Abstract
The ability to replace organs and tissues on demand could save or improve millions of lives each year globally and create public health benefits on par with curing cancer. Unmet needs for organ and tissue preservation place enormous logistical limitations on transplantation, regenerative medicine, drug discovery, and a variety of rapidly advancing areas spanning biomedicine. A growing coalition of researchers, clinicians, advocacy organizations, academic institutions, and other stakeholders has assembled to address the unmet need for preservation advances, outlining remaining challenges and identifying areas of underinvestment and untapped opportunities. Meanwhile, recent discoveries provide proofs of principle for breakthroughs in a family of research areas surrounding biopreservation. These developments indicate that a new paradigm, integrating multiple existing preservation approaches and new technologies that have flourished in the past 10 years, could transform preservation research. Capitalizing on these opportunities will require engagement across many research areas and stakeholder groups. A coordinated effort is needed to expedite preservation advances that can transform several areas of medicine and medical science.
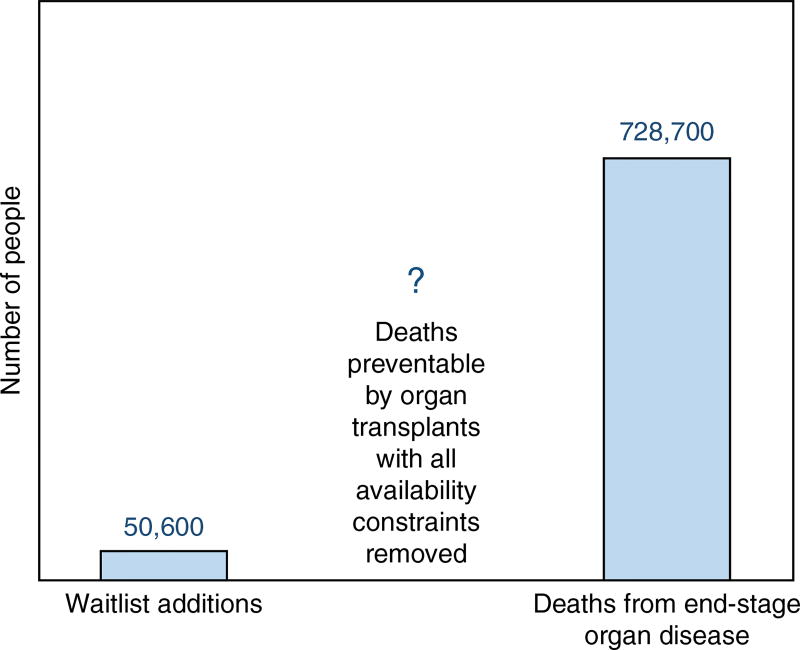
The global shortage of organs for transplantation has long been recognized as a major public health challenge, and the World Health Organization (Geneva) estimates that only 10% of the worldwide need for organ transplantation is being met1. The data suggest that the organ shortage is among the greatest crises facing biomedicine today. Although few estimates are available of the total number of patients who could benefit from organ transplantation if supply constraints were removed, the commonly cited transplant waiting lists clearly fail to capture the organ shortage’s true magnitude. For example, the number of patients added to US transplant waiting lists each year—roughly 50,000—is dwarfed by the ~730,000 annual US deaths attributable to end-stage organ disease (Fig. 1)2,3. As one example, the true need for heart transplantation in the United States has been estimated at more than ten times the heart transplant waiting list4,5. It has been suggested that with all supply constraints removed, organ replacement could theoretically prevent >30% of all deaths in the United States—doubling the average person’s likelihood of living to 80 years of age6–8. Similarly, estimates based on incidence of diseases that are potentially addressable by on-demand organ replacement place the true need at millions of transplant organs per year in the United States and Europe combined9.
Figure 1.
The true lifesaving potential of organ transplantation. The roughly 50,000 US patients added to transplant waiting lists in 2011 were outnumbered over 14-fold by those who died from end-stage organ disease (http://www.perfusix.com/impact-of-ex-vivo.html), without counting cases where malignancies could have been treated with organ replacement168. This suggests that the true size of the organ shortage could be many times larger than is reflected by transplant waiting lists (currently 120,000 US patients).
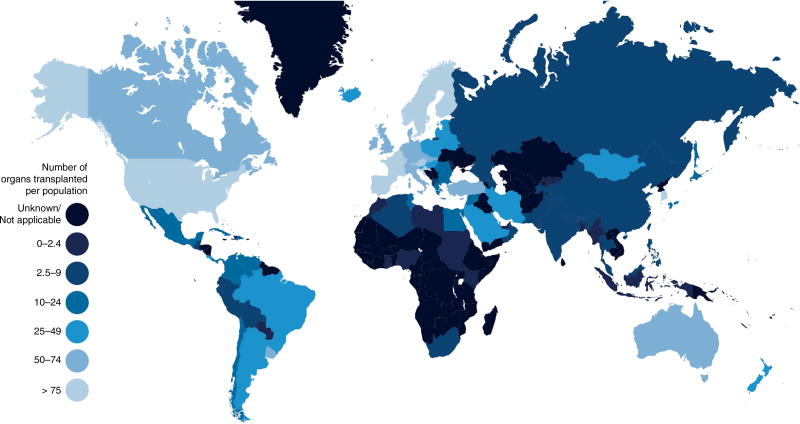
The organ shortage is markedly worse in many other countries. For instance, the continent of Africa holds 16% of the world’s population but performs only 0.5% of its organ transplants (Fig. 2). Moreover, in some of the countries with the least access to transplantation, a substantial fraction of transplant procedures are actually instances of transplant tourism10. In Pakistan, for instance, up to two-thirds of kidney transplants in 2005 are estimated to have been performed on foreigners10,11. The practice has widely been considered problematic because it creates opportunities for organ commodification and exploitation of vulnerable populations; the Declaration of Istanbul, signed by 157 representatives from countries across the globe, condemns the practice12. Healthcare infrastructure, national wealth, and sometimes cultural factors can each play a major role in the disparities in access to transplantation internationally. Yet over time, easing logistical burdens and increasing supply can lower the barriers to development of transplantation infrastructure4,5, and as discussed below, to equitable access to transplantation within countries.
Figure 2.
The global unmet need for transplantation greatly exceeds that of the United States (see Fig. 1), which contains roughly 4% of the world’s population but performs 25% of its organ transplants. By comparison, the continent of Africa contains roughly 16% of the world’s population but performs fewer than 0.5% of its organ transplants (http://www.transplant-observatory.org/summary/; https://esa.un.org/unpd/wpp/publications/files/key_findings_wpp_2015.pdf).
The above considerations should place technologies that can substantially increase the availability of transplant organs at the top of our scientific priority list. Moreover, the need for these technologies is shared with many other major public health challenges. Banking of viable organs and tissues can transform cancer treatment for young patients and have a dramatic impact on precision medicine and research on diseases such as heart disease, cancer, and Alzheimer’s disease. Ballooning costs in drug discovery are exacerbated by poor availability of human tissue models, which in many cases could provide more valuable data than the animal models currently used. Tissue transplantation faces enormous logistical barriers in emergency care because tissue is needed on such short notice. These challenges are magnified in contexts where large numbers of patients require care, such as the treatment of wounded service members and civilian victims of natural disasters or terrorist attacks. In these and many other areas, adequate techniques and treatments often already exist. However, their use is pervasively handicapped by the limited availability of organs and tissues, which are among the most precious resources in research and medicine. The aggregate toll on human health attributable to causes that could be addressed by increasing organ and tissue availability makes this problem one of the most important healthcare challenges of this era.
Developing an organ and tissue supply that can meet the healthcare demands of the twenty-first century requires the development of solutions to twin challenges: first, having enough of these lifesaving resources; and second, having the means to store and transport them for a variety of applications, each with distinct but overlapping logistical needs. Having enough organs and tissues to meet public health needs has been the subject of extensive efforts in science, medicine, and public policy aiming to increase organ donation13,14, improve donor organ utilization15–18, and gain the understanding needed to engineer laboratory-grown tissues19, bio-artificial organs20,21, and ‘humanized’ animal donor organs for transplantation22,23. The success of these efforts is intertwined with meeting the second challenge: preserving organs and tissues during procurement (or manufacturing), storage, transport, and other steps of the supply chain in order to meet logistical needs.
Despite its importance, the preservation challenge has received relatively little attention from funding agencies, the research community, and the general public. Taken together, preservation constraints place widespread burdens on efforts to use organs and tissues in transplantation, regenerative medicine, and research. Yet, although >80% of the budget of the US National Institutes of Health (NIH) goes to institutes with missions tied to unmet preservation needs24, and numerous other science agencies and stakeholder groups stand to benefit from preservation advances, no funding body has been charged with overcoming the remaining technical challenges common to the preservation of organ and tissue systems. As a result, the past half-century has seen only incremental and relatively ad hoc investments to advance preservation technologies.
By overcoming these institutional barriers and facilitating coordinated and cross-disciplinary research, it is now possible to dramatically accelerate progress in organ and tissue preservation using existing knowledge from a diverse array of fields. The past decade of progress has allowed us to understand and intervene in human physiology at the tissue and organ level as never before, with breakthroughs in nanotechnology, sequencing, imaging, omics approaches, and other areas. These technologies can be used to build on proofs of principle for organ cryopreservation6,7,25–28, discoveries from organisms that can enter ‘suspended animation’ at subzero temperatures29–32, rapid advances in perfusion technologies33–41, and other advances.
In light of these opportunities, a growing coalition of scientists, clinicians, policymakers, advocacy groups, academic institutions, and industry representatives is assembling to accelerate progress in organ and tissue preservation. This has led to an extensive dialog spanning more than a year, which has included the first global Organ Banking Summit at Stanford, NASA Research Park, and other locations7, a US National Science Foundation (NSF)-supported Roadmap to Organ Banking and Bioengineering Workshop6, a meeting hosted with the Defense Advanced Research Projects Agency (DARPA) leadership at the US Military Academy at West Point, New York, on a potential ‘Organs on Demand’ research program, a White House roundtable on organ banking and bioengineering, and a symposium and round-table on emerging organ preservation technologies on Capitol Hill. At these events, stakeholders have begun to outline the vast public health needs, remaining technological challenges, institutional and infrastructural barriers, and untapped research opportunities surrounding efforts to eliminate preservation constraints on the use of organs and tissues in biomedicine25. These efforts aim to facilitate the advancement of preservation platforms allowing us to transport, repair, assess, bank, and even enhance the health and function of organs and a variety of tissues used in research and medicine.
The diversity of authors of this article, with expertise spanning organ and tissue procurement and transplantation, preservation research, bioengineering, economics, trauma care, and regenerative medicine, reflects the breadth of need in this area—and the widespread concern that until preservation breakthroughs are pursued aggressively, many medical technologies will not come close to reaching their lifesaving potential. In the sections that follow, we describe how organ and tissue preservation can meet a variety of major public health needs. We also outline recent discoveries indicating that a revolution in organ and tissue preservation is now achievable, propose a novel paradigm for preservation involving convergence of a family of existing approaches, and describe how technologies have the potential to make a new generation of preservation technologies feasible. Finally, we suggest ways that the research community can overcome institutional barriers that hinder advances, and we highlight recent progress toward a coordinated research effort.
The unrealized potential of organ transplantation
Organ transplantation is one of the most impressive medical achievements of the past century. In the past 25 years, it has added over two million life-years to patients in the United States alone42. In the 60 years since its inception, researchers have made strides in drug-mediated immunosuppression43, achieved increasingly complex transplant operations44–46, and begun (recently) to move immune tolerance induction therapies into the clinic47–50.
