SUMMARY
Sustained virological response (SVR) to antiviral therapy for hepatitis C (HCV) reduces risk of hepatocellular carcinoma (HCC), but there is little information regarding how treatment failure (TF) compares to lack of treatment. We evaluated the impact of treatment status on risk of HCC using data from the Chronic Hepatitis Cohort Study (CHeCS–an observational study based in four large US health systems, with up to 7 years of follow-up on patients). Multivariable analyses were used to adjust for bias in treatment selection, as well as other covariates, followed by sensitivity analyses. Among 10 091 HCV patients, 3681 (36%) received treatment, 2099 (57%) experienced treatment failure (TF), and 1582 (43%) of these achieved sustained virological response (SVR). TF patients demonstrated almost twice the risk of HCC than untreated patients [adjusted hazard ratio (aHR) = 1.95, 95% confidence interval (CI) 1.50–2.53]; this risk persisted across all stages of fibrosis. Several sensitivity analyses validated these results. Although African Americans were at increased risk of treatment failure, they were at lower risk for HCC and all-cause mortality compared to White patients. SVR patients had lower risk of HCC than TF patients (aHR = 0.48, CI 0.31–0.73), whereas treatment – regardless of outcome – reduced all-cause mortality (aHR = 0.45, CI 0.34–0.60 for SVR patients; aHR = 0.78, CI 0.65–0.93 for TF patients).
Keywords: antiviral treatment, sustained virological response, treatment failure
INTRODUCTION
The benefits of sustained viral response (SVR) to hepatitis C antiviral therapy are well established. Many studies have confirmed that patients who achieve SVR are at reduced risk of hepatocellular carcinoma (HCC) compared to patients who do not receive treatment and those who do not achieve SVR. However, despite the high failure rate of interferon-based treatments (30–60%) [1], there are few reports that include comparisons of HCC risk between those who fail to achieve SVR – that is, ‘treatment failure’ (TF) patients – and untreated patients [2–7].
Among the few studies that include a third comparison group, results are contradictory. Most found no significant difference in rates of HCC between TF and untreated patients [3,4,6,7]; however, several of these [4,6] were hampered by small sample sizes (300–600 patients), which may have been insufficient to detect an effect. One study, limited to patients with compensated cirrhosis, found that TF reduced risk of HCC [2]. Conversely, a 1999 report found that ‘nonresponders’ were at significantly higher risk of HCC than untreated patients [5]. Notably, only one of these studies was performed in the United States [3] – a clinical trial that was limited to previous nonresponders. Such studies may not be generalizable to the diverse US population. For example, African Americans are at increased risk of HCV, less likely to receive treatment, and at greater risk of treatment failure than White patients; the persistence of such disparities underscores the importance of characterizing this risk in a diverse ‘real-world’ cohort.
The Chronic Hepatitis Cohort Study (CHeCS) is the first US study to characterize a diverse general population of over 10 000 HCV-infected patients. Although interferon-free regimens – including highly effective and well-tolerated direct-acting oral agents (DAAs) [8] – are transforming the landscape of HCV antiviral treatment, understanding the long-term impact of interferon therapy in the ‘real world’ will improve care for patients in the future. Our objective was to evaluate the impact of antiviral treatment on rates of hepatocellular carcinoma (HCC) in a large observational cohort with three groups –SVR, TF, and untreated patients.
METHODS
Study population
CHeCS [9] is a retrospective/prospective, observational multicentre study that includes patients from four large health systems. The study follows all guidelines of the US Department of Health and Human Services regarding the protection of human subjects; protocols are reviewed annually by the institutional review board at each site. CHeCS study methods have been previously described [9].
For each patient, observation commenced at an index date, defined as the latter date of either HCV diagnosis or initiation of first antiviral HCV treatment. This permitted sufficient follow-up to observe possible effects of treatment failure in patients receiving multiple courses of antiviral therapy. Patients were excluded if they were co-infected with HBV, were receiving ongoing HCV antiviral therapy, had completed therapy but had insufficient follow-up, had received a liver transplant prior to the index date, or had ever enrolled in an HCV antiviral clinical trial.
Adjustment for differences between treatment groups
Anticipating that treated and untreated patients would differ by pretreatment characteristics, we collected extensive electronic health record (EHR) data on baseline demographic and clinical variables (Table 1), including HIV co-infection, HCV genotype Oxford comma improves clarity, particularly in this instance. From this list, only laboratory test results were used for imputation of FIB4. and laboratory test results for imputation of the Fibrosis-4 (FIB4) score classified into one of three validated categories: ≤1.21; 1.21>5.88; >5.88) [10]. We calculated the Charlson/Deyo comorbidity index [11] from ICD-9 codes for 1 year prior to the index date. We also used ICD-9 and CPT-4 codes to assess contraindications to therapy (detailed in Table S1).
Table 1.
