Abstract
During periods of metabolic stress, animals must channel energy toward survival and away from processes such as reproduction. The reproductive axis, therefore, has the capacity to respond to changing levels of metabolic cues. The cellular and molecular mechanisms that link energy balance and reproduction, as well as the brain sites mediating this function, are still not well understood. This review focuses on the best characterized of the adiposity signals: leptin and insulin. We examine their reproductive role acting on the classic metabolic pathways of the arcuate nucleus, NPY/AgRP and POMC/CART neurons, and the newly identified kisspeptin network. In addition, other hypothalamic nuclei that may play a role in linking metabolic state and reproductive function are discussed. The nature of the interplay between these elements of the metabolic and reproductive systems presents a fascinating puzzle, whose pieces are just beginning to fall into place.
Keywords: gonadotropin-releasing hormone, leptin, insulin, kisspeptin
SINCE ANIMALS UNDER METABOLIC STRESS must invest energy in survival first and reproduction second, the reproductive axis has the capacity to respond to changes in caloric status. Indeed, every level of the reproductive axis, the hypothalamus, pituitary gland, and gonad, has the capacity to respond to metabolic cues. In humans, anorexia, cachexia, and excessive exercise can all shut down reproductive cyclicity and the secretion of gonadal steroids that are essential for the health of many organs and tissues (28, 57). On the opposite end of the spectrum, obesity and diabetes can also negatively affect fertility (74, 92). The mechanisms regulating these processes are not well understood, but recent work has begun to yield new insights.
While long recognized, the exact nature of the relationship between energy stores and fertility has been somewhat controversial. Work in rodent models (55) and human subjects (40, 41) gave rise to the idea that a female’s fat reserves must exceed a critical threshold for ovulation to occur. However, it has now become clear that the body allocates energy based on current energy balance as opposed to the absolute amount of stored adipose tissue. Ovulation is suppressed when a mammal is in negative energy balance whether that state is caused by inadequate food intake, excessive locomotor activity, or heavy thermoregulatory costs. In the mouse, ovulation occurs whenever extant energetic conditions permit, unless the process is blocked by nonmetabolic stress, social cues, or a predictive seasonal cue such as photoperiod. Mice in the wild often continue to ovulate and become pregnant during seasons of famine despite lacking the energy reserves to carry a litter to term (12). In the human, menstrual irregularities, amenorrhea, and infertility can result from inadequate food intake to compensate for energy demands; for instance, when a severe athletic training schedule is relaxed, luteinizing hormone (LH) pulses and menstrual cycles resume without a significant increase in body fat content (2, 31). The sensitivity of the reproductive axis to current energy availability has been highlighted by recent work suggesting that even subtle declines in energy availability can produce clinically recognized menstrual disturbances (29).
The hypothalamus plays a crucial role in maintaining fertility in all mammals. The GnRH neurons within the preoptic area control the secretion of pituitary LH via the pulsatile release of GnRH from their terminals in the median eminence into the hypophysial portal blood vessels. On a minute-to-hourly basis, the GnRH pulse generator is extremely sensitive to energetic stress. GnRH pulses are readily suppressed by food restriction, high or low ambient temperature, or excessive exercise, and GnRH pulsatility returns rapidly when the energetic challenge is alleviated, usually in one to two hours. Both males and females of a large number of species exhibit this suppression despite the fact that a temporary halt to spermatogenesis is unlikely to have any long-term reproductive consequences in the male (11, 39, 73).
