were determined based on the reference (33).
In experiments analyzing the interactions of Hsp47 and P4H with chemical compounds, purified Hsp47 or P4H protein was covalently immobilized on spots 1 and 5 of each flow cell in a Biacore CM5 sensor chip via standard amine coupling chemistry for a contact time of 10 min. The chemical compound was then injected, and binding to Hsp47 or P4H protein in HBS-P was detected.
Cell culture
hsp47+/+, hsp47+/−, and hsp47−/− MEFs (19) were cultured in high glucose Dulbecco's modified Eagle's medium (Gibco) containing 10% fetal bovine serum (FBS; Gibco), ascorbic acid phosphate, and antibiotics. Transfection with mouse Hsp47 expression vector was performed using Lipofectamine LTX (Invitrogen).
Sirius Red assay
hsp47+/− and hsp47−/− MEFs were plated into 24-well tissue culture plates. After culturing for 24 h, the cells were incubated with the test compounds for 72 h. The doses of highly toxic compounds were reduced; the actual doses of AN-232, AK-648, and AK-778 were 10, 50, and 50 μm, respectively. The cells were washed with phosphate-buffered saline (PBS) and fixed using Bouin solution (75% picric acid, 10% formalin, and 5% acetic acid). Collagen deposited in the wells was stained with 0.1% Sirius Red in picric acid for 1 h, as described previously (25). Unbound dye was removed by washing with 0.01 n HCl, and the bound complexes were dissolved in 0.1 n NaOH. Collagen deposition was quantified at 570 nm using a Biolumin960k spectrophotometer (Molecular Dynamics), and the results were expressed as a percentage.
Metabolic labeling experiment
MEFs were pre-incubated with the test compounds for 1 h and cultured with the compounds in Dulbecco's modified Eagle's medium (Gibco) lacking Met and Cys for 30 min. The cells were then cultured with 4.1 MBq/ml 35S-labeled Met and Cys (Express35S Protein Labeling Mixture, PerkinElmer Life Sciences) containing 10% dialyzed FBS (Gibco) for 20 min. For pulse-chase experiments, the labeled cells were chased for appropriate periods of time in medium containing the test compound and excess amounts of unlabeled Met and Cys. The cells were lysed on ice in buffer consisting of 50 mm Tris-HCl (pH 8.0), 150 mm NaCl, 5 mm EDTA, and 1% Nonidet P-40. After centrifugation at 18,000 × g for 20 min, the supernatant was collected and used for the analysis. Soluble proteins and proteins secreted into the culture medium were extracted in SDS-PAGE sample buffer and separated by SDS-PAGE using 5% gels. The gels were exposed to a storage phosphor screen and analyzed using a FLA-7000 laser scanner (GE Healthcare).
For trypsin digestion experiments, the labeled cells were chased for 3 h in Opti-MEM medium (Gibco) containing 5% FBS, the test compound, and excess amounts of unlabeled Met and Cys. For protease digestion, the culture medium was incubated with a mixture of 100 μg/ml trypsin and 250 μg/ml chymotrypsin at 37 °C for 1 min. As a positive control, a portion of the culture medium was treated with protease at 50 °C for 5 min. The reaction was stopped by the addition of phenylmethylsulfonyl fluoride and trichloroacetic acid. The trichloroacetic acid-insoluble fraction was extracted in SDS-PAGE sample buffer and separated by SDS-PAGE using 5% gels. The gels were exposed to phosphor imaging plates and analyzed using a FLA-7000 laser scanner (GE Healthcare).
Immunofluorescence
MEFs were incubated with the test compounds for 72 h and then fixed with 4% (w/v) paraformaldehyde for 15 min. After washing three times with PBS, the fixed cells were blocked with PBS containing 2% goat serum and 10% glycerol for 30 min. Rabbit polyclonal antibodies against mouse collagen type I (AB765P; Millipore) and laminin (a gift from Dr. M. Hayashi, University of Tokyo, Japan) were used as the primary antibodies. Alexa Fluor 488-conjugated anti-mouse or -rabbit IgG was used as the secondary antibody. Hoechst 33342 was used for nuclear staining. Fluorescent signals were analyzed using an LSM 700 confocal fluorescent microscope (Carl Zeiss).