Baseline treatment differences (before and after weighting)
| Unweighted
|
Weighted
|
||||||
|---|---|---|---|---|---|---|---|
| Parameter | Response | Untreated Treated (N = 6410) |
Treated (N = 3681) |
P-value | Untreated Treated (N = 6410) |
Treated (N = 3681) |
P-value |
| Site | KPNW | 2059 (32%) | 942 (26%) | <0.001 | (27%) | (26%) | 0.079 |
| KPHI | 596 (9%) | 315 (9%) | (9%) | (9%) | |||
| HFHS | 2492 (39%) | 1467 (40%) | (37%) | (40%) | |||
| GHS | 1263 (20%) | 957 (26%) | (27%) | (26%) | |||
| Age | <40 | 1153 (18%) | 634 (17%) | <0.001 | (17%) | (17%) | 0.820 |
| 40 < 50 | 2043 (32%) | 1475 (40%) | (39%) | (40%) | |||
| 50 < 60 | 2393 (37%) | 1290 (35%) | (35%) | (35%) | |||
| ≥60 | 821 (13%) | 282 (8%) | (8%) | (8%) | |||
| Sex | Female | 2626 (41%) | 1462 (40%) | 0.218 | (40%) | (40%) | 0.850 |
| Male | 3784 (59%) | 2219 (60%) | (60%) | (60%) | |||
| Race | Asian/other | 368 (6%) | 265 (7%) | <0.001 | (7%) | (7%) | 0.877 |
| Black | 1796 (28%) | 636 (17%) | (17%) | (17%) | |||
| Unknown | 379 (6%) | 140 (4%) | (4%) | (4%) | |||
| White | 3867 (60%) | 2640 (72%) | (72%) | (72%) | |||
| Index date | <2000 | 867 (14%) | 724 (20%) | <0.001 | (18%) | (20%) | 0.805 |
| 2000 < 2005 | 1859 (29%) | 1314 (36%) | (35%) | (36%) | |||
| 2005 < 2010 | 2780 (43%) | 1241 (34%) | (35%) | (34%) | |||
| ≥2010 | 904 (14%) | 402 (11%) | (11%) | (11%) | |||
| Insurance | Medicaid | 1039 (16%) | 382 (10%) | <0.001 | (11%) | (10%) | 0.965 |
| Medicare | 1184 (18%) | 550 (15%) | (14%) | (15%) | |||
| Private | 3976 (62%) | 2694 (73%) | (74%) | (73%) | |||
| None | 197 (3%) | 53 (1%) | (1%) | (1%) | |||
| Unknown | 14 (0%) | 2 (0%) | (0%) | (0%) | |||
| Median | <$30 | 1612 (25%) | 661 (18%) | <0.001 | (18%) | (18%) | 0.950 |
| household | $30 < 50 | 2999 (47%) | 1719 (47%) | (47%) | (47%) | ||
| income;(in thousands) | $50 < 75 | 1336 (21%) | 950 (26%) | (26%) | (26%) | ||
| ≥$75 | 290 (5%) | 273 (7%) | (7%) | (7%) | |||
| Not Reported | 173 (3%) | 78 (2%) | (2%) | (2%) | |||
| Alanine | Unknown | 1089 (17%) | 1275 (35%) | <0.001 | (33%) | (35%) | 0.809 |
| Aminotransferase | <LLN/Normal | 2183 (34%) | 838 (23%) | (23%) | (23%) | ||
| Category | ULN ≤ 2xULN | 1806 (28%) | 880 (24%) | (25%) | (24%) | ||
| >2xULN | 1332 (21%) | 688 (19%) | (20%) | (19%) | |||
| HIV | No | 6211 (97%) | 3622 (98%) | <0.001 | (98%) | (98%) | 0.992 |
| Yes | 199 (3%) | 59 (2%) | (2%) | (2%) | |||
| Weighted | 0 | 4114 (64%) | 2635 (72%) | <0.001 | (70%) | (72%) | 0.571 |
| Charlson/Deyo | 1 | 1296 (20%) | 642 (17%) | (19%) | (17%) | ||
| comorbidity score | 2 | 1000 (16%) | 404 (11%) | (11%) | (11%) | ||
| FIB4 | ≤1.21 | 1421 (22%) | 686 (19%) | <0.001 | (19%) | (19%) | 0.930 |
| (1.21–5.88) | 2124 (33%) | 1288 (35%) | (36%) | (35%) | |||
| >5.88 | 524 (8%) | 179 (5%) | (5%) | (5%) | |||
| Missing | 2341 (37%) | 1528 (42%) | (40%) | (42%) | |||
| HCV genotype | 1 | 3231 (50%) | 2034 (55%) | <0.001 | (57%) | (55%) | 0.154 |
| Other/Unknown | 3179 (50%) | 1647 (45%) | (43%) | (45%) | |||
| Diabetes | No | 5675 (89%) | 3450 (94%) | <0.001 | (94%) | (94%) | 0.637 |
| Yes | 735 (11%) | 231 (6%) | (6%) | (6%) | |||
| Substance abuse | No | 5545 (87%) | 3501 (95%) | <0.001 | (95%) | (95%) | 0.840 |
| Yes | 865 (13%) | 180 (5%) | (5%) | (5%) | |||
| Decompensated | No | 6247 (97%) | 3607 (98%) | 0.089 | (98%) | (98%) | 0.592 |
| cirrhosis | Yes | 163 (3%) | 74 (2%) | (2%) | (2%) | ||
| Absolute | No | 5018 (78%) | 3031 (82%) | <0.001 | (81%) | (82%) | 0.642 |
| contraindication to treatment | Yes | 1392 (22%) | 650 (18%) | (19%) | (18%) | ||
| Relative | No | 4926 (77%) | 3203 (87%) | <0.001 | (86%) | (87%) | 0.777 |
| contraindication to treatment | Yes | 1484 (23%) | 478 (13%) | (14%) | (13%) | ||
KPNW, Kaiser Permanente-Northwest; KPHI, Kaiser Permanente-Hawai’i; HFHS, Henry Ford Health System; GHS, Kiesinger Health System; LLN, lower limit of normal; ULN, upper limit of normal; FIB4, Fibrosis-4 index. All variables were included in the propensity score justification.
Propensity scores (PS) and inverse probability of treatment weighting [IPTW] [12] were estimated based on seventeen baseline covariates, using logistic regression to adjust for treatment selection bias [13].
Antiviral HCV therapy and its response
Routine viral RNA quantification data were obtained from the EHR. Patients were classified as having achieved SVR if RNA results ≥12 weeks’ post-therapy showed undetectable viral loads. Patients’ treatment/response status – treated with SVR, treated without SVR [‘treatment failure’ (TF)], or untreated – was considered a time-varying covariate.
Outcomes of interest
Patients were followed from their index date through 31 January 2013. Time-to-event outcomes included HCC, other cancer (excluding skin cancer), or death. Patients with outcome events occurring <6 months post-index were excluded to avoid possible prevalent conditions and possible misattribution of effects. Patients were followed until the outcome event or were censored at last observation within 15 years post-index date. Primary cancer diagnoses were ascertained using the Heath Care System Research Network tumour registry database [14]. Tumours were classified as primary liver cancer (HCC) or nonliver cancer based on ICD-O-3 codes. To assess screening bias, we collected information on HCC screening based on the presence of procedure codes for abdominal imaging (ultrasound, CT, or MRI). Death was ascertained by EHR data and a search against either national or state death indices. Use of all-cause mortality was based on our recent work showing that liver-related mortality is under-reported [15].
