Significance
Translational control is a cornerstone of gene-expression regulation in physiological and pathological contexts. The contribution of nonribosomal factors, including messenger RNAs (mRNAs) and mRNA-bound factors, to translational control have been extensively studied. Recently, the hypothesis of a ribosome-mediated regulation emerged, which proposes that cells produce ribosomes of different composition and displaying different translational properties. This work reveals that ribosomal RNA 2′-O-methylation can be modulated in human ribosomes, including at key functional sites for translation, and that changes in the 2′-O-methylation pattern control the intrinsic capabilities of ribosomes to translate mRNAs. This work directly demonstrates the existence of composition-modified ribosomes and their associated change in translational activity as conceptualized by the specialized ribosome concept.
Keywords: 2′-O-methylation, fibrillarin, ribosomal RNA, translational control, RNA epigenetics
Abstract
Ribosomal RNAs (rRNAs) are main effectors of messenger RNA (mRNA) decoding, peptide-bond formation, and ribosome dynamics during translation. Ribose 2′-O-methylation (2′-O-Me) is the most abundant rRNA chemical modification, and displays a complex pattern in rRNA. 2′-O-Me was shown to be essential for accurate and efficient protein synthesis in eukaryotic cells. However, whether rRNA 2′-O-Me is an adjustable feature of the human ribosome and a means of regulating ribosome function remains to be determined. Here we challenged rRNA 2′-O-Me globally by inhibiting the rRNA methyl-transferase fibrillarin in human cells. Using RiboMethSeq, a nonbiased quantitative mapping of 2′-O-Me, we identified a repertoire of 2′-O-Me sites subjected to variation and demonstrate that functional domains of ribosomes are targets of 2′-O-Me plasticity. Using the cricket paralysis virus internal ribosome entry site element, coupled to in vitro translation, we show that the intrinsic capability of ribosomes to translate mRNAs is modulated through a 2′-O-Me pattern and not by nonribosomal actors of the translational machinery. Our data establish rRNA 2′-O-Me plasticity as a mechanism providing functional specificity to human ribosomes.
Translational control is one of the most important regulators of gene expression (1). Translation is regulated through different mechanisms and coordinated with cell signaling. The best-described translational regulation pathways operate through nonribosomal elements, such as the messenger RNA (mRNA) sequence and modification, canonical translation factors, transfer RNAs (tRNAs), micro RNAs (miRNAs), and RNA binding proteins (2, 3). Recently, several studies have provided compelling evidence that regulation of ribosomal proteins or ribosome biogenesis factors was associated with selective regulation of mRNA subsets (4–7). These observations led to the hypothesis of a ribosome-mediated translational control through functionally “specialized ribosomes.” However, direct molecular evidence that ribosomes displaying a different ribosomal RNA (rRNA) or protein composition carry different translational capabilities remains to be provided to validate the concept of specialized ribosomes.
In eukaryotes, rRNAs undergo 12 different types of chemical modification, on at least 112 (of 5,475 nt) and 212 (of 7,184 nt) nucleotides in yeast and human, respectively (8). However, despite being one of the best-characterized, the role of the rRNA epitranscriptome remains largely unknown. Among the different types of chemical modifications, 2′-O-methylation (2′-O-Me) is the most abundant modification of eukaryotic rRNA, with 55 and 106 sites mapped in yeast and in human rRNA, respectively (9, 10). In human rRNA, 2′-O-Me is carried out by the methyl transferase fibrillarin (FBL) associated with the RNA-binding protein 15.5kDa and the core proteins NOP56 and NOP58. Methylation at each site is guided by small nucleolar RNAs (snoRNAs) from the C/D box snoRNA family, which carry a complementary sequence to the target rRNA.
A significant amount of data supports an essential role for rRNA 2′-O-Me in ribosomal activity. About 70% of 2′-O-Me sites are conserved from yeast to human, particularly those located within functional regions of rRNAs (11). Studies using snoRNA knockout yeast strains revealed the importance of 2′-O-Me for the molecular functioning of ribosomes and for cell fitness, and their potential impact on rRNA folding. In yeast, inhibition of 2′-O-Me at several positions was required to severely impair translation and cell growth (12, 13). In contrast, inhibition of 2′-O-Me at single sites in zebrafish was sufficient to induce embryonic lethality, indicating that the role of individual 2′-O-Me is dependent on the cellular context (14). Finally, dysregulations in C/D box snoRNA gene expression have been linked to human diseases, including cancer or inherited genetic disorders, such as the Prader-Willy syndrome (15). The mechanisms by which C/D box snoRNAs adversely impact human cell behavior remain to be determined, and a link with their 2′-O-Me guiding activity and ribosomal function needs to be established, since an impact of snoRNAs on other cellular functions cannot be excluded. FBL (encoded by NOP1 in yeast) is essential for rRNA 2′-O-Me in yeast and crucial for proper mouse development (16, 17). In addition, in yeast and mammals, FBL participates in pre-rRNA cleavage by association with C/D box snoRNAs, such as U3 or U14 (18), and regulates RNA Pol I activity on rDNA gene promoters by methylating a glutamine residue of histone H2A, by an unknown mechanism (19). FBL expression was recently shown to be highly modulated in physiological and pathological contexts, such as development (20), stem cell differentiation (21), viral infection (17), and cancer (7, 22). In cellular models of cancer, forced FBL up- or down-regulation modulated tumor progression (7). In addition, maintained expression of FBL in mouse embryonic stem cells prolonged their pluripotent state (21). In breast cancer cells, changes in FBL expression were correlated with alterations in the level of rRNA 2′-O-Me, with alterations in translational accuracy and with efficient translational initiation of mRNAs containing internal ribosome entry site (IRES) elements (7, 22, 23). However, due to the different activities of FBL, more data are needed to demonstrate that the effect of FBL modulation on translational activity is due to its impact on 2′-O-Me.