NMR spectroscopy
All experiments were performed on Bruker Avance-III 800-MHz spectrometers equipped with cryogenic probes. All spectra were collected at 298 K using a buffer consisting of 20 mm HEPES-NaOH (pH 7.4), 50 mm NaCl, 10% (v/v) deuterated glycerol, 4 mm DTT, 0.02% n-octyl-β-d-gucopyranoside, and 90% H2O, 10% D2O. The concentration of Leu/Val methyl-13C,1H-labeled Hsp47 was set to 0.1 mm. The collagen-like peptide (GPP)10 was suspended in deuterated DMSO at the trimeric concentration of 10 mm and directly added to the Hsp47 solution at the trimeric concentration of 133 μm. Col003 was dissolved in deuterated DMSO at a final concentration of 100 mm and then added to the Hsp47 solution at a final concentration of 200 μm. The spectra were processed using TopSpin software (Bruker) and analyzed with Sparky software (34). The normalized chemical shift perturbation values (Δδ) were calculated using Equation 1.
| (Eq. 1) |
Non-overlapping signals that had a normalized chemical shift change greater than the average plus standard deviation of all observed resonances, or a reduction in intensity less than half of the average of all observed resonances upon (GPP)10 or Col003 interaction, were judged to be substantially perturbed upon (GPP)10 or Col003 binding. Each spectrum was recorded for 24 h.
Docking simulation
Col003 was docked into the X-ray structure of Hsp47 (Protein Data Bank code 4AU2) (28) using the Glide SP program (Schrödinger). The grid center for docking simulation was defined around the druggable pocket near the Hsp47–collagen peptide interface. The druggable pocket analysis was performed using the SiteMap program (Schrödinger), and all molecular figures were generated using the PyMOL system (Schrödinger).
Statistical analysis
Statistically significant differences were determined using Student's t tests. p < 0.01 was considered significant.
Author contributions
S. I., T. N., and K. N. designed research; S. I., K. O., K. T., and T. H. performed research; M. T., M. Y., K. S., and T. D. contributed new reagents/computational tools; S. I., K. O., K. T., T. H., S. H., I. S., and N. G. analyzed data; and S. I., K. T., M Y., N. G., and K. N. wrote the paper.
Supplementary Material
Acknowledgments
We thank Dr. M. Hayashi for the laminin antibody and Dr. David S. Waugh for the generous gift of the Tev protease construct.
This work was supported by Grant-in-aid for Scientific Research (S) 24227009 from the Japan Society for the Promotion of Science (to K. N.), by Japan Society for the Promotion of Science Fellowship 11J05697 (to S. I.), and in part by the Platform for Drug Discovery, Informatics, and Structural Life Science from the Ministry of Education, Culture, Sports, Science and Technology, Japan and the ACT-MS from Japan Agency for Medical Research and Development (AMED). The authors declare that they have no conflicts of interest with the contents of this article.
This article contains supplemental Figs. S1–S3 and Methods.
- ECM
- extracellular matrix
- ER
- endoplasmic reticulum
- Hsp47
- heat-shock protein 47
- P4H
- prolyl 4-hydroxylase
- SPR
- surface plasmon resonance
- HMQC
- heteronuclear multiple quantum correlation
- MEF
- mouse embryonic fibroblast
- HSC
- hepatic stellate cell
- PDB
- Protein Data Bank
- RU
- response unit.
