Abstract
Introduction
Informal caregivers for people with dementia (hereafter: caregivers) often feel (over)burdened by the care for a loved one with dementia, and this can have various deleterious effects on both caregivers and patients. Support for caregivers is urgently needed, and for this reason, a dementia simulator (Into D’mentia) was developed in which caregivers experience what it is like to have dementia. The simulator attempts to heighten caregivers’ empathy and understanding for the patient and, in turn, diminish their own caregiver burden. The current study evaluates whether the simulator is effective on a number of outcomes.
Methods and analysis
A longitudinal, quasi-experimental study is ongoing in the Netherlands. We aim to recruit 142 caregivers in total divided over two groups: 71 caregivers in the intervention group and 71 caregivers in the control group. All participants will complete interviews and questionnaires at four time points: at baseline, 1 week, 2.5 months and 15 months after the training. The primary outcomes include empathy, caregiver burden, caregiver’s sense of competence, social reliance, anxiety, depression and caregivers’ subjective and objective health.
Ethics and dissemination
This study is being carried out in agreement with the Declaration of Helsinki, and the protocol has been approved by the local ethics committees.
Registration details
This study is registered with The Netherlands National Trial Register (NNTR5856).
Keywords: dementia, informal caregiver, virtual reality, caregiver burden, empathy, simulation
Strengths and limitations of the study.
It is a longitudinal, prospective design with multiple assessments. This is a useful addition to the existing effect studies into interventions for caregivers, which usually apply pre–post designs making it impossible to know if these interventions work in the longer term.
We include both quantitative (questionnaires) and qualitative (semi-structured interviews) measurements.
A control group is included which was not always the case in previous intervention studies with caregivers. The control group makes it possible to attribute the findings to the intervention, instead of to other variables such as elapsed time.
A potential limitation is that, due to practical reasons, the participants were not randomised. The simulator was available for free for 5 weeks only, in which we deemed it impossible to recruit enough caregivers for both the intervention and control groups. Instead, the groups are recruited consecutively, and we aim to statistically control for differing variables using covariates.
Background
The number of people living with dementia worldwide is currently estimated at 35.6 million. This number will double by 2030 and more than triple by 2050.1 In the Netherlands, 260 000 people were diagnosed with dementia in 2014. Seventy per cent of these people live at home and are dependent on informal caregivers (hereafter: caregivers) for their daily care.2 Caregivers are mostly unpaid spouses, sons, daughters, friends or relatives.
Although caregiving is satisfying for some caregivers,3–5 it can also be very burdensome.6 7 Caregivers often experience higher rates of depression,8 poorer physical and mental health,9–11 a lower sense of well-being, more social isolation12 and more financial burden13 than people who do not provide care. The likelihood of nursing home admission for the person with dementia rises when their caregiver becomes overburdened and can no longer cope.14 An intervention which supports caregivers in their caregiving role is therefore very desirable.
In the past 10–15 years, several interventions have been developed to support caregivers. These include training and education programmes, support groups, counselling and web-based and multicomponent interventions. These have been found to be moderately effective in improving the quality of care and competence of caregivers,15–17 diminishing caregiver burden,17 18 health-related problems,19 20 stress,20 21 improving the quality of life of both caregivers and their patients22 and diminishing the dependency on professionals.17 20 However, most of these interventions lack practical tips and advice on how to apply the knowledge gained in daily life. The idea came to us that if caregivers could actually experience symptoms of dementia themselves, they might understand their patients better and in turn have more empathy for them. With this hypothesis in mind, the mixed virtual reality simulator ‘Into D’mentia’ was developed in 2010.23 We also included education and the use of support groups in our training (these take place after the caregivers experience in the simulator) because these have been found to be beneficial in other interventions.24 25
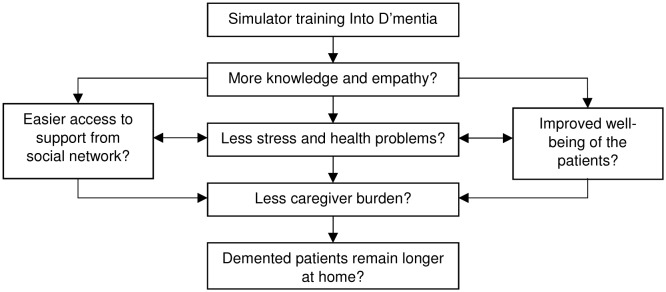
The simulator’s goal is to increase caregivers’ knowledge and empathy for the person with dementia. It is hypothesised that this will lead to decreased stress levels, caregiver burden and health problems associated with caregiving in the caregivers themselves and that this in turn will lead to the person with dementia living at home for longer before being institutionalised (see figure 1). A better understanding of dementia has been found to promote the well-being of caregivers in a previous study.26 In another study, when caregivers cared in a more empathetic way for the person with dementia, their own stress level was reduced.27 Professionals who have more (vs those who have less) empathy have also been found to have fewer burn-outs and are more satisfied with their work as a professional caregiver, while the people with dementia under their care adhere better to therapy and have better health-related outcomes.27 28
Figure 1.
The simulator’s goals.
