Significance
With more than 200 million cases per year worldwide and more than 400,000 deaths, mostly affecting children in sub-Saharan Africa, malaria is still one of the most prevalent infectious diseases. Infection with the malaria parasite Plasmodium falciparum is characterized by high inflammation but also the failure of the immune system to form efficient memory, leading to recurring infections. No efficient vaccine is available to date. Here we have studied the response of dendritic cells (DCs), an essential cell type in the orchestration of immune and vaccine responses. We found that P. falciparum induces a distinct transcriptional profile compared with a classical inflammatory stimulus in primary human DCs, leading to a highly atypical response, which may contribute to parasite immune evasion during malaria.
Keywords: Plasmodium falciparum, dendritic cells, malaria, activation, cytokines
Abstract
Dendritic cells (DCs) are activated by pathogens to initiate and shape immune responses. We found that the activation of DCs by Plasmodium falciparum, the main causative agent of human malaria, induces a highly unusual phenotype by which DCs up-regulate costimulatory molecules and secretion of chemokines, but not of cytokines typical of inflammatory responses (IL-1β, IL-6, IL-10, TNF). Similar results were obtained with DCs obtained from malaria-naïve US donors and malaria-experienced donors from Mali. Contact-dependent cross-talk between the main DC subsets, plasmacytoid and myeloid DCs (mDCs) was necessary for increased chemokine and IFN-α secretion in response to the parasite. Despite the absence of inflammatory cytokine secretion, mDCs incubated with P. falciparum-infected erythrocytes activated antigen-specific naïve CD4+ T cells to proliferate and secrete Th1-like cytokines. This unexpected response of human mDCs to P. falciparum exhibited a transcriptional program distinct from a classical LPS response, pointing to unique P. falciparum-induced activation pathways that may explain the uncharacteristic immune response to malaria.
Malaria is still one of the most prevalent infectious diseases worldwide, with more than 200 million cases per year and more than 400,000 deaths. Most of these cases are caused by the protozoan parasite Plasmodium falciparum (1). Malaria is characterized by cyclical fevers and high levels of inflammation, but even though an early inflammatory response is crucial for parasite clearance, excessive and persistent inflammation can contribute to severe forms of the disease (2). At the same time, repeated episodes of malaria fail to induce sterile immunity, indicating that the parasite is able to evade the host immune response (3). A more detailed understanding of how P. falciparum interacts with the human immune system is needed to accelerate malaria vaccine research and the development of novel adjuvant therapies aimed at decreasing malaria morbidity and mortality.
In particular, the role of dendritic cells (DCs) in initiating the adaptive immune response to P. falciparum is still unclear. DCs represent a critical component of the immune system because they are not only important for early cytokine responses but also essential for bridging and regulating the innate and adaptive immune responses to vaccines and pathogens (4). DCs reside in virtually all tissues throughout the body, where they sample their surroundings for pathogens. Upon encountering pathogenic material, they rapidly respond by undergoing a maturation process and migrating to secondary lymphoid organs to present antigens to naïve T cells (5, 6). DC maturation is typically triggered through pattern recognition receptors and is characterized by up-regulation of surface costimulatory molecules and secretion of immunomodulatory cytokines that are necessary for effective T cell activation (5). The two major human DC subsets, plasmacytoid DCs (pDCs) and myeloid DCs (mDCs), have distinct functions in initiating and coordinating the immune response. Whereas pDCs produce high levels of IFN-α upon activation, mDCs up-regulate maturation markers and are efficient antigen-presenting cells (7). Depending on their cytokine secretion profile, mDCs can induce tolerance or an inflammatory response by polarizing naïve T cells (8, 9).
Studies in humans and mouse models have been contradictory regarding the role of DCs in malaria (10, 11). Whereas some studies propose that Plasmodium induces effective activation of DCs (12–14), others point to inhibition of activation and/or apoptosis induced by the parasite (15–17). Possible sources of variation between these studies are the different host species (mouse vs. human), the source of DCs (primary vs. monocyte-derived), the ratio of Plasmodium-infected erythrocytes to DCs, and the different procedures for parasite preparation.
In the present study, we have used the most physiological in vitro system available, freshly isolated human primary DCs incubated with clinically relevant ratios of whole infected erythrocytes to DCs (18). Circulating primary human DCs are important to replenish tissue-resident DCs (5) and have a similar phenotype to spleen-resident DCs and DCs found in skin-draining lymph nodes (19, 20). Although low in numbers, they can be enriched from peripheral blood and used to analyze basic DC biology in vitro. We have performed a detailed analysis of the response of DCs to P. falciparum-infected red blood cells (RBCs; iRBCs) and found an atypical pattern of activation of DCs characterized by increased expression of surface maturation markers and chemokines with a near-complete absence of secretion of inflammatory cytokines. This unusual response of mDCs to P. falciparum was also demonstrated by genome-wide transcriptional profiling, which revealed a pattern distinct from LPS-induced activation and involved lipid synthesis-related pathways.
Results
P. falciparum Induces an Atypical Activation Profile in Human Primary DCs.
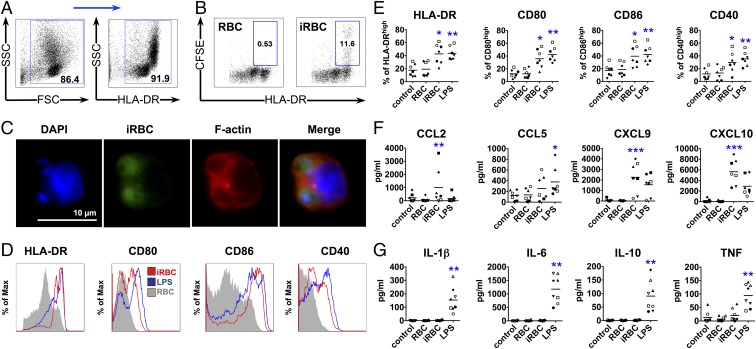
Primary human DCs more likely reflect DC function in vivo compared with murine or monocyte-derived human DCs generated by culturing precursors for several days with cytokines (21, 22). Therefore, we enriched lineage-negative and human leukocyte antigen-D related (HLA-DR)–positive primary human DCs from peripheral blood mononuclear cells (PBMCs) of healthy malaria-naïve donors by negative selection (Fig. 1A) to investigate the effect of P. falciparum blood stage parasites on DC maturation. Enriched DCs were incubated with intact P. falciparum-iRBCs during the late stages of erythrocyte infection. Uninfected RBCs were used as a negative control and LPS as a positive control. We then analyzed the ability of DCs to phagocytose iRBCs and observed efficient uptake of iRBCs by DCs expressing high levels of surface HLA-DR (Fig. 1 B and C).
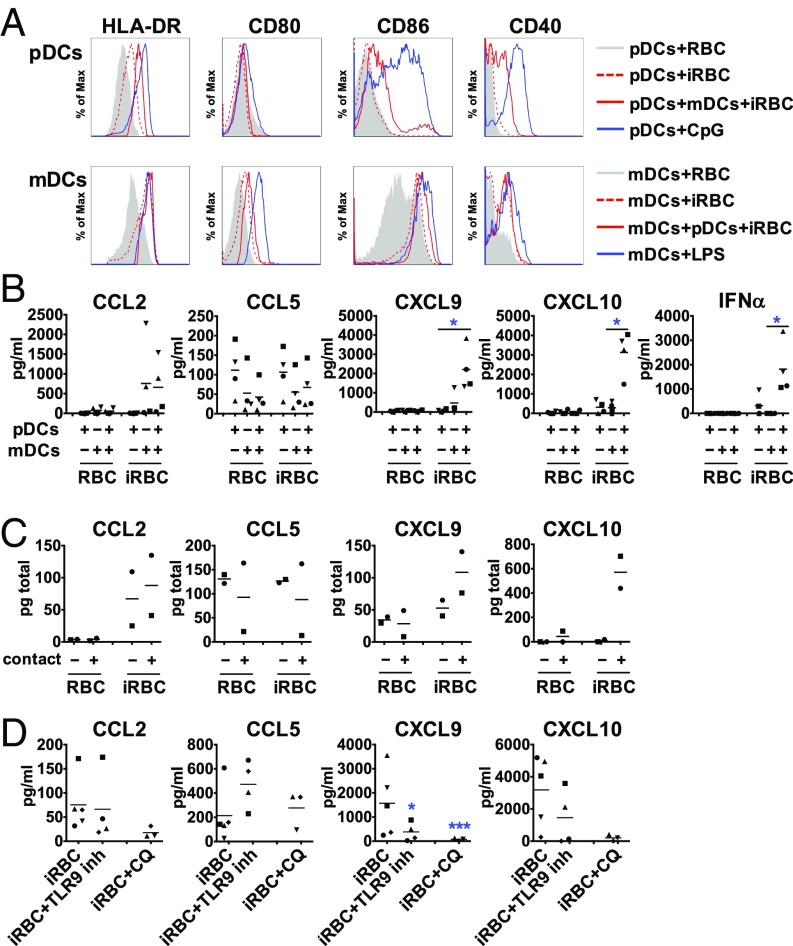
Fig. 1.
