Sir,
We have previously reported the use of rituximab as adjuvant therapy in 24 cases of pemphigus in 2014.[1] We would like to share our experience with continued use of the molecule in ninety additional patients in the following years and compare the two regimens used.
We retrospectively analyzed the data of 114 patients of pemphigus from October 2010 to December 2016. The diagnosis was made on the basis of clinical presentation and histopathological examination while the antidesmoglein antibody levels and direct immunofluorescence was done when needed. Rituximab was administered to refractory cases of pemphigus, patients with contraindications to steroid or other immunosuppressive agent (ISA) therapy and those unwilling for steroid therapy. With time and experience with the molecule, we offered the benefit of rituximab to all patients of moderate-to-severe pemphigus. The variables recorded were the duration of disease, previous therapy, remission rate on- and off-therapy, relapse rate, duration of follow-up and serious adverse effect if any.
The first 48 (42.11%) patients received rituximab according to a modification of the lymphoma regimen: 3 doses of 500 mg each 1 week apart followed by another dose of 500 mg 3 months later. Subsequently, due to good tolerance and for operational convenience, 66 (57.89%) patients were treated with the rheumatoid arthritis (RA) regimen of two infusions of 1 g each 15 days apart.
The study population included 114 patients, of which 99 (86.84%) were pemphigus vulgaris (PV) and 15 (13.16%) were pemphigus foliaceus, with 53 (46.49%) males and 61 (53.51%) females. The duration of disease prior to rituximab therapy ranged from 3 months to 4 years (mean: 28.8 months). All the patients had received some form of immunosuppressive treatment prior to rituximab, with 66 (57.89%) patients received dexamethasone cyclophosphamide pulse along with oral steroids and ISA, 43 (37.72%) received only oral steroids and ISA, 2 (1.75%) patients received only ISA, and 3 (2.63%) patients received only oral steroids. The ISA used was cyclophosphamide in 45 (39.47%) patients, azathioprine in 27 (23.68%) patients, mycophenolate mofetil in 12 patients (10.53%), methotrexate in 6 (5.26%) patients and a combination of ISA in 24 (21.05%) patients. Tapering of the steroid dose and stoppage of pulse therapy were attempted over 3 months following rituximab infusion. The patients were followed up for a mean duration of 29.3 months.
Response was classified as complete remission when the patient stopped getting new blisters and older lesions had healed completely with successful taper of steroid dose and pulse therapy. Patients who failed to show complete remission but were responding to treatment with <5 new lesions were considered to be in partial remission. Relapse was defined as more than 5 lesions requiring high-dose steroid and ISA (>20 mg prednisolone daily) or pulse therapy. The results are summarized in Figure 1. The patients were monitored for infusion-related reactions and followed up for any infectious complications [Figure 2].
Figure 1.
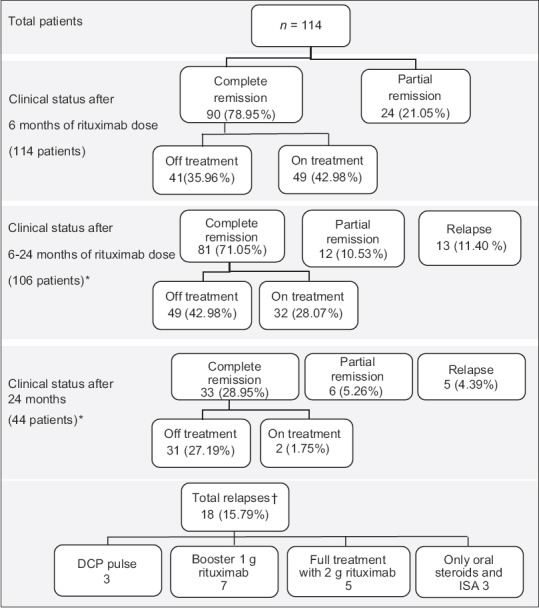
(Original): Patient response to rituximab. *Remaining patients are still under follow-up. †The mean duration of relapse requiring retreatment was 20.38 months
Figure 2.
