Abstract
Objectives
The objective of this systematic review was to study the impact of preoperative physical activity levels on adult cardiac surgical patients’ postoperative: (1) major adverse cardiac and cerebrovascular events (MACCEs), (2) adverse events within 30 days, (3) hospital length of stay (HLOS), (4) intensive care unit length of stay (ICU LOS), (5) activities of daily living (ADLs), (6) quality of life, (7) cardiac rehabilitation attendance and (8) physical activity behaviour.
Methods
A systematic search of MEDLINE, Embase, AgeLine and Cochrane library for cohort studies was conducted.
Results
Eleven studies (n=5733 patients) met the inclusion criteria. Only self-reported physical activity tools were used. Few studies used multivariate analyses to compare active versus inactive patients prior to surgery. When comparing patients who were active versus inactive preoperatively, there were mixed findings for MACCE, 30 day adverse events, HLOS and ICU LOS. Of the studies that adjusted for confounding variables, five studies found a protective, independent association between physical activity and MACCE (n=1), 30-day postoperative events (n=2), HLOS (n=1) and ICU LOS (n=1), but two studies found no protective association for 30-day postoperative events (n=1) and postoperative ADLs (n=1). No studies investigated if activity status before surgery impacted quality of life or cardiac rehabilitation attendance postoperatively. Three studies found that active patients prior to surgery were more likely to be inactive postoperatively.
Conclusion
Due to the mixed findings, the literature does not presently support that self-reported preoperative physical activity behaviour is associated with postoperative cardiac surgical outcomes. Future studies should objectively measure physical activity, clearly define outcomes and adjust for clinically relevant variables.
Registration
Trial registration number NCT02219815. PROSPERO number CRD42015023606.
Keywords: cardiac surgical procedures, exercise, prognosis, postoperative complications
Strengths and limitations of this study.
There were mixed findings regarding the impact of physical activity on postcardiac surgical outcomes.
Only self-reported physical activity tools were used.
The multiple tools to measure physical activity and the variety of definitions of outcomes did not allow for a quantitative synthesis (meta-analysis).
Introduction
Recent reports suggest that more than half of cardiac surgeries are being performed on older adults who are more likely to be frail and have multiple comorbidities.1 While cardiac surgery has been shown to improve the outcomes of these patients, more than 75% of major perioperative complications and deaths occur in older adults.2 3 Before surgery, many of these patients are deconditioned and have diminished resilience in the face of major stressors such as cardiac surgery, and it has been postulated that they could benefit from a therapeutic intervention prior to their major surgical procedure in order to reduce their operative risk. However, little information exists to evaluate the benefit of preoperative risk reduction strategies for the older cardiac surgery patient.
Adopting and sustaining a more physically active lifestyle is typically intended to be a part of an interdisciplinary rehabilitation plan that is instituted postoperatively and has been shown to reduce the risk of cardiac mortality and hospital admissions and improve health-related quality of life (QOL) in patients.4 Importantly, older adults who sustain a physically active lifestyle after a postoperative exercise-based rehabilitation programme can continue to improve their functional walking status.5 However, evidence suggests that cardiac surgery patients are highly sedentary during the preoperative period, especially in older adults.6 Furthermore, few randomised controlled trials exist that evaluate the therapeutic benefit of preoperative lifestyle modification in patients undergoing cardiac surgery.7–9 Information regarding the link between preoperative physical activity and postoperative health outcomes in cardiac surgery patients would be valuable for healthcare providers to assist them in selecting patients who might benefit from preoperative exercise therapy.
The purpose of this systematic review was to compare the following postoperative outcomes between cardiac surgery patients defined as physically active prior to surgery and those who were defined as physically inactive preoperatively: (1) major adverse cerebrovascular and cardiovascular events (MACCEs), (2) 30-day adverse events as defined by the Society of Thoracic Surgeons (STS),10 (3) hospital length of stay, (4) intensive care unit (ICU) length of stay, (5) health-related QOL, (6) activities of daily living (ADLs), (7) cardiac rehabilitation attendance and (8) physical activity levels postoperatively.
Material and methods
The protocol for this systematic review has been described in PROSPERO: CRD42015023606. Note the following ad hoc changes to the previous protocol: ICU length of stay and postoperative physical activity as additional outcomes were explored in this systematic review.
Eligibility criteria
Eligible studies included cohort studies that examined adult (>18 years) cardiac surgery patients undergoing coronary artery bypass grafting (CABG), aortic or mitral valve repair/replacement, transcatheter aortic valve implantation or combined procedures. Studies with patients undergoing congenital cardiac surgery, heart transplantation or left ventricular assist device implantation procedures were excluded. Studies could compare physically active versus inactive patients prior to cardiac surgery on the basis of subjective (eg, questionnaire) or objective (eg, pedometer and accelerometry) assessments of physical activity.
Eligible studies had to compare at least one of the following postoperative outcomes: MACCE defined as death, stroke, myocardial infarction and the need for emergency cardiac surgery; 30-day adverse events as defined by the STS,10 including an unexpected return to the operating room, complications due to pulmonary, cardiovascular, gastrointestinal, haematological, urological, infection, and neurological deficits, other important miscellaneous outcomes (eg, unexpected admission to ICU, or other events requiring admission to operating room requiring anaesthesia); hospital length of stay; ICU length of stay; health-related QOL with any assessment tool; ADLs using any evaluation strategy; cardiac rehabilitation attendance; and physical activity behaviour using either subjective or objective forms of assessment.
