Abstract
Background
Diabetic retinopathy (DR) is one of the most common and serious complications of diabetes mellitus (DM). The autophagy-lysosome pathway (ALP) is one of the main intracellular self-digestive degradation systems. Lysosomal impairment and autophagic dysfunction are early events in the pathogenesis of DR, suggesting autophagy might be a novel therapeutic strategy for DR treatment.
Material/Methods
In our study, we screened a differentially expressed miRNA, miR-1273g-3p, in streptozotocin (STZ)-injected DR rat retinal pigment epithelial (RPE) cells. miR-1273g-3p inhibitor and mimic were employed to treat RPE cells to assess the role of miR-1273g-3p. QRT-PCR and Western blot analysis were performed to examine the function of miR-1273g-3p on ALP-related and DR-related proteins.
Results
miR-1273g-3p was highly expressed in STZ-induced DM RPE cells. miR-1273g-3p mimic promoted the expression of DR-related MMP-2, MMP-9, and TNF-α proteins, and ALP-related LC3, cathepsin B, and cathepsin L factors, but miR-1273g-3p inhibitor suppressed the levels of these factors.
Conclusions
miR-1273g-3p is involved in the progression of DR by modulating the autophagy-lysosome pathway. These findings provided new evidence of the close relationship between DR and ALP, and reveal a new target for DR therapy.
MeSH Keywords: Diabetic Retinopathy, MicroRNAs, Retinal Pigment Epithelium
Background
The improved economic development and changes in lifestyle and diet have resulted in increased morbidity of diabetes mellitus (DM) worldwide [1]. DM can induce many severe complications, such as renal failure and diabetic retinopathy (DR). Recently, it was discovered that DR is a progressive vascular disease that is the main cause of blindness in Western developed countries, which is defined as neurodegenerative and microvascular disease [2]. Many biochemical and molecular mechanisms have been proposed to explain the pathology of DR, but the mechanism by which DR occurs and develops is quite complicated and is not fully understood [3]. Therefore, research on the timely prevention and treatment of DR has become very important.
The autophagy-lysosome pathway (ALP) is one of the main intracellular self-digestive degradation systems [4] for clearance of misfolded or damaged proteins. Damaged cell components are engulfed in autophagosomes and delivered to the lysosome for elimination [5]. The accumulation of damaged or misfolded proteins in retinal pigment epithelial (RPE) cells is considered a contributing factor for RPE dysfunction. RPE is an important tissue that maintains the metabolism of the neurosensory retina and ensures its sensitization [6]. However, the role of autophagy in DR is quite complicated. Autophagy promotes pericyte survival in early DR, whereas excessive autophagy causes excess stress and leads to necrosis [7]. Lysosomal impairment and autophagic dysfunction are early events in the pathogenesis of DR [8], suggesting autophagy might be a novel therapeutic strategy for DR treatment.
MicroRNAs (miRNAs) are small noncoding RNAs that regulate gene expression by post-transcriptional inhibition of mRNA translation [9]. Dysregulation of miRNAs has been reported to play an important role in the development of various disease [10]. Several miRNAs have been shown to be involved in diabetic diseases. The overexpression of miR-503 and miR-181d are responsible for the pathogenesis of diabetic nephropathy [11]. miR-21 [12] and miR-200b [13] have been identified to be involved in early DR (14). In our study, we constructed a streptozotocin (STZ)-induced DM rat model and screened a differentially expressed miRNA, miR-1273g-3p, for further investigation. We designed and synthesized miR-1273g-3p mimic and inhibitor, and detected the effect of 2 drugs on the expression of miR-1273g-3p. We examined the levels of DR-related factors (MMP-2, MMP-9, and TNF-α) and ALP-related factors (LC3, cathepsin B, and cathepsin L) after the treatment of miR-1273g-3p mimic or inhibitor to reveal the mechanism by which miR-1273g-3p is involved in the progression of DR. These finding provide a new target for DR therapy.
Material and Methods
Reagents
We used the following reagents: miR-1273g-3p mimic and miR-1273g-3p inhibitor (Sangon, Shanghai, China); Anti-mouse antibodies against microtubule-associated protein 1 light chain 3 (LC3), Cathepsin B, Cathepsin L, Matrix metalloproteinases 2 (MMP-2), Matrix metalloproteinases 9 (MMP-9) and tumor necrosis factor α (TNF-α) (Abcam, Cambridge, MA); Dulbecco’s modified Eagle’s medium (DMEM); and fetal bovine serum (FBS) (Gibco, USA).
