Abstract
Objectives
Poorly controlled type 2 diabetes mellitus (T2DM) is a major international health problem. Our aim was to assess the effectiveness of healthcare interventions, specifically targeting patients with poorly controlled T2DM, which seek to improve glycaemic control and cardiovascular risk in primary care settings.
Design
Systematic review.
Setting
Primary care and community settings.
Included studies
Randomised controlled trials (RCTs) targeting patients with poor glycaemic control were identified from Pubmed, Embase, Web of Science, Cochrane Library and SCOPUS. Poor glycaemic control was defined as HbA1c over 59 mmol/mol (7.5%).
Interventions
Interventions were classified as organisational, patient-oriented, professional, financial or regulatory.
Outcomes
Primary outcomes were HbA1c, blood pressure and lipid control. Two reviewers independently assessed studies for eligibility, extracted data and assessed study quality. Meta-analyses were undertaken where appropriate using random-effects models. Subgroup analysis explored the effects of intervention type, baseline HbA1c, study quality and study duration. Meta-regression analyses were undertaken to investigate identified heterogeneity.
Results
Forty-two RCTs were identified, including 11 250 patients, with most undertaken in USA. In general, studies had low risk of bias. The main intervention types were patient-directed (48%) and organisational (48%). Overall, interventions reduced HbA1c by −0.34% (95% CI −0.46% to −0.22%), but meta-analyses had high statistical heterogeneity. Subgroup analyses suggested that organisational interventions and interventions on those with baseline HbA1c over 9.5% had better improvements in HbA1c. Meta-regression analyses suggested that only interventions on those with population HbA1c over 9.5% were more effective. Interventions had a modest improvement of blood pressure and lipids, although baseline levels of control were generally good.
Conclusions
This review suggests that interventions for T2DM, in primary care, are better targeted at individuals with very poor glycaemic control and that organisational interventions may be more effective.
Keywords: diabetes & endocrinology, organisation of health services, primary care
Strengths and limitations of the study.
This systematic review adds to the evidence regarding the effectiveness of healthcare interventions, which specifically target patients with poor glycaemic control of type 2 diabetes mellitus, in community settings.
There is no specific definition for ‘poor control’ diabetes in the literature, but by including all studies that had patients with a HbA1c ≥59 mmol/mol (7.5%), we captured the full range of poor glycaemic control and also examined other key risk factors such as blood pressure and lipids.
Data were pooled from 42 studies across four continents, enhancing the generalisability of the findings.
We did not account for medication use in the studies, but given that all included studies were randomised controlled trials, which would balance out delivery of medications, we think that differences in underlying medication usage may relate to how different interventions promote intensification of medications.
An individual patient data meta-analysis may answer further questions not possible in this review.
Introduction
Worldwide, type 2 diabetes mellitus (T2DM) is rising in prevalence and will exceed 4.4% of the world’s population or 366 million by 2030.1 Despite a wealth of evidence regarding the importance of risk factor control in T2DM, many patients continue to have poor control of HbA1c, blood pressure and lipids. Up to 60% of the patients fail to meet target HbA1c levels.2 Similarly, over one-third of the patients with T2DM have inadequate blood pressure control.3 Poorly controlled T2DM—and its associated microvascular and macrovascular complications—is associated with higher morbidity, higher mortality, poorer quality of life and substantial economic burden.4
Several studies have examined interventions designed to support the delivery of diabetes care in the community to improve glycaemic and cardiovascular risk factor control.5–11 A 2011 review of community-based interventions including all patients with T2DM, comprising 68 studies, showed that only one-third had a statistically significant improvement in one of the relevant clinical outcomes for diabetes: HbA1c, blood pressure or lipids.8 The majority of the included studies targeted all patients with T2DM without focussing on those with poor control. Although no overall effect was noted, combining organisational with professional (multifaceted) interventions was concluded to be more beneficial than single interventions and the highest quality multifaceted randomised controlled trials (RCTs) tended to include decision support interventions and elements. A 2013 review looked at 48 cluster RCTs, assessing the effectiveness of quality improvement (QI) strategies on the management of diabetes (both T1DM and T2DM).11 It suggested that QI interventions, which intervened at a system level on diabetes management, were associated with the largest benefits in glycaemic control and that the effectiveness of interventions targeting healthcare practitioners varied with baseline glycaemic control, being more effective with patients with worse control.11 A 2016 review, of T1DM or T2DM in primary care, looked at the effects of Clinician Education, Clinician Reminders, Team Changes, Case Management, Electronic Patient Registry, Telemedicine and Audit and Feedback.10 Including 30 studies, it concluded that multifaceted interventions on multidisciplinary teams were most effective. Interventions targeting family physicians were only effective if computerised feedback on insulin prescribing was provided.
Four large RCTs from North America and the UK have investigated the effects of intensive management of hyperglycaemic and cardiac risk factors on mortality in T2DM across all settings.12–17 Uncertainty remains regarding intensive glycaemic management for all patients with T2DM, with concerns about aggressive reductions in HbA1c.18 Targeted reductions in cardiovascular and glycaemic risk factors in certain vulnerable populations (cognitively impaired, disabled and frail) have been advocated.19 Interventions that specifically target those with very poor control of risk factors may be more beneficial than those targeting all patients, achieving the benefits of cardiovascular and glycaemic control, but without the potential risks of intensively lowering HbA1c in all persons with T2DM. The effect of interventions specifically targeting patients with poorly controlled T2DM in primary care is unknown.
Our aim was to assess the effectiveness of healthcare interventions delivered in primary care and community settings, targeting poorly controlled T2DM, which seek to improve glycaemic control, blood pressure and lipids.
Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were used to standardise the conduct and reporting of the research and the protocol was registered on PROSPERO.20
Data sources and searches
We searched articles in all languages from the Cochrane Library, Pubmed, Embase, Web of Science and SCOPUS from 1990 to 31 December 2016. Reference lists of all included papers were searched. Secondary searching of all references from included studies was also conducted. Online supplementary appendix 1 outlines the search string.
bmjopen-2016-015135supp001.pdf (66.8KB, pdf)
Study selection
We considered RCTs, controlled clinical trials, controlled before and after studies and interrupted time series analyses meeting the Cochrane Effective Practice and Organisation of Care (EPOC) quality criteria.21 Studies published in all languages were eligible.
Population
Individuals with ‘poorly controlled’ T2DM were our population of interest. Though there is a broad consensus about the importance of achieving good glycaemic control for the reasons described, there are no validated cut-offs, which define ‘poor-control’ of T2DM for targeted interventions. Poorly controlled T2DM has been defined based on elevated glycated haemoglobin levels in the literature, with different thresholds of HbA1c described, from over 59 mmol/mol (7.5%), over 64 mmol/mol (8.0%) to over 75 mmol/mol (9.0%).22–24 In this review, we considered participants to have poorly controlled T2DM if their HbA1c was over 59 mmol/mol (7.5%) (or if over 80% of the population in a study had a HbA1c over 59 mmol/mol). Similarly, there is no defined cut-off as to what defines ‘poorly controlled’ blood pressure. We identified studies primarily based on poor glycaemic control and also included participants in these studies who had uncontrolled hypertension or elevated cholesterol/lipids, if the risk factor level was above that of an accepted international target, as designated by the study authors. Where studies included patients with ‘poor control’ based on a range of risk factor profiles, for consistency, we only included a study if 80% of the population had a HbA1c over 59 mmol/mol (7.5%).
Interventions
We included interventions delivered by healthcare professionals specifically aiming to target patients with poor control of T2DM, based in primary care or community settings. The primary healthcare setting was defined as providing ‘integrated, easy to access, healthcare services by clinicians who are accountable for addressing a large majority of personal healthcare needs, developing a sustained and continuous relationship with patients and practicing in the context of family and community’.25 We excluded drug trials though interventions could have involved treatment intensification. Interventions were defined as simple if they had one identifiable component and multifaceted if they had more than one element. We excluded trials performed within the hospital or the hospital-outpatient setting. The Cochrane EPOC taxonomy of interventions was used and the predominant intervention type was defined using five categories including organisational, patient-centred, regulatory, financial and professional. Examples of these intervention types are provided in online supplementary appendix 2.21
bmjopen-2016-015135supp002.pdf (39.6KB, pdf)
Comparison
Comparison groups were included if they received usual care in that setting for T2DM. Controls were also included if they received minor enhanced elements of care, such as education leaflets, which the study authors believed did not go beyond usual care in most settings.
Outcome measures
Primary outcomes included glycaemic control (HbA1c), blood pressure (systolic or diastolic) and lipid levels, but if studies did not include HbA1c, they were excluded. Secondary outcomes included patient-reported outcome measures (PROMs) (eg, health-related quality of life), utilisation of health services, behavioural outcomes such as medication adherence, provider behaviour, acceptability of service to patients and providers, economic outcomes and adverse events.
Data extraction and quality assessment
Two reviewers (MEM and RG) read the titles and/or abstracts of the identified references and eliminated irrelevant studies. Studies that were deemed eligible for inclusion were read in full and their suitability for inclusion in the systematic review was independently determined by two reviewers. Disagreements were managed by a third, independent reviewer (SMS). The following information was extracted: (a) details of intervention, (b) participants, (c) clinical setting, (d) study design, (e) outcomes, (f) author information. We contacted authors for missing data.
Risk of bias in articles was assessed using the Cochrane Handbook for systematic reviewing and EPOC criteria.26 Two review authors independently assessed the risk of bias of each included study against the criteria described in the Cochrane risk of bias tool. We explicitly judged each of these criteria using: low risk of bias, high risk of bias or unclear risk of bias (either lack of information or uncertainty over the potential for bias). We resolved disagreements by consensus and consulted a third review author to resolve disagreements if necessary. An overall assessment of a study’s risk of bias was determined using EPOC guidance, with judgement and consensus reached between two reviewers (MEM and SMS).26
Data analysis
For continuous data, we calculated the treatment effect using mean differences (MDs) and 95% CIs. No binary outcomes were included. Revman software was used to perform the analysis, determine heterogeneity and produce forest plots to illustrate pooled estimates.21 Stata version 13 was used to investigate publication bias by creating funnel plots and using Egger’s test to assess funnel plot asymmetry.27 A random-effects analysis was performed and heterogeneity across the studies was quantified using the I2 statistic. The I2 statistic describes the percentage of the variability in effect estimates which is due to heterogeneity rather than sampling error (chance).28 If the I2 statistic was >50%, it was deemed that there was significant heterogeneity between the studies.
Subgroup analyses were performed for primary outcomes based on a priori assumptions, as per the PROSPERO protocol.20 For HbA1c, we explored the possible effects of subgroups: (a) the type of intervention based on the EPOC taxonomy (see online supplementary appendix 2); (b) study quality and (c) baseline HbA1c in the study populations (HbA1c 7.5%–9.4% or ≥9.5%). After reviewing, the included studies we also included study duration as a subgroup (<12 months or ≥12 months), as a wide range in study duration was found. Subgroup analyses for systolic blood pressure (SBP) and diastolic blood pressure (DBP) explored the effects of intervention-type based on the EPOC taxonomy.
