Abstract
1α,25-Dihydroxyvitamin D3 (1,25(OH)2D3) is the active form of vitamin D, which is responsible for reducing the risk for diabetes mellitus (DM), decreasing insulin resistance, and improving insulin secretion. Previous studies have shown that 1,25(OH)2D3 inhibited the activity of FoxO1, which has been implicated in the regulation of glucose metabolism. However, its function and mechanism of action in DM-induced energy disorders and also in bone development remains unclear. Here, using in vitro and in vivo approaches including osteoblast-specific, conditional FoxO1-knock-out mice, we demonstrate that 1,25(OH)2D3 ameliorates abnormal osteoblast proliferation in DM-induced oxidative stress conditions and rescues the impaired glucose and bone metabolism through FoxO1 nuclear exclusion resulting from the activation of PI3K/Akt signaling. Using alizarin red staining, alkaline phosphatase assay, Western blot, and real-time qPCR techniques, we found that 1,25(OH)2D3 promotes osteoblast differentiation and expression of osteogenic phenotypic markers (i.e. alkaline phosphatase (1), collagen 1 (COL-1), osteocalcin (OCN), and osteopontin (OPN)) in a high-glucose environment. Moreover, 1,25(OH)2D3 increased both total OCN secretion and levels of uncarboxylated OCN (GluOC) by phosphorylating FoxO1 and promoting its nuclear exclusion, indicated by Western blot and cell immunofluorescence analyses. Taken together, our findings confirm that FoxO1 is a key mediator involved in glucose homeostasis and indicate that 1,25(OH)2D3 improves glucose metabolism and bone development via regulation of PI3K/Akt/FoxO1/OCN pathway.
Keywords: Akt PKB, diabetes, FOXO, osteoblast, vitamin D, forkhead transcription factor 1, PI3K/Akt pathway, high glucose, osteocalcin
Introduction
Diabetes mellitus (DM)3 is a chronic metabolic disorder that affects hundreds of millions of individuals. Apart from renal and cardiovascular complications, DM-induced hyperglycemia adversely affects bone metabolism, such as foot syndrome and Charcot neuroarthropathy (2–4). DM-induced oxidative stress causes osteoporosis, another skeletal complication that increases bone fragility (5–7). Studies suggested that insufficient peak bone mass and impaired bone formation in type 1 diabetes mellitus DM and the poor bone quality in type 2 DM (5, 8, 9) were proposed as major contributing factors in bone fragility. In addition, DM is also considered as one of contraindications to dental implant (10). Patients with well-controlled DM are acceptable, whereas those with poorly controlled DM are always declined for implant therapy. Consistent with this concern, lots of animal studies also demonstrated that delayed bone-implant healing and implant osseointegration were related to inadequate glycemia control (11–13).
Cellular oxidative stress and reactive oxygen species are suggested to be involved in DM development. FoxO (forkhead homeobox type O) transcription factors are regarded as mediators to oxidative stress because they can be regulated by reactive oxygen species (14). FoxO transcription factors induce apoptosis, stress resistance, and senescence (15, 16). As a member of FOXO transcription factors, FoxO1 orchestrates transcriptional actions regulating glucose metabolism (17). In addition, FoxO1 is highly expressed in skeleton that has, during the last few years, been identified as a transcriptional link between the skeleton and pancreas to regulate glucose homeostasis (18). In osteoblasts, FoxO1 regulates energy metabolism and inhibits insulin secretion and sensitivity. In the feedback regulation, insulin suppresses FoxO1 activity and promotes its nuclear exclusion by activating PI3K/Akt signaling pathway (19, 20). Sequence alignment shows that FoxOs proteins have highly conserved regions, including the N-terminal region containing an Akt phosphorylation site (21), through a combination of which FoxO1 can be phosphorylated by serine–threonine kinase Akt.
1,25(OH)2D3, an active form of vitamin D, is responsible for the balance of calcium and phosphorus, bone mineralization, and hormonal release (22). There is evidence showing a close relationship between DM and vitamin D. Vitamin D supplementation can reduce the risk for DM and insulin resistance (23, 24) and improve insulin secretion, insulin sensitivity, and HbA1c (25). Our previous research demonstrated that 1,25(OH)2D3 could promote insulin secretion and bone remodeling, maintain glucose homeostasis, and ameliorate implant osseointegration capacity in diabetic rats (26). The subsequent research4 further suggested that 1,25(OH)2D3 inactivated osteoblastic FoxO1 and promoted its nuclear exclusion in high-glucose environment, indicating that 1,25(OH)2D3 might play its role through FoxO1 inactivation.
Given FoxO1 has been regarded as a main target of vitamin D action in other tissues, including skeletal muscle (27), we investigated whether it fulfills its regulatory effect, at least in part, through its osteoblastic expression. In this report, we show herein that conditional knock-out FoxO1 in osteoblasts favors glucose metabolism and bone formation. Of note, 1,25(OH)2D3 prevents DM-induced bone loss and ameliorates osteoblastic capacity through PI3K/Akt/FoxO1/OCN pathway. That is, 1,25(OH)2D3 inactivates FoxO1 activity and promotes OCN expression and its uncarboxylated level, thus helping restrain the deleterious effects induced by high glucose, indicating that 1,25(OH)2D3 treatment and FoxO1 inhibition might be a novel therapeutic approach to enhance bone formation.
Results
1,25(OH)2D3 can rescue abnormal proliferation caused by high glucose
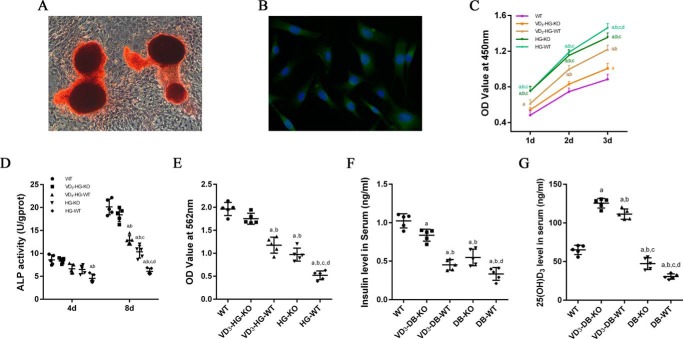
The successful isolation and culture of osteoblasts can be proved by alizarin red staining (Fig. 1A) and OCN (Fig. 1B) immunofluorescence. To evaluate the role of conditional FoxO1 knock-out in osteoblasts (FoxO1OB−/−) and 1,25(OH)2D3 treatment on osteoblasts growth in high-glucose environment, Cell Counting Kit-8 was conducted to detect cell proliferation at 1, 2, and 3 days, respectively. As shown in Fig. 1C, high glucose promoted cell proliferation throughout the whole experimental procedure. When compared with HG-WT group, FoxO1 knock-out reduced cell viability at 3 days in HG-KO group (p < 0.05). In addition, 1,25(OH)2D3 significantly reduced cell viability in the VD3-HG-WT and VD3-HG-KO groups, especially VD3-HG-KO group (p < 0.05).
Figure 1.
1,25(OH)2D3 (VD3) decreases abnormal proliferation and promotes osteogenic differentiation of osteoblasts in high-glucose environment. A, alizarin red staining of primary osteoblasts after 21-day osteogenic induction. B, immunofluorescent staining of OCN in osteoblasts. Alizarin red-positive nodules and obvious expression of OCN proves a successful isolation and culture of primary osteoblasts. C, cell viability determined by the CCK-8 assay at 1, 2, and 3 days in different groups (n = 6 specimens/group). D, cell differentiation assessed by ALP activity at 4 (4d) and 8 days (8d) (n = 5 specimens/group). E, quantification of mineralization nodules in different groups (n = 5 specimens/group). F and G, serum insulin (F) and serum 25(OH)D3 (G) were detected by ELISA (n = 5 specimens/group). The data are presented as means ± S.D. a, p < 0.05 for WT versus others; b, p < 0.05 for VD3-HG(DB)-WT or HG(DB)-KO or HG(DB)-WT versus VD3-HG(DB)-KO; c, p < 0.05 for HG(DB)-KO or HG(DB)-WT versus VD3-HG(DB)-WT; d, for HG(DB)-WT versus HG(DB)-KO. DB represents diabetes mellitus, and HG represents high glucose.