Search strategy
The search strategy was completed by a librarian and reviewed by a second librarian. The search included keywords and controlled vocabulary. English language limits were applied. Databases used included MEDLINE, Embase, AgeLine and Cochrane Library (CDSR, CENTRAL and DARE), and articles were searched from inception to December 2016. The MEDLINE strategy was registered and published online in PROSPERO (http://www.crd.york.ac.uk/PROSPEROFILES/23606_STRATEGY_20150518.pdf) and is also available as a online supplementary file 1. The search was validated through a cross-check of references of studies selected for inclusion. In addition, conference abstracts were hand searched using the internet. Attempts were made to contact authors of conference abstracts to determine if their findings were published in a peer-reviewed journal.
bmjopen-2016-015712supp001.pdf (8.3KB, pdf)
Study selection
The title, abstract and full-text article screening processes were independently completed by two reviewers. A training exercise for the title and abstract phase was conducted by the independent reviewers using a random sample of 100 titles and abstracts. Discrepancies in studies for inclusion were resolved by discussion of the two reviewers. The final observed agreement was 98% with a kappa statistic of 0.47 for the title and abstract screen. One training exercise of 10 randomly selected articles was completed for the full-text screen. Discrepancies for inclusion were resolved through discussion. The observed agreement for the full-text screen was 96% with a kappa statistic of 0.83.
Data abstraction
Two reviewers independently extracted relevant data for the selected outcomes described above. Discrepancies in the data extraction procedure were resolved through discussion. Data abstraction items included study characteristics (eg, authors, year of publication, sample size and follow-up time points if relevant), patient characteristics (eg, age, sex and surgery type), physical activity tool used and the outcomes that were measured.
Risk of bias assessment
Two reviewers independently reviewed the risk of bias of each included study using the Newcastle-Ottawa Scale.11 Items within this tool assess the risk of bias associated with selection of participants, comparability (eg, study authors controlled for patient demographics and clinical characteristics) and outcome assessment (eg, data collection method for outcome, sufficient follow-up and adequacy of follow-up of cohorts). Each study was given a score within each category (selection: 0–4, comparability: 0–2 and outcome: 0–3) and an overall score ranging from 0 to 9. A score of 0 suggests an increased risk of bias and a higher score suggests a lower risk of bias.
Quantitative synthesis
Due to the significant heterogeneity between studies in terms of physical activity assessment tools used and outcomes assessed, meta-analyses were not performed.
Results
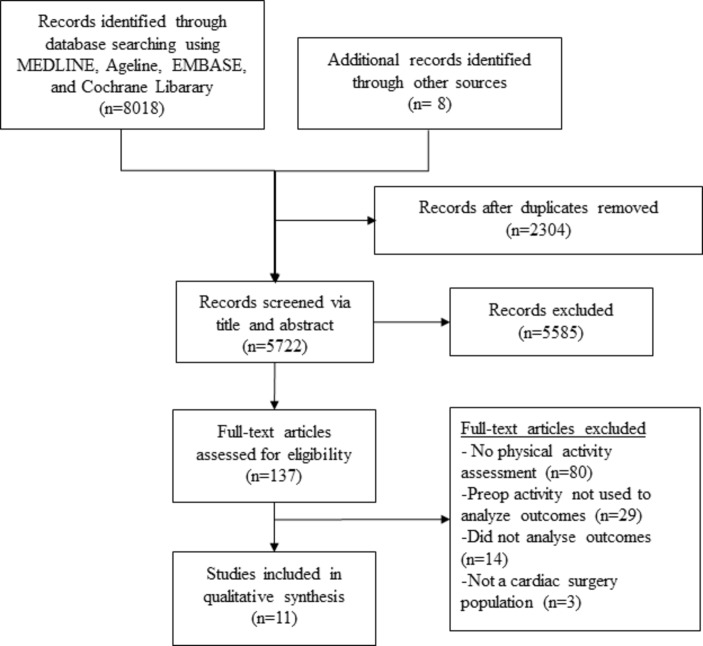
The literature search results are shown in figure 1. After removing duplicates, 5722 articles were title and abstract screened. A total of 137 articles were then assessed in full text. Eleven studies met the eligibility criteria for the final analysis, and they included a total of 5733 patients.12–22
Figure 1.
Study flow diagram.
An overview of the included studies can be viewed in table 1. In the studies by Markou et al,12 14 Nery et al13 16 and Martini et al,15 they evaluated CABG-only patients. Rengo et al,17 Giaccardi et al,18 Cacciatore et al,19 Noyez et al20 and Min et al21 evaluated both CABG and/or valve procedures, and van Laar et al22 evaluated isolated aortic valve repair patients. The average age of participants in different studies ranged from 60 years (Martini and Nery et al15 16) to 75 years (Rengo et al, Giaccardi et al, Min et al, van Laar et al17 18 21 22). Rengo et al,17 Giaccardi et al,18 Min et al21 and van Laar et al22 excluded patients with physical impairments or with New York Heart Association heart failure class IV symptoms (severe cardiac symptoms), but in general exclusion criteria were not explicitly reported. Studies were conducted in the Netherlands (Markou et al,12 14 Noyez et al20 and van Laar et al22), Brazil (Nery et al13 16 and Martini et al15), Italy (Rengo et al,17 Giaccardi et al18 and Cacciatore et al18) and the USA (Min et al21). Two studies by Nery et al13 and Martini et al15 used the same patient sample but examined different outcomes. The sample size of studies ranged from 35 in the Min et al21 study to 3150 in the Noyez et al20 study.
Table 1.
