Significance
Activated B lymphocytes produce different classes of antibodies that target specific pathogens. During this process, DNA double-strand breaks (DSBs) are generated at precise regions of the Ig gene and are joined by the classical nonhomologous end joining (C-NHEJ) pathway to allow class switch recombination (CSR). Defects occurring in these mechanisms are associated with impaired antibody diversification, genomic instability, and cancer. Here, we show that deletion of multiple myeloma SET domain (MMSET), a histone methyltransferase that is deregulated in various lymphoid malignancies, reduces DNA cleavage at the upstream Ig gene region without affecting joining of DSBs by the C-NHEJ pathway. These findings show an important role for the histone methyltransferase activity of MMSET in promoting activation-induced cytidine deaminase-mediated DNA breaks during CSR.
Keywords: class switch recombination, histone methyltransferase MMSET, C-NHEJ
Abstract
In B cells, Ig class switch recombination (CSR) is initiated by activation-induced cytidine deaminase (AID), the activity of which leads to DNA double-strand breaks (DSBs) within IgH switch (S) regions. Preferential targeting of AID-mediated DSBs to S sequences is critical for allowing diversification of antibody functions, while minimizing potential off-target oncogenic events. Here, we used gene targeted inactivation of histone methyltransferase (HMT) multiple myeloma SET domain (MMSET) in mouse B cells and the CH12F3 cell line to explore its role in CSR. We find that deletion of MMSET-II, the isoform containing the catalytic SET domain, inhibits CSR without affecting either IgH germline transcription or joining of DSBs within S regions by classical nonhomologous end joining (C-NHEJ). Instead, we find that MMSET-II inactivation leads to decreased AID recruitment and DSBs at the upstream donor Sμ region. Our findings suggest a role for the HMT MMSET in promoting AID-mediated DNA breaks during CSR.
Antigen-activated B lymphocytes further diversify their Ig genes by somatic hypermutation (SHM) and class switch recombination (CSR) (1). SHM introduces mutations in Ig heavy chain (IgH) and Ig light chain variable region exons to allow production of antibodies with higher affinity. IgH CSR promotes diversification of Ig effector functions by replacing the initially expressed constant Cµ region with one of six downstream constant regions, while retaining antibody specificity. CSR involves joining of DNA double-strand breaks (DSBs) in the switch (Sµ) region that is upstream of Cµ to DSBs in one of the S regions associated with downstream constant regions (except Cδ) (2). DSBs are initiated by activation-induced cytidine deaminase (AID), which deaminates cytidine (C) to uridine (U) in ssDNA S regions. AID activity generates U-G nucleotide mispairing, which co-opts base excision repair or mismatch repair (MMR) factors to convert mismatches into DSBs (1). Most DSBs occurring in two S regions targeted for CSR are end-joined by classical nonhomologous end joining (C-NHEJ), one of the two major mammalian DSB joining pathways (3). The critical role of C-NHEJ is emphasized by the reduction of CSR efficiency in B cells defective for the Ku70/80 heterodimer or DNA ligase complex XRCC4/ligase4 (LIG4), which are considered as “core” C-NHEJ factors (4). However, in the absence of C-NHEJ, CSR still occurs through alternative end joining (A-EJ) processes that predominantly use microhomology (MH)-mediated S-region joining (5–7). CSR is also substantially impaired by inactivation of ATM-dependent DNA DSB response factors, such as ATM, 53BP1, and H2AX (8–12); 53BP1 deficiency has a profound impact on CSR, as it potentially plays several roles in C-NHEJ, including inhibition of S-region DSB resections and promoting orientation-dependent joining of targeted S regions (13).
AID activity is preferentially targeted to Ig loci by transcription and transcription-related processes. Transcription through unrearranged (“germline”) repetitive G-rich S regions favors the formation of R-loop structures that expose ssDNA substrates for AID (14). Germline S-region transcription is also required for the recruitment of AID to S regions through the ability of AID to interact, directly or indirectly, with components of RNA polymerase II, such as Spt5 and Spt6, the Pol II elongation proliferating cell nuclear antigen (PCNA)-associated factor complex, or chromatin modifiers (15–18). The RNA exosome complex also contributes, apparently by removing nascent RNA that could block the transcribed strand from AID activity (19). Recent studies revealed that the majority of detectable AID off-target activity occurs within focal regions of overlapping sense/antisense transcription within intragenic super enhancers (20, 21). There also is evidence that AID targeting to S regions correlates with marks of open chromatin and the activity of histone-modifying enzymes (22–24).
The histone methyltransferase (HMT) multiple myeloma SET domain (MMSET) or Nuclear Suppressor of variegation, Enhancer of zeste, and Trithorax Domain-containing 2 (NSD2) is encoded by the Wolf Hirschhorn Syndrome Candidate 1 (WHSC1) gene, which is deleted in the developmental disorder Wolf Hirschhorn syndrome (WHS) (25). In mice, germline inactivation of Whsc1 leads to perinatal mortality, heart disease, growth retardation, and craniofacial defects similar to those in WHS patients (26). In humans, alterations in MMSET expression or function are associated with hematological disorders, which were first described in multiple myeloma, where about 15% of cases overexpress MMSET because of the t(4;14) translocation (27). The HMT activity of MMSET is catalyzed by the Suppressor of variegation, Enhancer of zeste, and Trithorax (SET) domain located at the C-terminal part of the protein. Initial studies suggested that MMSET could catalyze several histone modifications, such as H3K27me3, lysine 36 on histone H3 (H3K36me2), H3K36me3, and H4K20me2 (28–31). MMSET was also reported to be involved in the DNA damage response by promoting 53BP1 recruitment to DSBs through dimethylation of H4K20 (31). In line with this, knockdown of Mmset was shown to inhibit CSR in the mouse B-lymphoma cell line CH12F3 (32). However, the ability of MMSET to catalyze modifications on histone H4 has been somewhat controversial, with other studies suggesting that it may be more specifically required for dimethylation of H3K36me2 (29). Thus, potential functions of MMSET during CSR remain to be determined. In this study, we used gene-targeted inactivation to investigate the role of the HMT MMSET in Ig class switching in mouse B cells.
Results
MMSET Is Required for Efficient CSR in Mouse B Cells.
To elucidate the function of MMSET in mouse B cells, we first used gene-targeted inactivation to delete exons 15–17, which include the start of the SET domain, from both alleles of the Whsc1 locus in TC-1 ES cells (Fig. S1 A and B). Western blot analysis of two independent Whsc1-deficient (Mmset-II−/−) ES cell clones showed no expression of the MMSET-II protein, the isoform containing the SET domain, but normal expression of the shorter MMSET-I isoform lacking this region (Fig. S1C). We used the Rag2-deficient blastocyst complementation approach (33) with each of the Mmset-II−/− ES cell clones to generate chimera mice, in which all mature B and T cells are derived from the parental ES cells. Flow cytometric (FACS) analysis revealed similar levels of B and T cells in the spleen of Mmset-II−/− chimeras as in WT controls (Fig. S1 D and E). Thus, MMSET-II is not strictly required for the development of mature B and T cells in mice. To examine whether MMSET-II could be involved in the response of mature splenic B cells to activation for CSR, we monitored its expression levels in WT B cells after treatment with anti-CD40 and IL-4. Western blot analysis showed that both isoforms of MMSET were strongly induced in WT B cells, with up-regulation being readily detectable after 24 h of stimulation and maintained at 48 h, the time at which the AID protein was strongly induced (Fig. S1F).