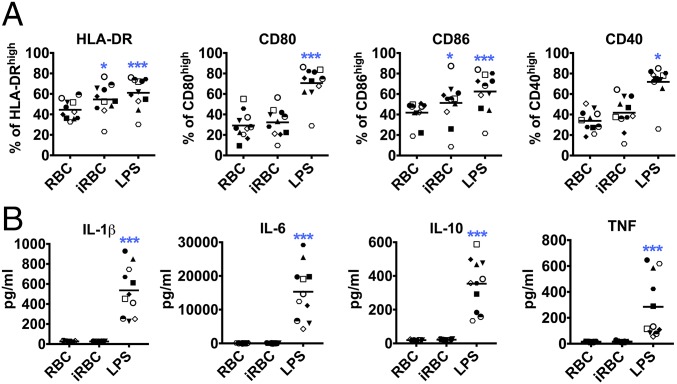
DCs phagocytose P. falciparum-iRBCs and up-regulate maturation markers and secretion of chemokines, but not cytokines. DCs were incubated with late-stage P. falciparum-iRBCs or uninfected RBCs at a ratio of 1:3 [DC:(i)RBC] for 24 h (A and D–G) or 1:3 [DC:carboxy-fluorescein succinimidyl ester (CFSE)-labeled (i)RBC] for 3 h (B and C) and analyzed for surface marker expression (A, D, and E), chemokine (F) and cytokine (G) secretion, or phagocytosis by FACS (B) and immunofluorescence microscopy (C). (A) DCs enriched by negative selection followed by positive selection for HLA-DR with magnetic beads from PBMCs were gated first by using forward scatter (FSC) and SSC, followed by selection of HLA-DR+ cells, and used for further analysis in B and D–G. (B and C) Phagocytosis of iRBCs is observed as CFSE-positive DCs. Data from one representative experiment of three are shown. An example of surface marker expression for one donor (D) and the analysis of seven (E and F) and eight (G) donors are shown (E–G), with each symbol representing results from one individual donor and experiment (*P < 0.05, **P < 0.01, and ***P < 0.001 by Friedman test vs. RBCs or control; line depicts grand mean).
Primary DCs obtained from seven donors were independently assayed and showed that iRBCs, as well as LPS, induced significant up-regulation of the surface maturation markers CD80, CD86, CD40, and HLA-DR on DCs as determined by FACS compared with control uninfected RBCs. Levels of marker up-regulation by P. falciparum were comparable to LPS-induced maturation (Fig. 1 D and E).
Secretion of chemokines implicated in inflammation, such as CCL2, CXCL9, and CXCL10, was also up-regulated after stimulation with iRBCs (Fig. 1F). However, iRBCs did not induce secretion of cytokines typical of inflammatory responses (IL-1β, IL-6, IL-10, and TNF) that are induced by LPS (Fig. 1G). The percentage of apoptotic and dead DCs in the culture was comparable to LPS control after 24 h incubation with iRBCs (SI Appendix, Fig. S1). IL-12p70 was not detected in the supernatant of DCs after stimulation with iRBCs or LPS.
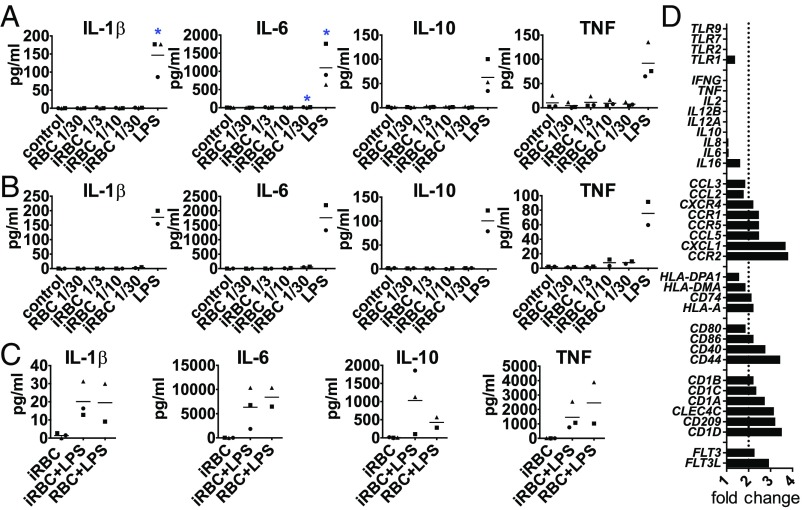
To determine whether the lack of cytokine secretion could be overcome by increased concentrations of iRBCs, we incubated DCs with ratios as high as 1:30 DC:iRBC, but still did not observe a notable cytokine response (Fig. 2A). We also determined that the DC response observed could not be interpreted as a low-intensity response induced by low concentrations of stimulus. A titration of LPS concentrations showed that lower concentrations do not induce a pattern similar to iRBCs (SI Appendix, Fig. S2 A–C). Because molecules responsible for innate immune activation are found inside the iRBCs and are released upon egress during late stages (23, 24), we also tested the capacity of iRBC lysates to induce cytokine secretion in DCs. Although iRBC lysates induced the secretion of chemokines similarly to intact iRBCs, they did not induce considerable cytokine secretion (SI Appendix, Fig. S2 D and E). We also observed that, in contrast to human primary DCs, monocyte-derived DCs showed a complete lack of maturation in response to iRBCs, that is, lack of costimulatory marker, cytokine, and chemokine up-regulation (SI Appendix, Fig. S3).
Fig. 2.
DCs fail to secrete and up-regulate cytokine gene expression upon activation by P. falciparum but are not blocked in their ability to secrete cytokines. (A–C) DCs were incubated alone (control), with P. falciparum-iRBCs without (A) or with knobs (B) at a ratio of 1:3, 1:10, or 1:30 (DC:iRBC), or with uninfected RBCs at a 1:30 ratio (DC:RBC) for 24 h and analyzed for cytokine secretion. (C) DCs were incubated with iRBCs or RBCs at a ratio of 1:3 for 24 h when LPS was added for an additional 24-h incubation. Each symbol represents one individual donor and experiment; line depicts grand mean (*P < 0.05 by Friedman test vs. control). (D) For gene expression analysis, DCs were enriched, positive-selected for HLA-DR, and incubated with iRBCs or RBCs at a ratio of 1:3 for 3 h. Total RNA was extracted and converted to cDNA, and gene expression was analyzed. Results from one donor are expressed as RNA expression fold change of DCs plus iRBCs over DCs plus RBCs.
P. falciparum-iRBCs in human patients express protein adhesion knobs on their surface that mediate interactions with host endothelial cells; however, these knobs are frequently lost during in vitro culture (25). We next used gelatin flotation to select iRBCs with knobs (26) and compared their capacity for stimulation of DCs with iRBCs lacking knobs. We observed that knob expression did not increase cytokine secretion in DCs (Fig. 2B). Taken together, these results point to an atypical activation of human primary DCs in response to P. falciparum-iRBCs, as classical maturation of DCs typically involves up-regulation of surface markers, as well as cytokine and chemokine secretion (27).
To determine whether iRBCs can inhibit the capacity of primary DCs to mature, LPS was added to the cocultures of DCs and iRBCs after 24 h. DCs could still secrete cytokines in response to LPS (Fig. 2C) despite exposure to iRBCs, indicating that DC cytokine secretion in response to LPS was not permanently inhibited upon parasite encounter.
Real-time PCR (RT-PCR) analysis of a panel of mRNAs related to DC activation confirmed our initial results showing increased expression of maturation markers and chemokines/chemokine receptors, but not cytokines (Fig. 2D), in response to iRBCs compared with uninfected RBCs.
P. falciparum-Activated DCs Prime CD4+ T Cells to Become Antigen-Specific Th1-Like Effector Cells.
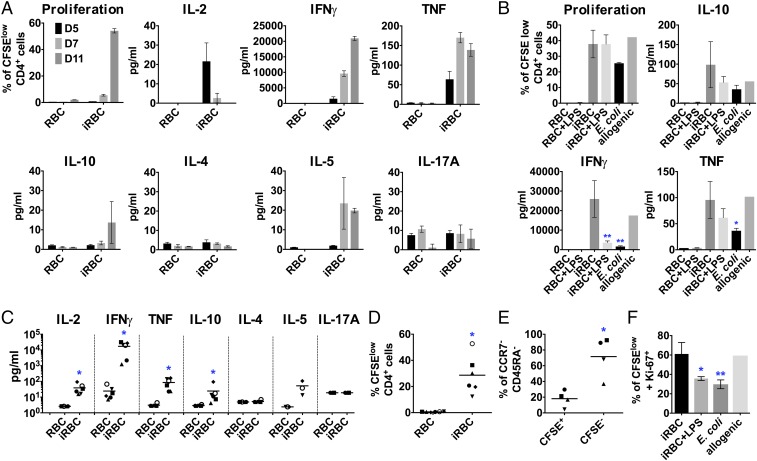
An essential function of DCs is to prime naïve T cells, shaping the immune response to be more tolerogenic or inflammatory depending on the DCs’ activation phenotype (28). To test if primary DCs coincubated with P. falciparum-iRBCs were able to prime autologous naïve CD4+ T cells, we analyzed T cell proliferation and cytokines secreted into the supernatant at different time points during the coincubation. Although parasite-activated DCs secrete only very low levels of cytokines, similar to control nonstimulated DCs (Fig. 2), they were able to activate and polarize autologous naïve CD4+ T cells into Th1-like effector cells with a robust proliferative response, comparable to the one induced by heat-killed Escherichia coli or by allogenic response (Fig. 3 A and B). Secretion of IL-2, IFN-γ and TNF (Fig. 3 A and C) and low levels of IL-10 and IL-5 could be detected in supernatants, whereas no significant increase of IL-4 or IL-17A was observed (Fig. 3 A and C). Although there is variability in the magnitude of the cytokines and proliferative responses among different donors/experiments (Fig. 3 A and C and SI Appendix, Fig. S4), proliferating CD4+ T cells down-regulated the typical naïve T cell markers (CCR7 and CD45RA; Fig. 3E), increased expression of the activation marker Ki-67+ (Fig. 3F), and presented high levels of viability (>96%; SI Appendix, Fig. S4), confirming a robust T cell response. T cells incubated with iRBCs alone did not proliferate or secrete cytokines, thereby excluding a mitogenic effect of the parasite in this assay (SI Appendix, Fig. S4). These results indicate that naïve T cells were efficiently activated by P. falciparum-primed DCs despite the lack of cytokine secretion.
Fig. 3.
