Abstract
Purpose
The research objectives of the Right to Care Clinical HIV Cohort analyses are to: (1) monitor treatment outcomes (including death, loss to follow-up, viral suppression and CD4 count gain among others) for patients on antiretroviral therapy (ART); (2) evaluate the impact of changes in the national treatment guidelines around when to initiate ART on HIV treatment outcomes; (3) evaluate the impact of changes in the national treatment guidelines around what ART regimens to initiate on drug switches; (4) evaluate the cost and cost-effectiveness of HIV treatment delivery models; (5) evaluate the need for and outcomes on second-line and third-line ART; (6) evaluate the impact of comorbidity with non-communicable diseases on HIV treatment outcomes and (7) evaluate the impact of the switch to initiating all patients onto ART regardless of CD4 count.
Participants
The Right to Care Clinical HIV Cohort is an open cohort of data from 10 clinics in two provinces within South Africa. All clinics include data from 2004 onwards. The cohort currently has data on over 115 000 patients initiated on HIV treatment and patients are followed up every 3–6 months for clinical and laboratory monitoring.
Findings to date
Cohort data includes information on demographics, clinical visit, laboratory data, medication history and clinical diagnoses. The data have been used to identify rates and predictors of first-line failure, to identify predictors of mortality for patients on second-line (eg, low CD4 counts) and to show that adolescents and young adults are at increased risk of unsuppressed viral loads compared with adults.
Future plans
Future analyses will inform national models of HIV care and treatment to improve HIV care policy in South Africa.
Keywords: HIV & AIDS, INFECTIOUS DISEASES, retention
Strengths and limitations of this study.
The biggest strength of the Right to Care Clinical cohort is its size. With over 115 000 patients ever initiated onto HIV treatment, precise evaluations can be conducted. This is particularly important when describing outcomes among subsets of the cohort that could not be conducted with much precision using individual clinic data.
Second, while the clinics use a similar treatment protocol and data collection strategies, they show geographic variation. While other cohort collaborations do have such geographic variation, few were designed to encompass clinics which shared a common software and approach to data collection. Further because we link the data to the National Health Laboratory Service and the National Population Registry, we have high-quality, nearly complete data on mortality (for citizens who provide an ID), viral load and CD4 counts as well as laboratory tests for antiretroviral monitoring such as haemoglobin and creatinine.
The main weakness of the data is the lack of standardised follow-ups. Because the cohort follows changing national guidelines and because specific research-based efforts to get patients to adhere to treatment visit schedules are not performed, we do not always have standard monitoring points for all patients within the cohort. This can make interpretation of results difficult and requires careful consideration of the meaning of missing data.
Introduction
As we enter the second decade of large-scale access to antiretroviral therapy (ART) in sub-Saharan Africa, there is little question about the role that clinical cohorts have played in both evaluating and shaping HIV policy within the continent.1–3 South Africa, with the largest HIV treatment programme in the world and over 3 million people on ART, has been a leader in this area. The numerous clinical cohorts that were established since 20044–7 have been used to evaluate the changes in national treatment policy. One of these cohorts, the Themba Lethu Clinical Cohort8 was established at the Helen Joseph Hospital in Johannesburg and has led to numerous insights into the effectiveness of the treatment roll-out and also participates in larger country-wide and region-wide evaluations through the International Epidemiologic Database to Evaluate AIDS (IeDEA) network.7 9 However, the Themba Lethu cohort is based at a large urban tertiary hospital and as the responsibility for HIV care and treatment shifts from hospital based programs to primary healthcare clinics, it has become clear that the Themba Lethu Clinical cohort is not sufficient for describing the HIV treatment programme in South Africa in its entirety. As such, the Right to Care Clinical Cohort, which includes the Themba Lethu cohort, has been established to provide a broader, more representative perspective on South Africa’s HIV care and treatment programme.
Cohort description
The Right to Care Clinical Cohort is a network of 10 clinical cohorts all established or expanded around 2004 as part of the public-sector roll-out of ART in South Africa under support from the US Agency for International Development from the President’s Emergency Plan for AIDS Relief programme. The clinics have all been supported by Right to Care, a South African NGO which partners with clinic-based HIV care and treatment programs throughout South Africa. As part of their support to clinics, Right to Care has provided both data entry and an electronic data capturing and patient management system called Therapy-Edge-HIV (TM). Thus, each clinic within the network follows a common data entry protocol and uses a standard database for data capture. Table 1 shows the major data fields captured within the database. To oversee the data and conduct analyses, Right to Care partners with both the individual clinics and the Health Economics and Epidemiology Research Office in South Africa, part of the Wits Health Consortium which functions as a collaboration between the University of the Witwatersrand and Boston University. Analysis of anonymised data has been approved by the University of the Witwatersrand Human Research Ethics Committee and the Boston University Institutional Review Board.
Table 1.
Routinely collected data for patients in the Right to Care Clinical HIV Cohort, South Africa
| Data fields | |
| Demographics | Clinic ID, name, national ID number, contact details, gender, date of birth, employment status, alcohol use, smoking history, ethnicity, education level |
| Clinical visit data | Date of visit (scheduled and actual), TB screening, urine analysis, vital signs, height, weight, description and duration of new symptoms, systems based clinical examination (eg, cardiology, neurology, respiratory, etc) |
| Laboratory results | ART initiation and monitoring bloods including CD4 count, HIV viral load, full blood counts, liver function tests, renal function tests, TB microscopy and culture results, pap smear screening results, lactate levels, glucose and lipid profiles |
| Medication history | Date of start and stop of ART and non-ART medications, reasons for treatment discontinuation, self-reported treatment adherence |
| Clinical diagnoses | Pregnancy, opportunistic infections including TB, hepatitis, PCP, AIDS-related malignancies including Kaposi sarcoma and cervical cancer, ART toxicities including peripheral neuropathy, anaemia, hyperlactataemia/lactic acidosis, lipoatrophy |
ART, antiretroviral therapy; TB, tuberculosis; PCP pneumocystis pneumonia.