Improved osteogenic differentiation capacity and mineralization in 1,25(OH)2D3-treated FoxO1OB−/− osteoblasts in high-glucose environment
To investigate the effects of 1,25(OH)2D3 and FoxO1OB−/− on osteogenic differentiation, alkaline phosphatase (ALP), a marker of early osteogenesis, was detected at 4 and 8 days after 1,25(OH)2D3 treatment (28) (Fig. 1D). The results showed that high glucose significantly reduced ALP activity in HG-WT group, whereas FoxO1OB−/− could improve ALP activity in HG-KO group when compared with HG-WT group (p < 0.05). 1,25(OH)2D3 up-regulated ALP activity in VD3-HG-KO and VD3-HG-WT group (p < 0.05). Notably, in high glucose condition, the expression of ALP activity in VD3-HG-KO mice is significantly higher than other groups (p < 0.05), which shows no significant difference compared with the WT group (p > 0.05).
To elucidate the capacity of osteoblastic mineralization in different groups, alizarin red staining was conducted to assess late osteogenic differentiation. As shown in Fig. 1E, after 3-week osteogenic induction, the OD value of alizarin red staining in HG-KO group was higher than that of the HG-WT group (p < 0.05). 1,25(OH)2D3 treatment promoted osteoblastic mineralization in the VD3-HG-KO and VD3-HG-WT groups. In addition, the VD3-HG-KO group showed no statistical difference in OD value when compared with the WT group (p > 0.05).
The results of the in vivo study showed that FoxO1OB−/− promoted insulin secretion (Fig. 1F). Although the expression of insulin level in VD3-DB-KO mice is lower than that in WT mice (p < 0.05), it is much higher than other three groups in high glucose condition (p < 0.05), which might be an explanation for the pro-osteogenic effect of 1,25(OH)2D3 on osteoblasts in as high-glucose environment. 25(OH)D3 acts as the indicator of VD3 nutritional status. We measured 25(OH)D3 by ELISA kit, and the results in Fig. 1G showed that the serum 25(OH)D3 level decreased significantly in untreated DB-WT mice when compared with other groups (p < 0.05). A slight increase of 25(OH)D3 was detected in DB-KO mice, indicating that FoxO1 knock-out favored serum 25(OH)D3 (p < 0.05). In addition, 1,25(OH)2D3 application resulted in a notable higher level of 25(OH)D3 in VD3-DB-KO and VD3-DB-WT mice when compared with other three groups (p < 0.05).
Increased osteogenic-related genes and proteins in 1,25(OH)2D3-treated FoxO1OB−/− osteoblasts in high-glucose environment
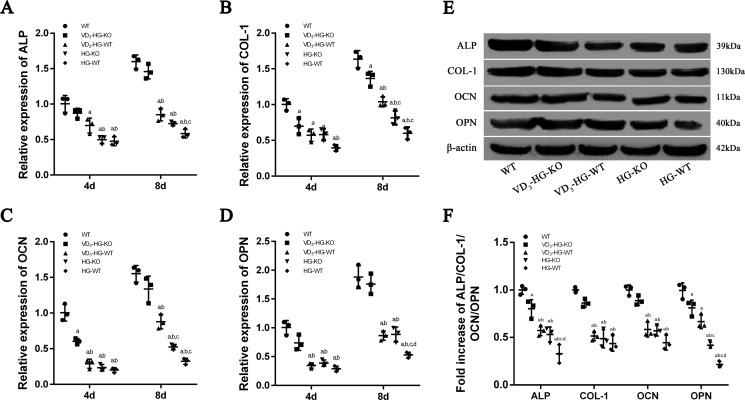
To further elucidate the osteogenic capacity of osteoblasts subjected to different treatment, we analyzed mRNA and protein expressions of osteogenic phenotype at 4 and 8 days, including ALP, COL-1, OCN, and OPN (29) (Fig. 2, A–D). When compared with WT cells, the mRNA levels of ALP, COL-1, OCN, and OPN in HG-WT cells reduced by ∼52.7, 60.7, 80.0, and 71.3% at 4 days and by 63.5, 63.3, 79.1, and 71.9% at 8 days (p < 0.05). Moreover, VD3-HG-WT cells showed an increase level of ALP, COL-1, OCN, and OPN at 8 days when compared with HG-WT group (p < 0.05). In addition, 1,25(OH)2D3 showed a remarkably beneficial effect of pro-osteogenesis in VD3-HG-KO, with no statistical difference in the expression of ALP, OCN, and OPN when compared with the WT group. Western blot analysis was also carried out to verify the protein level of osteogenic markers at 8 days. As shown in Fig. 2 (E and F), when compared with WT group, high glucose reduced the expression of ALP, COL-1, OCN, and OPN in HG-WT (p < 0.05). 1,25(OH)2D3 up-regulated these osteogenic markers in the VD3-HG-WT group. The results showed that there was no difference in COL-1 and OCN protein level between WT and VD3-HG-KO group (p > 0.05). Generally, the results of Western blot analysis were consistent with the RT-qPCR assays.
Figure 2.
1,25(OH)2D3 (VD3) increases the expression of osteogenic phenotype in high-glucose environment. A–D, relative expressions of ALP (A), COL-1 (B), OCN (C), and OPN (D) detected at day 4 (4d) and day 8 (8d) by RT-qPCR analysis. All data were normalized to GAPDH. The data are presented as means ± S.D. (n = 3 specimens/group). E and F, quantification of protein levels of ALP, COL-1, OCN, and OPN determined using Western blot (n = 3 specimens/group). a, p < 0.05 for WT versus others; b, p < 0.05 for VD3-HG-WT or HG-KO or HG-WT versus VD3-HG-KO; c, p < 0.05 for HG-KO or HG-WT versus VD3-HG-WT; d, for HG-WT versus HG-KO. DB represents diabetes mellitus, and HG represents high glucose.
FoxO1 might mediate the metabolic actions of uncarboxylated osteocalcin in vitro and in vivo
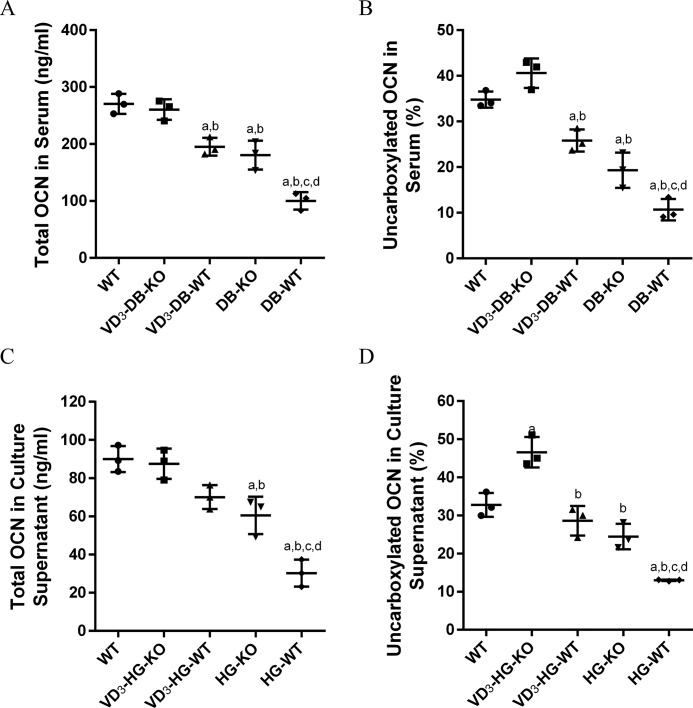
To understand the potential mechanism of 1,25(OH)2D3 regulatory process, we assayed total osteocalcin (tOCN) and the percentage of uncarboxylated osteocalcin (ucOCN%) in vivo and in vitro study. Mouse serum (Fig. 3, A and B) was harvested 2 months after surgery, and culture supernatant (Fig. 3, C and D) was collected after 8-day treatment in different conditions. Both of the in vivo and in vitro studies showed that tOCN and ucOCN% in hyperglycemia was significant lower than that in normal condition (p < 0.05). The expression of tOCN and ucOCN% in VD3-DB-KO mice increased by 2.6- and 3.8-fold, respectively, when compared with DB-WT mice (p < 0.05), which showed no significant difference with WT mice (p < 0.05). In addition, the level of tOCN and ucOCN% in VD3-DB-WT and DB-KO mice slightly increased when compared with DB-WT mice. In vitro study, the results were consistent with in vivo study. Of note, the ucOCN% in VD3-HG-KO was even much higher than WT group (Fig. 3D, p < 0.05).