Characteristics of included studies
| First author, year | Study population | Country | Participants at follow-up | Physical activity assessment | Longest follow-up | Main findings |
| Giaccardi, 201118 | All patients ≥65 years undergoing CABG and/or valve procedures (total sample: 74.1±5.8 years old); 43% female | Italy | 158 | Harvard Alumni Questionnaire | 4 weeks postoperatively | Physical activity had an independent association with postoperative atrial fibrillation within 30 days. |
| Markou, 200712 | Elective CABG patients (active: 64.4±9.4, inactive: 63.8±9.0 years old); % female not reported | The Netherlands | 428 | The Corpus Christi Heart Project | 1 year | Inactive versus active group had significantly more perioperative MIs, but not reoperations, ICU LOS, HLOS or postoperative complications at 1 year. Inactive group was more likely than active group to be physically active at 1 year. |
| Nery, 200713 | All patients undergoing CABG (active: 63±11, inactive 66±14 years old); 42% female | Brazil | 55 | Structured Questionnaire confirmed by Minnesota Leisure Time Physical Activity Questionnaire | 1 year | Inactive versus active groups had significantly longer HLOS and more postoperative events at 1 year. |
| Markou, 200814 | Elective CABG patients (64.3±9.04 years old); 18% female | The Netherlands | 568 | The Corpus Christi Heart Project | 1 year | Inactive versus active groups were more likely to be more physically active 1 year postoperatively. |
| Martini, 201015 | Elective CABG patients (active: 60±10, inactive: 62±10 years old); 34% female | Brazil | 185 | Baecke Usual Physical Activity Questionnaire | Two years | Inactive versus active group did not have significantly different MACCE outcomes at 2 years. |
| Nery, 201016 | Elective CABG patients (active: 60±10, inactive: 62±10 years old); 34% female | Brazil | 202 | Baecke Usual Physical Activity Questionnaire | Hospital discharge | Inactive versus active groups had more postoperative events within 30 days and a longer HLOS. |
| Rengo, 201017 | Acute or elective CABG patients ≥70 years (active: 72.3±3.2, inactive: 76.1±3.9 years old); 34% female | Italy | 587 | Physical Activity Scale for the Elderly | Mean 44.3±21.0 months | Physical activity had an independent and dose association with cardiac and all-cause mortality 5 years postoperatively. |
| Cacciatore, 201219 | All patients ≥65 years undergoing CABG and/or valve procedures (72.9±4.8 years old); 48% female | Italy | 250 | Physical Activity Scale for the Elderly | Hospital discharge | Physical activity was independently associated with reduced prolonged ICU LOS. Physical activity was not independently associated with postoperative ADLs. |
| Noyez, 201320 | Elective CABG and/or valve patients (69.7±10.1 years old); | The Netherlands | 3150 | The Corpus Christi Heart Project | 30 days postoperatively | Physical activity was not independently associated with hospital or 30 day mortality. Inactive vs. Active group had a significantly longer ICU LOS. |
| Min, 201521 | Elective CABG and/or valve patients ≥65 years (74.7±5.9 years old) | USA | 62 | The Health and Retirement Survey | 4–6 months | Inactive versus active groups had significantly higher postoperative physical activity up to 6 months postoperatively. |
| van Laar22 | Patients ≥75 years undergoing elective isolated aortic valve replacement (79.5±2.8 years old); 59% female | The Netherlands | 115 | The Corpus Christi Heart Project | 2 years postoperatively | Inactive versus active groups had significantly higher mortality rates 2 years postoperatively. |
ADL, activities of daily living; CABG, coronary artery bypass graft surgery; HLOS, hospital length of stay; ICU LOS, intensive care unit length of stay; MACCEs, major adverse cerebrovascular and cardiac events; MI, myocardial infarction.
Physical activity tools
The physical activity assessments in each study were based on self-reported assessment tools. The timing of the physical activity assessments prior to surgery was not reported by Cacciatore et al,19 Nery et al,13 16 Markou et al12 14 or Martini et al.15 Rengo et al17 reported the timing of their physical activity assessment, which was within 35±6 days prior to surgery. Noyez et al and van Laar et al measured activity the day before surgery.20 22 Min and colleagues measured physical activity 4 weeks prior to the patients’ surgical procedure.21 Finally, Giaccardi et al measured preoperative physical activity levels approximately 1 week following surgery.18
Four studies used the Corpus Christi Heart Project questionnaire,12 14 20 22 which asks participants about their typical physical activity behaviours over the past year during their leisure time. Participants were categorised into a sedentary group if they accumulated less than 30 min per day of light intensity activity, or into an active group if they accumulated at least one session per week of dynamic activity lasting ≥15 min marked by moderate intensity. Nery et al13 16 and Martini et al15 used a structured questionnaire confirmed by the Minnesota Leisure Time Physical Activity Questionnaire13 or the Baecke Usual Physical Activity Questionnaire.15 16 Both physical activity tools ask participants to recall their usual activities 12 months prior and determine the frequency, intensity and time of activity. Participants were categorised into an inactive group if they engaged only in light intensity (<3 metabolic equivalents) activity or into an active group if they achieved ≥3 metabolic equivalents. Rengo et al17 and Cacciatore et al19 used the Physical Activity Scale for the Elderly, which is a 7-day recall of a participant’s frequency, intensity, duration and type of activity. Participants receive a total score from 0 to 400. Rengo et al17 separated participants by inactive and active groups using the median score, whereas Cacciatore et al19 used the continuous measure. The Harvard Alumni Questionnaire was implemented by Giaccardi and colleagues,18 which measures the typical weekly amount and intensity of physical activity over the past year. Participants were categorised as inactive if they participated in <1 hour per week of light activity and as active if they participated in either ≥4 hours of light or more than 1–2 hours of moderate activity per week. In the study by Min et al,21 the physical activity-related questions were used from the Health and Retirement Survey, which determines a participant’s frequency and intensity of activity in a typical week. These authors used the continuous score in their study.
Major adverse cardiac and cerebrovascular events
Outcomes within the definition of MACCE were evaluated in four studies (table 2) by Nery et al,13 Martini et al,15 Rengo et al17 and van Laar et al.22 The follow-up periods were one (Nery et al13), two (Martini et al15 and van Laar et al22) and 5 years (Rengo et al17) postoperatively. Unadjusted differences between active versus inactive patients and MACCE (defined as atrial fibrillation, hospital admission, reoperation and myocardial infarction) were found 1 year postoperatively in the Nery et al13 study. The Martini et al15 study found no differences (defined as mortality, rehospitalisation, cerebrovascular accident and MI) at 2 years postoperatively. The unadjusted rates of mortality within 2 years postsurgery were significantly lower in the active versus inactive group in the study by van Laar and colleagues.22 The study by Rengo and colleagues found a significant and dose–response relationship between physical activity and postoperative cardiac and all-cause mortality after controlling for preoperative demographics, medical history, medications and clinical characteristics.17
Table 2.