Circulating Leptin and Insulin Affect Fertility
Insulin
Central control of reproduction requires the hypothalamus to receive information regarding the energy status of an animal, for example by sensing hormonal signals secreted into the circulation in proportion to body adipose stores. Woods and Porte originally suggested a role for insulin in the central regulation of energy homeostasis based on the observation that insulin levels circulate in proportion to adipose tissue in most mammals (97). They demonstrated that intracerebroventricular insulin administration results in a dose-dependent reduction in food intake and body weight. Following the advent of gene targeting techniques, neuron-specific deletion of insulin receptors (NIRKO mice) was found to lead to increased body fat deposition and hypothalamic hypogonadism (infertility due to reduced GnRH release) (13), confirming that insulin sensing in the brain is required for normal reproduction. These actions may be mediated by direct insulin action on GnRH neurons or by altering input from secondary insulin sensitive neurons. One critical, unsettled question is whether GnRH neurons express insulin receptors (IRs) in vivo. To date, the only evidence supporting that assertion comes from conditionally immortalized GnRH-expressing cell lines that have been reported to be insulin responsive (78). Given the inherent limitations of extrapolating data from cell lines to animals, in vivo data must be obtained to settle this issue. While standard approaches to this question are available, genetic techniques now allow the targeted deletion of receptors from specific neuronal subtypes. It is hoped that the phenotype of mice lacking IR expression in GnRH neurons will soon be reported.
Leptin
The cloning of the ob gene in 1994 by Friedman and associates resulted in the discovery of another physiologically important adiposity signal secreted by fat tissue: leptin (100). Mice and humans lacking leptin (ob/ob mice) or the leptin receptor (db/db mice) develop hyperphagic morbid obesity and insulin resistant diabetes (22). Moreover, sufficient levels of leptin are a prerequisite for successful reproduction. Leptin administration blunts the fasting-induced suppression of LH secretion and fertility (44, 58, 69, 70). In women with exercise-or anorexia-induced amenorrhea, leptin treatment increases pulse frequency and levels of LH, ovarian volume, number of dominant follicles, and estradiol levels (64, 95). Furthermore, ob/ob mice have low LH levels and are infertile, and leptin administration, but not weight loss alone, restores their fertility (5, 19, 68, 101). Expression of leptin receptor (LepR) in the brain of db/db mice or mice otherwise null for LepRs restores fertility completely in males and partially in females (26, 60).
These studies support a role for central LepRs in modulating GnRH release. However, despite initial reports showing expression of LepRs in immortalized GnRH cell lines, it is now recognized that GnRH neurons do not physiologically express LepRs (14, 38, 49). Instead, leptin is believed to act indirectly via interneurons impinging on GnRH-secreting cells in the hypothalamus (24, 90), although the identity of such interneurons is unclear. A better understanding of the neural circuitry underlying leptin signaling in the hypothalamus is critical not only for the advancement of our knowledge of the connection between metabolism and reproduction but also for future development of treatment strategies for hypothalamic hypogonadism.
Leptin and Insulin Sensing in Hypothalamic Neurons
The role of the arcuate nucleus of the hypothalamus in regulating energy balance is well established. The arcuate nucleus contains proopiomelanocortin/cocaine- and amphetamine-regulated transcript (POMC/CART)-expressing neurons, whose activation suppresses feeding. In contrast, the activation of a second population of arcuate neurons, neuropeptide Y/agouti-related protein (NPY/AgRP)-expressing neurons, stimulates feeding (6, 33). The coordinated regulation of these neurons and their downstream projections to key brain regions contributes to control of energy balance. These targets include the paraventricular nucleus of the hypothalamus, the lateral hypothalamic area, and other autonomic and neuroendocrine control sites (32).
IRs are expressed in the medial portion of the arcuate nucleus where NPY/AgRP-expressing neurons are located (8). Indeed, insulin affects the expression of NPY; in fasted animals, intracerebroventricular (icv) administration of insulin decreases NPY mRNA in the arcuate nucleus and NPY peptide in the paraventricular nucleus of the hypothalamus (79, 93). Insulin-deficient diabetic rats show increased hypothalamic levels of both NPY and its mRNA that are normalized by systemic insulin therapy (1, 80). High numbers of IRs are also found on POMC/CART neurons (9). Interestingly, no obvious metabolic or reproductive phenotype was seen in mice lacking IRs only in POMC neurons (59).
