Significance
The lack of available data, including written historical sources and natural proxy archives, has constrained us when disentangling the effects of climate change on the prevalence of infectious diseases. We first reconstructed human epidemics in China over the last two millennia and analyzed the impacts of climate change on the prevalence of human epidemics at various time scales. We show that long-term trends of cold and dry conditions indirectly facilitated the prevalence of epidemics through locusts and famines. Nevertheless, temperature showed unstable associations with epidemics on a small time scale. Our study highlights the urgent need to investigate scale-dependent impacts of climate change on the prevalence of diseases.
Keywords: epidemics, climate, scale dependent, natural disaster, disease
Abstract
A wide range of climate change-induced effects have been implicated in the prevalence of infectious diseases. Disentangling causes and consequences, however, remains particularly challenging at historical time scales, for which the quality and quantity of most of the available natural proxy archives and written documentary sources often decline. Here, we reconstruct the spatiotemporal occurrence patterns of human epidemics for large parts of China and most of the last two millennia. Cold and dry climate conditions indirectly increased the prevalence of epidemics through the influences of locusts and famines. Our results further reveal that low-frequency, long-term temperature trends mainly contributed to negative associations with epidemics, while positive associations of epidemics with droughts, floods, locusts, and famines mainly coincided with both higher and lower frequency temperature variations. Nevertheless, unstable relationships between human epidemics and temperature changes were observed on relatively smaller time scales. Our study suggests that an intertwined, direct, and indirect array of biological, ecological, and societal responses to different aspects of past climatic changes strongly depended on the frequency domain and study period chosen.
Several studies have suggested possible linkages between climatic changes and the outbreaks and range expansion of many infectious diseases, such as cholera (1–3), malaria (4), diarrhea (5), and plague (6–8) (for review, see refs. 9 and 10). Both the Intergovernmental Panel on Climate Change (IPCC) and the World Health Organization (WHO) report an increased risk of the prevalence of infectious diseases under the ongoing and predicted rise in global temperature and shifts in the hydrological cycle (9, 11, 12). Under a warmer climate, disease transmissions are facilitated, as vectors expand their range from tropical to temperate regions or reproduce more readily with increased rain or flooding (9–12). However, our current understanding of the impacts of climate change on the prevalence of diseases is mainly restricted to information from the past decades, as the above studies show.
Using short-term data to predict long-term changes may lead to incorrect results. The predicted ecological effects of climate change may differ when data represent different time scales (length of study interval) and frequency domains (short- or long-term trends) (4, 13, 14). Our understanding of the possible effects of climate variability on epidemics at time scales of several centuries to millennia is currently limited by the lack of written historical sources and natural proxy archives (but see refs. 7, 8, and 15). However, a few studies based on preindustrial long-term data suggest that cooling, rather than warming, increases the prevalence of epidemics in Europe (15, 16), and thus contradict results based on short-term data alone (17–19). As the apparent contradiction is mainly caused by the availability of data, this highlights the urgent need to investigate scale-dependent impacts of climate change on the prevalence of diseases over varying time scales. A long-term paleoperspective is broadly missing.
China has a long history of recording political events and natural disasters (20). Scientists have edited the records of natural and manmade disasters over the last 3,000 y, including explicit information on location and year (e.g., for locust outbreaks, see ref. 21; for natural and manmade disasters, see ref. 22; for meteorological records, see ref. 20). This was done by extracting the original records from standard histories and local gazetteers. These records have been successfully used to reconstruct outbreaks of locusts (13, 23) and human plague (7) in ancient China, and link them to climatic changes at different spatiotemporal scales (7, 13, 24, 25). However, such studies on the prevalence of human epidemics are still lacking.
In this study, we first reconstructed a time series of two millennia of human epidemic events in China (AD 1–1911). This was done using records extracted from the book A Compendium of Chinese Meteorological Records of the Last 3,000 Years (20), and using methods established by Tian et al. (13). Most of the records of human epidemic events (Yi, in Chinese) were characterized by reports of massive deaths of people over a short time period within several counties, mostly caused by unknown infectious diseases. The symptoms of reported human epidemics in history are similar to those of plague, cholera, or malaria (26, 27). However, the names of specific pathogens or diseases were not cited in most cases. Nevertheless, the records are still valuable when studying the effects of climate change on the prevalence of infectious diseases in general at a large spatial–temporal scale, and when assessing possible links between infectious diseases and social crisis (15).
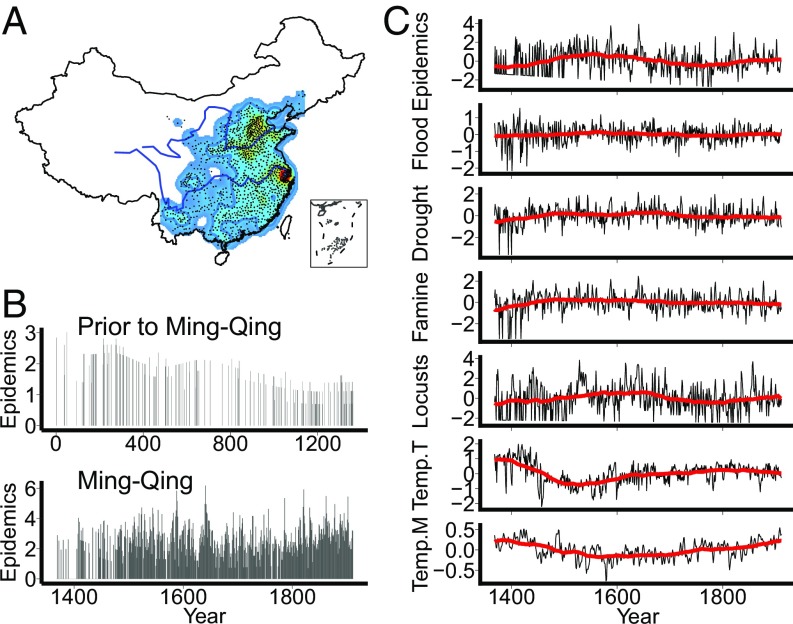
The historic records of human epidemic events over the past two millennia were mainly centralized on the mainland of eastern China, particularly the lower parts of the Yellow River and the Yangtze River regions, and along coastlines (Fig. 1A). Considering the quality and quantity of human epidemic records, we divided our data into two time periods: the Ming–Qing period (AD 1368–1911) and before the Ming–Qing period (AD 1–1367) (Fig. 1B). Climatic and biological time series were used in the analyses, from sources including temperature (28–33), drought, flood, locusts, and famine (see Methods and Fig. 1C and SI Appendix, Fig. S1 and Table S1). Abbreviations for Temp.G, Temp.L, Temp.M, Temp.T, Temp.W, and Temp.Y represent the reconstructed temperature series from refs. 28–33, respectively. As the epidemics data before the Ming–Qing period were too sparse, we only focused on analyzing the data of the Ming–Qing period, but presented analyses of the data before the Ming–Qing period in SI Appendix.