References
- 1. Friedman S. L., Sheppard D., Duffield J. S., and Violette S. (2013) Therapy for fibrotic diseases: nearing the starting line. Sci. Transl. Med. 5, 167sr1. [DOI] [PubMed] [Google Scholar]
- 2. Wynn T. A., and Ramalingam T. R. (2012) Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat. Med. 18, 1028–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sakaida I., Matsumura Y., Kubota M., Kayano K., Takenaka K., and Okita K. (1996) The prolyl 4-hydroxylase inhibitor HOE 077 prevents activation of Ito cells, reducing procollagen gene expression in rat liver fibrosis induced by choline-deficient l-amino acid-defined diet. Hepatology 23, 755–763 [DOI] [PubMed] [Google Scholar]
- 4. Bickel M., Baringhaus K. H., Gerl M., Günzler V., Kanta J., Schmidts L., Stapf M., Tschank G., Weidmann K., and Werner U. (1998) Selective inhibition of hepatic collagen accumulation in experimental liver fibrosis in rats by a new prolyl 4-hydroxylase inhibitor. Hepatology 28, 404–411 [DOI] [PubMed] [Google Scholar]
- 5. Higashi K., Tomigahara Y., Shiraki H., Miyata K., Mikami T., Kimura T., Moro T., Inagaki Y., and Kaneko H. (2011) A novel small compound that promotes nuclear translocation of YB-1 ameliorates experimental hepatic fibrosis in mice. J. Biol. Chem. 286, 4485–4492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu Y., Wang Z., Kwong S. Q., Lui E. L., Friedman S. L., Li F. R., Lam R. W., Zhang G. C., Zhang H., and Ye T. (2011) Inhibition of PDGF, TGF-β, and Abl signaling and reduction of liver fibrosis by the small molecule Bcr-Abl tyrosine kinase antagonist Nilotinib. J. Hepatol. 55, 612–625 [DOI] [PubMed] [Google Scholar]
- 7. Kivirikko K. I., and Myllyharju J. (1998) Prolyl 4-hydroxylases and their protein disulfide isomerase subunit. Matrix Biol. 16, 357–368 [DOI] [PubMed] [Google Scholar]
- 8. Boudko S. P., Engel J., and Bächinger H. P. (2012) The crucial role of trimerization domains in collagen folding. Int. J. Biochem. Cell Biol. 44, 21–32 [DOI] [PubMed] [Google Scholar]
- 9. Bourhis J. M., Mariano N., Zhao Y., Harlos K., Exposito J. Y., Jones E. Y., Moali C., Aghajari N., and Hulmes D. J. (2012) Structural basis of fibrillar collagen trimerization and related genetic disorders. Nat. Struct. Mol. Biol. 19, 1031–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lamandé S. R., Chessler S. D., Golub S. B., Byers P. H., Chan D., Cole W. G., Sillence D. O., and Bateman J. F. (1995) Endoplasmic reticulum-mediated quality control of type I collagen production by cells from osteogenesis imperfecta patients with mutations in the pro-α1(I) chain carboxyl-terminal propeptide which impair subunit assembly. J. Biol. Chem. 270, 8642–8649 [DOI] [PubMed] [Google Scholar]
- 11. Ishikawa Y., and Bächinger H. P. (2013) A molecular ensemble in the rER for procollagen maturation. Biochim. Biophys. Acta 1833, 2479–2491 [DOI] [PubMed] [Google Scholar]
- 12. Makareeva E., Aviles N. A., and Leikin S. (2011) Chaperoning osteogenesis: new protein-folding disease paradigms. Trends Cell Biol. 21, 168–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nagata K., Saga S., and Yamada K. M. (1986) A major collagen-binding protein of chick embryo fibroblasts is a novel heat-shock protein. J. Cell Biol. 103, 223–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tasab M., Batten M. R., and Bulleid N. J. (2000) Hsp47: a molecular chaperone that interacts with and stabilizes correctly-folded procollagen. EMBO J. 19, 2204–2211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ito S., and Nagata K. (2017) Biology of Hsp47 (Serpin H1), a collagen-specific molecular chaperone. Semin. Cell Dev. Biol. 62, 142–151 [DOI] [PubMed] [Google Scholar]
- 16. Koide T., Takahara Y., Asada S., and Nagata K. (2002) Xaa-Arg-Gly triplets in the collagen triple helix are dominant binding sites for the molecular chaperone HSP47. J. Biol. Chem. 277, 6178–6182 [DOI] [PubMed] [Google Scholar]