Major adverse and cerebrovascular events and postoperative events within 30 days
| Reference | Outcome definition | Adjustment variables | Number of events per group | OR or HR and 95% CI |
| Major adverse cerebrovascular and cardiac events | ||||
| Nery et al13 | 1-year postoperative AF, hospital readmission, new CABG, PCI, MI | None | Active: 8/25 (31%); inactive: 17/30 (57%)* | NR |
| Martini and Barbisan15 | 2-year postoperative death, re-hospitalisation, cerebrovascular accident, MI | None | Active: 9/66 (14%); inactive: 31/119 (26%) | NR |
| Rengo et al17 | 5-year postoperative cardiac and all-cause mortality | Demographics, medical history, medications, and clinical findings. | NR | Adjusted proportional hazard models: All-cause mortality: Exp(B) 0.248 (95% CI 0.141 to 0.434)* Cardiac mortality: Exp(B) 0.272 (0.133 to 0.555)* |
| van Laar et al22 | 2-year mortality | None | Active: 5/65 (13%); inactive: 11/50 (22%)* | NR |
| Postoperative events within 30 days | ||||
| Markou et al12 | Perioperative MI, reintervention, postoperative complications (wound, renal, neurological, pulmonary, gastrointestinal) | None | MI: active: 4/226 (2%); inactive: 11/202 (5%)* Reoperation: active: 15/226 (7%); inactive: 9/202 (5%), Wound infection: active: 3/226 (1%); inactive: 7/202 (3%), Renal: active: 3/226; inactive: 7/202 |
NR |
| Nery et al16 | Mortality, MI, reoperation | Age, smoking, PVD, COPD, Cleveland Risk Score. | Mortality: active: 0/66 (0%); inactive: 7/136 (5%) MI: active: 1/66 (2%); inactive: 6/136 (4%) Reoperation: active: 0/66 (0%); inactive: 1/136 (0.5%) |
Multivariate OR for being active: 0.22 (95% CI 0.09 to 0.51, p=0.001) |
| Rengo et al17 | Low-output syndromes, MI, cardiac support, stroke, bleedings, mediastinitis, pneumonia, dialysis | None | Any surgical complication: active: 53/267 (19.7%); inactive: 60/320 (18.6%) |
NR |
| Giaccardi et al18 | Atrial fibrillation | Age, episodes of AF 1 year preop, episodes of AF in the first week, β-blockers, amiodarone, left ventricular volume, left atrial emptying fraction | Postoperative atrial fibrillation: active: 6/74 (8.1%); inactive: 27/84 (32.1%)* | Multivariate OR for being inactive: 4.04 (95% CI 1.16 to 14.14, p=0.029) |
| Noyez et al20 | Mortality, reoperation, stroke, renal insufficiency, sternal wound, ventilation | ≥75 years, valve surgery, female, high operative risk, renal disease, obesity, NYHA IV, insulin, vascular pathology, poor LVEF, lung disease, MI, neurological event | Hospital mortality: active: 7/1815 (0.4%); inactive: 15/1335 (1.1%)* 30-day mortality: active: 10/1815 (0.6%); inactive: 20/1335 (1.5%)* Reoperation: active: 105/1815 (5.8%); inactive: 68/1335 (5%) Stroke: active: 9/1815 (0.5%); inactive: 12/1335 (0.9%) Renal insufficiency: active: 32/1815 (1.8%); inactive: 39/1335 (2.9%)* Sternal wound: active: 10/1815 (0.6%); inactive: 17/1335 (1.3%)* Ventilation >2 days: active: 31/1815 (1.7%); inactive: 54/1335 (4.0%)* |
Hospital mortality multivariate OR for being inactive: 1.20 (95% CI 0.4 to 3.5, p=0.617) 30 day mortality multivariate OR for being inactive: 1.10 (95% CI 0.5 to 2.7, p=0.70) |
*Indicates statistical significance (p<0.05).
AF, atrial fibrillation; BMI, body mass index; CABG, coronary artery bypass graft; COPD, chronic obstructive pulmonary disease; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NR, not reported; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; PVD, peripheral vascular disease.
30-day events
Five studies (Markou et al,12 Nery et al,16 Rengo et al,17 Giaccardi et al18 and Noyez et al20) evaluated postoperative events within 30 days of surgery (table 2). The postoperative events measured varied significantly between the studies. Three studies (Nery et al,16 Giaccardi et al18 and Noyez et al20) examined if physical activity was an independent protective factor against postoperative events. Physical activity was an independent protective factor against the combined outcome of mortality, MI and reoperation in the study by Nery et al16 as well as postoperative atrial fibrillation in the Giaccardi and colleagues study18 but was not significant for in-hospital or 30-day mortality in the Noyez et al20 study.
Postoperative health-related QOL
No studies evaluated postoperative health-related QOL.
Hospital and ICU length of stay
Three studies by Markou et al12 and Nery et al13 16 compared hospital length of stay between active and inactive cardiac surgery patients (table 3). Hospital length of stay was longer in the inactive group in two of three studies (both by Nery et al).13 16 One of the studies by Nery et al16 did not report hospital length of stay summary statistics between the active and inactive groups. However, that study reported an independent association between the preoperative active and inactive group and a reduced likelihood of prolonged hospital length of stay, though ‘prolonged’ was not defined in the study.
Table 3.