Fig. 1.
(A) Spatial distribution of human epidemic records. (B) Annually resolved time series of epidemic events before and during the Ming–Qing period. (C) All of the linearly detrended annually resolved time series during the Ming–Qing period. In A, the two blue lines show the Yellow River (Upper) and the Yangtze River (Lower), and the black dots represent locations with human epidemic records. The color ramp from light blue to red represents the density of records from low to high for the Ming–Qing period. In B, human epidemic time series were corrected by removing the trend of the recording effort and log transformed. In C, all time series were linearly detrended by regressions against year. Red lines are the 100-y moving average values.
To identify the scale-dependent impacts, the relationships between temperature and human epidemic events, drought, flood, locusts, and famine were analyzed by considering the frequency domains (short- or long-term trends) and time scales (i.e., length of study interval). Analyses of frequency domains aimed to study the associations of high frequency with rapid changes and low frequency with slow changes in the studied system of feedbacks to climate change. Data were analyzed at two time scales by using long-term data covering the Ming–Qing period (more than 500 y) and short-term data within 100-y moving windows, comparable to the time scale used in the literature. Using 100-y moving windows in our analyses helped us reveal the effects of time scale, and the stability of associations between epidemics and climate over time.
Results
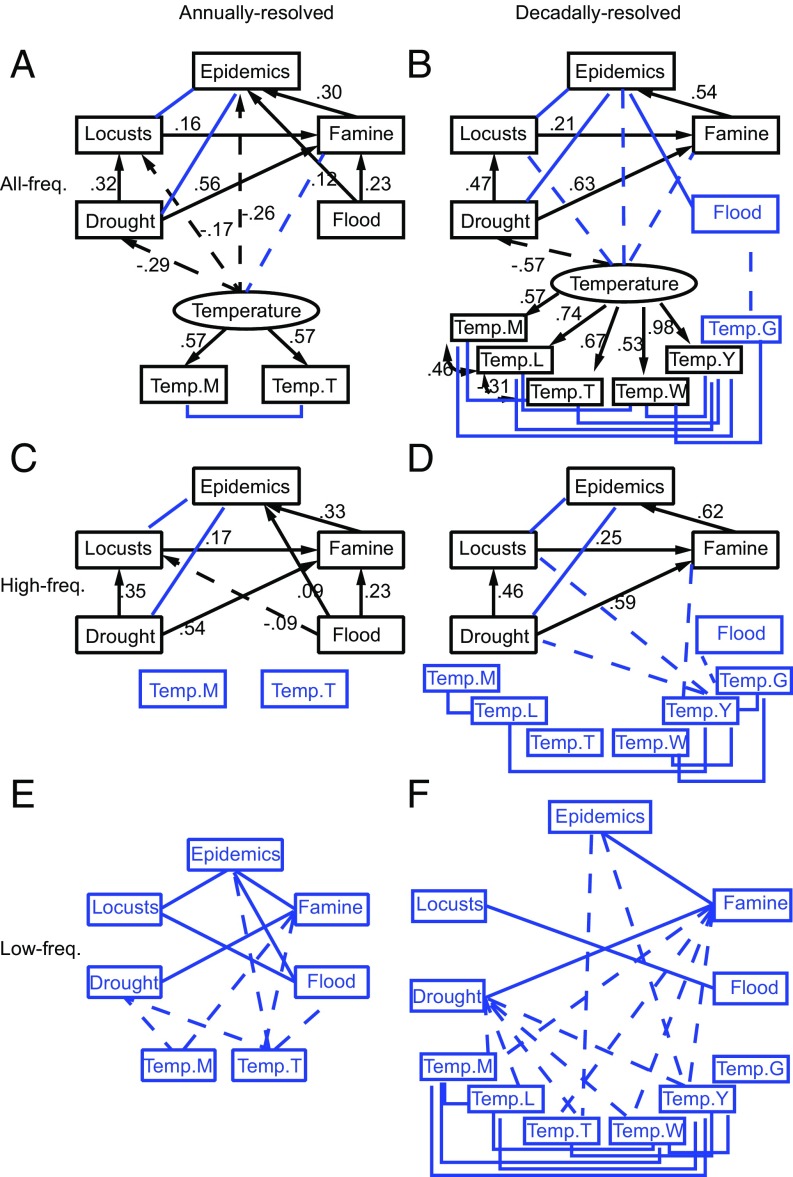
For the Ming–Qing period, correlation analyses for both annually resolved and decadally resolved data showed that most of the variables of epidemic events, locusts, famine, drought, and flood are significantly (P < 0.05) and positively correlated with each other; most temperature time series showed significant (P < 0.05) negative correlations with epidemic events via locusts, famine, drought, and flood, either directly or indirectly (Fig. 2 A and B and SI Appendix, Tables S2 and S3). For the Ming–Qing period, correlation analyses using high-frequency data also showed significant positive correlations among most of the variables of epidemic events, locusts, famine, drought, and flood (P < 0.05); but only Temp.Y showed significant negative associations (P < 0.05) with epidemic events via locusts, famine, and drought using decadally resolved data (Fig. 2 C and D). Correlation analyses using low-frequency data showed similar correlation patterns with those at all-frequency domains, except that the number of significant correlations became fewer; most temperature series (except for Temp.G) showed significant direct or indirect associations (P < 0.05) with epidemic events via drought and famine (Fig. 2 E and F).