The aim of the current study is to assess the effectivity of the Into D’mentia simulator on a number of variables over time including empathy, caregiver burden, feelings of competence of caregiving, depression and anxiety, the relationship between caregivers and their patients, and caregivers’ health. This will be the first study that evaluates an intervention which attempts to simulate dementia. Here, we describe the design and protocol of this study.
Methods and analyses
Design
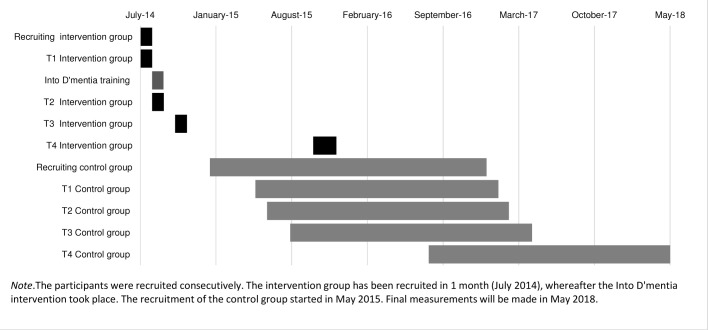
A longitudinal, quasi-experimental study with two groups is ongoing. The study began in 2014; the final measurements will be made in 2018. Participants are evaluated four times: 1 week before the Into D’mentia training (T1) and 1 week, 2.5 months and 15 months after the training (T2, T3 and T4, respectively). The control group is tested at the same time intervals, starting at T1. Figure 2 shows a graph of the time schedule and important dates.
Figure 2.
Time schedule of the study.
Study population
Two groups are created and consecutively recruited:
The intervention group. This group receives the Into D’mentia simulator training (and is not prohibited from usual care).
The group consists of informal caregivers of a relative, friend or spouse with dementia. The participants are recruited from de Wever in Tilburg, the Netherlands, an organisation for eldercare; elderly federations; Alzheimer Nederland; case managers; centres for daytime activities for people with dementia and via social media.
Inclusion criteria
An informal caregiver for a spouse, family member or friend with dementia, spending at least 8 hours a week caring for the patient who lives at home (not institutionalised).
At least 18 years old (no upper age limit).
Exclusion criteria
Physical disabilities which make entrance into the simulator impossible.
Severe communication disabilities which make understanding of the simulator impossible (eg, insufficient understanding of the Dutch language, blindness or deafness).
Self-reported severe psychological or medical disabilities which make the simulator too confusing (including self-reported dementia).
The control group. This group also consists of caregivers. The recruitment, inclusion and exclusion criteria are the same as for the intervention group. The only difference is that this group does not experience the intervention and as such is an attention-only group. This group is not prohibited from usual care. After completion of the study, a group meeting will be organised as a reward for participating in the study. During this meeting, professionals will provide information about dementia, and the participants will have the opportunity to ask questions.
Procedure
Eligible participants receive oral and written information about the study from case managers, nurses and supervisors at daytime activity centres or only written information on social media. Eligible participants are invited to contact the researchers (LJ) by phone or email if they have questions and to receive more information about the study. If they are interested in participating, the appointment for the first interview is scheduled, and the questionnaires are sent. For the intervention group, an appointment for the intervention training is made at the same time. Written consent is also obtained. For the follow-up assessments (T2–T4), participants are informed by letter, telephone or email and invited to participate after which an appointment is scheduled.
For both the intervention and control group, four measurements take place; for all four assessments, a semi-structured interview is conducted, and a questionnaire booklet is provided. The interviews are administered in a standardised way by trained neuropsychologists and take place either at the participant’s home or at Tilburg University depending on the caregivers’ preference. The questionnaire booklet is sent to the participants before the appointment for the interview with the request that they complete it at home and bring it with them to the interview when they can receive help should any problems arise.
The questionnaires and interviews are identical for the two groups. The only exception being for the control group, where questions about the simulator training are not relevant and therefore omitted.
Intervention
The intervention is a mixed-reality dementia simulator training. The training consists of three parts: the simulation, an individual conversation with the trainer immediately after the simulation and a group meeting with the other participants 1–2 weeks later. In the simulator, the participants experience what it is like to have dementia. The training was developed based on literature reviews and on talks with caregivers, professionals and a number of people with dementia.23 The caregivers, professionals and people with dementia were also involved in the process of developing, altering and improving the intervention. They all approved of the final simulator, which we are currently using in this study. The simulator training takes place in a portable unit in which a little front yard, a bathroom and a kitchen are built. After a short, individual introduction, the participant enters the simulator unit. The participant wears a speaker vest, with microphones from which their ‘inner voice’ tells the story. This inner voice gives them specific instructions, for example, to turn on the radio which then appears to not work properly. The participant’s ‘daughter’ is projected on a screen using a beamer, and she behaves like many caregivers do, for example, talking about the patient while the patient is in the room, getting frustrated and so on. Several audiovisual elements make the simulator interactive, allowing the participant to make choices and thereby influence the storyline. Empathic reactions of negative situations (like caring for a relative with pain or, in this case, dementia) can lead to stress or negative changes in neural networks.29 To ensure the safety and well-being of the participants, immediately after the training, an individual conversation with the trainer is organised. During this conversation, the participants discuss their experiences in the simulator, and the trainer comforts the participants if needed. If the participants are heavily distressed, they can also telephone the research team (all trained psychologists) for help. The participants are encouraged to discuss their experiences in the simulator with family members or friends regardless of immediate stress reactions. The participants can call the research team if they experience any negative reactions which cannot wait until the group meeting. A group meeting with 8–12 other participants is organised 1–2 weeks after the training in order to help them to better understand and to implement their experiences and new knowledge into their daily lives. During this group meeting, experiences in the simulator are described in more detail and are put into perspective. In addition, professionals give information about dementia, and some practical tips are shared. At the same time, the caregivers can learn from each other’s experiences.