Hospital length of stay, ICU length of stay and postoperative activities of daily living and physical activity
| First author, year | Adjustment variables | Length of stay/number of events per group | OR or HR and 95% CI |
| Hospital length of stay | |||
| Markou, 200712 | None | Active: 6.9±8.2 days; inactive: 7.3±7.1 days | NR |
| Nery, 200713 | None | Active: 12±5 days, median 9 days (IQR 8–15); inactive: 15±8 days, median 12 (IQR 9–19)* | NR |
| Nery, 201016 | Age, sex, Cleveland Risk Score, smoking, systemic arterial hypertension, stroke, MI and PVD. | NR | HR: 0.67 (95% CI 0.49 to 0.93)* |
| ICU length of stay | |||
| Markou, 200712 | None | Active: 2.2±5.3 days; inactive: 2.1±3.5 days | NR |
| Cacciatore, 201219 | For ICU length of stay >3 days: age, off-pump CABG, stroke, renal failure. | Active: 2.58±1.09 days; inactive: 3.33±1.68 days*† | For ICU length of stay >3 days Univariate OR: 0.984 (95% CI 0.977 to 0.992)* Multivariate OR: 0.992 (95% CI 0.983 to 1.000)* |
| Noyez, 201320 | None | Active: 1.3±1.9 days; inactive 3.0±41.8 days* ICU >5 days: Active: 19/1815 (1.0%); Inactive: 46/1335 (3.4%)* |
NR |
| Postoperative ADLs | |||
| Cacciatore, 201219 | Age, gender, CABG, NYHA ≥3, ICU length of stay ≥3 days, off-pump CABG, diabetes, renal failure, stroke, PVD, COPD, Cumulative Illness Rating Scale. | NR | Beta: 0.099 |
| Postoperative physical activity | |||
| Markou12 | Age ≥75 years, gender, neurological disease, vascular disease, diabetes and preoperative physical activity. | Better PA post-operatively: active: 48/226 (21.2 %), inactive: 129/202 (64%)* Equal PA postoperatively: active: 112/226 (49.6%), inactive: 59/202 (29.2%)* Worse PA postoperatively: active: 66/226 (29.2%), inactive: 14/202 (6.9%)* |
Decreased postoperative PA OR (inactive group as reference): 8.1 (95% CI 3.5 to 13.5)* |
| Markou14 | Diabetes, vascular disease, neurological disease, renal disease, MI, preoperative activity level. | NR | For becoming physically inactive postoperatively Male OR (inactive group as reference): 7.11 (95% CI 3.6 to 13.9)* Female OR (inactive group as reference): 11.0 (95% CI 2.2 to 55)* |
| Min21 | None | NR | Each weekly preoperative activity point was associated with a loss of 0.78 points at 6 weeks, p<0.001 and 0.65 points at 6 months* |
*Indicates statistical significance (p<0.05).
†Unpublished data obtained from Cacciatore et al.19
ADL, activities of daily living; CABG, coronary artery bypass graft; COPD, chronic obstructive pulmonary disease; ICU, intensive care unit; MI, myocardial infarction; NR, not reported; NYHA, New York Heart Association; PA, physical activity; PVD, peripheral vascular disease.
Three studies compared ICU length of stay between the preoperative physical activity groups (table 3) (Markou et al,12 Cacciatore et al19 and Noyez et al).20 Two studies (Markou et al12 and Noyez et al20) found that the inactive group had a significantly longer ICU length of stay compared with the active group. In the study by Cacciatore and colleagues, they found in their multivariate analysis that the active group was less likely to have a prolonged ICU length of stay >3 days compared with the inactive group after controlling for age, off-pump CABG, stroke and renal failure.
Postoperative ADLs
One study by Min et al19 examined the impact of preoperative physical activity and postoperative ADLs at the time of hospital discharge and revealed no statistically significant (p=0.079) association between the two after adjusting for preoperative demographics and clinical variables.
Cardiac rehabilitation attendance
No studies evaluated cardiac rehabilitation attendance postoperatively.
Postoperative physical activity behaviour
The impact of preoperative physical activity on postoperative physical activity levels was examined in the two studies by Markou et al12 14 and in the other study by Min et al21 (table 3). These studies found that the active group preoperatively was more likely to be physically inactive postoperatively. In both of the Markou et al12 14 studies, they completed a multivariate analyses and found that this association remained statistically significant after controlling for age, gender and preoperative clinical characteristics.
Risk of bias
The risk of bias assessment via the Newcastle-Ottawa Scale can be viewed in table 4. Since some studies assessed multiple outcomes, the risk of bias assessments were based on their highest possible score (eg, some outcomes were assessed with a multivariable analysis, while others were not in the same study). All studies scored at least 3 out of 4 for the selection of study groups. There was variability across studies for the ascertainment of exposure or outcome of interest. Total risk of bias scores ranged from 5 to 9, suggesting the studies were of moderate to high quality, respectively.
Table 4.
Newcastle-Ottawa Scale risk of bias scores
| Reference | Selection | Comparability | Outcome | Total |
| Markou et al12 | 3 | 2 | 3 | 8 |
| Nery et al13 | 3 | 0 | 2 | 5 |
| Markou et al14 | 3 | 2 | 2 | 7 |
| Martini and Barbisan15 | 3 | 0 | 2 | 5 |
| Nery and Barbisan16 | 3 | 2 | 2 | 7 |
| Rengo et al17 | 4 | 2 | 3 | 9 |
| Giaccardi et al18 | 3 | 2 | 2 | 7 |
| Cacciatore et al19 | 3 | 2 | 2 | 7 |
| Noyez et al20 | 3 | 2 | 3 | 8 |
| Min et al21 | 4 | 2 | 1 | 7 |
| van Laar et al22 | 3 | 0 | 3 | 6 |
| Average scores±SD | 3.18±0.40 | 1.45±0.93 | 2.27±0.65 | 6.91±1.22 |
Maximum scores are 4, 2 and 3 for selection, comparability and outcome, respectively. Maximum total score is 9. A lower score within each category and for a total score indicates a higher risk of bias.
Discussion
The purpose of this systematic review was to determine if physical activity before cardiac surgery was associated with postoperative health outcomes. Given the different self-reported physical activity tools used that prevented comparison across studies, the inconsistent use of adjustment for potential confounders, and the varying outcomes evaluated for MACCE and 30-day postoperative events, it cannot be concluded that preoperative physical activity is associated with postoperative outcomes in adult cardiac surgery patients. This systematic review highlights important gaps within the literature on this topic. Therefore, key recommendations for examining the impact of preoperative physical activity behaviour on postsurgical outcomes of cardiac patients are provided (table 5).
Table 5.