When important heterogeneity was identified, we investigated its causes using meta-regression. Meta-regression is an extension to subgroup analysis that allows the effect of continuous, as well as categorical, characteristics to be investigated.29 Meta-regression was performed to explore the effects of: (a) study quality (using the overall assessment risk of bias); (b) study population characteristics (eg, gender, age and baseline HbA1c and SBP); (c) intervention type (EPOC taxonomy) and (d) study duration on the primary outcomes.29 Random effects meta-regression was performed using Stata version 13.27
Results
Overall 18 829 titles were screened and 42 full text articles met the inclusion criteria (figure 1: PRISMA flow diagram). All 42 studies were RCTs, encompassing 50 interventions in total, comprising 11 250 patients.22–24 30–68 No other eligible study designs were identified.
Figure 1.
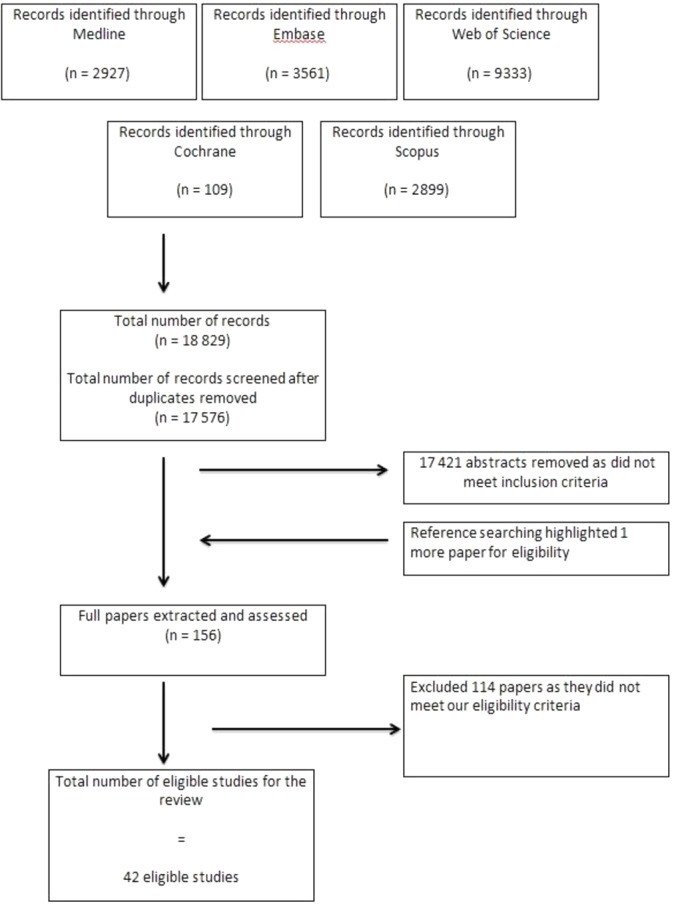
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow sheet.
Characteristics of studies
Twenty-nine of the 42 studies were conducted in USA, 9 in Europe, 2 in Australia, 1 in Mexico and 1 in Israel. Follow-up of outcomes in the studies varied in length from 353 to 36 months.46 The mean HbA1c at baseline across all studies was 9.5% (95% CI 9.3% to 9.8%). The mean age of patients in the studies was 58.0, varying from 47.9 (62) to 67.5 (41) partly reflecting different inclusion criteria (table 1). Thirty studies explicitly defined their study population as ‘poorly controlled’, ‘complicated’ or ‘persistently poorly controlled’, whereas the other 12 had poorly controlled T2DM with HbA1c ≥59 mmol/mol (7.5%) as per the review inclusion criteria. Twenty-seven of the 42 studies reported SBP results22–24 30–36 38 39 41 45 46 48–51 54 58–60 62 65 66 68 and of these, 23 reported DBP.22–24 31 32 34–36 38 39 41 45 46 48 49 51 54 58 59 62 65 66 68 Twenty of the studies reported a lipid outcome.23 24 30–32 35 36 38 39 41 45 46 48 51 56 58 62 65 66 68 All of the 42 studies reported at least one secondary outcome. Two studies were excluded from primary outcome analysis due to lack of appropriate data, despite efforts to contact authors.31 61
Table 1.
Characteristics of included studies
| Study ID Author Year Country |
Patient participants Total patients (n) Intervention (n) Control (n) Age (mean, unless stated) Gender (% male, unless stated) HbA1c cut-off of ‘poor control’ Baseline HbA1c level (mean) Baseline BP (mean) % on insulin at baseline Diabetes duration: (years) Practitioner and practice participants |
Brief intervention description | Predominant intervention type | Outcomes: Primary Secondary |
Study duration months |
| Anzaldo-Campos 2016 Mexico |
Patient participants 301 Patients (99 Intervention 1 (PD) and 102 in Intervention 2 (PD-TE) and 100 Control) Mean age: 51.5 % male: 33% T2DM with HbA1c≥8.0% Mean HbA1c: 11.16 Mean BP: 122/78 % insulin baseline: NR Mean diabetes duration: NR Practitioner and practice participants 81 medical offices within one Family Medical Unit Trained clinicians, nurses and peer educators |
Two interventions: Nurse care support and peer-led diabetes self-management education intervention (called Project Dulce). Nurse care support and peer-led diabetes self-management education intervention. A technology-enhanced intervention, using cell phone uploads of glucose and BP levels and text message support |
Patient-centred | Primary outcomes: HbA1c at 10 months Secondary outcomes: Lipid and TAG profile; BP; BMI Self-reported outcomes: Self-efficacy (Spanish self-efficacy); Depression (PHQ-9); Lifestyle (IMEVID); Quality of life (Diabetes 39); Diabetes knowledge (DKQ24) |
10 months |
| Basudev 2016 UK |
Patient participants 235 Patients (93 Intervention and 115 Control) Mean age: 59.9 % male: 57.4% T2DM with HbA1c>8.5% Mean HbA1c: 10.3 Mean BP: 135/78 % insulin baseline: 38% Mean diabetes duration: NR Practitioner and practice participants From six general practices in London |
Virtual clinic integrating primary and specialist care | Organisational | Primary outcomes: HbA1c at 12 months Secondary outcomes: BP; BMI; Lipids; Renal function (eGFR). |
12 months |
| Blackberry 2013 Victoria, Australia |
Patient participants 473 Patients (236 Intervention and 237 Control) Mean age: 62.8 % male: 57% T2DM with HbA1c>7.5% Mean HbA1c: 8.06 Mean BP: NR % insulin baseline: 27% Mean diabetes duration 10 (5–14 range) Practitioner and practice participants 59 practices Practice-based nurses |
Telephone coaching by nurses to support diabetes management and self-monitoring | Patient-centred | Primary outcomes: HbA1c at 18 months Secondary outcomes: Lipid and TAG profile; eGFR and urine ACR; BP; BMI; Waist circumference; Smoking status; Quality of life; Diabetes self-efficacy; Diabetes support; Depression status; Intensification of diabetes. Others: Health service utilisation; Physical activity; Nutrition |
18 months |
| Capozza 2015 USA |
Patient participants 93 patients (58 Intervention; 35 Control) Mean age: 58.7 % male: 35.5% T2DM with HbA1c>8% Mean baseline HbA1c 9.1% Mean baseline BP: NR % insulin baseline: NR Diabetes duration: NR Practitioner and practice participants Recruited from 18 primary clinics |
Text-message-based behavioural intervention for T2DM | Patient-centred | Primary outcome: Change in HbA1c from day 0 to day 180 Secondary outcomes: Patient interaction and satisfaction (CSQ8) with the programme |
6 months |
| Choe 2005 USA |
Patient participants 80 patients (41 Intervention and 39 Control) Age: 51.0 (all less 70) % male: 46% HbA1c≥8.o% Mean HbA1c 10.1 Mean BP: NR % insulin baseline: 30% Diabetes duration: NR Practitioner and practice participants one clinic one pharmacist case manager |
Pharmacist case management | Organisational. | Primary outcome: HbA1c level at 12 months Secondary outcomes: Rates of diabetes process measures (LDL, dilated retinal examination, urine ACR or use of ACE Inhibitors, monofilament testing for diabetic neuropathy, by chart review over 24 months); Rate of HbA1c measurement. |
12 month intervention with primary outcome reporting at 12 months and a further 24 month follow-up |
| Crowley 2015 USA |
Patient participants 50 patients (25 Intervention and 25 Control) Age: 60 % male: 24% HbA1c>9% Definition: Yes, defined as ‘persistently poor diabetes’ Mean HbA1c 10.5% Mean SBP: 127/80 % insulin baseline: NR Diabetes duration: 12 Practitioner and practice participants Patients all receiving care by Durham VA primary care and endocrinology |
Intensive telemedicine intervention for veterans | Organisational | Primary outcome: HbA1c Secondary outcomes: Diabetes self-management (self-care inventory revised); Depression (PHQ-9); Self-reported medication adherence (Morisky medication adherence); BP; Adverse events; Telephone encounters |
6 months |
| Dale 2009 England Exploratory RCT |
Patient participants 231 (90 (PS) Intervention 1, 44 (DSN) Intervention 2 and 97 Control) Age: No mean age provided, but wide spectrum of ages from below 50 to over 70 in each of the intervention and control groups. % male: 57% HbA1c≥7.5% Mean HbA1c: 8.6% Mean BP: NR % insulin baseline: 0% Diabetes duration: No mean, but between 1–15 years mostly. Practitioner and practice participants 29 practices Peer coaching or diabetes specialist nurse delivered |
Two intervention telecare groups: (a) Peer-support telecare intervention; (b) Diabetic specialist nurse telecare support | Patient-centred. | Primary outcome: Self-efficacy (DMSES) Secondary outcomes: HbA1c; Cholesterol; BMI. Diabetes distress (PAID) |
6 months |
| DePue 2013 US Territory of America Somoa Cluster RCT |
Patient participants 268 patients (104 Intervention and 164 Control) Age: 55 % male: 38% Intervention did not target poor control per se, mean baseline HbA1c of 9.6% (SD of 2.1%) was deemed eligible for inclusion Mean HbA1c 9.8 Mean BP: 133/84 % insulin baseline: NR Mean diabetes duration: NR Practitioner and practice participants Cluster RCT based on twelve village units Nurse care managers |
Nurse–Community Health Worker Team in American Somoa | Organisational. | Primary outcome: HbA1c Secondary outcomes: BP; BMI; Dietary intake; Medication adherence; Physical activity; Adapted measures of diabetes beliefs |
12 months |
| Edelman 2010 North Carolina and Virginia, USA. |
Patient participants 239 patients (133 Intervention and 106 Control) Age: 61.9 % male: 96% T2DM HbA1c>7.5 AND (SBP>140 DBP>90) Mean HbA1c: 9.2% Mean BP: 152/84 % insulin baseline: unclear Duration of diabetes: NR Practitioner and practice participants 2 VA centres A care team involving internist, pharmacist, a nurse and educator |
Enrolment into a GMC with an internist, pharmacist and a nurse or educator that met seven times over 12 months | Organisational. | Primary outcomes: HbA1c Secondary outcomes: Systolic blood pressure; Adherence to medications; Self-efficacy; Adverse events through structured self-report and medical record review; Health utilisation; Cost data |
12 months |
| Edelman 2015 USA |
Patient participants 377 patients (193 Intervention and 184 Control) Age: 58.7 % male: 45.4% HbA1c≥7.5 (and HTN) Mean HbA1c 9.1% Mean BP: 142.2/80.7 % insulin baseline: NR Diabetes duration: NR Practitioner and practice participants nine primary care practices in Duke. |
Nurse case management | Organisational | Primary outcome: HbA1c Secondary outcomes: BP; Weight; Physical activity; Self-efficacy; Health literacy; Medication adherence (via self-report) |
24 months |
| Farmer 2012 UK |
Patient participants 211 patients (126 Intervention and 85 Control) Age: 63.2 % male: 65% HbA1c≥7.5% Mean HbA1c: 8.3% Mean BP: 136.9/78.2 % insulin baseline: NR Mean diabetes duration: 6.8 years Practitioner and practice participants 13 practices Practice nurses |
Nurse-led, multilevel intervention to support medication adherence | Organisational | Primary outcome: % days over a 12-week period on which the correct number of doses of main glucose lowering medication was taken each day as prescribed. Secondary outcomes: Hba1c at 0 and 20 weeks (from protocol); Functional status as per SF 12 Physical and SF 12 Mental; Diabetes treatment satisfaction and satisfaction with nurse; MARS self-reported adherence (range 5–25); % reporting hypoglycaemia |