While the functional importance of 2′-O-Me is supported by genetic, developmental, cellular, and structural studies, whether the 2′-O-Me pattern represents an adjustable feature of ribosomes and a molecular basis of ribosome regulation is not yet determined. Initial proof supporting that 2′-O-Me could be modulated was provided in cellular models of breast cancer and in thalassemia patients using site-by-site analyses (7, 24, 25). However, a comprehensive view of 2′-O-Me within the four rRNAs, as well as a quantitative evaluation of the level of methylation at each site, is still missing. In the present study, we extensively characterize ribosomes following FBL down-regulation in HeLa cells. Using the recently developed RiboMethSeq approach, we show that the rRNA 2′-O-Me pattern can be qualitatively and quantitatively modulated. Mapping of the position of methylated nucleotides and their methylation frequency on the 3D structure of the human ribosome revealed an unsuspected 2′-O-Me plasticity within the critical functional domains of the ribosome, responsible for the ribosome translational activity. Using IRES-containing mRNAs as models coupled to hybrid in vitro translation assays, we demonstrate that the intrinsic capability of ribosomes to translate mRNAs is directly controlled by 2′-O-Me. Taken together, these studies establish rRNA 2′-O-Me and its plasticity as a molecular mechanism to regulate the translational activity of ribosomes.
Results
FBL Knockdown Decreases Ribosome Biogenesis and Global rRNA 2′-O-Me in Human Cells.
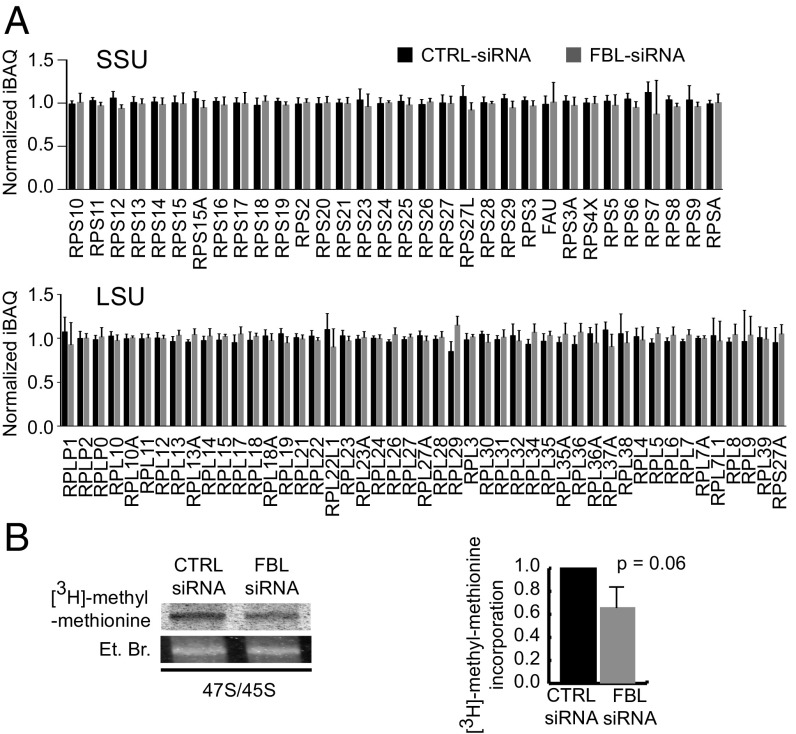
With the aim of altering global rRNA 2′-O-Me, we inhibited FBL expression in HeLa cells using small interfering RNA (siRNA). Transfection conditions were set up to obtain a 5- to 10-fold FBL knockdown over a period of 5 d to enable ribosome turnover (Fig. S1A). The decrease in FBL did not induce a widespread disorganization of nucleoli or instability of major nucleolar proteins (Fig. S1 B and C). FBL knockdown induced a clear, yet incomplete inhibition of the processing of the 5′-ETS region of the pre-rRNA, consequently inhibiting 18S rRNA maturation (Fig. S1D), an observation in agreement with previous studies on yeast NOP1 and with the association of FBL with C/D box snoRNAs involved in pre-rRNA folding and cleavage (18). In contrast, the processing of 5.8S and 28S rRNAs was not affected by FBL knockdown. Consistently, ribosome biogenesis was sufficient to maintain ribosome production at ∼80% of that of control cells (Fig. S1E). Since FBL participates in rRNA processing (Fig. S1D), we speculated that FBL knockdown could alter the assembly of ribosomal proteins (RPs). The assembly of newly synthesized ribosomal subunits appeared similar in FBL knockdown and control cells as evaluated using 2D-PAGE on ribosomes purified from isotope pulse-labeled cells (Fig. S1F). This observation was strengthened by label-free quantitative proteomics analysis, which showed no significant difference between ribosomes extracted from FBL knockdown cells compared with control cells (Fig. 1A and Dataset S1). Taken together, these findings indicate that FBL does not control the final stoichiometry of proteins in cytoplasmic ribosomes.
Fig. 1.
FBL knockdown impacts rRNA 2′-O-Me and not ribosome protein composition in human cells. (A) Label-free quantitative proteomic analysis of 0.5 M KCl-purified cytoplasmic ribosomes from siRNA transfected cells. Normalized Intensity-based absolute quantification (niBAQ) values are shown for RPs of the small subunit (SSU, Upper) and the large subunit (LSU, Lower). Values are presented as mean ± SD (n = 5) (see Dataset S1 for values). (B) Agarose gel electrophoresis (Left) of nuclear RNA purified from cells pulse labeled with [3H]-methyl-methionine. The gels show the [3H]-methyl-methionine incorporation in the 45S/47S pre-RNA (Upper), and the corresponding band stained with ethidium bromide as a loading control (Lower). The radioactive signal was normalized against the ethidium bromide signal (Right). Values are presented as mean ± SD (n = 2). See also Fig. S1 and Dataset S1.
Next, we investigated the impact of a decrease in FBL on levels of rRNA 2′-O-Me. Because 2′-O-Me was shown to be an early and primarily cotranscriptional event (26, 27), we first analyzed methylation of the pre-rRNA by pulse labeling (Fig. 1B). FBL knockdown induced a 33.8% (±19.2, P = 0.064) decrease in the level of pre-rRNA methylation. Thus, as could be anticipated, knockdown of the rRNA methyl-transferase fibrillarin induced a global decrease in methylation of the pre-rRNA.
Altogether, these findings revealed that altering FBL expression in HeLa cells impacted ribosome biogenesis, notably rRNA maturation. However, although 2′-O-Me had decreased, the cytoplasmic ribosomes presented a normal protein composition.
FBL Knockdown Impacts 2′-O-Me of Nucleotides in a Site-Specific Manner, Including Nucleotides at Key Positions Within the Ribosome.