P. falciparum-activated DCs prime autologous CD4+ T cells to become Th1-like effector cells in vitro. DCs were incubated with P. falciparum-iRBCs or uninfected RBCs at a ratio of 1:3 [DC:(i)RBC] for 3 h, harvested, and coincubated with autologous naïve CD4+ T cells at a ratio of 1:30 (DC:T cell). T cell proliferation was quantified by CFSE dilution, and cytokines were analyzed in the supernatants. (A) As a representative example, proliferation and cytokine levels are shown for days 5, 7, and 11 for one individual donor. Mean with SD is shown for duplicates and triplicates. (B) DCs were additionally incubated with uninfected RBCs plus LPS, infected RBCs plus LPS, and heat-killed E. coli at a ratio of 1:10 (DC:E. coli) or allogenic DCs. Cytokine levels (levels below detection limit were set to the detection limit; C) and proliferation (D) of different donors and experiments are shown at day 11 (except for IL-2, shown at day 5). (E) Naïve T cell marker expression (CCR7 and CD45RA) was analyzed in T cells primed by P. falciparum-activated DCs at day 11. (F) DCs were incubated with infected RBCs, infected RBCs plus LPS, and heat-killed E. coli at a ratio of 1:10 (DC:E. coli) or allogenic DCs, and CFSElow T cells were stained for the proliferation marker Ki-67 at day 11. Each symbol represents one individual experiment from at least four different donors; line depicts grand mean (C–E). *P < 0.05 by Wilcoxon test (C and D); *P < 0.05 by Student’s t test (E). Results from one donor and experiment are shown at day 11 in triplicates (except for allogenic; *P < 0.05 and **P < 0.01 by one-way ANOVA vs. iRBCs in B and F).
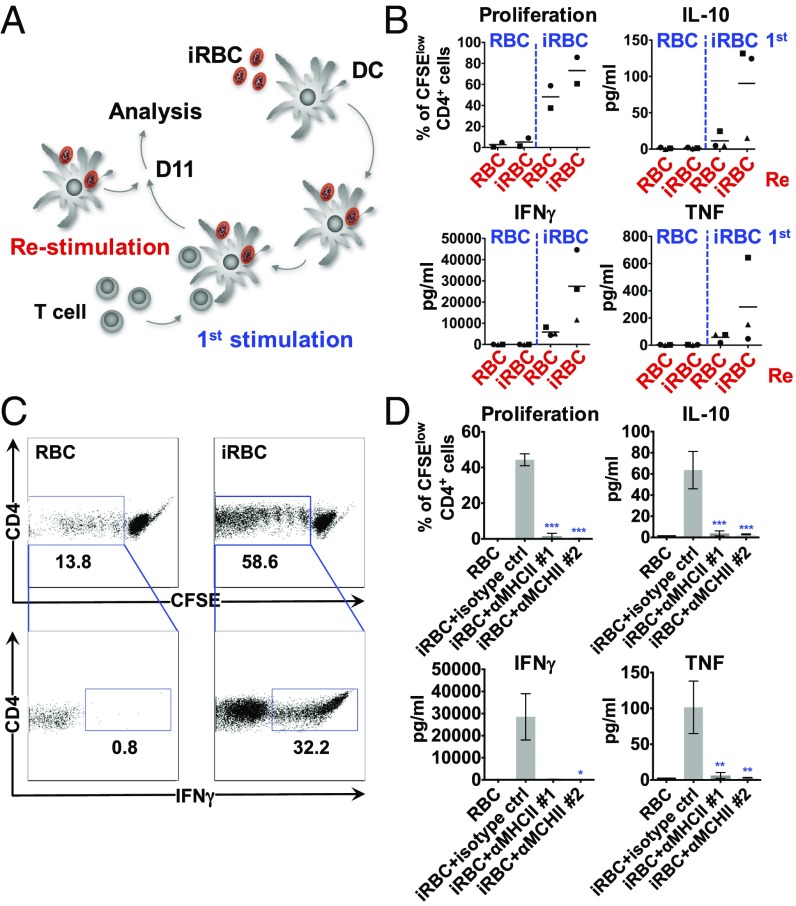
To determine if proliferating T cells induced by DCs preincubated with P. falciparum-iRBCs were specific for parasite antigens, the primed T cells were restimulated with autologous P. falciparum-primed DCs obtained from the same donor that had been previously frozen as PBMCs (Fig. 4A). Proliferation and cytokine secretion were analyzed in the cocultures, and Th1 cytokine levels in the supernatant increased rapidly (24 h) after restimulation of T cells. As a control, T cells restimulated with DCs incubated with uninfected RBCs showed lower levels of cytokine secretion compared with T cells restimulated with parasite-loaded DCs (Fig. 4 A and B). Intracellular staining of IFN-γ in CD4+ T cells confirmed that approximately one third of proliferating T cells were rapidly reactivated in response to P. falciparum-primed DCs, with less than 1% of T cells producing IFN-γ in uninfected RBC controls (Fig. 4C). To confirm that the observed T cell response is mediated by MHCII antigen presentation, blocking antibodies to HLA were added to DCs coincubated with P. falciparum-iRBCs and autologous naïve CD4+ T cells, and this demonstrated strong inhibition of T cell proliferation and cytokine secretion (Fig. 4D). Taken together, these results indicate that Th1-like CD4+ T cells primed by P. falciparum-activated DCs were specific to the parasite, confirming an antigen-specific response.
Fig. 4.
CD4+ T cells primed by P. falciparum are antigen-specific. DCs were incubated with P. falciparum-iRBCs or uninfected RBCs at a ratio of 1:3 [DC:(i)RBC] for 3 h, harvested, and coincubated with autologous naïve CD4+ T cells at a ratio of 1:30 (DC:T cell). Approximately 11 d after the first stimulation, the primed T cells were stained with CFSE and restimulated with autologous DCs incubated with iRBCs or uninfected RBCs as depicted in A. T cell proliferation was quantified after 2–3 d, and cytokines were analyzed in the supernatants after 24 h (B). Each symbol represents one individual donor and experiment; the line depicts the grand mean. Intracellular cytokine staining is shown for IFN-γ after 24 h of restimulation. One representative experiment of two is shown (C). Inhibitory anti-MHCII antibodies (1, anti–HLA-DR/DP/DQ, clone Tü39; 2, anti-HLA-DR, clone L243, 25 µg/mL) were added to the coculture every second day, and proliferation and cytokines were analyzed at day 11. One representative experiment of two is shown. Mean with SD is shown for triplicates (*P < 0.05, **P < 0.01, and ***P < 0.001 by Kruskal–Wallis test for IFN-γ and by one-way ANOVA for all others vs. iRBCs plus isotype control; D).
Cross-Talk Between pDCs and mDCs Is Essential for P. falciparum-Induced Secretion of IFN-α and Chemokines.
HLA-DR–positive and lineage-negative DCs in the aforementioned experiments contain the two major human DC subsets, mDCs and pDCs, which play distinct roles in the induction of the immune response. Whereas pDCs secrete high amounts of IFN-α and are important in innate immune responses to viral pathogens, mDCs shape cellular immune responses by activating antigen-specific T cells (7). pDCs can be activated by P. falciparum-iRBCs through TLR9 and produce IFN-α upon encounters with parasite-derived components (29).
To investigate the specific activation of each subset by iRBCs, we enriched pDCs and mDCs and incubated each subpopulation, independently or together, with P. falciparum-iRBCs (SI Appendix, Fig. S5). mDCs were negatively selected by depleting cells bound to magnetic beads with lineage-specific antibodies to enrich for CD141+ (BDCA-3) and CD1c+ (BDCA-1) mDCs. Alternatively, mDCs were positively selected for CD1c, with comparable results. pDCs were positively selected for CD304 (BDCA-4). We observed that mDCs express higher levels of HLA-DR compared with pDCs, which allowed for the identification of the phagocytic subset of DCs observed in Fig. 1B as mDCs. A drastic shift in the granularity of mDCs also suggested high levels of iRBC phagocytosis by this subtype. The median side scatter (SSC) increases twofold when mDCs are incubated with iRBCs, whereas pDCs SSC values remain constant (SI Appendix, Fig. S5C).
mDCs incubated independently with iRBCs moderately up-regulate all markers tested, but, when pDCs were independently incubated with iRBCs, we detected no up-regulation of surface costimulatory molecules (except for a moderate increase in HLA-DR; Fig. 5A). However, in the coculture of both subsets together after being obtained separately, pDCs increased the up-regulation of HLA-DR, CD86, and CD40, suggesting that mDCs play a role in the activation of pDCs. Likewise, mDCs show increased up-regulation of CD80 and CD86 in the presence of pDCs, but not of other surface markers tested (Fig. 5A).
Fig. 5.
Contact-mediated cross-talk between pDCs and mDCs is essential for P. falciparum-induced secretion of IFN-α and chemokines. Freshly purified pDCs and mDCs were incubated separately or together with P. falciparum-iRBCs or uninfected RBCs at a ratio of 1:3 [DC:(i)RBC] for 24 h and analyzed for surface marker expression (A), IFN-α (B), and chemokine (C and D) secretion. For contact-independent coculture, each subtype was seeded with iRBCs or uninfected RBCs in the upper or lower compartment of a Transwell system, and supernatants were analyzed for chemokines. Contact-independent (−) and cocultures in contact (+) (C). A TLR9 antagonist and chloroquine were used to inhibit TLR9 or intracellular TLR signaling, respectively (D). Each symbol represents results from one individual donor and experiment. Line depicts grand mean [*P < 0.05 by Friedman test vs. RBC or control (B) or *P < 0.05 and ***P < 0.001 by Student’s t test vs. iRBCs (D)].
Analysis of IL-1β, IL-6, IL-10, and TNF in supernatants of mDCs and pDCs cultured independently or together showed no secretion of these factors in response to iRBCs (SI Appendix, Fig. S5D). In contrast, IFN-α that was secreted at low levels by pDCs was greatly up-regulated when mDCs were present in the cultures (Fig. 5B). Similar results were obtained for the chemokines CXCL9 and CXCL10, with cocultures of mDCs and pDCs resulting in increased secretion of these factors (Fig. 5B), indicating that both subsets of DCs are required for these responses. These findings indicate that cross-talk between pDCs and mDCs is required for the efficient up-regulation of IFN-α and specific chemokine secretion upon stimulation with P. falciparum.