Although LepRs are expressed in many hypothalamic nuclei (34, 36, 65), significant attention has been given to neurons located in the arcuate nucleus. There, LepRs are expressed by both NPY/AgRP and POMC/CART neurons (7, 20, 34) and are required for maintaining a normal body weight (4, 67). It is important to note that insulin and leptin share some, but not all, overlapping intracellular signaling pathways and may thereby exert similar effects. Much ongoing work is dedicated to untangling the contribution of these pathways to the actions of leptin and insulin (71, 75, 98).
Evidence is accumulating that leptin has key targets outside the arcuate nucleus (47, 52). LepR is found in other hypothalamic nuclei, including the dorsomedial subdivision of the ventromedial nucleus (VMH), the caudal subdivision of the dorsomedial nucleus (DMH), the premammillary ventral nucleus (PMV), and, in a small extension, in the paraventricular nucleus (34, 36, 65). Indeed, neurons expressing LepR in the VMH also respond to glucose and insulin (15), but the pathways downstream of VMH neurons responsive to metabolic signals are not yet identified. In addition, the DMH and the PMV strongly innervate areas related to reproductive control, including the anteroventral periventricular nucleus (AVPV) and the medial preoptic area (16, 76, 88, 89). However, whether these projections originate from neurons responsive to leptin and are physiologically relevant to leptin action in reproductive control remains unsettled.
Mediobasal Hypothalamic Circuits Linking Metabolic State with Reproduction
Evidence suggests that the neurons involved in regulating energy metabolism can communicate with the hypothalamic-pituitary-gonadal (HPG) axis via interactions with GnRH neurons. NPY fibers are intimately associated with the dendrites and cell bodies of GnRH neurons in the medial preoptic area (48), and NPY fibers in the median eminence may also act on GnRH terminals (77). NPY neurons therefore have been considered good candidates to operate as neuroendocrine integrators, linking perturbations in energy balance and alterations in the activity of the reproductive axis. According to this model, NPY neurons are activated under conditions of negative energy balance, leading to an increase in NPY release from terminals in the paraventricular nucleus and preoptic area. Increased NPY release may then stimulate feeding behavior while inhibiting release of GnRH and activity in the pituitary-gonadal axis. In support of this hypothesis, a suppression of basal LH levels by fasting fails to occur in the NPY knockout female (50). In addition, ob/ob mice that are also NPY deficient display improved fertility compared with ob/ob controls (35).
However, pharmacological evidence in rats (23), rabbits (56), and monkeys (96) provides a compelling case for the existence of two mechanisms, one inhibitory and one stimulatory, through which endogenous NPY regulates the GnRH pulse generator. In numerous species, NPY has negative effects on GnRH levels, pulse amplitude, and pulse frequency in an environment of low estradiol and progesterone, such as in unprimed, ovariectomized animals (3). In these animals, steroid replacement switches the effect of NPY to a robust positive one (10, 91). Thus, surprisingly, NPY appears to be required for GnRH surge production; LH surges are attenuated after NPY immunoneutralization or Y1 receptor blockade on the day of proestrus (48, 53). In addition, NPY knockout females show a 70% reduction in their LH surges (99). This dual effect also explains why food deprivation initiated on the day of estrus is much less effective in suppressing ovulation than fasting begun on diestrus, when estrogen levels are low (12). Exciting new findings have begun to shed light on this paradox. In hypothalamic cell lines, the ratio of estrogen receptor-α (ERα) to -β (ERβ) regulates the expression of NPY gene in response to estrogen treatment. Whereas ERα favors the suppression of NPY gene, ERβ stimulates peptide expression (91). The critical next step is to determine whether the ratio of ER subtypes in the hypothalamus varies across the estrous cycle. Alternatively, GABA release by a subpopulation of NPY neurons (51) may affect GnRH secretion, as all GnRH neurons express GABAA receptors (21, 72). Interestingly, GABA release can hyperpolarize or depolarize GnRH neurons (30, 66) in agreement with opposing NPY effects on LH secretion. The origin of the GABAergic inputs to GnRH neurons is not known, and therefore further studies are necessary in order to explore NPY/GABA interaction modulating GnRH secretion.