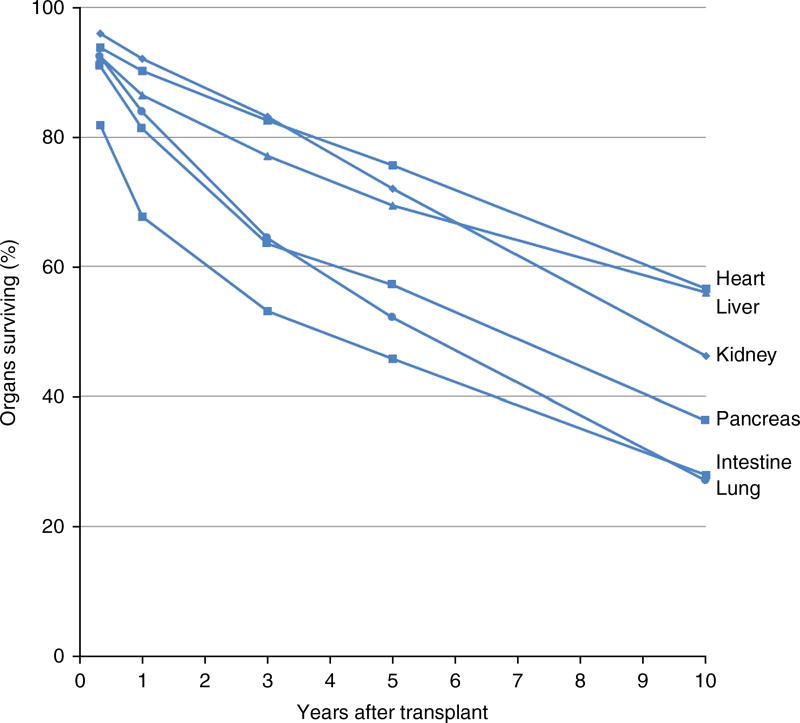
Yet access to transplantation and its efficacy are still fundamentally constrained. For the patients in need of organ replacement who are lucky enough to be placed on a transplant waiting list, morbidity and mortality are substantial51,52; one in five waiting-list patients in the United States will die or become too sick for a transplant before receiving a new organ52. In part because of the rare conditions that must exist for organs to be suitable for recovery and transplantation, today only 0.3% of those who die in the United States become organ donors3,53. Ideally, one organ donor can provide up to eight lifesaving organs to patients on transplant waiting lists, yet on average roughly only three are transplanted—despite decades of progress advancing organ procurement protocols and heroic efforts by organ procurement organizations2,53. Although advances in immunosuppression have greatly increased transplant success rates and graft survival54–56, half of these organs fail within 10 years of being transplanted, including as many as 75% of intestines and lungs (Fig. 3)57. To delay rejection, transplant recipients must adhere to lifelong immunosuppressant drug regimes, the side effects of which put patients at increased risk for life-threatening infections as well as cancer and other major age-related diseases58. Meanwhile, children, ethnic minorities, and other vulnerable patient populations have markedly reduced access to transplantation59–62. The toll on the economy of the unmet need for transplantation is immense; for instance, the worldwide cost of treating end-stage renal disease totals over $1 trillion in the course of a decade63, with over $40 billion spent by the United States in 2009 alone64.
Figure 3.
10-year graft survival for each of the six vital organs currently transplanted (single-organ, deceased donor transplant)49. 10-year survival rates for organs range from slightly over 50% (hearts and livers) to slightly over 25% (lungs and intestine). These data indicate that ensuring transplant organ quality and reducing susceptibility to chronic rejection are still major challenges in transplantation. Preservation advances present diverse opportunities to meet these challenges (Table 2).
These problems are fueled by severe logistical constraints related to organ preservation limits. Although leaps forward in machine perfusion33–41,65–67, organ cryopreservation26,27,68, understanding scientific mechanisms of ischemic injury and metabolic regulation29–32,41, and other areas have created a blueprint for transforming organ preservation, today maximum clinical organ preservation times are measured in hours, varying according to the organ transplanted, and necessitate transplantation almost immediately after the organ is recovered (http://www.nedonation.org/donation-guide/organ/acceptable-ischemic-times). Organs are rushed to their destinations, often by jet, or by helicopter flight straight to a landing pad on the transplant center rooftop. Speed is essential when arranging and performing the transplant surgery, leaving little room to adapt procedures to individual circumstances or deal with complications. Lengthy operations must be performed day or night with little advance warning. These factors contribute to high costs for organ transplantation, which in the United States can average well over $1 million (e.g., heart, intestine, and double lung transplant)69. During transplantation organs are exposed to a continuous barrage of inflammation and oxidative stress, both before and after organ procurement from the donor, contributing to immune rejection, delayed graft function, and other complications that harm transplant outcomes.
Donor organs and recipients must be matched over relatively short geographic distances and time periods, often resulting in the use of organs that are immunologically not well-matched to recipients. This puts patients at increased risk for organ rejection and contributes to the need for intensive immunosuppressive regimens70. The increased rate and severity of rejection limits organ life span, further exacerbating the organ shortage; within several years of transplantation, many patients are in need of a new organ all over again57. Limited matching distances leave waiting-list patients from different regions with unequal access to transplant organs as supply and need vary. These imbalances disproportionately affect patients with fewer resources, who cannot relocate to join more favorable waiting lists59,71. Matching limitations often fall hardest on populations with small pools of potential matching donors, particularly among children and ethnic minorities60,61,72,73.
Meanwhile, narrow windows of opportunity for organ assessment, allocation, and transplantation fuel organ discard. Organs are offered to individual patients on a waiting list, whose doctors must decide whether to recommend transplantation based on limited information about the organ’s suitability for transplantation; with little information on the organ’s functional status, some patients may turn it down when the donor’s history or other indicators are dubious, waiting until a less risky organ is (hopefully) made available52. Even a functional, transplantable organ may be turned down by the transplant centers of one patient after another until the organ’s preservation limits prevent further matching41,74. Each year, this phenomenon contributes to thousands of abdominal organs being discarded74,75, and the majority of thoracic organs from donors (~70% of heart and 80% of lung offers) going untransplanted2,76. Most likely, many of these organs could have been successfully transplanted under the right circumstances41,77–79. The resulting impact on waiting-list patients is profound. In the United States, if just 10% of the number of donor hearts currently left unused were transplanted, the number of additional hearts made available would equal the number of waiting-list patients who currently die or become too sick for a transplant before receiving one (Table 1)75.
Table 1.
Summary picture for four vital organs from deceased donors
Organ transplantation without preservation constraints
A successful large-scale organ preservation research effort would create a very different world for organ transplantation, creating a breadth of new capabilities that could make more organs available, improve transplant outcomes and mitigate risks, decrease costs, and complement and accelerate the development of other biomedical technologies that can alter the landscape of transplantation in the coming years (Table 2). For instance, preservation advances can build on promising strategies that use perfusion circuits to mimic healthy physiological conditions38,80,81. These platforms can allow the organ to recover from cellular stress and tissue injury during donor death and organ procurement, which can contribute to inflammation and organ rejection following transplantation80–83, and also enable therapeutic intervention before transplantation83–87. The advent of ex vivo organ perfusion shows promise to make larger pools of donor organs available by enabling rehabilitation of organs that would otherwise be unsuitable for transplantation38,41,82.
Table 2.
Preservation enables key transplant capabilities
| Goal | Capabilities |
|---|---|
| Increasing pool of donor organs | Reducing organ discard |
| Rescuing marginal organs | |
| Decreasing costs of transplantation | |
| New matching approaches in deceased donation | |
| Successive organ transplants in case of graft dysfunction | |
| Enhancing transplant viability and function | Repairing organ injury during removal and transport |
| Assessing organ function before transplant | |
| Enabling new immune-tolerance-induction strategies | |
| Transmissible disease screening for donors and organs | |
| Augmenting organs (e.g., gene therapy, immunomodulation) | |
| New donor-recipient compatibility assessment methods | |
| Preventing ischemic injury during transplant | |
| Expanding transplantation access | Extending live kidney donation chains |
| Enabling recipients with acute disease or trauma | |
| Flexible scheduling of transplant surgeries | |
| Galvanizing research | Accelerating progress in cryobiology and preservation |
| Accelerating progress in humanized xenotransplantation | |
| Accelerating progress toward lab-grown organs |
Perfusion-based preservation could be harnessed as a platform to functionally enhance organs. Any of several existing techniques might be part of the process to condition organs for transplantation or subsequent steps of the preservation process83; these include drug-mediated immunomodulation to apply treatments that block or alter sites recognized by the recipient’s immune system to mitigate rejection84,85; gene therapy86,87; antisense, or RNA interference. Similar interventions could even be used to improve organs’ health and function, making them in some ways healthier in the recipient than they were in the donor. For instance, perfusion platforms have allowed the ‘defatting’ of livers after removal in animal models of steatosis41, showing promise for mitigation or reversal of organ degeneration during the donor’s lifetime that could otherwise affect both transplant outcomes and, later, the recipient’s health15,88,89.
Perfusion platforms can allow transplantation of many organs that would otherwise be deemed too risky to transplant by allowing their health and function to be assessed outside the body37,41,87,90,91. For example, it has been proposed by the National Heart Lung and Blood Institute and found by other studies that many hearts that would provide substantial survival benefits to patients are going unused, largely owing to a lack of reliable methods to assess their suitability for transplantation79,92. By allowing organ function to be observed after procurement, perfusion platforms are enabling the investigation of new biomarkers that predict organ health and transplantability93–96.
A variety of preservation breakthroughs could enable transport of organs over longer distances7,40, opening up many new possibilities for organ allocation and therapeutic intervention. With distance no longer a factor in donor–recipient matching, closer matches could be achieved. This could decrease rejection and the need for immunosuppression70 and extend graft life span, while increasing access to transplantation for disadvantaged patient populations60,73. Organs could also be routed through specialized facilities, which have been suggested by several groups as a way to make technically challenging assessment, repair, functional augmentation, or banking procedures a clinical reality7,87,91. Thus, approaches that today would not be seriously considered could become practical and fruitful areas of innovation.
Preservation breakthroughs could allow organs to be banked in a state of suspended animation at subzero temperatures7,25–27,68, protected from ischemic injury and the damaging environment of the deceased donor body, for periods long enough to perform any assay needed on patients or tissue samples. This would enable more thorough screening for malignancies and transmissible diseases, such as rabies97,98 and HIV99. Currently, disease transmission rates in organ transplantation, although <1%, are estimated to be on the order of 10,000 times higher than in blood transfusion, where a maximum shelf life of 5–6 weeks enables the use of lengthy disease-screening assays100. Organ banking could also provide many new opportunities for matching, by uncoupling organ allocation from the narrow windows of time that currently constrain it.
Importantly, organ banking could prevent unnecessary loss of life by allowing any organs not immediately matched to be saved until a match could be found. This would make transplantation available to more patients, not only by offering a complementary organ supply but also by shifting the risk–benefit balance for patients and their transplant centers away from refusing transplantable organs. Primary graft dysfunction is a major cause of morbidity and mortality following transplantation101–103, and roughly 50–75% (depending on the organ transplanted) of all graft failure in the first year after transplantation occurs within the first 3 months57. Banked organs could provide a ‘backup’ supply in the case of early failure of the initial transplant; in cases where multiple matching organs were available, often multiple successive transplants would be feasible if the initial transplant fails. Thus, even when an organ individually carries a risk of early graft failure, the alternative supply of banked organs could substantially de-risk the overall process of transplantation, allowing transplant centers to accept the organ with substantially decreased patient risk.
Organ banking could also make transplantation a lifesaving treatment for heart attack victims, trauma patients, victims of accidental poisoning, and others with acute vital organ failure. For these patients, matching transplant organs need to be available within extremely short time periods, necessitating off-the-shelf solutions. Banking the substantial fraction of organs that go unused in the current allocation system could be lifesaving for these patients. The public health benefits of banking organs for emergency surgery could be vast; traumatic injury accounts for more deaths among adolescents and children than all other causes combined104. Similarly, an International Society of Heart and Lung Transplantation committee has estimated that a substantial proportion of heart attack victims could be saved if heart transplantation were available for these patients on demand4. This is a particularly attractive prospect given the large fraction of potentially transplantable hearts that currently cannot be matched79,92, which could be used to establish a heart bank. Further advancement of banking, assessment, and repair capabilities could allow this approach to benefit ever-larger patient populations.