Among the individual clinics within the Right to Care Clinical Cohort, two are stand-alone HIV clinics while the remaining eight are primary healthcare clinics, which have an HIV treatment programme. While the individual clinics provide fertile ground for evaluating the effects of specific drug regimens,10–14 treatment outcomes,15–21 opportunistic infections22–24 and adverse drug events,10 25 because the cohort is large and the clinics are diverse in location and staff mix but provide care according to a standard protocol,26–29 they also create an excellent environment for evaluating large policy changes like the recent move to treatment for all, South Africa’s national adherence strategy and South Africa’s national decanting strategy. In addition, such large collaborations are needed both to triangulate the results of other large collaborations such as IeDEA30 and to create cohorts large enough to evaluate future needs. These include the coming wave of patients failing first-line31 and second-line treatment12 32–34 and needing access to expensive third-line regimens or the needs of key populations such as pregnant women35 and adolescents in HIV care.17
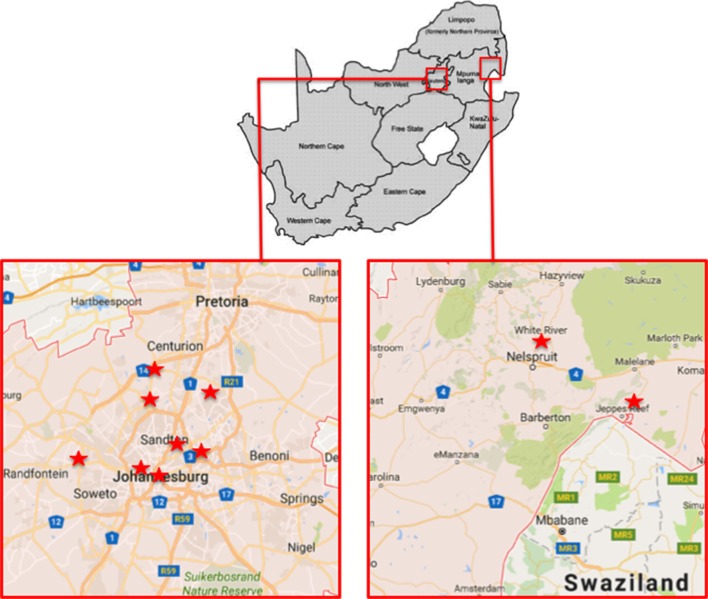
The Right to Care Clinical Cohort is an open cohort and includes all patients enrolled at one of the participating clinics since April 2004. The clinics included in the cohort are located in Gauteng and Mpumalanga provinces within South Africa as shown in figure 1. Of these, nine are urban sites while one is in a rural site. All the clinics follow the most recent version of the South African National HIV Treatment Guidelines,28 though the clinics vary in their staffing models.
Figure 1.
Location of clinics in Right to Care Clinical Cohort.
As of June 2016, across the 10 clinics, 155 144 patients have been enrolled, of which 116 490 have initiated ART. Currently, 46 241 are actively on ART. The clinics range in size from 4332 to 39 297 current patients. Table 2 summarises the demographic and clinical characteristics of the cohort stratified by last treatment regimen. About two-thirds of the cohort (63%) are female; most are black or of African ethnicity (95%) and the patients have a median age of 35 years (IQR 29–42 years). Average CD4 counts at ART initiation have been increasing over time as the treatment thresholds have increased from ≤200 cells/mm3 in 2004 to ≤350 cells/mm3 in 2011 to ≤500 cells/mm3 in 2015 to treatment for all in 2016. During that time, the median (IQR) CD4 count at ART initiation has increased from 118 (49–188 cells/mm3) to 241 (97–394 cells/mm3). South Africa has recently removed CD4 eligibility thresholds in line with recent WHO recommendations.
Table 2.