Measures
Tables 1 and 2 give an overview of the variables assessed and instruments used at each time point. Short questionnaires (or self-made questions) were specifically chosen in order to reduce the time (about 45 min in total) required to complete because caregivers are typically busy and 82% overburdened.30 The interviews take about 45 min to complete, leading to a time investment of approximately 90 min per measurement per caregiver.
Table 1.
Primary outcomes
| Primary outcomes | Variable/instrument | T1 | T2 | T3 | T4 |
| Empathy | Interpersonal Reactivity Index31 | X | X | X | X |
| Caregiver burden | Caregiver Reaction Assessment–Dutch33 | X | X | X | X |
| Depressive complaints | Hospital Anxiety and Depression Scale–subscale depression35 | X | X | X | X |
| Anxiety complaints | Hospital Anxiety and Depression Scale–subscale anxiety35 | X | X | X | X |
| Quality of the relationship | Relationship Quality Index37 | X | X | X | X |
| Quality of the Relationship38 | X | X | X | X | |
| Caregiver’s sense of competence | Short Sense of Competence Questionnaire39 | X | X | X | X |
Note. T1: 1 week before the simulator training; T2: 1 week after the training; T3: 2.5 months after the training, T4: 12 months after T3.
Table 2.
Secondary outcomes
| Variable/instrument | T1 | T2 | T3 | T4 | ||
| Social reliance | Inventory for Social Reliance40 | X | X | X | X | |
| Subjective health | Cognitive complaints | Self-made item: ‘Before the dementia of your spouse/friend/relative, did you experience cognitive complaints?’ | X | |||
| Self-made item: ‘In the previous month, have you experienced cognitive complaints?’ | X | X | X | X | ||
| Depressive complaints | Self-made item: ‘Before the dementia of your spouse/friend/relative, did you experience depressive complaints?’ | X | ||||
| Self-made item: ‘In the previous month, have you experienced depressive complaints?’ | X | X | X | X | ||
| Anxiety complaints | Self-made item: ‘Before the dementia of your spouse/friend/relative, did you experience anxiety complaints?’ | X | ||||
| Self-made item: ‘In the previous month, have you experienced anxiety complaints?’ | X | X | X | X | ||
| Objective health | Number of hospital admissions | X | X | X | X | |
| Number of hospital visits | X | X | X | X | ||
| Number of GP visits | X | X | X | X | ||
| Life events | Self-made item concerning the presence and impact of a positive of negative life event: ‘Last month, did something happen in your life which had a major impact on you? This may be something either pleasant or sad’ | X | X | X | X | |
| Quality of life | Self-made item concerning the quality of life of the caregivers: ‘How would you rate your quality of life on this point in your life?’ The subjects answers by putting an X on a line ranging from 0 (very bad) to 100 (very good) | X | X | X | X | |
| Quality of sleep | Self-made item about the quality of sleep: ‘Before the dementia of your spouse/friend/relative, how would you have rated your quality of sleep?’ The subjects answers by putting an X on a line ranging from 0 (very bad) to 100 (very good). | X | ||||
| Self-made item about the quality of sleep: ‘How would you have rated your quality of sleep on this point in your life?’ The subjects answers by putting an X on a line ranging from 0 (very bad) to 100 (very good). | X | X | X | X | ||
| Health and living situation patient | Self-made item concerning the progression of the dementia of the patient: ‘How is he or she doing compared with the time of the last interview?’ The possible answers are better, the same or worse, than the last interview. | X | X | X | ||
| Self-made item concerning the living situation of the patient: ‘Has something changed in the living situation of the patient since the last interview?’ | X | X | X | |||
| Self-made item about the concerns of the caregivers about the dementia of the patient: ‘Do you have any new concerns about the dementia since the last interview?’ | X | X | X | |||
Note. T1: 1 week before the simulator training; T2: 1 week after the training; T3: 2.5 months after the training, T4: 12 months after T3.
GP, general practitioner.
Outcomes
Primary outcomes
The primary outcomes chosen to assess how effective the Into D’mentia simulator are as follows: empathy, caregiver burden, depression and anxiety, the quality of the relationship between caregiver and patient, and caregiver’s sense of competence.