Animals and grouping
Male Sprague-Dawley (SD) rats weighing 200±20 g were purchased from Hunan SJA Laboratory Animal Co., LTD. The research protocol for all animals strictly conformed to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Rats were housed at a temperature of 24±1°C and relative humidity of 40–50% in a clean environment under 12: 12 h light and dark cycle. The animals had free access to food and purified water. All procedures were approved by the Animal Ethics Committee of the PLA General Hospital. The rats were randomly assigned to a diabetic model group (DM, n=10) or a normal control group (NC, n=10). The rats in the DM group were fed with a high-fat diet (1% cholesterol, 10% lard, 30% egg, and 59% sucrose, 2 mL/rat) for an initial period of 2 months [15]. Thereafter, rats received a 20 mg/kg intraperitoneal injection of streptozotocin (STZ) in freshly prepared sodium citrate buffer (0.1 mM, pH 4.5). Rats in the NC group were administered with the same volume of sodium citrate buffer. Rats with blood glucose levels after 72 h of STZ administration higher than 16.67 mM were diagnosed as diabetic and used in our experiments.
Primary culture of RPE cells and grouping
Rat retinas were stripped from the eye under sterile conditions. After being washed with Dulbecco’s Hanks balanced salt solution, the tissues were shredded and digested with 2% pancreatin (Sangon, Shanghai, China) at room temperature. After centrifugation, the tissues were digested with type I collagen (Sangon, Shanghai, China) for 30 min at 37°C. After being washed, the cells were suspended with DMEM supplemented with 10% FBS and seeded into flasks. About 1/3 medium was changed every other day to remove the disintegrated retinal fragments and dead cells. When cells were fused into a monolayer cell, they were digested and passaged after 0.25% pancreatin digestion. RPE cells from DM group were pretreated with miR-1273g-3p inhibitor (DM + inhibitor group) or miR-1273g-3p mimic (DM + mimic group), respectively. The control group from DM was incubated with the same amount of vehicle and marked with DM group. RPE cells isolated from NC group was named the NC group and treated with the same amount of vehicle.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was isolated from different groups and subjected to 1% native agarose gel for integrity and quantity detection. One μg of RNA was transcribed into cDNA with PrimeScript RT Reagent Kit (TaKaRa, Dalian, China) following the manufacturer’s instructions. The cDNA was used as the template using SYBR Green qRT-PCR SuperMix (Invitrogen, USA) and Real-Time System (Bio-Rad, USA) with the corresponding primers listed in Table 1. The relative expression of genes (MMP-2, MMP-9, TNF-α, LC3, cathespin B, and cathespin L) was calculated by normalizing to β-actin. U6 was used as an endogenous control to analyze the expression of miR-1273p-3g. All data were analyzed based on 3 independent biological replicates and at least 3 technical replicates.
Table 1.
Primers for qRT-PCR.
| Genes | Forward primers (5′-3′) | Reverse primers (5′-3′) |
|---|---|---|
| miR-1273-3g | tgcgaccactgcactccagcct | gcgagcacagaattaatacgac |
| CathepsinB | tggctgtaatggtggctat | tgttgcacttgggagtatct |
| CathepsinL | tcctggctgtcctctgct | tgcttcccgttgctgtac |
| MMP-2 | tggcaaggagtacaacagc | tggaagcggaatggaaac |
| MMP-9 | catcttccaaggccaatcc | ccatcaccgtcgagtcagc |
| TNF-α | ctgctcctctttcctcgtc | ccctctgggatctccttct |
| LC3 | ttgctgactgaccctcca | gctgcttctcacccttgtag |
| U6 | ctcgcttcggcagcaca | aacgcttcacgaatttgcgt |
| β-actin | ctccatcctggcctcgctgt | gctgtcaccttcaccgttcc |
Western blot
Cells were collected and lysed with RIPA buffer (Sigma, USA) for 30 min and then centrifuged at 10 000 rpm at 4°C for 10 min. Supernatant was collected and protein concentration was detected using the BCA kit (Beyotime Institute of Biotechnology, Shanghai, China) according to the manufacturer’s protocol. Equivalent protein was subjected to SDS-PAGE and transferred to a nitrocellulose membrane. The membrane was incubated with the corresponding primary antibodies at 4°C overnight, then probed with horseradish peroxidase-labelled secondary antibodies (1: 1000 dilution) for 1–2 h at room temperature. ECL reagents were employed for the target bands detection. The bands intensity was calculated with Quantity One software (Bio-Rad, USA) by normalization to that of β-actin.
Statistical analysis
All statistical analysis was performed using SPSS 18.0 software (SPSS, Chicago, IL). Experiment data were expressed as mean ± standard deviation (SD). The statistical analysis was carried out using one-way ANOVA. A P value of 0.05 or less was considered as a significant difference, where * P<0.05, ** P<0.01.