12 weeks (intervention was 8 weeks into a 20-week trial) |
| Forjouh 2014 USA |
Patient participants 376 patients (101 Intervention 1 (CDSMP), 81 Intervention 2 (PDA), 99 Intervention 3 (PDA, CDSMP and 95 Control) Age: 57.6 % male: 44.0% HbA1c>7.5% Mean HbA1c: 9.3 Mean BP: 134.8/77 % insulin baseline: NR Mean diabetes duration: NR Practitioner and practice participants seven practices involved Technology intervention |
Three intervention groups, reflecting the individual and combined effects of a behavioural and technology intervention; CDSMP and a diabetes self-care software on a PDA | Patient-centred | Primary outcome: HbA1c Secondary outcomes: BMI; BP; Self-management behavioural measures (eg, foot care) |
12 months |
| Frosch 2011 USA |
Patient participants 201 patients (100 Intervention and 101 Control) Age: 55.5 % male: 51.5% HbA1c>8.0 Mean HbA1c: 9.6% Mean BP: 127.7/74.0 % insulin baseline: NR Mean diabetes duration: 9.5 Practitioner and practice participants three academic primary care practices and one community based safety net clinic Nurse educators |
A video behavioural support intervention by nurse educators with a workbook followed by five sessions of telephone coaching | Patient-centred | Primary outcome: HbA1c Secondary outcomes: LDL cholesterol; BP; BMI; Prescribed medications; Diabetes knowledge (23 point diabetes knowledge test); Self-care behaviours (SDSCA) |
Unclear, possibly over 6 months |
| Guerci 2003 France |
Patient participants 988 patients (510 Intervention and 478 Control) Age: 60.6 % male: 53.7% HbA1c ≥ (7.5 and 11) diabetes. Mean HbA1c 8.95% Mean SBP: 139.6, 80.4 % insulin baseline: 0% Mean diabetes duration months: 96.6 Practitioner and practice participants 265 GPs involved, uncertain number of practices |
A self-monitoring of blood glucose intervention ASIA study |
Patient-centred | Primary outcome: HbA1c Secondary outcomes: Changes in fasting glucose; Symptomatic hypoglycaemia; BP; Weight; Diet; Drugs; Adverse drug event |
6 months |
| Heisler 2010 USA |
Patient participants 244 patients (126 Intervention and 119 Control (NCM)) Age: 62.0 % male: 100% HbA1c>7.5% Mean HbA1c 7.98 Mean BP: 138.4/76.5 % insulin baseline: 56% Diabetes duration: NR Practitioner and practice participants Two VA facilities Nurse and peer case managers |
Reciprocal peer support | Patient-centred | Primary outcome: HbA1c 6 months Secondary outcomes: Medication adherence; Diabetes emotional distress; Diabetes-specific social support; Medication changes; Attendance at clinics |
6 months |
| Jacobs 2012 USA |
Patient participants 396 patients (195 Intervention and 201 Control) Age: 62.9 % male: 50% HbA1c>8.0% Mean HbA1c 9.35 Mean BP: 138.7/78.9 % insulin baseline: NR Mean diabetes duration: NR Practitioner and practice participants five pharmacists, patients came from practices of 66 primary care physicians. |
A pharmacist assisted medication programme intervention | Organisational | Primary outcome: No specific primary outcome given or sample size Secondary outcomes: HbA1c<7%; LDL cholesterol<100 mg/dL; BP<130/80 mm Hg |
12 months |
| Jameson 2010 USA |
Patient participants 104 patients (52 Intervention and 52 Control) Age: 49.6 % male: 49% HbA1c≥9.0% (two of the population had T1DM) Mean HbA1c: 10.8% Mean BP: NR % insulin baseline: 49.6% Mean diabetes duration: NR Practitioner and practice participants one pharmacist. |
A pharmacist collaborative management intervention | Organisational | Primary outcome: HbA1c Secondary outcome: % of patients with a 1.0% decrease in HbA1c |
12 months |
| Jovanovic 2004 USA |
Patient participants 362 patients (186 Intervention and 172 Control) Age: 57.0 % male: 23.8% HbA1c>7.5 Mean HbA1c: 9.65% Mean BP: 135/79 % insulin baseline: NR Mean diabetes duration: 11.1 Practitioner and practice participants Unclear number of case managers and practices |
Diabetes case management by a nurse or dietician | Organisational | Primary outcome: HbA1c Secondary outcomes: % participants achieving HbA1c goals medication usage; BP; Lipids; BMI; Frequency of hypoglycaemia |
36 months |
| Keogh 2011 Ireland |
Patient participants 121 patients (60 Intervention and 61 Control) Age: 58.6 % male: 64% HbA1c≥8.0% Median HbA1c: 9.2 Mean BP: 138.8/76.8 % insulin baseline: 52% Mean diabetes duration: 9.4 Practitioner and practice participants One practice One psychologist |
Psychological family intervention | Organisational | Primary outcome: Hba1c Secondary outcomes: Illness perceptions (Brief illness Perception Questionnaire); Psychological well-being (12-item Well-Being questionnaire); BP; BMI; Diabetes self-management (Summary of Diabetes Self-care Activities Questionnaire); Self-Efficacy (UK version Diabetes Self-Efficacy Scale); Family support (Diabetes Family Behaviour Checklist) |
6 months |
| Kim 2009 USA |
Patient participants 83 patients (41 Intervention and 42 Control) Age: 56.4 % male: 55.4% HbA1c≥7.5% Mean HbA1c: 9.25% Mean BP 132.1/79.3 % insulin baseline: NR Mean diabetes duration: NR Practitioner and practice participants Uncertain number practices Community nurse delivered |
A community-based, culturally tailored behavioural intervention | Patient-centred | Primary outcome: HbA1c Secondary outcomes: DKT’ Self-efficacy (Stanford Chronic Disease Self-Efficacy scale); Self-care (Diabetes self-care activities (SDSCA); Depression (Kim Depression Scale for Korean Americans); Quality of Life (Diabetes Quality of Life Measure (DQOL); Lipids; BP; BMI |
30 weeks (7 months) 6-month intervention |
| Krein 2004 USA |
Patient participants 246 patients (123 Intervention and 123 Control) Age: 61 % male: 97% HbA1c≥7.5% Mean HbA1c 9.25 Mean BP: 145/86 % insulin baseline: 59% Mean diabetes duration: 11 Practitioner and practice participants One VA centre, unclear number of practices Two nurse case managers |
Case management by nurse practitioners | Organisational | Primary outcome: HbA1c Secondary outcomes: LDL; Cholesterol; BP; Health status; Patient satisfaction; Inpatient and outpatient encounters, pharmacy and laboratory use; Semistructured interviews also done |
18 months |
| Long 2012 USA |
Patient participants 118 patients (38 Intervention 1 (PM), 40 Intervention 2 (FI) and 39 Control) Age: 60 % male: 94% HbA1c>8.0% (two patients may have had T1DM) HbA1c Mean: 9.7 Mean BP: NR % insulin baseline: 74% Mean diabetes duration: NR Diabetes over 10 years: 58% Practitioner and practice participants Unclear number of practices Peer mentors |
Two interventions: Peer mentoring Financial incentivisation of patients |
Patient-centred | Primary outcome: Hba1c Secondary outcomes: Patient recollection of hypoglycaemic event |
6 months |
| Maislos 2002 Israel |
Patient participants 82 patients (48 Intervention and 34 Control) Age: 60.5 % male: 29.5% HbA1c≥10% Mean HbA1c 11.35 Mean BP: NR % insulin baseline: 20% Duration diabetes: 10 Practitioner and practice participants two practices involved via one mobile clinic |
A mobile clinic providing interdisciplinary care | Organisational | Primary outcome: Decrease of HbA1c of 0.5% at 6 months Secondary outcome: Compliance with study protocol at 6 months |
6 months |
| Mathers 2012 UK Cluster RCT |
Patient participants 175 patients (95 Intervention and 80 Control) Age: 64 % male: 54% HbA1c≥7.5 Mean HbA1c: 8.7% Mean BP: NR % insulin baseline: NR Duration diabetes: 7.8 Practitioner and practice participants 49 practices involved GPs and nurses from practices delivered intervention |
Patient decision aid to improve decision quality and glycaemic control | Professional | Primary outcome: HbA1c Secondary outcomes: Decisional conflict scale score- indicator of decision quality; Knowledge and realistic expectations of the risks and benefits; Regret scale |
6 months |
| McDermott 2015 Australia Cluster RCT |
Patient participants 213 patients (113 Intervention and 100 Control) Age: 47.9 % male: 37.6% HbA1c≥8.5 (69mmol/mol) Mean HbA1c 10.7 Mean BP: 131/79.3 % insulin baseline: 44.4% Diabetes duration: NR Practitioner and practice participants 12 remote communities in north Queensland. |
Community-based health-worker led case management approach to the care of Indigenous adults with poorly controlled T2DM in primary care services in remote northern Australia | Organisational | Primary outcome: HbA1c level at 18 months Secondary outcomes: BP; BMI; Lipids; Medications; ACR eGFR; TOFHLA; AQoL instrument; Implementation Fidelity |
18 months |
| McMahon 2005 USA |
Patient participants 104 patients (52 Intervention and 52 Control) Age: 63.5 % male: 99% HbA1c≥9% Mean HbA1c: 10.0% Mean BP: 140/81 % insulin baseline: 54% Duration diabetes: 12.3 years Practitioner and practice participants Practice number unclear Care manager available |
Web-based care management | Organisational | Primary outcome: HbA1c Secondary outcomes: SBP; DBP; TAG; LDL cholesterol; HDL cholesterol |
12 months |
| Mons 2013 Germany |
Patient participants 204 patients (103 Intervention and 101 Control) Age: 67.5 % male: 61% HbA1c>7.5% Mean HbA1c: 8.1% Mean BP: 137.5/80 % insulin baseline: NR Duration diabetes: NR Practitioner and practice participants 10 GP practices Practice nurses |
Supportive telephone counselling | Patient-centred | Primary outcome: HbA1c Secondary outcomes: SBP; DBP; Cholesterol; Health-related quality of life (Short Form General Health Survey: SF-12); Symptoms of depression: Geriatric depression scale |
18 months |
| O’Connor 2014 USA Cluster RCT |
Patient participants 1102 patients (569 Intervention and 533 Control) Age: 43%≥65 years.~61 mean % male: 51.3% HbA1c≥8% Mean HbA1c: 9.8% Mean BP: NR % insulin baseline: NR Diabetes duration: NR Practitioner and practice participants Large medical groups in California. Clusters defined on their linkage to primary care physicians. |
Telephone Outreach to Improve Medication Adherence and Metabolic Control in Adults With Diabetes | Organisational | Primary outcome: Medication adherence (at least one prescription fill within 60 days of prescription date) Secondary outcomes: Medication persistence (two or more prescription fills within 180 days); HbA1c; BP; Lipids |
6 months |
| Odegard 2005 USA |