POMC/CART neurons may also be involved in conveying metabolic status to GnRH neurons. POMC/CART-producing neurons in the arcuate nucleus project to the medial preoptic area, and terminals of POMC products (β-endorphin and α-MSH) and CART make apparent synaptic contact with GnRH-immunoreactive cells (63, 76), although the expression of melanocortin and/or opioid receptors in GnRH cell bodies is not well established. However, the various POMC gene products have differential effects on the reproductive axis; for example, α-MSH reduces food consumption and stimulates lordosis behavior in female rats (45, 81), whereas β-endorphin stimulates food consumption and inhibits GnRH/LH secretion (43, 62, 94). Further studies are needed to elucidate the precise role of these neuronal populations.
Finally, the overlapping intracellular signaling pathways of leptin and insulin raise the question of whether joint signaling by insulin and leptin in POMC and NPY cells contributes to the control of GnRH release. Although genetically ablating receptors for leptin or insulin in NPY or POMC neurons results in no obvious reproductive phenotypes (4, 59), these results may underrepresent the combined contribution of these adiposity signals to the maintenance of fertility. Double-knockout studies are needed to address this question.
Kisspeptin
Recently, the description of G protein-coupled receptor-54 (GPR54) and the cognate ligands, kisspeptins, has brought an avalanche of new findings into the reproductive field (37, 54, 83). KiSS-1 is expressed by neurons located in several hypothalamic nuclei, including the AVPV, a key site for the regulation of gonadotropin secretion, and the arcuate nucleus (46). The GPR54 receptor is expressed by GnRH neurons, and its mutation causes hypogonadotropic hypogonadism in humans and mice (25, 27, 42, 61, 82). Fasting causes a reduction in the amount of KiSS-1 mRNA (17), which precedes the fasting-induced decline of GnRH. In the mouse, icv administration of kisspeptin evokes LH and FSH secretion at remarkably low doses (46).
In both the AVPV and arcuate nucleus, KiSS-1 is regulated by sex steroids (83–85). Estrogen and androgen receptors (ERα and AR, respectively) are found in a high percentage of KiSS-1-expressing neurons, and estradiol and testosterone increase KiSS-1 expression in the AVPV and decrease KiSS-1 expression in the arcuate nucleus. It is believed that kisspeptin has distinct actions on GnRH release depending on the steroid hormone milieu, mediating the negative feedback of sex steroids on GnRH secretion via neurons in the arcuate nucleus and the positive feedback of sex steroids via AVPV neurons (85). However, the requirement for AR and ERα in mediating the feedback effects of sex steroids on Kiss-1 expression remains to be confirmed.
Kisspeptin appears to play a role in the reproductive effects of leptin. LepRs have been found in over 40% of Kiss-1 neurons in the arcuate nucleus. Compared with wild-type mice, obese ob/ob male mice show decreased expression of KiSS-1 in the arcuate nucleus, which is restored by leptin treatment (85). In a recent study, investigators showed that in diabetic rats KiSS-1 mRNA is decreased in the hypothalamus. These animals exhibit low circulating levels of leptin, insulin, and LH (18). Administration of kisspeptin restored LH and testosterone secretion and icv leptin, but not insulin, and normalized KiSS-1 mRNA levels in the hypothalamus as well as circulating levels of LH. Although KiSS-1 and LepR are coexpressed in the arcuate nucleus, the specific hypothalamic sites where leptin acts to stimulate KiSS-1 in this paradigm are not known.