Figure 3.
1,25(OH)2D3 (VD3) treatment and FoxO1 knock-out increases total osteocalcin and uncarboxylated osteocalcin level in serum and cellular supernatant by ELISA. A and B, total (A) and uncarboxylated OCN(%) (B) in serum. C and D, total (C) and uncarboxylated OCN(%) (D) in culture supernatant. The data are presented as means ± S.D. (n = 3 specimens/group). a, p < 0.05 for WT versus others; b, p < 0.05 for VD3-HG(DB)-WT or HG(DB)-KO or HG(DB)-WT versus VD3-HG(DB)-KO; c, p < 0.05 for HG(DB)-KO or HG(DB)-WT versus VD3-HG(DB)-WT; d, for HG(DB)-WT versus HG(DB)-KO. DB represents diabetes mellitus, and HG represents high glucose.
1,25(OH)2D3 induced a time-dependent Akt and FoxO1 phosphorylation
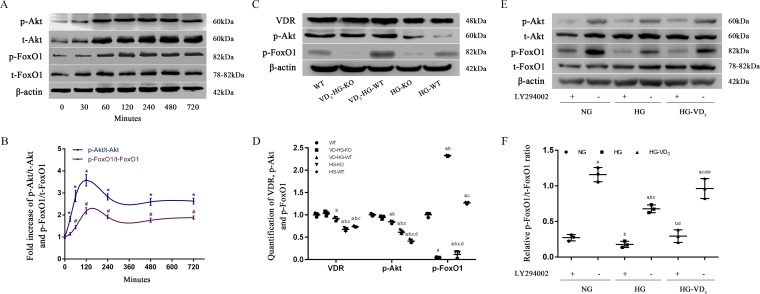
To further investigate the role of FoxO1 in 1,25(OH)2D3 regulation process, we evaluated the expression of p-Akt/t-Akt and p-FoxO1/t-FoxO1 at 0, 30, 60, 120, 240, 480, and 720 min after the treatment of 1,25(OH)2D3 using primary WT osteoblasts in a high-glucose environment (Fig. 4, A and B). p-Akt is the active form of Akt, whereas p-FoxO1 is the inactive form of FoxO1. When compared with 0 min, p-Akt/t-Akt ratio increased by 81.5% at 30 min, and p-FoxO1/t-FoxO1 ratio increased by 44.6% at 60 min, respectively (p < 0.05). The ratio of p-Akt/t-Akt reached its peak at 2 h after treatment and remained in a relatively high level in the next 10 h. The results suggested that p-Akt/p-FoxO1 might be involved in the regulation of 1,25(OH)2D3 on glucose and bone metabolism.
Figure 4.
PI3K/Akt/FoxO1 signaling pathway involved in the regulatory effect of 1,25(OH)2D3 (VD3) on osteoblasts. A and B, changes of protein levels of p-Akt, t-Akt, p-FoxO1, and t-FoxO1 (A) and the fold increase of p-Akt/t-Akt, p-FoxO1/t-FoxO1 at 0, 30, 60, 120, 240, 480, and 720 min (B) were determined by Western blot. C and D, quantification of VDR, p-Akt, and p-FoxO1 were detected by Western blot after 2-h VD3 treatment. E and F, changes of p-Akt/t-Akt (E) and quantification of p-FoxO1/FoxO1 (F) determined by Western blot. Whole cell protein was harvested with or without a 2-h incubation of PI3K inhibitor LY294002 (50 mol/liter). The data are presented as means ± S.D. (n = 3 specimens/group). A and B, *, p < 0.05 for 0 min versus other time points in p-Akt/t-Akt; #, p < 0.05, for 0 min versus other time points in p-FoxO1/t-FoxO1. C and D, a, p < 0.05 for WT versus others; b, p < 0.05 for VD3-HG-WT or HG-KO or HG-WT versus VD3-HG-KO; c, p < 0.05 for HG-KO or HG-WT versus VD3-HG-WT; d, for HG-WT versus HG-KO. E and F, a, p < 0.05 for NG-LY294002(+) versus others; b, p < 0.05, for HG-LY294002(±) or HG-VD3-LY294002(±) versus NG-LY294002(−); c, p < 0.05, for HG-LY294002(−) or HG-VD3-LY294002(±) versus HG-LY294002(+); d, p < 0.05, for HG-VD3-LY294002(±) versus HG-LY294002(−); e, p < 0.05, for HG-VD3-LY294002(−) versus HG-VD3-LY294002(+). HG represents high glucose, and NG represents normal glucose.
1,25(OH)2D3 promoted osteogenesis by activating vitamin D receptor (VDR)/PI3K/Akt signaling pathway in vivo and in vitro
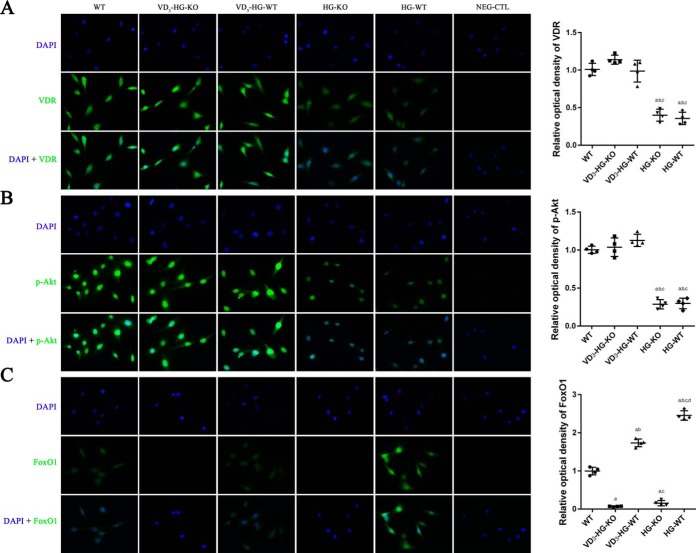
To confirm the role of VDR/PI3K/Akt pathway in the regulation of 1,25(OH)2D3 on glucose and bone metabolism, we examined the expression of VDR, p-Akt, p-FoxO1 using Western blot (Fig. 4, C–F), immunofluorescence (Fig. 5), and immunohistochemistry (Fig. 6). Given that the p-Akt/t-Akt ratio reached its peak at 2 h after the treatment of 1,25(OH)2D3, the whole cell protein was collected after a 2-h treatment of 1,25(OH)2D3 and subjected to Western blot analysis. The results manifested that high glucose inhibited the expression of VDR and p-Akt while promoting FoxO1 phosphorylation. 1,25(OH)2D3 treatment dramatically enhanced VDR expression by 53.0% in VD3-HG-KO group and 24.6% in VD3-HG-WT group (Fig. 4, C and D; p < 0.05). In addition, remarkable increase of p-Akt was detected in VD3-treated groups when compared with the non-treated high glucose groups (p < 0.05). 1,25(OH)2D3 increased FoxO1 phosphorylation in VD3-HG-WT group by 133 and 106.3% as compared with WT and HG-WT group, respectively. LY294002, inhibitor of PI3K, remarkably reduced p-FoxO1/FoxO1 ratio, demonstrating that FoxO1 was the downstream of Akt and could be phosphorylated by p-Akt (Fig. 4, E and F).
Figure 5.
1,25(OH)2D3 (VD3) activates VDR/PI3K/Akt signaling pathway. VDR (A), p-Akt (B), and FoxO1 (C) was detected by immunofluorescent staining (immunofluorescent staining, ×400), and the relative optical density was calculated by Image Pro Plus software. The data are presented as means ± S.D. (n = 3 specimens/group). a, p < 0.05 for WT versus others; b, p < 0.05 for VD3-HG-WT or HG-KO or HG-WT versus VD3-HG-KO; c, p < 0.05 for HG-KO or HG-WT versus VD3-HG-WT; d, for HG-WT versus HG-KO. HG represents high glucose, NG represents normal glucose, and NEG-CTL represents negative control.