Fig. 2.
Combined results of correlation and SEM analyses for the Ming–Qing period, when data were analyzed on a large time scale covering over 500 y and at three frequency domains, by using annually resolved and decadally resolved data. (A and B) All-frequency data, (C and D) high-frequency data, and (E and F) low-frequency data. SEM analysis is not applicable to highly correlated low-frequency data. Blue squared lines and variables indicate significant associations from correlation analysis, and black lines (labeled with standardized path coefficients) and variables indicate significant and causal associations (with arrows) selected by SEM analysis. Latent variables are indicated by ovals in the SEM analysis, and are used for simplification to show the correlations of temperature variables with other variables in A and B. Solid and dashed straight arrows and lines represent significant positive and negative relationships, respectively (P < 0.05). Autocorrelation of time series was adjusted in the correlation analyses following ref. 39.
For the Ming–Qing period, SEM analyses using all-frequency data showed that for both the annually and decadally resolved data, temperature variables had indirect negative effects on human epidemic events through two pathways: temperature→(−) drought→(+) famine→(+) epidemics; temperature→(−) drought→(+) locusts →(+) famine→(+) epidemics (+ indicates a positive effect, − a negative effect) (Fig. 2 A and B). In addition, when looking at the annually resolved data, temperature had a direct negative effect on human epidemic events and an indirect negative effect through the following pathway: temperature→(−) locusts→(+) famine→(+) epidemics. Floods had a direct positive effect on epidemic events (Fig. 2A). SEM analysis using high-frequency data showed that drought, flood (only for annually resolved data), locusts, and famine showed significant and positive effects on the prevalence of epidemics (Fig. 2 C and D), but temperature showed no significant effects on epidemic events. For the period before Ming–Qing, SEM results did not reveal an indirect pathway effect of temperature on epidemic events, but highlighted the direct negative effects of temperature on epidemic events for both annually and decadally resolved data (SI Appendix, Fig. S2).
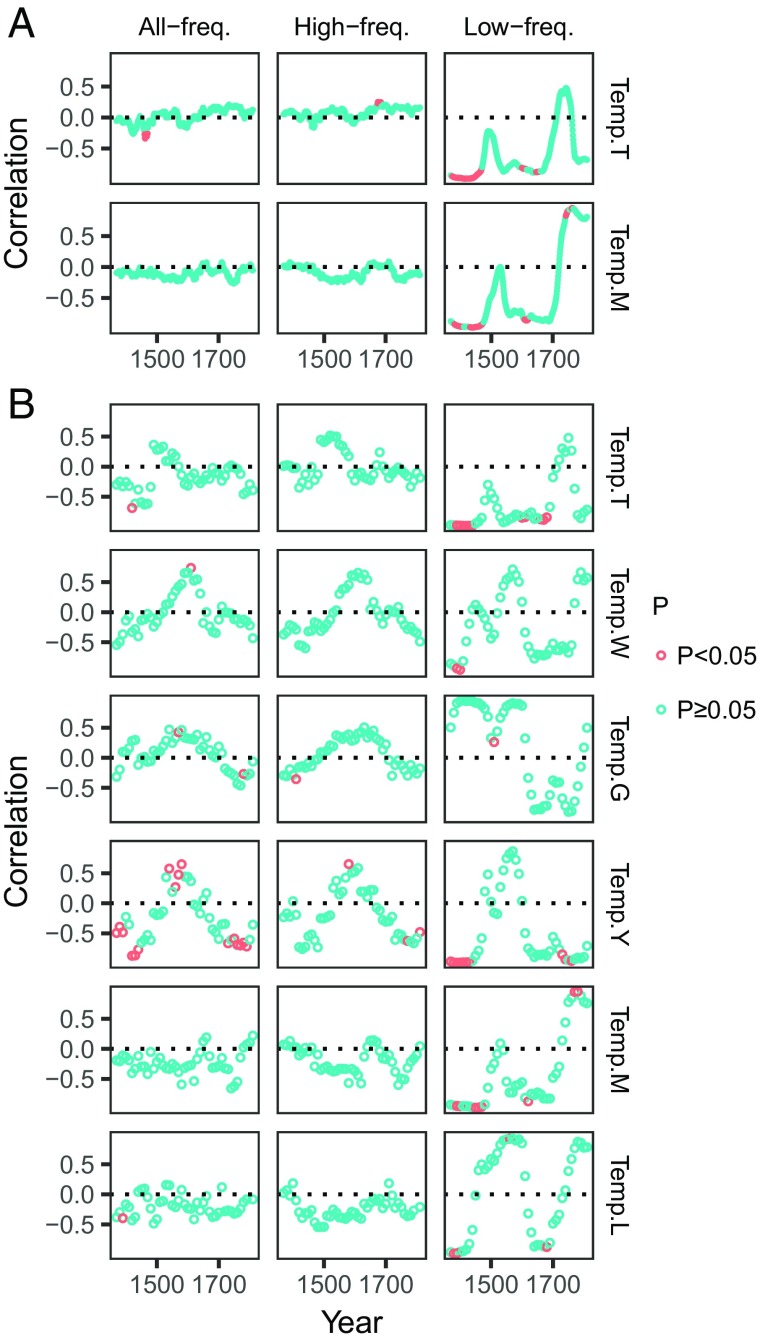
For the Ming–Qing period, when data were analyzed on a small time scale using 100-y moving windows, correlations between human epidemic events and temperature were unstable over time, but low-frequency data showed mainly negative associations (Fig. 3). Both positive and negative correlations between epidemic events and temperature were observed, but significant correlations were very few (Fig. 3). Both annually and decadally resolved data (Temp.T and Temp.M) showed similar correlation patterns. Global decadally resolved data (Temp.M and Temp.L) showed similar correlation patterns, but hardly differed from those of the four Chinese decadally resolved data series (they also had similar correlation patterns). The major similarity between those associations was that most of them had one or two positive correlation peaks around AD 1600 and 1800 (Fig. 3).