To measure empathy, the most important primary outcome, the Interpersonal Reactivity Index (IRI)31 is used. The IRI asks subjects to rate 28 items on several empathy-related statements on a 5-point Likert scale ranging from ‘does not describe me well’ to ‘describes me very well’. The 28 items are clustered into four subscales, each made up of seven different items: perspective taking, fantasy, empathic concern and personal distress, leading to a multidimensional approach to empathy. The Cronbach’s alpha for the subscales ranges from 0.70 to 0.76.32
Caregiver burden is evaluated by the Caregiver Reaction Assessment Dutch (CRA-D).33 The CRA-D measures both negative and positive reactions to caregiving. The questionnaire consists of 24 items, clustered into five dimensions: the impact of caregiving on disrupted schedule, financial problems, lack of family support, health problems and the impact of caregiving on caregiver’s self-esteem, with Cronbach’s alpha ranging from 0.62 to 0.83.34 The subject reports to what extent he or she agrees with the 24 statements on a 5-point scale.
Anxiety and depression are measured using the Hospital Anxiety and Depression Scale (HADS).35 The HADS comprises seven questions for anxiety and seven questions for depression and takes 2–5 min to complete. The items are rated on a 4-point scale (0–3) and concern anxiety and depression symptoms from the last week. The scores on the subscales are added up, and a cut-off score of 8 is used to indicate depressive or anxiety complaints. For the anxiety subscale, Cronbach’s alpha ranges from 0.76 to 0.93; for the depression subscale, it ranges from 0.72 to 0.90 in different studies.36
The quality of the relationship between caregiver and patient is evaluated using two questionnaires. The first is the Relationship Quality Index, which consists of five questions which can be answered on a 7-point Likert scale. The maximum score is 35. A higher score indicates a higher quality relationship.37
The second questionnaire to measure relationship quality is based on the Affectual Solidarity Questionnaire used for the Longitudinal Study of Generations,38 which in this study is named Quality of the Relationship (QoR). This questionnaire evaluates two domains: current relationship quality (QoR-current) (six items) and change in relationship quality (QoR-change) (five items). The six items of the QoR-current are evaluated on a 4-point scale. Scores range from 6 to 24, with a higher score indicating a better relationship quality. The five items of the QoR-change are statements regarding how much things have changed since the dementia diagnosis of a loved one. The statements are evaluated on a 5-point scale; the total score ranges from 5 to 25, with a higher score indicating a lower relationship quality.
Caregiver’s sense of competence is assessed by the Short Sense of Competence Questionnaire, which consists of seven items, rated according to a 5-point Likert scale (1–5). The items are clustered into three domains: lack of satisfaction with the person with dementia as a recipient of care, lack of satisfaction with one’s own performance as a carer and consequences of involvement in care for the personal life of the carer. The total score ranges from 0 to 35, with a Cronbach’s alpha of 0.76.39
Secondary outcomes
Secondary outcomes for the caregivers include the following: social reliance (use of social networks and participation), subjective and objective health, life events, quality of life and quality of sleep. The living situation of the person with dementia will also be assessed.
Social reliance is measured by the Dutch version of the Inventory for Social Reliance. The questionnaire evaluates both the quantitative and qualitative aspects of social support. The quantitative part consists of two items: the number of good friends and the number of acquaintances in the participants’ neighbourhood. The qualitative part entails 11 items, rated according to a 4-point Likert scale, which cover three aspects of social support: perceived emotional support, actual emotional support, mutual visiting and one rest item.40 41
Subjective health is evaluated by asking the caregivers if they had cognitive, depressive or anxiety complaints in the last 4 weeks. Objective health in the caregivers is assessed during the semi-structured interviews using the following (separate) measures (relying on self-report): the number of medications the caregiver personally uses, the number of hospital admissions, visits to the general practitioner and visits to the hospital in the last month.
To assess life events, the participants answer the following self-made written question concerning the presence and impact of a positive or negative life event ‘In the past month, did something happen in your life which had a major impact on you? This may be something either pleasant or sad’. The subjects can choose between ‘no’ and ‘yes’. If the answer is yes, the next question is what the total impact of the experience is, which the subject can rate according to a 5-point Likert scale ranging from ‘very negative impact’ to ‘very positive impact’.
Quality of life and quality of sleep are both evaluated using one self-made Likert scale. The subject is asked to rate their quality of sleep and quality of life at ‘this’ moment in their lives, by putting a cross on this line:
 The living situation of the person with dementia is assessed by asking the caregivers if the person still lives at home or if he or she has been institutionalised.
The living situation of the person with dementia is assessed by asking the caregivers if the person still lives at home or if he or she has been institutionalised.
Possible determinants/confounders
A wide range of possible determinants/confounders (factors in the prediction model and/or covariates) are additionally taken into account, based on what is currently known from the literature about caregivers. These include sociodemographic variables, medicine use of both caregivers and the people with dementia they care for, and clinical variables regarding the dementia such as the type and time since diagnosis. These data rely on self-report of the informal caregiver. Finally, a couple of qualitative variables are also assessed, for example, subjective experiences with the simulator (for the intervention group only). Table 3 lists the specific variables assessed and instruments used.
Table 3.