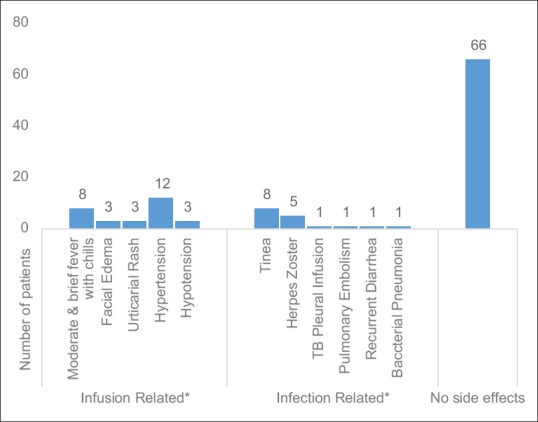
(Original): Side effects of rituximab. *The infusion-related side effects were limited to the first infusion and easily controlled with antihistamines and adjusting the infusion rate while the infections responded well to appropriate therapy
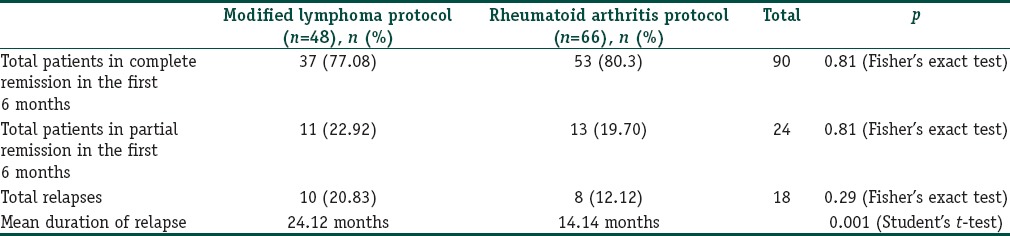
The lymphoma protocol, RA protocol, and various modifications of these protocols have been used in the past with good results.[2,3,4] There is currently no consensus on the optimal dosage and schedule of rituximab in the treatment of pemphigus. The advantages of the RA protocol over the lymphoma protocol are operational convenience, less cost and fewer infusions. Zakka et al. reported that the lymphoma protocol produced lower response rates, relapse rates and serious infections but higher mortality and there were nonresponders whereas the RA protocol had higher response rates, relapse rates, serious infections but lower mortality and there were no nonresponders.[5] From Table 1, it is clear that no remarkable difference was observed in the patients’ responses from the two different protocols used. The relapse rate was higher in the modified lymphoma protocol but was found to be statistically insignificant (P = 0.29). This could be attributed to the longer duration of follow-up. A significant difference was found in the relapse duration with earlier relapses in the RA protocol compared to the modified lymphoma protocol (P = 0.001). Of particular interest is that there were no nonresponders to rituximab in either protocol.
Table 1.
Comparison of patient responses in the two treatment protocols
In conclusion, rituximab is a newer modality of treatment with the ability to induce prolonged remissions in patients of pemphigus. It has the potential of being used as an adjuvant therapy routinely in cases of pemphigus to bring down the dose and duration of steroids and other ISAs.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Londhe PJ, Kalyanpad Y, Khopkar US. Intermediate doses of rituximab used as adjuvant therapy in refractory pemphigus. Indian J Dermatol Venereol Leprol. 2014;80:300–5. doi: 10.4103/0378-6323.136832. [DOI] [PubMed] [Google Scholar]
- 2.Joly P, Mouquet H, Roujeau JC, D’Incan M, Gilbert D, Jacquot S, et al. A single cycle of rituximab for the treatment of severe pemphigus. N Engl J Med. 2007;357:545–52. doi: 10.1056/NEJMoa067752. [DOI] [PubMed] [Google Scholar]
- 3.Heelan K, Al-Mohammedi F, Smith MJ, Knowles S, Lansang P, Walsh S, et al. Durable remission of pemphigus with a fixed-dose rituximab protocol. JAMA Dermatol. 2014;150:703–8. doi: 10.1001/jamadermatol.2013.6739. [DOI] [PubMed] [Google Scholar]
- 4.Cianchini G, Corona R, Frezzolini A, Ruffelli M, Didona B, Puddu P. Treatment of severe pemphigus with rituximab: Report of 12 cases and a review of the literature. Arch Dermatol. 2007;143:1033–8. doi: 10.1001/archderm.143.8.1033. [DOI] [PubMed] [Google Scholar]
- 5.Zakka LR, Shetty SS, Ahmed AR. Rituximab in the treatment of pemphigus vulgaris. Dermatol Ther. 2012;2:17. doi: 10.1007/s13555-012-0017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