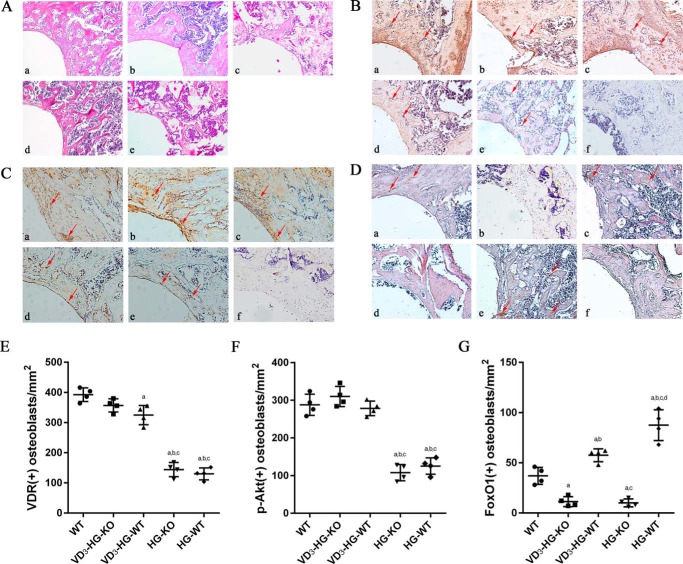
Figure 6.
Histomorphometric analysis of trabecular changes and immunochemistry of VDR/p-Akt/FoxO1. A, HE staining of the trabecular near the implant (×100). B–G, IHC staining and semiquantification for VDR (B and E), p-Akt (C and F), and FoxO1 (D and G) around implant in different groups (×100). Positive osteoblasts are marked with red arrows. A–D, panels a, WT; panels b, 1,25(OH)2D3 (VD3)-DB-KO; panels c, VD3-DB-WT; panels d, DB-KO; panels e, DB-WT; panels f, negative control. E–G, data are presented as means ± S.D. (n = 3 specimens/group). a, p < 0.05 for WT versus others; b, p < 0.05 for VD3-HG-WT or HG-KO or HG-WT versus VD3-HG-KO; c, p < 0.05 for HG-KO or HG-WT versus VD3-HG-WT; d, for HG-WT versus HG-KO.
In cell immunofluorescent staining, 1,25(OH)2D3 enhanced VDR and p-Akt expression in VD3-HG-KO, with no obvious difference compared with WT group (Fig. 5, A and B). A relatively weaker green fluorescence was detected in HG-KO and HG-WT groups. High glucose obviously promoted FoxO1 expression and its nuclear location, whereas 1,25(OH)2D3 down-regulated FoxO1 by promoting its nuclear exclusion. Results in immunohistochemistry were consistent with immunofluorescence. Positive staining of VDR and p-Akt was detected mainly in osteoblasts grown along the bone surface in WT, VD3-HG-KO, and VD3-HG-WT mice (Fig. 6, B–G), whereas the staining of VDR and p-Akt in the HG-KO and HG-WT groups was weaker than in the WT group (p < 0.05). In addition, 1,25(OH)2D3 also attenuated the high expressed FoxO1 induced by diabetes in vivo study.
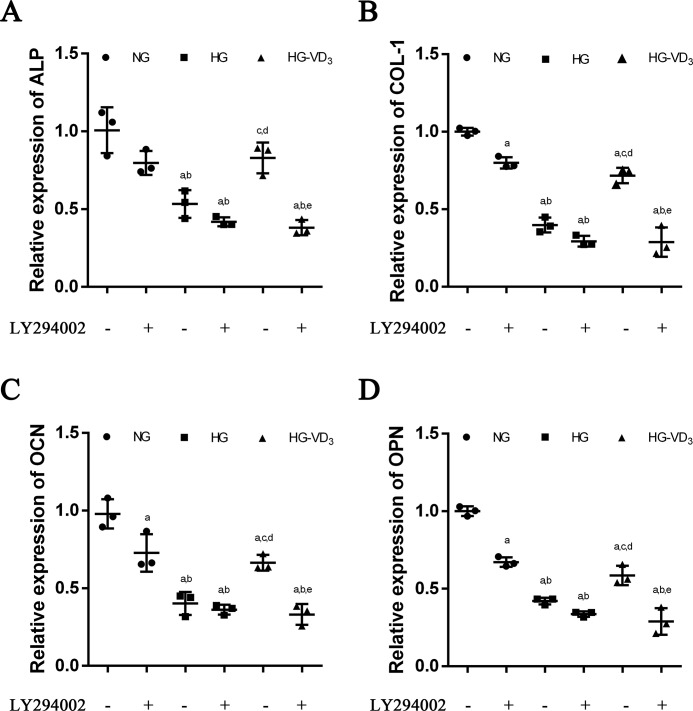
To verify the pro-osteogenic effect of 1,25(OH)2D3 on osteoblasts was achieved through PI3K/Akt signaling pathway, we applied PI3K inhibitor LY294002 to block PI3K/Akt pathway in WT osteoblasts treated with NG, HG, and VD3-HG separately (Fig. 7, A–D). 1,25(OH)2D3 ameliorated the reduced expression of the ALP, COL-1, OCN, and OPN in high-glucose medium, although LY294002 abolished the pro-osteogenic effect of 1,25(OH)2D3 on osteoblasts, with no difference with HG-LY294002(+) group (p > 0.05). The evidence demonstrated that the pro-osteogenic effect of 1,25(OH)2D3 on osteoblasts was achieved through PI3K/Akt signaling pathway.
Figure 7.
PI3K/Akt inhibitor LY294002 blocks the pro-osteogenic effect of 1,25(OH)2D3 (VD3) on osteoblasts in a high-glucose environment. A–D, relative expressions of ALP (A), COL-1 (B), OCN (C), and OPN (D) detected at day 8 by RT-qPCR analysis. The cells were pretreated with a 2-h incubation of PI3K inhibitor LY294002 (50 mol/liter). All data were normalized to GAPDH. The data are presented as means ± S.D. (n = 3 specimens/group). a, p < 0.05 for WT versus others; b, p < 0.05 for VD3-HG-WT or HG-KO or HG-WT versus VD3-HG-KO; c, p < 0.05 for HG-KO or HG-WT versus VD3-HG-WT; d, for HG-WT versus HG-KO. HG represents high glucose, and NG represents normal glucose.
Discussion
The oxidative stress caused by DM has negative effects on bone quality and strength (30). It has recently been suggested that FoxO1 is linked to prevent osteoblasts from oxidative stress (31, 32). Based on this evidence, we hypothesize that this molecule may mediate bone metabolism in DM. Moreover, the beneficial effects of vitamin D on glucose metabolism and bone formation may be achieved through this transcription factor. To support our hypothesis, we present genetic evidence showing that 1,25(OH)2D3 promotes glucose metabolism4 and bone formation through, at least in part, inactivation of FoxO1 and the aroused elevation of OCN and ucOCN%. Through this mechanism, 1,25(OH)2D3 inhibits the abnormal proliferation induced by high glucose and promotes osteoblastic differentiation and bone formation. High glucose interferes osteoblastic gene expression (33), thus inhibiting the process of osteoblast maturation and bone mineralization. These results are consistent with extensive evidence of Almeida and co-workers (34), who suggested that FoxOs resulted in the loss of cancellous bone mass, whereas deletion of FoxOs could ameliorate the adverse effects resulted from DM-induced oxidative stress, thereby promoting bone formation. They further demonstrated that this beneficial effect on bone formation in FoxO-deficient mice was due to up-regulation of Wnt/β-catenin signaling and cyclin D1 expression (35). However, these observations are challenged by some other publications suggesting that conditional knock-out of FoxOs increased skeletal oxidative stress and osteoblast apoptosis and thereby attenuated bone formation rate and bone mass (31). Because FoxOs family has four isoforms, and each one has its own functions, part of this discrepancy can be attributed to different transgenic strategies. In addition, different mice strains and its disease models may be other possibilities.
As a specific secreted protein in osteoblast, OCN has recently been regarded as a hormone that is involved in glucose and energy metabolism. Two forms of OCN exists in circulation: γ-carboxylated GlaOC and uncarboxylated GluOC. Karsenty and co-workers (36) first reported that OCN-null mice exhibited severe damage in glucose metabolism, indicating that GluOC functions as a hormone (37, 38). In this study, 1,25(OH)2D3 treatment facilitated secretion of total OCN and GluOC% level. Particularly, when compared with DB-WT mice, FoxO1OB−/− diabetic mice without any treatment showed a significant increase in OCN and GluOC%, which may be explained by the elevated insulin in circulation. Evidence showed that OCN knock-out reduced β-cell number, insulin secretion, and impaired glucose metabolism (37). In addition, inactivation of GPRC6A, receptor of GluOC, especially in β-cells resulted in glucose intolerance caused by a notable decrease in β-cell number and insulin secretion in circulation (39), suggesting GluOC directly activates GPRC6A and triggers β-cell proliferation and insulin production (40). Our work was also consistent with the result of Kousteni and co-workers (20), who claimed that FoxO1 could bind to OCN promoter and inhibit its expression. Taking all the evidence published and what we found in our study, we suggest that oxidative stress induced by hyperglycemia in vivo or high glucose in vitro stimulates FoxO1 activity and promotes its nuclear location, which inhibits the expression of OCN and its GluOC level. Conversely, FoxO1 knock-out in osteoblasts or 1,25(OH)2D3-induced FoxO1 inactivation could rescue the impairment via favoring OCN production and GluOC level.