Guidelines for physical activity measurement and outcome assessment in cardiac surgery patients: limitations and opportunities for future research
| Drawbacks | Opportunity |
| Physical activity | |
| 1. Heterogeneity in tools used across studies |
|
| 2. Only subjective measures were used | |
| 3. Time of preoperative physical activity assessment was unclear in most studies | |
| Outcomes | |
| 4. Heterogeneity in MACCE and postoperative events within 30 days definitions |
|
| 5. No patient-oriented outcomes were assessed |
|
| Statistical procedures | |
| 6. Shortage of studies addressing confounders |
|
ICU, intensive care unit; MACCE, major adverse cerebrovascular and cardiac events; STS, Society of Thoracic Surgeons.
The different self-reported physical activity tools used across the studies makes it difficult to compare the preoperative physical activity levels of patients prior to cardiac surgery. Even so, it is important to note that in the studies included in this systematic review, most of the studies identified a subsample of cardiac surgery patients who were more vulnerable to poor health outcomes by categorising patients as active or inactive prior to surgery using their self-reported physical activity measures. However, the way the physical activity tools measured physical activity (eg, over the past year or in the past week; see the Methods section) could have influenced the outcomes of the study. There seems to be no universally accepted tool to measure self-reported physical activity levels,23 and it is unclear if any of the physical activity tools identified by this review have been validated in the cardiac surgery patient. One advantage of using self-reported physical activity measures in studies is their ease of administration compared with other objectively measured physical activity tools. Furthermore, self-reported physical activity tools appear to provide some value when assessing the independent association between activity levels and poor outcomes. In fact, most physical activity guideline recommendations for health benefits, including those in North America, are based on self-reported measures.24 25 Another strength of using a subjective physical activity tool in the preoperative cardiac surgery patient is that it would capture a patient’s physical activity behaviour before they are placed on a waiting list, when they might refrain from being physically active in fear of making their condition worse. However, cardiac surgery patients and other patient populations tend to misreport their physical activity levels compared with objectively measured physical activity.6 26 Nevertheless, this systematic review found no studies that evaluated objectively measured physical activity before cardiac surgery and its link to postoperative health outcomes. Evidence suggests there is a stronger association between objective measures of physical activity and various cardiovascular and metabolic biomarkers as compared with subjective measures of physical activity.27 28 While it is unclear which objective measures of physical activity are most appropriate in the complex cardiac surgical patients, future studies should use a physical activity tools such as accelerometers or pedometers.
There were inconsistent findings across studies assessing the same outcomes, and many studies did not adjust for clinically relevant variables that could influence the health outcomes of cardiac surgery patients. It is possible that most of the included studies were not statistically powered to detect changes between inactive and active groups. The study by Rengo et al17 had the largest sample size of the four studies that assessed MACCE outcomes, which found a significant protective association between preoperative physical activity and cardiac and all-cause mortality 5 years postoperatively after controlling for clinically relevant variables (table 2). In contrast, the largest study examined in this systematic review by Noyez and colleagues20 found no association between preoperative activity and hospital and 30-day mortality after controlling for covariates (table 2). It is difficult to determine if patient-level factors influence outcomes (eg, elective or acute patients, surgery type, older vs younger, females vs males) as the samples were somewhat heterogeneous. Even so, some of the results of this systematic review are promising. Specifically, of the studies that controlled for confounding variables, five studies found a protective, independent association between higher preoperative physical activity levels when assessing clinical outcomes, including MACCE,1730-day postoperative events,16 18hospital length of stay16 and ICU length of stay,19 whereas only two studies found no protective association for 30-day postoperative events20 and postoperative ADLs.19 Yet, more studies are needed to elucidate the impact of preoperative physical activity on postcardiac surgical outcomes that control for clinically relevant variables. Clinical variables included in the cardiac surgical risk models (eg, EuroSCORE and STS score) could attenuate or mitigate the relationship between preoperative physical activity behaviour and postoperative outcomes. Collectively, future studies are needed to determine if preoperative physical activity is a protective factor for health outcomes after cardiac surgery that control for clinically relevant variables known to impact cardiac surgery outcomes.
An unanticipated finding was that patients who were active before surgery had a higher likelihood of being physically inactive postoperatively, after controlling for comorbidities.12 14 21 Healthcare providers may have advised patients with more severe symptomology prior to surgery to refrain from physical activity. Also, the relief of cardiac symptoms after surgery among inactive patients could have led them to become more active postoperatively. However, these possibilities were not explored in the included studies.
While outside the scope of this systematic review, future studies should investigate if changes to physical activity levels prior to cardiac surgery impact long-term patient health outcomes. Cardiac rehabilitation programmes are intended to support cardiac patients in becoming more physically active postoperatively, and it has been shown that patients who attend such programmes reduce their risk for cardiac-related mortality and hospitalisation rates.29 Evidence suggests that among those referred to cardiac rehabilitation after cardiac surgery, only 40% attend.6 However, the literature is less clear on whether patients who attend cardiac rehabilitation are more physically active compared with those who do not attend. It is possible that patients who adopt and sustain a more physically active lifestyle on their own after cardiac surgery could yield similar health benefits compared with those who attend an exercise-based rehabilitation programme, but this hypothesis requires further investigation.
Previous randomised controlled trials comparing an exercise programme to standard care prior to elective cardiac surgery (ie, ‘Prehab’) demonstrate reductions in hospital length of stay and improvements in walking ability postoperatively.7–9 However, there were mixed findings from this systematic review when comparing preoperative physical activity behaviour and hospital stay.12 16 These divergent findings suggest either that a medically supervised and individualised physical activity programme is needed to derive the health benefits of physical activity prior to cardiac surgery, or that patients are misreporting their physical activity behaviours. Future cohort studies in this area should address the drawbacks of the included studies in this systematic review included in table 5, while randomised trials should focus on whether preoperative exercise therapy programmes are feasible and efficacious in clinical practice.