Possible determinants/confounders
| Variable/instrument | T1 | T2 | T3 | T4 | |
| Sociodemographic and clinical variables of the caregivers | |||||
| Age, gender, education, employment status | X | ||||
| Medicine use | X | ||||
| Presence and severity of physical disabilities | Self-made question: ‘Do you have any physical disabilities and if so, to what extent do these interfere with caregiving?’ | X | |||
| Presence and severity of psychological disabilities | Self-made question: ‘Do you have any psychological disabilities and if so, to what extent do these interfere with caregiving?’ | X | |||
| Variables concerning caregiving | Relationship with the patient with dementia (spouse/daughter/son/something else) | X | |||
| Distance to the patient (shares household/walking distance/in the same city/in a different city) | X | ||||
| Days providing care a week | X | ||||
| Hours providing care a week | X | ||||
| Years since first time providing care for this patient | X | ||||
| Support of professionals (eg, housekeeper, case manager) | X | ||||
| Perceived support of friends or family | X | ||||
| Clinical variables of the patient with dementia | |||||
| Diagnosis | Alzheimer’s disease/vascular dementia/Parkinson’s disease Dementia/frontotemporal dementia/other/unknown | X | |||
| Time since diagnosis (in years) | X | ||||
| Medicine use | X | ||||
| Comorbidities | Physical comorbidities | X | |||
| Psychological comorbidities | X | ||||
| Support of professional (eg, physiotherapist) | X | ||||
| Self-made items regarding the subjective effectivity of the training* | |||||
| ‘Does the simulator give an accurate reflection of what a demented person goes through?’ | X | X | X | ||
| ‘Did the simulator meet your expectations?’ | X | X | X | ||
| ‘Do you think the simulator is useful?’ | X | X | X | ||
| ‘Did you feel supported by the experiences and stories of the other participants in the group meeting?’ | X | X | X | ||
| ‘Did the group meeting meet your expectations?’ | X | X | X | ||
| ‘Do you think the group meeting is useful?’ | X | X | X | ||
| ‘Did the whole training (simulator and group meeting together) had a personal impact on you?’ | X | X | X | ||
| ‘Do you think that the whole training helps you to be a more effective caregiver?’ | X | X | X | ||
| ‘Do you think the whole training has helped you to understand your spouse/relative/friend?’ | X | X | X | ||
| ‘Do you think that you are better prepared for what is going to happen in the future?’ | X | X | X | ||
| ‘Are you surer of your qualities because of the training?’ | X | X | X | ||
| ‘Did you learn anything from the training? And if yes, what?’ | X | X | X | ||
| ‘Do you do anything different in caring because of the training? And if yes, what? | X | X | X | ||
| ‘Do you think the training missed anything? And if yes, what?’ | X | X | |||
Note. T1: 1 week before the simulator training; T2: 1 week after the training; T3: 2.5 months after the training, T4: 12 months after T3.
*Questions for the intervention group only.
Planned statistical analyses
SPSS Statistics 22 will be used for the statistical analyses. Parametric and non-parametric tests will be used to determine if the two groups are comparable at baseline on four variables, three caregiver variables (gender, age and level of education) and one person with dementia variable (time since diagnosis). Variables that differ will be used as covariates in the subsequent analyses.
Cross-sectional analyses will be used to evaluate group differences at each of the individual time points (T2–T4) and include χ2 for categorical variables, the Mann-Whitney U test for ordinal data and the Student t-test or multivariate analysis of variance for continuous dependent variables.
Differences across the time points will be analysed using multilevel analysis, which allows inclusion of all available data (ie, also those from participants with missing data).
The predictive value of the determinants for the primary and secondary outcome measures at T2, T3 and T4 will be determined using multivariate regression analysis (two time points) or multilevel analysis (>2 time points). Potential predictors are defined as variables with at least a marginally significant association (p<0.10) with the outcome. Only these variables will be included in the subsequent analyses to determine the most important predictors. Effects with a two-tailed p<0.05 are considered statistically significant. Missing data will be imputed where possible. We will use the Bonferroni correction to correct for multiple comparisons.
A prediction model will be developed to define the most valuable variables for the effectivity of this intervention. Possible predictors are age, gender, relationship with the patient and hours of care.
The qualitative questions in the interviews will be analysed using descriptive statistics and frequencies.
Sample size and power calculation
The sample size needed is calculated with G*Power, based on the main research question: does the simulator training increase the empathy of informal caregivers? Based on an alpha level of 0.05 and a power of 0.80, 64 participants per group are needed to be able to detect a medium difference (d=0.5) between the groups. We expect about 10% drop-out during the 1-year follow-up period due to mortality of the caregivers or the person the caregivers care for or due to refusal to continue participation. Therefore, we aim to include at least 71 participants in each group; 2×71=142 participants in total.
Ethics and dissemination
Ethical considerations
This study is non-invasive and imposes no risk on either the participating caregivers or the people with dementia. This protocol has been approved by the psychological ethical committees of both the Tilburg School of Social and Behavioural Sciences, Tilburg University, and De Wever (a care organisation for eldercare) in Tilburg, the Netherlands. Written informed consent is obtained from all participants, in accordance with the Declaration of Helsinki (Seoul Revision, 2008). The data are stored anonymously, and only the primary researchers (LHJ, REM and MMS) have access to the data. This study has been registered by The Dutch National Trial Register (NTR) number (TC): NTR5856. There is a mismatch in the dates between the start of the study (see figure 2; July 2014) and the registry date (1 December 2015). This is because the Into D’mentia simulator was available for 5 weeks in July 2014 for free. At that time, it was not certain we could continue the study due to lack of funding. The inclusion of the control group started later when financial support was obtained. The study was registered after this financial support was received, with the corresponding date.