In addition, because insulin receptors have been shown to be expressed in osteoblasts (41), and evidence also showed the beneficial effects of insulin on osteoblasts production and differentiation (42–44), the elevated insulin level mediated by GluOC in our study could, in turn, stimulate insulin signaling in osteoblasts, thereby 1) promoting osteoblast differentiation and bone matrix (45) and 2) inhibiting FoxO1 activation (46), which forms a feed forward regulatory loop.
In vitro studies have shown that high glucose weakened osteoblast response to 1,25(OH)2D3 and indirectly down-regulated VDR expression (47). Likewise, our previous study also showed that high glucose attenuated the expression of insulin receptor and VDR, whereas these adverse effects can be reversed by 1,25(OH)2D3 treatment (48). In this study, we found that 1,25(OH)2D3 rescued the decreased VDR expression induced by high glucose. In addition, we verified that this beneficial effects of 1,25(OH)2D3 on bone metabolism and glucose homeostasis under oxidative stress condition were achieved through a combination with VDR. Phosphorylation is one of the main post-translational modifications of FoxO1, the process of which can be induced by the activation of PI3K/Akt signaling (49). Based on this evidence, we speculated that the effect of 1,25(OH)2D3 on FoxO1/OCN expression might be achieved through PI3K/Akt signaling. In our study, Western blot and immunofluorescence staining verified the involvement of PI3K/Akt signaling in the regulation of 1,25(OH)2D3. In other words, 1,25(OH)2D3 combined VDR in osteoblasts subsequently activated and phosphorylated Akt, and then the increased p-Akt phosphorylated FoxO1 and promoted its nuclear exclusion, which indirectly promoted OCN expression and ucOCN% level in the circulation in hyperglycemia environment.
As for Akt phosphorylation, professor Huhtakangas et al. (50) suggested that the anti-apoptosis effect of 1,25(OH)2D3 might include a pertussis toxin-sensitive G protein upstream of PI3K activation, which interacts with the PI3K catalytic subunit, resulting in stimulation of PI3K. In some other cases, 1,25(OH)2D3 has also been reported to associate with the activation of some novel membrane receptors that bind to a G protein (51, 52). This evidence might explain how 1,25(OH)2D3 phosphorylates Akt, but further research is needed to elucidate the details of the mechanism.
It should be noted that VD3-HG-KO showed better pro-osteogenic effect than HG-KO, indicating that there existed other pathways involving in the regulation process of 1,25(OH)2D3 on bone and glucose metabolism. Apart from FoxO1/OCN pathway in osteoblasts, VDR found in pancreatic β-cells, and 1a-hydroxylase expressed in pancreatic islet tissues (53, 54) also suggests that vitamin D directly influences insulin production. On the other hand, vitamin D insufficient causes low muscle mass and muscle weakness. Given the cross-talk between muscle and bone (55, 56), 1,25(OH)2D3 may also regulate bone metabolism indirectly via affecting skeletal muscle. This evidence may account for the better effects found in the VD3-KO-HG group in our study.
In summary, the results of this study expand our understanding of the endocrine function of the skeleton in a new perspective. We show that 1,25(OH)2D3 treatment rescues the energy and bone metabolism disorder induced by diabetes. We further confirm that 1,25(OH)2D3 activates PI3K/Akt through combination with VDR in vivo and in vitro, which subsequently phosphorylates FoxO1 and thus promotes GlaOC and GluOC secretion. In addition, sole FoxO1 knock-out in osteoblasts without 1,25(OH)2D3 treatment can, in part, promote osteogenic phenotype and ameliorate oxidative stress. Our data demonstrate that FoxO1 is a critical mediator of glucose homeostasis and bone metabolism and suggest that FoxO1 may be a potential target for treatment of diabetes and diabetes-induced osteoporosis.
Experimental procedures
Animal study
FoxO1OB−/− mice were obtained by crossing FVB.129S6 (Cg)-FoxO1<tm1Rdp>/J (FoxO1fl/fl) mice and B6N.FVB-Tg(BGLAP-cre)1Clem/J (Cre+/−) mice (57, 58), which were both purchased from Jackson Laboratories. Phenotypes of mice were identified using PCR. FoxO1fl/fl mice were first crossed with Cre± mice to produce FoxO1fl/− Cre+/− mice, which were subsequently crossed with FoxO1fl/fl mice. FoxO1OB−/− mice and their WT littermates were used for the following experiments. All animal care and experiments were conducted in accordance with international standards on animal welfare and approved by the Animal Research Committee of Sichuan University (Chengdu, China). Mouse DM models were established using repeated low doses of (40 mg/kg) streptozotocin (Sigma) via intraperitoneal injection (59). One week after the final injection, mice with a blood glucose>150 mg/dl higher than control mice were validated for further study.
Forty mice in total were divided into following five groups: normal WT mice (WT); 1,25(OH)2D3-treated diabetic FoxO1OB−/−mice (VD3-DB-KO); 1,25(OH)2D3-treated diabetic WT mice (VD3-DB-WT); untreated diabetic FoxO1OB−/− mice (DB-KO); and untreated diabetic WT mice (DB-WT). The implant surgery has been previously described (60). 1,25(OH)2D3 was dissolved in propylene glycol and alcohol (4:1) and given at a dose of 5 μg/kg via intraperitoneal injection every other day for 4 weeks. The mice were then sacrificed 2 months post-operation. Serum and femurs with implants were collected for further study.
Cell culture and identification
Calvaria from 3-day-old mice were dissected aseptically and placed in tissue culture dish containing PBS and 5× penicillin and streptomycin (pH 7.4). After stripping off the periosteum, the calvaria were cut into 0.5 × 0.5-mm2 pieces with scissors and removed into a culture flask containing 5 ml of αMEM supplemented with 10% FBS (Gibco), 100 units/ml penicillin, and 100 mg/ml streptomycin sulfate. The inverted flask was placed in incubator and turned over 2 h later. The calvaria were routinely incubated at 37 °C in 5% CO2. Medium was replaced every 3 days until cells were subconfluent. OBs were identified via alizarin red staining and osteocalcin (OCN) immunofluorescence before being used in the following experiments.
OBs in their third passage were detached and reseeded. After a 2-day culture to subconfluence in αMEM containing 10% FBS and 22 mmol/liter glucose, cells from FoxO1OB−/− mice and WT mice were respectively trypsinized, reseeded, and treated as follows: normal glucose (5.5 mmol/liter)-treated WT OBs (WT), high glucose (22 mmol/liter), and 1,25(OH)2D3 (10−8 mol/liter; Sigma)-treated FoxO1OB−/− OBs (VD3-HG-KO), high glucose–treated and 1,25(OH)2D3-treated WT OBs (VD3-HG-WT), high glucose–treated FoxO1OB−/− OBs (HG-KO), and high glucose–treated WT OBs (HG-WT).
Cell viability and differentiation assay
For viability assay, the cells were seeded at a density of 5 × 103/well in 96-well plates and allowed to adhere for 24 h with αMEM containing 10% FBS. Serum-free medium was applied for another 24-h culture before cells were treated in different conditions described previously. Cell Counting Kit-8 (CCK8, Dojindo, Japan) was employed to detect cell proliferation at 24, 48, and 72 h, respectively. Cell viability was measured by absorbance at 450 nm using a microplate reader SpectroMAX spectrophotometer (NanoDrop). For osteogenic differentiation, alizarin red (Sigma–Aldrich) staining was conducted. Briefly, after a 3-week osteogenic induction with mineralized solution (10 mm β-glycerophosphate, 50 mg/ml ascorbic acid, and 10 nm dexamethasone), medium in a 6-well plates was removed, and cells were fixed with 4% paraformaldehyde for 30 min. The induced deposition was stained using 1% alizarin red S solution at pH 4.2 for 1 h at room temperature. Mineralization of nodules were dissolved with 10% cetylpyridinium chloride, and the absorbance was examined at 562 nm. For ALP assay, cells were respectively harvested at time point of 4 and 8 days and lysed according to four standard freeze-thaw cycles and ultrasonication. Alkaline phosphatase assay kit (Beyotime) was used to evaluate the absorbance at 405 nm of p-nitrophenyl, which was a hydrolysate of p-nitrophenyl phosphate catalyzed by ALP. The same samples were used to measure total protein levels by the BCA protein assay kit (Beyotime) following the manufacturer's instructions. The results were expressed in nmol/min/mg protein of produced p-nitrophenol (61).