The ability to save organs not immediately matched could be useful in live donation as well. In the recent innovation of live-kidney-donor chains, in which a patient who has found a willing donor (e.g., a friend or relative) whose kidney is not compatible exchanges their donor kidney for a compatible one, transplants are arranged in long chains, so that each patient in the chain receives a compatible kidney. However, chains end when no appropriate recipient can be immediately found for the last donor in the chain, who instead donates to an individual on the deceased-donor waiting list without a corresponding donor to continue the chain105,106. The opportunity to delay transplantation could create wider opportunities to find a donor–recipient pair who can continue the chain, allowing longer chains to be assembled.
Moreover, the ability to bank organs can aid in the development of technologies that could be game-changers for transplantation. For instance, a diverse array of immune tolerance induction approaches could overcome graft rejection while largely eliminating the need for immune suppression. Currently, all successful clinical trials involve living donors, so that tolerance induction treatments can be initiated before transplantation47–50. Temporary banking of donor organs could enable tolerance induction for deceased donor organs (the vast majority of transplants) as well, by allowing the required pretransplant preparative regimen to be administered to recipients before the organ transplant. This could give the patient’s immune system time to adapt to the donor’s antigens before transplantation, and it would also provide time to evaluate the success of tolerance induction protocols in a matched patient before transplantation.
Meanwhile, longer-term efforts to create lab-grown organs by tissue engineering or xenotransplantation of ‘humanized’ donor animal organs would be aided by the ability to bank inventories to make these approaches practical and cost effective at scale. With advances in immune tolerance induction22 and the advent of CRISPR–Cas9 gene editing methods that open the possibility of more complex genetic engineering of donor organs to make them less vulnerable to recipient immune rejection107, xenotransplantation could potentially offer a vast new source of transplant organs. But attainment of an engineered organ capable of engraftment and survival remains years away, and the investments required for clinical xenotransplantation are tremendous; large, centralized facilities would be required to produce transplant organs at scale, making cost-effective manufacturing and distribution a major concern unless these organs can be banked23. Similarly, shelf life has been widely recognized as a key bottleneck in the progress of tissue engineering6,108,109.
Challenges in complex tissue preservation
The same technologies that promise to transform vital organ preservation also advance the preservation of a vast array of tissue systems and address a large breadth of public health needs. Inadequate tissue preservation capabilities are a constraint on basic and preclinical biomedical research aimed at addressing major illnesses, drug testing and drug development, trauma care, stockpiling of medical countermeasures for large-scale public health emergencies, fertility restoration, as well as the advancement of tissue engineering and regenerative medicine (Table 3).
Table 3.
Unmet needs for organ preservation
| Area of biomedicine | Example of public health need |
|---|---|
| Organ transplantation | Almost 70% of US donor hearts go untransplanted, largely due to preservation limits on assessment and matching2,38,79,92,170 |
| Cancer treatment and fertility | Ovary banking can save fertility/hormone balance in 140,000 girls and young women diagnosed with cancer and potentially exposed to chemo- and radiotherapy in the United States each year117 |
| Emergency preparedness | Banked bone marrow and cord blood could benefit >10,000 patients after a nuclear accident or attack130,171 as well as 14,000 US patients each year suffering acute injury who would benefit from a transplant172 |
| Limb recovery and transplantation | 30,000 traumatic amputations per year in the United States; two-thirds of victims are children and young adults133 |
| Basic medical research | Human tissue would be a superior model to the 100 million mice and rats used in research each year173; tissue banking advances are critically needed to aid approaches seeking to treat malignancies174, neurodegenerative diseases175, and other disorders |
| Trauma care | 30,000 patients admitted to specialized US burn units each year176. After a nuclear accident/attack, and estimated ~3% of the skin grafts required would currently be available177 |
| Tissue engineering and regenerative medicine Drug discovery | Shelf life of regenerative medicine products, a sector with a predicted >$500-billion market by 2025178 Banked human tissue would benefit pre-clinical drug testing and potentially improve low efficiency of drug development179,180 |
Tissue engineering and regenerative medicine
Current preservation limits present major challenges for the clinical translation of tissue engineering breakthroughs. Without the ability to lengthen shelf life, any firm attempting to develop biomanufactured tissue products lacks capabilities for batch manufacturing and distribution, while also facing difficulties adjusting to changing demand8,19,24,110,111. Short product shelf life also prevents implementation of some methods for quality control for tissue and organ products, adding substantial cost and risk8,19,24,111.
Opportunities abound to enhance banking capabilities for tissue engineering. For instance, successful cryopreservation of a 2.3 liter biomass consisting of encapsulated liver spheroids for use in a bioartificial liver device may stimulate research on other large-volume tissue-engineered products112. These challenges have led the US Commerce Department, US Department of Defense (DoD), and the US government’s Multi-Agency Tissue Engineering Sciences working group coordinating tissue engineering research support across the NIH, NSF, the US National Institute of Standards and Technology, the DoD, and other science agencies, to identify preservation as one of the key bottlenecks in tissue engineering efforts6,108,109. For instance, a new Advanced Tissue Biofabrication Manufacturing Innovation Institute announced at the June 2016 White House Organ Summit has a major focus on advancing organ preservation113; the solicitation calls for preservation technologies to enable biomanufacturing to move from unscalable, just-in-time manufacturing to scalable models using off-the-shelf tissues109. Likewise, potential future initiatives under the Defense Innovation Unit-Experimental (http://www.diux.mil) in the areas of biofabrication, tissue engineering, regenerative medicine, and tissue-based chip devices will all likely require capabilities emerging from organ and tissue preservation advances.
Protection of reproductive tissue in cancer patients
Organ and tissue banking could also become a staple of cancer care for children and young adults. Ovary, uterus, and testis banking could be used to restore fertility and hormone balance to the 140,000 childhood and young adult cancer survivors in the United States each year27,114–117 and hundreds of thousands more each year worldwide. Reproductive organs are highly sensitive to injury from chemotherapy and radiation, often leaving survivors of childhood and young adult cancer infertile and with altered endocrine function, resulting in lifelong sexual and psychological side effects27,117,118. These complications could be prevented by saving and banking reproductive organs and tissue before treatment, then re-implanting them afterward27,28,119,120.
Thus far, >60 healthy offspring have been born to women who banked ovarian tissue before their first sterilizing cancer treatment121–123. Recently, whole sheep ovaries have been cryopreserved and reimplanted, and the sheep have gone on to produce healthy offspring27. Additional research can make ovary banking clinically feasible and yield insights applicable to banking testicular tissue, whole testes, and larger organs. With >1 million survivors of childhood and young adult cancer living in the United States alone (spanning roughly two generations)118,124, it is reasonable to estimate that reproductive organ and tissue banking could become the standard of care for millions of future cancer patients worldwide in the coming decades.
Countermeasures for public health emergencies
Advances in the preservation of many tissues are needed for trauma care, particularly to incorporate regenerative medicine therapies into strategic national stockpiles maintained by the United States’ interagency Public Health Emergency Medical Countermeasures Enterprise for natural disasters, nuclear accidents or attacks, chemical and biological weapons, and other large-scale public health threats. For instance, radiological threats stemming from nuclear accidents or attacks have led the US Biomedical Advanced Research and Development Authority (BARDA), charged with procuring medical countermeasures for the strategic national stockpile, to search for measures that can treat radiation injury125. A 10-kiloton nuclear blast could cause acute radiation injury in >1 million people across a >10-mile radius. Large quantities of stockpiled bone marrow, cord blood, and other sources of hematopoietic stem cells could be used either to permanently replace irreparably damaged bone marrow or to serve as a ‘bridge’ until the recovery of autologous hematopoiesis126. The blast from such an event could cause burns and/or trauma combined with radiation exposure in over 45,000 victims127, necessitating skin grafts for severe burns128,129, and blood vessel grafts for extremity injuries130. Similar need for on-demand bone marrow and skin could also arise from the use of mustard gas or other exfoliants. Similarly, a large supply of banked human tissue—particularly liver, kidney, brain, or heart slices—could be a critical resource for the rapid study of novel bioagents and development of medical countermeasures for biological and chemical terrorism threats.
Tissue preservation and banking advances are needed to incorporate these and other treatments into strategic national stockpiles. For instance, precision-cut tissue slices can currently be cultured only for a matter of days, precluding standardization in preparation and ondemand use to address bioterrorism threats131. Shortages of skin for use on demand have led both BARDA and the US military to look for biomanufacturing solutions to stockpile large quantities of skin for combat or emergencies, yet for both groups short tissue shelf life has been cited as a limiting factor25,129,130. Cryopreservation has enabled the banking and subsequent transplantation of both bone marrow and skin, but the current state of the art results in some loss of viability128,132. In the case of bone marrow banking, improvements to cryopreservation methods can also reduce the incidence of complications after transfusion132. Thus, preservation advances would help address large public health needs for these tissues. For entities such as the DoD and BARDA to successfully leverage advances in regenerative medicine, preservation research is a necessity; the nature of emergency response dictates that banked tissues must be available for off-the-shelf use.
Transplantation for acute injuries
Preservation advances could also dramatically increase patient access to transplantation or recovery of vascularized composite tissues, such as limbs, hands, or faces after traumatic injury. For example, roughly 30,000 traumatic amputations occur per year in the United States, over two-thirds in children and young adults; it has been estimated that there will be >900,000 survivors of traumatic amputation living in the United States by 2020133.
Extending preservation capabilities for recovered limbs can allow a greater number to be reattached, and in the past 15 years it has become possible to transplant hands, faces, and whole limbs from deceased donors44–46,134. Although ample donor pools are already available, these procedures are still not routine—largely because matching must be done very quickly (for both cosmetic and immunological criteria) and patients face complications from immunosuppression134. As discussed, preservation can address both of these challenges, playing a pivotal role in providing access to hand, limb, and face transplantation for tens of thousands of new patients each year.
An integrated approach to preservation
A growing body of evidence indicates that a transformation in organ and tissue preservation is now achievable. Recent promising discoveries include organ cryopreservation and subzero cooling, perfusion, interventions before organ and tissue recovery, and adaptations that allow dozens of species in nature to enter suspended animation at subfreezing temperatures (Table 4). Together, these approaches form a blueprint for a leap forward in preservation capabilities, centered on a combination of two promising strategies:
Providing organ ‘life support’ by recapitulating aspects of the organ’s healthy physiological environment.
Effectively controlling biological time by slowing or halting metabolism to decrease the rate of deterioration.
Table 4.
Proofs of principle already exist for each pillar of organ and tissue preservation
| Approach | Examples of proof-of-principle discoveries |
|---|---|

|
|

|
|

|
|

|
|

|
|
Photo credits: “Programmed metabolic suppression”: J.M. Storey, Carleton University; “Cryopreservation”: G.M.F.
Progress on both fronts is needed because each preservation approach involves tradeoffs often requiring the application of combined strategies in the same organ or tissue. For instance, slowing organ deterioration for extended preservation periods can be achieved by lowering organ temperature and metabolic rates, but this also entails the loss of normal organ function and opportunities for beneficial interventions, such as organ assessment, repair, and functional augmentation (Table 2).