Patient participants 77 patients (43 Intervention and 34 Control) Age: 51.8 % male: 57% HbA1c≥9.0% Mean HbA1c: 10.4% Mean BP: NR % insulin baseline: 32% Duration diabetes: 7.6 Practitioner and practice participants seven primary care clinics Pharmacists: Unclear number |
A pharmacist intervention care management intervention | Organisational | Primary outcome: HbA1c 12 months Secondary outcomes: Medication appropriateness (Medication Appropriate Index/MAI); Self-reported adherence by questionnaire |
6 month intervention but HbA1c at 12 months |
| Palmas 2014 USA |
Patient participants 360 patients (181 Intervention and 179 Control) Age: 57.6 % male: 38% HbA1c≥8.0% Mean HbA1c: 8.7% Mean BP: 136/81 % insulin baseline: NR Duration diabetes: NR Practitioner and practice participants Unclear number GP practices Two community health workers |
CHW intervention in an Hispanic population | Patient-centred | Primary outcome: HbA1c Secondary outcomes: SBP; DBP; LDL cholesterol; Medication adherence; Dosage and intensity; Physical activity; Diet; Depression |
12 months |
| Phillis- Tsimikas 2011 USA |
Patient participants 207 patients (104 Intervention and 103 Control) Age: 50.7 % male: 29.5% HbA1c>8.0% Mean HbA1c: 10.4% Mean BP: 122.6/75 Duration diabetes: NR % insulin baseline: NR Practitioner and practice participants Unclear number GP practices participating Peer educators |
Peer-led diabetes education programs in high-risk Mexican Americans | Patient-centred | Primary outcome: HbA1c Secondary outcomes: Lipids; BP; BMI; Self-management behaviours and depression (in separate publication) |
10-month intervention was 4 months and primary outcome was 6 months after this |
| Polonsky 2011 USA Cluster RCT |
Patient participants 499 patients (256 Intervention and 227 Control) Age: 55.8 % male: 53.2% HbA1c>7.5% Mean HbA1c: 8.9 Mean BP: NR % on insulin: 0% Duration diabetes: 7.6 Practitioner and practice participants 34 GP practices participating |
Self blood glucose monitoring | Patient-centred | Primary outcome: Hba1c Secondary outcomes: Treatment intensification; Total number of visits with medication or lifestyle modifications; Time to the first treatment change; Frequency of SMBG; GWB from WHO-5 Well-Being Index |
12 months |
| Protheroe 2016 UK Feasibility study |
Patient participants 76 Patients (37 Intervention and 39 Control) Mean age: 63.1 % male: 50.3% T2DM with HbA1c>7.5% Mean HbA1c: 9.3 Mean BP: NR % insulin baseline: NR Mean diabetes duration: 61%>5 years Practitioner and practice participants From six family doctor practices |
LHT interviews with patients, creating a self-management plan, with supportive phone calls | Organisational | Feasibility study Outcomes included: Deprivation; Health literacy; Diabetes self-care; Diabetes Quality of Life; Diabetes UK Scale Items; Health-related Quality of Life; Warwick- Edinburgh Mental Well-Being; Illness perception; Health status measure; Resource use, HbA1c |
7 months |
| Quinn 2011 USA Cluster RCT |
Patient participants Cluster trial, three intervention groups, one control 163 patients (Intervention 1 (CO) 23, Intervention 2 (CPP) 22, Intervention 3 (CPDS) 62 and Control 56) Age: 52.9 (weighted average) % male: 52.5% (weighted average) HbA1c≥7.5% Mean HbA1c: 9.4 Mean SBP: 131/NR % insulin baseline: NR Duration diabetes: 8.2 Practitioner and practice participants 26 GP practices participating |
Mobile phone-based treatment/behavioural coaching intervention | Patient-centred | Primary outcome: HbA1c Secondary outcomes: PHQ-9 questionnaire for depressive symptoms; Self-completion patient outcome instrument; Diabetes Distress Scale; BP; Lipids; Hypoglycaemic events; Hospitalisations and ED visits |
12 months |
| Rothman 2005 USA |
Patient participants 217 patients (112 Intervention and 105 Control) Age: 55.5 % male: 44% HbA1c≥8.0% Mean HbA1c: 11 Mean BP: 138.5/81 % insulin baseline: 39% Duration diabetes: 8.5 Practitioner and practice participants Three pharmacists |
A primary care-based disease management programme delivered by trained pharmacists | Organisational | Primary outcome: HbA1c Secondary outcomes: BP; Aspirin; Lipids; Diabetes Knowledge Satisfaction (Diabetes Treatment Satisfaction Questionnaire); Use of clinical services; Adverse events; Process measures (time spent with patients and medication changes) |
12 months |
| Schillinger 2009 USA |
Patient participants 339 patients (112 intervention 1 (ATSM), 113 intervention 2 (GVC) and 114 Control) Age: 56.1 % male: 41% HbA1c≥8.0% Mean HbA1c: 9.5% Mean BP: 140/77.3 % insulin baseline: 38% Duration diabetes: 9.5 Practitioner and practice participants Uncertain number GPs—in a safety net health system |
Two interventions: Self-Management Support via (a) ATSM and (b) GMVs |
Patient-centred | Primary outcome: Self-management behaviour Secondary outcomes: PACIC; Diabetes Quality Improvement Programme; Interpersonal Processes of Care for Diverse Populations (IPC) instrument; Self-management behaviour (foods, diets, exercise, self-monitoring); SF-12 instrument for QoL; Functional status-Likert scale; HbA1c; SBP; DBP; BMI |
12 months |
| Sen 2014 USA |
Patient participants 75 patients (21 Intervention 1 (low), 26 Intervention 2 (high) and 28 Control) Age: 54.3 % male: 36% HbA1c≥7.5% (90%–95% had T2DM from personal correspondence with author) Mean HbA1c 9.5% Mean BP: 132.9/86.1 % insulin baseline: NR Mean diabetes duration: NR Practitioner and practice participants one practice |
Financial incentives for home-based monitoring—two interventions | Financial | Primary outcome: Adherence over 3 months Secondary outcome: HbA1c |
12 weeks |
| Sugiyama 2015 USA |
Patient participants 516 patients (258 Intervention and 258 Control) Age: 63 % male: 30% HbA1c≥8.0% Mean HbA1c: 9.7 Mean BP: NR % insulin baseline: NR Diabetes duration: NR Practitioner and practice participants Participants were recruited from senior centres, churches, community clinics and Los Angeles County Community and Senior Service Centres |
Diabetes self-management education by trained health educators. | Patient-centred | Primary outcome: HbA1c Secondary outcomes: Change Mental Component Summary Score (MCS-12) from the SF-12; Social support score from the Diabetes Care Profile |
6 months |
| Tang 2013 USA |
Patient participants 415 patients (203 Intervention and 213 Control) Age: 54 % male: 60% HbA1c≥7.5% Mean HbA1c: 9.3 Mean BP: 126.6/72.7 % insulin baseline: NR Mean diabetes duration: NR Practitioner and practice participants Uncertain number practices |
Online disease management of diabetes | Patient-centred | Primary outcome: HbA1c Secondary outcomes: SBP; DBP; LDL; 10-year Framingham risk; Satisfaction; Psychosocial well-being; Healthcare utilisation |
12 months |
| Taylor 2003 USA |
Patient participants 169 patients (84 intervention and 85 control) Age: 55.2 % male: 52.7% HbA1c>10.0% Mean HbA1c: 9.5% Mean BP: 127.5/72.8 % insulin baseline: NR Mean diabetes duration NR Practitioner and practice participants Uncertain number practices Nurse care managers |
NCM | Organisational | Primary outcome: % of patients in ‘target’ HbA1c Secondary outcomes: Total cholesterol; HDL cholesterol; LDL cholesterol; TAGs; Glucose; Microalbuminuria; SBP; DBP; Processes of care (foot, eye, dental examination and influenza shot); Psychosocial (SF 26 for QoL and Duke Activity Status); Patient and physician satisfaction; Medical utilisation (physician visits) |
12 months |
| Thom 2013 USA |
Patient participants 299 patients (151 Intervention and 148 Control) Age: 55.2 % male: 47.8% HbA1c≥8.0% Mean HbA1c: 10.0 Mean BP: 143.2/NR % insulin baseline: 55% Mean diabetes duration: 8.9 Practitioner and practice participants six practices included Peer coaches |
Peer health coaching | Patient-centred | Primary outcome: HbA1c Secondary outcomes: % patients whose HbA1c dropped 1%; % patients with a HbA1c less 7.5; LDL; SBP; BMI |
6 months |
| Wild 2016 UK |
Patient participants 231 Patients (160 Intervention and 161 Control) Mean age: 61 % male: 66.8% T2DM with HbA1c>7.5% Mean HbA1c: 8.9 Mean BP: 134/79 % insulin baseline: 26% Mean diabetes duration 7.4 Practitioner and practice participants From 44 practices from four UK regions |
Supported telemonitoring involving twice-weekly self-measurement of glucose and transmission to a general practitioner | Patient-centred | Primary outcome: HbA1c at 9 months Secondary outcomes: BP; BMI; Lipid and TAG profile; eGFR and urine ACR; UKPDS risk score; Anxiety and Depression Score; Quality of life; Diabetes Self-efficacy; Self-reported physical activity, alcohol intake, exercise tolerance and diabetes knowledge; Healthcare utilisation |
9 months |
ACR, albumin–creatinine ratio; AQoL, assessment of quality of life; ASIA, auto-surveillance intervention active; ATSM, automated telephone self-management support; BMI, body mass index; BP, blood pressure; CDSMP, chronic disease self-management programme; CHW, community health worker; CO, coach-only; CPDS, coach primary care provider portal with decision support; CPP, coach primary care physician portal; CSQ8, client satisfaction scale 8; DBP, diastolic blood pressure; DKT, diabetes knowledge test; DMSES, diabetes management self efficacy scale; DQOL, diabetes quality of life measure; DSN, diabetes specialist nurse; ED, emergency department; eGFR, estimated glomerular filtration rate; FI, financial incentivisation; GMC, general medical clinic; GMV, group medical visits; GWB, global well-being; HDL, high-density lipoprotein; HTN, hypertension; IPC, Interpersonal Processes of Care for Diverse Populations; LDL, low-density lipoprotein; LHT, lay health trainer; MAI, medication appropriate index; MARS, medication adherence rating scale; MCS-12, mental component summary score; NCM, Nurse care management; NR, not recorded; PACIC, Patient assessment of chronic illness care; PAID, problems areas in diabetes scale; PDA, personal digital assistant; PD-TE, Project Dulce technology-enhanced; PHQ-9, Patient Health Questionnaire 9; PM, peer mentoring; PS, peer support; SBP, systolic blood pressure; SDSCA, Summary of Diabetes Self-care Behaviours Scale; SF-12, Short-Form general health survey; SMBG, self-monitoring blood glucose; TAG, Triacylglycerides; T2DM, type 2 diabetes mellitus; TOFHLA, test of functional health literacy for adults; UKPDS, UK prospective diabetes study; VA, veteran’s affairs.