Results
miR-1273g-3p was highly expressed in DR RPE cells
We incubated DR RPE cells with clusterin and screened some differential expressed genes shown in Figure 1, including miR-1273g-3p and complement component 5a receptor 2 (C5AR2). To evaluate the role of miR-1273g-3p and C5AR2 in DR, we constructed the DM model by intraperitoneal injection of STZ into rats fed with high fat and isolated the STZ-induced DR RPE cells for further analysis. After 3 days of STZ injection, the glucose level was detected. The level of glucose in normal rat (4.3±1.21 mM) was significantly lower than that in STZ-injected rats (25.1±4.58 mM, P < 0.01). These data revealed that the DM rat model was constructed successfully. We examined the expression profile of miR-1273g-3p in STZ-induced DR RPE cells. miR-1273g-3p was expressed in both the NC group and DM group, but the level of miR-1273g-3p in the DM group was dramatically increased than that in the NC group (P<0.01). Furthermore, the supplement of miR-1273g-3p inhibitor suppressed the upregulation of miR-1273g-3p in DR RPE cells, but miR-1273g-3p mimic enhanced the elevation. We further investigated the expression of C5AR2 in different groups. Compared with NC group, the expression of C5AR2 in the DM group was significantly suppressed (P<0.01). However, miR-1273g-3p inhibitor enhanced the level of C5AR2, while miR-1273g-3p mimic suppressed the expression of miR-1273g-3p. These data showed that miR-1273g-3p and C5AR2 might be associated with the progression of DR, but there was a significant negative correlation between miR-1273g-3p and C5AR2 expression. These results might reveal a new target for DR treatment.
Figure 1.
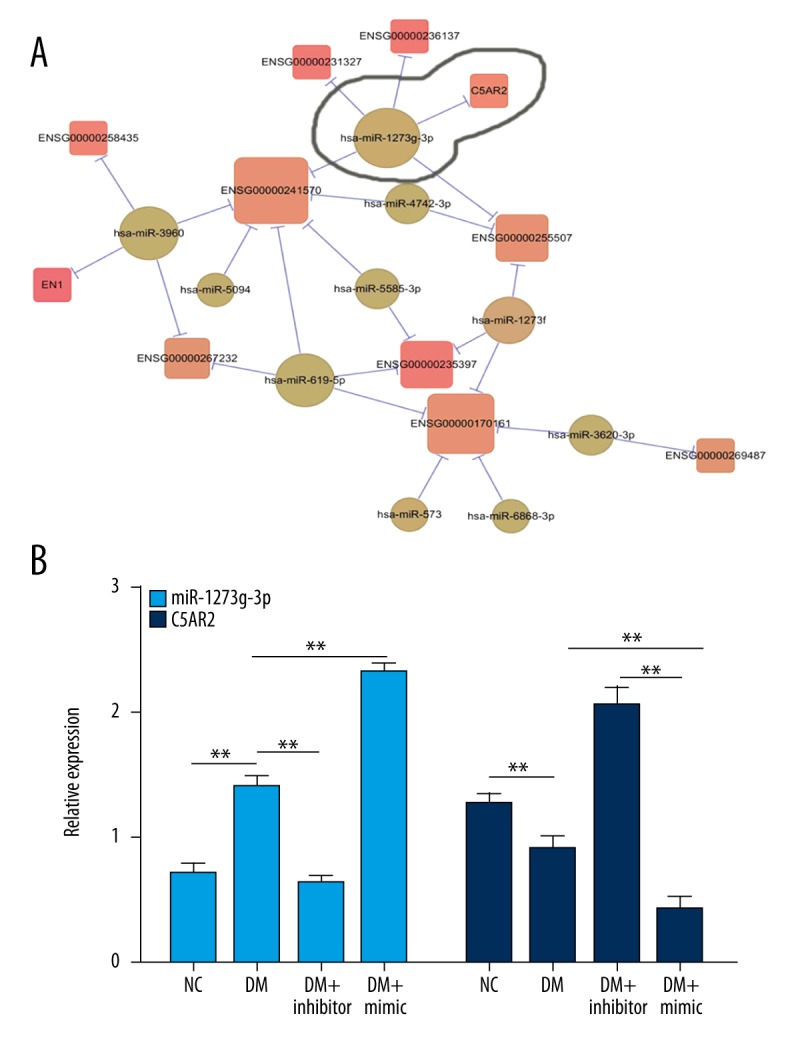
miR-1273g-3p was highly expressed in STZ-induced DR RPE cells. (A) Differential expression analysis was performed using Cuffdiff. RPE cells were induced by clusterin and an mRNA library was constructed. Protein-coding transcripts and lncRNAs with fold change (FC) >1.5 or FC <0.67 and P value <0.05 were considered to be differentially expressed in RPM cells treated by clusterin; miRNAs with FC >2 or FC <0.5 were considered aberrantly expressed. (B) miR-1273g-3p was highly expressed in STZ-induced DR RPE cells. Total RNA was isolated from NC, DM, DM + inhibitor, and DM + mimic groups for qRT-PCR. NC, RPE cells from normal SD rats; DM, RPE cells from STZ-induced DM rats; DM + inhibitor, RPE cells from STZ-induced DM rats pretreated with miR-1273g-3p inhibitor; DM + mimic, RPE cells from STZ-induced DM rats pretreated with miR-1273g-3p mimic. U6 was used as the internal control to miR-1273g-3p. The mRNA level of C5AR2 was normalized to β-actin. * P<0.05, ** P<0.01.