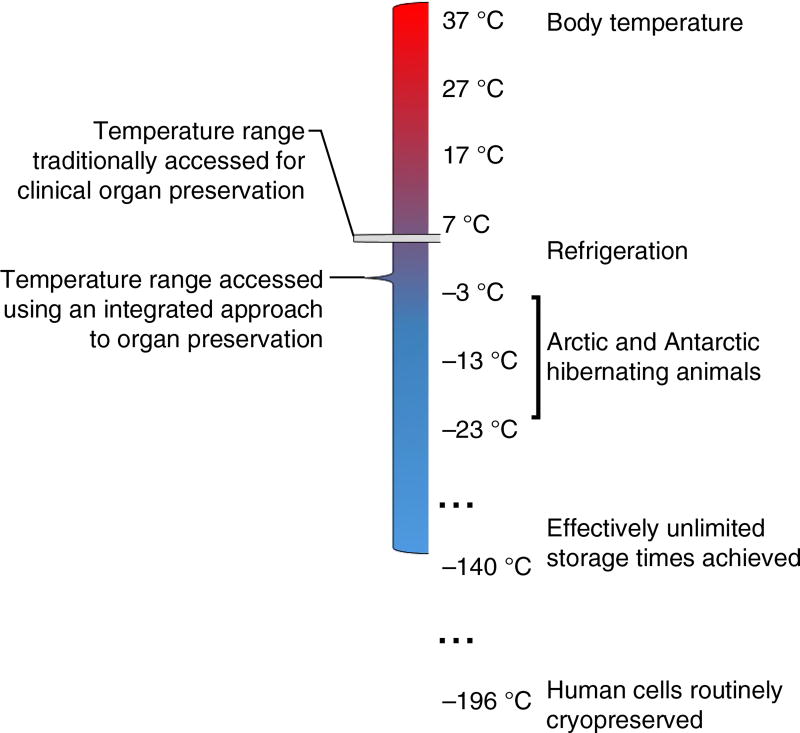
Thus, we must begin to think about the aim of preservation not as the pursuit of a singular ‘best’ environment to keep a particular organ or tissue healthy on its way to transplantation (or use in research), but as an ‘integrated’ process during which the organ or tissue traverses multiple preservation conditions and temperature ranges that are used synergistically (Fig. 4). To make an integrated approach to preservation successful, we must combine and advance a family of research areas that includes cryopreservation7, programmed metabolic suppression31, subzero preservation and supercooling135, and perfusion and ex vivo maintenance at a variety of temperatures, ranging from hypothermia (refrigeration) to normothermia (body temperature)34,35,37,136, and donor management before organ and tissue recovery137–139. The discoveries noted in Table 4 have provided various proofs of principle for using these approaches in organ and tissue preservation. They have historically been relatively siloed, despite the fact that they are complementary and often synergistic6,7,26,31,40,87,91,137.
Figure 4.
An integrated approach to organ and tissue preservation would combine multiple preservation conditions and temperature ranges, drawing on the strategies found in Table 4. Thus, when called for, differing preservation modalities could be used during successive stages of the preservation process, accessing a much wider range of temperatures and conditions than are currently used in conventional organ preservation. For instance, transplant organs could be held at subnormothermic temperatures during pharmacological pre-conditioning for cryopreservation, then cooled to cryogenic temperatures for transport or banking, then returned to near-normothermic temperatures for functional assessment. Many combinations are conceivable based on the diverse proof-of-principle discoveries; the optimal preservation protocol will most likely vary according to tissue type and application.
Advancing organ and tissue preservation through an integrated approach has become an achievable goal, as the past decade has seen an explosion of technologies enabling us to understand and intervene in human physiology at the tissue and organ level. Advances in cellular and tissue imaging140–142, organoids, organs on a chip and regenerative medicine13, high-throughput assays and sequencing readouts143, miniaturization and microfluidics144, nanotechnology145,146, and molecular engineering and gene editing147–152 can all be harnessed to galvanize research into the fundamental biology of tissue and organ cryopreservation, discover novel cryoprotectants, and develop new preservation strategies. This creates exciting prospects for translating the ‘suspended animation’ programs of animals, such as the arctic ground squirrel153 and even tardigrade154, into tools for organ and tissue preservation. This decade has also seen rapid advances in ex vivo perfusion platforms33,34,36–38,41,66, which can be adapted to recapitulate aspects of an organ’s in vivo environment, condition it for storage or transport in a hypometabolic state, or enhance recovery from stresses experienced during donor death or the preservation period40,155–158. By building on, and combining, these innovations from different disciplines, we are now poised to create a new generation of organ and tissue preservation capabilities driven by both public and private sector funding (Box 1).
Box 1 Burgeoning public and private sector interest in preservation.
The White House recently announced an upcoming Summit on Organ Banking through Converging Technologies to be held at the Harvard Medical School’s Martin Conference Center in August (http://obs2017.obs2017.org/en/). This will be the first scientific consensus-building conference to map out how these and other technologies can be applied systematically to overcome remaining organ preservation challenges. Both basic and translational preservation research seems to be positioned to benefit from rapidly advancing platform technologies. Indeed, the past 3 years have seen a wave of new biotech companies in the organ and tissue preservation space, capitalizing on a small fraction of the opportunities that have emerged. Supported by multiple grant solicitations from the DoD and substantial funding from NIH, these firms are pursuing strategies such as programmed hypometabolism, biomimetic nanoscience, radiofrequency-based ‘nanowarming,’ isochoric preservation, subnormothermic perfusion, and high-subzero temperature preservation (http://firstround.com/; http://x-therma.com/; https://www.sbir.gov/sbc/sylvatica-biotech-inc)196–198. Much of the current focus is on banking and subzero preservation; in this respect, the synergy between these technologies and ex vivo perfusion platforms (Tables 2 and 4)6,7,26,40,41,87,91 means they benefit from—and enhance the value of—the substantial investments made in ex vivo perfusion in recent years199–202.
Catalyzing breakthroughs
So far, the very features that make preservation a foundational and high-impact research area have hindered its progress in the absence of coordinated support. The vast need to increase organ and tissue availability is spread across many areas of medicine and public health (Table 3)—and ultimately stakeholder groups. For instance, over 80% of NIH’s budget goes to institutes with missions tied to unmet preservation needs, encompassing 15 different institutes24. This makes organ and tissue preservation research a nearly universal concern, yet it is not the focus of any major funding body.
The research expertise needed is similarly dispersed. Organ and tissue preservation is quintessentially a ‘convergence technology’, integrating device engineering, applied mathematics, organic and inorganic chemistry, thermodynamics and biophysics, biochemistry and chemical biology, materials science, nanotechnology and molecular engineering, as well as molecular and cell biology6,143,159. This creates special challenges, as the institutions supporting science and engineering research have historically been divided according to research discipline159. For example, it can be inherently difficult to fund an ambitious preservation project because of the inability to assemble an NIH study section that can address all aspects of such a cross-disciplinary grant proposal—even if all the research expertise to propose such a project comes together in the first place. The nature of organ and tissue preservation demands coordination among a large number of actors spanning many research communities, science agencies, industries, and stakeholder organizations.
This coordination is beginning to take shape. For instance, the NSF-supported technology roadmapping process for organ banking and bioengineering, involving representatives from multiple agencies, including NIH, NSF, the US Food and Drug Administration, the DoD, and other agencies, along with dozens of academic institutions, identified >20 surrounding research areas that can be applied to accelerate progress on organ cryopreservation and recommended scientific and institutional strategies to enable organ banking6. Similarly, two US Health Resources and Services Administration–funded consensus conferences recently identified untapped opportunities for in situ preservation of organs through donor management160. This set the stage for a National Academy of Medicine (Washington, DC, USA) study this year aiming to develop a national infrastructure that will foster donor management research in the United States (http://www.ishlt.org/ContentDocuments/2016DecLinks_Nelson.html). At the June 2016 White House Organ Summit113, the Organ Preservation Alliance (of which S.G. and J.K.L. are directors) announced that the alliance is leading a coalition of organizations to study the public health needs, scientific opportunities, and institutional challenges in advancing organ and tissue preservation. The stakeholder groups, which to date include the Association of Organ Procurement Organizations (McLean, VA, USA), the American Society of Mechanical Engineers (New York), the Society for Cryobiology (Luton, UK), and others, will work together to craft a cohesive strategy to advance organ and tissue preservation on all fronts161. The need for a concerted effort to remove logistical barriers in organ and tissue replacement has also been emphasized in international efforts, for instance, in a recent strategic plan for organ and tissue donation in Canada developed by more than 140 stakeholder organizations162.
Initiatives aiming to encourage coordination have already borne fruit in the form of collaborative research efforts that have sprung up around them. In 2015, three DoD small business grant solicitations, targeted toward complex tissue preservation and banking163–165 (to the authors’ knowledge, the first US grant solicitations on this topic), yielded applications from 35 teams consisting of >100 laboratories across industry and academia—a virtually unprecedented response for the funding mechanism used. The DoD increased its support with three more grant solicitations in 2016130,166,167, largely as a result of the abundance of strong proposals during the previous year from cross-disciplinary teams.
Although this demonstrates the wealth of untapped opportunities in organ and tissue preservation research, active and centralized networking among research laboratories also played a substantial role in the strong response. Another successful effort to bolster research coordination is the Charlotte Banks research initiative at the University of North Carolina, Charlotte, which developed out of discussions at the first global Organ Banking Summit in 2015 in Washington, DC. The initiative aims to cryopreserve living thick tissues by coordinating research among almost a dozen laboratories in vascular biology, nanotechnology, materials science, machine perfusion, computational physics, thermodynamics, and other areas (https://eng-resources.uncc.edu/charlottebanks/). In response to the 2016 White House Organ Summit and Emerging Technologies in Organ Preservation roundtable on Capitol Hill, the American Society of Transplantation has launched a community of practice to advance organ and tissue preservation, in partnership with the Organ Preservation Alliance (https://www.myast.org/about-ast/white-house-highlights-asts-new-initiative-organ-preservation-alliance).
Beyond these promising first steps, several additional mechanisms could be used to accelerate progress. Ambitious but achievable preservation challenges, such as large tissue cryopreservation, may be a good fit for high-stakes, high-publicity incentive prize funding. A standing committee comprising experts and stakeholders from diverse fields is needed to coordinate organ and tissue preservation research. The broader challenge of increasing organ and tissue availability (which includes donation, preservation, manufacturing, and transplantation) is ideally suited for a national or international initiative on the scale of the NIH BRAIN Initiative or Human Genome Project, given the inherent complexity of the remaining scientific challenges and the coordination needed, the increasingly important role of regenerative medicine, and the enormous potential of organ and tissue replacement to improve human health.
Organ and tissue preservation has become fertile ground for the application of existing knowledge, talent and research tools. Opportunities abound for diverse (and often converging) fields to provide innovative solutions, but institutional challenges remain and mechanisms to facilitate wider collaboration are needed. If we meet these challenges and build on the scientific proofs of principle that already exist, we may enter a new era of organ and tissue preservation in the coming years—benefiting millions of patients globally and changing the course of many domains of public health.
Acknowledgments
The lead authors owe thanks to many who helped make this article possible: the Thiel Foundation, the Society for Cryobiology, the American Society of Mechanical Engineers, Virginia Commonwealth University, and New Organ for supporting the events that helped develop the growing dialog; the NSF for funding the development of a beta Roadmap to Organ Banking and Bioengineering, and the DoD for funding the development of an Organ Banking Report, each of which has contributed heavily to the content of this article; L.A. acknowledges DARPA’s Service Chief Fellows Program for enabling a major ‘Organs on Demand’ workshop, integral to developing the conclusions in this article. The authors thank M. Severs, J. Horwitz, C. Mallory, and J. Lewis for valuable feedback on the presentation of the information herein; the hundreds of participants of the first global Organ Banking Summit, the Roadmap to Organ Banking and Bioengineering Workshop, the White House Organ Banking Roundtable, the DARPA Organs-on-Demand workshop at the US Military Academy, and the Emerging Technologies in Organ Preservation symposium and roundtable discussion on Capitol Hill, along with many other supporters who have each helped outline the needs and opportunities in biopreservation. U.D. acknowledges NIH grant R01EB015776-05. K.U. and J.F.M. acknowledge NIH grant R01 DK107875. J.A.W.E. holds a Canada Research Chair in Thermodynamics. The authors also thank K. Caldwell, V. Morigi, R. Farmanfarmaian, E. Mohamed, M. Rostad, R. El Assal, P. Kilbride, and the rest of the Organ Preservation Alliance team for their support and dedication to making leaps forward in the ability to preserve organs and tissues.