Interventions were all complex with multiple components. Studies were categorised based on the predominant intervention element using the EPOC taxonomy. The included interventions were categorised as predominantly patient-centred (n=20, 48%), organisational (n=20, 48%), financial (n=1, 2%) or professional (n=1, 2%). One study44 comprised two intervention arms with a patient-centred and financial intervention (included as a patient-centred predominant intervention in our analysis). Descriptions of the interventions are outlined in table 1.
The 20 patient-centred interventions in our review included 4 telephone-based,34 41 56 58 5 computerised/mobile phone-based,32 36 52 61 68 1 video-based,51 5 peer-support-based,30 38 44 49 65 3 self-monitoring-based37 50 64 and 2 -culturally supportive self-management interventions.39 45 The 20 organisational interventions included 5 pharmacist interventions performing case management,35 40 47 48 57 6 nurse case management interventions,23 31 46 53 55 60 3 web-based/telemedicine/telephone case management interventions,24 59 63 3 new-clinic-based interventions,43 54 66 1 community health-worker intervention,62 1 psychological intervention22 and 1 lay health worker intervention.67 Eight interventions had an mHealth or telehealth component.33 36 45 52 56 59 65 68 More detailed descriptions of the interventions are outlined in online supplementary appendix 3.
bmjopen-2016-015135supp003.pdf (182.1KB, pdf)
Risk of bias
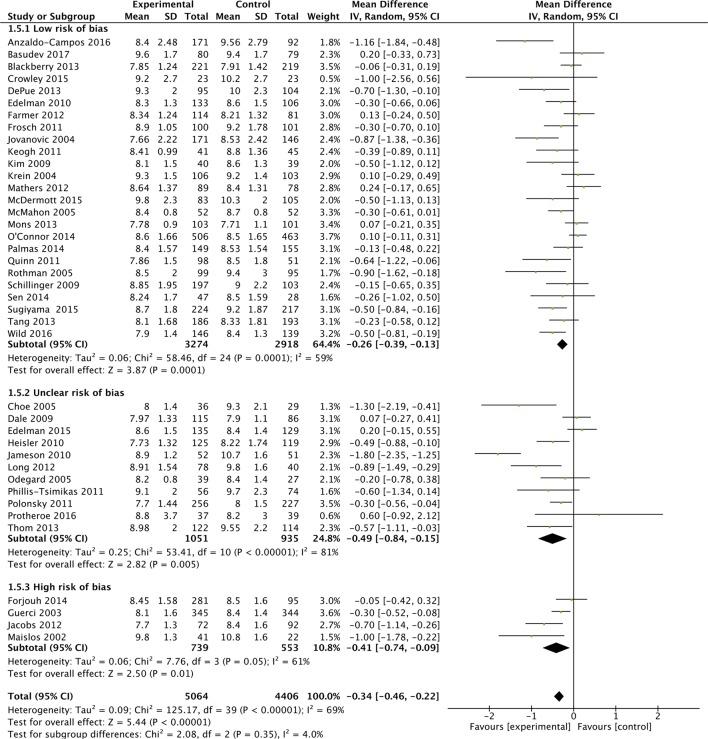
All 42 studies were RCTs, with six being cluster RCTs. Overall, 25 studies were classified as having a predominant low-risk of bias (59.5%),22–24 32–36 39 41 42 45 46 51 53–55 58 59 62–66 68 13 studies had an unclear-risk (31%)30 31 37 38 40 44 47 49 56 57 60 61 67 and 4 RCTs were classified as having a high-risk of bias (9.5%)43 48 50 52 (see online supplementary appendix 4). Blinding of outcome assessment was classified as low-risk in all studies. Attrition bias was evident in seven studies. Online supplementary appendix 5 outlines the summary judgements for both overall risk of bias and predominant intervention type, which were used in the meta-regression analysis.
bmjopen-2016-015135supp004.jpg (455.4KB, jpg)
bmjopen-2016-015135supp005.pdf (55.1KB, pdf)
There was no evidence of publication bias in the studies included in the HbA1c (p=0.37) or SBP analysis (p=0.54). However, there was some evidence of publication bias in the studies included in the DBP analysis (p<0.01) (see online supplementary appendixes 6(a) and 6(b)).
bmjopen-2016-015135supp006.jpg (265.1KB, jpg)
bmjopen-2016-015135supp007.jpg (249.5KB, jpg)
Primary outcomes
HbA1c
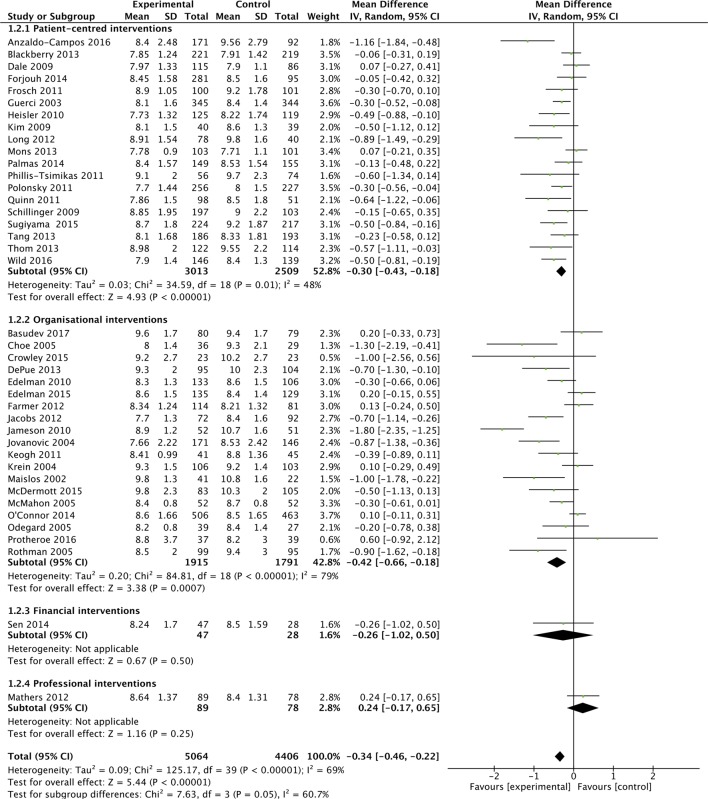
Overall 40 of the 42 studies were included in a meta-analysis, which found a MD in HbA1c of −3.7 mmol/mol (−0.34%; 95% CI −0.46% to −0.22%) favouring intervention groups, but with statistical heterogeneity (I2=69%). Figure 2A outlines the overall effect of interventions on HbA1c, across EPOC categories.
Figure 2A.
Effects of interventions on HbA1c, with intervention-type subgroups.
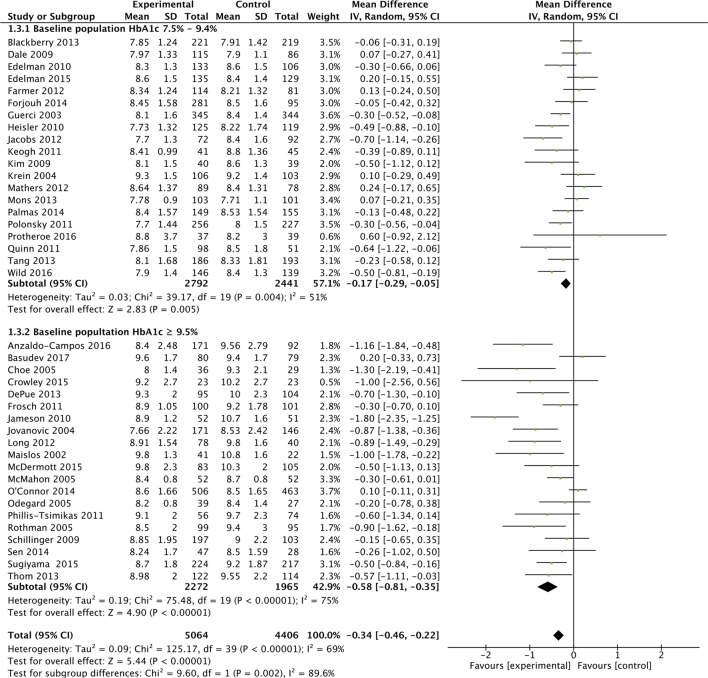
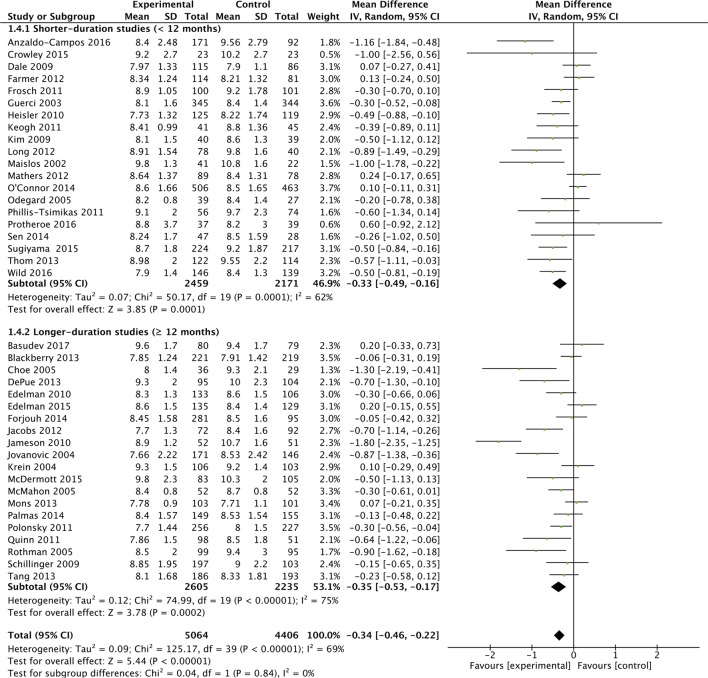
Subgroup analyses were performed based on the predominant intervention type (figure 2A), the baseline HbA1c level (figure 2B), study duration (figure 2C) and study quality (figure 2D).
Figure 2B.
Effects of interventions on HbA1c, with baseline HbA1c subgroup.
Figure 2C.
Effects of interventions on HbA1c, with baseline study duration subgroups.
Figure 2D.
Effects of interventions on HbA1c, with baseline study quality subgroup.