Characteristics of patients in the Right to Care Clinical HIV Cohort in South Africa by last treatment regimen
| Demographic characteristics | First line (N=1 10 452) | Second line (N=5909) | Third line (N=129) | |
| Gender | Female, n (%) | 70 590 (63.9) | 3822 (64.7) | 65 (50.4) |
| Male, n (%) | 39 860 (36.1) | 2087 (35.3) | 64 (49.6) | |
| Missing, n (%) | 2 (0) | 0 (0) | 0 (0) | |
| Nationality | South African, n (%) | 96 977 (88.0) | 5354 (90.6) | 109 (84.5) |
| Non-South African, n (%) | 13 176 (12.0) | 555 (9.4) | 20 (15.5) | |
| Missing, n (%) | 299 (0) | 2 (0) | 0 (0) | |
| Education level | No education, n (%) | 7372 (6.7) | 384 (6.5) | 1 (0.8) |
| Primary, n (%) | 18 520 (16.8) | 886 (15.0) | 33 (25.6) | |
| Secondary, n (%) | 56 156 (50.8) | 3058 (51.8) | 66 (51.2) | |
| Tertiary, n (%) | 2179 (2.0) | 124 (2.1) | 1 (0.8) | |
| Missing, n (%) | 26 225 (23.7) | 1457 (24.7) | 28 (21.7) | |
| Employment status | Unemployed, n (%) | 58 862 (53.3) | 3378 (57.2) | 48 (37.2) |
| Employed, n (%) | 43 261 (39.2) | 2274 (38.5) | 74 (57.4) | |
| Missing, n (%) | 8329 (7.5) | 257 (4.3) | 7 (5.4) | |
| Characteristics at ART initiation | ||||
| Age (years) | Median (IQR) | 35.6 (29.4–42.3) | 33.9 (28.4–40.1) | 36.1 (29.8–41.2) |
| Body mass index (kg/m2) | <18.5, n (%) | 12 640 (11.4) | 754 (12.8) | 14 (10.9) |
| 18.5–24.9, n (%) | 36 258 (32.8) | 1948 (33.0) | 35 (27.1) | |
| 25–29.9, n (%) | 13 289 (12.0) | 650 (11.0) | 9 (7.0) | |
| 30, n (%) | 7394 (6.7) | 340 (5.8) | 11 (8.5) | |
| Missing | 40 871 (37.0) | 2217 (37.5) | 60 (46.5) | |
| Median (IQR) | 22.2 (19.4–25.8) | 22.0 (19.1–25.3) | 22.2 (18.9–26.2) | |
| CD4 count category (cells/mm3) | <50, n (%) | 18 651 (16.9) | 1344 (22.7) | 20 (15.5) |
| 50–100, n (%) | 13 915 (12.6) | 858 (14.5) | 16 (12.4) | |
| 100–200, n (%) | 26 426 (23.9) | 1227 (20.8) | 17 (13.2) | |
| 200–350, n (%) | 17 046 (15.4) | 578 (9.8) | 15 (11.6) | |
| >350, n (%) | 7573 (6.9) | 262 (4.4) | 10 (7.8) | |
| Missing, n (%) | 26 841 (24.3) | 1640 (27.8) | 51 (39.5) | |
| Median (IQR) | 137 (58–222) | 97 (36–180) | 113 (43–257) | |
| HIV viral load (copies/mL3) | ≤1 00 000, n (%) | 17 710 (16.0) | 1100 (18.6) | 28 (21.7) |
| >1 00 000, n (%) | 10 087 (9.1) | 786 (13.3) | 12 (9.3) | |
| Missing*, n (%) | 82 655 (74.8) | 4023 (68.1) | 89 (69.0) | |
| Haemoglobin level (g/dL) | Median (IQR) | 11.5 (10.0–13.0) | 11.4 (10.0–12.8) | 11.9 (10.7–13.2) |
| Tuberculosis | Yes, n (%) | 9871 (8.9) | 632 (10.7) | 9 (6.9) |
| No, n (%) | 1 00 381 (91.1) | 5258 (89.3) | 120 (93.1) | |
| Current status | Alive and in care, n (%) | 42 542 (38.5) | 3605 (61.0) | 94 (72.9) |
| Deceased, n (%) | 10 561 (9.6) | 261 (4.4) | 2 (1.6) | |
| Lost to follow-up, n (%) | 28 561 (25.9) | 1010 (17.1) | 25 (19.4) | |
| Transferred out, n (%) | 28 788 (26.1) | 1033 (17.5) | 8 (6.2) | |
*HIV viral load was only completed at baseline in the early years of the programme.
Since the large-scale roll-out of ART in South Africa in 2004, the recommended drug regimens for both first-line and second-line ART have evolved (first-line regimens shown in figure 2). Within the cohort, 39 244 patients are currently on first-line ART. Since 2004, three-drug first-line therapy has been non-nucleoside reverse transcriptase inhibitor (NNRTI) based, with efavirenz preferred, but nevirapine is also available. In addition to lamivudine, stavudine was the favoured nucleoside reverse transcriptase inhibitor in the early years but in 2010 tenofovir was recommended.
Figure 2.
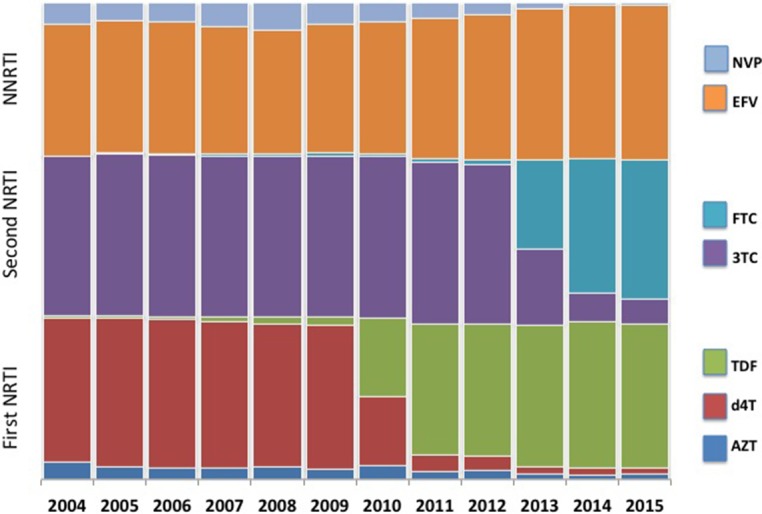
Distribution of first-line antiretroviral therapy regimen component drugs by calendar year. 3TC, lamivudine; AZT, zidovudine; d4T, stavudine; EFV, efavirenz; FTC, emtricitabine; NRTI, nucleoside reverse transcriptase inhibitor; NVP, nevirapine; TDF, tenofovir.