Footnotes
AUTHOR CONTRIBUTIONS
S.G., J.K.L., and L.A. developed the main conclusions of the article. S.G. and J.K.L. developed the key points discussed with input from all other co-authors. S.G. developed the original concepts that led to the article’s creation. J.K.L. developed the structure of the article and wrote the manuscript. S.G., J.K.L., L.A., R.L., A.E.R., G.M.C., J.F.M., D.H.S., A.C., J.A.W., M.R., E.S.B., E.E., B.P., W.P.A.L., G.B., D.M.W., G.E., D.N., J.P.A., K.U., B.S., B.P.W., A.T., G.M.F., K.B.S., B.R., J.B., J.A.W.E., T.K.W., G.J.M., U.D., K.G.M.B., E.J.W., R.N.B., J.G.B., D.G., B.F., Y.R., D.C.K., M.J.T., and M.T. added conclusions, content, and references, critically reviewed the manuscript, and provided input on structure, style and content. S.G., J.K.L., B.S., B.W., and A.T. created the figures and tables.
COMPETING FINANCIAL INTERESTS
The authors declare competing financial interests: details are available in the online version of the paper.
References
- 1.Jones B, Bes M. Keeping kidneys. Bull. World Health Organ. 2012;90:718–719. doi: 10.2471/BLT.12.021012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Israni AK, Zaun D, Rosendale JD, Snyder JJ, Kasiske BL. OPTN/SRTR 2012 Annual Data Report: deceased organ donation. Am. J. Transplant. 2014;14(Suppl. 1):167–183. doi: 10.1111/ajt.12585. [DOI] [PubMed] [Google Scholar]
- 3.Hoyert DL, Xu J. Deaths: preliminary data for 2011. Natl. Vital Stat. Rep. 2012;61:1–51. [PubMed] [Google Scholar]
- 4.Cooper DK, et al. Report of the Xenotransplantation Advisory Committee of the International Society for Heart and Lung Transplantation: the present status of xenotransplantation and its potential role in the treatment of end-stage cardiac and pulmonary diseases. J. Heart Lung Transplant. 2000;19:1125–1165. doi: 10.1016/s1053-2498(00)00224-2. [DOI] [PubMed] [Google Scholar]
- 5.Evans RW. In: Xenotransplantation. Platt JL, editor. American Society of Microbiology; 2001. pp. 29–51. [Google Scholar]
- 6.Anonymous. Solving Organ Shortage through Organ Banking and Bioengineering. Organ Preservation Alliance; 2015. https://www.organpreservationalliance.org/roadmap. [Google Scholar]
- 7.Lewis JK, et al. The grand challenges of organ banking: proceedings from the first global summit on complex tissue cryopreservation. Cryobiology. 2016;72:169–182. doi: 10.1016/j.cryobiol.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Fahy GM, Wowk B, Wu J. Cryopreservation of complex systems: the missing link in the regenerative medicine supply chain. Rejuvenation Res. 2006;9:279–291. doi: 10.1089/rej.2006.9.279. [DOI] [PubMed] [Google Scholar]
- 9.Rothblatt M. Your Life or Mine: How Geoethics Can Resolve the Conflict Between Public and Private Interests in Xenotransplantation. Ashgate Publishing Company; 2004. [DOI] [PubMed] [Google Scholar]
- 10.Shimazono Y. The state of the international organ trade: a provisional picture based on integration of available information. Bull. World Health Organ. 2007;85:955–962. doi: 10.2471/BLT.06.039370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anonymous. Second Global Consultation in Human Transplantation: Towards a Common Attitude to Transplantation. World Health Organization; 2007. http://apps.who.int/medicinedocs/documents/s15437e/s15437e.pdf. [Google Scholar]
- 12.International Summit on Transplant Tourism and Organ Trafficking. The declaration of Istanbul on organ trafficking and transplant tourism. Clin. J. Am. Soc. Nephrol. 2008;3:1227–1231. doi: 10.2215/CJN.03320708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson J. Increasing Organ Donation Through Education. AAMC Report. 2014 [Google Scholar]
- 14.Kumar K, et al. A smartphone app for increasing live organ donation. Am. J. Transplant. 2016 doi: 10.1111/ajt.13961. http://dx.doi.org/10.1111/ajt.13961. [DOI] [PubMed]
- 15.Loinaz C, González EM. Marginal donors in liver transplantation. Hepatogastroenterology. 2000;47:256–263. [PubMed] [Google Scholar]
- 16.Saidi RF, Hejazii Kenari SK. Challenges of organ shortage for transplantation: solutions and opportunities. Int. J. Organ Transplant. Med. 2014;5:87–96. [PMC free article] [PubMed] [Google Scholar]
- 17.Maggiore U, et al. Strategies to increase the donor pool and access to kidney transplantation: an international perspective. Nephrol. Dial. Transplant. 2015;30:217–222. doi: 10.1093/ndt/gfu212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jadlowiec CC, Taner T. Liver transplantation: current status and challenges. World J. Gastroenterol. 2016;22:4438–4445. doi: 10.3748/wjg.v22.i18.4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khademhosseini A, Langer R. A decade of progress in tissue engineering. Nat. Protoc. 2016;11:1775–1781. doi: 10.1038/nprot.2016.123. [DOI] [PubMed] [Google Scholar]
- 20.Yagi H, et al. Human-scale whole-organ bioengineering for liver transplantation: a regenerative medicine approach. Cell Transplant. 2013;22:231–242. doi: 10.3727/096368912X654939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barkai U, et al. Enhanced oxygen supply improves islet viability in a new bioartificial pancreas. Cell Transplant. 2013;22:1463–1476. doi: 10.3727/096368912X657341. [DOI] [PubMed] [Google Scholar]
- 22.Vagef PA, Shah JA, Sachs DH. Progress towards inducing tolerance of pig-to-primate xenografts. Int. J. Surg. 2015;23(Pt B):291–295. doi: 10.1016/j.ijsu.2015.07.720. [DOI] [PubMed] [Google Scholar]
- 23.Reardon S. New life for pig-to-human transplants. Nature. 2015;527:152–154. doi: 10.1038/527152a. [DOI] [PubMed] [Google Scholar]
- 24.Samadikuchaksaraei A. Scientific and industrial status of tissue engineering. Afr. J. Biotechnol. 2007;6:2897–2909. [Google Scholar]
- 25.Alvarez LM. Compendium of organ & tissue banking concepts. DODlive. 2015 http://science.dodlive.mil/files/2015/01/Organ-and-Tissue-Banking-Compendium-2015-Jan.pdf.
- 26.Fahy GM, et al. Cryopreservation of organs by vitrification: perspectives and recent advances. Cryobiology. 2004;48:157–178. doi: 10.1016/j.cryobiol.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 27.Campbell BK, et al. Restoration of ovarian function and natural fertility following the cryopreservation and autotransplantation of whole adult sheep ovaries. Hum. Reprod. 2014;29:1749–1763. doi: 10.1093/humrep/deu144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arav A, et al. Oocyte recovery, embryo development and ovarian function after cryopreservation and transplantation of whole sheep ovary. Hum. Reprod. 2005;20:3554–3559. doi: 10.1093/humrep/dei278. [DOI] [PubMed] [Google Scholar]
- 29.Larson DJ, et al. Wood frog adaptations to overwintering in Alaska: new limits to freezing tolerance. J. Exp. Biol. 2014;217:2193–2200. doi: 10.1242/jeb.101931. [DOI] [PubMed] [Google Scholar]
- 30.Storey KB, Storey JM. Metabolic rate depression in animals: transcriptional and translational controls. Biol. Rev. Camb. Philos. Soc. 2004;79:207–233. doi: 10.1017/s1464793103006195. [DOI] [PubMed] [Google Scholar]
- 31.Storey KB, Storey JM. Metabolic rate depression: the biochemistry of mammalian hibernation. Adv. Clin. Chem. 2010;52:77–108. [PubMed] [Google Scholar]
- 32.Storey KB, Storey JM. Tribute to P. L. Lutz: putting life on ‘pause’--molecular regulation of hypometabolism. J. Exp. Biol. 2007;210:1700–1714. doi: 10.1242/jeb.02716. [DOI] [PubMed] [Google Scholar]
- 33.Wszola M, et al. One-year results of a prospective, randomized trial comparing two machine perfusion devices used for kidney preservation. Transpl. Int. 2013;26:1088–1096. doi: 10.1111/tri.12169. [DOI] [PubMed] [Google Scholar]
- 34.Guarrera JV, et al. Hypothermic machine preservation in human liver transplantation: the first clinical series. Am. J. Transplant. 2010;10:372–381. doi: 10.1111/j.1600-6143.2009.02932.x. [DOI] [PubMed] [Google Scholar]
- 35.Moers C, Pirenne J, Paul A, Ploeg RJ. Machine perfusion or cold storage in deceased-donor kidney transplantation. N. Engl. J. Med. 2012;366:770–771. doi: 10.1056/NEJMc1111038. [DOI] [PubMed] [Google Scholar]
- 36.Cypel M, et al. Experience with the first 50 ex vivo lung perfusions in clinical transplantation. J. Thorac. Cardiovasc. Surg. 2012;144:1200–1207. doi: 10.1016/j.jtcvs.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 37.Machuca TN, Cypel M. Ex vivo lung perfusion. J. Thorac. Dis. 2014;6:1054–1062. doi: 10.3978/j.issn.2072-1439.2014.07.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Messer S, Ardehali A, Tsui S. Normothermic donor heart perfusion: current clinical experience and the future. Transpl. Int. 2015;28:634–642. doi: 10.1111/tri.12361. [DOI] [PubMed] [Google Scholar]
- 39.Berendsen TA, et al. A simplified subnormothermic machine perfusion system restores ischemically damaged liver grafts in a rat model of orthotopic liver transplantation. Transplant. Res. 2012;1:6. doi: 10.1186/2047-1440-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berendsen TA, et al. Supercooling enables long-term transplantation survival following 4 days of liver preservation. Nat. Med. 2014;20:790–793. doi: 10.1038/nm.3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ravikumar R, Leuvenink H, Friend PJ. Normothermic liver preservation: a new paradigm? Transpl. Int. 2015;28:690–699. doi: 10.1111/tri.12576. [DOI] [PubMed] [Google Scholar]
- 42.Rana A, et al. Survival benefit of solid-organ transplant in the United States. JAMA Surg. 2015;150:252–259. doi: 10.1001/jamasurg.2014.2038. [DOI] [PubMed] [Google Scholar]
- 43.Calne RY, et al. Cyclosporin A in patients receiving renal allografts from cadaver donors. Lancet. 1978;312:1323–1327. doi: 10.1016/s0140-6736(78)91970-0. [DOI] [PubMed] [Google Scholar]
- 44.Dubernard JM, et al. Human hand allograft: report on first 6 months. Lancet. 1999;353:1315–1320. doi: 10.1016/S0140-6736(99)02062-0. [DOI] [PubMed] [Google Scholar]
- 45.Dubernard JM, et al. Functional results of the first human double-hand transplantation. Ann. Surg. 2003;238:128–136. doi: 10.1097/01.SLA.0000078945.70869.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Devauchelle B, et al. First human face allograft: early report. Lancet. 2006;368:203–209. doi: 10.1016/S0140-6736(06)68935-6. [DOI] [PubMed] [Google Scholar]
- 47.Scandling JD, et al. Tolerance and chimerism after renal and hematopoietic-cell transplantation. N. Engl. J. Med. 2008;358:362–368. doi: 10.1056/NEJMoa074191. [DOI] [PubMed] [Google Scholar]
- 48.Scandling JD, Busque S, Shizuru JA, Engleman EG, Strober S. Induced immune tolerance for kidney transplantation. N. Engl. J. Med. 2011;365:1359–1360. doi: 10.1056/NEJMc1107841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leventhal JR, et al. Immune reconstitution/immunocompetence in recipients of kidney plus hematopoietic stem/facilitating cell transplants. Transplantation. 2015;99:288–298. doi: 10.1097/TP.0000000000000605. [DOI] [PubMed] [Google Scholar]