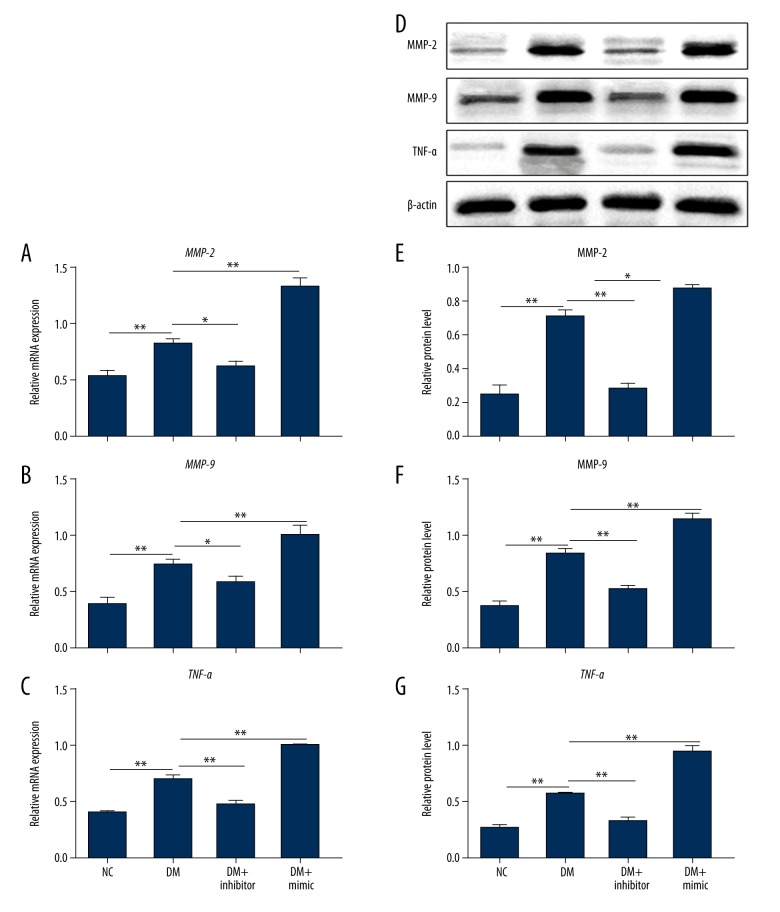
MiR-1273g-3p mimic enhanced the expression of MMP-2, MMP-9, and TNF-α
To further examine the mechanism underlying the role of miR-1273g-3p in the development of DR, we detected the effect of miR-1273g-3p mimic or inhibitor on the levels of DR-related factors in Figure 2. The mRNA and protein levels of MMP-2, MMP-9, and TNF-α were increased obviously in the DM group compared to the NC group, suggesting the above molecules play roles in DR. Furthermore, the application of miR-1273g-3p inhibitor inhibited the upregulation of MMP-2, MMP-9, and TNF-α on mRNA and protein levels, while the administration of miR-1273g-3p mimic enhanced the elevation of MMP-2, MMP-9, and TNF-α (P<0.01). These findings reveal that miR-1273g-3p is involved in the progression of DR by regulating the expression of DR-related factors.
Figure 2.
miR-1273g-3p modulated the expression of MMP-2, MMP-9, and TNF-α on mRNA and protein levels. Total RNA was isolated from NC, DM, DM + inhibitor, and DM + mimic groups for qRT-PCR. The mRNA levels of MMP-2 (A), MMP-9 (B), and TNF-α (C) were detected normalized to β-actin. Proteins were extracted from the above 4 groups for Western blot analysis (D). The protein levels of MMP-2 (E), MMP-9 (F), and TNF-α (G) were detected normalized to β-actin. NC, RPE cells from normal SD rats; DM, RPE cells from STZ-induced DM rats; DM + inhibitor, RPE cells from STZ-induced DM rats pretreated with miR-1273g-3p inhibitor; DM + mimic, RPE cells from STZ-induced DM rats pretreated with miR-1273g-3p mimic. * P<0.05, ** P<0.01.