Thus far, the results are consistent with a model whereby leptin and perhaps other adiposity and satiety factors stimulate KiSS-1 expression, triggering production of kisspeptin and stimulation of GnRH release. It is tempting to speculate that reproductive deficits associated with leptin-deficient states may be attributable to diminished expression patterns of KiSS-1 or its receptor. Nevertheless, whether leptin is solely acting on KiSS-1 neurons in the central control of GnRH or whether additional peripheral regulators cooperate with leptin in the control of KiSS-1 for the integration of energy balance remains to be elucidated. The use of genetic mouse models that allow the deletion or reactivation of KiSS-1 gene in specific neurons will provide crucial access to this pathway.
Summary
We have entered an exciting era for the study of metabolic regulation of reproductive function. Our knowledge of the hypothalamic circuitry involved in monitoring energy balance and providing input to GnRH neurons continues to expand rapidly. We have discussed only a few of the neuropeptides and hormones involved, and no doubt more are waiting to be discovered. A major goal of future research should therefore be not only to discover individual players communicating energy status to the reproductive axis but also to understand how each fits within the neuronal network connecting these two critical systems. Many relationships remain to be elucidated (Fig. 1). Pursuit of these questions will yield a greater understanding of the central control of reproduction and holds out the hope of addressing the clinical impact of impaired fertility and steroid production due to metabolic causes.
Fig. 1.
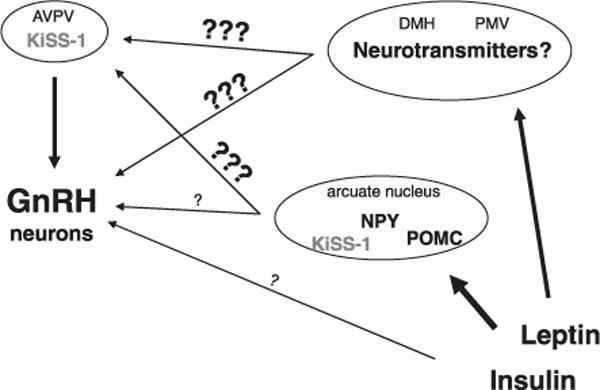
Schematic representation of candidate brain pathways mediating leptin and insulin actions in reproductive control. Leptin and insulin receptors are distributed in a variety of brain nuclei, but the connections with areas related to reproductive control including the anteroventral periventricular nucleus (AVPV) and GnRH neurons are unknown. Moreover, although the arcuate nucleus neurons expressing proopiomelanocortin (POMC) or neuropeptide Y (NPY) have been extensively investigated, their projections to the AVPV are not described, and the innervation of GnRH neurons is still controversial. A few studies have also suggested the direct action of insulin in GnRH neurons, but these findings need to be replicated. DMH, dorsomedial nucleus; PMV, premammillary ventral nucleus.
References
- 1.Abe M, Saito M, Ikeda H, Shimazu T. Increased neuropeptide Y content in the arcuato-paraventricular hypothalamic neuronal system in both insulin-dependent and non-insulin-dependent diabetic rats. Brain Res. 1991;539:223–227. doi: 10.1016/0006-8993(91)91624-a. [DOI] [PubMed] [Google Scholar]
- 2.Abraham SF, Beumont PJ, Fraser IS, Llewellyn-Jones D. Body weight, exercise and menstrual status among ballet dancers in training. Br J Obstet Gynaecol. 1982;89:507–510. doi: 10.1111/j.1471-0528.1982.tb03649.x. [DOI] [PubMed] [Google Scholar]
- 3.Allen L, Wilson FJ, Macdonald GJ. Neuropeptide Y-containing nerves in rat gonads: sex difference and development. Biol Reprod. 1989;40:371–378. doi: 10.1095/biolreprod40.2.371. [DOI] [PubMed] [Google Scholar]
