Abstract
Undifferentiated carcinoma of the pancreas with osteoclast-like giant cells (UC-OGC) is a rare and poorly described pancreatic malignancy. It is comprised of mononuclear, pleomorphic, and undifferentiated cells as well as osteoclast-like giant cells (OGC’s). It constitutes less than 1% of pancreatic non-endocrine neoplasia and is twice as likely to occur in females as in males. Its histopathologic properties remain poorly understood. It is suspected that UC-OGC is of epithelial origin that can then transition to mesenchymal elements. As part of this study, we describe a case of a malignant pancreatic neoplasm that was discovered in a 69-year old patient as an incidental finding. We also provide an overview of previously published data to highlight UC-OGC’s clinical and pathologic features.
Keywords: Adenocarcinoma; Carcinoma, Pancreatic Ductal; Osteoclasts; Pancreatic Neoplasms
INTRODUCTION
Pancreatic carcinoma is the second most common type of gastrointestinal malignancy after colorectal carcinoma.1,2 Undifferentiated carcinoma of the pancreas (UCP) and undifferentiated carcinoma of the pancreas with osteoclast-like giant cells (UC-OGC) are rare neoplasms that make up 2% of pancreatic malignancies.3,4 UCP can be further sub classified into sarcomatoid carcinoma and carcinosarcoma.5 UCP has a much poorer prognosis than typical ductal adenocarcinoma of the pancreas.1,6 Survival in some reports has been on average as low as 5 months from the time of diagnosis.3,6 Based on histologic features, UCP subtypes and UC-OGC have notable overlap, such as the presence of heterologous elements including osteoid, muscle and cartilage.6 However, the presence of OGC’s is a defining characteristic of UC-OGC in the World Health Organization (WHO) classification of pancreatic malignancies.7 This entity makes up less than 1% of pancreatic malignancies and has a distinct histopathological and clinical profile.1,8 We describe a case of UC-OGC with sarcomatoid features and abundant osteoid production in a previously asymptomatic patient.
CASE REPORT
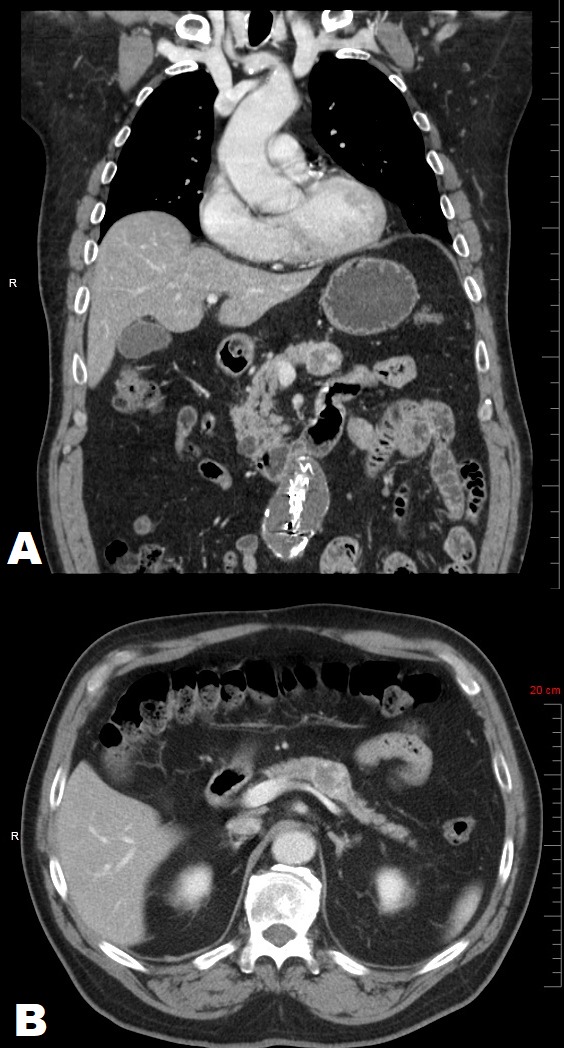
A 69-year old male with a past medical history of chronic hypercholesterolemia, hypertension and morbid obesity and remote history of alcohol and tobacco use was incidentally discovered to have a 2.5 cm partially calcified, cystic mass within the body of the pancreas. The mass was discovered as an incidental finding on CT angiogram of the abdomen (Figures 1A and 1B) during the evaluation for an abdominal aortic aneurysm. Follow-up esophagogastroduodenoscopy/endoscopic-ultrasound highlighted a 2.7 × 2.6 cm hypoechoic, heterogeneous, partially calcified lesion abutting the splenic vein (Figure 1).