Fig. 3.
The correlations between the temperature proxies and human epidemic events at all-, high-, and low-frequency domains for the Ming–Qing period when the (A) annually resolved and (B) decadally resolved data were analyzed on a small time scale using 100-y moving windows. Each dot represents a correlation coefficient between temperature and a variable within a 100-y time window. Red dots indicate that the correlation coefficients are statistically significant (P < 0.05). Autocorrelation of time series was adjusted in the correlation analyses by following ref. 39.
Discussion
Our results indicate that the effects of climate on the prevalence of human epidemic events are likely scale dependent, which may help explain various contradicting observations in the literature. Using long-term data on a scale of centuries to millennia, we found that a cold climate was associated with more human epidemic events in ancient China via increased frequencies of drought and locusts, followed by famine. Low-frequency (or long-term trend) temperature changes mainly contributed to the observed negative associations between human epidemic events and temperature, suggesting that climate cooling could have resulted in collapsed agricultural production and reduced health conditions due to famine, thereby increasing the prevalence of human epidemic events. Our results on disease prevalence derived from long-term data are inconsistent with previous research results derived from short-term data (often of only several decades). Further, our results from a small time scale (i.e., 100 y) indicated that the associations between human epidemic events and temperature were unstable over time. This suggests that the impacts of climate on human epidemic events are scale dependent (i.e., dependent on data frequency and time scale). We urge additional in-depth studies to look at the scale-dependent effects of climate change on the prevalence of human diseases.
Climate change is expected to produce profound effects on both the prevalence and severity of infectious diseases that are transmitted by their hosts and vectors, as many host or vector species of pathogens are sensitive to temperature and rainfall (9, 10, 34–36). When looking at short-term data, climate warming reportedly increases the risk of disease transmission due to vectors expanding their range from tropical to temperate regions or reproducing more readily with increased rain or flooding (9–12). In the case of malaria, tropical mosquitoes are vectors for the disease and more actively transmit it under warm and wet conditions (17). Malaria tends to occur during the rainy seasons because the mosquitoes prefer to lay their eggs in aquatic habitats or containers. In contrast, periods of drought may promote the transmission of the West Nile virus, as under these conditions mosquitoes and birds carrying the virus are more likely to approach people looking for water. In China, warm temperature has been found to increase the prevalence of several mosquito-borne diseases, such as malaria and dengue (18) and human plague (19).
However, in a few studies using long-term data, a cold climate has been proposed to increase the prevalence of human epidemic events in ancient Europe (15, 16). By using tree-ring chronologies from the Russian Altai and European Alps over the past two millennia, Büntgen et al. (16) identified the Late Antique Little Ice Age (LALIA) as occurring from AD 536 to around 660. The authors further suggested that the Justinian plague might be related to the LALIA. However, the quantitative relationship between climate and human epidemic events was not analyzed. By using historical records, Zhang et al. (15) found that climate cooling in the Little Ice Age (LIA) caused the collapse of agriculture, increasing both the incidence of famine and human epidemic events during AD 1500–1800 in Europe. Specifically, climate cooling led to a poor food supply, poor human nutritional status, and more migration, and hence an increase in the incidence of human epidemic events. However, the roles of other climatic factors (e.g., drought and flood) were not assessed. The resulting impacts of precipitation on human epidemic events are not consistent in the literature. The second and third pandemics of human plague are linked to increased precipitation, based on paleoclimatic reconstruction from AD 1280–1350 (6, 37). Flooding was found to facilitate the spatial transmission of human plague during the third pandemic in China (AD 1772–1969) (7). However, drought events in central Asia were found to have triggered the spread of the Black Death of AD 1347–1353 to Europe (8).
In our study, using long-term data, we have demonstrated that a cold climate had an overall positive effect on the prevalence of human epidemic events. Both annually and decadally resolved data showed similar temperature-induced responses of human epidemic events via drought, locusts, and famine (Fig. 2). This is consistent with results (15, 16) obtained from long-term data in Europe, but contradicts more current observations using short-term data (17–19). Furthermore, we revealed three indirect pathways from temperature to human epidemic events via drought, flood, locusts, and famine.
In this study, the negative bottom-up effects between temperature, drought, flood, and locusts were consistent with our previous studies showing that cold climates lead to more droughts, floods, and locusts over long-term periods (13, 24, 25). These temperature-induced climatic and biological disasters could destroy agricultural production, resulting in more famine, an increase in the price of rice, and triggering social crisis (15, 25). In many historical documents, famines are often recorded as linked to outbreaks of locusts, drought, and flood, causing the collapse of agricultural production (20). Our results show that climate change can increase the incidence of human epidemic events through an increased incidence of famine. Famine has been found to be linked to the poor nutritional conditions of people in Europe (15); it may have reduced immunity, hence increasing the risk of infection from various diseases. Famine induced by cool temperatures has been reported to trigger wars and large-scale immigration, further accelerating the transmission of disease (38).
Our results suggest that the observed negative effects of temperature on human epidemic events (as well as droughts and locusts) were mainly attributed to its low-frequency components. However, both positive and negative associations were detected when data were analyzed on a small time scale using 100-y moving windows (Fig. 3). The observed differences in the associations of human epidemic events with temperature, in relation to different frequency domains and time scales, may partially explain why the results of our study contradict those found in the literature. High-frequency components of climate, which represent the short-term trends of climate, should be more associated with direct effects on epidemic events, while low-frequency data, which represents the long-term trends of climate, should be more associated with indirect, delayed, or accumulative effects. Short-term data, which represent a short study interval, mainly capture high-frequency components, while long-term data, which represent a long study interval, should capture both the low-frequency and high-frequency features of variables. Thus, the ecological effects of climate on diseases and their vectors may differ when investigated at different frequency domains and time scales (14).