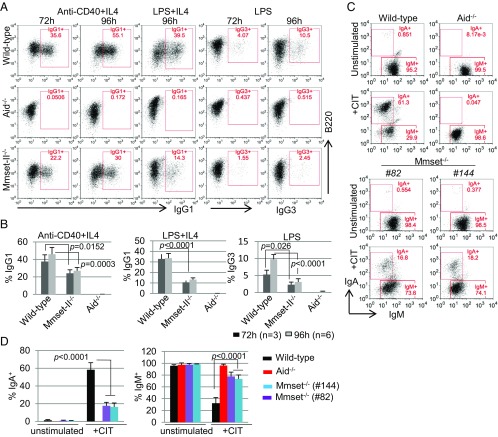
To investigate the effect of Mmset-II inactivation on CSR, we purified splenic B cells from Mmset-II−/− chimeras and assayed their ability to undergo class switching to IgG1 after culture in the presence of anti-CD40 and IL-4 (anti–CD40/IL-4) or LPS and IL-4 (LPS/IL-4). Switching to IgG1 was assayed at 72 and 96 h by staining B cells with anti-IgG1–specific antibodies followed by FACS analyses. In response to anti–CD40/IL-4 stimulation, Mmset-II−/− B cells exhibited a reduction of ∼50% in IgG1 expression compared with WT B cells (Fig. 1 A and B). Similarly, after stimulation with LPS/IL-4, we observed ∼60% reduction in IgG1 expression compared with WT control. As expected, no class switching was observed with stimulated AID−/− B cells. We next tested whether CSR to other IgH constant regions was also impaired in Mmset-II−/− B cells by stimulating purified B220+ B cells with LPS alone to induce switching to IgG3. LPS-stimulated WT B cells generated ∼10% of IgG3-positive cells, whereas only about 3% of treated Mmset-II−/− B cells stained for IgG3 (Fig. 1 A and B). These results show that MMSET-II plays an important role during IgH CSR in mouse B cells.
Fig. 1.
MMSET is required for efficient CSR in stimulated B cells and CH12F3 cells. (A) Flow cytometry analysis of switching to IgG1 or IgG3 in WT, Aid−/−, and Mmset-II−/− B cells stimulated with anti–CD40/IL-4 or LPS/IL-4 for 72 and 96 h. (B) Percentage (+SD) of CSR to different isotypes tested after 72 and 96 h in WT, Aid−/−, and Mmset-II−/− B cells. Average and SD were calculated from at least four independent experiments. (C) Flow cytometry analysis of switching to IgA in WT, Aid−/−, and two independent Mmset−/− CH12F3 clones that were unstimulated or stimulated with anti–CD40/IL-4/TGF-β (CIT) for 48 h. The percentages of different cell subsets are indicated in each gate. (D) Bar graphs showing the summarized data from four independent experiments of IgA switching (Left) and the IgM+ subset (Right) of various genotypes of CH12F3 cells without and with CIT for 48 h. P values were calculated by the unpaired two-tailed t test.
To examine MMSET-II function in a different cellular model, we developed a CRISPR vector (34) to delete exon 15 of Whsc1 in CH12F3 cells, a cell line that undergoes CSR to IgA in response to stimulation with CD40 ligation, IL-4, and TGF-β (CIT) (35). Excision of exon 15, referred to as Mmset−/−, forces splicing of exon 14 to exon 16 and introduces an earlier STOP codon as the result of a coding frameshift (Fig. S2 A and B). To avoid possible off-target effects, we designed two different pairs of CRISPR-Cas9 vectors (pair 1 and pair 2) and obtained two independent clones (82 and 144) homozygous for deletion of exon 15 (Fig. S2 A and C). Both Mmset−/− CH12F3 clones are, as expected, defective for expression of the MMSET-II isoform, which contains the SET domain, but have normal levels of the MMSET-I protein (Fig. S2D).
We compared the efficiency of IgH CSR to IgA between WT and Mmset−/− CH12F3 clones in response to CIT by staining the cells with anti-IgA antibodies followed by FACS analyses. We also included CH12F3 cells deficient for AID (AID−/−), which are defective in the initiation of CSR. While ∼58% of WT CH12F3 cells expressed IgA after 48 h of CIT treatment, only ∼17% of both Mmset−/− CH12F3 clones stained for IgA after stimulation (Fig. 1 C and D). No IgA expression was detected in CIT-stimulated AID−/− cells. Of note, Mmset-deficient CH12F3 cells display ∼70% of IgM+ cells after 48 h of CIT treatment, a level significantly higher than WT (30%) CH12F3 cells (Fig. 1D). These results are consistent with the defect in CSR to other IgH constant regions that we observed in MMSET-II–deficient primary B cells and support a critical role for MMSET-II in CSR.
Inactivation of MMSET-II Does Not Affect B-Cell Proliferation, Germline Transcription, or AID Expression.
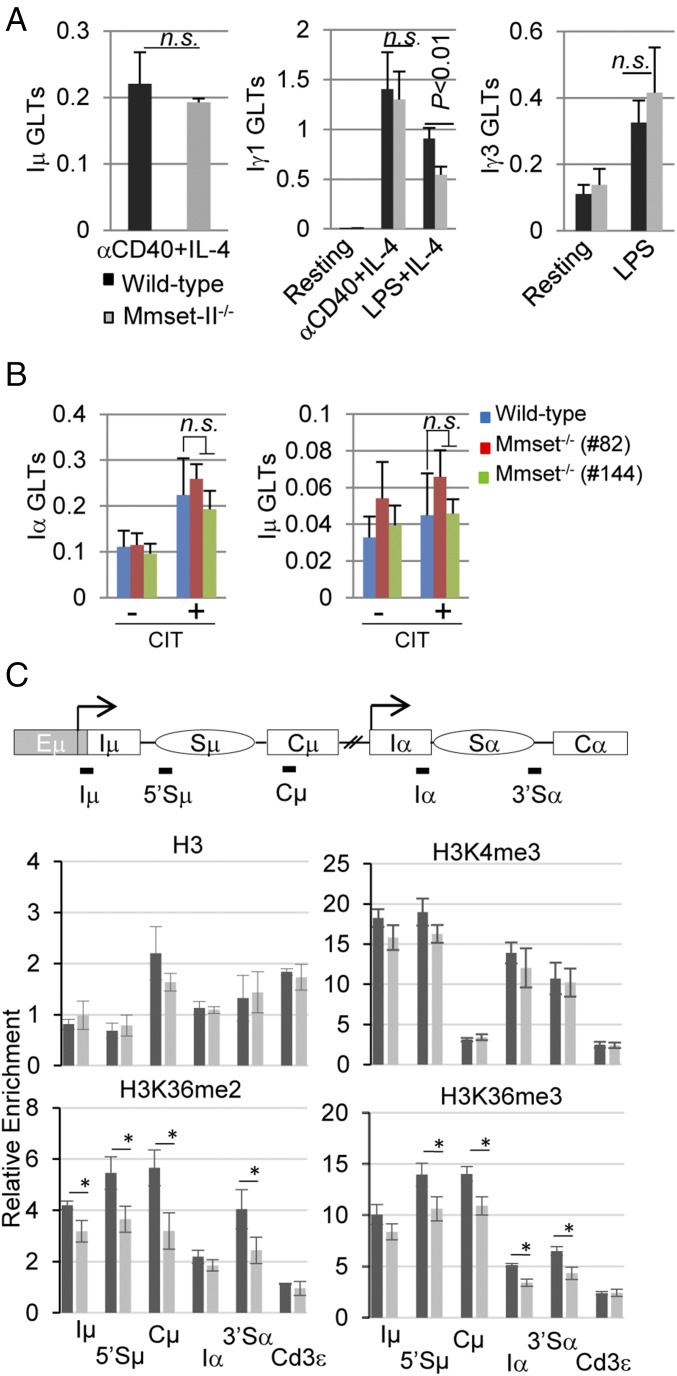
To assay whether lack of MMSET-II could have an indirect effect on CSR, we analyzed AID expression in response to activation signals. After stimulation with anti-CD40 and IL-4, WT and Mmset−/− B cells showed similar induction of AID (Fig. S3A). AID was also efficiently up-regulated in Mmset−/− CH12F3 clones compared with controls (Fig. S3B). To gain additional insights into the role of MMSET in CSR, we assessed whether proliferation, which is required for efficient switching (36), is altered in Mmset-II−/−–stimulated B cells. We observed similar levels of expansion of B cells from MMSET-II–deficient and WT mice after in vitro activation of CSR (Fig. S3C). Similarly, proliferation rates were unaffected in Mmset−/− CH12F3 clones (Fig. S3D). We conclude that impaired CSR of Mmset−/− B cells is not mediated through a proliferation defect. We also measured expression levels of the germline transcripts (GLTs) initiated from the upstream I promoters (I) of activated C-region genes, a process which is a prerequisite for CSR (2). qPCR analysis show that, while Iγ1 transcripts were reduced by ∼40% in mutant B cells after activation with LPS/IL-4, Iμ and Iγ1 transcripts were expressed at similar levels in Mmset-II−/− B cells stimulated with anti–CD40/IL-4 compared with the WT (Fig. 2A). In line with this, the levels of IgG3 GLTs were unaffected in response to LPS stimulation (Fig. 2A). Similarly, we did not find differences in the levels of Iα and Iµ GLTs in stimulated Mmset−/− compared with WT CH12F3 cells (Fig. 2B). Inactivation of MMSET-II also did not affect expression of the three GLTs produced by the Iα promoter or Iµ GLTs after CIT stimulation of CH12F3 cells as assayed by semi-qPCR (Fig. S3E). Thus, defects in germline transcription of the targeted CH regions do not fully account for the reduced CSR in Mmset-II−/− B cells.