South Africa monitors the effectiveness of HIV treatment using viral loads. For patients who have documented first-line failure (defined as two consecutive viral loads≥1000 copies/mL3 at least 2 weeks apart), switching to second-line protease inhibitor-based therapy (typically lopinavir-ritonavir) is recommended. A total of 5909 patients are currently on second-line ART, making it one of the largest second-line cohorts in Africa. More recently, access to third-line ART has become available for patients failing second-line. Access is managed by application to a national third-line committee that will order resistance testing and prescribe an appropriate third-line regimen based on the results. Third-line consists of any of darunavir, raltegravir and etravirine. Currently, 129 patients have been initiated on third-line within the cohort.
Follow-up measures
Protocols for follow-up of patients on ART within the clinics follow national HIV treatment guidelines that have changed over the years. While initially patients were required to be seen each month to collect antiretrovirals (ARVs), as the programme has matured, patients who have been demonstrated to be adherent and stable on treatment can be prescribed 2 or 3 months of ART at a time, allowing for fewer visits, a reduced burden on the patient and lower clinic volume on a daily basis. A total of 116 490 patients who had ART within the cohort have contributed a total of 3 42 931 person-years on ART. The median (IQR) duration of follow-up per person has been 2.17 (0.81–4.41) person-years with a range of 0.04 to 12.5 person-years. This equates to 1 597 690 total medical and 2 262 626 pharmacy visits at a rate of 6.6 per year.
Laboratory investigations for all the clinics in the cohort are conducted by the National Health Laboratory Service (NHLS). While NHLS sends back individual reports to the clinics with the results of each investigation, within the cohort, data from the NHLS are downloaded directly and are integrated into the individual patient database on a daily basis, allowing complete and accurate data on lab investigations.
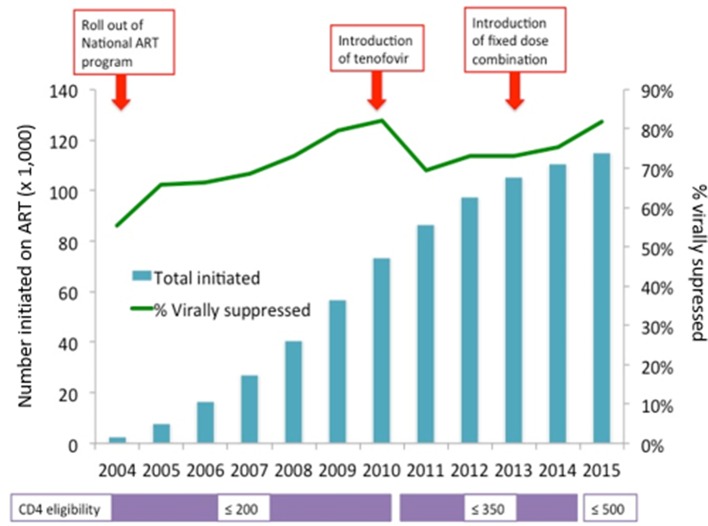
Since the beginning of the programme, CD4 counts have been used to determine ART eligibility, with the first viral load monitoring being conducted at between 4 and 6 months on treatment. The interval for monitoring has changed from every 6 months after the initial viral load, to a 6-month and 12-month viral load and then a repeat viral load every year as of 2013. Cohort patients had a median (IQR) of 2.1 (0.48–4.6) viral loads per year and a median (IQR) of 3 (2–7) viral loads per person. Figure 3 shows the increase in numbers on ART and the proportion of patients virally suppressed with changes in the national guidelines.
Figure 3.
Numbers on antiretroviral therapy and viral load suppression over time in the Right to Care HIV Cohort. This analysis was cross-sectional, and missing viral loads are not included.
Because of the richness and size of the cohort, the data have been used to conduct important evaluations of the impact of second-line treatment,13 36 of transfer between clinics,15 of the impact of changes in CD4 thresholds on clinic crowding,37 as well as of the impacts of the shift to tenofovir in replacement of stavudine11 38 in the national programme. In addition, when warranted, analyses of subsets of the data are used to conduct urgent evaluations such as the impact of pregnancy on retention,35 outcomes within patients on second-line ART33 and outcomes for adolescents on ART.39 The data have also been successfully used by doctoral students for dissertation work.37
Data collection
As noted, all clinics within the cohort have employed a common patient data collection system since 2004, the TherapyEdge-HIV (TM) patient management system. The system is a longitudinal database designed to capture essential information from all HIV-related clinic visits.40 Data are captured on patients both prior to ART initiation and after. While the CD4 threshold for ART initiation has changed over time, the protocol for patients prior to initiation has been 6-monthly monitoring of CD4 count until eligibility is established.
For each patient interaction at a cohort clinic, the date of the encounter is recorded allowing for longitudinal follow-up. At the first visit, demographics data are collected including key identifiers and information on age, sex, race, education, alcohol use and smoking. When possible national identifiers are collected to allow linkage with national registries and phone numbers are collected to allow for patient tracing should patients become lost to follow-up. At each follow-up visit, data on clinical conditions that are identified (such as side effects, TB and so on) are captured. At each visit, information is also recorded on any medication dispensed including ARVs and other medications.
Overall retention in HIV care in sub-Saharan Africa is less than ideal.41–43 Because one of the main uses of these data has been to describe the cascade of HIV care,44 45 no attempts are made to influence retention for research purposes. Instead, all measures are taken to reduce attrition as part of usual clinical care. All the clinics within the cohort attempt to trace patients who are lost to follow-up, though with differing intensities. Each clinic uses its own definition of loss for triggering tracing activities, though a common definition of 3 months late for an appointment is used for research purposes and standardising loss rates across clinics. At all clinics, tracing is initially done through calling the phone number collected at enrolment. Some of the clinics also employ tracers or use community outreach workers to attempt to trace patients who do not return to care.