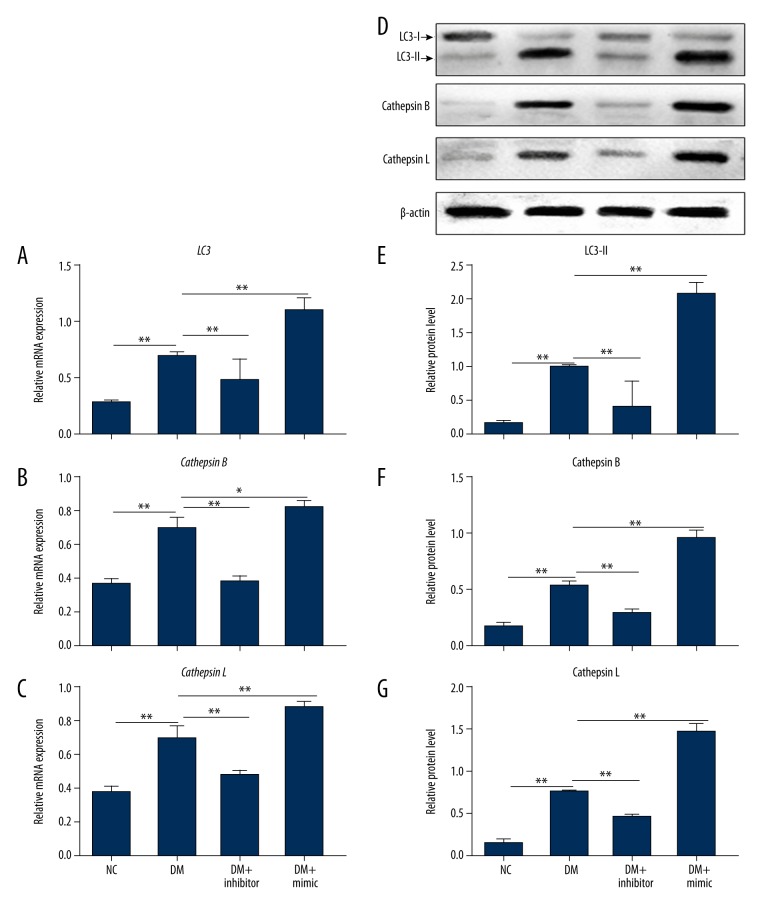
miR-1273g-3p mimic promoted the expression of LC3, cathepsin B, and cathepsin L
Abnormal ALP was closely associated with the progression of DR [16]. To further explore the mechanism by which miR-1273g-3p is involved in DR, we examined the effect of miR-1273g-3p on the expression of ALP-related factors. The qRT-PCR results revealed that LC3, cathepsin B, and cathepsin L were markedly overexpressed in the DM group compared with the NC group (Figure 3) (P<0.01). The supplement of miR-1273g-3p inhibitor largely suppressed the levels of LC3 II, cathepsin B, and cathepsin L, but the addition of miR-1273g-3p mimic enhanced the expression of these 3 genes. A similar trend was observed in the protein levels of LC3, cathepsin B, and cathepsin L by Western blot analysis. These results show that miR-1273g-3p regulates ALP-related factors expression, and provides a new link between DR and ALP.
Figure 3.
miR-1273g-3p modulated the expression of LC, cathepsin B, and cathepsin L on mRNA and protein levels. Total RNA was isolated from NC, DM, DM + inhibitor, and DM + mimic groups for qRT-PCR. The mRNA levels of LC3 (A), cathepsin B (B) and cathepsin L (C) were detected normalized to β-actin. Proteins were extracted from the above 4 groups for Western blot analysis (D). The protein levels of LC3 II (E), cathepsin B (F), and cathepsin L (G) were detected normalized to β-actin. NC, RPE cells from normal SD rats; DM, RPE cells from STZ-induced DM SD rats; DM + inhibitor, RPE cells from STZ-induced DM rats pretreated with miR-1273g-3p inhibitor; DM + mimic, RPE cells from STZ-induced DM rats pretreated with miR-1273g-3p mimic. * P<0.05, ** P<0.01.