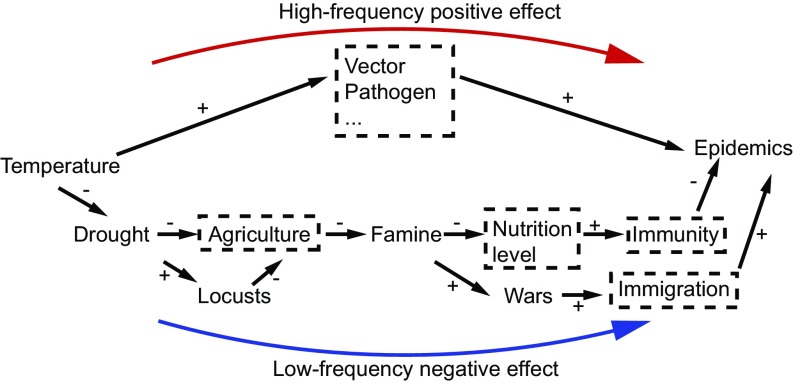
Koelle et al. (3) found that the low-frequency transmission rate of cholera was negatively correlated with rainfall and river water levels in India, but the high-frequency transmission rate of cholera was positively correlated with the El Niño Southern Oscillation (ENSO) or sea surface temperature (SST) and rainfall. Tian et al. (13) demonstrated that locust occurrences were negatively associated with temperature when data were analyzed using long-term data covering over two millennia, contradicting the positive association observed between locusts and temperature using short-term data covering only several decades (23). In China, a warm climate would benefit the growth and overwintering survival of many insect and rodent vectors directly at small time scales of several decades, but at large time scales of centuries or millennia, cold climate would lead to an increase in drought and locusts (25). This in turn would lead to more famine (as shown in this study), due to collapses in agricultural production. Climate cooling-induced famine would facilitate disease infection and transmission by weakening both health conditions and immunity, due to the large-scale immigrations of people (15). Based on this study and previous observations in the literature, we proposed a scale-dependent mechanism of temperature-induced occurrences of human epidemic events (Fig. 4).
Fig. 4.
The hypothesized scale-dependent mechanisms for the temperature-induced prevalence of human epidemic events in our study. +, positive effect; −, negative effect; and dashed rectangle, variables that are not available in this study. The blue line indicates the low-frequency effects of temperature with slow changes, and the red line indicates the high-frequency effects of temperature with rapid changes.
First, a slowly cooling climate (i.e., low-frequency temperature) showed a positive effect on the prevalence of epidemic events via increased droughts and locusts which destroyed agriculture, caused more famine, and then reduced human immunity. People with low immunity and poor health conditions would be more susceptible to infection by pathogens. Such an indirect effect was often detected by using long-term data (i.e., studies using large time scales). Second, a rapidly warming climate (i.e., high-frequency temperature) would benefit the reproduction and development of vectors, which would increase the risk of disease infection. Such a direct effect was often captured by using short-term data (i.e., studies using small time scales). It was also possible to detect the indirect negative associations at low-frequency domains using short-term data (Fig. 3). However, data transformation by smoothing or detrending often removed the low-frequency trend, which significantly reduced the possibility of finding a negative relationship between human epidemic events and temperature (39).
When data were analyzed on a small time scale, we found two obvious correlation peaks (most of the correlations were positive) around AD 1600 and 1800 (Fig. 3), corresponding to the cold phases (AD 1600, all temperature series) or relatively cold periods (AD 1800, except for Temp.T) (SI Appendix, Fig. S1). We did not know the exact reasons for this, but we speculated that these were likely caused by the direct positive effects of high-frequency temperature (Fig. 4). Time series of epidemic events and temperature are often composed of both high-frequency and low-frequency components. Long-term trends of climate cooling would increase epidemic events indirectly via drought, flood, locusts, famine, and wars (Fig. 4). However, at the cold phases around AD 1600 and AD 1800, an increase of temperature would benefit the vectors of pathogens, which would in turn increase the transmission of diseases, resulting in a direct positive association between temperature and epidemic events (SI Appendix, Fig. S3). Thus, the unstable associations seen on the small time scale were more likely caused by the conflicting effects of the high-frequency (direct and positive) and low-frequency (indirect and negative) components of temperature (Fig. 4 and SI Appendix, Fig. S3). Future studies should pay more attention to the scale-dependent effects of temperature on human epidemic events.
It is also notable that some differences existed among different temperature series in their relationships with epidemics. In the SEM analysis and correlation analysis at low-frequency domains, only Temp.G showed no significant effects on the other variables (Fig. 2). This was because Temp.G was obviously different from the other temperature proxies; the major difference was that Temp.G showed a trend of increasing temperature during AD 1400–1700 (SI Appendix, Fig. S1). For the decadally resolved temperature variables, all series showed unstable associations (most of them were insignificant) with human epidemic events, but there were more negative correlations at low-frequency domains (Fig. 3). Two global temperature series (Temp.M and Temp.L) showed similar correlation patterns, while all Chinese temperature series except for Temp.T showed similar correlation patterns (Fig. 3). These results suggested that the effects of global and regional temperature on epidemic events were similar for the large time scale (Fig. 2 A and B), but different for the small time scale (Fig. 3). This is reasonable, because climate would differ greatly at regional levels, but the long-term trends of climate change would be similar under the global drivers.
The results using annually resolved and decadally resolved data were similar for the large time scale (Fig. 2), but the correlation patterns between them were different for the small time scale using 100-y time windows (Fig. 3). The difference observed may be due to the removal of high-frequency data of less than 10 y from the decadally resolved data. The sample size of annually resolved data (over 500 y) was much larger than the decadally resolved one (over 50 decades). The analysis of decadally resolved data allowed us to use more temperature series.