These analyses suggested that organisational interventions (MD in HbA1c of −5.2 mmol/mol (−0.42%; 95% CI −0.66% to −0.18%; I2=79%) had better improvements in HbA1c than patient-centred interventions (−0.30%; 95% CI −0.43% to −0.18%; I2=48%) (p=0.05). Similarly interventions performed when the baseline population-HbA1c was over 80 mmol/mol (9.5%) (MD in HbA1c of −6.3 mmol/mol (−0.58%; 95% CI −0.81% to −0.35%; I2=75%) had better improvements in HbA1c than populations with a baseline-HbA1c<9.5% (−0.17%%; 95% CI −0.29% to −0.05%%; I2=51%) (p=0.002). Study duration did not appear to affect HbA1c (figure 2C). Lastly, studies with a low-risk of bias (MD in HbA1c was −2.8 mmol/mol (−0.26%; 95% CI −0.39% to −0.13%; I2=59%) appeared to have a smaller reduction in HbA1c compared with unclear (−0.49%%; 95% CI −0.84%% to −0.15%; I2=81%) and high-risk studies (−0.41%; 95% CI −0.74% to −0.09%; I2=61%), but there was no evidence of a statistically significant difference (p=0.35). Though not considered in our original protocol, subgroup analysis did not highlight additional benefit from those interventions (included in both organisational and patient-centred intervention types), which had a telemedicine or mHealth component (see online supplementary appendix 7).33 36 45 52 56 59 65 68
bmjopen-2016-015135supp008.jpg (807.5KB, jpg)
As the overall results showed statistical heterogeneity, meta-regression analysis was also conducted to explore the components of this heterogeneity. As with the meta-analyses, higher baseline HbA1c was associated with a greater reduction in HbA1c (β-Coefficient: −0.27; 95% CI −0.41 to –0.13; p<0.001). The predominant-intervention type, risk of bias and study-duration were not associated with improved glycaemic control.
Blood pressure
Overall there was small improvement in SBP in the 26 interventions included in the meta-analysis, (MD SBP: –1.13 mm Hg (95% CI −2.19 to –0.08)) with moderate heterogeneity (I2=47%) (see online supplementary appendix 8).22–24 30–36 38 39 41 45 46 48–51 54 58–60 62 65 66 68 DBP improved modestly in the 22 studies included in the meta-analysis (MD DBP: –1.37 mm Hg (95% CI −2.25 to –0.50)) with moderate heterogeneity (I2=44%) (see online supplementary appendix 9).22–24 31 32 34–36 38 39 41 45 46 48 49 51 54 58 59 62 65 66 68
bmjopen-2016-015135supp009.jpg (645.1KB, jpg)
bmjopen-2016-015135supp010.jpg (549.2KB, jpg)
In the subgroup analysis, organisational interventions appeared to improve SBP modestly (MD SBP: –2.69 mm Hg; 95% CI −5.11 to –0.26; I2=57%) compared with patient-centred interventions (MD SBP: –0.52 mm Hg; 95% CI −1.41 to 0.38; I2=20%) which showed no statistically significant improvement (see online supplementary appendix 8). However, there was no evidence of a statistically significant difference between intervention types. Similarly with DBP, organisational interventions appeared to improve DBP modestly (MD DBP: −2.87 mm Hg; 95% CI −4.29 to –1.45; I2=30%) compared with patient-centred interventions (MD DBP: −1.37 mm Hg; 95% CI −1.42 to 0.2; I2=30%) (see online supplementary appendix 9) and there was evidence of a statistically significant difference (p=0.007). Meta-regression analysis was not conducted for SBP or DBP, as significant heterogeneity was not present on the overall effect sizes.
Lipids
Twenty of the 42 studies reported total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol or triacylglicerides.23 24 30–32 35 36 38 39 41 45 46 48 51 56 58 62 65 66 68 Statistically significant improvements in lipids were only demonstrated in 4 of these 20 studies.31 32 45 48 Baseline lipid levels were generally not reported. Eleven of the 20 studies reported data relating to total cholesterol. Meta-analysis was undertaken on these studies, which indicated a modest improvement in total cholesterol, favouring intervention groups (MD total cholesterol – 4.29 mg/dL (95% CI −7.68 to –0.89); I2=0%) (see online supplementary appendix 10).35 36 38 41 45 46 58 62 65 66 68
bmjopen-2016-015135supp011.jpg (285.3KB, jpg)
Secondary outcomes
All but 1 of the 42 included studies reported at least one of the eligible secondary outcomes (see online supplementary appendix 11). Overall, interventions had very limited effect on secondary outcomes. Twenty-six studies reported other physical outcomes (eg, body mass index (BMI) and estimated glomerular filtration rate). Of the 15 studies that reported on weight or BMI, only one showed significant improvement.56 Ten studies reported mental health outcomes36 38 41 45 58 59 64 with two showing a significant improvement in the Change Mental Component Summary Score and the Short Form-12 Mental Health Score.64 67 Twenty-eight studies reported PROMs, with 11 showing an improvement with the intervention. Ten studies reported medication adherence outcomes, with two showing improvement. Eighteen studies reported utilisation outcomes, with four improving processes of care.
bmjopen-2016-015135supp012.pdf (138.5KB, pdf)
Discussion
Statement of principle findings
Healthcare interventions have positive, although modest, effects on HbA1c in poorly controlled T2DM. Interventions targeting those with a higher baseline HbA1c (≥80 mmol/mol (9.5%)) show the greatest effects. There was also evidence of a modest impact on both blood pressure and lipids, though baseline control of these risk factors was generally good. Generally, little effect on secondary outcomes was found. Our results suggest that a targeted approach to T2DM management, focussing on individuals with very poor glycaemic control, may represent a prudent strategy for future management.
Strengths and weaknesses of the study
The methodology of our systematic review addresses key credibility issues.69 70 The research question was sensible, our search of the literature was exhaustive and our results are outlined clearly for primary and secondary outcomes. The effect of baseline HbA1c was consistent across studies, biologically plausible and was an a priori hypothesis.70
We performed meta-regression to explore the heterogeneity, which also confirmed the increased effectiveness of interventions on those with HbA1c ≥80 mmol/mol (9.5%). However, a major limitation is that meta-regression is usually underpowered to detect anything but very large associations. Meta-regression considers the interactions between trial level covariates and the treatment effect, but it inherits difficulties of interpretation attached to non-randomised studies, as it is not possible to randomise patients to one covariate value or another, so causality cannot be attached its findings.71 Though we do not believe the subgroup findings occurred by chance, there remained high heterogeneity and we explored between-study comparisons rather than within-study comparisons.70 There was some evidence of publication bias in the DBP analysis, but this was not present for the 22 studies reporting SBP. It should also be noted that the power of Egger’s test is low when the number of studies is small and should only be used if the analysis includes a range of study sizes.
This study will inform researchers regarding the range of interventions that have been deployed to target patients with poorly controlled T2DM. There is no specific definition for ‘poor control’ of T2DM in the literature, but by including all studies that had patients with a HbA1c >59 mmol/mol (7.5%), we captured the full range of poor glycaemic control. Studies examining poor control of HbA1c possess a risk of regression towards the mean. However, all included studies were RCTs with control groups, which should have accounted for this. Targeted interventions in poorly controlled T2DM need to be distinguished from interventions, which are designed to intensively reduce HbA1c in all patients. Though persons with very poor glycaemic control are also at risk of the adverse effects of hypoglycaemic agents, targeting this population is more likely to reach the right balance of reducing harms of overtreatment and maximising potential benefits.18 The relative importance of targeting glycaemic or cardiovascular risk has been debated in the literature.17 We did not account for medication use in the studies, but given that all included studies were RCTs, which would balance out delivery of medications, we think that differences relating to underlying medication usage relate to how different interventions types promote the intensification of medications.
Comparison with other studies
The existing literature examining healthcare interventions to improve glycaemic control has focused on a range of approaches. There have been systematic reviews of interventions including QI initiatives, education, self-management support, case-management, adherence to medication and professional interventions, though as outlined previously, most have not specifically targeted patients with poor glycaemic control.8 10 11
A synthesis of 27 systematic reviews and 347 RCTs identified the cost-effectiveness of self-management interventions in T2DM in all patients with T2DM.72 This overview included studies that targeted all patients with T2DM and found very good evidence that education improves blood glucose control in patients with T2DM in the short term (less than 12 months) and that behavioural and psychological interventions are associated with modest improvements in blood glucose control (HbA1C).72 73 A review of computer-based diabetes self-management interventions to manage T2DM reported a small beneficial effect on blood glucose control (MD of −0.2%).74 Another recent systematic review of 118 self-management interventions found improvements in HbA1c in 62% of studies. The overall mean effect was to reduce HbA1c by −0.57%, although patients with persistently elevated HbA1c over 9 had greater improvements.75 In our review, patient-orientated interventions, such as self-monitoring of blood glucose and self-management interventions, seemed to be less effective than organisational interventions.
Case management by nurses and other professionals and case management in socially disadvantaged have been shown to be beneficial when targeted at all patients with T2DM and our review supports this conclusion for poorly controlled populations.5 76–78 Pharmacist-based interventions have been studied, mainly in outpatient settings or in US primary care and have been found to be effective and cost-effective.79 80 The five pharmacist interventions in our review, targeting patients with poorly controlled T2DM, showed mixed results, but overall had predominantly positive effects on HbA1c.
Attention to, and reporting of, intensification of antidiabetic medications and patient’s adherence to treatment regimens are needed to achieve optimal glycaemic control.81 82 Evidence regarding adherence in T2DM is mixed. A previous systematic review of 21 studies that included 14 RCTs to enhance T2DM treatment adherence in community and hospital settings found that few studies measured or assessed adherence and that interventions to improve adherence did not show benefits or harms.83 A review by Farmer et al found limited evidence of effect for interventions promoting the monitoring of medication use and brief messaging to support medication adherence in patients with T2DM, though the included studies did not specifically target patients with poorly controlled diabetes.84 Only 10 of the 42 included studies in our review looked at adherence to medications as an outcome and only 2 of these 9 studies had a statistically significant effect on adherence.49 62 The baseline level of adherence varied considerably and studies used different scale ranges.
Our review identified only one professional-based interventions in poorly controlled T2DM, through a physician decision aid.42 Two systematic reviews have examined the impact of clinical decision support systems (CDSS) on the management of T2DM in primary care, between them looking at 28 trials, with varying results but none of these CDSS interventions were designed to promote intensification of prescribing in persons with poor glycaemic control.85 86
Future research
There is a need for further research examining professional-based interventions in poorly controlled T2DM, such as CDSS, which promote intensification of medications.81 Studies from jurisdictions outside North America on poorly controlled populations would also be welcome. An individual patient data meta-analysis would answer further questions not possible in this review and future research should attempt to obtain individual-level patient data. It is likely that most successful interventions have their impact as a result of intensification of medicines and/or improving adherence to medicines.81 As adherence was not measured in most of the studies and intensification poorly documented, it is important that future interventions report on these findings. Furthermore organisational interventions could incur significant costs to a health system, so cost-effectiveness analyses on future interventions should be undertaken to ensure the modest improvements in HbA1c are beneficial for the health systems.
In conclusion, clinicians and policy makers, when considering organisation of care for T2DM, should focus their effects on those patients with very poor glycaemic control (≥80 mmol/mol (9.5%)). Prioritising interventions that emphasise structured organisation of care, which can include intensification and adherence to medications, also seem more likely to deliver optimal results in terms of glycaemic control for T2DM patients.
Supplementary Material
Footnotes
Contributors: All authors contributed to the drafting of the paper. MEM and RG independently assessed studies for eligibility, extracted data and assessed study quality. Decisions or disagreements were brought to SMS. SMS, TF and FB provided methodological and statistical support to the paper.
Funding: This work was supported by the HRB Centre for Primary Care Research (Research Grant: HRC-2014-1), a publicly funded body. Four of the six study authors are employed by this agency.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: All collected data has been supplied as Supplementary Files. Please contact the corresponding author (MEM) if there are queries regarding this data.