Fig. 2.
Germline transcription and histone modification in MMSET-II–deficient cells. (A) qPCR analysis of Iµ, Iγ1, and Iγ3 transcripts in WT and Mmset-II−/− unstimulated B cells and B cells stimulated by the indicated conditions. Results were normalized to hypoxanthine-guanine phosphoribosyltransferase (HPRT) expression. Mean and SD are shown for three independent experiments. P values were calculated by the unpaired two-tailed t test. (B) Analysis of Iα and Iμ transcripts in WT and Mmset−/− CH12F3 cells stimulated with CIT as measured by qPCR. Nonsignificant variations are indicated as n.s. (C) ChIP analysis of the IgH locus. (Top) Shown is a representation of the IgH locus with the position of transcription start sites (arrows) and PCR amplicons used in the assay (black lines). (Middle and Bottom) ChIP assays were performed on WT and Mmset−/− CH12F3 cells (clone 82) stimulated with anti–CD40/IL-4/TGF-β (CIT) for 20 h and immunoprecipitated with anti-H3, anti-H3K4me3, anti-H3K36me, or anti-H3K36me3 antibodies. qPCR analysis of the indicated IgH regions was performed in duplicate with the primers listed in Table S1. The Cd3ε gene was used as a negative control. The relative enrichment amounts were calculated by dividing DNA amounts from immunoprecipitated by inputs for each sample. Mean and SD are shown for three independent experiments. P values were calculated by the unpaired two-tailed t test. *P < 0.05.
Since MMSET is an HMT, we further analyzed histone modifications at the IgH locus in CIT-treated Mmset−/− CH12F3 cells. In line with the methyltransferase activity of MMSET on H3K36, ChIP assay showed reduced levels of H3K36me2 and H3K36me3 marks, notably at 5′Sµ, Cµ, and 3′Sα regions, in MMSET-deficient compared with WT CH12F3 cells (Fig. 2C). In contrast, H3K4me3, a mark previously shown to be enriched at transcribed I and S regions, was not significantly reduced in Mmset−/− cells. Together, these results indicate that disruption of MMSET-II in CH12F3 cells reduces the levels of H3K36me2 and H3K36me3 at IgH regions targeted for class switching without significantly decreasing their germline transcription.
MMSET Is Not Required for AID Downstream DNA DSB Repair by the C-NHEJ Pathway During CSR.
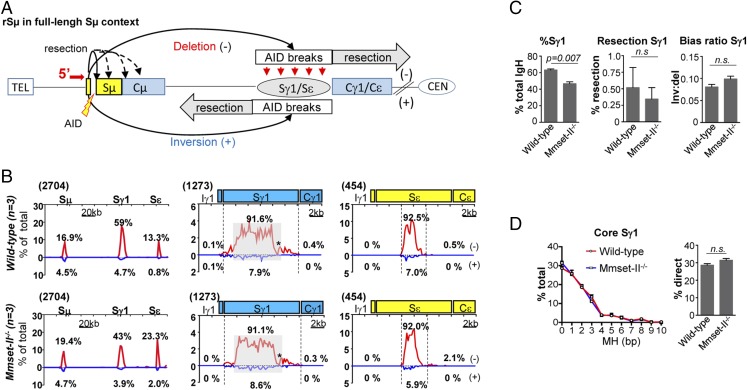
It has previously been shown that B cells deficient for the DSB response factor 53BP1 are substantially impaired for CSR and display Sµ-Sγ1 or Sµ-Sε junctions with longer resections and impaired orientation-dependent joining (13). As recruitment of 53BP1 at DNA breaks was suggested to be facilitated by MMSET via dimethylation on H4K20 (31), we used high-throughput genome-wide translocation sequencing (HTGTS) (Fig. 3A) to compare joining of AID-initiated 5′Sµ DSBs with Sγ1 and Sε DSBs in anti–CD40/IL-4–activated WT and Mmset-II−/− splenic B cells. Mmset-II–deficient B cells showed a significantly reduced percentage of Sγ1 junctions compared with WT B cells (Fig. 3 B and C). There was no evidence of an increase in long resections in activated Mmset-II−/− B cells, where the HTGTS profile at Sγ1 and Sε junctions appeared very similar to that of WT B cells, with more than 90% of the events falling within the S regions (Fig. 3 B and C), which is in sharp contrast to patterns observed in 53BP1−/− cells (13). Furthermore, lack of MMSET-II did not affect orientation-dependent joining, and the ratio of inversion/deletion seemed normal compared with WT B cells (Fig. 3C). Finally, analysis of more than 1,000 Sμ-Sγ1 junctions showed that the level of MH was similar in Mmset-II−/−– and WT-activated B cells (Fig. 3D and Fig. S4). Lack of significant junction numbers in Sε region precludes us from analyzing Sμ-Sε junctions (Fig. S4A). Thus, based on S-region large resection patterns and end joining pathway usage, end joining in Mmset-II−/− B cells appears similar to that observed in the WT. This suggests that MMSET-II is dispensable for the C-NHEJ pathway downstream of AID activity during CSR.
Fig. 3.
MMSET-deficient B cells exhibit a normal pattern of junctions at S regions. (A) Junctional outcomes from 5′ Sμ AID-initiated broken end joining to AID-initiated DSBs in Sγ1 and Sε, including deletion (−) or inversion (+); long resections are indicated by gray arrows. Break site 5′ Sμ resections are also depicted. The red arrow denotes 5′ Sμ primer as described (13). The centromere (CEN) and telomere (TEL) of the chromosome are shown. (B) Linear distribution of pooled junctions along the 200-kb IgH constant (CH) locus (Left) or at 20-kb regions surrounding Sγ1 and Sε (Center and Right, respectively) recovered from anti–CD40/IL-4 stimulated WT (n = 3) and Mmset-II−/− (n = 3) B cells. Libraries were randomly sampled and normalized to the minimal number of junctions mapped to the 200-kb CH region or 20-kb regions surrounding Sγ1 and Sε from all six experiments. Numbers in parentheses denote total junctions analyzed from experimental repeats after normalization. Red lines represent deletional joins, and blue lines represents inversional joins. Gray boxes indicate repetitive sequences with junctions mapping to multiple locations in Sγ1. *G-rich Sγ1 region devoid of AID motifs and junctions. (C) Bar graphs show the percentage of junctions mapping to the Sγ1 region relative to the total amount in the IgH region in WT and Mmset-II−/− B cells (Left), the percentage of Sγ1 resection at junctions mapping to deletional (−) resection region in WT and Mmset-II−/− B cells (Center), and the ratios of inversional to deletional 5′ Sµ joins to Sγ1 of WT and Mmset-II−/− B cells (Right). D, Left shows the percentage of MHs at core Sγ1 junctions recovered from three independent and normalized HTGTS libraries in WT or Mmset-II−/− B cells stimulated with anti–CD40/IL-4. Mean and SEM are shown. D, Right shows the percentage of direct Sμ-Sγ1 joins (mean ± SEM) in WT and Mmset-II−/− B cells. n.s., Nonsignificant variations.