To study whether the cross-talk between the two subpopulations of DCs required direct contact between the cells, we separated each subpopulation by a semipermeable membrane that allows the flow of soluble factors, but not of cells. Under these conditions, we observed that, although expression levels of surface costimulatory molecules were similar, the secretion of CXCL9 and especially CXCL10 was greatly reduced (Fig. 5C), suggesting that cell-to-cell contact is necessary to induce specific chemokine secretion.
In human DC subsets, unlike in mice, only pDCs express TLR9 (30). Because pDCs can be activated by P. falciparum-iRBCs through TLR9 (29), we specifically inhibited the activation of this receptor with an antagonistic CpG oligodeoxynucleotide in pDC/mDC cocultures. A significant decrease of CXCL9 secretion was observed, suggesting that activation of pDCs through TLR9 contributes to the chemokine response. To inhibit intracellular TLR signaling more broadly, we used chloroquine, resulting in decreased chemokine levels in the supernatants (Fig. 5D).
P. falciparum-Activated mDCs Are Sufficient to Prime Naïve CD4+ T Cells to Become Th1-Like Effector Cells.
To further characterize the role of DC subpopulation cross-talk, we analyzed its effects on T cell activation. We found that P. falciparum-activated mDCs, but not pDCs, efficiently drive proliferation and cytokine secretion in CD4+ T cells (Fig. 6A).
Fig. 6.
mDCs are sufficient for CD4+ T cell activation and Th1-like polarization. (A) pDCs and mDCs were purified by positive selection using magnetic beads and incubated separately or together (both) with P. falciparum-iRBCs or uninfected RBCs at a ratio of 1:3 [DC:(i)RBC] for 3 h, harvested, and coincubated with autologous naïve CD4+ T cells at a ratio of 1:30 (DC:T cell) for 7 d. T cell proliferation was quantified by CFSE dilution, and cytokines were analyzed in the supernatants. (B) Purified mDCs were incubated with iRBCs at a ratio of 1:3 [DC:(i)RBC] or LPS for 24 h, washed, and analyzed with light microscopy. (Scale bar,10 µm.) (C) Purified mDCs were incubated with P. falciparum-iRBCs or uninfected RBCs at a ratio of 1:3 [DC:(i)RBC] for 3 h, harvested, and coincubated with autologous naïve CD4+ T cells at a ratio of 1:30 (DC:T cell) with isotype control or blocking antibodies for CD40L or IL-12p70 for 7 d. SD of triplicates from one experiment is shown (*P < 0.05 by Kruskal–Wallis test vs. iRBCs plus isotype control). One representative experiment of two is shown.
As mDCs alone are able to effectively induce CD4+ T cell activation, we focused next on these cells to further dissect the specific requirements for P. falciparum-induced T cell activation. We had observed that mDCs alone do not secrete significant levels of inflammatory cytokines (SI Appendix, Fig. S5) or the CXCR3-attractant chemokines CXCL9 and -10 in response to iRBCs (Fig. 5B); however, these cells respond by up-regulating costimulatory surface markers (Fig. 5A) and secreting CCL2 (Fig. 5B).
First, we observed that the morphology of mDCs did not change after incubation with iRBCs. Whereas mDCs activated by LPS were spread and exhibited typical protrusions, mDCs incubated with P. falciparum-iRBCs maintained a more rounded morphology typical of immature DCs (Fig. 6B). In addition to the lack of secretion of the main inflammatory cytokines by mDCs (SI Appendix, Fig. S5C), this finding further corroborates the atypical activation of mDCs by P. falciparum.
CD4+ T cell activation by mDCs in response to iRBCs was further characterized with a panel of monoclonal antibodies specifically inhibiting essential components required for this activation. Blocking of CD40L resulted in inhibition of the secretion of typical Th1 cytokines (IFN-γ and TNF), but not T cell proliferation. Although we were not able to detect IL-12p70 in supernatants, blocking of IL-12p70 also led to lower IFN-γ but did not affect TNF secretion (Fig. 6C), suggesting that levels below the detection limit of this cytokine might promote Th1 polarization in our system.
Taken together, our findings indicate that the atypical activation of DCs by P. falciparum still leads to a strong Th1-like response in naïve CD4+ T cells, despite DCs immature morphology and lack of IL-1β, IL-6, IL-10, and TNF cytokine production.
mDCs Respond Similarly to P. falciparum-iRBCs in Malaria-Naïve and Malaria-Experienced Adults.
Adults living in areas of intense malaria transmission are often infected with P. falciparum blood-stage parasites without symptoms (31, 32). This immunity, which is gradually acquired over years of repeated infections, is known to be antibody-mediated (33), but may also involve immune regulation that limits parasitemia and inflammation (34, 35). To investigate whether repeated malaria infections influence mDC responses relative to responses observed in malaria-naïve donors, we analyzed mDCs from 11 adults residing in a rural village in Mali with lifelong exposure to intense P. falciparum transmission (36). The donors were PCR-negative for Plasmodium at the time of the blood draw, which occurred at the end of the malaria season. Freshly purified PBMCs were used to enrich for mDCs, which were then incubated with RBCs or iRBCs. It has been reported that spontaneous apoptosis is increased in DCs of P. falciparum-infected individuals (16, 17). Although unexpected in the uninfected subjects in this study, we investigated DC apoptosis and subset percentages in freshly isolated PBMCs (SI Appendix, Fig. S6B). The subset percentages were similar to those in healthy US adults and, except for two donors, DCs showed very low annexin V binding. Similar to what we observed in malaria-naïve US donors (Fig. 5), mDCs from Malian donors showed moderately increased surface maturation marker expression (Fig. 7A) and chemokine secretion (SI Appendix, Fig. S6C), but did not secrete significant amounts of cytokines (Fig. 7B) when incubated with the parasite.
Fig. 7.
mDCs from individuals living in an endemic area up-regulate markers but fail to secrete cytokines upon stimulation with P. falciparum. mDCs were enriched from PBMCs from Malian adults and incubated with P. falciparum-iRBCs or uninfected RBC lysates at a ratio of 1:3 [DC:(i)RBC] for 24 h and analyzed for surface marker expression (A) and cytokine secretion (B). Each symbol represents one individual donor and experiment. Line depicts grand mean (*P < 0.05, **P < 0.01, and ***P < 0.001 by Friedman test vs. uninfected RBC control; n = 11).
RNA Sequencing Analysis of mDC After Incubation with P. falciparum-iRBCs Reveals a Distinct Transcriptional Profile.
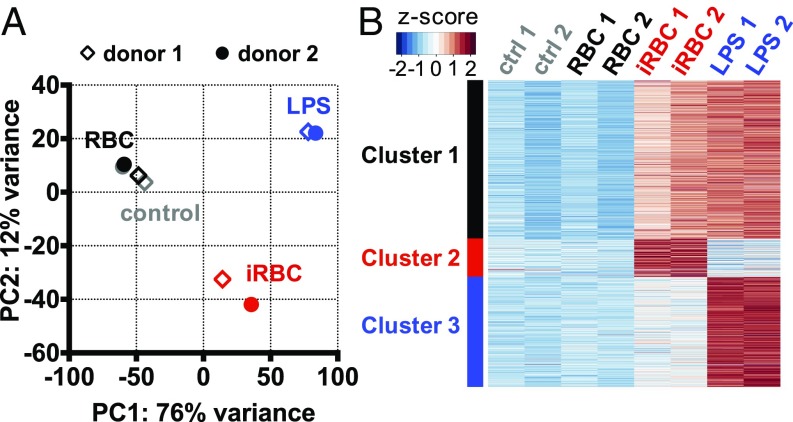
A detailed analysis of the transcriptional profiles in mDCs, the DC subtype responsible for T cell activation (Fig. 6A), was performed by using RNA sequencing (RNA-seq) to better understand the response of this cell type to P. falciparum-iRBCs. Principal component analysis using all expressed genes (N = 25,087 genes) demonstrated the reproducibility of our results and showed that P. falciparum-iRBCs and LPS activation led to very different transcriptional profiles in mDCs (Fig. 8A). We partitioned the union set of genes up-regulated by P. falciparum-iRBCs and LPS (n = 4,626 genes) into three clusters by using k-means clustering: cluster 1 included genes commonly activated by both stimuli (n = 2,388 genes), whereas clusters 2 and 3 included genes that were predominantly activated by P. falciparum-iRBCs (n = 574 genes) and LPS (n = 1,664 genes), respectively (Fig. 8B).
Fig. 8.
CD1c+ mDCs show distinct gene expression upon stimulation with P. falciparum-iRBCs. CD1c+ mDCs were purified by positive selection using magnetic beads and incubated with P. falciparum-iRBCs or uninfected RBCs at a ratio of 1:3 [DC:(i)RBC] for 6 h before RNA extraction and whole-genome RNA-seq of two individual donors and experiments. (A) Principal component analysis of all 25,087 genes showed that DCs stimulated with LPS or iRBCs have different gene expression profiles. Scatter diagram of the first two principal components is shown. (B) K-means clustering partitioning the union set of 4,626 up-regulated genes from LPS or iRBCs stimulations into three distinct sets of genes. Cluster 1, genes commonly up-regulated by LPS and iRBCs; cluster 2, genes predominantly up-regulated by iRBCs; cluster 3, genes predominantly up-regulated by LPS.
Pathway enrichment analysis of the genes activated commonly by LPS and P. falciparum (cluster 1) found typical maturation pathways, such as IFN and TLR signaling and DC maturation, as described previously for LPS activation of human monocyte-derived DCs (37). In contrast, the genes predominantly activated by iRBCs (cluster 2) were enriched with lipid synthesis-related pathways, such as cholesterol biosynthesis and adipogenesis pathway (SI Appendix, Fig. S7). By using alternative methods such as hierarchical clustering and set operations to define genes predominantly or uniquely up-regulated by iRBCs (n = 434 genes defined by using hierarchical clustering and n = 597 genes defined by using set operations), we consistently found lipid synthesis-related pathways to be enriched in this set of genes (SI Appendix, Fig. S7).