Mortality is ascertained in multiple ways within the cohort. All sites use passive follow-up to identify patients who have died and record the information when it is reported back by a family member or friend. In addition, data on all patients who have provided a national identification number and who are lost to follow-up are cross-referenced with South Africa’s National Population Register15 (NPR) to determine final outcomes. The proportion of patients who are either in possession of, or report their national ID number varies from clinic to clinic, ranging from 35% to 74%. Prior to the initial linkage between Themba Lethu Clinic and the NPR, 17% of patients within the clinic were considered lost to follow-up and 4% were known to have died. After linkage, the proportion who were considered to be lost dropped to 10% and the proportion who were recorded to have died increased to 11%.15
Another way patients leave the cohort is through transfer to another treatment facility. When the patient formally requests this it is noted in the database as a patient outcome. The rate of transfer within the cohort is 12% and varies from 11% to 50%, though those at the higher end see this as intentional transfers of patients who are ‘down-referred’ to smaller clinics once stable.46 47 However, as is common within HIV treatment programmes within the region, transfers are often ‘silent transfers’ where patients transfer without informing their current clinic. Patients who transfer to a new clinic outside our network without informing us will appear as lost to follow-up. We have shown that this can bias estimates of retention in care,48 and therefore the clinics within the cohort request patients notify the clinic if they wish to receive care elsewhere so that care can be coordinated.
Findings to date
The Right to Care Clinical Cohort is used to monitor the continued roll-out of ART in South Africa and to evaluate the impact of changes in the national treatment programme as they are made. Current areas of research include: (1) monitoring treatment outcomes (including death, loss to follow-up, viral suppression, CD4 count gain, etc) for patients on ART; (2) evaluating the impact of changes in the national treatment guidelines around when to initiate ART on HIV treatment outcomes; (3) evaluating the impact of changes in the national treatment guidelines around what ART regimens to initiate on drug switches; (4) evaluating the cost and cost-effectiveness of HIV treatment delivery models; (5) evaluating the need for and outcomes on second-line and third-line ART; (6) evaluating the impact of comorbidity with non-communicable diseases on HIV treatment outcomes and (7) evaluating the impact of the switch to initiating all patients onto ART regardless of CD4 count.
To date, we have used the Right to Care cohort to evaluate various aspects of the national HIV treatment programme. Three examples of this work include:
Treatment outcomes. While numerous models have found important predictors of failing first-line therapy, few have had the size to be able to develop a precise predictive model of treatment failure.49 Using data on 71 154 individuals, we showed that age, sex, interactions between age and sex, first-line NNRTI, CD4 count, mean corpuscular volume, haemoglobin level, history of TB and missed visits during the first 6 months on ART were predictive of failure. After stratifying into risk groups, failure in the highest risk group was 24.4% over 5 years on ART but only 9.4% among the lowest risk group, allowing stratification of risk for clinics.49
Second-line ART outcomes. The need for second-line treatment has been growing as treatment scale up has continued, but there is little robust data on outcomes among patients who have already failed a first-line regimen.34 With data on 1435 patients on second-line ART between 2004 and 2013, we found that a low CD4 count (<50 cells/mm3) at the time of switch was strongly predictive of mortality (adjusted HR (aHR) vs ≥200 cells/mm3: 3.76; 95% CI: 1.87 to 7.57) as was a high viral load (≥50 000 copies/mL vs 1000–4999 copies/mL aHR: 2.01; 95% CI: 1.07 to 3.77).33 The results suggest that earlier switch would likely benefit patients failing first-line before disease progresses to severe immunosuppression.
Outcomes for adolescents on HIV treatment. While having been identified as a key population for ending the AIDS epidemic, few cohorts have been able to measure outcomes among adolescents on HIV treatment in resource-limited settings because there are not sufficient numbers. Comparing 310 adolescents 10–14 years, 342 adolescents 15–19 and 1599 young adults 20–24 years to adults ≥25 years, we found both older adolescents (adjusted risk ratio (RR) 1.75 95% CI 1.25 to 2.47) and young adults (RR 1.33 95% CI 1.10 to 1.60) were at increased risk of an unsuppressed viral load compared with adults17 suggesting they need to be targeted for additional intervention.
Perhaps the biggest strength of the Right to Care Clinical cohort is its size. With over 1 15 000 patients ever initiated onto HIV treatment, precise evaluations can be conducted. This is particularly important when describing outcomes among subsets of the cohort that could not be conducted with much precision using individual clinic data. For example, by pooling data across 10 clinics, we are able to conduct precise analyses on a large cohort of patients initiating second-line therapy.
Second, while the clinics use a similar treatment protocol and data collections strategies, they show geographic variation. While other cohort collaborations do have such geographic variation, few were designed to encompass clinics which shared a common software and approach to data collection. Further because we link the data to the NHLS and the National Population Registration, we have high-quality, nearly complete data on mortality (for citizens who provide an ID), viral load and CD4 counts as well as ARV monitoring labs such as haemoglobin and creatinine.
Perhaps the main weakness of the data is the lack of standardised follow-ups. Because the cohort follows changing national guidelines and because specific research-based efforts to get patients to adhere to treatment visit schedules are not performed, we do not always have standard monitoring points for all patients within the cohort. This can make interpretation of results difficult and requires careful consideration of the meaning of missing data. It also calls for the use of missing data methods50 and formal quantitative bias analysis51–54 to explore the impact of any systematic errors. In addition, because the cohort is not designed as a research cohort, biological samples are not collected at routine intervals (beyond what is required for clinical care) and these samples are not stored. Therefore, we cannot use the cohort to study topics such as molecular epidemiology or biomarker research. Because the cohort is meant to reflect what is happening in actual clinic care, such samples will continue to not be stored going forward.