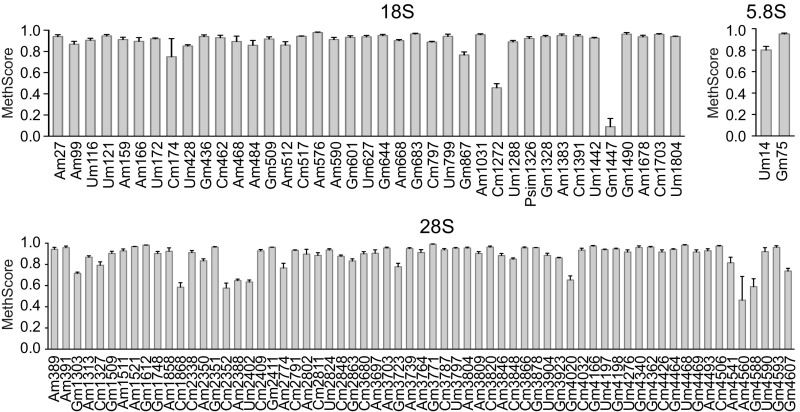
To identify potential site-specific methylation events and to quantify individual variations following FBL knockdown, we analyzed the methylation frequency of every nucleotide known to be ribose-methylated in human ribosomes (10). Several RNA-Seq–based 2′-O-Me mapping methods have been developed and used to refine the map of rRNA 2′-O-Me; however, these methods have so far not been applied to studying the dynamics of individual rRNA 2′-O-Me (9, 10, 28). We modified and applied our recently developed high-throughput RiboMethSeq technology (9) to human rRNA. RiboMethSeq is based on the protection of RNA hydrolysis provided by the methyl group, and on high-throughput sequencing to quantify the fraction of methylated nucleotides. The calculated MethScore represents the fraction of methylated rRNA at a given nucleotide in the ribosomal population (see Materials and Methods for details). We first established a reference map of rRNA 2′-O-Me in HeLa cells, using three independent biological replicates (Fig. 2). All of the 106 previously validated 2′-O-methylated nucleotides were highly methylated in rRNA of HeLa cells, except the 18S-Gm1447 nucleotide (MethScore = 0.09 ± 0.08). This was likely not due to a technical bias, since high MethScore values for this position were obtained in other cell lines. The majority of 2′-O-Me sites were methylated in over 80% of ribosomes, and only 16 sites (15%) were less-frequently methylated (MethScore ranging from 0.2 to 0.8). Among the sites conserved between yeast and human, all except one belonged to the highly methylated category (MethScore > 0.8), which is consistent with a high frequency of methylation of these nucleotides in yeast rRNA (9, 27). MethScore of individual sites displayed low dispersion among biological replicates, with a mean SD of 2.9%. Of the 106 known sites, 100 sites showed a level of variability below 5%, whereas only two sites in the 18S rRNA (Gm1447 and Cm174) and one in the 28S rRNA (Am4560) showed variability exceeding 10% (Fig. S2 and Dataset S2). This indicates that RiboMethSeq provides a robust measurement of 2′-O-Me levels. Altogether, these results demonstrate that the rRNA 2′-O-Me pattern is heterogeneous among human ribosomes.
Fig. 2.
Quantitative mapping of rRNA 2′-O-Me in human cells. The 2′-O-Me levels at each site of 18S, 28S, and 5.8S rRNA, evaluated by RiboMethSeq on nontreated HeLa cell rRNA. Data are expressed as mean MethScore values ± SD (n = 3 independent biological replicates) for each known methylated nucleotide in 18S, 5.8S, and 28S human rRNA. See also Fig. S2.
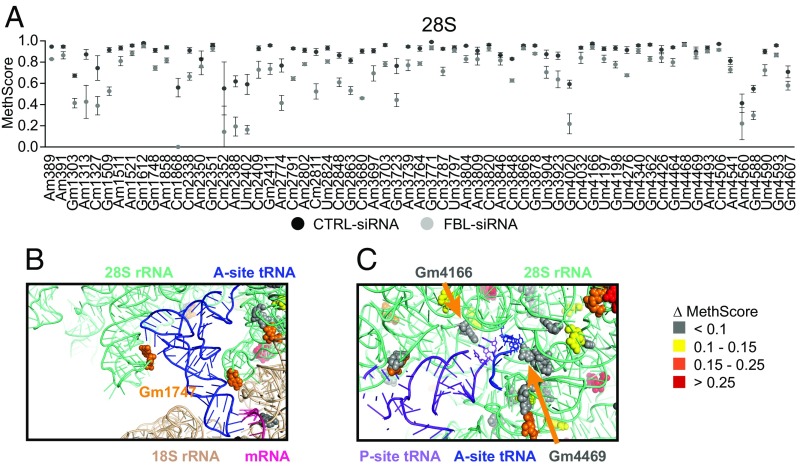
RiboMethSeq was then applied to analyze the rRNA 2′-O-Me pattern upon FBL knockdown. Importantly, the transfection procedure did not introduce any experimental bias (Fig. S3A). Upon FBL knockdown, the frequency of 2′-O-Me decreased at almost all sites, although this variation was not statistically significant for all of the positions (Fig. 3A, Fig. S3 B and C, and Dataset S2). Surprisingly, the decrease in methylation was very different among sites, ranging from 0.2 to 57% (Fig. S3C), indicating that 2′-O-Me is likely controlled in a site-specific manner rather than systemically. The level of methylation significantly decreased for 59 sites (P < 0.05; 10 in 18S rRNA, 1 in 5.8S rRNA and 48 in 28S rRNA) (Dataset S2). Interestingly, each site with an initial methylation level below 80% decreased by at least an additional 10% upon FBL knockdown, suggesting that partial methylation might render these sites more sensitive to FBL knockdown, or that they are intrinsically prone to variation (Fig. S3D). Of note, the decrease in 2′-O-Me was greater for 28S rRNA than for 18S rRNA, which we attributed to the lower turnover of the 18S rRNA in FBL knockdown cells (Fig. S1D).
Fig. 3.