Discussion
DR still remains the major cause of blindness among young adults. Exploring the pathogenesis of DR and investigating the key target of DR are critical for the treatment of DR. STZ-induced diabetic rats provide a good model for the study of DR. In our study, we used RPE cells to explore the role of miR-1273g-3p during the development of DR. The expression of miR-1273g-3p was upregulated in STZ-induced diabetic rats. miR-1273g-3p mimic promoted the levels of DR- and ALP-related factors, but miR-1273g-3p inhibitor suppressed the expression of DR- and ALP-related factors. These results show that miR-1273g-3p participates in the progression of DR via the autophagy-lysosome pathway.
miR-1273g-3p is involved in the progression of DR
Several miRNAs members of the miR-1273 family with different features have been identified, such as miR-1273a, miR-1273c, miR-1273d, miR-1273e, miR-1273f, miR-1273g-5p, miR-1273h-3p, and miR-1273h-5p [17]. In our study, a new member, miR-1273g-3p, was screened from the RPE cells of STZ-induced DM rats. So far, little has been reported on the function of miR-1273g-3p. In human umbilical vein endothelial cells, miR-1273g-3p is involved in acute glucose fluctuation-induced autophagy, dysfunction, and proliferation attenuation [18]. In hepatic stellate cells, miR-1273g-3p monitored cell activation and apoptosis by targeting PTEN [19]. In our study, we found that miR-1273g-3p was highly expressed in RPE cells of DR rats. miR-1273g-3p mimic promoted the expression of miR-1273g-3p, and miR-1273g-3p inhibitor had the opposite effect. These results show that miR-1273g-3p inhibitor or mimic was successfully synthesized for further experiments, and revealed some functions of miR-1273g-3p in DR. The complement cascade is an ancient part of our innate immune system that can be activated following tissue damage [20]. C5a has been associated with a number of autoimmune, inflammatory, and neurodegenerative disorders [21]. C5AR2, a receptor of C5a, has both pro- and anti-inflammatory activities [22]. In our study, we found that C5AR2 was expressed at low levels in the DM group compared with the NC group, and miR-1273g-3p inhibitor or mimic affected the mRNA level of C5AR2, suggesting that C5AR2 is associated with the development of DR and the negative association between C5AR2 and miR-1273g-3p. These results also show some link between the progression of DR and inflammation and provide good targets for the study of this link. However, further study is needed to reveal the mechanism. MMPs, a family of Zn2+-dependent endoproteases, have been shown to act as fine regulators of both health and disease [23]. Studies have documented the important proinflammatory roles of MMPs, including MMP-2 and MMP-9, in the retinas of diabetic animals [24]. MMP-2 and MMP-9 are increased in diabetic animals, and drugs targeting MMP-2 and MMP-9 can effectively inhibit DR in diabetes [25]. In this study, we found that the mRNA and protein levels of MMP-2 and MMP-9 were elevated in STZ-induced DR RPE cells. These results are in agreement with previous reports. Furthermore, miR-1273g-3p inhibitor suppressed the upregulation of MMP-2 and MMP-9, but mimic promoted the elevation. These data indicate that miR-1273g-3p is involved in the progression of DR by the regulation of MMP-2 and MMP-9. A variety of studies have found critical roles of some signals in the development of DR, including proinflammatory cytokines TNF-α [26]. Anti-inflammatory drugs prevent the development of DR via the suppression of TNF-α, an independent serum marker for DR [27]. In our study, the mRNA and protein levels of TNF-α were enhanced in DR RPE cells, which was similar to previous results. miR-1273g-3p mimic or inhibitor had opposite effects on the expression of TNF-α. These data provide new evidence of a link between DR and inflammation, and show that miR-1273g-3p participates in the development of DR by regulating the expression of MMP-2, MMP-9, and TNF-α.
miR-1273g-3p regulates the expression of ALP-related LC3, cathepsin B, and cathepsin L
The activation of autophagy is directly linked to the development of eye diseases such as DR, ocular tumor, and cataracts [28]. In diabetic rats, the level of LC3, an autophagy marker, was increased [29], which is in accordance with our findings. Autophagy is an intracellular pathway for bulk protein degradation and the removal of damaged organelles by lysosomes [30]. Lysosomal cathepsins regulate an exquisite range of biological functions, and their disorder is associated with inflammatory, metabolic, and degenerative diseases in humans [31]. The activities of cathepsins B and L, 2 autophagosome-associated lysosomal proteases, contribute to the progression of DR [32]. In our study, we observed that the mRNA and protein levels of cathepsins B and L in DR RPE cells were higher than those in normal RPE cells, which was in agreement with the above report. Furthermore, miR-1273g-3p inhibitor or mimic affected the expression of LC3 II and cathepsins B and L in DR RPE cells. These data reveal that miR-1273g-3p modulates the expression of ALP-related factors. These findings show that miR-1273g-3p might be a link between DR and ALP.