Real-time PCR and Western blot analysis
Total RNA from each sample was extracted using TRIzol reagent (Invitrogen) according to the manufacturer's protocol. cDNA was synthesized from 1-μg purified RNA templates using PrimeScript RT reagent kit with gDNA Eraser (Takara Bio, Inc., Shiga, Japan). The expression of ALP, COL-1, OCN, and OPN at 4 and 8 days of culture in different treatment was subsequently quantified with ABI 7300 real-time PCR system (Applied Biosystems, Foster City, CA) and SYBR Premix Ex Taq (Takara, Japan). Primers of ALP, COL-1, OCN, OPN, and GAPDH are listed in Table 1. The relative expression levels of the target genes were normalized to the expression of endogenous housekeeping gene GAPDH using 2−ΔΔCt method.
Table 1.
Primer sequences for RT-qPCR
| Gene (mouse) | Forward (5′ → 3′) | Reverse (5′ → 3′) | Accession no. |
|---|---|---|---|
| FoxO1fl | ACCACTCTGGACGGCATACT | TGAGTCTGGGGCTAGTTTGA | |
| BGLAP-cre | CAAATAGCCCTGGCAGATTC | TGATACAAGGGACATCTTCC | |
| ALP | AACCCAGACACAAGCATTCC | GCCTTTGAGGTTTTTGGTCA | NM_007431.3 |
| COL-1 | GATGACGTGCAATGCAATGAA | CCCTCGACTCCTACATCTTCTGA | NM_007742.4 |
| OCN | GCGCTCTGTCTCTCTGACCT | ACCTTATTGCCCTCCTGCTT | NM_031368.5 |
| OPN | CCCGGTGAAAGTGACTGATT | TTCTTCAGAGGACACAGCATTC | NM_009263.3 |
| GAPDH | AAGGCCGGGGCCCACTTGAA | GGACTGTGGTCATGAGCCCTTCCA | NM_008084.3 |
Cell protein was obtained using KeyGEN whole cell lysis assay (KeyGEN) as per the manufacturer's instructions. 50 μg of protein extracts were separated by 10% SDS-PAGE gels, transferred onto a PVDF membrane (Millipore Corp., Bedford, MA), and then incubated at 4 °C overnight with rabbit polyclonal anti-VDR (1:100, Abcam), rabbit polyclonal anti-pAkt (1:500, Cell Signaling Technology), rabbit monoclonal anti-Akt (1:500, Cell Signaling Technology), rabbit monoclonal anti-pFoxO1 (1:100, Santa Cruz), rabbit polyclonal anti-FoxO1 (1:100, Santa Cruz), and mouse polyclonal anti-β-actin (1:1000, Cell Signaling Technology), respectively. After the successful binding of HRP-conjugated secondary antibody (1:1000) to primary antibodies, immunoreactive bands were detected using an ECL kit (Millipore, Billerica, MA). The density of the bands were measured by Quantity One (Bio-Rad).
ELISA
To detect the concentration of tOCN and ucOCN, ELISA (Abcam) was conducted according to the manufacturer's instructions. Blood samples and culture supernatants were collected 2 months and 8 days after 1,25(OH)2D3 treatment, respectively. For quantification of uncarboxylated osteocalcin, both serum and supernatant samples were incubated with hydroxyapatite (calcium phosphate tribasic; Sigma) for 1 h. The supernatant containing the ucOC was removed and analyzed by the ELISA kit. The percentage of ucOC (%ucOC) was calculated as the ratio of unadsorbed tOC (i.e. the amount remaining in the supernatant fluid to total osteocalcin multiplied by 100) (62). The insulin (Mercodia, Sweden) level and serum value of 25(OH)D3 (R&D System) were measured by ELISA following the manufacturer's instructions.
Immunofluorescence and immunohistochemistry
OBs in different groups were seeded on coverslips until confluent. After 4% paraformaldehyde fixing for 20 min and 2% Triton X-100 permeabilizing for 5 min, the cells were then blocked with 5% bovine serum albumin (Sigma) for 30 min and incubated with primary antibodies of VDR (1:50, Santa Cruz Biotechnology), p-Akt (1:50, Cell Signaling Technology), and FoxO1 (1:50, Santa Cruz Biotechnology) respectively at 4 °C overnight, whereas negative control was incubated with PBS. Samples were subsequently incubated with fluorescein isothiocyanate-conjugated secondary antibodies (1:50; Zsjq Bio Co., Beijing, China) for 1 h. The nuclei were further stained with DAPI (1:1000; Beyotime, Shanghai, China). The cells were observed using a fluorescent microscopy (Olympus, Tokyo, Japan).
For histology and immunohistochemistry, femurs were harvested 2 months postoperatively and maintained in 4% neutral paraformaldehyde for 2 days. Bones were washed with running water for 12 h before being subjected to decalcification in 10% EDTA-buffered solution for 4 weeks. The samples were then dehydrated using graded ethanol, vitrified by dimethylbenzene, and embedded in paraffin. 5-μm longitudinal sections were obtained. Bones from different groups were stained with hematoxylin and eosin. Additionally, bone sections were immunostained for VDR (Abcam), p-Akt (Cell Signaling Technology), and FoxO1 (Santa Cruz) using rabbit anti-VDR monoclonal antibody, rabbit anti-pAkt monoclonal antibody, and rabbit anti-FoxO1 polyclonal antibody, respectively. All the immunostained sections were quenched in 3% H2O2 for 5 min. Antigens were retrieved in boiling citrate buffer (pH 6.0) for 8 min, three times. After being blocked in 5% goat serum for 30 min to avoid nonspecific staining, sections were incubated with primary antibodies overnight at 4 °C. Specimens were rewarmed at room temperature for 30 min before secondary antibodies were applied to combine primary antibodies. Sections were finally developed using 3,3′-diaminobenzidine (63) and counterstained with hematoxylin.
Statistical analysis
Statistical analysis was conducted by SPSS 17.0 (SPSS, Inc., Chicago, IL). The results are given as means ± S.D. The data were analyzed using one-way analysis of variance followed by Newman–Keuls Student's t test. A significant difference was assumed at p < 0.05 (without specific explanation: a, p < 0.05 for WT versus others; b, p < 0.05 for VD3-HG-WT or HG-KO or HG-WT versus VD3-HG-KO; c, p < 0.05 for HG-KO or HG-WT versus VD3-HG-WT; and d, for HG-WT versus HG-KO.
Author contributions
Y. W. and P. G. designed the research. Y. X. and Y. Z. performed the in vitro and in vivo experiments and collected the data; N. X., Y. Y., and Q. Z. assisted with animal study; and Y. X. and Y. Z. analyzed the data and wrote the paper. All the authors read and approved the final version of the manuscript.
This work was supported by National Natural Science Foundation of China Grants NSFC 81400543 and NSFC 81571008 and Outstanding Scholars Research Funding of Sichuan University (2017SCU04A21). The authors declare that they have no conflicts of interest with the contents of this article.
Y. Xiong, Y. Zhang, N. Xin, Y. Yuan, Q. Zhang, P. Gong, and Y. Wu, unpublished results.
- DM
- diabetes mellitus
- 1,25(OH)2D3
- 1α,25-dihydroxyvitamin D3
- COL-1
- collagen 1
- OCN
- osteocalcin
- GluOC
- uncarboxylated OCN
- OPN
- osteopontin
- ALP
- alkaline phosphatase
- tOCN
- total osteocalcin
- ucOCN%
- the percentage of uncarboxylated osteocalcin
- VDR
- vitamin D receptor
- OB
- osteoblast
- αMEM
- α minimum Eagle's medium.