Figure 1. Abdominal Computed Tomography with intravenous and oral contrast: Sagittal (A) and Coronal (B) views demonstrating a well-circumscribed heterogeneous mass within the body of the pancreas (arrowheads), apparently without vascular invasion.
EUS aspiration revealed a high grade malignant neoplasm of uncertain origin. The patient was not jaundiced and his total and direct bilirubin, lipase, amylase and CA19-9 were within normal limits. He denied having nausea, vomiting, diarrhea, constipation, abdominal pain, appetite changes or bloating. The patient subsequently underwent endovascular repair of his abdominal aortic aneurysm and his recovery remained unremarkable for any gastrointestinal symptoms. Six weeks later the patient underwent a fine needle aspiration (FNA) of the pancreatic mass which demonstrated numerous malignant cells with enlarged hyperchromatic nuclei with prominent nucleoli, as well as many multinucleated cells. Four weeks later the patient underwent an elective exploratory laparotomy that consisted of a subtotal pancreatectomy, splenectomy and omentectomy. The pancreatic mass was found to have dense adhesions around the celiac trunk and splenic vessels which necessitated resection of the spleen and a portion of the inferior mesenteric vein. The lesion was a 3.5 × 2.5 cm firm, brown to yellow, well-circumscribed, heterogeneous nodule with focal areas of hemorrhage. There was no evidence of tumor extending beyond the pancreas.
On microscopic examination, the tumor was predominated by pleomorphic, spindle cells associated with hemorrhage and abundant hemosiderin pigment (Figures 2A and 2B). The spindle cell sarcomatous component was diffusely immunoreactive with vimentin (Figure 2C). Notably, the tumor contained several well-formed ductal structures (Figure 2D). With immunohistochemical staining the glandular structures were reactive with CK AE1/AE3 and CK Cam5.2 (Figures 3A and 3B). Osteoclast-like giant cells were present individually and in clusters (Figure 3C), staining positive for CD68 (Figure 3D), and were admixed within a background of numerous foamy histiocytes (Figure 4A). Areas of irregular calcification as well as malignant osteoid were evident within the tumor (Figure 4B). The foamy macrophages throughout the tumor as well as the osteoclast-like giant cells were immunoreactive with CD68 and CD31. The malignant spindle cells were positive for CD34, while CD31 highlighted adjacent foamy macrophages and giant cells (Figures 4C and 4D). Several large vacuolated malignant cells were admixed with the numerous foamy macrophages that resembled lipoblasts. This morphology and immunohistochemistry staining pattern was consistent with undifferentiated carcinoma of the pancreas with osteoclast-like giant cells. No premalignant lesions, including pancreatic intraepithelial neoplasia, intraductal papillary mucinous neoplasms and mucinous cystic neoplasm were identified.
Figure 2. Photomicrography of the pancreatic mass showing in A – Pleomorphic giant cells and malignant spindle cells (H&E, 200X); B – hemosiderin and red blood cells (H&E, 200X); C – staining positive for vimentin (200X); and D – Ductal carcinoma component (H&E, 200X).