To further characterize specific mDC functions activated by the two different activating conditions, we extracted expression values of genes annotated with different Gene Ontology (GO) terms. We found that, whereas genes involved in TLR signaling pathway and chemokine genes were expressed at similar levels of abundance in both iRBC- and LPS-activated mDCs, the genes involved in T cell costimulation and cytokine genes showed greater variability in expression levels between iRBC- and LPS-activated mDCs (SI Appendix, Fig. S7F).
To identify potential mechanisms regulating the activation of a major contributor to inflammation during infection, the TLR signaling pathway, we used the annotations for this pathway in the curated Ingenuity database. We first identified genes included in this pathway and showed that genes within the TLR2/MyD88/IRAK1 axis were highly up-regulated with iRBCs. Upon visualization of the pathway, we demonstrated that genes commonly induced by both LPS and the parasite were natural downstream effectors of TLR signaling, such as NF-κB and TNF receptor-associated factor 6 (TRAF6), which usually leads to inflammatory cytokine secretion (SI Appendix, Fig. S8). However, addition of an inhibitor of NF-κB, IKK inhibitor VII, had no effect in cytokine secretion or surface marker expression in DCs (SI Appendix, Fig. S9), suggesting that NF-κB signaling might not be involved in the observed DC activation by iRBCs. Inhibitors for other candidate genes and pathways, such as peroxisome proliferator-activated receptor-γ (PPAR-γ), lipid signaling nuclear receptor RXR, and the autophagy pathway, showed no effect in DC cytokine secretion and surface marker expression (SI Appendix, Fig. S9).
Although we did not detect cytokine secretion by mDCs incubated with P. falciparum-iRBCs (SI Appendix, Fig. S5D), genes up-regulated by parasite and LPS include genes and gene subunits for some cytokines, specifically TNF (SI Appendix, Fig. S8A). It is possible that the TNF gene is transcriptionally up-regulated by P. falciparum-iRBCs but posttranslational regulation of TNF result in little to no secretion of the cytokine at the protein level (SI Appendix, Fig. S5). This discrepancy specifically between TNF transcription and secreted protein levels has been described before in human monocyte-derived DCs activated with LPS (38). Our analysis also revealed several cytokine genes that were induced only by the parasite and not by LPS, one of them being the regulatory cytokine TGF-β1 (SI Appendix, Fig. S10).
mDCs Activated by P. falciparum-iRBCs Share Expression Profiles with mDCs Activated by Four Viral Vaccines.
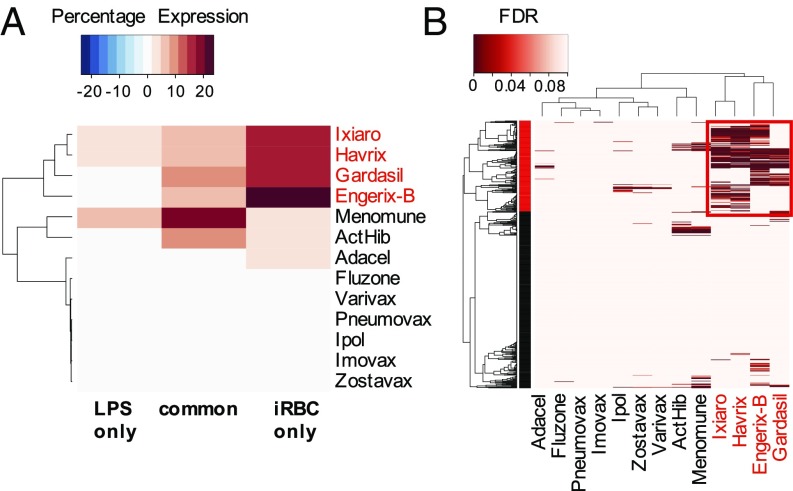
To obtain more insight into the distinct features of P. falciparum-iRBC activation, we decided to compare our RNA-seq transcriptional profiling results with a set of microarray-based transcriptional profiling data by Banchereau et al. (39), in which primary human CD1c+ DCs were stimulated with 13 different microbial vaccines, including inactivated bacterial, inactivated viral, and live attenuated viral vaccines. By using a similar strategy, we determined the “percentage expression” value for each gene of the gene cluster from our data set under each vaccine stimulation condition. We found the iRBC-specific gene cluster to be most highly expressed in CD1c+ DCs stimulated with Ixiaro and Havrix, which are vaccines based on inactivated Japanese encephalitis and hepatitis A viruses, respectively; Gardasil, based on human papilloma virus-like particles; and Engerix-B, based on recombinant protein hepatitis B surface antigen (Fig. 9A). This indicates that the activation state of CD1c+ DCs stimulated with P. falciparum-iRBCs shares similarities with the activation state of CD1c+ DCs activated by these four vaccines, suggesting common regulatory mechanisms in how P. falciparum and these four vaccines activate CD1c+ DCs. The cluster containing genes commonly up-regulated by LPS and iRBCs was most highly expressed with stimulation by Menomune and ActHib, which are both LPS-containing vaccines (Fig. 9A).
Fig. 9.
The P. falciparum-iRBC–specific genes are most highly expressed in CD1c+ DCs activated with the vaccines Ixiaro, Gardasil, Havrix, and Engerix-B. (A) The iRBC-specific gene cluster is most highly induced with the vaccines Ixiaro, Gardasil, Havrix, and Engerix-B (names in red). Values shown here are percentage expression, where a positive value indicates up-regulation and a negative value indicates down-regulation. (B) The high expression of the iRBC-specific gene cluster in the four vaccines is contributed by 158 genes (boxed in red). Values shown here represent statistical significance, as determined by the calculation of false discovery rate (FDR). Darker shades of red represent a low FDR value and therefore statistical significance compared with expression levels under control medium.
The similarities in activation profiles between iRBCs and the four viral vaccines were contributed by 158 iRBC-specific genes, whereas the remaining iRBC-specific genes were largely unchanged across the data set (Fig. 9B). Most of these genes were up-regulated upon stimulation with the vaccines (n = 132 genes), but a small subset of these iRBC-specific genes was down-regulated instead (n = 26 genes; SI Appendix, Fig. S11A). The genes commonly up-regulated by iRBCs and the vaccines are associated with different metabolic pathways, such as l-serine and n-acetylglucosamine degradation, adipogenesis, and triacylglycerol degradation (SI Appendix, Fig. S11B). Additionally, the expression of several genes inferred to be regulated by TREM-1 (using the upstream regulator analysis in Ingenuity Pathway Analysis) in DCs stimulated with iRBCs, Ixiaro, Gardasil, Havrix, and Engerix-B suggests a shared pathway in DC activation (SI Appendix, Fig. S11 C and D and Table S1). Another set of genes with similar expression profiles during activation by iRBCs and the four vaccines were genes associated with murine models of “alternatively activated macrophages.” These included ALDH1A2 (an enzyme that can produce retinoic acid), PPAR-γ, and EGR2 (40–42).
We also compared the capabilities of the Ixiaro, Gardasil, Havrix, and Engerix-B vaccines to produce cytokines by examining the expression levels of genes annotated with the GO term “cytokine activity” when stimulated by each of these vaccines in an independent analysis. Although the four vaccines do not induce the expression of most cytokines, a small subset of cytokines was transcriptionally induced (SI Appendix, Fig. S12A). Some of these cytokines were also uniquely expressed during iRBC activation, which include OSM, TNFSF14, and GREM1 (SI Appendix, Fig. S10). This further suggests a similar DC activation state by these vaccines and iRBCs.
Discussion
Our results indicate that human primary DCs undergo a distinctive activation in response to P. falciparum-iRBCs, which includes up-regulation of HLA-DR and costimulatory molecules and chemokines, but not secretion of significant amounts of cytokines. Previous studies had shown a direct inhibition of human monocyte-derived DC maturation induced by the parasite at high concentrations (15) and a lack of typical maturation features such as up-regulation of HLA-DR and costimulatory molecules at lower concentrations (14). This discrepancy with the results presented here is most likely explained by the origin of DCs, as previous studies had used monocyte-derived DCs, which we also found to lack up-regulation of HLA-DR and costimulatory molecules at similar concentrations of parasite. Although working with human primary DCs is an experimental hurdle because of their low frequency in peripheral blood, these results highlight the importance of using primary cells in these studies.
The low inflammatory cytokine response observed in DCs in response to P. falciparum contrasts strongly with the high levels of inflammation observed in patients with malaria (43). This low inflammatory cytokine response to the parasite in vitro has previously been noticed in several in vitro studies that analyzed the response of mice and human macrophages to Plasmodium-infected erythrocytes (44, 45) and suggests that additional inflammatory stimuli may be generated by the host upon infection, which may explain the extreme differences in disease tolerance that are observed in malaria-infected individuals (46). It is also possible that the lack of an inflammatory cytokine response from individual cell types, such as shown in the present work for DCs, or in macrophages (44, 45), is overcome in the presence of other immune cells, as cytokine responses tended to be higher when cultures of PBMCs were stimulated with iRBCs (47).
The observed lack of an inflammatory cytokine response to iRBCs is also unusual in the context of DC activation, in which, typically, HLA-DR and costimulatory molecules, chemokines, and cytokines are simultaneously up-regulated in response to a stimulus (5). Similar to our results, a functionally distinct pathway of DC activation has been described as a result of cluster disruption in human stem cell-derived DCs, which results in up-regulation of HLA-DR and costimulatory molecules and certain chemokines, but a lack of secretion of inflammatory cytokines. However, these DCs also induce a tolerogenic phenotype in T cells that is mediated by activation of β-catenin in DCs (48, 49), which is not observed after incubation of DCs with iRBCs. Ligation of CD47 also limits cytokine secretion by DCs, suggesting a possible mechanism, but it simultaneously inhibits DC up-regulation of costimulatory molecules and their capacity to stimulate T cell proliferation and IFN-γ production (50). We also identified cytokine genes specifically induced only by P. falciparum in DCs, i.e., not by LPS, with TGFB1 being an interesting candidate that is secreted by DCs and suppresses liver stage CD8+ T cell responses in a malaria mouse model (51).