- 50.Szabolcs P, Burlingham WJ, Thomson AW. Tolerance after solid organ and hematopoietic cell transplantation. Biol. Blood Marrow Transplant. 2012;18(Suppl.):S193–S200. doi: 10.1016/j.bbmt.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goldberg D, French B, Abt P, Feng S, Cameron AM. Increasing disparity in waitlist mortality rates with increased model for end-stage liver disease scores for candidates with hepatocellular carcinoma versus candidates without hepatocellular carcinoma. Liver Transpl. 2012;18:434–443. doi: 10.1002/lt.23394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lai JC, et al. Frailty predicts waitlist mortality in liver transplant candidates. Am. J. Transplant. 2014;14:1870–1879. doi: 10.1111/ajt.12762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Anonymous. Organ Procurement and Transplantation Network (OPTN). National Data Reports. [accessed 8 July 2016];US Dept. of Health and Human Services. https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/
- 54.Knechtle SJ, et al. Renal transplantation at the University of Wisconsin in the cyclosporine era. Clin. Transpl. 1993:211–218. [PubMed] [Google Scholar]
- 55.Knoll G. Trends in kidney transplantation over the past decade. Drugs. 2008;68(Suppl. 1):3–10. doi: 10.2165/00003495-200868001-00002. [DOI] [PubMed] [Google Scholar]
- 56.Dunn J, et al. Causes of graft loss beyond two years in the cyclosporine era. Transplantation. 1990;49:349–353. doi: 10.1097/00007890-199002000-00024. [DOI] [PubMed] [Google Scholar]
- 57.Anonymous. OPTN/SRTR Annual Report 2011. US Department of Health and Human Services; 2011. https://srtr.transplant.hrsa.gov/annual_reports/2011/pdf/2011_SRTR_ADR.pdf. [Google Scholar]
- 58.Morrissey PE, Flynn ML, Lin S. Medication noncompliance and its implications in transplant recipients. Drugs. 2007;67:1463–1481. doi: 10.2165/00003495-200767100-00007. [DOI] [PubMed] [Google Scholar]
- 59.Ladner DP, Mehrotra S. Methodological challenges in solving geographic disparity in liver allocation. JAMA Surg. 2016;151:109–110. doi: 10.1001/jamasurg.2015.3937. [DOI] [PubMed] [Google Scholar]
- 60.Saunders MR, et al. Racial disparities in reaching the renal transplant waitlist: is geography as important as race? Clin. Transplant. 2015;29:531–538. doi: 10.1111/ctr.12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Arce CM, Goldstein BA, Mitani AA, Lenihan CR, Winkelmayer WC. Differences in access to kidney transplantation between Hispanic and non-Hispanic whites by geographic location in the United States. Clin. J. Am. Soc. Nephrol. 2013;8:2149–2157. doi: 10.2215/CJN.01560213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thabut G, et al. Geographic disparities in access to lung transplantation before and after implementation of the lung allocation score. Am. J. Transplant. 2012;12:3085–3093. doi: 10.1111/j.1600-6143.2012.04202.x. [DOI] [PubMed] [Google Scholar]
- 63.Lysaght MJ. Maintenance dialysis population dynamics: current trends and long-term implications. J. Am. Soc. Nephrol. 2002;13(Suppl. 1):S37–S40. [PubMed] [Google Scholar]
- 64.Collins AJ, et al. United States Renal Data System 2011 Annual Data Report: Atlas of chronic kidney disease and end-stage renal disease in the United States. Am. J. Kidney Dis. 2012;59(Suppl. 1):e1–420. doi: 10.1053/j.ajkd.2011.11.015. A7. [DOI] [PubMed] [Google Scholar]
- 65.Bruinsma BG, et al. Supercooling preservation and transplantation of the rat liver. Nat. Protoc. 2015;10:484–494. doi: 10.1038/nprot.2015.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bruinsma BG, et al. Subnormothermic machine perfusion for ex vivo preservation and recovery of the human liver for transplantation. Am. J. Transplant. 2014;14:1400–1409. doi: 10.1111/ajt.12727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vogel T, Brockmann JG, Friend PJ. Ex-vivo normothermic liver perfusion: an update. Curr. Opin. Organ Transplant. 2010;15:167–172. doi: 10.1097/MOT.0b013e328337349d. [DOI] [PubMed] [Google Scholar]
- 68.Fahy GM, et al. Physical and biological aspects of renal vitrification. Organogenesis. 2009;5:167–175. doi: 10.4161/org.5.3.9974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bentley TS. 2014 US Organ and Tissue Transplant Cost Estimates and Discussion. Milliman; 2014. [Google Scholar]
- 70.Abboudi H, Macphee IA. Individualized immunosuppression in transplant patients: potential role of pharmacogenetics. Pharmgenomics Pers. Med. 2012;5:63–72. doi: 10.2147/PGPM.S21743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schwartz A, Schiano T, Kim-Schluger L, Florman S. Geographic disparity: the dilemma of lower socioeconomic status, multiple listing, and death on the liver transplant waiting list. Clin. Transplant. 2014;28:1075–1079. doi: 10.1111/ctr.12429. [DOI] [PubMed] [Google Scholar]
- 72.Brierley J, Hasan A. Aspects of deceased organ donation in paediatrics. Br. J. Anaesth. 2012;108(Suppl. 1):92–i95. doi: 10.1093/bja/aer405. [DOI] [PubMed] [Google Scholar]
- 73.Vranic GM, Ma JZ, Keith DS. The role of minority geographic distribution in waiting time for deceased donor kidney transplantation. Am. J. Transplant. 2014;14:2526–2534. doi: 10.1111/ajt.12860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sack K. In discarding of kidneys, system reveals its flaws. [19 September, 2012];The New York Times. http://www.nytimes.com/2012/09/20/health/transplant-experts-blame-allocation-system-for-discarding-kidneys.html.
- 75.Matas AJ. OPTN/SRTR 2013 Annual Data Report: kidney. Am. J. Transplant. 2015;15(Suppl. 2):1–34. doi: 10.1111/ajt.13195. [DOI] [PubMed] [Google Scholar]
- 76.Wigfield CH, et al. Successful emergent lung transplantation after remote ex vivo perfusion optimization and transportation of donor lungs. Am. J. Transplant. 2012;12:2838–2844. doi: 10.1111/j.1600-6143.2012.04175.x. [DOI] [PubMed] [Google Scholar]
- 77.Vilca Melendez H, Rela M, Murphy G, Heaton N. Assessment of graft function before liver transplantation: quest for the lost ark? Transplantation. 2000;70:560–565. doi: 10.1097/00007890-200008270-00002. [DOI] [PubMed] [Google Scholar]
- 78.Burdelski M, et al. Evaluation of quantitative liver function tests in liver donors. Transplant. Proc. 1987;19:3838–3839. [PubMed] [Google Scholar]
- 79.Khush KK, Menza R, Nguyen J, Zaroff JG, Goldstein BA. Donor predictors of allograft use and recipient outcomes after heart transplantation. Circ Heart Fail. 2013;6:300–309. doi: 10.1161/CIRCHEARTFAILURE.112.000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hoffmann T, Minor T. New strategies and concepts in organ preservation. Eur. Surg. Res. 2015;54:114–126. doi: 10.1159/000369455. [DOI] [PubMed] [Google Scholar]
- 81.Reddy SP, Brockmann J, Friend PJ. Normothermic perfusion: a mini-review. Transplantation. 2009;87:631–632. doi: 10.1097/TP.0b013e3181995e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cypel M, Yeung JC, Keshavjee S. Novel approaches to expanding the lung donor pool: donation after cardiac death and ex vivo conditioning. Clin. Chest Med. 2011;32:233–244. doi: 10.1016/j.ccm.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 83.Lv X, Tan J, Liu D, Wu P, Cui X. Intratracheal administration of p38α short-hairpin RNA plasmid ameliorates lung ischemia-reperfusion injury in rats. J. Heart Lung Transplant. 2012;31:655–662. doi: 10.1016/j.healun.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 84.Jamieson RW, Friend PJ. Organ reperfusion and preservation. Front. Biosci. 2008;13:221–235. doi: 10.2741/2672. [DOI] [PubMed] [Google Scholar]
- 85.Mohamed MSA. Translational insights on lung transplantation: learning from immunology. Iran. J. Immunol. 2015;12:156–165. [PubMed] [Google Scholar]
- 86.Cypel M, et al. Functional repair of human donor lungs by IL-10 gene therapy. Sci. Transl. Med. 2009;1:4ra9. doi: 10.1126/scitranslmed.3000266. [DOI] [PubMed] [Google Scholar]
- 87.Hosgood SA, van Heurn E, Nicholson ML. Normothermic machine perfusion of the kidney: better conditioning and repair? Transpl. Int. 2015;28:657–664. doi: 10.1111/tri.12319. [DOI] [PubMed] [Google Scholar]
- 88.Xue M, et al. Donor liver steatosis: a risk factor for early new-onset diabetes after liver transplantation. J. Diabetes Investig. 2017;8:181–187. doi: 10.1111/jdi.12560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Avolio AW, et al. Successful use of extended criteria donor grafts with low to moderate steatosis in patients with model for end-stage liver disease scores below 27. Transplant. Proc. 2009;41:208–212. doi: 10.1016/j.transproceed.2008.10.050. [DOI] [PubMed] [Google Scholar]
- 90.Bruinsma BG, et al. Metabolic profiling during ex vivo machine perfusion of the human liver. Sci. Rep. 2016;6:22415. doi: 10.1038/srep22415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Whitson BA, Black SM. Organ assessment and repair centers: The future of transplantation is near. World J. Transplant. 2014;4:40–42. doi: 10.5500/wjt.v4.i2.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shah MR, Starling RC, Schwartz Longacre L, Mehra MR. Heart transplantation research in the next decade—a goal to achieving evidence-based outcomes: National Heart, Lung, And Blood Institute Working Group. J. Am. Coll. Cardiol. 2012;59:1263–1269. doi: 10.1016/j.jacc.2011.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Perk S, et al. A metabolic index of ischemic injury for perfusion-recovery of cadaveric rat livers. PLoS One. 2011;6:e28518. doi: 10.1371/journal.pone.0028518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cypel M, Keshavjee S. The clinical potential of ex vivo lung perfusion. Expert Rev. Respir. Med. 2012;6:27–35. doi: 10.1586/ers.11.93. [DOI] [PubMed] [Google Scholar]
- 95.van Smaalen TC, Hoogland ERP, van Heurn LWE. Machine perfusion viability testing. Curr. Opin. Organ Transplant. 2013;18:168–173. doi: 10.1097/MOT.0b013e32835e2a1b. [DOI] [PubMed] [Google Scholar]
- 96.Machuca TN, et al. Protein expression profiling predicts graft performance in clinical ex vivo lung perfusion. Ann. Surg. 2015;261:591–597. doi: 10.1097/SLA.0000000000000974. [DOI] [PubMed] [Google Scholar]
- 97.US Centers for Disease Control and Prevention. Investigation of rabies infections in organ donor and transplant recipients—Alabama, Arkansas, Oklahoma, and Texas, 2004. MMWR Morb. Mortal. Wkly. Rep. 2004;53:586–589. [PubMed] [Google Scholar]
- 98.Anonymous. CDC confirms rabies death in organ transplant recipient. CDC Newsroom. 2013 https://www.cdc.gov/media/releases/2013/s0315_rabies_organs.html.