Statistical analysis
Baseline patient characteristics were compared between treatment groups using logistic regression.
We used Cox regression adjusted for IPTW to test the effect of time-dependent treatment variables, with estimation of adjusted hazard ratios (aHR) and 95% confidence intervals (CI) for each outcome of interest. Unadjusted Kaplan–Meier survival curves were used for data illustration. A similar approach was used to study baseline covariate effects, which included: baseline age; sex; race; HCV genotype [GT]; Charlson/Deyo index; diabetes diagnosis; recent drug/alcohol abuse; HIV status; and FIB4. Any variable with univariate effects was considered a candidate for initial multivariable modelling. Covariate-by-treatment interactions were considered if there was a univariate effect. The final model retained treatment variables, baseline variables, and possible treatment-by-covariate interactions with P values <0.05. Study site was used as a stratification variable for all analyses.
Primary results were based on the entire cohort to ensure study integrity. To address limitations inherent to observational studies, several sensitivity analyses were performed, including: (i) a one-to-one treated/untreated matched cohort [16]; (ii) the subgroup of patients with available FIB4 data at index (to eliminate ‘FIB4 unknowns’); (iii) exclusion of patients with SVR responses, to validate the TF effect. We also performed a fourth sensitivity analysis in which several important treatment and prognostic factors were removed from the PS weighting to assess whether there were changes in effect estimates, a strategy that addresses unmeasurable confounders [17]. Finally, a multiple imputation strategy [18,19] was performed to impute unknown baseline variables.
RESULTS
Characteristics of the study population
Our analytic sample included 10 091 of the 11 276 confirmed HCV patients our cohort. We excluded 1185 patients for enrolment in a clinical trial (n = 264), ongoing therapy (n = 367), HBV co-infection (n = 155), or cancer ≤6 months’ post-index/insufficient follow-up (n = 109); these criteria were not mutually exclusive. Among the analytic sample, 3681 (36%) were treated, 2844 received a single course, and 837 received more than one course of therapy. Median course duration was 11 months. Of the 3681 treated patients, 1582 (43%) achieved SVR (Table S.2a). Median follow-up was 6.9 years (interquartile range 3.6–10.5 years). We observed 351 HCC (3.5%), 456 nonliver cancer (4.5%), and 1074 death (11.0%) events (Table S.2b). Estimated median time from infection to first treatment was 1.6 years (interquartile range 0.3–5.9 years).
Table 1 displays patient baseline characteristics. We initially observed differences in likelihood to receive treatment by most baseline variables. After IPTW adjustment, all baseline covariates were well-balanced; these adjustments were included in outcome analyses.
Effects of treatment and SVR
A significant effect of treatment on HCC was detected in both univariate (Table S.2c) and multivariate analyses after IPTW (Table 2). Patients with TF had almost twice the risk of HCC than untreated patients (aHR = 1.95, CI 1.50–2.53). Notably, this was true even though screening rates were lower in the treated vs untreated groups (0.23 vs 0.28 overall screenings/person-year). Sensitivity analyses demonstrated consistent effects of TF on risk of HCC (Table 2).
Table 2.
Multivariate analysis: Impact of HCV treatment and SVR on clinical outcomes (Mortality, HCC and Non-HCC Cancer)
| Mortality
|
HCC
|
Non-HCC cancer
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Value | Comparator group | aHR (CI) | P value | Overall P value |
aHR (CI) | P value | Overall P value |
aHR (CI) | P value | Overall P value |
|
| Age (years) | 40 < 50 | <40 | 1.93 (1.39–2.68) | <0.0001 | <0.0001 | 1.96 (1.08–3.55) | 0.0262 | <0.0001 | 2.27 (1.19–4.32) | 0.0124 | <0.0001 | |
| 50 < 60 | 3.66 (2.62–5.12) | <0.0001 | 4.55 (2.5–8.27) | <0.0001 | 4.36 (2.3–8.26) | <0.0001 | ||||||
| ≥60 | 4.54 (3.06–6.74) | <0.0001 | 5.86 (3.01–11.41) | <0.0001 | 7.89 (3.99–15.62) | <0.0001 | ||||||
| Sex | Male | Female | 1.53 (1.29–1.83) | <0.0001 | <0.0001 | 2.13 (1.59–2.85) | <0.0001 | <0.0001 | ||||
| Race | Asian/other | White | 1.05 (0.75–1.47) | 0.7603 | <0.0001 | 1.16 (0.75–1.78) | 0.5012 | 0.0007 | 0.97 (0.62–1.53) | 0.9117 | 0.0056 | |