Conclusions
We found that miR-1273g-3p was overexpressed and C5AR2 was suppressed in STZ-induced DR RPE cells, suggesting that miR-1273g-3p and C5AR2 are related to the progression of DR, and there is a negative association between miR-1273g-3p and C5AR2. miR-1273g-3p inhibitor or mimic showed an opposite effect on the levels of DR-related MMP-2, MMP-9, TNF-α, ALP-related LC3 II, and cathepsins B and L. ALP contributes to the development of DR; thus, miR-1273g-3p is involved in STZ-induced DR by modulating ALP. These results provide a new link between DR and ALP, and reveal a new target for DR therapy.
Abbreviation
- DR
diabetic retinopathy
- ALP
autophagy-lysosome pathway
- STZ
streptozotocin
- DM
diabetes mellitus
- miRNAs
microRNAs
- C5AR2
complement component 5a receptor 2
- MMP-2
matrix metalloproteinases 2
- MMP-9
matrix metalloproteinases 9
- TNF-α
tumor necrosis factor α
- LC3
microtubule-associated protein 1 light chain 3
- RPE
retinal pigment epithelial
Footnotes
Source of support: This research was supported by China Social Assistance Fund (No. BJ-LM2015001J)
Compliance with ethical standards
This study has received ethics approval from the Institutional Committee.
Disclosure/conflict of interest
The authors declare no conflict of interest.
References
- 1.van Dooren FE, Nefs G, Schram MT, et al. Depression and risk of mortality in people with diabetes mellitus: A systematic review and meta-analysis. PLoS One. 2013;8:e57058. doi: 10.1371/journal.pone.0057058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ting DS, Cheung GC, Wong TY. Diabetic retinopathy: Global prevalence, major risk factors, screening practices and public health challenges: A review. Clin Exp Ophthalmol. 2016;44:260–77. doi: 10.1111/ceo.12696. [DOI] [PubMed] [Google Scholar]
- 3.Yau JW, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556–64. doi: 10.2337/dc11-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ott C, Konig J, Hohn A, et al. Reduced autophagy leads to an impaired ferritin turnover in senescent fibroblasts. Free Radic Biol Med. 2016;101:325–33. doi: 10.1016/j.freeradbiomed.2016.10.492. [DOI] [PubMed] [Google Scholar]
- 5.Riffelmacher T, Simon AK. Mechanistic roles of autophagy in hematopoietic differentiation. FEBS J. 2017;284(7):1008–20. doi: 10.1111/febs.13962. [DOI] [PubMed] [Google Scholar]
- 6.Zhao C, Zhang Z, Chen L, et al. Effectiveness of intravitreal injection of ranibizumab for neovascular age-related macular degeneration with serous pigment epithelial detachment. Med Sci Monit. 2016;22:833–39. doi: 10.12659/MSM.895528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Du M, Wu M, Fu D, et al. Effects of modified LDL and HDL on retinal pigment epithelial cells: A role in diabetic retinopathy? Diabetologia. 2013;56:2318–28. doi: 10.1007/s00125-013-2986-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lopes de Faria JM, Duarte DA, Montemurro C, et al. Defective autophagy in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2016;57:4356–66. doi: 10.1167/iovs.16-19197. [DOI] [PubMed] [Google Scholar]
- 9.Cai Y, Yu X, Hu S, Yu J. A brief review on the mechanisms of miRNA regulation. Genomics Proteomics Bioinformatics. 2009;7:147–54. doi: 10.1016/S1672-0229(08)60044-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12:861–74. doi: 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
- 11.Zhu X, Zhang C, Fan Q, et al. Inhibiting microRNA-503 and microRNA-181d with losartan ameliorates diabetic nephropathy in KKAy mice. Med Sci Monit. 2016;22:3902–9. doi: 10.12659/MSM.900938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Usui-Ouchi A, Ouchi Y, Kiyokawa M, et al. Upregulation of mir-21 levels in the vitreous humor is associated with development of proliferative vitreoretinal disease. PLoS One. 2016;11:e0158043. doi: 10.1371/journal.pone.0158043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McArthur K, Feng B, Wu Y, et al. MicroRNA-200b regulates vascular endothelial growth factor-mediated alterations in diabetic retinopathy. Diabetes. 2011;60:1314–23. doi: 10.2337/db10-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kovacs B, Lumayag S, Cowan C, Xu S. MicroRNAs in early diabetic retinopathy in streptozotocin-induced diabetic rats. Invest Ophthalmol Vis Sci. 2011;52:4402–9. doi: 10.1167/iovs.10-6879. [DOI] [PubMed] [Google Scholar]