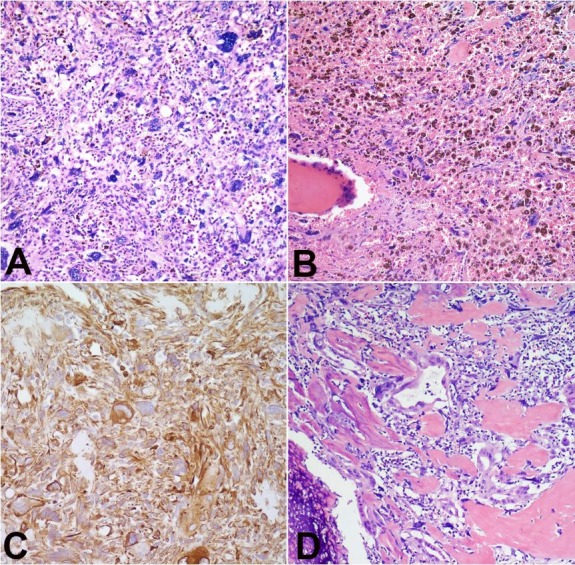
Figure 3. Photomicrography of the pancreatic mass showing in A – Ductal carcinoma component staining positive for CK AE1/AE3 (400X); and Cam 5.2 in B (200X); C – Osteoclast-like giant cells (400X) staining positive for CD68 in D (400X).

Figure 4. Photomicrography of the pancreatic mass showing in A – Foamy histiocytes (H&E, 100X) and osteoid with partial calcification in B (H&E, 100X); C – Tumor staining positive for CD31 (200X) and CD 34 diffusely in D (200X).

Microscopically, the tumor did not involve the major vessels, including the inferior mesenteric vein, celiac trunk or the splenic vessels. These areas, however, were surrounded by dense fibrosis.
DISCUSSION
Tumors with osteoclast-like giant cells have been documented within a variety of organs including the kidney, breast, thyroid gland, heart, parotid and skin.1,7-10 Within the pancreas, they are typically large, usually greater than 3.5 cm.3,7,9-12 The mean tumor size in reported cases was 5-8 cm.6,7,9-12 The tumor is usually a heterogeneous mass that can cause compression of adjacent normal structures, or constitutional symptoms such as fatigue and weight loss.3,7,9-12 Two-thirds of cases in the literature reported abdominal or back pain, one-third presented with painless jaundice.6,7,9-12 One third also reported nausea.6,7,9-12 Weight loss and anorexia were also reported in half of the cases.6,7,9-12 CA19-9 was elevated in two-thirds of cases described by Muraki et al.6 Our patient was asymptomatic and the tumor was discovered incidentally during evaluation of an abdominal aortic aneurysm.
UC-OGC is more likely to occur within the body and tail of the pancreas, account for about 70% of reported cases.6,7,9-12 The majority of cases are diagnosed in the sixth to seventh decade of life, when the tumor is in late progression. This is mostly due to its asymptomatic profile in its early stages.6,7
Radiographically, unlike typical adenocarcinoma of the pancreas which is uniformly hypoechoic, UC-OGC commonly appears as a heterogeneous mass with distinct hyper- and hypoechoic regions.4,7,12 Pancreatic FNA or biopsy is usually performed in cases of pancreatic malignancies. In Muraki et al’s6 study, 90% of the cases that underwent preoperative biopsy demonstrated malignancy, with a quarter having the diagnosis of OGC. Preoperative biopsy showed the OGC component was identified in a third of cases by Muraki et al.6 Our case demonstrated a diagnosis of malignant neoplasm on FNA.