The Level of DSBs Is Reduced at the Sµ Region in MMSET-II–Deficient CH12F3 Cells.
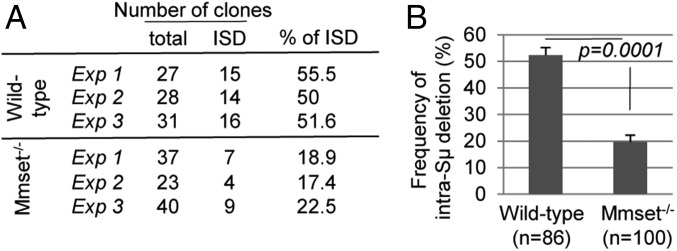
AID activity generates multiple DSBs within S regions. In addition to joining to a downstream DSB in an acceptor S region to mediate CSR, these DSBs can undergo end processing (e.g., resections and insertions) and either be religated to themselves or be ligated to separate DSBs in the same S region to generate intra-S recombination. After 48 h of CIT treatment, Mmset−/− CH12F3 clones remained IgM-positive (∼70%) cells, while this population was dramatically reduced in WT cells (Fig. 1D). In the absence of a defect in C-NHEJ, this phenomenon could have two explanations: (i) deletion of Mmset triggers higher frequency of intra-S recombination, or (ii) it reduces the levels of DSBs at S regions. To directly quantify intra-Sµ deletions, we subcloned 86 WT and 100 Mmset−/− CH12F3 cells retaining IgM expression after CIT stimulation (Fig. S5A). Southern blot analysis with a Cµ probe showed that the frequency of intra-Sµ deletions was reduced in Mmset−/− (∼20%) compared with the WT (∼50%) (Fig. 4 and Fig. S5B). These results indicate that intra-Sµ deletions are reduced in Mmset−/− CH12F3 cells, suggesting that the accumulation of IgM-positive cells in absence of MMSET-II after CIT stimulation is rather caused by defects in DSB generation at the Sµ region.
Fig. 4.
MMSET deletion decreases the frequency of intra-Sμ recombination. (A) Table summarizing the results of intraswitch deletion (ISD) in WT and Mmset-deficient CH12F3 cells. WT and Mmset−/− cells were stimulated with CIT for 48 h, and IgM+ cells were sorted by flow cytometry, subcloned, and analyzed by Southern blot in three independent experiments. The number of clones with ISD, the total number of clones, and percentages of ISD are shown. (B) The bar graph represents the frequency of intra-S deletions of the coding allele from IgM+ WT and Mmset−/− CH12F3 subclones. Mean and SD were calculated from three independent experiments, with total numbers of clones analyzed in the parentheses. P values were calculated by the unpaired two-tailed t test.
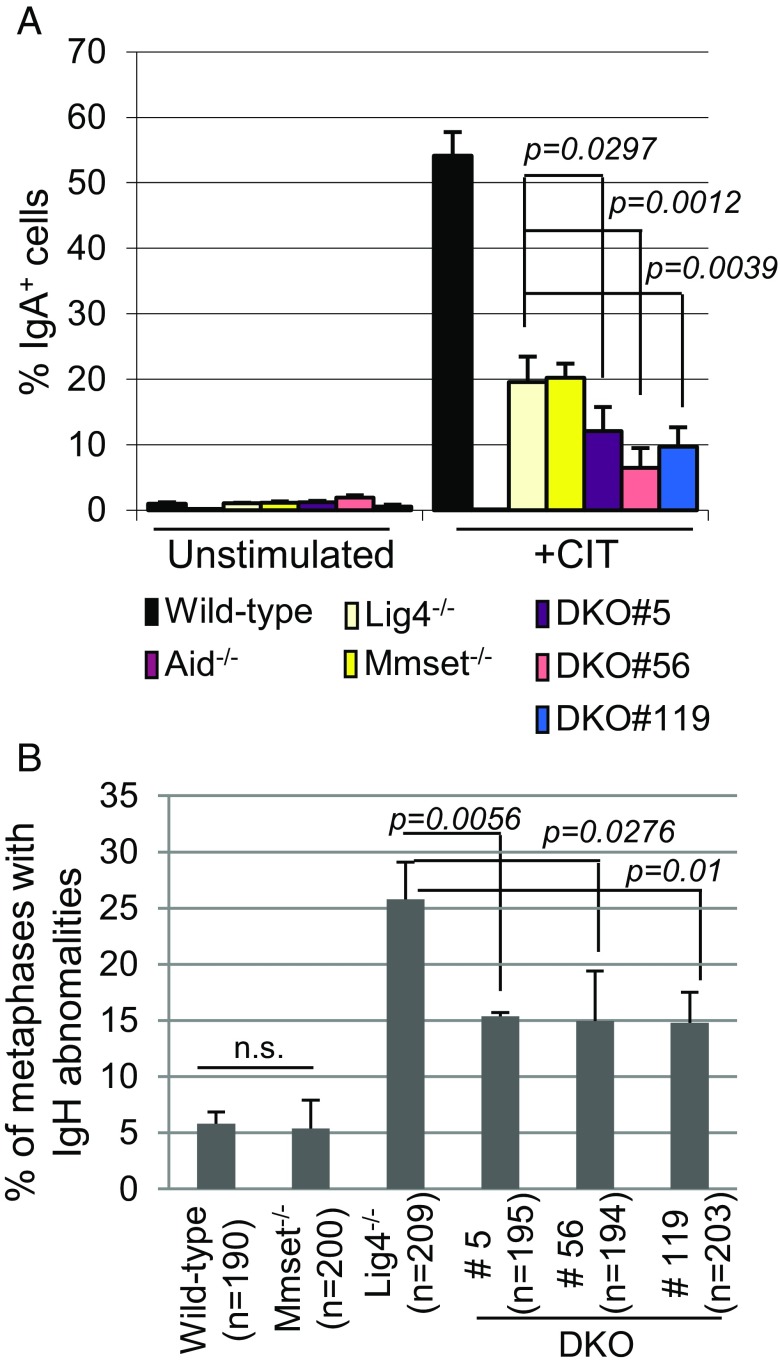
To test the potential role of MMSET in promoting generation of S-region DSBs, we developed a CH12F3-based model to evaluate the level of DSBs occurring within the IgH locus as a result of AID activity. Given that Lig4−/− B cells poorly undergo CSR and show increased frequency of unresolved chromosomal breaks involving the IgH locus caused by defective C-NHEJ (6, 7), we reasoned that, if MMSET-II promoted DSBs, its inactivation in Lig4-deficient cells should not only further reduce CSR compared with Lig4 single mutants but also, decrease the frequency of unresolved chromosomal breaks within the IgH locus. We used the exon15-Cas9 vector (pair 1) (Fig. S2A) to inactivate MMSET-II in Lig4−/− CH12F3 cells (Fig. S6A) and isolated three double-deficient (DKO) clones (5, 56, and 119), which expressed neither the MMSET-II nor the Ligase4 protein (Fig. S6B). After CIT treatment, Lig4−/− CH12F3 cells poorly underwent CSR and showed a 50% reduction of IgA-expressing cells as assayed by FACS compared with the WT control (Fig. 5A and Fig. S6C). DKO clones displayed a 50% reduction of IgA-positive cells compared with both Lig4−/− and Mmset−/− CH12F3 clones, and as in AID−/−, the great majority of them retained expression of surface IgM (Fig. 5A and Fig. S6C).
Fig. 5.