- 17. Saga S., Nagata K., Chen W. T., and Yamada K. M. (1987) pH-dependent function, purification, and intracellular location of a major collagen-binding glycoprotein. J. Cell Biol. 105, 517–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nakai A., Satoh M., Hirayoshi K., and Nagata K. (1992) Involvement of the stress protein HSP47 in procollagen processing in the endoplasmic reticulum. J. Cell Biol. 117, 903–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nagai N., Hosokawa M., Itohara S., Adachi E., Matsushita T., Hosokawa N., and Nagata K. (2000) Embryonic lethality of molecular chaperone hsp47 knockout mice is associated with defects in collagen biosynthesis. J. Cell Biol. 150, 1499–1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ishida Y., Kubota H., Yamamoto A., Kitamura A., Bächinger H. P., and Nagata K. (2006) Type I collagen in Hsp47-null cells is aggregated in endoplasmic reticulum and deficient in N-propeptide processing and fibrillogenesis. Mol. Biol. Cell 17, 2346–2355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Matsuoka Y., Kubota H., Adachi E., Nagai N., Marutani T., Hosokawa N., and Nagata K. (2004) Insufficient folding of type IV collagen and formation of abnormal basement membrane-like structure in embryoid bodies derived from Hsp47-null embryonic stem cells. Mol. Biol. Cell 15, 4467–4475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Masuda H., Fukumoto M., Hirayoshi K., and Nagata K. (1994) Coexpression of the collagen-binding stress protein HSP47 gene and the α1(I) and α1(III) collagen genes in carbon tetrachloride-induced rat liver fibrosis. J. Clin. Invest. 94, 2481–2488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sunamoto M., Kuze K., Tsuji H., Ohishi N., Yagi K., Nagata K., Kita T., and Doi T. (1998) Antisense oligonucleotides against collagen-binding stress protein HSP47 suppress collagen accumulation in experimental glomerulonephritis. Lab. Invest. 78, 967–972 [PubMed] [Google Scholar]
- 24. Honzawa Y., Nakase H., Shiokawa M., Yoshino T., Imaeda H., Matsuura M., Kodama Y., Ikeuchi H., Andoh A., Sakai Y., Nagata K., and Chiba T. (2014) Involvement of interleukin-17A-induced expression of heat-shock protein 47 in intestinal fibrosis in Crohn's disease. Gut 63, 1902–1912 [DOI] [PubMed] [Google Scholar]
- 25. Sato Y., Murase K., Kato J., Kobune M., Sato T., Kawano Y., Takimoto R., Takada K., Miyanishi K., Matsunaga T., Takayama T., and Niitsu Y. (2008) Resolution of liver cirrhosis using vitamin A-coupled liposomes to deliver siRNA against a collagen-specific chaperone. Nat. Biotechnol. 26, 431–442 [DOI] [PubMed] [Google Scholar]
- 26. Ishiwatari H., Sato Y., Murase K., Yoneda A., Fujita R., Nishita H., Birukawa N. K., Hayashi T., Sato T., Miyanishi K., Takimoto R., Kobune M., Ota S., Kimura Y., Hirata K., Kato J., and Niitsu Y. (2013) Treatment of pancreatic fibrosis with siRNA against a collagen-specific chaperone in vitamin A-coupled liposomes. Gut 62, 1328–1339 [DOI] [PubMed] [Google Scholar]
- 27. Ono T., Miyazaki T., Ishida Y., Uehata M., and Nagata K. (2012) Direct in vitro and in vivo evidence for interaction between Hsp47 protein and collagen triple helix. J. Biol. Chem. 287, 6810–6818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Widmer C., Gebauer J. M., Brunstein E., Rosenbaum S., Zaucke F., Drögemüller C., Leeb T., and Baumann U. (2012) Molecular basis for the action of the collagen-specific chaperone Hsp47/SERPINH1 and its structure-specific client recognition. Proc. Natl. Acad. Sci. U.S.A. 109, 13243–13247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Friedman S. L. (2008) Mechanisms of hepatic fibrogenesis. Gastroenterology 134, 1655–1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Raghu G., Johnson W. C., Lockhart D., and Mageto Y. (1999) Treatment of idiopathic pulmonary fibrosis with a new antifibrotic agent, pirfenidone: results of a prospective, open-label phase II study. Am. J. Respir. Crit. Care Med. 159, 1061–1069 [DOI] [PubMed] [Google Scholar]
- 31. Hisatomi K., Mukae H., Sakamoto N., Ishimatsu Y., Kakugawa T., Hara S., Fujita H., Nakamichi S., Oku H., Urata Y., Kubota H., Nagata K., and Kohno S. (2012) Pirfenidone inhibits TGF-β1-induced over-expression of collagen type I and heat-shock protein 47 in A549 cells. BMC Pulm. Med. 12, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kawasaki K., Ushioda R., Ito S., Ikeda K., Masago Y., and Nagata K. (2015) Deletion of the collagen-specific molecular chaperone Hsp47 causes endoplasmic reticulum stress-mediated apoptosis of hepatic stellate cells. J. Biol. Chem. 290, 3639–3646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nordin H., Jungnelius M., Karlsson R., and Karlsson O. P. (2005) Kinetic studies of small molecule interactions with protein kinases using biosensor technology. Anal. Biochem. 340, 359–368 [DOI] [PubMed] [Google Scholar]
- 34. Goddard T. D., and Kneller D. G. (2014) SPARKY 3, University of California, San Francisco [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.