A number of P. falciparum-derived molecules have been described as TLR or inflammasome activators based on their capacity to induce cytokine secretion in immune cells in vitro (23). In this context, it is intriguing that whole infected erythrocytes or their lysates do not induce potent cytokine responses in DCs in vitro. It is possible that P. falciparum-infected RBCs also carry inhibitory molecules that could modulate the activation phenotype of DCs. Indeed, we have observed that P. falciparum-iRBCs induce increased transcription of genes, such as PPARA and PPARG, that are involved in the modulation of DC responses (52). Hemozoin has been shown to increase PPAR-γ expression and inhibit DC maturation when human monocytes were loaded with the pigment before differentiation (53). A possible mechanism for the suppression of cytokine responses by PPARs is their inhibition of the transcription factor NF-κB (54), which regulates inflammatory cytokine expression. However, inhibition of PPAR-γ or NF-κB did not affect the DC cytokine secretion in response to iRBCs, suggesting that these factors may not be involved in the observed low-cytokine DC response. Accordingly, it has been shown that costimulatory marker up-regulation in the absence of increased cytokine secretion was independent of NF-κB in DC activation (55). Costimulatory markers and chemokines can also be induced through IFN-α (56) or β-catenin signaling (48).
We have observed that a large number of the specific signaling pathways activated by P. falciparum are different from the ones induced by a classical TLR activator such as LPS. We found a high number of lipid synthesis-related pathways, in particular cholesterol biosynthesis, which may contribute to the atypical activation phenotype of mDCs (57). In the list of pathways up-regulated uniquely by P. falciparum, we also found the TLR2 pathway that may be induced by parasite glycosylphosphatidylinositol anchors, which are known to activate TLR2 and induce an inflammatory response in vitro (58) and in mice (59). TLR2 is also up-regulated in peripheral blood of patients with malaria (60), and a polymorphism in this gene is associated with protection from cerebral malaria (61), suggesting an important role for this receptor in the human response to malaria.
Despite the low inflammatory cytokine secretion by DCs, iRBC-primed DCs induced strong activation of T cells characterized by proliferation and secretion of Th1 cytokines such as IFN-γ and TNF. This response was found to be typically antigen-specific, as observed in the recall experiments, and mediated by CD40L and IL-12p70.
In addition to the classical Th1 cytokines, T cells incubated with iRBC-primed DCs also secrete IL-5 and IL-10, although it is unknown whether these cytokines are a reflection of different subtypes of T cells in the cultures or coexpression of multiple cytokines in a particular subtype. CD4+ T cells coproducing IL-10 and IFN-γ, or T regulatory-1 cells, correlate with exposure to malaria and have been proposed to play a role in the modulation of immunity in vivo (35, 36, 63).
It seems unusual that malaria-naïve donors present large numbers of T cells specific for P. falciparum antigens. However, high frequencies of T cells specific for P. falciparum antigens in nonexposed donors has been reported before (14, 64–66). Because malaria is considered the strongest evolutionary-selective force in recent human history (67), it is possible that having a large number of T cells specific for this parasite may be under positive selection.
Distinct functional capabilities have been described for each of the two main subtypes of DCs, pDCs and mDCs (7). Because the characteristics of these DC subpopulations are significantly different in mice and humans (68), our analysis of the interactions of primary DCs subtypes with iRBCs are particularly relevant. As described for other pathogens, mDCs are the subset that predominantly phagocytoses iRBCs and the one solely responsible for the activation of T cells (7, 69). On the contrary, pDCs differentially secrete IFN-α, but not TNF, in response to iRBCs, as previously described (29), suggesting that P. falciparum activates different signaling pathways compared with classical activators of pDCs that induce both IFN-α and TNF.
Maturation of pDCs in response to bacterial stimulation or TLR ligands requires cooperation from mDCs, whereby both cell contact and soluble factors play essential roles (70, 71). On the contrary, mDCs are not able to be activated by HIV but need bystander activation facilitated by the secretion of TNF and type I IFNs by pDCs (72). We also observed that the presence of mDCs increases the secretion of IFN-α and the up-regulation of costimulatory molecules by pDCs, which indicates a possible role for mDCs in the activation of pDCs. This is particularly relevant because recent studies have highlighted the importance of type I IFN in regulating the immune response to malaria (63, 73).
Also, a significant increase in CXCL10 secretion that requires direct contact between both subpopulations of DCs is observed in the cocultures. Because CXCL10 is a potent T cell recruiter, this probably leads to the recruitment of naïve T cells and ultimately heightens priming in vivo. A possible advantage for this cooperation between pDCs and mDCs might be an increased response even with inefficient activation of either one of the subsets. mDCs are most likely not able to sense parasite DNA because of the lack of TLR9 expression, whereas pDCs do not respond to TLR2 ligands as a result of the lack of expression of this receptor (70, 74). If parasites like Plasmodium evolved to escape the immune system by inhibiting DC activation, the cross-talk between pDCs being primed by parasite DNA through TLR9 and mDCs being activated through TLR2 could still lead to a functional, although maybe not maximally efficient, response against the parasite. Indeed, CXCL10 has a detrimental effect on the development of immunity and promotes severe outcomes in murine (75, 76) and human (77) malaria. In agreement with our findings, a recent study investigating immune cell responses in murine spleens during Plasmodium chabaudi infection also reported increased expression of CXCL9 and CXCL10 in classical DCs but no increased cytokine gene expression when analyzing the transcriptomes of single DCs (78). Despite the differences between human and murine DC subsets, this suggests that a dominant chemokine response, specifically CXCL9 and -10, rather than inflammatory cytokines, might be a conserved response to Plasmodium.
To gain additional insight into possible pathways involved in DC activation by P. falciparum-iRBCs, we compared our RNA-seq data set with one of the only other high-quality transcriptional profiling experiments performed with primary human DCs that were incubated with different vaccines (39). We found that, although the majority of genes induced by the parasite were not differentially expressed in DCs stimulated with the different vaccines, four viral vaccines share a subset of genes induced by P. falciparum-iRBCs. These four vaccines induce only low to intermediate levels of DC maturation, while down-regulating CD40, confirming that crucial pathways in the response are not shared between the vaccines and iRBCs. However, our analyses provided hints that certain pathways may be shared, including TREM-1 activation, as well as “alternative activation” pathways similar to M2 alternatively activated macrophages in mice. In malaria, TREM-1 has been described to have a role in severe disease outcomes (79). We have also identified cytokines like OSM and TNFSF14 (LIGHT) that seem to be expressed in human primary DC activation states that lack expression of typical inflammatory cytokines. Our findings provide insights into the complexity of DC activation, showing that specific viral vaccines (which also act through unknown mechanisms) share distinctive features of iRBC activation. The viral origin of the four vaccines together with the strong induction of IFN-α secretion in pDCs by the parasite suggest an activation state similar to the one induced by viruses. Interestingly, Zika virus activates primary human mDCs in a similar way as P. falciparum-iRBCs, namely the lack of cytokine secretion accompanied by a relatively high CXCL9 and -10 response (80). These analyses highlight the complexity of DC response to different microbial stimuli, suggesting that DC maturation should not be cataloged as a yes-or-no response, but as a complex and nuanced reaction that is tailored to each specific microbe.
It is remarkable that a pathogen causing such a highly inflammatory disease as malaria fails to induce a potent cytokine response in DCs. The particular response of DCs we have observed may underlie some of the unconventional features of the malaria-adaptive immune response whereby DCs are expected to have an important influence, such as the lack of sterilizing immunity and its rapid decrease in the absence of exposure (81).
Materials and Methods
P. falciparum Culture and Isolation.
Erythrocyte asexual stage cultures of the P. falciparum strain 3D7 were maintained as described in SI Appendix, SI Materials and Methods.
US and Malian Donors.
Peripheral venous blood from healthy donors in New York (United States) or Kambila (Mali) was obtained. Approval was obtained from the institutional review board (IRB) at New York University School of Medicine; the ethics committee of the Faculty of Medicine, Pharmacy and Dentistry at the University of Sciences, Technique and Technology of Bamako; and the National Institute of Allergy and Infectious Diseases/National Institutes of Health (NIH). Informed consent was obtained from each donor before donation.
DC Enrichment, Activation, and Phagocytosis.
The different cell types were enriched with magnetic beads. To analyze DC activation, DCs were cultured with intact or lysed P. falciparum-iRBCs at a ratio of 1:3 (DC:iRBC) for 24 h. For phagocytosis, P. falciparum-infected RBCs were labeled and coincubated with the DCs at a ratio of 1:3 (DC:iRBC) for 3 h. Details are provided in SI Appendix, SI Materials and Methods.
Autologous CD4+ T Cell Activation and Restimulation.
The naïve CD4+ T cell activation was described before (82) and is detailed in SI Appendix, SI Materials and Methods.
Cytokine and Chemokine Analysis.
Cytokines and chemokines were quantified by using BD Cytometric Bead Arrays (BD Biosciences) and acquired on a FACSCalibur device (BD Biosciences).
Quantitative RT-PCR Array, RNA-Seq, and Comparison of Data with Published CD1c+ Vaccine Data Set.
DCs were enriched with negative selection followed by HLA-DR positive selection and CD1c (BDCA-1)+ DC isolation kit, respectively. Cells were then incubated with P. falciparum-iRBCs at a ratio of 1:3 (DC:iRBC). Analysis and comparison of RNA-seq data were performed as detailed in SI Appendix, SI Materials and Methods.
Statistical Analysis.