MMSET is required for efficient generation of DSB in Sµ. (A) Bar graphs showing the average percentages of switching to IgA in WT, Aid−/−, Mmset−/− (82), Lig4−/−, and three independent Mmset-Lig4−/− DKO CH12F3 clones unstimulated or stimulated with anti–CD40/IL-4/TGF-β (CIT) for 48 h. Results are from four independent experiments. SD and P values calculated by the unpaired two-tailed t test are shown. (B) Bar graphs showing percentages of IgH breaks on metaphases from WT, Mmset−/−, Lig4−/−, and three DKO clones. Total numbers of metaphases analyzed are in parentheses below each bar graph. Mean and SD are shown for the three independent experiments, with P values determined by the unpaired two-tailed t test.
We also performed IgH-FISH assays to quantify the frequency of chromosome abnormalities of the IgH locus after 2 d of CIT stimulation (Fig. S6D). Unligated chromosomal breaks within IgH occurred in a large proportion (25% on average) of metaphases from activated Lig4−/− CH12F3 cells but were detected at lower levels (5%) in Mmset−/− or WT controls (Fig. 5B). Chromosomal breaks within IgH were significantly reduced (15%) among the metaphases from DKO (Mmset-Lig4−/−) clones compared with Lig4−/− cells. During CSR, MMR activity has been proposed to convert AID DNA damage into blunt broken DNA substrates for C-NHEJ and A-EJ (37, 38). Deficiency for MMR activity in cells deficient for C-NHEJ core factor, such as XRCC4, suppresses IgH locus instability, consistent with its role in promoting DNA cleavage upstream of the end-joining phase of CSR (39). To test if, in Lig4-deficient CH12F3 cells, loss of MMR activity, as loss of MMSET-II, suppressed IgH locus instability, we inactivated the MMR gene Msh2 in WT or Lig4−/− CH12F3 cells using CRISPR-Cas9 and isolated two clones, which were null for both LIG4 and MSH2 proteins (Msh2-Lig4−/−) (Fig. S7A). CIT stimulation of both Msh2−/− and Mmset−/− CH12F3 cells triggered similar levels of switching to IgA and represented about one-half of that detected in WT cells (Fig. S7 B and C). In addition, DKO Msh2-Lig4−/− and DKO had a more pronounced reduction of CSR to IgA compared with single-deficient clones (Fig. S7 B and C). Similarly, as shown in XRCC4 and Msh2 mutant B cells (39), IgH-FISH assay revealed marked decreased levels (approximately twofold) of chromosomal breaks in DKO Msh2-Lig4−/− and DKO compared with the Lig4−/− control (Fig. S7D). Thus, reduced DSBs resulting from either defective MMR pathway or deletion of MMSET-II generated similar phenotypes regarding CSR and chromosomal aberration in Lig4−/− CH12F3 cells. Together, these results strongly suggest the role of MMSET-II in promoting AID-mediated DSBs within the IgH locus during CSR.
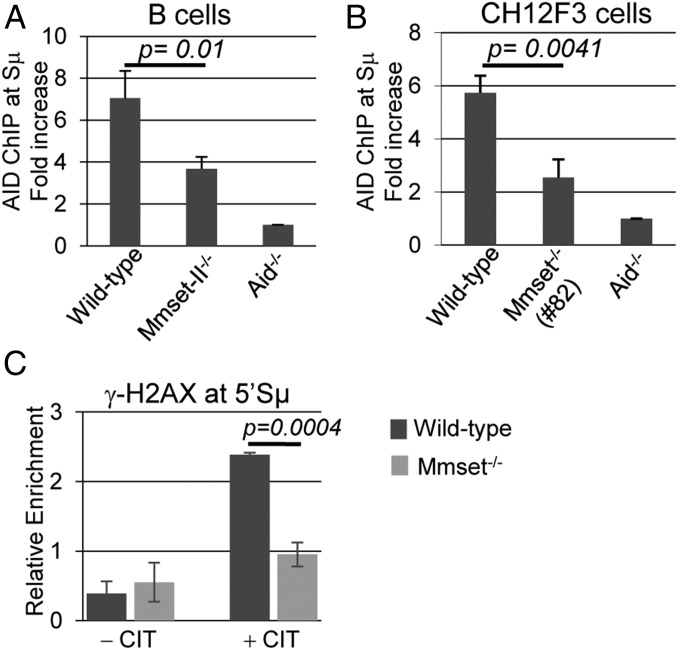
Deletion of MMSET-II Reduces both Recruitment of AID and Phosphorylation of H2AX at 5′Sµ in Activated CH12F3 Cells.
To investigate the mechanism underlying decreased DSBs at the IgH locus during CSR, we performed ChIP experiments to analyze the recruitment of AID at the 5′ sequences of the Sµ region. AID was readily detected at the Sµ region in anti-CD40– and IL-4–activated WT B cells, while binding was reduced by 50% in Mmset-II−/− B cells (Fig. 6A). We observed similar results in activated CH12F3 cells, where inactivation of MMSET-II triggered a twofold decrease in AID recruitment at 5′Sµ (Fig. 6B). In line with this, ChIP revealed that phosphorylation of H2AX (γ-H2AX), which occurs at sites of DSBs as a result of AID activity, was reduced by about twofold at the IgH locus in activated Mmset−/− CH12F3 cells compared with the WT (Fig. 6C). These results suggest that reduced AID recruitment at the Sµ region could contribute to the decreased level of DSBs in MMSET-II–deficient B cells.
Fig. 6.
Reduced recruitment of AID and γ-H2AX at 5′Sµ in MMSET-II–deficient cells. ChIP analysis of AID occupancy at the 5′Sμ region in WT, Mmset−/−, and Aid−/− splenic B cells cultured with (A) anti–CD40/IL-4 for 48 h or (B) CH12F3 cells stimulated with anti–CD40/IL-4/TGF-β (CIT) for 20 h. For each sample, AID-ChIP values were normalized to the input control and then normalized to AID-ChIP signal in Aid−/− B cells or CH12F3 cells that was assigned an arbitrary value of one. (C) ChIP analysis of γ-H2AX occupancy at the 5′Sμ region in CH12F3 cells stimulated (or not) with anti–CD40/IL-4/TGF-β (CIT) for 20 h. The relative enrichment amounts were calculated by dividing DNA amounts from immunoprecipitation by inputs for each sample. For each experiment, mean and SD are shown for three independent experiments; P values were calculated by the unpaired two-tailed t test.
Discussion
In this study, we showed that MMSET-II is largely dispensable for mouse B- and T-cell development but that it positively regulates CSR in activated B cells. Importantly, our results indicate that the HMT activity of MMSET-II is not required for the end-joining steps of AID-mediated DSBs by the C-NHEJ pathway.
MMSET-II Is Dispensable for C-NHEJ Pathway During CSR.
Based on shRNA knockdown approaches in cell lines, MMSET has been proposed to catalyze H4K20me2 at DSB sites, facilitate 53BP1 recruitment, and promote CSR (31, 32). We show here that inactivation of MMSET-II inhibits CSR in mouse B cells (Fig. 1); however, our data do not support a role of MMSET-II activity in controlling the function of components of the C-NHEJ pathway or recruitment of DSB response factor 53BP1 during IgH switching. Our HTGTS analysis shows that long resections at S junctions, which are the hallmark of impaired 53BP1 function (13), are not detected in MMSET-II–deficient B cells (Fig. 3). Similarly, MHs, which are frequently found at S junctions in B cells deficient for core C-NHEJ factors (6, 7), occur at a normal frequency when analyzed on a large scale in MMSET-deficient activated B cells (Fig. 3D). In addition, 53BP1 deficiency is associated with marked IgH locus chromosomal breaks (40), but we did not detect increased IgH locus chromosomal aberrations in Mmset−/− CH12F3 cells (Fig. 5). Thus, our results suggest that the impaired CSR in Mmset-II−/− B cells is not caused by a failure to recruit 53BP1 to sites of DSB. As our gene-targeting strategies specifically inactivate the MMSET-II protein, it remains possible that the MMSET-I isoform, which is readily expressed in Mmset-II−/− B cells, could play a role in DSB response during CSR. Assuming that knockdown approaches used in previous works equally inhibited both MMSET isoforms in CH12F3 cells, the retention of MMSET-I in our study could provide a rationale as to why we did not observe similar defects of DSB junctions as those previously reported (32). However, since the MMSET-I isoform lacks the SET domain, it is unlikely that it could recruit 53BP1 at DSBs through HMT activity. While additional work is needed to elucidate the specific role of MMSET-I in DNA repair, our results strongly support a C-NHEJ–independent role of MMSET-II in promoting CSR in B cells.