- 99.Anonymous. HIV transmitted from a living organ donor—New York City, 2009. MMWR Morb. Mortal. Wkly. Rep. 2011;60:297–301. [PubMed] [Google Scholar]
- 100.Fishman JA, Grossi PA. Donor-derived infection—the challenge for transplant safety. Nat. Rev. Nephrol. 2014;10:663–672. doi: 10.1038/nrneph.2014.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lee JC, Christie JD. Primary graft dysfunction. Clin. Chest Med. 2011;32:279–293. doi: 10.1016/j.ccm.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 102.Chen X-B, Xu M-Q. Primary graft dysfunction after liver transplantation. Hepatobiliary Pancreat. Dis. Int. 2014;13:125–137. doi: 10.1016/s1499-3872(14)60023-0. [DOI] [PubMed] [Google Scholar]
- 103.Kobashigawa J, et al. Report from a consensus conference on primary graft dysfunction after cardiac transplantation. J. Heart Lung Transplant. 2014;33:327–340. doi: 10.1016/j.healun.2014.02.027. [DOI] [PubMed] [Google Scholar]
- 104.Mendelson KG, Fallat ME. Pediatric injuries: prevention to resolution. Surg. Clin. North Am. 2007;87:207–228. doi: 10.1016/j.suc.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 105.Ashlagi I, Gilchrist DS, Roth AE, Rees MA. Nonsimultaneous chains and dominos in kidney-paired donation—revisited. Am. J. Transplant. 2011;11:984–994. doi: 10.1111/j.1600-6143.2011.03481.x. [DOI] [PubMed] [Google Scholar]
- 106.Anderson R, Ashlagi I, Gamarnik D, Roth AE. Finding long chains in kidney exchange using the traveling salesman problem. Proc. Natl. Acad. Sci. USA. 2015;112:663–668. doi: 10.1073/pnas.1421853112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cowan PJ, Tector AJ. The resurgence of xenotransplantation. Am. J. Transplant. 2017 doi: 10.1111/ajt.14311. http://dx.doi.org/10.1111/ajt.14311. [DOI] [PubMed]
- 108.Anonymous. Advancing Tissue Science and Engineering. Multi-Agency Tissue Engineering Sciences (MATES) Interagency Working Group; 2007. https://webarchive.library.unt.edu/eot2008/20080916012251/http://tissueengineering.gov/advancing_tissue_science_&_engineering.pdf. [DOI] [PubMed] [Google Scholar]
- 109.Anonymous. Funding opportunity announcement: Advanced Tissue Biofabrication Manufacturing Innovation Institute (ATB-MII) US Dept. of Defense, Dept. of the Army—Materiel Command; 2016. https://www.grants.gov/web/grants/view-opportunity.html?oppId=284612. [Google Scholar]
- 110.Bradbury J. Of tardigrades, trehalose, and tissue engineering. Lancet. 2001;358:392. doi: 10.1016/S0140-6736(01)05595-7. [DOI] [PubMed] [Google Scholar]
- 111.Ratner B. What are the opportunities in the field of tissue engineering/regenerative medicine? Workshop on tissue engineering and regenerative medicine. US Department of Health and Human Services; Mar 28, 2003. [Google Scholar]
- 112.Kilbride P, et al. Spatial considerations during cryopreservation of a large volume sample. Cryobiology. 2016;73:47–54. doi: 10.1016/j.cryobiol.2016.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pondrom S. White House holds summit on organ transplantation. Am. J. Transplant. 2016;16:2241–2242. doi: 10.1111/ajt.13942. [DOI] [PubMed] [Google Scholar]
- 114.Jeruss JS, Woodruff TK. Preservation of fertility in patients with cancer. N. Engl. J. Med. 2009;360:902–911. doi: 10.1056/NEJMra0801454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.De Vos M, Smitz J, Woodruff TK. Fertility preservation in women with cancer. Lancet. 2014;384:1302–1310. doi: 10.1016/S0140-6736(14)60834-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tomao F, et al. Special issues in fertility preservation for gynecologic malignancies. Crit. Rev. Oncol. Hematol. 2016;97:206–219. doi: 10.1016/j.critrevonc.2015.08.024. [DOI] [PubMed] [Google Scholar]
- 117.Letourneau JM, et al. Acute ovarian failure underestimates age-specific reproductive impairment for young women undergoing chemotherapy for cancer. Cancer. 2012;118:1933–1939. doi: 10.1002/cncr.26403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Woodruff TK. The emergence of a new interdiscipline: oncofertility. Cancer Treat. Res. 2007;138:3–11. doi: 10.1007/978-0-387-72293-1_1. [DOI] [PubMed] [Google Scholar]
- 119.Grazul-Bilska AT, et al. Morphology and function of cryopreserved whole ovine ovaries after heterotopic autotransplantation. Reprod. Biol. Endocrinol. 2008;6:16. doi: 10.1186/1477-7827-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Courbiere B, et al. Difficulties improving ovarian functional recovery by microvascular transplantation and whole ovary vitrification. Fertil. Steril. 2009;91:2697–2706. doi: 10.1016/j.fertnstert.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 121.Donnez J, Dolmans M-M. Fertility preservation in women. Nat. Rev. Endocrinol. 2013;9:735–749. doi: 10.1038/nrendo.2013.205. [DOI] [PubMed] [Google Scholar]
- 122.Donnez J, et al. Ovarian tissue cryopreservation and transplantation: a review. Hum. Reprod. Update. 2006;12:519–535. doi: 10.1093/humupd/dml032. [DOI] [PubMed] [Google Scholar]
- 123.Donnez J, Dolmans M-M. Transplantation of ovarian tissue. Best Pract. Res. Clin. Obstet. Gynaecol. 2014;28:1188–1197. doi: 10.1016/j.bpobgyn.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 124.Anonymous. Cancer Treatment and Survivorship: Facts & Figures 2014–2015. Special Section: Cancer in Children & Adolescents. American Cancer Society; 2014. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/cancer-treatment-and-survivorship-facts-and-figures/cancer-treatment-and-survivorship-facts-and-figures-2014-2015.pdf. [Google Scholar]
- 125.US Department of Health & Human Services. HHS boosts stockpile of products to treat acute radiation syndrome. [26 September 2013];EMSWORLD. http://www.emsworld.com/news/11178384/hhs-boosts-stockpile-of-products-to-treat-acute-radiation-syndrome.
- 126.Stewart DL. Preparing Communities for Nuclear Terrorism. Lawrence Livermore National Laboratory; 2013. https://e-reports-ext.llnl.gov/pdf/761273.pdf. [Google Scholar]
- 127.Knebel AR, et al. Allocation of scarce resources after a nuclear detonation: setting the context. Disaster Med. Public Health Prep. 2011;5(Suppl. 1):S20–S31. doi: 10.1001/dmp.2011.25. [DOI] [PubMed] [Google Scholar]
- 128.Ben-Bassat H. Performance and safety of skin allografts. Clin. Dermatol. 2005;23:365–375. doi: 10.1016/j.clindermatol.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 129.Crosse M. National Preparedness: Countermeasures for Thermal Burns GAO-12–304. US Government Accountability Office; 2012. http://www.gao.gov/assets/590/588738.pdf. [Google Scholar]
- 130.Anonymous. On-demand cell and tissue biologics for mass casualty response. A16-050. Army SBIR; 2016. http://www.acq.osd.mil/osbp/sbir/solicitations/sbir20161/army161.pdf. [Google Scholar]
- 131.de Graaf IAM, et al. Preparation and incubation of precision-cut liver and intestinal slices for application in drug metabolism and toxicity studies. Nat. Protoc. 2010;5:1540–1551. doi: 10.1038/nprot.2010.111. [DOI] [PubMed] [Google Scholar]
- 132.Shu Z, Heimfield S, Gao D. Hematopoietic SCT with cryopreserved grafts: adverse reactions after transplantation and cryoprotectant removal before infusion. Bone Marrow Transplant. 2014;49:469–476. doi: 10.1038/bmt.2013.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ziegler-Graham K, MacKenzie EJ, Ephraim PL, Travison TG, Brookmeyer R. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch. Phys. Med. Rehabil. 2008;89:422–429. doi: 10.1016/j.apmr.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 134.Kueckelhaus M, et al. Vascularized composite allotransplantation: current standards and novel approaches to prevent acute rejection and chronic allograft deterioration. Transpl. Int. 2016;29:655–662. doi: 10.1111/tri.12652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zachariassen KE, Kristiansen E. Ice nucleation and antinucleation in nature. Cryobiology. 2000;41:257–279. doi: 10.1006/cryo.2000.2289. [DOI] [PubMed] [Google Scholar]
- 136.Barbas AS, Goldaracena N, Dib MJ, Selzner M. Ex-vivo liver perfusion for organ preservation: Recent advances in the field. Transplant. Rev. (Orlando) 2016;30:154–160. doi: 10.1016/j.trre.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 137.Niemann CU, Malinoski D. Therapeutic hypothermia in deceased organ donors and kidney-graft function. N. Engl. J. Med. 2015;373:2687. doi: 10.1056/NEJMc1511744. [DOI] [PubMed] [Google Scholar]
- 138.Schnuelle P, et al. Effects of donor pretreatment with dopamine on graft function after kidney transplantation: a randomized controlled trial. J. Am. Med. Assoc. 2009;302:1067–1075. doi: 10.1001/jama.2009.1310. [DOI] [PubMed] [Google Scholar]
- 139.Singbartl K, et al. Intensivist-led management of brain-dead donors is associated with an increase in organ recovery for transplantation. Am. J. Transplant. 2011;11:1517–1521. doi: 10.1111/j.1600-6143.2011.03485.x. [DOI] [PubMed] [Google Scholar]
- 140.Lee JH, et al. Highly multiplexed subcellular RNA sequencing in situ. Science. 2014;343:1360–1363. doi: 10.1126/science.1250212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chen KH, Boettiger AN, Mofftt JR, Wang S, Zhuang X. RNA imaging. Spatially resolved, highly multiplexed RNA profiling in single cells. Science. 2015;348:aaa6090. doi: 10.1126/science.aaa6090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chung K, et al. Structural and molecular interrogation of intact biological systems. Nature. 2013;497:332–337. doi: 10.1038/nature12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sharp P. Meeting global challenges: discovery and innovation through convergence. Science. 2014;346:1468–1471. [Google Scholar]
- 144.Huang Y, Williams JC, Johnson SM. Brain slice on a chip: opportunities and challenges of applying microfluidic technology to intact tissues. Lab Chip. 2012;12:2103–2117. doi: 10.1039/c2lc21142d. [DOI] [PubMed] [Google Scholar]
- 145.Manuchehrabadi N, et al. Improved tissue cryopreservation using inductive heating of magnetic nanoparticles. Sci. Transl. Med. 2017;9:eaah4586. doi: 10.1126/scitranslmed.aah4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bischof J. 11. Nanowarming: a new concept in tissue and organ preservation. Cryobiology. 2015;71:167. [Google Scholar]
- 147.Boyden ES. A history of optogenetics: the development of tools for controlling brain circuits with light. F1000 Biol. Rep. 2011;3:11. doi: 10.3410/B3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhang F, et al. The microbial opsin family of optogenetic tools. Cell. 2011;147:1446–1457. doi: 10.1016/j.cell.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mali P, et al. RNA-guided human genome engineering via Cas9. Science. 2013;339:823–826. doi: 10.1126/science.1232033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cong L, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mojica FJM, Díez-Villaseñor C, García-Martínez J, Soria E. Intervening sequences of regularly spaced prokaryotic repeats derive from foreign genetic elements. J. Mol. Evol. 2005;60:174–182. doi: 10.1007/s00239-004-0046-3. [DOI] [PubMed] [Google Scholar]
- 152.Jinek M, et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337:816–821. doi: 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Liu Y, et al. Genomic analysis of miRNAs in an extreme mammalian hibernator, the Arctic ground squirrel. Physiol. Genomics. 2010;42A:39–51. doi: 10.1152/physiolgenomics.00054.2010. [DOI] [PubMed] [Google Scholar]
- 154.Hashimoto T, et al. Extremotolerant tardigrade genome and improved radiotolerance of human cultured cells by tardigrade-unique protein. Nat. Commun. 2016;7:12808. doi: 10.1038/ncomms12808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Limkemann A, et al. Donor gluconate rescues livers from uncontrolled donation after cardiac death. Surgery. 2016;159:852–861. doi: 10.1016/j.surg.2015.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Parrish D, et al. New low-volume resuscitation solutions containing PEG-20k. J. Trauma Acute Care Surg. 2015;79:22–29. doi: 10.1097/TA.0000000000000682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Magliocca JF, et al. Extracorporeal support for organ donation after cardiac death effectively expands the donor pool. J. Trauma. 2005;58:1095–1102. doi: 10.1097/01.ta.0000169949.82778.df. [DOI] [PubMed] [Google Scholar]
- 158.Brasile L, et al. Overcoming severe renal ischemia: the role of ex vivo warm perfusion. Transplantation. 2002;73:897–901. doi: 10.1097/00007890-200203270-00011. [DOI] [PubMed] [Google Scholar]
- 159.Sharp P, Jacks T, Hockfield S. Convergence: The Future of Health. Massachusetts Institute of Technology; 2016. http://www.convergencerevolution.net/2016-report/ [Google Scholar]
- 160.Nelson DP. Donor Management Research Consensus Conference. Int. Soc. Heart Lung Transplant. Links Newsletter. 2013 Nov;5 https://www.ishlt.org/ContentDocuments/2013Nov_Links.html. [Google Scholar]
- 161.White House. Fact sheet: Obama administration announces key actions to reduce the organ waiting list. 2016 https://obamawhitehouse.archives.gov/the-press-office/2016/06/13/fact-sheet-obama-administration-announces-key-actions-reduce-organ.