Collaborations
Investigators wishing to work with the data should contact the team at the Health Economics and Epidemiology Research Office (information@heroza.org) and send a concept sheet for the analyses they are interested in performing and the variables that would be required. Anyone wishing to work with the data from the Right to Care Cohort must seek Institutional Review Board approval from both their own institutions and from the Human Research Ethics Committee (Medical) of the University of the Witwatersrand. After receiving such approvals, those wishing to work with the data must sign a data-use agreement. Those wishing to find out more about the Right to Care cohort can visit the website of the Health Economics and Epidemiology Research Office in Johannesburg, South Africa, at http://www.heroza.org/.
bmjopen-2016-015620supp001.doc (85KB, doc)
Acknowledgments
The authors wish to thank Babatyi Malope-Kgokong for her efforts and input in developing the database. We also thank Ms. Imogen Jaffray and the TherapyEdge-HIV team as well as the data capturers who helped collect the data. Most importantly, we thank the patients who contributed to this cohort.
Footnotes
Contributors: MPF and MM conceptualised the study. MPF and MM wrote the first draft. GM conducted the data analysis. MPF, MM, ATB, DE, DO, GM, PM, JB, OE, DM, SM, LL and IS contributed to interpreting the data and to the writing and revising of the manuscript.
Funding: This study is made possible by the generous support of the American people through Cooperative Agreement AID 674-A-12-00029 from the US Agency for International Development (USAID).
Disclaimer: The contents are the responsibility of the authors and do not necessarily reflect the views of USAID or the US Government. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Competing interests: None declared.
Ethics approval: Boston University Institutional Review Board, University of the Witwatersrand Human Research Ethics Committee.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Investigators wishing to work with the data should contact the team at the Health Economics and Epidemiology Research Office and send a concept sheet for the analyses they are interested in performing and the variables that would be required. Anyone wishing to work with the data from the Right to Care Cohort must seek IRB approval from both their own institutions and from the Human Research Ethics Committee (Medical) of the University of the Witwatersrand. After receiving such approvals those wishing to work with the data must sign a data-use agreement. Those wishing to find out more about the Right to Care cohort can visit the website of the Health Economics and Epidemiology Research Office in Johannesburg, South Africa at.
Correction notice: This paper has been amended since it was published Online First. Owing to a scripting error, some of the publisher names in the references were replaced with ‘BMJ Publishing Group’. This only affected the full text version, not the PDF. We have since corrected these errors and the correct publishers have been inserted into the references. Mhairi Maskew was correctly listed as the second author but then listed again at the end of the author list. This has been corrected so he only appears once in the author list.
References
- 1. Lowrance DW, Ndamage F, Kayirangwa E, et al. . Adult clinical and immunologic outcomes of the national antiretroviral treatment program in Rwanda during 2004-2005. J Acquir Immune Defic Syndr 2009;52:49–55. 10.1097/QAI.0b013e3181b03316 [DOI] [PubMed] [Google Scholar]
- 2. Pujades-Rodríguez M, Schramm B, Som L, et al. . Immunovirological outcomes and resistance patterns at 4 years of antiretroviral therapy use in HIV-infected patients in Cambodia. Trop Med Int Health 2011;16:205–13. 10.1111/j.1365-3156.2010.02689.x [DOI] [PubMed] [Google Scholar]
- 3. Holmes CB, Sanne I. Changing models of care to improve progression through the HIV treatment cascade in different populations. Curr Opin HIV AIDS 2015;10:447–50. 10.1097/COH.0000000000000194 [DOI] [PubMed] [Google Scholar]
- 4. Coetzee D, Boulle A, Hildebrand K, et al. . Promoting adherence to antiretroviral therapy: the experience from a primary care setting in Khayelitsha, South Africa. AIDS 2004;18(Suppl 3):S2: 27–31. [DOI] [PubMed] [Google Scholar]
- 5. Boulle A, Van Cutsem G, Hilderbrand K, et al. . Seven-year experience of a primary care antiretroviral treatment programme in Khayelitsha, South Africa. AIDS 2010;24:563–72. 10.1097/QAD.0b013e328333bfb7 [DOI] [PubMed] [Google Scholar]
- 6. Geng EH, Nash D, Kambugu A, et al. . Retention in care among HIV-infected patients in resource-limited settings: emerging insights and new directions. Curr HIV/AIDS Rep 2010;7:234–44. 10.1007/s11904-010-0061-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Egger M, Ekouevi DK, Williams C, et al. . Cohort Profile: the international epidemiological databases to evaluate AIDS (IeDEA) in sub-Saharan Africa. Int J Epidemiol 2012;41:1256–64. 10.1093/ije/dyr080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fox MP, Maskew M, MacPhail AP, et al. . Cohort profile: the Themba Lethu clinical cohort, Johannesburg, South Africa. Int J Epidemiol 2013;42:430–9. 10.1093/ije/dys029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boulle A, Schomaker M, May MT, et al. . Mortality in patients with HIV-1 infection starting antiretroviral therapy in South Africa, Europe, or North America: a collaborative analysis of prospective studies. PLoS Med 2014;11:e1001718 10.1371/journal.pmed.1001718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brennan A, Evans D, Maskew M, et al. . Relationship between renal dysfunction, nephrotoxicity and death among HIV adults on tenofovir. AIDS 2011;25:1603–9. 