There is much debate in the literature as to the origin of the tumor, with many authors favoring mesenchymal origin, and others favoring epithelial origin.4,6,9-12 Consensus appears to be leaning toward an epithelial origin with some authors purporting that components of vimentin-positive carcinoma are mesenchymal transition from ductal cells.3,6 In reported cases, cytokeratin-positive ductal structures have been reported to comprise < 5% to 80% of the tumor.6 Our case was characterized by rare ductal structures and focal immunoreactivity with CK AE1/AE3 and Cam 5.2 (Figures 2B and 2C), and an atypical mesenchymal component with immunoreactivity with vimentin (Figure 3B). In a study by Luchini et al.,13 it was purported that UC-OGC’s are variants of pancreatic ductal carcinoma (PDC) due to the presence of shared mutations in KRAS and other critical tumor suppressor genes commonly associated with PDC (TP53, CDKN2A and SMAD4).
OGCs in UC-OGC consist of benign giant cells within a background of infiltrating anaplastic mononuclear malignant cells.1,3 They are commonly considered to be of benign histiocytic origin, which is supported in our case by a lack of atypia, and immunoreactivity with CD68. It is hypothesized that OGC recruitment is a result of chemotactic factors produced by neoplastic cells.7 The presence of large sheets of foamy histiocytes within the tumor supports the concept of a histiocytic chemotactic factor.7 Within the OGCs are phagocytized neoplastic cell remnants.7 OGCs themselves may be few or may comprise the bulk of the tumor, occurring in sheets or cell clusters.6 OGCs are typically found in nodules associated with areas of hemorrhage. They can fill and replace ducts within the pancreas.6 The OGC component can comprise the majority of the tumor and even resemble an osteoclastoma pattern with minimal amounts of the malignant components.6 With a greater osteoclast component, a more protracted clinical course is expected.6 One third of cases reported in the study by Muraki et al.6 had presence of metaplastic, mature bone tissue. Many of the cases in the study showed associated pancreatitis. OGC and pancreatic intraepithelial lesions were observed in 47% of the UC-OGC cases.6
UC-OGC usually demonstrates lymph-vascular and perineural invasion. In a study by Muraki et al.,6 lymph-vascular invasion was present in 63% of the time in UC-OGC cases. Perineural invasion was present in 32% of cases as compared to 86% in PDCs (p < 0.0001).6 Our case showed no evidence of lymph-vascular or perineural invasion. Muraki et al.6 found that lymph node metastasis in UC-OGC was less as compared to PDC. It was present in 23% of cases of UC-OGC and 64% in PDC (p < 0.0001).6 Our case, however, showed no regional lymph node metastasis.
UC-OGCs behave differently to other tumors of the pancreas. They appear to occur close to a decade earlier than PDCs with a mean age of 58 at diagnosis compared to 65 years in PDC.6 Although UCP has a very poor prognosis with average survival less than 1 year, compared to PDC,6 UC-OGC has a more favorable prognosis with a 5-year survival rate of 59.1% compared to 15.6% for PDC.3,6 Median survival in Muraki et al.6 study was 7.7 years compared to 1.6 years in patients with PDCs (p<0.0009). In our case, the patient has remained clinically free of tumor thirteen months post resection. UC-OGC has a propensity to invade adjacent structures, although, complete resection of the tumor can prove curative when the tumor is limited to the pancreas.3,6 Further research on this rare entity may help establish reliable management guidelines.
CONCLUSION
It is important to differentiate UC-OGC from other pancreatic malignancies due to the relative improvement in prognosis. Due to its rarity, therapeutic guidelines are limited. Further studies may help establish treatment modalities and possible molecular biomarkers.