| Black | 0.69 (0.53–0.89) | 0.0048 | 0.53 (0.38–0.74) | 0.0002 | 1.83 (1.29–2.59) | 0.0007 | ||||||
| Unknown | 2.22 (1.52–3.23) | <0.0001 | 1.42 (0.79–2.54) | 0.2387 | 0.76 (0.35–1.68) | 0.5015 | ||||||
| Insurance | Medicare | Medicaid | 0.92 (0.69–1.23) | 0.5571 | <0.0001 | |||||||
| Type | None | 1.6 (0.91–2.83) | 0.1036 | |||||||||
| Private | 0.58 (0.45–0.75) | <0.0001 | ||||||||||
| Unknown | NA | 0.9533 | ||||||||||
| Median | $30 < 50K | <$30K | 0.87 (0.71–1.07) | 0.1933 | 0.014 | |||||||
| Household | $50 < 75K | 0.64 (0.49–0.84) | 0.0011 | |||||||||
| Income | ≥$75K | 0.67 (0.45–1.01) | 0.0548 | |||||||||
| Not Reported | 1.03 (0.55–1.9) | 0.9372 | ||||||||||
| HIV co-infection | Yes | No | 2.33 (1.19–4.55) | 0.0136 | 0.0136 | |||||||
| Charlson/Deyo | 1 | 0 | 1.37 (1.11–1.7) | 0.0038 | <0.0001 | |||||||
| comorbidity index | ≥2 | 2.40 (1.95–2.96) | <0.0001 | |||||||||
| FIB4 | 1.21–5.88 | <1.21 | 1.90 (1.43–2.53) | <0.0001 | <0.0001 | 5.81 (2.38–14.21) | 0.0001 | <0.0001 | ||||
| >5.88 | 6.20 (4.39–8.75) | <0.0001 | 27.23 (10.72–69.18) | <0.0001 | ||||||||
| Missing | 1.02 (0.76–1.38) | 0.8889 | 6.07 (2.5–14.71) | <0.0001 | ||||||||
| Diabetes | Yes | No | 1.65 (1.09–2.49) | 0.0178 | 0.0178 | |||||||
| Substance abuse | Yes | No | 1.48 (1.08–2.04) | 0.0156 | 0.0156 | |||||||
| HCV Genotype | 2 | 1 | 0.64 (0.37–1.12) | 0.1177 | 0.0031 | |||||||
| 3 | 1.60 (1.04–2.44) | 0.0306 | ||||||||||
| Other/Unknown | 0.73 (0.54–0.98) | 0.0362 | ||||||||||
| HCV Treatment/Response | TF | No Trtmt | 0.78 (0.65–0.93) | 0.0064 | <0.0001 | 1.95 (1.50–2.53) | <0.0001 | <0.0001 | 0.88 (0.66–1.16) | 0.3609 | 0.6386 | |
| SVR | 0.45 (0.34–0.60) | <0.0001 | 0.93 (0.60–1.43) | 0.7286 | 0.92 (0.63–1.34) | 0.6474 | ||||||
| SVR | TF | 0.58 (0.43–0.79) | 0.0005 | 0.48 (0.31–0.73) | 0.0008 | 1.05 (0.69–1.59) | 0.8323 | |||||
| Sensitivity analyses* (adjusted for the other covariates in the multivariable model*) | ||||||||||||
| 1:1 matched cohort | TF | No Trtmt | 0.84 (0.69–1.01) | 0.0665 | <0.0001 | 1.96 (1.47–2.61) | <0.0001 | <0.0001 | 0.85 (0.63–1.14) | 0.2823 | 0.5202 | |
| SVR | 0.48 (0.35–0.64) | <0.0001 | 0.86 (0.53–1.38) | 0.5335 | 0.89 (0.60–1.3) | 0.5327 | ||||||
| FIB4 available subgroup | TF | 0.65 (0.51–0.82) | 0.0003 | <0.0001 | 1.66 (1.12–2.47) | 0.0121 | 0.0049 | 1.09 (0.76–1.58) | 0.6285 | 0.778 | ||
| SVR | 0.39 (0.28–0.55) | <0.0001 | 0.74 (0.42–1.30) | 0.2948 | 0.92 (0.58–1.45) | 0.7167 | ||||||
| Two-group comparison | TF | 0.88 (0.73–1.07) | 0.206 | 0.206 | 2.1 (1.56–2.83) | <0.0001 | <0.0001 | 0.96 (0.69–1.32) | 0.782 | 0.782 | ||
| Key variables for | TF | 0.78 (0.65–0.93) | 0.0061 | <0.0001 | 1.97 (1.52–2.55) | <0.0001 | <0.0001 | 0.90 (0.68–1.20) | 0.484 | 0.7546 | ||
| PS calculation omitted | SVR | 0.45 (0.34–0.60) | <0.0001 | 0.96 (0.62–1.49) | 0.8544 | 0.92 (0.63–1.34) | 0.6662 | |||||
| Multiple imputation of missing values | TF | 0.67 (0.56–0.80) | <0.0001 | 1.90 (1.46–2.49) | <0.0001 | 0.85 (0.64–1.14) | 0.2836 | |||||
| SVR | 0.44 (0.33–0.59) | <0.0001 | 0.89 (0.58–1.38) | 0.6001 | 0.88 (0.60–1.29) | 0.5262 | ||||||
HCC, hepatocellular carcinoma; aHR, adjusted hazard ratio; CI, 95% confidence interval; FIB4, fibrosis-4 index; SVR, sustained virological response; TF, treatment failure.
Detailed in online supplements, Tables S2–S6.
Patients who achieved SVR had lower risk of HCC (aHR = 0.48, CI 0.31–0.73) than patients with treatment failure. There was also a significant difference (P=0.01) between treated and untreated patients in HCC stage; treated patients were more likely to be diagnosed at an earlier stage than untreated patients (local: 65% treated vs 50% untreated; regional: 19% treated vs 26% untreated; metastatic: 2% treated vs 7% untreated). No interaction was found between treatment/response and fibrosis category for risk of HCC, indicating that treatment was influential across all fibrosis categories.
As shown in other studies [20], treatment reduced risk of all-cause mortality – regardless of SVR (SVR: aHR = 0.45, CI 0.34–0.60; TF: aHR = 0.78, CI 0.65–0.93) (Table 2, Fig. 1). Again, no interaction was found between treatment/response and fibrosis category for all-cause mortality. Similar treatment effects on mortality were observed in the sensitivity analyses (Table 2B).
Fig. 1.
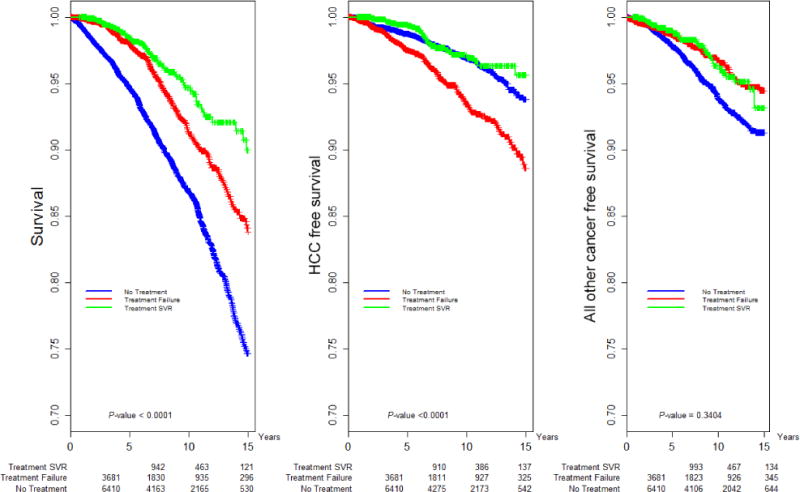
Kaplan–Meier analysis: Overall, HCC-free and non-HCC cancer-free survival by response to HCV antiviral treatment.