10.1097/QAD.0b013e32834957da [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Takuva S, Maskew M, Brennan AT, et al. . Anemia among HIV-Infected patients initiating antiretroviral therapy in South Africa: improvement in hemoglobin regardless of degree of immunosuppression and the initiating ART regimen. J Trop Med 2013;2013:1–6. 10.1155/2013/162950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fox MP, Cutsem GV, Giddy J, et al. . Rates and predictors of failure of first-line antiretroviral therapy and switch to second-line ART in South Africa. J Acquir Immune Defic Syndr 2012;60:428–37. 10.1097/QAI.0b013e3182557785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Long L, Fox M, Sanne I, et al. . The high cost of second-line antiretroviral therapy for HIV/AIDS in South Africa. AIDS 2010;24:915–9. 10.1097/QAD.0b013e3283360976 [DOI] [PubMed] [Google Scholar]
- 14. Maskew M, Westreich D, Fox MP, et al. . Effectiveness and safety of 30 mg versus 40 mg stavudine regimens: a cohort study among HIV-infected adults initiating HAART in South Africa. J Int AIDS Soc 2012;15:13 10.1186/1758-2652-15-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fox MP, Brennan A, Maskew M, et al. . Using vital registration data to update mortality among patients lost to follow-up from ART programmes: evidence from the Themba Lethu Clinic, South Africa. Trop Med Int Health 2010;15:405–13. 10.1111/j.1365-3156.2010.02473.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rosen S, Larson B, Brennan A, et al. . Economic outcomes of patients receiving antiretroviral therapy for HIV/AIDS in South Africa are sustained through three years on treatment. PLoS One 2010;5:e12731 10.1371/journal.pone.0012731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Evans D, Menezes C, Mahomed K, et al. . Treatment outcomes of HIV-infected adolescents attending public-sector HIV clinics across Gauteng and Mpumalanga, South Africa. AIDS Res Hum Retroviruses 2013;29:892–900. 10.1089/aid.2012.0215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Maskew M, Brennan AT, Westreich D, et al. . Gender differences in mortality and CD4 count response among virally suppressed HIV-positive patients. J Womens Health 2013;22:113–20. 10.1089/jwh.2012.3585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wallis CL, Papathanasopolous MA, Fox M, et al. . Low rates of nucleoside reverse transcriptase inhibitor resistance in a well-monitored cohort in South Africa on antiretroviral therapy. Antivir Ther 2012;17:313–20. 10.3851/IMP1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Budgell EP, Maskew M, Long L, et al. . Brief Report: does most mortality in patients on ART Occur in Care or after lost to Follow-Up? evidence from the Themba Lethu Clinic, South Africa. J Acquir Immune Defic Syndr 2015;70:323–8. 10.1097/QAI.0000000000000755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Maskew M, Brennan AT, MacPhail AP, et al. . Poorer ART outcomes with increasing age at a large public sector HIV clinic in Johannesburg, South Africa. J Int Assoc Physicians AIDS Care 2012;11:57–65. 10.1177/1545109711421641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Maskew M, MacPhail AP, Whitby D, et al. . Kaposi sarcoma-associated herpes virus and response to antiretroviral therapy: a prospective study of HIV-infected adults. J Acquir Immune Defic Syndr 2013;63:442–8. 10.1097/QAI.0b013e3182969cc1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Maskew M, Fox MP, van Cutsem G, et al. . Treatment response and mortality among patients starting antiretroviral therapy with and without kaposi sarcoma: a cohort study. PLoS One 2013;8:e64392 10.1371/journal.pone.0064392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Maskew M, Macphail AP, Whitby D, et al. . Prevalence and predictors of kaposi sarcoma herpes virus seropositivity: a cross-sectional analysis of HIV-infected adults initiating ART in Johannesburg, South Africa. Infect Agent Cancer 2011;6:22 10.1186/1750-9378-6-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shearer K, Fox MP, Maskew M, et al. . The impact of choice of NNRTI on short-term treatment outcomes among HIV-infected patients prescribed tenofovir and lamivudine in Johannesburg, South Africa. PLoS One 2013;8:e71719 10.1371/journal.pone.0071719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. National Department of Health SA. National Antiretroviral Treatment Guidelines. 1st edn Pretoria, South Africa, 2004. [Google Scholar]
- 27. National Department of Health South Africa. Clinical Guidelines for the Managment of HIV & AIDS in Adults and Adolescents. South Africa, 2010. [Google Scholar]
- 28. National Department of Health Republic of South Africa. The South African antiretroviral treatment guidelines. South Africa, 2013. [Google Scholar]
- 29. South African National Department of Health. National Consolidated guidelines for the prevention of mother-to-child transmission of HIV (PMTCT) and the management of HIV in children, adolescents and adults. Pretoria, South Africa, 2015. [Google Scholar]
- 30. Egger M, Ekouevi DK, Williams C, et al. . Cohort Profile: the international epidemiological databases to evaluate AIDS (IeDEA) in sub-Saharan Africa. Int J Epidemiol 2012;41:1256–64. 10.1093/ije/dyr080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fox MP, Shearer K, Maskew M, et al. . HIV treatment outcomes after seven years in a large public-sector HIV treatment program in Johannesburg, South Africa. AIDS 2012;26:1823–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fox MP, Ive P, Long L, et al. . High rates of survival, immune reconstitution, and virologic suppression on second-line antiretroviral therapy in South Africa. J Acquir Immune Defic Syndr 2010;53:500–6. 10.1097/QAI.0b013e3181bcdac1 [DOI] [PubMed] [Google Scholar]