References
- 1. Wild S, Roglic G, Green A, et al. . Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004;27:1047–53. 10.2337/diacare.27.5.1047 [DOI] [PubMed] [Google Scholar]
- 2. Spann SJ, Nutting PA, Galliher JM, et al. . Management of type 2 diabetes in the primary care setting: a practice-based research network study. Ann Fam Med 2006;4:23–31. 10.1370/afm.420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Campbell DJ, McGrady M, Prior DL, et al. . Most individuals with treated blood pressures above target receive only one or two antihypertensive drug classes. Intern Med J 2013;43:137–43. 10.1111/j.1445-5994.2012.02927.x [DOI] [PubMed] [Google Scholar]
- 4. Stratton IM, Adler AI, Neil HA, et al. . Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000;321:405–12. 10.1136/bmj.321.7258.405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stellefson M, Dipnarine K, Stopka C. The chronic care model and diabetes management in US primary care settings: a systematic review. Prev Chronic Dis 2013;10:E26 10.5888/pcd10.120180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mays N. Reducing unwarranted variations in healthcare in the English NHS. BMJ 2011;342:d1849 10.1136/bmj.d1849 [DOI] [PubMed] [Google Scholar]
- 7. Simmons RK, Carlsen AH, Griffin SJ, et al. . Variation in prescribing of lipid-lowering medication in primary care is associated with incidence of cardiovascular disease and all-cause mortality in people with screen-detected diabetes: findings from the ADDITION-Denmark trial. Diabet Med 2014;31:1577–85. 10.1111/dme.12574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Seitz P, Rosemann T, Gensichen J, et al. . Interventions in primary care to improve cardiovascular risk factors and glycated haemoglobin (HbA1c) levels in patients with diabetes: a systematic review. Diabetes Obes Metab 2011;13:479–89. 10.1111/j.1463-1326.2010.01347.x [DOI] [PubMed] [Google Scholar]
- 9. Renders CM, Valk GD, Griffin SJ, et al. . Interventions to improve the management of diabetes in primary care, outpatient, and community settings: a systematic review. Diabetes Care 2001;24:1821–33. 10.2337/diacare.24.10.1821 [DOI] [PubMed] [Google Scholar]
- 10. Seidu S, Walker NS, Bodicoat DH, et al. . A systematic review of interventions targeting primary care or community based professionals on cardio-metabolic risk factor control in people with diabetes. Diabetes Res Clin Pract 2016;113:1–13. 10.1016/j.diabres.2016.01.022 [DOI] [PubMed] [Google Scholar]
- 11. Tricco AC, Ivers NM, Grimshaw JM, et al. . Effectiveness of quality improvement strategies on the management of diabetes: a systematic review and meta-analysis. Lancet 2012;379:2252–61. 10.1016/S0140-6736(12)60480-2 [DOI] [PubMed] [Google Scholar]
- 12. Patel A, MacMahon S, Chalmers J, et al. . Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358:2560–72. 10.1056/NEJMoa0802987 [DOI] [PubMed] [Google Scholar]
- 13. Gerstein HC, Miller ME, Byington RP, et al. . Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–59. 10.1056/NEJMoa0802743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Duckworth W, Abraira C, Moritz T, et al. . Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009;360:129–39. 10.1056/NEJMoa0808431 [DOI] [PubMed] [Google Scholar]
- 15. Turnbull FM, Abraira C, Anderson RJ, et al. . Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia 2009;52:2288–98. 10.1007/s00125-009-1470-0 [DOI] [PubMed] [Google Scholar]
- 16. Skyler JS, Bergenstal R, Bonow RO, et al. . Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA Diabetes Trials: a position statement of the american Diabetes Association and a Scientific Statement of the American College of Cardiology Foundation and the american Heart Association. J Am Coll Cardiol 2009;53:298–304. 10.1016/j.jacc.2008.10.008 [DOI] [PubMed] [Google Scholar]
- 17. Hayward RA, Reaven PD, Wiitala WL, et al. . Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015;372:2197–206. 10.1056/NEJMoa1414266 [DOI] [PubMed] [Google Scholar]
- 18. Hayward RA. Excessive testing of adults with type 2 diabetes. BMJ 2015;351:h6549 10.1136/bmj.h6549 [DOI] [PubMed] [Google Scholar]
- 19. Mossello E. Targeting vascular risk factors in older adults: from Polypill to Personalized Prevention. JAMA Intern Med 2015;175:1949–50. 10.1001/jamainternmed.2015.5941 [DOI] [PubMed] [Google Scholar]
- 20. Murphy M, Galvin R, Fahey T, et al. . Effectiveness of interventions in primary care to improve glycated haemoglobin (HbA1c) and cardiovascular risk factor levels in patients with poorly-controlled type 2 diabetes mellitus: a systematic review. PROSPERO 2014. CRD42014014442. [Google Scholar]
- 21. Effective practice and Organisation of Care. EPOC Intervention types. Norwegian Knowledge Centre for the Health Services. 2015. accessed on 13 April 2016 https://epoc.cochrane.org/sites/epoc.cochrane.org/files/uploads/EPOC.
- 22. Keogh KM, Smith SM, White P, et al. . Psychological family intervention for poorly controlled type 2 diabetes. Am J Manag Care 2011;17:105–13. [PubMed] [Google Scholar]
- 23. Krein SL, Klamerus ML, Vijan S, et al. . Case management for patients with poorly controlled diabetes: a randomized trial. Am J Med 2004;116:732–9. 10.1016/j.amjmed.2003.11.028 [DOI] [PubMed] [Google Scholar]
- 24. McMahon GT, Gomes HE, Hickson Hohne S, Hohne SH, et al. . Web-based care management in patients with poorly controlled diabetes. Diabetes Care 2005;28:1624–9. 10.2337/diacare.28.7.1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vanselow NA. A New Definition of Primary Care. JAMA: The Journal of the American Medical Association 1995;273:192 10.1001/jama.1995.03520270026023 7807653 [DOI] [Google Scholar]
- 26. Effective practice and Organisation of Care (EPOC). Summary assessments of the risk of Bias. EPOC Resources for review authors Oslo: Norwegian Knowledge Centre for the Health Services 20132016. http://epoc.cochrane.org/sites/epoc.cochrane.org/files/uploads/16. [Google Scholar]
- 27. StataCorp. Stata Statistical Software: release 13. College Station, TX: StataCorp, 2013. LP. [Google Scholar]
- 28. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 29. Thompson SG, Higgins JP. How should meta-regression analyses be undertaken and interpreted? Stat Med 2002;21:1559–73. 10.1002/sim.1187 [DOI] [PubMed] [Google Scholar]
- 30. Thom DH, Ghorob A, Hessler D, et al. . Impact of peer health coaching on glycemic control in low-income patients with diabetes: a randomized controlled trial. Ann Fam Med 2013;11:137–44. 10.1370/afm.1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Taylor CB, Miller NH, Reilly KR, et al. . Evaluation of a nurse-care management system to improve outcomes in patients with complicated diabetes. Diabetes Care 2003;26:1058–63. 10.2337/diacare.26.4.1058 [DOI] [PubMed] [Google Scholar]
- 32. Tang PC, Overhage JM, Chan AS, et al. . Online disease management of diabetes: engaging and motivating patients online with enhanced resources-diabetes (EMPOWER-D), a randomized controlled trial. J Am Med Inform Assoc 2013;20:526–34. 10.1136/amiajnl-2012-001263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sen AP, Sewell TB, Riley EB, et al. . Financial incentives for home-based health monitoring: a randomized controlled trial. J Gen Intern Med 2014;29:770–7. 10.1007/s11606-014-2778-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schillinger D, Handley M, Wang F, et al. . Effects of self-management support on structure, process, and outcomes among vulnerable patients with diabetes: a three-arm practical clinical trial. Diabetes Care 2009;32:559–66. 10.2337/dc08-0787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rothman RL, Malone R, Bryant B, et al. . A randomized trial of a primary care-based disease management program to improve cardiovascular risk factors and glycated hemoglobin levels in patients with diabetes. Am J Med 2005;118:276–84. 10.1016/j.amjmed.2004.09.017 [DOI] [PubMed] [Google Scholar]
- 36. Quinn CC, Shardell MD, Terrin ML, et al. . Cluster-randomized trial of a mobile phone personalized behavioral intervention for blood glucose control. Diabetes Care 2011;34:1934–42. 10.2337/dc11-0366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Polonsky WH, Fisher L, Schikman CH, et al. . A structured self-monitoring of blood glucose approach in type 2 diabetes encourages more frequent, intensive, and effective physician interventions: results from the STeP study. Diabetes Technol Ther 2011;13:797–802. 10.1089/dia.2011.0073 [DOI] [PubMed] [Google Scholar]
- 38. Philis-Tsimikas A, Fortmann A, Lleva-Ocana L, et al. . Peer-led diabetes education programs in high-risk mexican Americans improve glycemic control compared with standard approaches: a project Dulce Promotora randomized trial. Diabetes Care 2011;34:1926–31. 10.2337/dc10-2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Palmas W, Findley SE, Mejia M, et al. . Results of the Northern Manhattan Diabetes community outreach project: a Randomized trial studying a community health worker intervention to improve diabetes care in Hispanic adults. Diabetes Care 2014;37:963–9. 10.2337/dc13-2142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Odegard PS, Goo A, Hummel J, et al. . Caring for poorly controlled diabetes mellitus: a randomized pharmacist intervention. Ann Pharmacother 2005;39:433–40. 10.1345/aph.1E438 [DOI] [PubMed] [Google Scholar]
- 41. Mons U, Raum E, Krämer HU, et al. . Effectiveness of a Supportive Telephone counseling intervention in type 2 Diabetes Patients: randomized Controlled Study. PLoS One 2013;8:e77954 10.1371/journal.pone.0077954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mathers N, Ng CJ, Campbell MJ, et al. . Clinical effectiveness of a patient decision aid to improve decision quality and glycaemic control in people with diabetes making treatment choices: a cluster randomised controlled trial (PANDAs) in general practice. BMJ Open 2012;2:e001469 10.1136/bmjopen-2012-001469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Maislos M, Weisman D. Multidisciplinary approach to patients with poorly controlled type 2 diabetes mellitus: a prospective, randomized study. Acta Diabetol 2004;41:44–8. 10.1007/s00592-004-0143-1 [DOI] [PubMed] [Google Scholar]
- 44. Long JA, Jahnle EC, Richardson DM, et al. . Peer mentoring and financial incentives to improve glucose control in african american veterans: a randomized trial. Ann Intern Med 2012;156:416–24. 10.7326/0003-4819-156-6-201203200-00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kim MT, Han HR, Song HJ, et al. . A community-based, culturally tailored behavioral intervention for korean Americans with type 2 diabetes. Diabetes Educ 2009;35:986–94. 10.1177/0145721709345774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jovanovic L. California Medi-Cal Type 2 Diabetes Study Group. Closing the gap: effect of diabetes case management on glycemic control among low-income ethnic minority populations: the California Medi-Cal type 2 diabetes study. Diabetes Care 2004;27:95–103. [DOI] [PubMed] [Google Scholar]