Dissemination
The results obtained will be disseminated to the scientific and general public by publication in national and international (peer reviewed) scientific and professional journals, as well as by presentations at conferences and meetings with professionals dealing with (informal caregivers of people with) dementia. First, a manuscript with the results of the primary study outcome (empathy) will be published in a peer-reviewed journal. Separate manuscripts will be written on the secondary research outcomes, and these will also be submitted for publication in peer-reviewed journals. The data will not be made public, assuring the study participants’ privacy. Requests for data sharing will be considered on an individual basis, for appropriate research purposes only, after completion of the trial and after publication of the primary manuscript.
Discussion
This is the first study in which the effectivity of a mixed virtual reality dementia simulator is extensively tested in caregivers in a controlled trial. While multiple interventions for caregivers have been designed and tested,42 this is the first dementia simulator in which caregivers actually experience what it is like to have dementia, on a functional level and emotionally and socially. The focus on experience-based learning makes this intervention very practical.
Strong elements of this study are its longitudinal prospective design with multiple assessments. This is a useful addition to the existing effect studies into interventions for caregivers, which usually apply pre–post designs which makes it impossible to know if these interventions work in the longer term. In addition, we include both quantitative (questionnaires) and qualitative (semi-structured interviews) measurements. We are aware that there are many variables, but we feel that it is necessary to take them all into account because many factors are involved in caregiver burden and need to be considered in any attempt to ultimately figure out which are important. Also, a control group is included which was not always the case in previous intervention studies with caregivers. The control group makes it possible to attribute the findings to the intervention, instead of to other variables such as elapsed time. A potential limitation is that due to practical reasons, the participants were not randomised. The simulator was available for free for 5 weeks only (after which it was again made available for a financial compensation), in which we deemed it impossible to include enough caregivers for both the intervention and control groups. Instead, the groups are recruited consecutively, and we aim to statistically control for differing variables using covariates. These practical reasons were mainly of a financial nature; the intervention is freely available for the public at a cost.
The recruitment of the control group took longer than the recruitment of the intervention group (see figure 2). This is partly due to the fact that our existing networks were depleted once we started the recruitment of the control group, so new networks had to be formed. Another potential reason was that these (control) participants may have been less eager to participate because they had to wait until the end of the study for their ‘reward’ (the group meeting).
In conclusion, we hope that this study will determine how effective (or not) the Into D’mentia training is on a variety of variables including empathy and caregiver burden. Furthermore, we believe that it has the potential to contribute to existing knowledge about caregivers. The dementia simulator is expected to be specifically effective in enhancing the quality of life of both caregivers and the people with dementia they care for by helping caregivers understand dementia better in a more personal way.
More informal caregivers than ever before are involved in the care for a family member or friend living with dementia. Helping them in their task should be a priority in healthcare services around the world. At the moment, the Into D’mentia training is too expensive for many individual caregivers (the training costs €240 per person). If it proves to be effective (on one or more outcomes), the next step would be to do a cost-effectiveness analyses and get it implemented into standard care, making it available for all caregivers and also for care professionals. The ultimate goal is to assist caregivers in the best possible way in their task of caring for their loved ones with dementia, a task most come unprepared to and a task that no one asks for.
Supplementary Material
Acknowledgments
The authors thank the VU University Medical Centre Amsterdam, De Wever Tilburg, Ijsfontein Amsterdam, Ideon Amersfoort and Stichting Into D’mentia for their help developing the Into D’mentia simulator training.
Footnotes
Contributors: LHJ, REM, BWJMJ, JR, RMD and MMS contributed to study concept, planning and data acquisition; LHJ, REM and MMS will contribute to the statistical analyses, publication and dissemination of findings. LHJ wrote the first manuscript, and all other authors provided critical feedback during the manuscript development and approved of the final manuscript. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding: This work was supported by ZonMw’s Memorabel program (part of NWO, The Netherlands) and Alzheimer Nederland, project number: 733050608. However, the authors are solely responsible for the design and conduct of this study as well as all study analyses and the drafting and editing of this article.
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: Psychologisch Ethische Toetsingscommissie (PETC), Tilburg University.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Data are sensitive, and the first priority in sharing data will be protection of study participants’ privacy. Therefore, this will not be a public use dataset. The authors will consider requests for data sharing on an individual basis, for appropriate research purposes, after publication of the major findings of the study.
References
- 1. World Health Organization. Dementia: a Public Health Priority. Geneva: World Health Organization, 2012. [Google Scholar]
- 2. Alzheimer Nederland. Neemt het aantal mensen met dementie toe of af? http://www.alzheimer-nederland.nl/nieuws/onderzoek/2014/februari/aantal-mensen-met-dementie.aspx (accessed 18 Nov 2016).