Statistical analysis is detailed in SI Appendix, SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank the residents of Kambila, Mali, and the donors at New York University for participating in this study; and Jeff Skinner and Julio Gallego-Delgado for help with statistical analysis. New York University School of Medicine Clinical and Translational Science Institute (CTSI) is supported by NIH Grant 3UL1 TR001445. The New York University School of Medicine Genome Technology Center is supported by NIH Grant P30CA016087. Field studies in Mali are funded by the Division of Intramural Research, National Institutes of Allergy and Infectious Diseases, NIH. This work was supported by a fellowship within the Postdoc-Programme of the German Academic Exchange Service and the American Association of Immunology Careers in Immunology Fellowship Program (M.S.T.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The RNAseq data (fastq and count files) reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, https://www.ncbi.nlm.nih.gov/geo (accession no. GSE89087).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1708383114/-/DCSupplemental.
References
- 1. World Health Organization (2016) World Malaria Report 2016 (World Health Organization, Geneva)
- 2.Storm J, Craig AG. Pathogenesis of cerebral malaria–Inflammation and cytoadherence. Front Cell Infect Microbiol. 2014;4:100. doi: 10.3389/fcimb.2014.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stanisic DI, Barry AE, Good MF. Escaping the immune system: How the malaria parasite makes vaccine development a challenge. Trends Parasitol. 2013;29:612–622. doi: 10.1016/j.pt.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 5.Banchereau J, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 6.Randolph GJ, Angeli V, Swartz MA. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat Rev Immunol. 2005;5:617–628. doi: 10.1038/nri1670. [DOI] [PubMed] [Google Scholar]
- 7.Collin M, McGovern N, Haniffa M. Human dendritic cell subsets. Immunology. 2013;140:22–30. doi: 10.1111/imm.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lutz MB, Schuler G. Immature, semi-mature and fully mature dendritic cells: Which signals induce tolerance or immunity? Trends Immunol. 2002;23:445–449. doi: 10.1016/s1471-4906(02)02281-0. [DOI] [PubMed] [Google Scholar]
- 9.van den Broek M. Dendritic cells break bonds to tolerize. Immunity. 2007;27:544–546. doi: 10.1016/j.immuni.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Cockburn IA, Zavala F. Dendritic cell function and antigen presentation in malaria. Curr Opin Immunol. 2016;40:1–6. doi: 10.1016/j.coi.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 11.Wykes MN, Good MF. What really happens to dendritic cells during malaria? Nat Rev Microbiol. 2008;6:864–870. doi: 10.1038/nrmicro1988. [DOI] [PubMed] [Google Scholar]
- 12.Perry JA, Rush A, Wilson RJ, Olver CS, Avery AC. Dendritic cells from malaria-infected mice are fully functional APC. J Immunol. 2004;172:475–482. doi: 10.4049/jimmunol.172.1.475. [DOI] [PubMed] [Google Scholar]
- 13.Pouniotis DS, et al. Dendritic cells induce immunity and long-lasting protection against blood-stage malaria despite an in vitro parasite-induced maturation defect. Infect Immun. 2004;72:5331–5339. doi: 10.1128/IAI.72.9.5331-5339.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elliott SR, et al. Inhibition of dendritic cell maturation by malaria is dose dependent and does not require Plasmodium falciparum erythrocyte membrane protein 1. Infect Immun. 2007;75:3621–3632. doi: 10.1128/IAI.00095-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Urban BC, et al. Plasmodium falciparum-infected erythrocytes modulate the maturation of dendritic cells. Nature. 1999;400:73–77. doi: 10.1038/21900. [DOI] [PubMed] [Google Scholar]
- 16.Pinzon-Charry A, et al. Apoptosis and dysfunction of blood dendritic cells in patients with falciparum and vivax malaria. J Exp Med. 2013;210:1635–1646. doi: 10.1084/jem.20121972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woodberry T, et al. Low-level Plasmodium falciparum blood-stage infection causes dendritic cell apoptosis and dysfunction in healthy volunteers. J Infect Dis. 2012;206:333–340. doi: 10.1093/infdis/jis366. [DOI] [PubMed] [Google Scholar]
- 18.Scholzen A, Mittag D, Rogerson SJ, Cooke BM, Plebanski M. Plasmodium falciparum-mediated induction of human CD25Foxp3 CD4 T cells is independent of direct TCR stimulation and requires IL-2, IL-10 and TGFbeta. PLoS Pathog. 2009;5:e1000543. doi: 10.1371/journal.ppat.1000543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mittag D, et al. Human dendritic cell subsets from spleen and blood are similar in phenotype and function but modified by donor health status. J Immunol. 2011;186:6207–6217. doi: 10.4049/jimmunol.1002632. [DOI] [PubMed] [Google Scholar]
- 20.Segura E, et al. Characterization of resident and migratory dendritic cells in human lymph nodes. J Exp Med. 2012;209:653–660. doi: 10.1084/jem.20111457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lundberg K, et al. Transcriptional profiling of human dendritic cell populations and models–Unique profiles of in vitro dendritic cells and implications on functionality and applicability. PLoS One. 2013;8:e52875. doi: 10.1371/journal.pone.0052875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robbins SH, et al. Novel insights into the relationships between dendritic cell subsets in human and mouse revealed by genome-wide expression profiling. Genome Biol. 2008;9:R17. doi: 10.1186/gb-2008-9-1-r17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gazzinelli RT, Kalantari P, Fitzgerald KA, Golenbock DT. Innate sensing of malaria parasites. Nat Rev Immunol. 2014;14:744–757. doi: 10.1038/nri3742. [DOI] [PubMed] [Google Scholar]
- 24.Ndungu FM, Urban BC, Marsh K, Langhorne J. Regulation of immune response by Plasmodium-infected red blood cells. Parasite Immunol. 2005;27:373–384. doi: 10.1111/j.1365-3024.2005.00771.x. [DOI] [PubMed] [Google Scholar]
- 25.Langreth SG, Reese RT, Motyl MR, Trager W. Plasmodium falciparum: Loss of knobs on the infected erythrocyte surface after long-term cultivation. Exp Parasitol. 1979;48:213–219. doi: 10.1016/0014-4894(79)90101-2. [DOI] [PubMed] [Google Scholar]
- 26.Goodyer ID, Johnson J, Eisenthal R, Hayes DJ. Purification of mature-stage Plasmodium falciparum by gelatine flotation. Ann Trop Med Parasitol. 1994;88:209–211. doi: 10.1080/00034983.1994.11812859. [DOI] [PubMed] [Google Scholar]
- 27.Blanco YC, et al. Hyperbaric oxygen prevents early death caused by experimental cerebral malaria. PLoS One. 2008;3:e3126. doi: 10.1371/journal.pone.0003126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Swafford D, Manicassamy S. Wnt signaling in dendritic cells: Its role in regulation of immunity and tolerance. Discov Med. 2015;19:303–310. [PMC free article] [PubMed] [Google Scholar]
- 29.Pichyangkul S, et al. Malaria blood stage parasites activate human plasmacytoid dendritic cells and murine dendritic cells through a Toll-like receptor 9-dependent pathway. J Immunol. 2004;172:4926–4933. doi: 10.4049/jimmunol.172.8.4926. [DOI] [PubMed] [Google Scholar]
- 30.Kastenmüller W, Kastenmüller K, Kurts C, Seder RA. Dendritic cell-targeted vaccines–hope or hype? Nat Rev Immunol. 2014;14:705–711. doi: 10.1038/nri3727. [DOI] [PubMed] [Google Scholar]
- 31.Langhorne J, Ndungu FM, Sponaas AM, Marsh K. Immunity to malaria: More questions than answers. Nat Immunol. 2008;9:725–732. doi: 10.1038/ni.f.205. [DOI] [PubMed] [Google Scholar]
- 32.Tran TM, et al. An intensive longitudinal cohort study of Malian children and adults reveals no evidence of acquired immunity to Plasmodium falciparum infection. Clin Infect Dis. 2013;57:40–47. doi: 10.1093/cid/cit174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cohen S, McGregor IA, Carrington S. Gamma-globulin and acquired immunity to human malaria. Nature. 1961;192:733–737. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- 34.Jagannathan P, et al. Loss and dysfunction of Vδ2+γδ T cells are associated with clinical tolerance to malaria. Sci Transl Med. 2014;6:251ra117. doi: 10.1126/scitranslmed.3009793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Portugal S, et al. Exposure-dependent control of malaria-induced inflammation in children. PLoS Pathog. 2014;10:e1004079. doi: 10.1371/journal.ppat.1004079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crompton PD, et al. Sickle cell trait is associated with a delayed onset of malaria: Implications for time-to-event analysis in clinical studies of malaria. J Infect Dis. 2008;198:1265–1275. doi: 10.1086/592224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Castiello L, et al. Monocyte-derived DC maturation strategies and related pathways: A transcriptional view. Cancer Immunol Immunother. 2011;60:457–466. doi: 10.1007/s00262-010-0954-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Messmer D, Messmer B, Chiorazzi N. The global transcriptional maturation program and stimuli-specific gene expression profiles of human myeloid dendritic cells. Int Immunol. 2003;15:491–503. doi: 10.1093/intimm/dxg052. [DOI] [PubMed] [Google Scholar]