Apart from climatic and biological factors, social factors such as wars and population density may also affect the prevalence of human epidemic events. It is expected that wars and population density facilitate the prevalence of infectious diseases; wars would disturb agricultural production and medical aid, while a high population density would increase the risk and spread of infection. By introducing war frequency (38) and population density (40) data into our analysis, we found a negative effect of temperature on human epidemic events via war frequency (SI Appendix, Fig. S4). Our previous studies have demonstrated that climate cooling could increase war frequency via agricultural collapse due to more droughts and locusts (38). In fact, it is well documented that wars have caused social chaos, large-scale immigrations and famine, increasing the prevalence of human epidemic events (41). However, we did not find a pathway effect from population density to human epidemic events; instead, we found that high-frequency population density was negatively correlated with human epidemic events (r = −0. 16, P < 0.05, SI Appendix, Table S3). We speculated that the prevalence of human epidemic events led to human population declines. Indeed, high mortality was frequently reported in the historical records of epidemic events.
In summary, we found that temperature had negative effects on the prevalence of human epidemic events on a large time scale, mainly at low-frequency domains. However, these effects were unstable on a small time scale; positive associations were more likely detected at high-frequency domains. The positive effects of drought, flood, locust, and famine events on the prevalence of human epidemic events were consistent at both high- and low-frequency domains. Our results suggest that, over short-term periods, we should pay more attention to the effects of climate warming and drought, flood, locust, and famine events; over long-term periods we should be cautious as to the effects of climate cooling on the prevalence of disease transmission. The effects of temperature on human epidemic events were often scale dependent and far more complex than expected. Using short-term data to predict the long-term trend of disease prevalence should be done with caution.
Methods
Data Sources.
The time series of human epidemic events, drought, flood, famine, and locusts were extracted from the book entitled A Compendium of Chinese Meteorological Records of the Last 3,000 Years (20). The compendium was compiled over a period of more than 20 y by a team of Chinese scientists and historians (42). It presents records of significant weather events dating back to over 3,000 y ago. Over 8,000 historical documents were used and over 100,000 weather-related records were identified. Each record was carefully cross-checked for consistency with other reports and presented by date and location. Most of the records came from standard histories and local gazetteers in the Ming–Qing period. Standard histories were compiled by governments, while local gazetteers (including historical, geographical, economic, administrative, and natural events) were compiled by local officials. By the end of the Ming dynasty, almost every county or prefecture had its own gazetteer.
Time Series Reconstruction of Human Epidemic Events.
A total of 250 and 5,131 human epidemic records were extracted from the compendium for the period before Ming–Qing (AD 1–1367) and from the Ming-Qing period (AD 1368–1911), respectively. Most of the records were not identified as linked to specific kinds of diseases, and only some records were able to be linked to rodent-borne plague, malaria, and cholera. Human epidemic records vary greatly spatially in China (Fig. 1). Some provinces (e.g., Xinjiang Autonomous Region, Xizang Autonomous Region, Hainan province, and Taiwan province) have a small number of records. In this study, we mainly reconstructed time series of human epidemic events from eastern China (shadowed region in Fig. 1).
Our analysis focused on the data from the Ming–Qing period, because there were far more records of epidemic events (over 5,000 records) from this period. For the Ming–Qing period, we defined the prevalence of epidemic events for years with no epidemic records as zero, as the recording efforts of this period were extensive. For the period before Ming–Qing, there were only 250 epidemic records, due to the poor recording system at this time. Thus, for the period before Ming–Qing, we defined the prevalence of epidemic events in years with no epidemic records as missing values.
For the Ming–Qing period, the description of epidemic events was brief and informative, reporting the extent of infection and mortality. All of these descriptions were classified into ranks from 1–5 according to their severity (for details, see SI Appendix, Text S1). During this period, the total number of counties experiencing epidemics did not change too much, and the areas of the counties were relatively stable. Therefore, the county was used as the basic spatial unit in reconstructing the time series. The prevalence of human epidemics in a year was defined as the sum of the rank of human epidemic events of the reporting county. For the period before Ming–Qing, the descriptions of human epidemic events were very simple, and the number of administrative units (prefecture and county) and areas varied greatly. For this period, the prevalence of epidemic events in a year was defined as the number of administrative units with human epidemic records.
Climatic and Biological Variables.
Temperature, drought, flood, locusts, and famine were used to represent the potential influencing factors. Records of famine, drought, flood, and locusts were also extracted from the book entitled A Compendium of Chinese Meteorological Records of the Last 3,000 Years (20). The number of counties or prefectures with records of drought, flood, and famine in a year were used to reconstruct the time series of drought, flood, and famine. The locust time series during AD 1–1911 was obtained from Tian et al. (13).
We have only two annually resolved temperature series, but six decadally resolved ones (SI Appendix, Table S1). Therefore, both annually and decadally resolved data were used in the analysis. Temp.T is the only available long-term annually resolved temperature proxy inside our study region. The decadally resolved temperature proxy Temp.W matches our research region best, while Temp.Y and Temp.G represent the temperature of China in its entirety, and Temp.M and Temp.L are two temperature proxies representing the northern hemisphere. Annually resolved data had a much larger sample size (over 500 y) than the decadally resolved ones (over 50 decades). However, the decadally resolved series helped us detect the regional or global differences in the associations between epidemic events and temperature.
Statistical Analysis.
Data preparation.
All time series of drought, flood, locusts, famine, and epidemic events could be biased, mainly due to the differences in recording efforts in the study period. Following the method of Tian et al. (13), we used the total number of meteorologically related records to represent the recording effort (SI Appendix, Fig. S5). By fitting the trend of the recording effort using a generalized additive model, we adjusted the reconstructed time series using the formula: T = (Gmax + G)/(2G) × T.orig, where T is the adjusted time series, T.orig is the original time series, G is the smoothed meteorological record number, and Gmax is the maximum of G (13).