- 15.Dong H, Li Q, Wang M, Wan G. Association between IL-10 gene polymorphism and diabetic retinopathy. Med Sci Monit. 2015;21:3203–8. doi: 10.12659/MSM.894371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tagawa A, Yasuda M, Kume S, et al. Impaired podocyte autophagy exacerbates proteinuria in diabetic nephropathy. Diabetes. 2016;65:755–67. doi: 10.2337/db15-0473. [DOI] [PubMed] [Google Scholar]
- 17.Ivashchenko A, Berillo O, Pyrkova A, Niyazova R. Binding sites of miR-1273 family on the mRNA of target genes. Biomed Res Int. 2014;2014:620530. doi: 10.1155/2014/620530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo J, Sang Y, Yin T, et al. miR-1273g-3p participates in acute glucose fluctuation-induced autophagy, dysfunction, and proliferation attenuation in human umbilical vein endothelial cells. Am J Physiol Endocrinol Metab. 2016;310:E734–43. doi: 10.1152/ajpendo.00444.2015. [DOI] [PubMed] [Google Scholar]
- 19.Niu X, Fu N, Du J, et al. miR-1273g-3p modulates activation and apoptosis of hepatic stellate cells by directly targeting PTEN in HCV-related liver fibrosis. FEBS Lett. 2016;590:2709–24. doi: 10.1002/1873-3468.12309. [DOI] [PubMed] [Google Scholar]
- 20.Barbu A, Hamad OA, Lind L, et al. The role of complement factor C3 in lipid metabolism. Mol Immunol. 2015;67:101–7. doi: 10.1016/j.molimm.2015.02.027. [DOI] [PubMed] [Google Scholar]
- 21.Fattahi F, Ward PA. Complement and sepsis-induced heart dysfunction. Mol Immunol. 2017;84:57–64. doi: 10.1016/j.molimm.2016.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Croker DE, Monk PN, Halai R, et al. Discovery of functionally selective C5aR2 ligands: novel modulators of C5a signalling. Immunol Cell Biol. 2016;94:787–95. doi: 10.1038/icb.2016.43. [DOI] [PubMed] [Google Scholar]
- 23.Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15:786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhatt LK, Addepalli V. Attenuation of diabetic retinopathy by enhanced inhibition of MMP-2 and MMP-9 using aspirin and minocycline in streptozotocin-diabetic rats. Am J Transl Res. 2010;2:181–89. [PMC free article] [PubMed] [Google Scholar]
- 25.Yang R, Liu H, Williams I, Chaqour B. Matrix metalloproteinase-2 expression and apoptogenic activity in retinal pericytes: Implications in diabetic retinopathy. Ann NY Acad Sci. 2007;1103:196–201. doi: 10.1196/annals.1394.000. [DOI] [PubMed] [Google Scholar]
- 26.Gustavsson C, Agardh E, Bengtsson B, Agardh CD. TNF-alpha is an independent serum marker for proliferative retinopathy in type 1 diabetic patients. J Diabetes Complications. 2008;22:309–16. doi: 10.1016/j.jdiacomp.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 27.Chen DY, Su GF. Tumor necrosis factor-like weak inducer of apoptosis association with proliferative diabetic retinopathy and promotes proliferation and collagen synthesis in retinal ARPE-19 cells. Gen Mol Res. 2016;15(1) doi: 10.4238/gmr.15016920. [DOI] [PubMed] [Google Scholar]
- 28.Chai P, Ni H, Zhang H, Fan X. The evolving functions of autophagy in ocular health: A double-edged sword. Int J Biol Sci. 2016;12:1332–40. doi: 10.7150/ijbs.16245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mao XB, You ZP, Wu C, Huang J. Potential suppression of the high glucose and insulin-induced retinal neovascularization by Sirtuin 3 in the human retinal endothelial cells. Biochem Biophys Res Commun. 2017;482(2):341–45. doi: 10.1016/j.bbrc.2016.11.065. [DOI] [PubMed] [Google Scholar]
- 30.Wang DW, Peng ZJ, Ren GF, Wang GX. The different roles of selective autophagic protein degradation in mammalian cells. Oncotarget. 2015;6:37098–116. doi: 10.18632/oncotarget.5776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olson OC, Joyce JA. Cysteine cathepsin proteases: Regulators of cancer progression and therapeutic response. Nat Rev Cancer. 2015;15:712–29. doi: 10.1038/nrc4027. [DOI] [PubMed] [Google Scholar]
- 32.Kim LA, Amarnani D, Gnanaguru G, et al. Tamoxifen toxicity in cultured retinal pigment epithelial cells is mediated by concurrent regulated cell death mechanisms. Invest Ophthalmol Vis Sci. 2014;55:4747–58. doi: 10.1167/iovs.13-13662. [DOI] [PMC free article] [PubMed] [Google Scholar]