References
- 1. Ghosh P., Sahoo R., Vaidya A., Chorev M., and Halperin J. A. (2015) Role of complement and complement regulatory proteins in the complications of diabetes. Endocr. Rev. 36, 272–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. O'Loughlin A., McIntosh C., Dinneen S. F., and O'Brien T. (2010) Basic concepts to novel therapies: A review of the diabetic foot. Int. J. Low. Extrem. Wounds 9, 90–102 [DOI] [PubMed] [Google Scholar]
- 3. Carnevale V., Romagnoli E., and D'Erasmo E. (2004) Skeletal involvement in patients with diabetes mellitus. Diabetes Metab. Res. Rev. 20, 196–204 [DOI] [PubMed] [Google Scholar]
- 4. Schwartz A. V. (2003) Diabetes mellitus: does it affect bone? Calcif. Tissue Int. 73, 515–519 [DOI] [PubMed] [Google Scholar]
- 5. Vestergaard P., Rejnmark L., and Mosekilde L. (2009) Diabetes and its complications and their relationship with risk of fractures in type 1 and 2 diabetes. Calcif. Tissue Int. 84, 45–55 [DOI] [PubMed] [Google Scholar]
- 6. Janghorbani M., Van Dam R. M., Willett W. C., and Hu F. B. (2007) Systematic review of type 1 and type 2 diabetes mellitus and risk of fracture. Am. J. Epidemiol. 166, 495–505 [DOI] [PubMed] [Google Scholar]
- 7. Ahmed L. A., Joakimsen R. M., Berntsen G. K., Fønnebø V., and Schirmer H. (2006) Diabetes mellitus and the risk of non-vertebral fractures: the Tromso study. Osteoporos. Int. 17, 495–500 [DOI] [PubMed] [Google Scholar]
- 8. Strotmeyer E. S., and Cauley J. A. (2007) Diabetes mellitus, bone mineral density, and fracture risk. Curr. Opin. Endocrinol. Diabetes Obes. 14, 429–435 [DOI] [PubMed] [Google Scholar]
- 9. Schwartz A. V., and Sellmeyer D. E. (2004) Women, type 2 diabetes, and fracture risk. Curr. Diab. Rep. 4, 364–369 [DOI] [PubMed] [Google Scholar]
- 10. Oikarinen K., Raustia A. M., and Hartikainen M. (1995) General and local contraindications for endosseal implants: an epidemiological panoramic radiograph study in 65-year-old subjects. Community Dent. Oral Epidemiol. 23, 114–118 [DOI] [PubMed] [Google Scholar]
- 11. Fiorellini J. P., Nevins M. L., Norkin A., Weber H. P., and Karimbux N. Y. (1999) The effect of insulin therapy on osseointegration in a diabetic rat model. Clin. Oral Implants Res. 10, 362–368 [DOI] [PubMed] [Google Scholar]
- 12. Hasegawa H., Ozawa S., Hashimoto K., Takeichi T., and Ogawa T. (2008) Type 2 diabetes impairs implant osseointegration capacity in rats. Int. J. Oral Maxillofac. Implants 23, 237–246 [PubMed] [Google Scholar]
- 13. Gerritsen M., Lutterman J. A., and Jansen J. A. (2000) Wound healing around bone-anchored percutaneous devices in experimental diabetes mellitus. J. Biomed. Mater. Res. 53, 702–709 [DOI] [PubMed] [Google Scholar]
- 14. Ponugoti B., Dong G., and Graves D. T. (2012) Role of forkhead transcription factors in diabetes-induced oxidative stress. Exp. Diabetes Res. 2012, 939751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brunet A., Sweeney L. B., Sturgill J. F., Chua K. F., Greer P. L., Lin Y., Tran H., Ross S. E., Mostoslavsky R., Cohen H. Y., Hu L. S., Cheng H. L., Jedrychowski M. P., Gygi S. P., Sinclair D. A., et al. (2004) Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 303, 2011–2015 [DOI] [PubMed] [Google Scholar]
- 16. Essers M. A., Weijzen S., de Vries-Smits A. M., Saarloos I., de Ruiter N. D., Bos J. L., and Burgering B. M. (2004) FOXO transcription factor activation by oxidative stress mediated by the small GTPase Ral and JNK. EMBO J. 23, 4802–4812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ferron M., Wei J., Yoshizawa T., Del Fattore A., DePinho R. A., Teti A., Ducy P., and Karsenty G. (2010) Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell 142, 296–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kousteni S. (2012) FoxO1, the transcriptional chief of staff of energy metabolism. Bone 50, 437–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. van der Horst A., and Burgering B. M. (2007) Stressing the role of FoxO proteins in lifespan and disease. Nat. Rev. Mol. Cell Biol. 8, 440–450 [DOI] [PubMed] [Google Scholar]
- 20. Rached M. T., Kode A., Silva B. C., Jung D. Y., Gray S., Ong H., Paik J. H., DePinho R. A., Kim J. K., Karsenty G., and Kousteni S. (2010) FoxO1 expression in osteoblasts regulates glucose homeostasis through regulation of osteocalcin in mice. J. Clin. Invest. 120, 357–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Obsil T., and Obsilova V. (2008) Structure/function relationships underlying regulation of FOXO transcription factors. Oncogene 27, 2263–2275 [DOI] [PubMed] [Google Scholar]
- 22. Pérez-López F. R. (2007) Vitamin D and its implications for musculoskeletal health in women: an update. Maturitas 58, 117–137 [DOI] [PubMed] [Google Scholar]
- 23. Schöttker B., Herder C., Rothenbacher D., Perna L., Müller H., and Brenner H. (2013) Serum 25-hydroxyvitamin D levels and incident diabetes mellitus type 2: a competing risk analysis in a large population-based cohort of older adults. Eur. J. Epidemiol. 28, 267–275 [DOI] [PubMed] [Google Scholar]
- 24. Afzal S., Bojesen S. E., and Nordestgaard B. G. (2013) Low 25-hydroxyvitamin D and risk of type 2 diabetes: a prospective cohort study and metaanalysis. Clin. Chem. 59, 381–391 [DOI] [PubMed] [Google Scholar]
- 25. Jehle S., Lardi A., Felix B., Hulter H. N., Stettler C., and Krapf R. (2014) Effect of large doses of parenteral vitamin D on glycaemic control and calcium/phosphate metabolism in patients with stable type 2 diabetes mellitus: a randomised, placebo-controlled, prospective pilot study. Swiss Med. Wkly. 144, w13942. [DOI] [PubMed] [Google Scholar]
- 26. Wu Y. Y., Yu T., Yang X. Y., Li F., Ma L., Yang Y., Liu X. G., Wang Y. Y., and Gong P. (2013) Vitamin D3 and insulin combined treatment promotes titanium implant osseointegration in diabetes mellitus rats. Bone 52, 1–8 [DOI] [PubMed] [Google Scholar]
- 27. Chen S., Villalta S. A., and Agrawal D. K. (2016) FOXO1 mediates vitamin D deficiency-induced insulin resistance in skeletal muscle. J. Bone Miner. Res. 31, 585–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee D. H., Lim B. S., Lee Y. K., and Yang H. C. (2006) Effects of hydrogen peroxide (H2O2) on alkaline phosphatase activity and matrix mineralization of odontoblast and osteoblast cell lines. Cell Biol. Toxicol. 22, 39–46 [DOI] [PubMed] [Google Scholar]
- 29. Xiang L., Ma L., He Y., Wei N., and Gong P. (2014) Osteogenic differentiation of human periodontal ligament cells after transfection with recombinant lentiviral vector containing follicular dendritic cell secreted protein. J. Periodontal Res. 49, 554–562 [DOI] [PubMed] [Google Scholar]
- 30. Kurra S., Fink D. A., and Siris E. S. (2014) Osteoporosis-associated fracture and diabetes. Endocrinol. Metab. Clin. North Am. 43, 233–243 [DOI] [PubMed] [Google Scholar]
- 31. Rached M. T., Kode A., Xu L., Yoshikawa Y., Paik J. H., Depinho R. A., and Kousteni S. (2010) FoxO1 is a positive regulator of bone formation by favoring protein synthesis and resistance to oxidative stress in osteoblasts. Cell Metab. 11, 147–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ambrogini E., Almeida M., Martin-Millan M., Paik J. H., Depinho R. A., Han L., Goellner J., Weinstein R. S., Jilka R. L., O'Brien C. A., and Manolagas S. C. (2010) FoxO-mediated defense against oxidative stress in osteoblasts is indispensable for skeletal homeostasis in mice. Cell Metab. 11, 136–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Botolin S., and McCabe L. R. (2006) Chronic hyperglycemia modulates osteoblast gene expression through osmotic and non-osmotic pathways. J. Cell. Biochem. 99, 411–424 [DOI] [PubMed] [Google Scholar]