After correcting for recording effort, all data were log transformed and further detrended using linear regression to remove long-term linear trends in time, without removing the true long-term variations (low-frequency components) of these variables (SI Appendix, Datasets S1 and S2). Temperature variables were also detrended using linear regressions. The time series of temperature, drought, flood, locusts, famine, and epidemic events used for statistical analysis are shown in Fig. 1 and SI Appendix, Fig. S1. Time series often are composed of both high-frequency (rapid changing) and low-frequency (slow changing) components. The slow-changing environmental processes are also important in studying population dynamics of biological organisms, but they are often ignored by differencing or smoothing methods (39). To identify the frequency-dependent effects, the time series described above were split into high-frequency and low-frequency series by using a 100-y moving average filter; that is, the low-frequency series was the data smoothed by the 100-y moving average filter, and the high-frequency series was the residuals of the 100-y moving average filter. To identify the differences between results analyzed at large and small time scales (i.e., length of study interval), all-frequency, high-frequency, and low-frequency data were also analyzed on a small time scale using a 100-y moving window. This allowed comparison with analyses conducted at the large time scale of over 500 y.
Correlation and structural equation models.
Correlation analysis was applied to map the relationships among these variables by following an adjusted significance testing method accounting for autocorrelation (13, 39), because autocorrelation in ecological time series often violates assumptions of independence in correlation tests. Structural equation models (SEMs) were conducted to test the direct and indirect causal pathway assumptions as to how climatic variables affected the prevalence of epidemic events (SI Appendix, Fig. S6). The SEM analyses were performed in SPSS AMOS (version 21.0, IBM). We assumed that base climatic variables would affect all upstream variables in the SEMs, except for locusts, which we assumed would have no direct effects on human epidemic events. The best-fitting path diagrams were selected by keeping the significant path coefficients (P < 0.05), and taking into consideration the χ2 test (43), comparative fit index (44), and root mean square error of approximation (43). Details are shown in SI Appendix, Table S4. A latent variable representing several temperature variables was introduced into the SEMs to simplify the presentation of the results. SEM analysis on low-frequency data were not conducted due to strong correlations among these variables, but correlation results were presented to infer potential direct and indirect associations among variables. The results of both the correlation and SEM analyses are combined in Fig. 2. To demonstrate the temporal consistency of associations between epidemic events and temperature on a small time scale, the correlations of temperature with these variables were analyzed by using 100-y moving windows (Fig. 3).
Supplementary Material
Acknowledgments
We thank Sari C. Cunningham for improving the English of this manuscript. We are grateful to the editors and anonymous reviewers for their valuable comments to improve the manuscript. This work is supported by the External Cooperation Program of Bureau of International Cooperation (BIC), Chinese Academy of Sciences Grant 152111KYSB20150023; the Key International Cooperation of National Natural Science Foundation of China Grant 31420103913; and the scientific programs of the Biological Consequences of Global Change, the International Society of Zoological Sciences, and the International Union of Biological Sciences. U.B. received funding from the Ministry of Education, Youth and Sports of the Czech Republic within the National Sustainability Program I (NPU I Grant LO1415).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 12845.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1706470114/-/DCSupplemental.
References
- 1.Pascual M, Rodó X, Ellner SP, Colwell R, Bouma MJ. Cholera dynamics and El Niño-southern oscillation. Science. 2000;289:1766–1769. doi: 10.1126/science.289.5485.1766. [DOI] [PubMed] [Google Scholar]
- 2.Rodo X, Pascual M, Fuchs G, Faruque AS. ENSO and cholera: A nonstationary link related to climate change? Proc Natl Acad Sci USA. 2002;99:12901–12906. doi: 10.1073/pnas.182203999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koelle K, Rodó X, Pascual M, Yunus M, Mostafa G. Refractory periods and climate forcing in cholera dynamics. Nature. 2005;436:696–700. doi: 10.1038/nature03820. [DOI] [PubMed] [Google Scholar]
- 4.Hay SI, et al. Climate change and the resurgence of malaria in the East African highlands. Nature. 2002;415:905–909. doi: 10.1038/415905a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mac Kenzie WR, et al. A massive outbreak in Milwaukee of cryptosporidium infection transmitted through the public water supply. N Engl J Med. 1994;331:161–167. doi: 10.1056/NEJM199407213310304. [DOI] [PubMed] [Google Scholar]
- 6.Stenseth NC, et al. Plague dynamics are driven by climate variation. Proc Natl Acad Sci USA. 2006;103:13110–13115. doi: 10.1073/pnas.0602447103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu L, et al. Nonlinear effect of climate on plague during the third pandemic in China. Proc Natl Acad Sci USA. 2011;108:10214–10219. doi: 10.1073/pnas.1019486108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmid BV, et al. Climate-driven introduction of the Black Death and successive plague reintroductions into Europe. Proc Natl Acad Sci USA. 2015;112:3020–3025. doi: 10.1073/pnas.1412887112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shuman EK. Global climate change and infectious diseases. N Engl J Med. 2010;362:1061–1063. doi: 10.1056/NEJMp0912931. [DOI] [PubMed] [Google Scholar]
- 10.Altizer S, Ostfeld RS, Johnson PT, Kutz S, Harvell CD. Climate change and infectious diseases: From evidence to a predictive framework. Science. 2013;341:514–519. doi: 10.1126/science.1239401. [DOI] [PubMed] [Google Scholar]