The findings of this systematic review suggest that the literature would benefit from standardisation of the definition of measures such as MACCE and postoperative events within 30 days. The heterogeneity in reporting of outcomes can lead to considerably different conclusions across studies.30 Attempts should also be made to ensure other clinically important outcomes are captured, such as the addition of 30 day events. Only one study in this review compared physically active versus inactive patients preoperatively and reported on the individual postoperative events within 30 days.20 Collectively, uniform outcome reporting and appropriate outcome definitions are recommended when examining the outcomes of cardiac surgery.30
Patient-oriented outcomes should also be captured to ensure that cardiac surgery is improving other outcomes that patients value. No studies in this review determined if there was a link between preoperative physical activity behaviour and postoperative health-related QOL, and only one study evaluated postoperative ADLs.19 QOL postoperatively tends to improve in some older patients, while others tend to decline.31 Importantly, the preoperatively physical activity and overall functional status of cardiac surgery patients could play a role in the postoperative trajectory of these outcomes such as QOL. Other patient-oriented outcomes, including postoperative pain and cardiac symptoms, could also be investigated.
If physical activity is to be assessed in the preoperative period, the extent of missing data may also be a concern, especially with objective physical activity measures. The possibility of missing data from individual studies included in this systematic review was outside the objectives of the present study but is a salient point that should be considered for future investigations. It is also important to understand patient-level factors associated with missing data. The use of statistical techniques that address missing data, such as multiple imputation, is one approach to address missing physical activity data. Importantly, it has been shown that multiple imputation leads to precise estimates of predicting 30-day mortality risk in cardiac surgery patients when important clinical variables are missing, as compared with estimating risk with a complete case analysis.32
Limitations
One limitation to consider is that the patients included across the studies evaluated in this systematic review may have been different, as the recruitment criteria were not always clearly stated. A small sample of studies explicitly stated that they excluded those with physical limitations and healthcare providers may have advised higher risk patients to not participate in physical activity. There is also a limitation associated with the methodology of this systematic review: only studies written in English were included, raising the possibility that some studies were missed.
Conclusion
Due to the mixed findings in this systematic review, it cannot be concluded that self-reported physical activity behaviour before cardiac surgery is associated with health outcomes after surgery. The mixed findings could be due to the heterogeneity in physical activity tools used, definitions of outcomes and the few studies adjusting for other potentially confounding variables. These findings highlight the need for more research in this area.
Supplementary Material
Acknowledgments
Dr Francesco Cacciatore and his research group provided additional data on ICU length of stay. TAD was supported by a Manitoba Health Research Council (MHRC) Establishment Grant. DSK was supported by an MHRC Studentship, a CIHR Frederick Banting and Charles Best Canada Graduate Scholarship, a CIHR Strategic Training Initiative in Health Research (STIHR) Knowledge Translation Canada Student Fellowship, a CIHR STIHR Population Intervention for Chronic Disease Prevention Fellowship and the Heart and Stroke Foundation Dr. Dexter Harvey Award. ANS was supported by a CIHR Canada Graduate Scholarship.
Footnotes
Contributors: DSK was responsible for (1) analysis and interpretation of data, (2) drafting and revising the manuscript and (3) consenting for manuscript submission. ANS, BH, NT, RF, ASHH, NG, AH, J-FL were responsible for (1) analysis and interpretation of data, (2) revising the manuscript and (3) consenting for manuscript submission. KM was responsible for (1) developing the systematic review literature search, (2) analysis and interpretation of data, (3) drafting and revising the manuscript and (4) consenting for manuscript submission. RCA and TAD were responsible for (1) the conception and design, and analysis and interpretation of data, (2) revising the manuscript and (3) consenting for manuscript submission. RCA and TAD are cosenior authors.
Funding: The work was supported by an Operating Grant from the Canadian Institutes of Health Research.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: There are no additional unpublished data to share as a part of this systematic review.
References
- 1. Ferguson TB, Hammill BG, Peterson ED, et al. . A decade of change--risk profiles and outcomes for isolated coronary artery bypass grafting procedures, 1990-1999: a report from the STS National Database Committee and the Duke clinical Research Institute. Society of Thoracic Surgeons. Ann Thorac Surg 2002;73:480–9. 10.1016/S0003-4975(01)03339-2 [DOI] [PubMed] [Google Scholar]
- 2. Pfisterer M, Buser P, Osswald S, et al. . Outcome of elderly patients with chronic symptomatic coronary artery disease with an invasive vs optimized medical treatment strategy: one-year results of the randomized TIME trial. JAMA 2003;289:1117–23. 10.1001/jama.289.9.1117 [DOI] [PubMed] [Google Scholar]
- 3. Alexander KP, Anstrom KJ, Muhlbaier LH, et al. . Outcomes of cardiac surgery in patients > or = 80 years: results from the National Cardiovascular Network. J Am Coll Cardiol 2000;35:731–8. 10.1016/S0735-1097(99)00606-3 [DOI] [PubMed] [Google Scholar]
- 4. Anderson L, Oldridge N, Thompson DR, et al. . Exercise-Based Cardiac Rehabilitation for Coronary Heart disease: cochrane Systematic Review and Meta-Analysis. J Am Coll Cardiol 2016;67:1–12. 10.1016/j.jacc.2015.10.044 [DOI] [PubMed] [Google Scholar]
- 5. Macchi C, Polcaro P, Cecchi F, et al. . One-year adherence to exercise in elderly patients receiving postacute inpatient rehabilitation after cardiac surgery. Am J Phys Med Rehabil 2009;88:727–34. 10.1097/PHM.0b013e3181b332a1 [DOI] [PubMed] [Google Scholar]
- 6. Horne D, Kehler DS, Kaoukis G, et al. . Impact of physical activity on depression after cardiac surgery. Can J Cardiol 2013;29:1649–56. 10.1016/j.cjca.2013.09.015 [DOI] [PubMed] [Google Scholar]
- 7. Arthur HM, Daniels C, McKelvie R, et al. . Effect of a preoperative intervention on preoperative and postoperative outcomes in low-risk patients awaiting elective coronary artery bypass graft surgery. A randomized, controlled trial. Ann Intern Med 2000;133:253–62. 10.7326/0003-4819-133-4-200008150-00007 [DOI] [PubMed] [Google Scholar]
- 8. Sawatzky JA, Kehler DS, Ready AE, et al. . Prehabilitation program for elective coronary artery bypass graft surgery patients: a pilot randomized controlled study. Clin Rehabil. 2014;28:648–57. 10.1177/0269215513516475 [DOI] [PubMed] [Google Scholar]
- 9. Herdy AH, Marcchi PL, Vila A, et al. . Pre- and postoperative cardiopulmonary rehabilitation in hospitalized patients undergoing coronary artery bypass surgery: a randomized controlled trial. Am J Phys Med Rehabil 2008;87:714–9. 10.1097/PHM.0b013e3181839152 [DOI] [PubMed] [Google Scholar]
- 10. Society of Thoracic Surgeons. General Thoracic Surgery Database http://www.sts.org/sts-national-database/database-managers/general-thoracic-surgery-database (accessed 17 Feb 2015).