Additional risk factors
Compared to a baseline FIB4 score of <1.21, scores of 1.21–5.88 and >5.88 were independent risk factors for both HCC and death (Table 2, Fig. 2). HCV genotype (GT) 3 patients demonstrated increased risk of HCC than GT1 patients (aHR = 1.60, CI 1.04–2.44); there was no genotype effect on nonliver cancer or death (Table 2, Fig. 3). Men were at higher risk of both HCC (aHR = 2.31, CI 1.59–2.85) and death (aHR = 1.53, CI 1.29–1.83; Table 2).
Fig. 2.
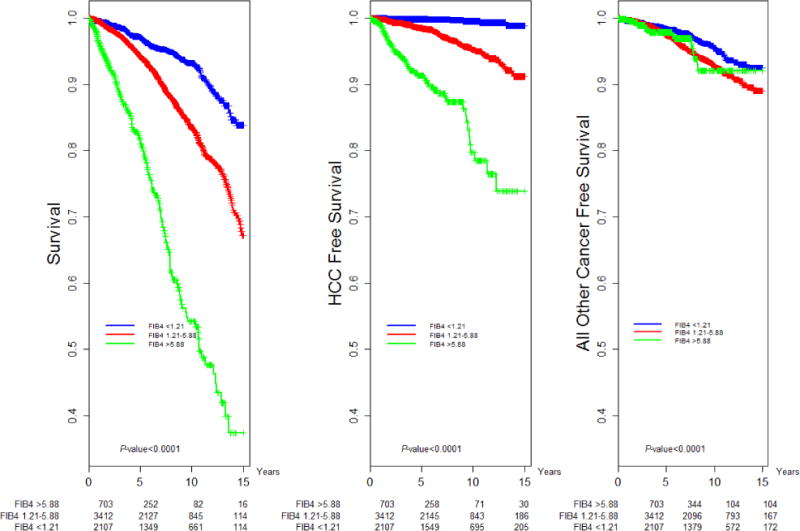
Kaplan–Meier Analysis: Overall, HCC-free and non-HCC cancer-free survival by FIB4 Score Category.
Fig. 3.
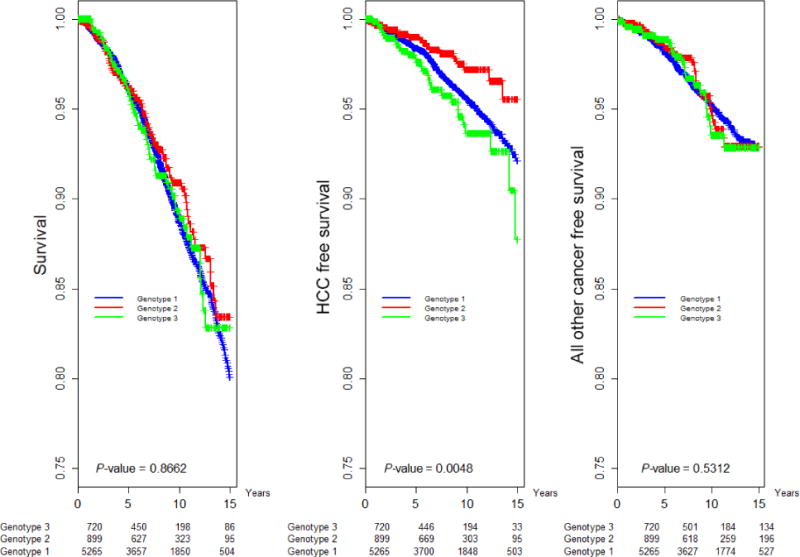
Kaplan–Meier analysis of overall survival, HCC-free and non-HCC cancer-free survival by HCV genotype.
African American patients had a higher risk of nonliver cancers than whites (aHR = 1.83, CI 1.29–2.59), but lower risk of HCC (aHR = 0.53, CI 0.38–0.74) and death (aHR = 0.69, CI 0.53–0.89). Privately insured patients demonstrated reduced mortality (aHR = 0.58, CI 0.45–0.75) independent of treatment. As expected, age was a risk factor for all outcomes (Table 2). Diabetes had a significant effect on risk of HCC (aHR = 1.65 CI 1.09–2.49, Table 2, Fig. 4). Substance abuse was a risk factor for death (aHR = 1.48 CI 1.08–2.04, Table 2). HIV had a significant effect on risk of non-HCC cancer (aHR = 2.33, CI 1.19–4.55, Table 2).
Fig. 4.
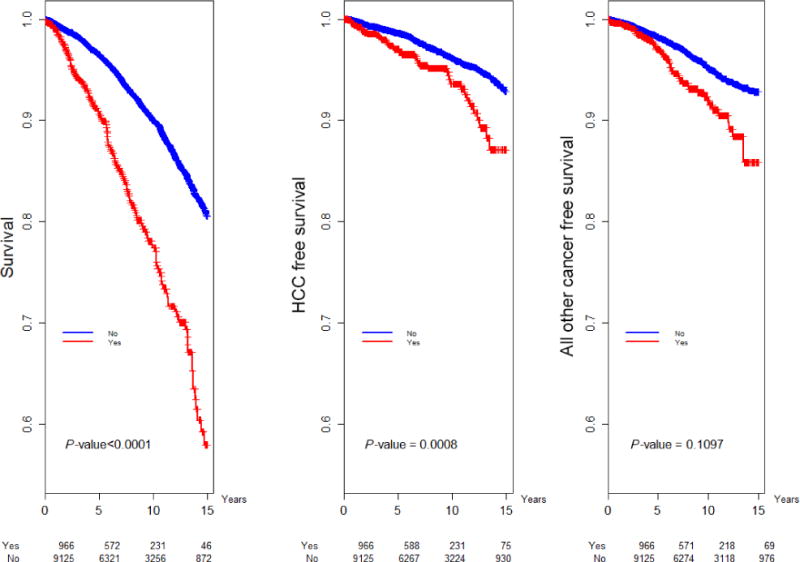
Kaplan–Meier analysis of overall survival, HCC-free and non-HCC cancer-free survival by presence of diabetes.