FBL knockdown impacts 2′-O-Me of nucleotides in a site-specific manner, including nucleotides at key positions within the ribosome. (A) Mean MethScore values ± SD (n = 3 independent biological replicates) for each methylated nucleotide in 28S rRNA from HeLa cells transfected with CTRL-siRNA (black circle) or FBL-siRNA (gray circle). (B) View of the A-site in a HeLa cell ribosome 3D structure. Methylation sites are color coded according to the variation in MethScore comparing FBL siRNA cells with CTRL siRNA cells, as indicated on the right. The Gm1747 methylation site (orange, methylation decreased by 16.7%), is oriented with the 2′OH group close to the D-loop of the A-site tRNA (blue). (C) View of the PTC showing the tRNAs in the A-site (blue) and P-site (purple). Methylation frequency of nucleotides Gm4469 and Gm4166 (Gray) was not altered by FBL knockdown. See also Fig. S3 and Datasets S2 and S3.
Because a majority of 2′-O-methylated nucleotides are localized within functional domains of the rRNA, as evidenced by 2D maps of rRNAs (11), we investigated whether the nucleotides displaying an altered 2′-O-Me upon FBL knockdown were localized in particular domains within the ribosome structure. Each 2′-O-Me site was mapped on the 3D structure of the HeLa cell 80S ribosome recently obtained by cryo-EM (29), and was assigned a color based on the decrease in methylation in FBL knockdown cells, according to four different groups (Fig. 3 B and C and Dataset S3). Affected sites (yellow, orange, and red in Fig. S3E) were distributed throughout the ribosome structure, including in the “core” of the ribosome, the most conserved region compared with bacterial ribosomes (30). Strikingly, several affected 2′-O-Me sites were located in regions that are known to be involved in the translational process, in particular close to the A and P-sites, the intersubunit bridges, and the peptide exit tunnel (Fig. 3B and Fig. S3F), demonstrating that these important regions are subjected to variations in methylation. In contrast, 2′-O-Me sites close to the peptidyl transferase center (PTC) were not affected, indicating that this functional region might be protected from variations in methylation (Fig. 3C). The decoding center within 18S rRNA was also devoid of altered sites (Fig. S3G).
In conclusion, these data demonstrate that the down-regulation of FBL, a factor of the general ribose methylation machinery, induces site-specific modulation of the 2′-O-Me pattern. While several functional domains of the ribosome are subjected to 2′-O-Me variation, other key domains might be protected.
2′-O-Me Inhibition Selectively Modifies the Intrinsic Capability of Ribosomes to Initiate Translation from Dicistrovirus IRES Elements and Not from the m7G-Cap.
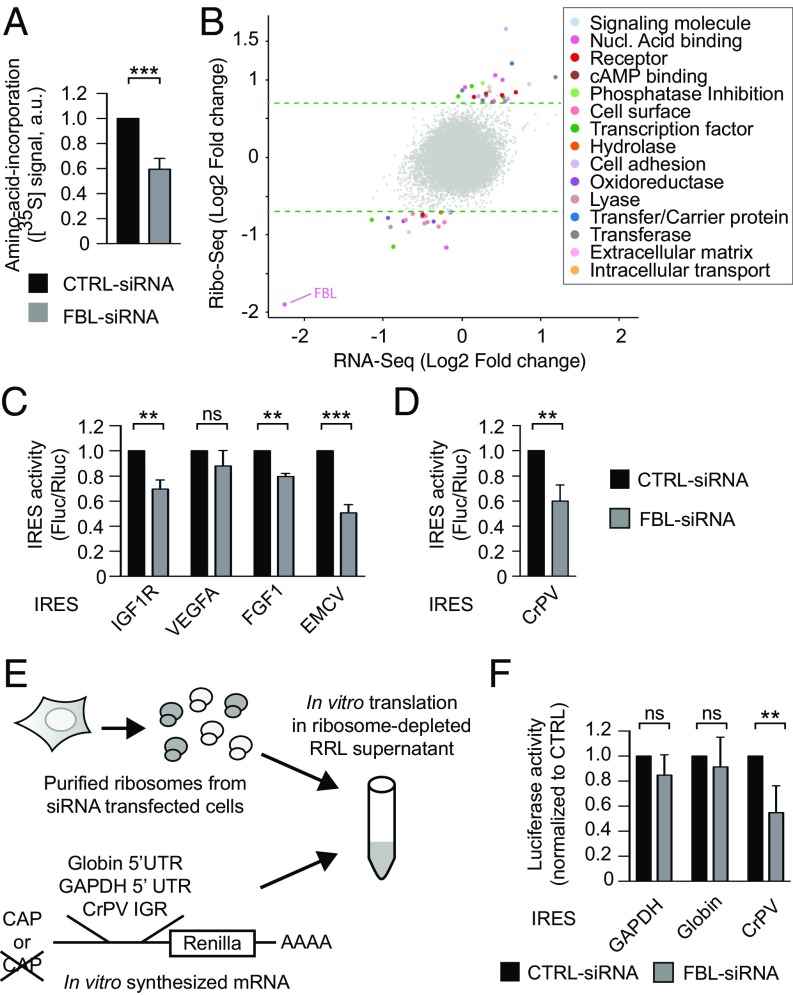
To evaluate whether FBL knockdown impacts protein synthesis at a global level, we performed both a puromycylation assay (31), the signal produced by which represents the number of nascent peptides (Fig. S4 A and B), and an isotope pulse labeling with [35S]-labeled amino acids, to evaluate the rate of amino acid incorporation (Fig. 4A and Fig. S4C). The results show a decrease in global synthesis of proteins, which indicates a reduction in the number of actively translating ribosomes, and is consistent with a decrease in ribosome production (Fig. S1E). Next, we sought whether FBL knockdown selectively altered mRNA translation. For this we applied ribosome profiling on HeLa cell lines expressing a FBL shRNA or a CTRL shRNA in an inducible manner (Fig. S4 D, F, and G). Notably, change in the 2′-O-Me pattern, analyzed by RiboMethSeq, was similar after FBL knockdown induced by shRNA compared with the one induced by siRNA (Fig. S4E). Upon FBL knockdown, several genes were translationally altered (Fig. 4B). Translation efficiency of altered genes was either higher (n = 28) or lower (n = 22). This observation further supported that FBL, and possibly 2′-O-Me, could selectively regulate the translation efficiency of particular mRNA, although in this cellular model, there was no enrichment in particular molecular or cellular function (Fig. 4B).
Fig. 4.