- 33. Rohr JK, Ive P, Horsburgh CR, et al. . Marginal structural models to assess delays in Second-Line HIV treatment initiation in South Africa. PLoS One 2016;11:e0161469 10.1371/journal.pone.0161469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shearer K, Evans D, Moyo F, et al. . Treatment outcomes of over 1000 patients on second-line, protease inhibitor-based antiretroviral therapy from four public-sector HIV treatment facilities across Johannesburg, South Africa. Trop Med Int Health 2017;22 10.1111/tmi.12804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Clouse K, Pettifor A, Shearer K, et al. . Loss to follow-up before and after delivery among women testing HIV positive during pregnancy in Johannesburg, South Africa. Trop Med Int Health 2013;18:451–60. 10.1111/tmi.12072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rosen S, Long L, Sanne I, et al. . The net cost of incorporating resistance testing into HIV/AIDS treatment in South Africa: a Markov model with primary data. J Int AIDS Soc 2011;14:24 10.1186/1758-2652-14-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kluberg SA, Fox MP, LaValley M, et al. . 23rd Conference on Retroviruses and Opportunistic Infections (CROI) Do ART Eligibility Expansions Crowd out the Sickest? Evidence From South Africa. Boston, 2016. (Abstract 1012 Themed Discussion). [Google Scholar]
- 38. Brennan AT, Maskew M, Ive P, et al. . Increases in regimen durability associated with the introduction of tenofovir at a large public-sector clinic in Johannesburg, South Africa. J Int AIDS Soc 2013;16:18794 10.7448/IAS.16.1.18794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Evans D, Menezes C, Mahomed K, et al. . Treatment outcomes of HIV-infected adolescents attending public-sector HIV clinics across Gauteng and Mpumalanga, South Africa. AIDS Res Hum Retroviruses 2013;29:892–900. 10.1089/aid.2012.0215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Brennan AT, Maskew M, Sanne I, et al. . The importance of clinic attendance in the first six months on antiretroviral treatment: a retrospective analysis at a large public sector HIV clinic in South Africa. J Int AIDS Soc 2010;13:49 10.1186/1758-2652-13-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fox MP, Rosen S. Systematic review of retention of pediatric patients on HIV treatment in low and middle-income countries 2008-2013. AIDS 2015;29:493–502. 10.1097/QAD.0000000000000559 [DOI] [PubMed] [Google Scholar]
- 42. Fox MP, Rosen S. Retention of adult patients on antiretroviral therapy in low- and Middle-Income countries: systematic review and Meta-analysis 2008-2013. J Acquir Immune Defic Syndr 2015;69:98–108. 10.1097/QAI.0000000000000553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fox MP, Larson B, Rosen S. Defining retention and attrition in pre-antiretroviral HIV care: proposals based on experience in Africa. Trop Med Int Health 2012;17:1235–44. 10.1111/j.1365-3156.2012.03055.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bor J, Fox MP, Rosen S, et al. , 2016. The Real-World Impact of CD4-Eligibility Criteria on Retention in HIV Care. 23rd Conference on Retroviruses and Opportunistic Infections (CROI) Boston: (Abstract 1011) [Google Scholar]
- 45. Fox MP, Shearer K, Maskew M, et al. . Attrition through multiple stages of pre-treatment and ART HIV care in South Africa. PLoS One 2014;9:e110252 10.1371/journal.pone.0110252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Brennan AT, Long L, Maskew M, et al. . Outcomes of stable HIV-positive patients down-referred from a doctor-managed antiretroviral therapy clinic to a nurse-managed primary health clinic for monitoring and treatment. AIDS 2011;25:2027–36. 10.1097/QAD.0b013e32834b6480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Long L, Brennan A, Fox MP, et al. . Treatment outcomes and cost-effectiveness of shifting management of stable ART patients to nurses in South Africa: an observational cohort. PLoS Med 2011;8:e1001055 10.1371/journal.pmed.1001055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fox M, Bor J, MacLeod W, et al. , 2016. Is retention on ART underestimated due to patient transfers? estimating system-wide retention using a national labs database in South Africa. 21st International AIDS Conference Durban, South Africa: [Google Scholar]
- 49. Rohr JK, Ive P, Horsburgh CR, et al. . Developing a predictive risk model for first-line antiretroviral therapy failure in South Africa. J Int AIDS Soc 2016;19 10.7448/IAS.19.1.20987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Greenland S. Bayesian perspectives for epidemiologic research: iii. Bias analysis via missing-data methods. Int J Epidemiol 2009;38:1662–73. 10.1093/ije/dyp278 [DOI] [PubMed] [Google Scholar]
- 51. Lash TL, Fox M, Fink AK. Applying Quantitative Bias analysis to Epidemiologic Data. New York: Springer, 2009. [Google Scholar]
- 52. Lash TL, Fox MP, MacLehose RF, et al. . Good practices for quantitative Bias analysis. Int J Epidemiol 2014;43:1969–85. 10.1093/ije/dyu149 [DOI] [PubMed] [Google Scholar]
- 53. Fox MP. Creating a demand for Bias analysis in epidemiological research. J Epidemiol Community Health 2009;63:91 10.1136/jech.2008.082420 [DOI] [PubMed] [Google Scholar]
- 54. Fox MP, Lash TL, Greenland S. A method to Automate probabilistic sensitivity analyses of misclassified binary variables. Int J Epidemiol 2005;34:1370–6. 10.1093/ije/dyi184 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2016-015620supp001.doc (85KB, doc)