- 4.Balthasar N, Coppari R, McMinn J, Liu SM, Lee CE, Tang V, Kenny CD, McGovern RA, Chua SC, Jr, Elmquist JK, Lowell BB. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 2004;42:983–991. doi: 10.1016/j.neuron.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Barash IA, Cheung CC, Weigle DS, Ren H, Kabigting EB, Kuijper JL, Clifton DK, Steiner RA. Leptin is a metabolic signal to the reproductive system. Endocrinology. 1996;137:3144–3147. doi: 10.1210/endo.137.7.8770941. [DOI] [PubMed] [Google Scholar]
- 6.Baskin D, Breininger JF, Schwartz MW. SOCS-3 expression in leptin-sensitive neurons of the hypothalamus of fed and fasted rats. Regul Pept. 2000;92:9–15. doi: 10.1016/s0167-0115(00)00143-9. [DOI] [PubMed] [Google Scholar]
- 7.Baskin D, Schwartz MW, Seeley RJ, Woods SC, Porte D, Jr, Breininger JF, Jonak Z, Schaefer J, Krouse M, Burghardt C, Campfield LA, Burn P, Kochan JP. Leptin receptor long-form splice-variant protein expression in neuron cell bodies of the brain and co-localization with neuropeptide Y mRNA in the arcuate nucleus. J Histochem Cytochem. 1999;47:353–362. doi: 10.1177/002215549904700309. [DOI] [PubMed] [Google Scholar]
- 8.Baskin DG, Figlewicz DP, Woods SC, Porte D, Jr, Dorsa DM. Insulin in the brain. Annu Rev Physiol. 1987;49:335–347. doi: 10.1146/annurev.ph.49.030187.002003. [DOI] [PubMed] [Google Scholar]
- 9.Benoit SC, Air EL, Coolen LM, Strauss R, Jackman A, Clegg DJ, Seeley RJ, Woods SC. The catabolic action of insulin in the brain is mediated by melanocortins. J Neurosci. 2002;22:9048–9052. doi: 10.1523/JNEUROSCI.22-20-09048.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berria M, Pau KY, Spies HG. Evidence for alpha 1-adrenergic involvement in neuropeptide Y-stimulated GnRH release in female rabbits. Neuroendocrinology. 1991;53:480–486. doi: 10.1159/000125761. [DOI] [PubMed] [Google Scholar]
- 11.Bronson FH, Heideman PD. Short-term hormonal responses to food intake in peripubertal female rats. Am J Physiol Regul Integr Comp Physiol. 1990;259:R25–R31. doi: 10.1152/ajpregu.1990.259.1.R25. [DOI] [PubMed] [Google Scholar]
- 12.Bronson FH, Marsteller FA. Effect of short-term food deprivation on reproduction in female mice. Biol Reprod. 1985;33:660–667. doi: 10.1095/biolreprod33.3.660. [DOI] [PubMed] [Google Scholar]
- 13.Bruning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Muller-Wieland D, Kahn CR. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289:2122–2125. doi: 10.1126/science.289.5487.2122. [DOI] [PubMed] [Google Scholar]
- 14.Burcelin R, Thorens B, Glauser M, Gaillard RC, Pralong FP. Gonadotropin-releasing hormone secretion from hypothalamic neurons: stimulation by insulin and potentiation by leptin. Endocrinology. 2003;144:4484– 4491. doi: 10.1210/en.2003-0457. [DOI] [PubMed] [Google Scholar]
- 15.Canabal D, Song Z, Potian JG, Beuve A, McArdle JJ, Routh VH. Glucose, insulin, and leptin signaling pathways modulate nitric oxide synthesis in glucose-inhibited neurons in the ventromedial hypothalamus. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1418–R1428. doi: 10.1152/ajpregu.00216.2006. [DOI] [PubMed] [Google Scholar]
- 16.Canteras N, Simerly RB, Swanson LW. Projections of the ventral premammillary nucleus. J Comp Neurol. 1992;324:195–212. doi: 10.1002/cne.903240205. [DOI] [PubMed] [Google Scholar]