2′-O-Me inhibition selectively modifies the intrinsic capability of ribosomes to initiate translation from dicistrovirus IRES elements and not from the m7G-cap. (A) Global protein synthesis was measured by incorporation of [35S]-methionine–[35S]cysteine labeling following SDS/PAGE and counting of radioactive signals. Values are presented as mean ± SD (n = 4). (B) Comparative mRNA translation by ribosome profiling on HeLa cells expressing either a CTRL-shRNA or a FBL-shRNA. Fold-changes at mRNA and translation levels are plotted along the y and the x axes, respectively. Translationally altered mRNA are colored according to their molecular function. Dotted green lines represent the significance threshold. (C) IRES-dependent translation (Fluc/Rluc) from several IRES elements was measured in HeLa cells transfected with CTRL-siRNA (black bars) or FBL-siRNA (gray bars). Values are presented as mean ± SD (n = 3). (D) Identical experimental set-up as in D using a reporter construct carrying the CrPV-IGR IRES element. Values are presented as mean ± SD (n = 3). (E) Schematic representation of the hybrid in vitro translation assay. (F) In vitro translation was evaluated by measuring luciferase activity produced with 1 µg of ribosomes. Cap-dependent translation was evaluated using reporter constructs containing the 5′UTR of GAPDH or globin mRNA. IRES-dependent translation was evaluated using a Cap-less mRNA containing the CrPV-IGR IRES as 5′UTR. ns, not significant; **P ≤ 0.01; ***P ≤ 0.001. See also Fig. S4.
Changes in FBL expression have been associated with alterations in IRES-dependent translation initiation (7, 22, 23). Within the subset of translationally altered mRNAs, 8% (four mRNAs) were previously identified in a large-scale screen for mRNAs able to drive Cap-independent translation (32). As a readout of changes in ribosome behavior, we analyzed IRES-dependent translational initiation in cellulo for a panel of cellular and viral IRESs using bicistronic constructs that code for two luciferases, the translation of which is either driven by the m7G-cap (Renilla luciferase) or by an IRES structure (firefly luciferase) (Fig. S4H). The firefly/Renilla ratio revealed that FBL knockdown induced a decrease in translation initiation from cellular IRESs of FGF1, IGF-1R, and from the type II encephalomyocarditis virus (EMCV) IRES, but not from VEGFA IRES (Fig. 4C). Consistently, luciferase activity/mRNA ratios, which reflect translation efficiency, showed a decrease in Cap-dependent translation consistent with the global protein synthesis reduction observed in FBL-siRNA cells (Fig. 4A), and a stronger decrease in IRES-dependent translation (Fig. S4I). Thus, FBL knockdown alters IRES-dependent translational initiation with a selective impact depending on the nature of the IRES.
To determine whether altering the pattern of rRNA methylation directly contributes to the FBL-induced reduction in IRES-dependent translation, we analyzed the impact of FBL knockdown on translation initiation using the cricket paralysis virus intergenic region (CrPV-IGR) IRES, which is able to trigger the assembly of an active 80S ribosome in the absence of any cellular translation initiation factor (33). First, we observed that translation initiation from the CrPV-IGR IRES was significantly reduced upon FBL knockdown compared with control cells using a bicistronic construct (Fig. 4D). Second, to consolidate these data and further exclude the contribution of other factors involved in translation, such as tRNA, mRNA, or miRNA, we analyzed the translational capability of ribosomes extracted from FBL knocked-down cells in a hybrid in vitro translation assay, which we developed recently (34) (Fig. 4E). In this assay all of the translation machinery components, except for the ribosomes, are provided as purified products so that the cell-extracted ribosomes are the only variable components (see Materials and Methods for details). In this context, translation initiation from a Cap-less mRNA containing the CrPV-IGR IRES was severely impaired using ribosomes from FBL knockdown cells (Fig. 4F). In contrast, m7G-cap–driven translation from mRNAs containing the GAPDH or globin 5′UTR, was not significantly affected (Fig. 4F). In addition to the CrPV-IGR IRES element, translation from the Drosophila C virus (DCV) IRES, another dicistrovirus type IV IRES, and from the type II IRES EMCV was also strongly impaired (Fig. S4J). Any artifact due to nonspecific binding of mRNA to ribosomes was excluded by reproducing the experiment using a range of mRNA:ribosome ratios (Fig. S4 K and L). In conclusion, these data demonstrate that modulation of the 2′-O-Me pattern alters the intrinsic capability of ribosomes to initiate translation from IRES elements, but not from the m7G-cap structure of mRNAs.
Discussion
The most abundant modification in human rRNA, 2′-O-Me, is a highly complex and specific posttranscriptional modification, which is present in functionally important domains of the ribosome, indicating a significant contribution to ribosome functioning. However, existence of distinct 2′-O-Me patterns and the direct contribution of 2′-O-Me on the translational activity of ribosomes remain to be demonstrated. Here we show that rRNA 2′-O-Me patterns can be extensively modulated, although in a site-specific manner, including sites present in known functional regions of the ribosome, demonstrating that 2′-O-Me is a regulated, complex, and plastic feature of human ribosomes, and a molecular mechanism controlling ribosome functioning.
RiboMethSeq represents a unique method to simultaneously map and quantify 2′-O-Me on each site present in human rRNA, and was used here to explore the dynamics of 2′-O-Me. In HeLa cells, addition of 2′-O-Me appeared to be highly efficient since the majority of sites were methylated in almost 100% of the ribosomes. However, in contrast to yeast rRNA (9), a subset of sites was partially methylated, which has several conceptual implications: first, 2′-O-Me is not constitutively added at all sites in each ribosome; second, cells tolerate the production of ribosomes lacking some 2′-O-Me; and third, 2′-O-Me is a source of heterogeneity for the ribosomal population. In addition, a decrease in methylation was observed as a consequence of FBL knockdown (Fig. 3), and establishes 2′-O-Me as an adjustable and dynamic process, and a source of ribosome diversity. Subsequently, 2′-O-Me sites unaffected or weakly affected by FBL knockdown may represent sites for which methylation is highly efficient, or for which absence of methylation cannot be tolerated during ribosome biogenesis and subsequent quality control of ribosome fitness. The presence of 12 sites with a decrease in methylation exceeding 30% implies that FBL knocked-down cells contain ribosomes lacking 2′-O-Me at several sites. Consequently, 2′-O-Me should be considered and studied as a combination of sites, and not only individually, consistent with data obtained using snoRNA knockout yeast strains (12, 13).