- 11.Pachauri R, Reisinger A. 2007. Climate change 2007: Synthesis report—contribution of Working Groups I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change (IPCC, Geneva)
- 12.World Health Organization . Quantitative Risk Assessment of the Effects of Climate Change on Selected Causes of Death, 2030s and 2050s. WHO; Geneva: 2014. [Google Scholar]
- 13.Tian H, et al. Reconstruction of a 1,910-y-long locust series reveals consistent associations with climate fluctuations in China. Proc Natl Acad Sci USA. 2011;108:14521–14526. doi: 10.1073/pnas.1100189108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Z, Yan C, Krebs CJ, Stenseth NC. Ecological non-monotonicity and its effects on complexity and stability of populations, communities and ecosystems. Ecol Modell. 2015;312:374–384. [Google Scholar]
- 15.Zhang DD, et al. The causality analysis of climate change and large-scale human crisis. Proc Natl Acad Sci USA. 2011;108:17296–17301. doi: 10.1073/pnas.1104268108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Büntgen U, et al. Cooling and societal change during the Late Antique Little Ice Age from 536 to around 660 AD. Nat Geosci. 2016;9:231–236. [Google Scholar]
- 17.Lafferty KD. The ecology of climate change and infectious diseases. Ecology. 2009;90:888–900. doi: 10.1890/08-0079.1. [DOI] [PubMed] [Google Scholar]
- 18.Bai L, Morton LC, Liu Q. Climate change and mosquito-borne diseases in China: A review. Global Health. 2013;9:10. doi: 10.1186/1744-8603-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li H-R, Wang W-Y, Yang L-S, Tan J-A. Coupling analysis of climate change with human plague prevalence. Chin J Zoonoses. 2005;21:887–891. [Google Scholar]
- 20.Zhang D. A Compendium of Chinese Meteorological Records of the Last 3000 Years. Jiangsu Education Publishing House; Nanjing, Jiangsu: 2004. [Google Scholar]
- 21.Chen J. Outbreaks of locusts recorded in Chinese literatures. Bull Zhejiang Entomol Bureau. 1936;5:188–241. [Google Scholar]
- 22.Chen G. China Successive Natural and Manmade Disasters Table. Jinan University Book Series; Guangzhou, China: 1939. [Google Scholar]
- 23.Ma S. The population dynamics of the oriental migratory locust (Locusta migratoria manilensis Meyen) in China. Acta Entomol Sin. 1958;8:1–40. [Google Scholar]
- 24.Stige LC, Chan K-S, Zhang Z, Frank D, Stenseth NC. Thousand-year-long Chinese time series reveals climatic forcing of decadal locust dynamics. Proc Natl Acad Sci USA. 2007;104:16188–16193. doi: 10.1073/pnas.0706813104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Z, et al. Periodic temperature-associated drought/flood drives locust plagues in China. Proc Biol Sci. 2009;276:823–831. doi: 10.1098/rspb.2008.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang JG. Epidemics of Three Thousands of Years. Jiangxi High Education Publisher; Nanchang, China: 1999. [Google Scholar]
- 27.Li W. 2004. Historical Records of Infectious Disease in China (Chemical Industrial Press, Beijing). Chinese.
- 28.Ge Q, Hao Z, Zheng J, Shao X. Temperature changes over the past 2000 yr in China and comparison with the Northern Hemisphere. Clim Past. 2013;9:1153–1160. [Google Scholar]
- 29.Ljungqvist FC. A new reconstruction of temperature variability in the extra-tropical Northern Hemisphere during the last two millennia. Geogr Ann A. 2010;92:339–351. [Google Scholar]
- 30.Moberg A, et al. Highly variable Northern Hemisphere temperatures reconstructed from low- and high-resolution proxy data. Nature. 2005;433:613–617 and correction (2006) 439:1014. doi: 10.1038/nature03265. [DOI] [PubMed] [Google Scholar]
- 31.Tan M, et al. Cyclic rapid warming on centennial‐scale revealed by a 2650‐year stalagmite record of warm season temperature. Geophys Res Lett. 2003;30 [Google Scholar]
- 32.Wang S, et al. Reconstruction of temperature series of China for the last 1000 years. Chin Sci Bull. 2007;52:3272–3280. [Google Scholar]
- 33.Yang B, Braeuning A, Johnson KR, Yafeng S. General characteristics of temperature variation in China during the last two millennia. Geophys Res Lett. 2002;29:38-1–38-4. [Google Scholar]
- 34.Garrett KA, et al. The effects of climate variability and the color of weather time series on agricultural diseases and pests, and on decisions for their management. Agric For Meteorol. 2013;170:216–227. [Google Scholar]
- 35.Baker-Austin C, et al. Emerging Vibrio risk at high latitudes in response to ocean warming. Nat Clim Change. 2013;3:73–77. [Google Scholar]
- 36.Burge CA, et al. Climate change influences on marine infectious diseases: Implications for management and society. Annu Rev Mar Sci. 2014;6:249–277. doi: 10.1146/annurev-marine-010213-135029. [DOI] [PubMed] [Google Scholar]
- 37.Kausrud KL, et al. Modeling the epidemiological history of plague in Central Asia: Palaeoclimatic forcing on a disease system over the past millennium. BMC Biol. 2010;8:112. doi: 10.1186/1741-7007-8-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Z, et al. Periodic climate cooling enhanced natural disasters and wars in China during AD 10–1900. Proc Biol Sci. 2010;277:3745–3753. doi: 10.1098/rspb.2010.0890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pyper BJ, Peterman RM. Comparison of methods to account for autocorrelation in correlation analyses of fish data. Can J Fish Aquat Sci. 1998;55:2127–2140. [Google Scholar]
- 40.Li X, et al. Human impact and climate cooling caused range contraction of large mammals in China over the past two millennia. Ecography. 2015;38:74–82. [Google Scholar]
- 41.Prinzing F. Epidemics Resulting from Wars. Clarendon Press, Oxford; H. Milford; London: 1916. [Google Scholar]
- 42.Bradley RS. Book review: A compendium of Chinese meteorological records of the last 3,000 years. Holocene. 2006;16:621–622. [Google Scholar]
- 43.Bollen KA, Long JS. Testing Structural Equation Model. Sage; Newbury Park, CA: 1993. pp. 209–227. [Google Scholar]
- 44.Bentler PM. Comparative fit indexes in structural models. Psychol Bull. 1990;107:238–246. doi: 10.1037/0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.