- 17.Castellano J, Navarro VM, Fernandez-Fernandez R, Nogueiras R, Tovar S, Roa J, Vazquez MJ, Vigo E, Casanueva FF, Aguilar E, Pinilla L, Dieguez C, Tena-Sempere M. Changes in hypothalamic KiSS-1 system and restoration of pubertal activation of the reproductive axis by kisspeptin in undernutrition. Endocrinology. 2005;146:3917–3925. doi: 10.1210/en.2005-0337. [DOI] [PubMed] [Google Scholar]
- 18.Castellano J, Navarro VM, Fernandez-Fernandez R, Roa J, Vigo E, Pineda R, Dieguez C, Aguilar E, Pinilla L, Tena-Sempere M. Expression of hypothalamic KiSS-1 system and rescue of defective gonadotropic responses by kisspeptin in streptozotocin-induced diabetic male rats. Diabetes. 2006;55:2602–2610. doi: 10.2337/db05-1584. [DOI] [PubMed] [Google Scholar]
- 19.Chehab FF, Lim ME, Lu R. Correction of the sterility defect in homozygous obese female mice by treatment with the human recombinant leptin. Nat Genet. 1996;12:318–320. doi: 10.1038/ng0396-318. [DOI] [PubMed] [Google Scholar]
- 20.Cheung C, Clifton DK, Steiner RA. Proopiomelanocortin neurons are direct targets for leptin in the hypothalamus. Endocrinology. 1997;138:4489–4492. doi: 10.1210/endo.138.10.5570. [DOI] [PubMed] [Google Scholar]
- 21.Clarkson J, Herbison AE. Development of GABA and glutamate signaling at the GnRH neuron in relation to puberty. Mol Cell Endocrinol. 2006;254–255:32–38. doi: 10.1016/j.mce.2006.04.036. [DOI] [PubMed] [Google Scholar]
- 22.Coleman DL. Obese and diabetes: two mutant genes causing diabetes-obesity syndromes in mice. Diabetologia. 1978;14:141–148. doi: 10.1007/BF00429772. [DOI] [PubMed] [Google Scholar]
- 23.Crowley W, Kalra SP. Neuropeptide Y stimulates the release of luteinizing hormone-releasing hormone from medial basal hypothalamus in vitro: modulation by ovarian hormones. Neuroendocrinology. 1987;46:97–103. doi: 10.1159/000124804. [DOI] [PubMed] [Google Scholar]
- 24.Cunningham M, Clifton DK, Steiner RA. Leptin’s actions on the reproductive axis: perspectives and mechanisms. Biol Reprod. 1999;60:216– 222. doi: 10.1095/biolreprod60.2.216. [DOI] [PubMed] [Google Scholar]
- 25.d’Anglemont de Tassigny X, Fagg LA, Dixon JPC, Day K, Leitch HG, Hendrick AG, Zahn D, Franceschini I, Caraty A, Carlton MBL, Aparicio SAJR, Colledge WH. Hypogonadotropic hypogonadism in mice lacking a functional Kiss1 gene. Proc Natl Acad Sci USA. 2007;104:10714–10719. doi: 10.1073/pnas.0704114104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Luca C, Kowalski TJ, Zhang Y, Elmquist JK, Lee C, Kilimann MW, Ludwig T, Liu SM, Chua SC., Jr Complete rescue of obesity, diabetes, and infertility in db/db mice by neuron-specific LEPR-B transgenes. J Clin Invest. 2005;115:3484–3493. doi: 10.1172/JCI24059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA. 2003;100:10972–10976. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Souza M, Miller B, Loucks A, Luciano A, Pescatello L, Campbell C, Lasley BL. High frequency of luteal phase deficiency and anovulation in recreational women runners: blunted elevation in follicle-stimulating hormone observed during luteal-follicular transition. J Clin Endocrinol Metab. 1998;83:4220–4232. doi: 10.1210/jcem.83.12.5334. [DOI] [PubMed] [Google Scholar]