FBL knockdown induced an unexpected site-specific alteration of 2′-O-Me (Fig. 3) by mechanisms that need to be further studied. Changes in single 2′-O-Me sites were not correlated with the global level of the corresponding snoRNA guide, further supporting that snoRNA expression by itself is not the main mechanism regulating 2′-O-Me (10). Possibly, the efficiency of methylation might be disproportionate among snoRNPs. The site-specific impact of FBL knockdown shows that modulating the expression of common components of the methylation machinery represents a means of regulating 2′-O-Me patterns. It follows that the steep down-regulation of FBL observed during neurogenesis and stem cell differentiation (20, 21) may affect rRNA 2′-O-Me patterns, with a direct impact on ribosome function. Conversely, overexpression of FBL in tumors and cancer cells might increase 2′-O-Me at selected sites, as suggested by our previous data (7). The moderate impact on ribosome production (Fig. S1E) and absence of detectable consequences on ribosomal protein assembly and stoichiometry provides additional quantitative biochemical evidence that FBL regulates protein synthesis through its impact on 2′-O-Me plasticity (7). Therefore, FBL regulation may represent a means of modulating the 2′-O-Me pattern of rRNA without adversely impacting overall ribosome production.
In this study, we used translation initiation from Cap and IRES structures as functional assays to assess changes in behavior of ribosomes. The decrease in CrPV IRES activity in in cellulo and in vitro assays demonstrates that ribosomes with an altered 2′-O-Me pattern become intrinsically less efficient at initiating translation from IRES elements, in a manner independent of translation initiation factors. The decrease in EMCV IRES activity in our in vitro assay (Fig. S4J) reveals that 2′-O-Me impacts different types of IRESs, and further supports that 2′-O-Me is responsible for the FBL-dependent regulation of IRES-containing cellular mRNAs (7) (Fig. 4C). IRES elements recruit the 40S subunit through different interacting pathways involving eIF, but also ribosomal proteins, such as RPS25 (35). This raises the possibility that 2′-O-Me controls IRES translation via RPs, although our proteomic analysis demonstrates that 2′-O-Me alterations did not induce significant changes in RP composition, thus excluding that the decrease in IRES translation originated from a loss of RP, such as RPS25. Cap-independent translation of cellular mRNAs appears more widespread than anticipated, and comprises mechanisms based on direct interaction between mRNAs and 18S rRNA, in a Shine d’Algarno-like manner (32). Such a mechanism might thus be more sensitive to chemical modifications of rRNA. Importantly, rRNA 2′-O-Me provides selectivity to the translation machinery toward a subset of mRNAs (Fig. 4 B and C). Additional studies are necessary to characterize mRNAs, the translation of which is regulated through rRNA 2′-O-Me.
The limited impact observed on translation from globin and GAPDH 5′UTR in the in vitro translation assay indicates that 2′-O-Me does not significantly modulate the ability of ribosomes to initiate Cap-dependent translation. This suggests that the decrease in global protein synthesis observed in cellular assays (Fig. 4A and Fig. S4C) is related to the lower ribosome production in FBL knockdown cells. Nevertheless, at this point we cannot exclude that 2′-O-Me affects some of the Cap-dependent pathways, and additional studies will be necessary to evaluate the impact of 2′-O-Me on the different mechanisms of Cap-dependent translation initiation. In addition, the limited impact of 2′-O-Me on Cap-dependent translation in the in vitro translation assay, also indicates that there was no major defect in translation elongation. Data from [35S]-methionine–[35S]cysteine pulse labeling, which reflect the rate of amino acid incorporation, and data from puromycylation assays, which reveal the number of ribosomes engaged in translation, both showed similar alterations upon FBL knockdown, and further indicate that elongation rate is similar in FBL knockdown cells compared with control cells. The impact of 2′-O-Me on synthesis of proteins, which are sensitive to translation elongation rate, remains to be studied. These observations unambiguously demonstrate that 2′-O-Me contributes to the translational activity of the ribosome.
The role of 2′-O-Me on ribosome structure and function is not known. Mapping of 2′-O-Me sites onto the ribosome structure revealed that 2′-O-Me can be modulated in several regions involved in intermolecular interactions, such as between tRNA and the A-site (Fig. 3B), intersubunit bridges (Fig. S3F), or around the peptide exit tunnel. The importance of 2′-O-Me at these locations was demonstrated in yeast and should now be explored in human models (11). Equally important are functional regions that did not display variations in 2′-O-Me. In particular, the 2′-O-Me sites of the PTC were unaltered, strongly indicating that this region is protected from 2′-O-Me variation. Therefore, our study supports the notion that 2′-O-Me comprises constitutively modified sites and regulated sites. How 2′-O-Me contributes to the molecular structure of the ribosome remains to be determined. Recent high-resolution crystal structures of the Thermus thermophilus ribosome and cryo-EM structures of human ribosomes, showed that the ribose 2′-O positions of several nucleotides are directly involved in molecular interactions, both in a methylated and unmethylated state (29, 36). It can be anticipated that these interactions would be disrupted upon changes in methylation of these nucleotides, and may impact elongation and termination, in addition to initiation. However, technological advances in structural tools available today, such as cryo-EM and X-crystallography, are required to obtain a finer view of the structure–function relationship of human 2′-O-Me patterns.
In conclusion, 2′-O-Me plasticity reported herein expands the repertoire of ribosome composition and further demonstrates the existence of diversity in ribosome populations. The impact on the intrinsic ribosomal functioning establishes 2′-O-Me plasticity as a molecular mechanism modulating ribosomal activity, and further supports that modifications in rRNA chemical patterns, including pseudouridylation and base modifications, mediate ribosome functional specialization. These data expose the ribosomal RNA epitranscriptome as a new level of regulation of gene expression.
Materials and Methods
Detailed experimental procedures are described in SI Materials and Methods.
Ribosome Protein Composition.
Ribosomes composition was analyzed by label-free quantitative proteomics as described previously (37, 38).
Analysis of rRNA Methylation.
Site-specific rRNA methylation was determined by RiboMethSeq, as previously described (9).
Ribosome Structure Analysis.
Methylated nucleotides were mapped on the cryo-EM structure of the human ribosome (PDB ID code 4UG0) (29). The reference structure of prokaryotic ribosome containing A-, P-, and E-site tRNAs plus mRNA was from T. thermophilus (PDB ID code 4V5C) (39).
Translation Assay.
Global protein synthesis was performed as previously described (40). In cellulo translation assays using bicistronic vectors and in vitro translation were performed as described previously (7, 34, 41).
Ribosome Profiling.