DISCUSSION
We found that patients who received antiviral treatment but failed to achieve sustained virological response were significantly more likely to develop HCC than untreated patients (aHR = 1.95, CI 1.50–2.53), despite the fact that HCC screening rates were lower in treated than untreated patients. It is not clear why treatment failure increases HCC risk. At least one study has noted that TF patients experience higher rates of fibrotic progression than untreated patients [21], suggesting that treatment failure may accelerate fibrotic progression (a known risk factor for HCC). It is also possible that minority variants emerging after TF [22] may also contribute to development of HCC. Due to the observational nature of our study, we cannot offer mechanistic explanations for our finding. However, consistent results across five sensitivity analyses indicate that this finding is robust. In particular, our findings were similar after IPTW adjustment for treatment selection bias (the two-group comparison sensitivity analysis); we also found only negligible difference in hazard ratios between the last sensitivity analysis (which removed several key variables) and the main analysis (Table 2). These results indicate that unmeasurable confounders are unlikely to have influenced our findings [17].
As noted in previous studies, we found that SVR reduced risk of HCC across all levels of fibrosis [10]; this effect remained significant regardless of known risk factors. Advanced fibrosis and cirrhosis increased risk of HCC by roughly 5- and 27-fold, respectively, regardless of treatment status. Although African Americans were at increased risk of treatment failure, they were at lower risk for HCC and all-cause mortality compared to White patients; this is consistent with results from a large VA-based study [23]. Finally, we found that diabetes is an independent risk factor for HCC, consistent with studies in non-HCV populations [24].
GT3 patients had twice the risk of HCC (but not a higher risk of death) than GT1 patients, a finding also reported in other cohorts [25,26]. Notably, we found that GT3 was associated with HCC independent of diabetes. These findings assume importance in the light of recent estimates that GT3 is the second most prevalent genotype globally [27], as well as recent studies that show it to be the most difficult to treat of all HCV genotypes [28–30].
Neither treatment nor achievement of SVR resulted in lower rates of nonliver cancers, although HIV infection was an independent risk factor for non-HCC cancer. This finding is not unexpected; previous studies have shown that HIV raises the risk of several types of cancer with known infectious causes [31].
SVR reduced the risk of all-cause mortality by 55% vs lack of treatment and by 42% vs treatment failure. This confirms our previous finding that SVR reduced risk of all-cause mortality in a cohort of treated patients with advanced fibrosis [25]. We also found that treatment (regardless of SVR) consistently reduced risk of all-cause mortality across FIB4 categories and all sensitivity analyses.
Although confounding is always a challenge in observational research, we designed our study by following guidance from Stuart et al. [32] and the STROBE Statement [33] to ensure that appropriate inferences could be drawn and that results would be reliable. We also performed a number of analyses to account for treatment selection bias (propensity score calculations), unmeasurable covariates (sensitivity analyses) [17], and missing data (subgroup analysis and sensitivity analysis using multiple imputation). We also conducted a subgroup analysis with a two-treatment group comparison (TF vs untreated) to confirm the TF effect. Based on consistent results from these analyses (see details in Tables S2–6), we are confident in our estimated treatment effects and that unobserved confounding did not significantly influence our results [17].
Duration of HCV infection in this population is estimated. However, we used several levels of data to confirm first indication of infection/date of diagnosis, including: (i) patient self-report; (ii) medical chart abstraction; (iii) EHR-based HCV medication prescriptions/fills; and (iv) ICD9/CPT codes from the EHR.
In conclusion, patients who fail interferon-based therapy demonstrate a higher risk of HCC than untreated patients, possibly due to interferon-related acceleration of fibrosis in the absence of successful viral eradication. Regardless of whether this finding reflects an impaired innate immune responsiveness or an interferon-mediated acceleration of fibrosis, we suggest that such treatment failure patients may represent a cohort of individuals for whom retreatment with new, highly effective direct-acting all-oral antiviral therapies should become a priority.
Supplementary Material
Table S1. Possible contraindications to antiviral therapy, used to adjust for possible confounding.
Table S2a. Treatment and SVR detail.
Table S2b. Outcomes.
Table S2c. Univariate analysis of the impact of HCV treatment and achievement of SVR on clinical outcomes (mortality, HCC, and non-HCC cancer).
Table S3a. Outcomes in the 1:1 matched cohort.
Table S3b. Treatment differences at baseline in the 1:1 matched cohort.
Table S3c. Univariate analysis of the impact of HCV treatment and achievement of SVR on clinical outcomes (mortality, HCC, and non-HCC cancer) in the 1:1 matched cohort.
Table S3d. Multivariate analysis of the impact of HCV treatment and achievement of SVR on clinical out comes (mortality, HCC, and non-HCC cancer) in the 1:1 matched cohort.
Table S4a. Outcomes in the FIB4 subgroup.
Table S4b. Treatment differences at baseline in the FIB4 subgroup, before and after weighting.
Table S4c. Univariate analysis of the impact of HCV treatment and achievement of SVR on clinical outcomes (mortality, HCC, and non-HCC cancer) in the FIB4 subgroup.
Table S4d. Multivariate analysis of the impact of HCV treatment and achievement of SVR on clinical outcomes (mortality, HCC, and non-HCC cancer) in the FIB4 subgroup.
Table S5a. Outcomes in the two-group comparison subgroup.
Table S5b. Treatment differences at baseline in the two-group comparison subgroup, before and after weighting.
Table S5c. Univariate analysis of the impact of HCV treatment and achievement of svr on clinical outcomes (mortality, HCC, and non-HCC cancer) in the two-group comparison subgroup.
Table S5d. Multivariate analysis of the impact of HCV treatment and achievement of SVR on clinical outcomes (mortality, HCC, and non-HCC cancer) in the two-group comparison subgroup.
Table S6a. Outcomes in the analysis with key variables omitted.
Table S6b. Treatment differences at baseline (before/after weighting, allowing for a six-month window of treatment exposure) with key variables omitted.
Table S6c. Univariate analysis of the impact of HCV treatment and achievement of SVR on clinical outcomes (mortality, HCC, and non-HCC cancer) with key variables omitted.