- 39.Banchereau R, et al. Transcriptional specialization of human dendritic cell subsets in response to microbial vaccines. Nat Commun. 2014;5:5283. doi: 10.1038/ncomms6283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chawla A. Control of macrophage activation and function by PPARs. Circ Res. 2010;106:1559–1569. doi: 10.1161/CIRCRESAHA.110.216523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gundra UM, et al. Alternatively activated macrophages derived from monocytes and tissue macrophages are phenotypically and functionally distinct. Blood. 2014;123:e110–e122. doi: 10.1182/blood-2013-08-520619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jablonski KA, et al. Novel markers to delineate murine M1 and M2 macrophages. PLoS One. 2015;10:e0145342. doi: 10.1371/journal.pone.0145342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crompton PD, et al. Malaria immunity in man and mosquito: Insights into unsolved mysteries of a deadly infectious disease. Annu Rev Immunol. 2014;32:157–187. doi: 10.1146/annurev-immunol-032713-120220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Couper KN, et al. Parasite-derived plasma microparticles contribute significantly to malaria infection-induced inflammation through potent macrophage stimulation. PLoS Pathog. 2010;6:e1000744. doi: 10.1371/journal.ppat.1000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou J, Ludlow LE, Hasang W, Rogerson SJ, Jaworowski A. Opsonization of malaria-infected erythrocytes activates the inflammasome and enhances inflammatory cytokine secretion by human macrophages. Malar J. 2012;11:343. doi: 10.1186/1475-2875-11-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Galatas B, Bassat Q, Mayor A. Malaria parasites in the asymptomatic: looking for the hay in the haystack. Trends Parasitol. 2016;32:296–308. doi: 10.1016/j.pt.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 47.Orengo JM, et al. Uric acid is a mediator of the Plasmodium falciparum-induced inflammatory response. PLoS One. 2009;4:e5194. doi: 10.1371/journal.pone.0005194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jiang A, et al. Disruption of E-cadherin-mediated adhesion induces a functionally distinct pathway of dendritic cell maturation. Immunity. 2007;27:610–624. doi: 10.1016/j.immuni.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Manicassamy S, et al. Activation of beta-catenin in dendritic cells regulates immunity versus tolerance in the intestine. Science. 2010;329:849–853. doi: 10.1126/science.1188510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Demeure CE, et al. CD47 engagement inhibits cytokine production and maturation of human dendritic cells. J Immunol. 2000;164:2193–2199. doi: 10.4049/jimmunol.164.4.2193. [DOI] [PubMed] [Google Scholar]
- 51.Ocaña-Morgner C, et al. Role of TGF-beta and PGE2 in T cell responses during Plasmodium yoelii infection. Eur J Immunol. 2007;37:1562–1574. doi: 10.1002/eji.200737068. [DOI] [PubMed] [Google Scholar]
- 52.Jakobsen MA, Petersen RK, Kristiansen K, Lange M, Lillevang ST. Peroxisome proliferator-activated receptor alpha, delta, gamma1 and gamma2 expressions are present in human monocyte-derived dendritic cells and modulate dendritic cell maturation by addition of subtype-specific ligands. Scand J Immunol. 2006;63:330–337. doi: 10.1111/j.1365-3083.2006.01745.x. [DOI] [PubMed] [Google Scholar]
- 53.Skorokhod OA, Alessio M, Mordmüller B, Arese P, Schwarzer E. Hemozoin (malarial pigment) inhibits differentiation and maturation of human monocyte-derived dendritic cells: A peroxisome proliferator-activated receptor-gamma-mediated effect. J Immunol. 2004;173:4066–4074. doi: 10.4049/jimmunol.173.6.4066. [DOI] [PubMed] [Google Scholar]
- 54.Laganà AS, et al. Pleiotropic actions of peroxisome proliferator-activated receptors (PPARs) in dysregulated metabolic homeostasis, inflammation and cancer: Current evidence and future perspectives. Int J Mol Sci. 2016;17:E999. doi: 10.3390/ijms17070999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vander Lugt B, et al. Transcriptional determinants of tolerogenic and immunogenic states during dendritic cell maturation. J Cell Biol. 2017;216:779–792. doi: 10.1083/jcb.201512012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Parlato S, et al. Expression of CCR-7, MIP-3beta, and Th-1 chemokines in type I IFN-induced monocyte-derived dendritic cells: Importance for the rapid acquisition of potent migratory and functional activities. Blood. 2001;98:3022–3029. doi: 10.1182/blood.v98.10.3022. [DOI] [PubMed] [Google Scholar]
- 57.Fessler MB. Regulation of adaptive immunity in health and disease by cholesterol metabolism. Curr Allergy Asthma Rep. 2015;15:48. doi: 10.1007/s11882-015-0548-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhu J, Krishnegowda G, Li G, Gowda DC. Proinflammatory responses by glycosylphosphatidylinositols (GPIs) of Plasmodium falciparum are mainly mediated through the recognition of TLR2/TLR1. Exp Parasitol. 2011;128:205–211. doi: 10.1016/j.exppara.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Krishnegowda G, et al. Induction of proinflammatory responses in macrophages by the glycosylphosphatidylinositols of Plasmodium falciparum: Cell signaling receptors, glycosylphosphatidylinositol (GPI) structural requirement, and regulation of GPI activity. J Biol Chem. 2005;280:8606–8616. doi: 10.1074/jbc.M413541200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yamagishi J, et al. Interactive transcriptome analysis of malaria patients and infecting Plasmodium falciparum. Genome Res. 2014;24:1433–1444. doi: 10.1101/gr.158980.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Greene JA, et al. Toll-like receptor polymorphisms and cerebral malaria: TLR2 Δ22 polymorphism is associated with protection from cerebral malaria in a case control study. Malar J. 2012;11:47. doi: 10.1186/1475-2875-11-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jagannathan P, et al. IFNγ/IL-10 co-producing cells dominate the CD4 response to malaria in highly exposed children. PLoS Pathog. 2014;10:e1003864. doi: 10.1371/journal.ppat.1003864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Montes de Oca M, et al. Type I interferons regulate immune responses in humans with blood-stage Plasmodium falciparum infection. Cell Rep. 2016;17:399–412. doi: 10.1016/j.celrep.2016.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fell AH, Silins SL, Baumgarth N, Good MF. Plasmodium falciparum-specific T cell clones from non-exposed and exposed donors are highly diverse in TCR beta chain V segment usage. Int Immunol. 1996;8:1877–1887. doi: 10.1093/intimm/8.12.1877. [DOI] [PubMed] [Google Scholar]
- 65.Geiger R, Duhen T, Lanzavecchia A, Sallusto F. Human naive and memory CD4+ T cell repertoires specific for naturally processed antigens analyzed using libraries of amplified T cells. J Exp Med. 2009;206:1525–1534. doi: 10.1084/jem.20090504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zevering Y, et al. High frequency of malaria-specific T cells in non-exposed humans. Eur J Immunol. 1992;22:689–696. doi: 10.1002/eji.1830220311. [DOI] [PubMed] [Google Scholar]
- 67.Kwiatkowski DP. How malaria has affected the human genome and what human genetics can teach us about malaria. Am J Hum Genet. 2005;77:171–192. doi: 10.1086/432519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mestas J, Hughes CC. Of mice and not men: Differences between mouse and human immunology. J Immunol. 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 69.Voisine C, Mastelic B, Sponaas AM, Langhorne J. Classical CD11c+ dendritic cells, not plasmacytoid dendritic cells, induce T cell responses to Plasmodium chabaudi malaria. Int J Parasitol. 2010;40:711–719. doi: 10.1016/j.ijpara.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 70.Piccioli D, et al. Human plasmacytoid dendritic cells are unresponsive to bacterial stimulation and require a novel type of cooperation with myeloid dendritic cells for maturation. Blood. 2009;113:4232–4239. doi: 10.1182/blood-2008-10-186890. [DOI] [PubMed] [Google Scholar]
- 71.van Beek JJ, et al. Human blood myeloid and plasmacytoid dendritic cells cross activate each other and synergize in inducing NK cell cytotoxicity. OncoImmunology. 2016;5:e1227902. doi: 10.1080/2162402X.2016.1227902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fonteneau JF, et al. Human immunodeficiency virus type 1 activates plasmacytoid dendritic cells and concomitantly induces the bystander maturation of myeloid dendritic cells. J Virol. 2004;78:5223–5232. doi: 10.1128/JVI.78.10.5223-5232.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mooney JP, Wassmer SC, Hafalla JC. Type I interferon in malaria: A balancing act. Trends Parasitol. 2017;33:257–260. doi: 10.1016/j.pt.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 74.Kadowaki N, et al. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J Exp Med. 2001;194:863–869. doi: 10.1084/jem.194.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ioannidis LJ, et al. Monocyte- and neutrophil-derived CXCL10 impairs efficient control of blood-stage malaria infection and promotes severe disease. J Immunol. 2016;196:1227–1238. doi: 10.4049/jimmunol.1501562. [DOI] [PubMed] [Google Scholar]
- 76.Nie CQ, et al. IP-10-mediated T cell homing promotes cerebral inflammation over splenic immunity to malaria infection. PLoS Pathog. 2009;5:e1000369. doi: 10.1371/journal.ppat.1000369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wilson NO, et al. CXCL4 and CXCL10 predict risk of fatal cerebral malaria. Dis Markers. 2011;30:39–49. doi: 10.3233/DMA-2011-0763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lönnberg T, et al. Single-cell RNA-seq and computational analysis using temporal mixture modelling resolves Th1/Tfh fate bifurcation in malaria. Sci Immunol. 2017;2:eaal2192. doi: 10.1126/sciimmunol.aal2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Adukpo S, et al. Triggering receptor expressed on myeloid cells 1 (TREM-1) and cytokine gene variants in complicated and uncomplicated malaria. Trop Med Int Health. 2016;21:1592–1601. doi: 10.1111/tmi.12787. [DOI] [PubMed] [Google Scholar]
- 80.Bowen JR, et al. Zika virus antagonizes type I interferon responses during infection of human dendritic cells. PLoS Pathog. 2017;13:e1006164. doi: 10.1371/journal.ppat.1006164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wykes MN, Stephens R, Cockburn IA. Adaptive immunity to Plasmodium blood stages. In: Mota MM, Rodriguez A, editors. Malaria: Immune Response to Infection and Vaccination. Springer; New York: 2017. pp. 47–66. [Google Scholar]
- 82.Moser JM, et al. Optimization of a dendritic cell-based assay for the in vitro priming of naïve human CD4+ T cells. J Immunol Methods. 2010;353:8–19. doi: 10.1016/j.jim.2009.11.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.