Ribosome profiling was performed as previously described (42). Gene Ontology (GO) terms were identified for genes showing a significant expression variation using Panther (43).
Supplementary Material
Acknowledgments
We thank A.-C. Prats and D. Ruggero for kindly providing internal ribosome entry site-containing vectors; D. Lafontaine for discussion; and Brigitte Manship for editing the manuscript. Microscopic imaging was performed using the Centre de Recherche en Cancérologie de Lyon imaging platform. The project was funded by Agence Nationale pour la Recherche (RIBOMETH ANR-13-BSV8-0012-01); the “Programmes d'Actions Intégrées de Recherche” RiboTEM (PAIR, Institut National du Cancer, Fondation ARC pour la Recherche sur le Cancer, Ligue Nationale Contre le Cancer); the “Ligue Nationale Contre le Cancer”; the “Ligue Contre le Cancer, Comité Départemental de la Drôme, du Rhône, du Puy-de-Dôme, et de l'Allier”; and Fondation ARC pour la Recherche sur le Cancer. F.C. is a CNRS research fellow. J.-J.D., and V. Marcel are INSERM research fellows. M.G. was supported by Agence Nationale pour la Recherche Grant “INVADE” PC201507. F.L. received a PhD fellowship from the French Ministry for Research and Education. V. Marchand and Y.M. were supported by joint Agence Nationale de la Recherche -Deutsche Forschungsgemeinschaft Grant HTRNAMod (ANR-13-ISV8-0001/HE 3397/8-1). Y.C. and A.A. were supported by the bottom-up platform and informatics group at Exploring the Dynamics of Proteomes (EDyP). Proteomic experiments were partly supported by the ProFi Grant ANR-10-INBS-08-01. M.M. and M.Y. were supported by European Research Council advanced Grant 294312. M.Y. was supported by the Russian Government Program of Competitive Growth of Kazan Federal University. This work has benefited from the platform and expertise of the High-throughput Sequencing Platform of Institute for Integrative Biology of the Cell (I2BC).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The mass spectrometry proteomics data have been deposited in the ProteomeXchange Consortium via the PRIDE partner repository (accession no. PXD00724). RNA-Seq data of RiboMethSeq data have been deposited in the European Nucleotide Archive database (accession no. PRJEB43738). The RNA-Seq data of Ribosome Profiling have been deposited in the Gene Expression Omnibus (GEO) database, https://www.ncbi.nlm.nih.gov/geo (accession no. GSE105248).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1707674114/-/DCSupplemental.
References
- 1.Schwanhäusser B, et al. Global quantification of mammalian gene expression control. Nature. 2011;473:337–342. doi: 10.1038/nature10098. [DOI] [PubMed] [Google Scholar]
- 2.Hinnebusch AG, Ivanov IP, Sonenberg N. Translational control by 5′-untranslated regions of eukaryotic mRNAs. Science. 2016;352:1413–1416. doi: 10.1126/science.aad9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoernes TP, Erlacher MD. Translating the epitranscriptome. Wiley Interdiscip Rev RNA. 2017;8:e1375. doi: 10.1002/wrna.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xue S, Barna M. Specialized ribosomes: A new frontier in gene regulation and organismal biology. Nat Rev Mol Cell Biol. 2012;13:355–369. doi: 10.1038/nrm3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xue S, et al. RNA regulons in Hox 5′ UTRs confer ribosome specificity to gene regulation. Nature. 2015;517:33–38. doi: 10.1038/nature14010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Majzoub K, et al. RACK1 controls IRES-mediated translation of viruses. Cell. 2014;159:1086–1095. doi: 10.1016/j.cell.2014.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marcel V, et al. p53 acts as a safeguard of translational control by regulating fibrillarin and rRNA methylation in cancer. Cancer Cell. 2013;24:318–330. doi: 10.1016/j.ccr.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma S, Lafontaine DL. ‘View from a bridge’: A new perspective on eukaryotic rRNA base modification. Trends Biochem Sci. 2015;40:560–575. doi: 10.1016/j.tibs.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 9.Marchand V, Blanloeil-Oillo F, Helm M, Motorin Y. Illumina-based RiboMethSeq approach for mapping of 2′-O-Me residues in RNA. Nucleic Acids Res. 2016;44:e135. doi: 10.1093/nar/gkw547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krogh N, et al. Profiling of 2′-O-Me in human rRNA reveals a subset of fractionally modified positions and provides evidence for ribosome heterogeneity. Nucleic Acids Res. 2016;44:7884–7895. doi: 10.1093/nar/gkw482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Decatur WA, Fournier MJ. rRNA modifications and ribosome function. Trends Biochem Sci. 2002;27:344–351. doi: 10.1016/s0968-0004(02)02109-6. [DOI] [PubMed] [Google Scholar]
- 12.Liang XH, Liu Q, Fournier MJ. rRNA modifications in an intersubunit bridge of the ribosome strongly affect both ribosome biogenesis and activity. Mol Cell. 2007;28:965–977. doi: 10.1016/j.molcel.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 13.Baudin-Baillieu A, et al. Nucleotide modifications in three functionally important regions of the Saccharomyces cerevisiae ribosome affect translation accuracy. Nucleic Acids Res. 2009;37:7665–7677. doi: 10.1093/nar/gkp816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higa-Nakamine S, et al. Loss of ribosomal RNA modification causes developmental defects in zebrafish. Nucleic Acids Res. 2012;40:391–398. doi: 10.1093/nar/gkr700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bortolin-Cavaillé ML, Cavaillé J. The SNORD115 (H/MBII-52) and SNORD116 (H/MBII-85) gene clusters at the imprinted Prader-Willi locus generate canonical box C/D snoRNAs. Nucleic Acids Res. 2012;40:6800–6807. doi: 10.1093/nar/gks321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tollervey D, Lehtonen H, Jansen R, Kern H, Hurt EC. Temperature-sensitive mutations demonstrate roles for yeast fibrillarin in pre-rRNA processing, pre-rRNA methylation, and ribosome assembly. Cell. 1993;72:443–457. doi: 10.1016/0092-8674(93)90120-f. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez-Corona U, Sobol M, Rodriguez-Zapata LC, Hozak P, Castano E. Fibrillarin from Archaea to human. Biol Cell. 2015;107:159–174. doi: 10.1111/boc.201400077. [DOI] [PubMed] [Google Scholar]