- 11. Wells GA, Shea B, O’Connell D, et al. . The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2011. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed 25 Jan 2015).
- 12. Markou AL, Lasten PJ, Noyez L. Physical activity post myocardial revascularization. ‘’will surgery improve my mobility? J Cardiovasc Surg 2007;48:201–6. [PubMed] [Google Scholar]
- 13. Nery RM, Barbisan JN, Mahmud MI. Influence of the practice physical activity in the coronary artery bypass graft surgery results. Rev Bras Cir Cardiovasc 2007;22:297–302. [DOI] [PubMed] [Google Scholar]
- 14. Markou AL, Evers M, van Swieten HA, et al. . Gender and physical activity one year after myocardial revascularization for stable angina. Interact Cardiovasc Thorac Surg 2008;7:96–101. 10.1510/icvts.2007.160382 [DOI] [PubMed] [Google Scholar]
- 15. Martini MR, Barbisan JN. Influence of physical activity during leisure time in patients in the follow-up two years after CABG. Rev Bras Cir Cardiovasc 2010;25:359–64. [DOI] [PubMed] [Google Scholar]
- 16. Nery RM, Barbisan JN. Effect of leisure-time physical activity on the prognosis of coronary artery bypass graft surgery. Rev Bras Cir Cardiovasc 2010;25:73–8. [DOI] [PubMed] [Google Scholar]
- 17. Rengo G, Galasso G, Vitale DF, et al. . An active lifestyle prior to coronary surgery is associated with improved survival in elderly patients. J Gerontol A Biol Sci Med Sci 2010;65:758–63. 10.1093/gerona/glp216 [DOI] [PubMed] [Google Scholar]
- 18. Giaccardi M, Macchi C, Colella A, et al. . Postacute rehabilitation after coronary surgery: the effect of preoperative physical activity on the incidence of paroxysmal atrial fibrillation. Am J Phys Med Rehabil 2011;90:308–15. 10.1097/PHM.0b013e31820f9535 [DOI] [PubMed] [Google Scholar]
- 19. Cacciatore F, Belluomo Anello C, Ferrara N, et al. . Determinants of prolonged intensive care unit stay after cardiac surgery in the elderly. Aging Clin Exp Res 2012;24:627–34. 10.3275/8521 [DOI] [PubMed] [Google Scholar]
- 20. Noyez L, Biemans I, Verkroost M, et al. . Is a sedentary lifestyle an independent predictor for hospital and early mortality after elective cardiac surgery? Neth Heart J 2013;21:439–45. 10.1007/s12471-013-0444-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Min L, Mazzurco L, Gure TR, et al. . Longitudinal functional recovery after geriatric cardiac surgery. J Surg Res 2015;194:25–33. 10.1016/j.jss.2014.10.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van Laar C, Kievit PC, Noyez L. Surgical aortic valve replacement in patients older than 75 years: is there really a quality of life benefit? Neth Heart J 2015;23:174–9. 10.1007/s12471-015-0660-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. van Poppel MN, Chinapaw MJ, Mokkink LB, et al. . Physical activity questionnaires for adults: a systematic review of measurement properties. Sports Med Auckl NZ 2010;40:565–600. [DOI] [PubMed] [Google Scholar]
- 24. Tremblay MS, Warburton DER, Janssen I, et al. . New Canadian physical activity guidelines. Appl Physiol Nutr Metab 2011;36:36–46. 10.1139/H11-009 [DOI] [PubMed] [Google Scholar]
- 25. Physical activity guidelines Advisory Committee. Physical Activity Guidelines Advisory Committee Report. Washington, DC: U.S: Department of Health and Human Services, 2008. [Google Scholar]
- 26. Lee PH, Macfarlane DJ, Lam TH, et al. . Validity of the International Physical Activity Questionnaire Short form (IPAQ-SF): a systematic review. Int J Behav Nutr Phys Act 2011;8:115 10.1186/1479-5868-8-115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schmidt MD, Cleland VJ, Thomson RJ, et al. . A comparison of subjective and objective measures of physical activity and fitness in identifying associations with cardiometabolic risk factors. Ann Epidemiol 2008;18:378–86. 10.1016/j.annepidem.2008.01.005 [DOI] [PubMed] [Google Scholar]
- 28. Atienza AA, Moser RP, Perna F, et al. . Self-reported and objectively measured activity related to biomarkers using NHANES. Med Sci Sports Exerc 2011;43:815–21. 10.1249/MSS.0b013e3181fdfc32 [DOI] [PubMed] [Google Scholar]
- 29. Anderson L, Thompson DR, Oldridge N, et al. . Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev 2016;1:CD001800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Goldfarb M, Drudi L, Almohammadi M, et al. . Outcome Reporting in cardiac surgery trials: systematic review and critical appraisal. J Am Heart Assoc 2015;4:e002204 10.1161/JAHA.115.002204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Abah U, Dunne M, Cook A, et al. . Does quality of life improve in octogenarians following cardiac surgery? A systematic review. BMJ Open 2015;5:e006904 10.1136/bmjopen-2014-006904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Karim MN, Reid CM, Tran L, et al. . Missing Value Imputation improves mortality risk prediction following cardiac surgery: an investigation of an australian patient Cohort. Heart Lung Circ 2017;26:301–8. 10.1016/j.hlc.2016.06.1214 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2016-015712supp001.pdf (8.3KB, pdf)