- 3. Schulz R, Newsom J, Mittelmark M, et al. . Health effects of caregiving: the caregiver health effects study: an ancillary study of the Cardiovascular Health Study. Ann Behav Med 1997;19:110–6. 10.1007/BF02883327 [DOI] [PubMed] [Google Scholar]
- 4. Koerner SS, Kenyon DB, Shirai Y. Caregiving for elder relatives: which caregivers experience personal benefits/gains? Arch Gerontol Geriatr 2009;48:238–45. 10.1016/j.archger.2008.01.015 [DOI] [PubMed] [Google Scholar]
- 5. Cohen CA, Colantonio A, Vernich L. Positive aspects of caregiving: rounding out the caregiver experience. Int J Geriatr Psychiatry 2002;17:184–8. 10.1002/gps.561 [DOI] [PubMed] [Google Scholar]
- 6. Pinquart M, Sörensen S. Correlates of physical health of informal caregivers: a meta-analysis. J Gerontol B Psychol Sci Soc Sci 2007;62:P126–P137. 10.1093/geronb/62.2.P126 [DOI] [PubMed] [Google Scholar]
- 7. Schulz R, Beach SR. Caregiving as a risk factor for mortality: the Caregiver Health Effects Study. JAMA 1999;282:2215–9. [DOI] [PubMed] [Google Scholar]
- 8. D’Aoust RF, Brewster G, Rowe MA. Depression in informal caregivers of persons with dementia. Int J Older People Nurs 2015;10:14–26. 10.1111/opn.12043 [DOI] [PubMed] [Google Scholar]
- 9. Schulz R, Sherwood PR. Physical and mental health effects of family caregiving. Am J Nurs 2008;108:23–7. 10.1097/01.NAJ.0000336406.45248.4c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wills W, Soliman A. Understanding the needs of the family carers of people with dementia. Mental Health Review Journal 2001;6:25–8. 10.1108/13619322200100017 [DOI] [Google Scholar]
- 11. Schulz R, O’Brien AT, Bookwala J, et al. . Psychiatric and physical morbidity effects of dementia caregiving: prevalence, correlates, and causes. Gerontologist 1995;35:771–91. 10.1093/geront/35.6.771 [DOI] [PubMed] [Google Scholar]
- 12. Brodaty H, Hadzi-Pavlovic D. Psychosocial effects on carers of living with persons with dementia. Aust N Z J Psychiatry 1990;24:351–61. 10.3109/00048679009077702 [DOI] [PubMed] [Google Scholar]
- 13. Max W, Webber P, Fox P. Alzheimer’s disease. The unpaid burden of caring. J Aging Health 1995;7:179–99. 10.1177/089826439500700202 [DOI] [PubMed] [Google Scholar]
- 14. Afram B, Stephan A, Verbeek H, et al. . Reasons for institutionalization of people with dementia: informal caregiver reports from 8 European countries. J Am Med Dir Assoc 2014;15:108–16. 10.1016/j.jamda.2013.09.012 [DOI] [PubMed] [Google Scholar]
- 15. Van Mierlo LD, Meiland FJ, Van de Ven PM, et al. . Evaluation of DEM-DISC, customized e-advice on health and social support services for informal carers and case managers of people with dementia; a cluster randomized trial. Int Psychogeriatr 2015;27:1365–78. 10.1017/S1041610215000423 [DOI] [PubMed] [Google Scholar]
- 16. Lewis ML, Hobday JV, Hepburn KW. Internet-based program for dementia caregivers. Am J Alzheimers Dis Other Demen 2010;25:674–9. 10.1177/1533317510385812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chiu M, Pauley T, Wesson V, et al. . Evaluation of a problem-solving (PS) techniques-based intervention for informal carers of patients with dementia receiving in-home care. Int Psychogeriatr 2015;27:937–48. 10.1017/S1041610214002798 [DOI] [PubMed] [Google Scholar]
- 18. Cristancho-Lacroix V, Wrobel J, Cantegreil-Kallen I, et al. . A web-based psychoeducational program for informal caregivers of patients with Alzheimer’s disease: a pilot randomized controlled trial. J Med Internet Res 2015;17:e117 10.2196/jmir.3717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. van Mierlo LD, Meiland FJ, Dröes RM. Dementelcoach: effect of telephone coaching on carers of community-dwelling people with dementia. Int Psychogeriatr 2012;24:212–22. 10.1017/S1041610211001827 [DOI] [PubMed] [Google Scholar]
- 20. Marziali E, Garcia LJ. Dementia caregivers’ responses to 2 Internet-based intervention programs. Am J Alzheimers Dis Other Demen 2011;26:36–43. 10.1177/1533317510387586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chiu T, Marziali E, Colantonio A, et al. . Internet-based caregiver support for Chinese Canadians taking care of a family member with alzheimer disease and related dementia. Can J Aging 2009;28:323–36. 10.1017/S0714980809990158 [DOI] [PubMed] [Google Scholar]
- 22. Haberstroh J, Neumeyer K, Krause K, et al. . TANDEM: communication training for informal caregivers of people with dementia. Aging Ment Health 2011;15:405–13. 10.1080/13607863.2010.536135 [DOI] [PubMed] [Google Scholar]