- 18.Watkins NJ, Bohnsack MT. The box C/D and H/ACA snoRNPs: Key players in the modification, processing and the dynamic folding of ribosomal RNA. Wiley Interdiscip Rev RNA. 2012;3:397–414. doi: 10.1002/wrna.117. [DOI] [PubMed] [Google Scholar]
- 19.Tessarz P, et al. Glutamine methylation in histone H2A is an RNA-polymerase-I-dedicated modification. Nature. 2014;505:564–568. doi: 10.1038/nature12819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Recher G, et al. Zebrafish midbrain slow-amplifying progenitors exhibit high levels of transcripts for nucleotide and ribosome biogenesis. Development. 2013;140:4860–4869. doi: 10.1242/dev.099010. [DOI] [PubMed] [Google Scholar]
- 21.Watanabe-Susaki K, et al. Biosynthesis of ribosomal RNA in nucleoli regulates pluripotency and differentiation ability of pluripotent stem cells. Stem Cells. 2014;32:3099–3111. doi: 10.1002/stem.1825. [DOI] [PubMed] [Google Scholar]
- 22.Su H, et al. Elevated snoRNA biogenesis is essential in breast cancer. Oncogene. 2014;33:1348–1358. doi: 10.1038/onc.2013.89. [DOI] [PubMed] [Google Scholar]
- 23.Basu A, et al. Requirement of rRNA methylation for 80S ribosome assembly on a cohort of cellular internal ribosome entry sites. Mol Cell Biol. 2011;31:4482–4499. doi: 10.1128/MCB.05804-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Belin S, et al. Dysregulation of ribosome biogenesis and translational capacity is associated with tumor progression of human breast cancer cells. PLoS One. 2009;4:e7147. doi: 10.1371/journal.pone.0007147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sornjai W, et al. Hypermethylation of 28S ribosomal RNA in beta-thalassemia trait carriers. Int J Biol Macromol. 2017;94:728–734. doi: 10.1016/j.ijbiomac.2016.10.039. [DOI] [PubMed] [Google Scholar]
- 26.Kos M, Tollervey D. Yeast pre-rRNA processing and modification occur cotranscriptionally. Mol Cell. 2010;37:809–820. doi: 10.1016/j.molcel.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Birkedal U, et al. Profiling of ribose methylations in RNA by high-throughput sequencing. Angew Chem Int Ed Engl. 2015;54:451–455. doi: 10.1002/anie.201408362. [DOI] [PubMed] [Google Scholar]
- 28.Incarnato D, et al. High-throughput single-base resolution mapping of RNA 2'-O-methylated residues. Nucleic Acids Res. 2016;45:1433–1441. doi: 10.1093/nar/gkw810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khatter H, Myasnikov AG, Natchiar SK, Klaholz BP. Structure of the human 80S ribosome. Nature. 2015;520:640–645. doi: 10.1038/nature14427. [DOI] [PubMed] [Google Scholar]
- 30.Ben-Shem A, et al. The structure of the eukaryotic ribosome at 3.0 Å resolution. Science. 2011;334:1524–1529. doi: 10.1126/science.1212642. [DOI] [PubMed] [Google Scholar]
- 31.David A, et al. Nuclear translation visualized by ribosome-bound nascent chain puromycylation. J Cell Biol. 2012;197:45–57. doi: 10.1083/jcb.201112145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weingarten-Gabbay S, et al. Comparative genetics. Systematic discovery of cap-independent translation sequences in human and viral genomes. Science. 2016;351:aad4939. doi: 10.1126/science.aad4939. [DOI] [PubMed] [Google Scholar]
- 33.Fernández IS, Bai XC, Murshudov G, Scheres SH, Ramakrishnan V. Initiation of translation by cricket paralysis virus IRES requires its translocation in the ribosome. Cell. 2014;157:823–831. doi: 10.1016/j.cell.2014.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Panthu B, Décimo D, Balvay L, Ohlmann T. In vitro translation in a hybrid cell free lysate with exogenous cellular ribosomes. Biochem J. 2015;467:387–398. doi: 10.1042/BJ20141498. [DOI] [PubMed] [Google Scholar]
- 35.Hertz MI, Landry DM, Willis AE, Luo G, Thompson SR. Ribosomal protein S25 dependency reveals a common mechanism for diverse internal ribosome entry sites and ribosome shunting. Mol Cell Biol. 2013;33:1016–1026. doi: 10.1128/MCB.00879-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Polikanov YS, Melnikov SV, Söll D, Steitz TA. Structural insights into the role of rRNA modifications in protein synthesis and ribosome assembly. Nat Struct Mol Biol. 2015;22:342–344. doi: 10.1038/nsmb.2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Penzo M, et al. Human ribosomes from cells with reduced dyskerin levels are intrinsically altered in translation. FASEB J. 2015;29:3472–3482. doi: 10.1096/fj.15-270991. [DOI] [PubMed] [Google Scholar]
- 38.Casabona MG, Vandenbrouck Y, Attree I, Couté Y. Proteomic characterization of Pseudomonas aeruginosa PAO1 inner membrane. Proteomics. 2013;13:2419–2423. doi: 10.1002/pmic.201200565. [DOI] [PubMed] [Google Scholar]
- 39.Voorhees RM, Weixlbaumer A, Loakes D, Kelley AC, Ramakrishnan V. Insights into substrate stabilization from snapshots of the peptidyl transferase center of the intact 70S ribosome. Nat Struct Mol Biol. 2009;16:528–533. doi: 10.1038/nsmb.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bash-Imam Z, et al. Translational reprogramming of colorectal cancer cells induced by 5-fluorouracil through a miRNA-dependent mechanism. Oncotarget. 2017;8:46219–46233. doi: 10.18632/oncotarget.17597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jack K, et al. rRNA pseudouridylation defects affect ribosomal ligand binding and translational fidelity from yeast to human cells. Mol Cell. 2011;44:660–666. doi: 10.1016/j.molcel.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baudin-Baillieu A, et al. Genome-wide translational changes induced by the prion [PSI+] Cell Rep. 2014;8:439–448. doi: 10.1016/j.celrep.2014.06.036. [DOI] [PubMed] [Google Scholar]
- 43.Mi H, et al. PANTHER version 11: Expanded annotation data from gene ontology and reactome pathways, and data analysis tool enhancements. Nucleic Acids Res. 2017;45:D183–D189. doi: 10.1093/nar/gkw1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.