MMSET-II Promotes the Generation of AID-Mediated DSBs at Switch Regions Independent of Germline Transcription.
Direct analysis of histone modifications or of AID recruitment at core S regions has been hampered by technical difficulties because of their abundant repetitive DNA sequences. Nevertheless, using molecular and genetic approaches, we show here that stimulated MMSET-II–deficient CH12F3 cells have reduced levels of intra-Sµ deletions (Fig. 4) and decreased levels of γ-H2AX at the 5′Sµ region (Fig. 6). Furthermore, we show that, in a C-NHEJ–deficient background (Lig4−/−), lack of MMSET-II reduces the amounts of both CSR and unrepaired IgH breaks that directly arise from AID activity in CH12F3 cells (Fig. 5). Together, the data strongly suggest decreased levels of DSBs at Sμ region in MMSET-II–deficient B cells. Several levels of regulation contribute to trigger AID-mediated DSBs at S regions. Transcription through repetitive G-rich S regions has been proposed to favor the formation of R-loop structures that expose ssDNA substrates for AID activity and to promote the recruitment of AID through interactions with components of RNA polymerase II (14). Transcribed S regions are enriched for specific histone marks, such as H3K9/K14ac, H3K27ac, H3K4me3, H3K36me2, and H3K36me3, that are generally found at open chromatin (41). Chromatin remodeling factors operate at different levels during activation of the IgH locus that precedes CSR. PTIP, a key component of the mixed lineage leukemia (MLL)-like complex, is essential for deposition of H3K4me3 at acceptor S regions, and its inactivation decreases germline transcription of IgG1, IgG2b, and IgG3 and inhibits CSR in activated B splenocytes (22, 42). We found that, although MMSET-II is required to achieve high levels of H3K36me2 and H3K36me3 at the IgH regions targeted for CSR, H3K4me3 and germline transcription (after most stimulations) are largely unaffected by its deletion (Fig. 2). Thus, MMSET-II seems to function at a different stage of CSR than the MLL-like complex. Conversely, inactivation of the facilitates chromatin transcription (FACT) core components (SSRP1 and SPT16) reduces CSR and decreases the levels of H3K4me3 at targeted S regions without affecting their germline transcription (23). It was suggested that FACT-mediated H3K4me3 may serve as a marker of DNA cleavage during CSR. In agreement with previous studies showing the association of MMSET activity with methylation of K36 on histone H3 (26, 29, 43), we observed reduced levels of H3K36me2/3 at the IgH locus in MMSET-II–deficient cells (Fig. 2). Thus, as with FACT-mediated H3K4me3, MMSET could provide specific histone marks (H3K36me2 and/or H3K36me3) that could stabilize or enhance AID activity at S regions, thereby ensuring optimal levels of DNA breaks. Although we cannot exclude that MMSET-II could act indirectly by affecting the expression (or activity) of other factor(s) required for the generation of AID-mediated DSBs, taken together, our results suggest that the HMT function of MMSET promotes AID activity at the donor Sµ region during CSR in B cells.
Materials and Methods
Generation of Mmset-Deficient ES Cells.
The targeting construct was designed to replace exons 15–17 of the Whsc1 gene with the NeoR gene cassette. A 4.2-kb 5′ arm and a 3.9-kb 3′ arm were amplified by PCR separately from TC1 ES cell DNA (129 strain), cloned into pTOPO vector (Invitrogen), sequenced, and subcloned into the pLNTK vector. The targeting construct was then electroporated into TC1 ES cells, and successful targeting was assessed by Southern blot analyses using EcoRV-digested genomic DNA and 3′/5′ genomic probes (Fig. S1A). Correctly targeted TC1 clones (Mmset+/−) were identified by the appearance of a unique 13.9-kb band as determined by Southern blot with the 5′ genomic probe (probe a). Two independent clones containing one targeted Whsc1 allele were obtained. One clone was exposed to increased levels of Neomycin-G418 to select for homozygous mutant ES cells. With the probe a, the homozygous clones were identified by disappearance of the 17.8-kb germline band and retention of only the 13.9-kb band. Three independent clones were obtained. The 3′ probe was used to confirm all clones (Fig. S1B). To generate Mmset−/− chimera, two homozygous clones were used for injection in Rag-2–deficient blastocysts. Chimeric mice were analyzed at 8–16 wk of age. All animal experiments were performed under protocols approved by the Institutional Animal Care and Use Committee of Boston Children’s Hospital.
Gene Targeting by Using Cas9.
Several guide RNAs were designed to target exon 15 of the Whsc1 gene (Fig. S2A). Annealed oligonucleotides were cloned into the pX330 vector (Addgene plasmid ID 42230) as described (34) and verified by sequencing. For Cas9 deletion, 2 × 106 unswitched IgM-positive CH12F3 cells were nucleofected with 2 µg of each Cas9 vector using the 4D-Nucleofector Kit (solution SF–protocol CA-137; Lonza). Immediately after transfection, cells were incubated for 12 h in CH12 medium, and single-cell subclones were seeded into 96-well plates. Genomic DNA from single-cell subclones was used for PCR screening. For each positive clone, we synthesized cDNA to verify mRNA sequence by Sanger sequencing. Finally, the level of MMSET expression was determined by Western blot. To generate Msh2-deficient CH12F3 cells, two Cas9 vectors were designed to delete exon 12 of Msh2 gene using the protocol described above. All of primers used for guide RNA CRISPR-Cas9 vector cloning and the primers used for PCR screening are shown in Table S1.
Southern Blot Analysis.
For Mmset deletion ES cells screening, at least 5 µg genomic DNA was digested with EcoRV overnight. For intra–S-region recombination experiment, 10 µg genomic DNA from each single CH12F3 clone was digested with EcoRI overnight. Digested genomic DNA was run on a 1% agarose gel. DNA was transferred from the gels to membranes that were hybridized with corresponding probes, washed, and exposed to XAR film (Kodak Biomax) according to standard procedures.
In Vitro Cell Culture and CSR Assays.
Splenic naive B cells from Mmset-II−/− chimera and age-matched WT 129 mice were purified by CD43-negative depletion (murine CD43 microbeads; Miltenyi Biotec). B cells were cultured in RPMI 1640 supplemented with 10% FBS, 10 mM β-mercaptoethanol, and 20 mM Hepes. Naive B cells were stimulated at 0.5 × 106/mL density to induce CSR to IgG3 (20 µg/mL LPS) or IgG1 (1 µg/mL anti-CD40 or 20 µg/mL LPS and 20 ng/mL recombinant IL-4). CSR efficiency was analyzed by FACS at 72 and 96 h poststimulation. All of CH12F3 cell lines in this study were maintained in RPMI 1640 supplement with 10% FBS, 10 mM β-mercaptoethanol, 20 mM Hepes, and 5% NCTC-109 (CH12 medium). For the CSR assays, cells were stimulated with 1 µg/mL anti-CD40 (eBiosciences), 20 ng/mL recombinant murine IL-4, and 1 ng/mL TGF-β (Peprotech), referred to as CIT treatment, for 48 h and then analyzed by FACS.
Proliferation.
In total, 106 primary B cells from 129 WT or Mmset-II−/− chimera mice were grown in a six-well plate in 4 mL medium containing stimulation conditions to induce CSR to IgG1 or IgG3. Live cells were collected and counted at various time points using trypan blue dye, with counts performed in triplicate. For CH12F3 cell lines, 5 × 104 cells were cultured under condition with anti–CD40/IL-4/TGF-β, and live cells were counted as above. The results shown were obtained from three independent stimulations with each genotype.