- 34. Iyer S., Han L., Ambrogini E., Yavropoulou M., Fowlkes J., Manolagas S. C., and Almeida M. (2017) Deletion of FoxO1, 3, and 4 in osteoblast progenitors attenuates the loss of cancellous bone mass in a mouse model of type 1 diabetes. J. Bone Miner. Res. 32, 60–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Iyer S., Ambrogini E., Bartell S. M., Han L., Roberson P. K., de Cabo R., Jilka R. L., Weinstein R. S., O'Brien C. A., Manolagas S. C., and Almeida M. (2013) FOXOs attenuate bone formation by suppressing Wnt signaling. J. Clin. Invest. 123, 3409–3419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ducy P., Desbois C., Boyce B., Pinero G., Story B., Dunstan C., Smith E., Bonadio J., Goldstein S., Gundberg C., Bradley A., and Karsenty G. (1996) Increased bone formation in osteocalcin-deficient mice. Nature 382, 448–452 [DOI] [PubMed] [Google Scholar]
- 37. Lee N. K., Sowa H., Hinoi E., Ferron M., Ahn J. D., Confavreux C., Dacquin R., Mee P. J., McKee M. D., Jung D. Y., Zhang Z., Kim J. K., Mauvais-Jarvis F., Ducy P., and Karsenty G. (2007) Endocrine regulation of energy metabolism by the skeleton. Cell 130, 456–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zoch M. L., Clemens T. L., and Riddle R. C. (2016) New insights into the biology of osteocalcin. Bone 82, 42–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wei J., Hanna T., Suda N., Karsenty G., and Ducy P. (2014) Osteocalcin promotes beta-cell proliferation during development and adulthood through Gprc6a. Diabetes 63, 1021–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mizokami A., Kawakubo-Yasukochi T., and Hirata M. (2017) Osteocalcin and its endocrine functions. Biochem. Pharmacol. 132, 1–8 [DOI] [PubMed] [Google Scholar]
- 41. Thomas D. M., Hards D. K., Rogers S. D., Ng K. W., and Best J. D. (1996) Insulin receptor expression in bone. J. Bone Miner. Res. 11, 1312–1320 [DOI] [PubMed] [Google Scholar]
- 42. Kanazawa I., Yamaguchi T., and Sugimoto T. (2011) Serum insulin-like growth factor-I is a marker for assessing the severity of vertebral fractures in postmenopausal women with type 2 diabetes mellitus. Osteoporos. Int. 22, 1191–1198 [DOI] [PubMed] [Google Scholar]
- 43. Klein G. L. (2014) Insulin and bone: recent developments. World J. Diabetes 5, 14–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yang J., Zhang X., Wang W., and Liu J. (2010) Insulin stimulates osteoblast proliferation and differentiation through ERK and PI3K in MG-63 cells. Cell Biochem. Funct. 28, 334–341 [DOI] [PubMed] [Google Scholar]
- 45. Canalis E. (1993) Systemic and local factors and the maintenance of bone quality. Calcif. Tissue Int. 53, S90–S93 [DOI] [PubMed] [Google Scholar]
- 46. Kousteni S. (2011) FoxO1: a molecule for all seasons. J. Bone Miner. Res. 26, 912–917 [DOI] [PubMed] [Google Scholar]
- 47. Ghodsi M., Larijani B., Keshtkar A. A., Nasli-Esfahani E., Alatab S., and Mohajeri-Tehrani M. R. (2016) Mechanisms involved in altered bone metabolism in diabetes: a narrative review. J. Diabetes Metab. Disord. 15, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wu Y. Y., Yu T., Zhang X. H., Liu Y. S., Li F., Wang Y. Y., Wang Y. Y., and Gong P. (2012) 1,25(OH)2D3 inhibits the deleterious effects induced by high glucose on osteoblasts through undercarboxylated osteocalcin and insulin signaling. J. Steroid Biochem. Mol. Biol. 132, 112–119 [DOI] [PubMed] [Google Scholar]
- 49. Cheng Z., and White M. F. (2011) Targeting forkhead box O1 from the concept to metabolic diseases: lessons from mouse models. Antioxid. Redox Signal. 14, 649–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Huhtakangas J. A., Olivera C. J., Bishop J. E., Zanello L. P., and Norman A. W. (2004) The vitamin D receptor is present in caveolae-enriched plasma membranes and binds 1α,25(OH)2-vitamin D3 in vivo and in vitro. Mol. Endocrinol. 18, 2660–2671 [DOI] [PubMed] [Google Scholar]
- 51. Thomas P., Pang Y., Filardo E. J., and Dong J. (2005) Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology 146, 624–632 [DOI] [PubMed] [Google Scholar]
- 52. Srivastava D. P., Yu E. J., Kennedy K., Chatwin H., Reale V., Hamon M., Smith T., and Evans P. D. (2005) Rapid, nongenomic responses to ecdysteroids and catecholamines mediated by a novel Drosophila G-protein-coupled receptor. J. Neurosci. 25, 6145–6155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mathieu C., Gysemans C., Giulietti A., and Bouillon R. (2005) Vitamin D and diabetes. Diabetologia 48, 1247–1257 [DOI] [PubMed] [Google Scholar]
- 54. Littorin B., Blom P., Schölin A., Arnqvist H. J., Blohmé G., Bolinder J., Ekbom-Schnell A., Eriksson J. W., Gudbjörnsdottir S., Nyström L., Ostman J., and Sundkvist G. (2006) Lower levels of plasma 25-hydroxyvitamin D among young adults at diagnosis of autoimmune type 1 diabetes compared with control subjects: results from the nationwide Diabetes Incidence Study in Sweden (DISS). Diabetologia 49, 2847–2852 [DOI] [PubMed] [Google Scholar]
- 55. Brotto M., and Bonewald L. (2015) Bone and muscle: interactions beyond mechanical. Bone 80, 109–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Brotto M., and Johnson M. L. (2014) Endocrine crosstalk between muscle and bone. Curr. Osteoporos. Rep. 12, 135–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Paik J. H., Kollipara R., Chu G., Ji H., Xiao Y., Ding Z., Miao L., Tothova Z., Horner J. W., Carrasco D. R., Jiang S., Gilliland D. G., Chin L., Wong W. H., Castrillon D. H., et al. (2007) FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell 128, 309–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dacquin R., Starbuck M., Schinke T., and Karsenty G. (2002) Mouse α1(I)-collagen promoter is the best known promoter to drive efficient Cre recombinase expression in osteoblast. Dev. Dyn. 224, 245–251 [DOI] [PubMed] [Google Scholar]
- 59. Furman B. L. (2015) Streptozotocin-induced diabetic models in mice and rats. Curr. Protoc. Pharmacol. 70, 5.47.1–5.47.20 [DOI] [PubMed] [Google Scholar]
- 60. Xiang L., Ma L., Wei N., Wang T., Yao Q., Yang B., Xiong Y., Wu Y., and Gong P. (2017) Effect of lentiviral vector overexpression α-calcitonin gene-related peptide on titanium implant osseointegration in α-CGRP-deficient mice. Bone 94, 135–140 [DOI] [PubMed] [Google Scholar]
- 61. Gao A., Hang R., Huang X., Zhao L., Zhang X., Wang L., Tang B., Ma S., and Chu P. K. (2014) The effects of titania nanotubes with embedded silver oxide nanoparticles on bacteria and osteoblasts. Biomaterials 35, 4223–4235 [DOI] [PubMed] [Google Scholar]
- 62. Liu G., and Peacock M. (1998) Age-related changes in serum undercarboxylated osteocalcin and its relationships with bone density, bone quality, and hip fracture. Calcif. Tissue Int. 62, 286–289 [DOI] [PubMed] [Google Scholar]
- 63. Wasielica-Berger J., Dlugosz J. W., Laszewicz W., Baniukiewicz A., Werpachowska I., Mroczko B., and Dabrowski A. (2007) Exocrine pancreatic function in biliary tract pathology treated with the endoscopic methods. Adv. Med. Sci. 52, 222–227 [PubMed] [Google Scholar]