- 23. Hattink BJJ, Meiland FJM, Campman CAM, et al. . Zelf dementie ervaren: ontwikkeling en evaluatie van de Into D?D?mentia simulator. Tijdschr Gerontol Geriatr 2015;46:262–81. 10.1007/s12439-015-0130-8 [DOI] [PubMed] [Google Scholar]
- 24. Thompson CA, Spilsbury K, Hall J, et al. . Systematic review of information and support interventions for caregivers of people with dementia. BMC Geriatr 2007;7:18 10.1186/1471-2318-7-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lopez-Hartmann M, Wens J, Verhoeven V, et al. . The effect of caregiver support interventions for informal caregivers of community-dwelling frail elderly: a systematic review. Int J Integr Care 2012;12:133 10.5334/ijic.845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Graham C, Ballard C, Sham P. Carers’ knowledge of dementia, their coping strategies and morbidity. Int J Geriatr Psychiatry 1997;12:931–6. [DOI] [PubMed] [Google Scholar]
- 27. Lamothe M, Boujut E, Zenasni F, et al. . To be or not to be empathic: the combined role of empathic concern and perspective taking in understanding burnout in general practice. BMC Fam Pract 2014;15:1 10.1186/1471-2296-15-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Neumann M, Bensing J, Mercer S, et al. . Analyzing the "nature" and "specific effectiveness" of clinical empathy: a theoretical overview and contribution towards a theory-based research agenda. Patient Educ Couns 2009;74:339–46. 10.1016/j.pec.2008.11.013 [DOI] [PubMed] [Google Scholar]
- 29. Simons LE, Goubert L, Vervoort T, et al. . Circles of engagement: childhood pain and parent brain. Neurosci Biobehav Rev 2016;68:537–46. 10.1016/j.neubiorev.2016.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Prince M, Wimo A, Guerchet M, et al. . World Alzheimer Report The global impact of Dementia: an analysis of prevalence, incidence, cost and trends. London: Alzheimer’s Disease International (ADI), 2015. [Google Scholar]
- 31. Davis MH. A multidimensional approach to individual differences in empathy. Journal of Southern African Studies 1980;10:85–104. [Google Scholar]
- 32. Fernández AM, Dufey M, Kramp U. Testing the psychometric properties of the Interpersonal reactivity index (IRI) in Chile. Eur J Psychol Assess 2011. [Google Scholar]
- 33. Given CW, Given B, Stommel M, et al. . The caregiver reaction assessment (CRA) for caregivers to persons with chronic physical and mental impairments. Res Nurs Health 1992;15:271–83. 10.1002/nur.4770150406 [DOI] [PubMed] [Google Scholar]
- 34. Nijboer C, Triemstra M, Tempelaar R, et al. . Measuring both negative and positive reactions to giving care to cancer patients: psychometric qualities of the Caregiver Reaction Assessment (CRA). Soc Sci Med 1999;48:1259–69. 10.1016/S0277-9536(98)00426-2 [DOI] [PubMed] [Google Scholar]
- 35. Spinhoven P, Ormel J, Sloekers PP, et al. . A validation study of the Hospital Anxiety and Depression Scale (HADS) in different groups of Dutch subjects. Psychol Med 1997;27:363–70. 10.1017/S0033291796004382 [DOI] [PubMed] [Google Scholar]
- 36. Beljouw van I, Verhaak P. Geschikte uitkomstmaten voor routinematige registratie door eerstelijnspsychologen. Nivel Utrecht, 2010. [Google Scholar]
- 37. Norton R. Measuring marital quality: a critical look at the dependent variable. J Marriage Fam 1983;45:141–51. 10.2307/351302 [DOI] [Google Scholar]
- 38. Bengtson VL. Longitudinal study of generations, 1971, 1985, 1988, 1991, 1994, 1997, 2000, 2005 (California). Ann Arbor, MI: Inter-university Consortium for Political and Social Research, 2009. [Google Scholar]
- 39. Vernooij-Dassen MJ, Felling AJ, Brummelkamp E, et al. . Assessment of caregiver’s competence in dealing with the burden of caregiving for a dementia patient: a Short Sense of Competence Questionnaire (SSCQ) suitable for clinical practice. J Am Geriatr Soc 1999;47:256–7. 10.1111/j.1532-5415.1999.tb04588.x [DOI] [PubMed] [Google Scholar]
- 40. van Dam-Baggen C, Huiskes C, Kraaimaat F. Inventory for Measuring Social Involvement.[Inventarisatielijst Sociale Betrokkenheid: ISB]. Utrecht: Academic Hospital Utrecht, 1986. [Google Scholar]
- 41. Van Dam-Baggen R, Kraaimaat F. De Inventarisatielijst Sociale betrokkenheid (ISB): een zelfbeoordelingslijst om sociale steun te meten [The inventarisation inventory to measure sociale integration: a self report inventory to assess social support]. Gedragstherapie 1992;25:27–45. [Google Scholar]
- 42. Dickinson C, Dow J, Gibson G, et al. . Psychosocial intervention for carers of people with dementia: What components are most effective and when? A systematic review of systematic reviews. Int Psychogeriatr 2017;29:31–43. 10.1017/S1041610216001447 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.