- 47. Jameson JP, Baty PJ. Pharmacist collaborative management of poorly controlled diabetes mellitus: a randomized controlled trial. Am J Manag Care 2010;16:250–5. [PubMed] [Google Scholar]
- 48. Jacobs M, Sherry PS, Taylor LM, et al. . Pharmacist Assisted Medication Program enhancing the regulation of Diabetes (PAMPERED) study. Journal of the American Pharmacists Association 2012;52:613–21. 10.1331/JAPhA.2012.10183 [DOI] [PubMed] [Google Scholar]
- 49. Heisler M, Vijan S, Makki F, et al. . Diabetes control with reciprocal peer support versus nurse care management: a randomized trial. Ann Intern Med 2010;153:507–15. 10.7326/0003-4819-153-8-201010190-00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Guerci B, Drouin P, Grangé V, et al. . Self-monitoring of blood glucose significantly improves metabolic control in patients with type 2 diabetes mellitus: the Auto-Surveillance intervention active (ASIA) study. Diabetes Metab 2003;29:587–94. 10.1016/S1262-3636(07)70073-3 [DOI] [PubMed] [Google Scholar]
- 51. Frosch DL. Evaluation of a Behavior Support Intervention for Patients With Poorly Controlled Diabetes. Arch Intern Med 2011;171:2011–7. 10.1001/archinternmed.2011.497 [DOI] [PubMed] [Google Scholar]
- 52. Forjuoh SN, Bolin JN, Huber JC, et al. . Behavioral and technological interventions targeting glycemic control in a racially/ethnically diverse population: a randomized controlled trial. BMC Public Health 2014;14:71 10.1186/1471-2458-14-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Farmer A, Hardeman W, Hughes D, et al. . An explanatory randomised controlled trial of a nurse-led, consultation-based intervention to support patients with adherence to taking glucose lowering medication for type 2 diabetes. BMC Fam Pract 2012;13:30 10.1186/1471-2296-13-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Edelman D, Fredrickson SK, Melnyk SD, et al. . Medical clinics versus usual care for patients with both diabetes and hypertension: a randomized trial. Ann Intern Med 2010;152:689–96. 10.7326/0003-4819-152-11-201006010-00001 [DOI] [PubMed] [Google Scholar]
- 55. DePue JD, Dunsiger S, Seiden AD, et al. . Nurse-community health worker team improves diabetes care in American Samoa: results of a randomized Controlled trial. Diabetes Care 2013;36:1947–53. 10.2337/dc12-1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dale J, Caramlau I, Sturt J, et al. . Telephone peer-delivered intervention for diabetes motivation and support: the telecare Exploratory RCT. Patient Educ Couns 2009;75:91–8. 10.1016/j.pec.2008.09.014 [DOI] [PubMed] [Google Scholar]
- 57. Choe HM, Mitrovich S, Dubay D, et al. . Proactive case management of high-risk patients with type 2 diabetes mellitus by a clinical pharmacist: a randomized controlled trial. Am J Manag Care 2005;11:253–60. [PubMed] [Google Scholar]
- 58. Blackberry ID, Furler JS, Best JD, et al. . Effectiveness of general practice based, practice nurse led telephone coaching on glycaemic control of type 2 diabetes: the patient engagement and coaching for Health (PEACH) pragmatic cluster randomised controlled trial. BMJ 2013;347:f5272 10.1136/bmj.f5272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Crowley MJ, Edelman D, McAndrew AT, et al. . Effectiveness of a scalable telemedicine intervention for veterans with persistent poor diabetes control. Diabetes 2015;64:A80. [Google Scholar]
- 60. Edelman D, Dolor RJ, Coffman CJ, et al. . Nurse-led behavioral management of diabetes and hypertension in community practices: a randomized trial. J Gen Intern Med 2015;30:626–33. 10.1007/s11606-014-3154-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Capozza K, Woolsey S, Georgsson M, et al. . Going mobile with diabetes support: a randomized study of a text message-based personalized behavioral intervention for type 2 diabetes self-care. Diabetes Spectr 2015;28:83–91. 10.2337/diaspect.28.2.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. McDermott RA, Schmidt B, Preece C, et al. . Community health workers improve diabetes care in remote Australian Indigenous communities: results of a pragmatic cluster randomized controlled trial. BMC Health Serv Res 2015;15:68 10.1186/s12913-015-0695-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. O’Connor PJ, Schmittdiel JA, Pathak RD, et al. . Randomized trial of telephone outreach to improve medication adherence and metabolic control in adults with diabetes. Diabetes Care 2014;37:3317–24. 10.2337/dc14-0596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sugiyama T, Steers WN, Wenger NS, et al. . Effect of a community-based diabetes self-management empowerment program on mental health-related quality of life: a causal mediation analysis from a randomized controlled trial. BMC Health Serv Res 2015;15:115 10.1186/s12913-015-0779-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Anzaldo-Campos MC, Contreras S, Vargas-Ojeda A, et al. . Dulce Wireless Tijuana: a Randomized Control Trial evaluating the impact of Project Dulce and Short-Term Mobile Technology on Glycemic Control in a Family Medicine Clinic in Northern Mexico. Diabetes Technol Ther 2016;18:240–51. 10.1089/dia.2015.0283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Basudev N, Crosby-Nwaobi R, Thomas S, et al. . A prospective randomized controlled study of a virtual clinic integrating primary and specialist care for patients with type 2 diabetes mellitus. Diabet Med 2016;33:768–76. 10.1111/dme.12985 [DOI] [PubMed] [Google Scholar]
- 67. Protheroe J, Rathod T, Bartlam B, et al. . The feasibility of Health Trainer Improved Patient Self-Management in patients with Low Health literacy and poorly controlled Diabetes: a pilot Randomised Controlled Trial. J Diabetes Res 2016;2016:1–11. 10.1155/2016/6903245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wild SH, Hanley J, Lewis SC, et al. . Supported Telemonitoring and Glycemic Control in People with Type 2 Diabetes: The Telescot Diabetes Pragmatic Multicenter Randomized Controlled Trial. PLoS Med 2016;13:e1002163 10.1371/journal.pmed.1002163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Murad MH, Montori VM, Ioannidis JP, et al. . How to read a systematic review and meta-analysis and apply the results to patient care: users’ guides to the medical literature. JAMA 2014;312:171–9. 10.1001/jama.2014.5559 [DOI] [PubMed] [Google Scholar]
- 70. Sun X, Ioannidis JP, Agoritsas T, et al. . How to use a subgroup analysis: users’ guide to the medical literature. JAMA 2014;311:405–11. 10.1001/jama.2013.285063 [DOI] [PubMed] [Google Scholar]
- 71. Dias S, Sutton AJ, Welton NJ, et al. . Heterogeneity: subgroups, meta-regression, Bias and bias-adjustment. NICE Decision Support Unit Technical Support Document [Internet] 2012. [PubMed] [Google Scholar]
- 72. Health and Information and Quality Authority. Health technology assessment of chronic disease self- management support interventions, 2015. [Google Scholar]
- 73. Ismail K, Winkley K, Rabe-Hesketh S. Systematic review and meta-analysis of randomised controlled trials of psychological interventions to improve glycaemic control in patients with type 2 diabetes. Lancet 2004;363:1589–97. 10.1016/S0140-6736(04)16202-8 [DOI] [PubMed] [Google Scholar]
- 74. Pal K, Eastwood SV, Michie S, et al. . Computer-based interventions to improve self-management in adults with type 2 diabetes: a systematic review and meta-analysis. Diabetes Care 2014;37:1759–66. 10.2337/dc13-1386 [DOI] [PubMed] [Google Scholar]
- 75. Chrvala CA, Sherr D, Lipman RD. Diabetes self-management education for adults with type 2 diabetes mellitus: A systematic review of the effect on glycemic control. Patient Educ Couns 2016;99:926–43. 10.1016/j.pec.2015.11.003 [DOI] [PubMed] [Google Scholar]
- 76. Norris SL, Nichols PJ, Caspersen CJ, et al. . The effectiveness of disease and case management for people with diabetes. A systematic review. Am J Prev Med 2002;22(4 Suppl):15–38. 10.1016/S0749-3797(02)00423-3 [DOI] [PubMed] [Google Scholar]
- 77. Glazier RH, Bajcar J, Kennie NR, et al. . A systematic review of interventions to improve diabetes care in socially disadvantaged populations. Diabetes Care 2006;29:1675–88. 10.2337/dc05-1942 [DOI] [PubMed] [Google Scholar]
- 78. Saxena S, Misra T, Car J, et al. . Systematic review of primary healthcare interventions to improve diabetes outcomes in minority ethnic groups. J Ambul Care Manage 2007;30:218–30. 10.1097/01.JAC.0000278982.65063.5c [DOI] [PubMed] [Google Scholar]
- 79. Wang Y, Yeo QQ, Ko Y. Economic evaluations of pharmacist-managed services in people with diabetes mellitus: a systematic review. Diabet Med 2016;33:421–7. 10.1111/dme.12976 [DOI] [PubMed] [Google Scholar]
- 80. Santschi V, Chiolero A, Paradis G, et al. . Pharmacist interventions to improve cardiovascular disease risk factors in diabetes: a systematic review and meta-analysis of randomized controlled trials. Diabetes Care 2012;35:2706–17. 10.2337/dc12-0369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Krass I, Schieback P, Dhippayom T. Adherence to diabetes medication: a systematic review. Diabet Med 2015;32:725–37. 10.1111/dme.12651 [DOI] [PubMed] [Google Scholar]
- 82. Cramer JA. A systematic review of adherence with medications for diabetes. Diabetes Care 2004;27:1218–24. 10.2337/diacare.27.5.1218 [DOI] [PubMed] [Google Scholar]
- 83. Vermeire E, Wens J, Van Royen P, et al. . Interventions for improving adherence to treatment recommendations in people with type 2 diabetes mellitus. Cochrane Database Syst Rev 2005;2:Cd003638 10.1002/14651858.CD003638.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Farmer AJ, McSharry J, Rowbotham S, et al. . Effects of interventions promoting monitoring of medication use and brief messaging on medication adherence for people with Type 2 diabetes: a systematic review of randomized trials. Diabet Med 2016;33:565–79. 10.1111/dme.12987 [DOI] [PubMed] [Google Scholar]
- 85. Cleveringa FG, Gorter KJ, van den Donk M, et al. . Computerized decision support systems in primary care for type 2 diabetes patients only improve patients’ outcomes when combined with feedback on performance and case management: a systematic review. Diabetes Technol Ther 2013;15:180–92. 10.1089/dia.2012.0201 [DOI] [PubMed] [Google Scholar]
- 86. Jeffery R, Iserman E, Haynes RB; CDSS Systematic Review Team. Can computerized clinical decision support systems improve diabetes management? A systematic review and meta-analysis. Diabet Med 2013;30:739–45. 10.1111/dme.12087 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2016-015135supp001.pdf (66.8KB, pdf)
bmjopen-2016-015135supp002.pdf (39.6KB, pdf)
bmjopen-2016-015135supp003.pdf (182.1KB, pdf)
bmjopen-2016-015135supp004.jpg (455.4KB, jpg)
bmjopen-2016-015135supp005.pdf (55.1KB, pdf)
bmjopen-2016-015135supp006.jpg (265.1KB, jpg)
bmjopen-2016-015135supp007.jpg (249.5KB, jpg)
bmjopen-2016-015135supp008.jpg (807.5KB, jpg)
bmjopen-2016-015135supp009.jpg (645.1KB, jpg)
bmjopen-2016-015135supp010.jpg (549.2KB, jpg)
bmjopen-2016-015135supp011.jpg (285.3KB, jpg)
bmjopen-2016-015135supp012.pdf (138.5KB, pdf)