FACS Analysis and Antibodies.
To determine switching efficiency, allophycocyanin (APC)-conjugated anti-B220 and fluorescein isothiocyanate (FITC)-conjugated anti-IgG1 or anti-IgG3 were used to stain surface B220, IgG1,and IgG3 (eBiosciences), respectively. For CH12F3 cells, the cells cultured without or with anti–CD40/IL-4/TGF-β were analyzed at 48 h by flow cytometry by staining surface with FITC-conjugated or phycoerythrin (PE)-conjugated anti-IgA antibodies and APC-conjugated or phycoerythrin-cyanine7 (PECy7)-conjugated anti-IgM antibodies (eBiosciences). All analyses were performed on an FACS Calibur or Canto II (Becton Dickinson), and data were collected from at least 2 × 104 viable cells.
ChIP Assay.
ChIP assays were performed using the Magna ChIP Immunoprecipitation Kit (Millipore) according to the manufacturer’s instruction. Briefly, 106 cells were fixed by adding formaldehyde (1% final concentration) for 10 min at room temperature, and reactions were stopped by adding glycine (final concentration 0.125 M). Cell lysates were sonicated to reduce the DNA length, and the soluble chromatin fraction was obtained after centrifugation. This fraction was diluted 10-fold in ChIP dilution buffer and incubated with 4 μg of anti–γ-H2AX (05–636; Millipore), anti-H3K4me3 (07–473; Millipore), anti-H3K36me2 (39255; Active Motive), anti-H3K36me3 (ab194677; Abcam), or anti-H3 (06–755; Millipore) overnight at 4 °C, and the immunocomplexes were collected by adding Magna Protein A/G bead by an incubation of 1 h at 4 °C. Precipitates were washed successively with low-salt, high-salt, and lithium chloride washing buffer and then once with in TE buffer (10 mM Tris·HCl, 1 mM EDTA, pH 8.0). The bound immunocomplex was treated with proteinase K and reverse cross-linked by heating at 65 °C for 10–12 h. The DNA was purified and recovered with the provided columns and subjected to PCR. Real-time PCR was performed using the ABI 7300 System (Applied Biosystems). The relative enrichment amounts were calculated by dividing DNA amounts from immunocomplexes to inputs. For AID binding at Sμ region, the fold changes were next calculated by normalizing WT, Mmset−/−- vs. Aid−/−-activated B cells, or activated CH12F3 cells.
Real-Time PCR Analysis.
Total RNA was extracted from B cells or CH12F3 cells using TRIzol (Invitrogen). The cDNA was synthesized using oligo d(T) primer and M-MLV reverse transcriptase (Invitrogen) followed by a real-time PCR using the FastStart Universal SYBR Green Master (Roche) on an ABI 7300 System (Applied Biosystems). Primer sequences are shown in Table S1.
SDS/PAGE and Western Blot.
Cells were lysed in lysis buffer (50 mM Tris, pH 8, 150 mM NaCl, 1% IGEPAL-CA630, 0.1% SDS, protease inhibitors mixture, 1 mM PMSF). Total cell lysates were separated by SDS/PAGE (8% acrylamide or Tris-Glycine gradient gel), transferred to polyvinylidene difluoride (PVDF) membranes (PerkinElmer), and probed with indicated antibodies. Primary antibodies used were NSD2 (clone 29D1; Abcam), β-Actin (clone I-19; Santa Cruz), MSH2 (clone FE11; Thermo Fisher), and AID (gift from Jayanta Chaudhuri, Memorial Sloan Kettering Cancer Center, New York). LIG4 antibody was a gift from David Schatz, Yale University, New Haven, CT. Western blot was detected by chemiluminescence (Amersham ECL Western Blotting Detection Reagents) using a Fusion Solo camera (Vilber Lourmat).
HTGTS.
HTGTS libraries were generated by the linear amplification-mediated PCR method, and the data analysis of MiSeq sequencing reads was performed as previously described in ref. 13. To account for the potential impact of differential recovery of junctions from different libraries from WT and Mmset−/− samples, the libraries were randomly sampled and normalized to the lowest number of Sμ-Sγ1 or Sμ-Sε junctions among all libraries before being used for plotting of junction distribution and MH analysis. Junctions with insertions have been excluded from MH analysis as described (6, 44, 45).
FISH.
Metaphases were prepared from different genotypes of CH12F3 cells stimulated with anti–CD40/IL-4/TGF-β to induce CSR to IgA. After 48 h of culture, cells were blocked in metaphase by incubating with Colcemid (Life Technologies), then swollen in 70 mM KCl, and fixed in 3:1 methanol:acetic acid. Metaphases were then dropped on glass slides to be used for FISH analysis (7). For IgH locus instability, the 3′ region of the IgH locus is detected using bacterial artificial chromosome (BAC)199, and the 5′ region of the IgH locus is detected using BAC207. BACs were labeled with either biotin or digoxigenin by nick translation and then hybridized to slides overnight. All probes used for FISH have been described previously (7). For each experiment, more than 50 metaphases were analyzed for each sample.
Statistics.
Statistical analysis was performed using GraphPad Prism. Data are reported as mean and SD. Differences between groups of research subjects were analyzed for statistical significance with unpaired two-tailed t test.
Supplementary Material
Acknowledgments
We thank M. Goodhardt and D. Garrick for criticism of the manuscript. AID−/− CH12 cells and Lig4−/− CH12 cells were provided by Dr. Kefei Yu. H.V.N. was supported by fellowships from French Agence National pour la Recherche, Programme d’Investissements d’avenir ANR-10-IDEX-03-02. V.K. is supported by Ruth L. Kirchstein National Service Research Award F30-CA189740-02. F.W.A. is an investigator with the Howard Hughes Medical Institute. This work was supported by NIH Grant AI077595 (to F.W.A.) and a grant from the Fondation Française pour la Recherche contre le Myélome et les Gammapathies monoclonales (to J.-C.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1701366114/-/DCSupplemental.