Footnotes
How to cite: Sakhi R, Hamza A, Khurram MS, Ibrar W, Mazzara P. Undifferentiated carcinoma of the pancreas with osteoclast-like giant cells reported in an asymptomatic patient: a rare case and literature review. Autops Case Rep [Internet]. 2017;7(4):51-57. http://dx.doi.org/10.4322/acr.2017.042
Financial support: None
REFERENCES
- 1.Loya AC, Ratnakar KS, Shastry RA. Combined osteoclastic giant cell and pleomorphic giant cell tumor of the pancreas: a rarity: an immunohistochemical analysis and review of the literature. JOP. 2004;5(4):220-4. [PubMed] [Google Scholar]
- 2.Moore JC, Bentz JS, Hilden K, Adler DG. Osteoclastic and pleomorphic giant cell tumors of the pancreas diagnosed via EUS-guided FNA: unique clinical, endoscopic, and pathologic findings in a series of 5 patients. World J Gastrointest Endosc. 2010;2(1):15-9. http://dx.doi.org/10.4253/wjge.v2.i1.15. [DOI] [PubMed] [Google Scholar]
- 3.Jo S. Huge undifferentiated carcinoma of the pancreas with osteoclast-like giant cells. World J Gastroenterol. 2014;20(10):2725-30. http://dx.doi.org/10.3748/wjg.v20.i10.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maksymov V, Khalifa MA, Bussey A, Carter B, Hogan M. Undifferentiated (anaplastic) carcinoma of the pancreas with osteoclast-like giant cells showing various degree of pancreas duct involvement: a case report and literature review. JOP. 2011;12(2):170-6. [PubMed] [Google Scholar]
- 5.Kane JR, Laskin WB, Matkowskyj KA, Villa C, Yeldandi AV. Sarcomatoid (spindle cell) carcinoma of the pancreas: a case report and review of the literature. Oncol Lett. 2014;7(1):245-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muraki T, Reid MD, Basturk O, et al. Undifferentiated carcinoma with osteoclastic giant cells of the pancreas. Am J Surg Pathol. 2016;40(9):1203-16. http://dx.doi.org/10.1097/PAS.0000000000000689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sah SK, Li Y, Li Y. Undifferentiated carcinoma of the pancreas with osteoclast-like giant cells: a rare case report and review of the literature. Int J Clin Exp Pathol. 2015;8(9):11785-91. [PMC free article] [PubMed] [Google Scholar]
- 8.Burkadze G, Turashvili G. A case of osteoclast-like giant cell tumor of the pancreas associated with borderline mucinous cystic neoplasm. Pathol Oncol Res. 2009;15(1):129-31. http://dx.doi.org/10.1007/s12253-008-9053-9. [DOI] [PubMed] [Google Scholar]
- 9.Farah F, Mlika M, Eddiba T, Zermani R, Jilani SBB. Undifferentiated carcinoma with osteoclast-like giant cells of the pancreas. Sci Rep. 2012;1:179 http://dx.doi.org/10.4172/scientificreports.179. [Google Scholar]
- 10.Kawamoto Y, Ome Y, Terada K, Hashida K, Kawamoto K, Ito T. Undifferentiated carcinoma with osteoclast-like giant cells of the ampullary region: Short term survival after pancreaticoduodenectomy. Int J Surg Case Rep. 2016;24:199-202. http://dx.doi.org/10.1016/j.ijscr.2016.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hur YH, Kim HH, Seoung JS, et al. Undifferentiated carcinoma of the pancreas with osteoclast-like giant cells. J Korean Surg Soc. 2011;81(2):146-50. http://dx.doi.org/10.4174/jkss.2011.81.2.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Georgiou GK, Balasi E, Siozopoulou V, Tsili A, Fatouros M, Glantzounis G. Undifferentiated carcinoma of the head of pancreas with osteoclast-like giant cells presenting as a symptomatic cystic mass, following acute pancreatitis: case report and review of the literature. Int J Surg Case Rep. 2016;19:106-8. http://dx.doi.org/10.1016/j.ijscr.2015.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luchini C, Pea A, Lionheart G, et al. Pancreatic undifferentiated carcinoma with osteoclast‐like giant cells is genetically similar to, but clinically distinct from, conventional ductal adenocarcinoma. J Pathol. 2017;243(2):148-54. http://dx.doi.org/10.1002/path.4941. [DOI] [PMC free article] [PubMed] [Google Scholar]