Table S6d. Multivariate analysis of the impact of HCV treatment and achievement of SVR on clinical outcomes (mortality, HCC, and non-HCC cancer) with key variables omitted.
Acknowledgments
In addition to the authors, CHeCS investigators include Jim Xing and Cindy Tong, Division of Viral Hepatitis, National Centers for HIV, Viral Hepatitis, STD, and TB Prevention, Centers for Disease Control and Prevention (CDC), Atlanta, GA; David R. Nerenz, Nonna Akkerman, Nancy Oja-Tebbe and Talan Zhang, Henry Ford Health System, Detroit, MI; Zahra S. Daar and Robert E. Smith, Center for Health Research, Geisinger Health System, Danville PA; John V. Parker, Center for Health Research, Kaiser Permanente-Hawai’i, Honolulu, HI; Judy L. Donald and Erin M. Keast, Center for Health Research, Kaiser Permanente-Northwest, Portland, OR.
FUNDING
CHeCS is funded by the CDC Foundation, which receives grants from AbbVie, Gilead Sciences and Janssen Pharmaceuticals. Past funders include Genentech and Vertex Pharmaceuticals. Past partial funders include Bristol–Myers Squibb. Granting corporations do not have access to CHeCS data and do not contribute to data analysis or manuscripts.
Stuart C. Gordon receives grant/research support from AbbVie Pharmaceuticals, Bristol–Myers Squibb, Gilead Pharmaceuticals, GlaxoSmithKline, Intercept Pharmaceuticals, Merck and Vertex Pharmaceuticals. He is also a consultant for Amgen, Bristol–Myers Squibb, CVS Care-mark, Gilead Pharmaceuticals, Merck, Novartis and Vertex and is on the Data Monitoring Board for Janssen Pharmaceuticals
Abbreviations
- aHR
adjusted hazard ratio
- EHR
electronic health record
- FIB4
fibrosis-4 Index
- GT
genotype
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- IPTW
inverse probability treatment weighting
- PS
propensity score
- SVR
sustained virological response
- TF
treatment failure
Footnotes
CONFLICT OF INTEREST DISCLOSURES
Other authors have no conflict of interest to declare.
SUPPORTING INFORMATION
Additional Supporting Information may be found online in the supporting information tab for this article:
References
- 1.Yau AH, Yoshida EM. Hepatitis C drugs: the end of the pegylated interferon era and the emergence of all-oral interferon-free antiviral regimens: a concise review. Can J Gastroenterol Hepatol. 2014;28(8):445–451. doi: 10.1155/2014/549624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aleman S, Rahbin N, Weiland O, et al. A risk for hepatocellular carcinoma persists long-term after sustained virologic response in patients with hepatitis C-associated liver cirrhosis. Clin Infect Dis. 2013;57(2):230–236. doi: 10.1093/cid/cit234. [DOI] [PubMed] [Google Scholar]
- 3.Di Bisceglie AM, Shiffman ML, Everson GT, et al. Prolonged therapy of advanced chronic hepatitis C with low-dose peginterferon. N Engl J Med. 2008;359(23):2429–2441. doi: 10.1056/NEJMoa0707615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Imai Y, Kawata S, Tamura S, et al. Relation of interferon therapy and hepatocellular carcinoma in patients with chronic hepatitis C. Osaka Hepatocellular Carcinoma Prevention Study Group. Ann Intern Med. 1998;129(2):94–99. doi: 10.7326/0003-4819-129-2-199807150-00005. [DOI] [PubMed] [Google Scholar]
- 5.Shindo M, Ken A, Okuno T. Varying incidence of cirrhosis and hepatocellular carcinoma in patients with chronic hepatitis C responding differently to interferon therapy. Cancer. 1999;85(9):1943–1950. [PubMed] [Google Scholar]
- 6.Shiratori Y, Ito Y, Yokosuka O, et al. Antiviral therapy for cirrhotic hepatitis C: association with reduced hepatocellular carcinoma development and improved survival. Ann Intern Med. 2005;142(2):105–114. doi: 10.7326/0003-4819-142-2-200501180-00009. [DOI] [PubMed] [Google Scholar]
- 7.Yoshida H, Tateishi R, Arakawa Y, et al. Benefit of interferon therapy in hepatocellular carcinoma prevention for individual patients with chronic hepatitis C. Gut. 2004;53(3):425–430. doi: 10.1136/gut.2003.030353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Association for the Study of Liver Diseases IDSoA. Recommendations for Testing, Managing, and Treating Hepatitis C. 2014;2015 [Google Scholar]
- 9.Moorman AC, Gordon SC, Rupp LB, et al. Baseline characteristics and mortality among people in care for chronic viral hepatitis: the chronic hepatitis cohort study. Clin Infect Dis. 2013;56(1):40–50. doi: 10.1093/cid/cis815. [DOI] [PubMed] [Google Scholar]
- 10.Li J, Gordon SC, Rupp LB, et al. The validity of serum markers for fibrosis staging in chronic hepatitis B and C. J Viral Hepatitis. 2014;21(12):930–937. doi: 10.1111/jvh.12224. [DOI] [PubMed] [Google Scholar]
- 11.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 12.Austin PC. The performance of different propensity-score methods for estimating relative risks. J Clin Epidemiol. 2008;61(6):537–545. doi: 10.1016/j.jclinepi.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 13.Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11(5):550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Steiner JF, Paolino AR, Thompson EE, Larson E. Sustaining research networks: the Twenty-Year Experience of the HMO research network. eGEMS. 2014;2(2):1067. [PMC free article] [PubMed] [Google Scholar]
- 15.Mahajan R, Xing J, Liu SJ, et al. Mortality among persons in care with hepatitis C virus infection: the Chronic Hepatitis Cohort Study (CHeCS), 2006–2010. Clin Infect Dis. 2014;58(8):1055–1061. doi: 10.1093/cid/ciu077. [DOI] [PubMed] [Google Scholar]
- 16.SAS Institute Inc. Proceedings of the Twenty-Sixth Annual SAS Users Group International Conference. Cary, NC: SAS Institute Inc; 2001. [Google Scholar]
- 17.Steventon A, Grieve R, Sekhon J. A comparison of alternative strategies for choosing control populations in observational studies. Health Serv Outcomes Res Method. 2015;15(3):157–181. doi: 10.1007/s10742-014-0135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rubin DB. Inference and missing data. Biometrika. 1976;63:581–592. [Google Scholar]