- 162.Anonymous. A strategic plan to improve organ and tissue donation and transplantation performance for Canadians. Canadian Blood Services; 2011. https://blood.ca/sites/default/files/otdt-indx-final-c2a.pdf. [Google Scholar]
- 163.Anonymous. Cryopreservation for regenerative medical applications. A15-059. Army SBIR; 2015. http://www.acq.osd.mil/osbp/sbir/solicitations/sbir20151/army151.pdf. [Google Scholar]
- 164.Anonymous. Optimal rewarming solutions for cryopreserved tissue systems. DHP15–014. SBIRSource. 2015 https://sbirsource.com/sbir/topics/91484.
- 165.Anonymous. Optimization of cryoprotectants, cryotherapeutics, and protocols for cryopreservation of large tissue systems. DHP15–013. Defense Health Program SBIR; 2015. http://www.acq.osd.mil/osbp/sbir/solicitations/sbir20151/dhp151.pdf. [Google Scholar]
- 166.Anonymous. Conservation and maintenance of trauma injured tissues for autologous repair and reconstruction. A16-049. Army SBIR; 2016. http://www.acq.osd.mil/osbp/sbir/solicitations/sbir20161/army161.pdf. [Google Scholar]
- 167.Anonymous. Genitourinary tissue repair, restoration and protection: preserving fertility and function in wounded warriors. DHP16–012. Defense Health Program SBIR; 2016. http://www.acq.osd.mil/osbp/sbir/solicitations/sbir20161/dhp161.pdf. [Google Scholar]
- 168.Mathew J, Kratzke RA. Lung cancer and lung transplantation: a review. J. Thorac. Oncol. 2009;4:753–760. doi: 10.1097/JTO.0b013e31819afdd9. [DOI] [PubMed] [Google Scholar]
- 169.Anonymous. How organs are matched. [accessed: 13 October 2016];UNOS. https://www.unos.org/transplantation/matching-organs/?gclid=CjwKEAjwsuK_BRDD9ISR1bawwUwSJACbOiixRLbb3ISa3IAx_Otd5EB83n6BYvoUYWkeSid6bg3UKBoC3CLw_wcB.
- 170.Khush KK, Zaroff JG, Nguyen J, Menza R, Goldstein BA. National decline in donor heart utilization with regional variability: 1995–2010. Am. J. Transplant. 2015;15:642–649. doi: 10.1111/ajt.13055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Waselenko JK, et al. Medical management of the acute radiation syndrome: recommendations of the Strategic National Stockpile Radiation Working Group. Ann. Intern. Med. 2004;140:1037–1051. doi: 10.7326/0003-4819-140-12-200406150-00015. [DOI] [PubMed] [Google Scholar]
- 172.Anonymous. Blood marrow and cord blood donation and transplantation. [accessed: 1 January 2016];HRSA. http://bloodcell.transplant.hrsa.gov/
- 173.Carbone L. What Animals Want: Expertise and Advocacy in Laboratory Animal Welfare Policy. Oxford University Press; 2004. [Google Scholar]
- 174.Rogers J, Carolin T, Vaught J, Compton C. Biobankonomics: a taxonomy for evaluating the economic benefits of standardized centralized human biobanking for translational research. J. Natl. Cancer Inst. Monogr. 2011;2011:32–38. doi: 10.1093/jncimonographs/lgr010. [DOI] [PubMed] [Google Scholar]
- 175.Dragunow M. The adult human brain in preclinical drug development. Nat. Rev. Drug Discov. 2008;7:659–666. doi: 10.1038/nrd2617. [DOI] [PubMed] [Google Scholar]
- 176.Anonymous. Burn incidence and treatment in the United States: 2016. [accessed: 1 January 2016];American Burn Association. http://www.ameriburn.org/resources_factsheet.php.
- 177.DiCarlo AL, et al. Radiation injury after a nuclear detonation: medical consequences and the need for scarce resources allocation. Disaster Med. Public Health Prep. 2011;5(Suppl. 1):S32–S44. doi: 10.1001/dmp.2011.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Anonymous. 2020: A New Vision. A Future for Regenerative Medicine. Interagency Federal Working Group on Regenerative Medicine; 2005. https://medicine.osu.edu/regenerativemedicine/documents/2020vision.pdf. [Google Scholar]
- 179.Hay M, Thomas DW, Craighead JL, Economides C, Rosenthal J. Clinical development success rates for investigational drugs. Nat. Biotechnol. 2014;32:40–51. doi: 10.1038/nbt.2786. [DOI] [PubMed] [Google Scholar]
- 180.Cummings JL, Morstorf T, Zhong K. Alzheimer’s disease drug-development pipeline: few candidates, frequent failures. Alzheimers Res. Ther. 2014;6:37. doi: 10.1186/alzrt269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Taylor MJ, et al. Asanguineous whole body perfusion with a new intracellular acellular solution and ultraprofound hypothermia provides cellular protection during 3.5 hours of cardiac arrest in a canine model. ASAIO J. 1994;40:M351–M358. doi: 10.1097/00002480-199407000-00022. [DOI] [PubMed] [Google Scholar]
- 182.Taylor MJ, et al. A new solution for life without blood. Asanguineous low-flow perfusion of a whole-body perfusate during 3 hours of cardiac arrest and profound hypothermia. Circulation. 1995;91:431–444. doi: 10.1161/01.cir.91.2.431. [DOI] [PubMed] [Google Scholar]
- 183.Rhee P, et al. Induced hypothermia during emergency department thoracotomy: an animal model. J. Trauma. 2000;48:439–450. doi: 10.1097/00005373-200003000-00011. [DOI] [PubMed] [Google Scholar]
- 184.Munshi L, Keshavjee S, Cypel M. Donor management and lung preservation for lung transplantation. Lancet Respir. Med. 2013;1:318–328. doi: 10.1016/S2213-2600(12)70064-4. [DOI] [PubMed] [Google Scholar]
- 185.Azevedo M. Donor lungs ready for transplant is goal of Toronto start-up supported by $2.6M from XENiOS. [26 February 2016];Pulmonary Fibrosis News. https://pulmonaryfibrosisnews.com/2016/02/26/xenios-invests-2-6-million-in-xor-labs-toronto/
- 186.Lowe CH, Lardner PJ, Halpern EA. Supercooling in reptiles and other vertebrates. Comp. Biochem. Physiol. A. 1971;39:125–135. doi: 10.1016/0300-9629(71)90352-5. [DOI] [PubMed] [Google Scholar]
- 187.Blackstone E, Morrison M, Roth MB. H2S induces a suspended animationlike state in mice. Science. 2005;308:518. doi: 10.1126/science.1108581. [DOI] [PubMed] [Google Scholar]
- 188.Donnez J, Dolmans MM. Ovarian cortex transplantation: 60 reported live births brings the success and worldwide expansion of the technique towards routine clinical practice. J. Assist. Reprod. Genet. 2015;32:1167–1170. doi: 10.1007/s10815-015-0544-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Silber S, Pineda J, Lenahan K, DeRosa M, Melnick J. Fresh and cryopreserved ovary transplantation and resting follicle recruitment. Reprod. Biomed. Online. 2015;30:643–650. doi: 10.1016/j.rbmo.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 190.Arav A, Friedman O, Natan Y, Gur E, Shani N. Rat hindlimb cryopreservation and transplantation - A step towards “organ banking”. Am. J. Transplant. 2017 doi: 10.1111/ajt.14320. [DOI] [PubMed] [Google Scholar]
- 191.Wang Z, et al. Cryopreservation and replantation of amputated rat hind limbs. Eur. J. Med. Res. 2014;19:28. doi: 10.1186/2047-783X-19-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Song YC, Khirabadi BS, Lightfoot F, Brockbank KG, Taylor MJ. Vitreous cryopreservation maintains the function of vascular grafts. Nat. Biotechnol. 2000;18:296–299. doi: 10.1038/73737. [DOI] [PubMed] [Google Scholar]
- 193.Taylor MJ, Brockbank KGM, Song YC. In: Life in the Frozen State. Fuller BJ, Lane N, Benson EE, editors. CRC Press; 2004. pp. 603–641. [Google Scholar]
- 194.Abazari A, Jomha NM, Elliott JAW, McGann LE. Cryopreservation of articular cartilage. Cryobiology. 2013;66:201–209. doi: 10.1016/j.cryobiol.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 195.Jomha NM, et al. Vitrification of intact human articular cartilage. Biomaterials. 2012;33:6061–6068. doi: 10.1016/j.biomaterials.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 196.Anonymous. Tissue Testing Technologies. [accessed: 1 January 2016];SBIR-STTR. https://www.sbir.gov/sbc/tissue-testing-technologies-llc-3.
- 197.Anonymous. Ossium Health Inc. [accessed: 1 January 2016];Buzzfile. http://www.buzzfile.com/business/Ossium-Health-317-370-7293.
- 198.Anonymous. Organ Solutions, LLC. [accessed: 1 January 2016];Buzzfile. http://www.buzzfile.com/business/Organ-Solutions,-LLC-617-643-3800.
- 199.Anonymous. TransMedics, Inc. announces closing of $51.2 million growth financing to transform organ transplant therapy. [accessed: 28 September 2016];PRNewswire. http://www.prnewswire.com/news-releases/transmedics-inc-announces-closing-of-512-million-growth-financing-to-transform-organ-transplant-therapy-300274937.html.
- 200.Anonymous. TransMedics, Inc. secures $36 million round of financing led by Abrams Capital Management. [accessed: 28 September 2016];TransMedics. http://www.transmedics.com/wt/page/pr_1354044406.
- 201.Mathis KB. Mayo Clinic adding new lung center. [5 August 2015];The Daily Record. http://www.jaxdailyrecord.com/showstory.php?Story_id=545906.
- 202.Anonymous. Company profiles: organ recovery systems. [accessed: 28 September 2016];PitchBook. http://pitchbook.com/profiles/organ-recovery-systems-profile-investors-funding-valuation-and-analysis.