References
- 1.Hwang JK, Alt FW, Yeap LS. Related mechanisms of antibody somatic hypermutation and class switch recombination. Microbiol Spectr. 2015;3:MDNA3-0037-2014. doi: 10.1128/microbiolspec.MDNA3-0037-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chaudhuri J, et al. Evolution of the immunoglobulin heavy chain class switch recombination mechanism. Adv Immunol. 2007;94:157–214. doi: 10.1016/S0065-2776(06)94006-1. [DOI] [PubMed] [Google Scholar]
- 3.Alt FW, Zhang Y, Meng FL, Guo C, Schwer B. Mechanisms of programmed DNA lesions and genomic instability in the immune system. Cell. 2013;152:417–429. doi: 10.1016/j.cell.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boboila C, Alt FW, Schwer B. Classical and alternative end-joining pathways for repair of lymphocyte-specific and general DNA double-strand breaks. Adv Immunol. 2012;116:1–49. doi: 10.1016/B978-0-12-394300-2.00001-6. [DOI] [PubMed] [Google Scholar]
- 5.Boboila C, et al. Alternative end-joining catalyzes robust IgH locus deletions and translocations in the combined absence of ligase 4 and Ku70. Proc Natl Acad Sci USA. 2010;107:3034–3039. doi: 10.1073/pnas.0915067107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar V, Alt FW, Frock RL. PAXX and XLF DNA repair factors are functionally redundant in joining DNA breaks in a G1-arrested progenitor B-cell line. Proc Natl Acad Sci USA. 2016;113:10619–10624. doi: 10.1073/pnas.1611882113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yan CT, et al. IgH class switching and translocations use a robust non-classical end-joining pathway. Nature. 2007;449:478–482. doi: 10.1038/nature06020. [DOI] [PubMed] [Google Scholar]
- 8.Lumsden JM, et al. Immunoglobulin class switch recombination is impaired in Atm-deficient mice. J Exp Med. 2004;200:1111–1121. doi: 10.1084/jem.20041074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manis JP, et al. 53BP1 links DNA damage-response pathways to immunoglobulin heavy chain class-switch recombination. Nat Immunol. 2004;5:481–487. doi: 10.1038/ni1067. [DOI] [PubMed] [Google Scholar]
- 10.Reina-San-Martin B, Chen HT, Nussenzweig A, Nussenzweig MC. ATM is required for efficient recombination between immunoglobulin switch regions. J Exp Med. 2004;200:1103–1110. doi: 10.1084/jem.20041162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reina-San-Martin B, et al. H2AX is required for recombination between immunoglobulin switch regions but not for intra-switch region recombination or somatic hypermutation. J Exp Med. 2003;197:1767–1778. doi: 10.1084/jem.20030569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ward IM, et al. 53BP1 is required for class switch recombination. J Cell Biol. 2004;165:459–464. doi: 10.1083/jcb.200403021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong J, et al. Orientation-specific joining of AID-initiated DNA breaks promotes antibody class switching. Nature. 2015;525:134–139. doi: 10.1038/nature14970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matthews AJ, Zheng S, DiMenna LJ, Chaudhuri J. Regulation of immunoglobulin class-switch recombination: Choreography of noncoding transcription, targeted DNA deamination, and long-range DNA repair. Adv Immunol. 2014;122:1–57. doi: 10.1016/B978-0-12-800267-4.00001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nambu Y, et al. Transcription-coupled events associating with immunoglobulin switch region chromatin. Science. 2003;302:2137–2140. doi: 10.1126/science.1092481. [DOI] [PubMed] [Google Scholar]
- 16.Okazaki IM, et al. Histone chaperone Spt6 is required for class switch recombination but not somatic hypermutation. Proc Natl Acad Sci USA. 2011;108:7920–7925. doi: 10.1073/pnas.1104423108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pavri R, et al. Activation-induced cytidine deaminase targets DNA at sites of RNA polymerase II stalling by interaction with Spt5. Cell. 2010;143:122–133. doi: 10.1016/j.cell.2010.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willmann KL, et al. A role for the RNA pol II-associated PAF complex in AID-induced immune diversification. J Exp Med. 2012;209:2099–2111. doi: 10.1084/jem.20112145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Basu U, et al. The RNA exosome targets the AID cytidine deaminase to both strands of transcribed duplex DNA substrates. Cell. 2011;144:353–363. doi: 10.1016/j.cell.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meng FL, et al. Convergent transcription at intragenic super-enhancers targets AID-initiated genomic instability. Cell. 2014;159:1538–1548. doi: 10.1016/j.cell.2014.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qian J, et al. B cell super-enhancers and regulatory clusters recruit AID tumorigenic activity. Cell. 2014;159:1524–1537. doi: 10.1016/j.cell.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daniel JA, et al. PTIP promotes chromatin changes critical for immunoglobulin class switch recombination. Science. 2010;329:917–923. doi: 10.1126/science.1187942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stanlie A, Aida M, Muramatsu M, Honjo T, Begum NA. Histone3 lysine4 trimethylation regulated by the facilitates chromatin transcription complex is critical for DNA cleavage in class switch recombination. Proc Natl Acad Sci USA. 2010;107:22190–22195. doi: 10.1073/pnas.1016923108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang L, Wuerffel R, Feldman S, Khamlichi AA, Kenter AL. S region sequence, RNA polymerase II, and histone modifications create chromatin accessibility during class switch recombination. J Exp Med. 2009;206:1817–1830. doi: 10.1084/jem.20081678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stec I, et al. WHSC1, a 90 kb SET domain-containing gene, expressed in early development and homologous to a Drosophila dysmorphy gene maps in the Wolf-Hirschhorn syndrome critical region and is fused to IgH in t(4;14) multiple myeloma. Hum Mol Genet. 1998;7:1071–1082. doi: 10.1093/hmg/7.7.1071. [DOI] [PubMed] [Google Scholar]
- 26.Nimura K, et al. A histone H3 lysine 36 trimethyltransferase links Nkx2-5 to Wolf-Hirschhorn syndrome. Nature. 2009;460:287–291. doi: 10.1038/nature08086. [DOI] [PubMed] [Google Scholar]
- 27.Chesi M, et al. The t(4;14) translocation in myeloma dysregulates both FGFR3 and a novel gene, MMSET, resulting in IgH/MMSET hybrid transcripts. Blood. 1998;92:3025–3034. [PubMed] [Google Scholar]
- 28.Kim JY, et al. Multiple-myeloma-related WHSC1/MMSET isoform RE-IIBP is a histone methyltransferase with transcriptional repression activity. Mol Cell Biol. 2008;28:2023–2034. doi: 10.1128/MCB.02130-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuo AJ, et al. NSD2 links dimethylation of histone H3 at lysine 36 to oncogenic programming. Mol Cell. 2011;44:609–620. doi: 10.1016/j.molcel.2011.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martinez-Garcia E, et al. The MMSET histone methyl transferase switches global histone methylation and alters gene expression in t(4;14) multiple myeloma cells. Blood. 2011;117:211–220. doi: 10.1182/blood-2010-07-298349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pei H, et al. MMSET regulates histone H4K20 methylation and 53BP1 accumulation at DNA damage sites. Nature. 2011;470:124–128. doi: 10.1038/nature09658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pei H, et al. The histone methyltransferase MMSET regulates class switch recombination. J Immunol. 2013;190:756–763. doi: 10.4049/jimmunol.1201811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen J, Lansford R, Stewart V, Young F, Alt FW. RAG-2-deficient blastocyst complementation: An assay of gene function in lymphocyte development. Proc Natl Acad Sci USA. 1993;90:4528–4532. doi: 10.1073/pnas.90.10.4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cong L, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakamura M, et al. High frequency class switching of an IgM+ B lymphoma clone CH12F3 to IgA+ cells. Int Immunol. 1996;8:193–201. doi: 10.1093/intimm/8.2.193. [DOI] [PubMed] [Google Scholar]
- 36.Hodgkin PD, Lee JH, Lyons AB. B cell differentiation and isotype switching is related to division cycle number. J Exp Med. 1996;184:277–281. doi: 10.1084/jem.184.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schrader CE, Edelmann W, Kucherlapati R, Stavnezer J. Reduced isotype switching in splenic B cells from mice deficient in mismatch repair enzymes. J Exp Med. 1999;190:323–330. doi: 10.1084/jem.190.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stavnezer J, Schrader CE. Mismatch repair converts AID-instigated nicks to double-strand breaks for antibody class-switch recombination. Trends Genet. 2006;22:23–28. doi: 10.1016/j.tig.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 39.Eccleston J, Yan C, Yuan K, Alt FW, Selsing E. Mismatch repair proteins MSH2, MLH1, and EXO1 are important for class-switch recombination events occurring in B cells that lack nonhomologous end joining. J Immunol. 2011;186:2336–2343. doi: 10.4049/jimmunol.1003104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Franco S, et al. H2AX prevents DNA breaks from progressing to chromosome breaks and translocations. Mol Cell. 2006;21:201–214. doi: 10.1016/j.molcel.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 41.Zan H, Casali P. Epigenetics of peripheral B-cell differentiation and the antibody response. Front Immunol. 2015;6:631. doi: 10.3389/fimmu.2015.00631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schwab KR, Patel SR, Dressler GR. Role of PTIP in class switch recombination and long-range chromatin interactions at the immunoglobulin heavy chain locus. Mol Cell Biol. 2011;31:1503–1511. doi: 10.1128/MCB.00990-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jaffe JD, et al. Global chromatin profiling reveals NSD2 mutations in pediatric acute lymphoblastic leukemia. Nat Genet. 2013;45:1386–1391. doi: 10.1038/ng.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frock RL, et al. Genome-wide detection of DNA double-stranded breaks induced by engineered nucleases. Nat Biotechnol. 2015;33:179–186. doi: 10.1038/nbt.3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wei PC, et al. Long neural genes harbor recurrent DNA break clusters in neural stem/progenitor cells. Cell. 2016;164:644–655. doi: 10.1016/j.cell.2015.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.