- 19.Rubin DB, Schenker N. Multiple imputation in health-care databases: an overview and some applications. Stat Med. 1991;10(4):585–598. doi: 10.1002/sim.4780100410. [DOI] [PubMed] [Google Scholar]
- 20.Wen Y, Zheng YX, de Tan M. A comprehensive long-term prognosis of chronic hepatitis C patients with antiviral therapy: a meta-analysis of studies from 2008 to 2014. Hepat Mon. 2015;15(5):e27181. doi: 10.5812/hepatmon.15(5)2015.27181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baran B, Gulluoglu M, Soyer OM, et al. Treatment failure may lead to accelerated fibrosis progression in patients with chronic hepatitis C. J Viral Hepatitis. 2014;21(2):111–120. doi: 10.1111/jvh.12127. [DOI] [PubMed] [Google Scholar]
- 22.Abdelrahman T, Hughes J, Main J, McLauchlan J, Thursz M, Thomson E. Next-generation sequencing sheds light on the natural history of hepatitis C infection in patients who fail treatment. Hepatology (Baltimore, MD) 2015;61(1):88–97. doi: 10.1002/hep.27192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El-Serag HB, Kramer J, Duan Z, Kanwal F. Racial differences in the progression to cirrhosis and hepatocellular carcinoma in HCV-infected veterans. Am J Gastroenterol. 2014;109(9):1427–1435. doi: 10.1038/ajg.2014.214. [DOI] [PubMed] [Google Scholar]
- 24.Hassan MM, Curley SA, Li D, et al. Association of diabetes duration and diabetes treatment with the risk of hepatocellular carcinoma. Cancer. 2010;116(8):1938–1946. doi: 10.1002/cncr.24982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van der Meer AJ, Veldt BJ, Feld JJ, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308(24):2584–2593. doi: 10.1001/jama.2012.144878. [DOI] [PubMed] [Google Scholar]
- 26.Kanwal F, Kramer JR, Ilyas J, Duan Z, El-Serag HB. HCV genotype 3 is associated with an increased risk of cirrhosis and hepatocellular cancer in a national sample of U.S. Veterans with HCV Hepatology. 2014;60(1):98–105. doi: 10.1002/hep.27095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Messina JP, Humphreys I, Flaxman A, et al. Global distribution and prevalence of hepatitis C virus genotypes. Hepatology (Baltimore, MD) 2015;61(1):77–87. doi: 10.1002/hep.27259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aghemo A, Colombo M. Hepatitis C genotype 3: a tough match for interferon-free regimens. Gastroenterology. 2014;146(4):1125–1127. doi: 10.1053/j.gastro.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 29.Jacobson IM, Gordon SC, Kowdley KV, et al. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med. 2013;368(20):1867–1877. doi: 10.1056/NEJMoa1214854. [DOI] [PubMed] [Google Scholar]
- 30.Lawitz E, Mangia A, Wyles D, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368(20):1878–1887. doi: 10.1056/NEJMoa1214853. [DOI] [PubMed] [Google Scholar]
- 31.Pierangeli A, Antonelli G, Gentile G. Immunodeficiency-associated viral oncogenesis. Clin Microbiol Infect. 2015;21(11):975–983. doi: 10.1016/j.cmi.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 32.Stuart EA, DuGoff E, Abrams M, et al. Estimating causal effects in observational studies using electronic health data: challenges and (some) solutions. eGEMS. 2013;1(3):4. doi: 10.13063/2327-9214.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Int J Surg. 2014;12(12):1500–1524. doi: 10.1016/j.ijsu.2014.07.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Possible contraindications to antiviral therapy, used to adjust for possible confounding.
Table S2a. Treatment and SVR detail.
Table S2b. Outcomes.
Table S2c. Univariate analysis of the impact of HCV treatment and achievement of SVR on clinical outcomes (mortality, HCC, and non-HCC cancer).
Table S3a. Outcomes in the 1:1 matched cohort.
Table S3b. Treatment differences at baseline in the 1:1 matched cohort.
Table S3c. Univariate analysis of the impact of HCV treatment and achievement of SVR on clinical outcomes (mortality, HCC, and non-HCC cancer) in the 1:1 matched cohort.
Table S3d. Multivariate analysis of the impact of HCV treatment and achievement of SVR on clinical out comes (mortality, HCC, and non-HCC cancer) in the 1:1 matched cohort.
Table S4a. Outcomes in the FIB4 subgroup.
Table S4b. Treatment differences at baseline in the FIB4 subgroup, before and after weighting.
Table S4c. Univariate analysis of the impact of HCV treatment and achievement of SVR on clinical outcomes (mortality, HCC, and non-HCC cancer) in the FIB4 subgroup.
Table S4d. Multivariate analysis of the impact of HCV treatment and achievement of SVR on clinical outcomes (mortality, HCC, and non-HCC cancer) in the FIB4 subgroup.
Table S5a. Outcomes in the two-group comparison subgroup.
Table S5b. Treatment differences at baseline in the two-group comparison subgroup, before and after weighting.
Table S5c. Univariate analysis of the impact of HCV treatment and achievement of svr on clinical outcomes (mortality, HCC, and non-HCC cancer) in the two-group comparison subgroup.
Table S5d. Multivariate analysis of the impact of HCV treatment and achievement of SVR on clinical outcomes (mortality, HCC, and non-HCC cancer) in the two-group comparison subgroup.
Table S6a. Outcomes in the analysis with key variables omitted.
Table S6b. Treatment differences at baseline (before/after weighting, allowing for a six-month window of treatment exposure) with key variables omitted.
Table S6c. Univariate analysis of the impact of HCV treatment and achievement of SVR on clinical outcomes (mortality, HCC, and non-HCC cancer) with key variables omitted.
Table S6d. Multivariate analysis of the impact of HCV treatment and achievement of SVR on clinical outcomes (mortality, HCC, and non-HCC cancer) with key variables omitted.