- 29.De Souza MJ, Lee DK, VanHeest JL, Scheid JL, West SL, Williams NI. Severity of energy-related menstrual disturbances increases in proportion to indices of energy conservation in exercising women. Fertil Steril. 2007;88:971–975. doi: 10.1016/j.fertnstert.2006.11.171. [DOI] [PubMed] [Google Scholar]
- 30.DeFazio R, Heger S, Ojeda SR, Moenter SM. Activation of A-type gamma-aminobutyric acid receptors excites gonadotropin-releasing hormone neurons. Mol Endocrinol. 2002;16:2872–2891. doi: 10.1210/me.2002-0163. [DOI] [PubMed] [Google Scholar]
- 31.Dueck CA, Matt KS, Manore MM, Skinner JS. Treatment of athletic amenorrhea with a diet and training intervention program. Int J Sport Nutr. 1996;6:24–40. doi: 10.1123/ijsn.6.1.24. [DOI] [PubMed] [Google Scholar]
- 32.Elias C, Saper CB, Maratos-Flier E, Tritos NA, Lee C, Kelly J, Tatro JB, Hoffman GE, Ollmann MM, Barsh GS, Sakurai T, Yanagisawa M, Elmquist JK. Chemically defined projections linking the mediobasal hypothalamus and the lateral hypothalamic area. J Comp Neurol. 1998;402:442–459. [PubMed] [Google Scholar]
- 33.Elias CF, Aschkenasi C, Lee C, Kelly J, Ahima RS, Bjorbaek C, Flier JS, Saper CB, Elmquist JK. Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area. Neuron. 1999;23:775–786. doi: 10.1016/s0896-6273(01)80035-0. [DOI] [PubMed] [Google Scholar]
- 34.Elmquist JK, Bjorbaek C, Ahima RS, Flier JS, Saper CB. Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol. 1998;395:535–547. [PubMed] [Google Scholar]
- 35.Erickson JC, Hollopeter G, Palmiter RD. Attenuation of the obesity syndrome of ob/ob mice by the loss of neuropeptide Y. Science. 1996;274:1704–1707. doi: 10.1126/science.274.5293.1704. [DOI] [PubMed] [Google Scholar]
- 36.Fei H, Okano HJ, Li C, Lee GH, Zhao C, Darnell R, Friedman JM. Anatomic localization of alternatively spliced leptin receptors (Ob-R) in mouse brain and other tissues. Proc Natl Acad Sci USA. 1997;94:7001–7005. doi: 10.1073/pnas.94.13.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fernandez-Fernandez R, Martini AC, Navarro VM, Castellano JM, Dieguez C, Aguilar E, Pinilla L, Tena-Sempere M. Novel signals for the integration of energy balance and reproduction. Mol Cell Endocrinol. 2006;254–255:127–132. doi: 10.1016/j.mce.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 38.Finn PD, Cunningham MJ, Pau KY, Spies HG, Clifton DK, Steiner RA. The stimulatory effect of leptin on the neuroendocrine reproductive axis of the monkey. Endocrinology. 1998;139:4652–4662. doi: 10.1210/endo.139.11.6297. [DOI] [PubMed] [Google Scholar]
- 39.Foster DL, Ebling FJ, Micka AF, Vannerson LA, Bucholtz DC, Wood RI, Suttie JM, Fenner DE. Metabolic interfaces between growth and reproduction. I. Nutritional modulation of gonadotropin, prolactin, and growth hormone secretion in the growth-limited female lamb. Endocrinology. 1989;125:342–350. doi: 10.1210/endo-125-1-342. [DOI] [PubMed] [Google Scholar]
- 40.Frisch RE. Critical fatness hypothesis. Am J Physiol Endocrinol Metab. 1997;273:E231–E232. doi: 10.1152/ajpendo.1997.273.1.E231. [DOI] [PubMed] [Google Scholar]
- 41.Frisch RE, McArthur JW. Menstrual cycles: fatness as a determinant of minimum weight for height necessary for their maintenance or onset. Science. 1974;185:949–951. doi: 10.1126/science.185.4155.949. [DOI] [PubMed] [Google Scholar]
- 42.Funes S, Hedrick JA, Vassileva G, Markowitz L, Abbondanzo S, Golovko A, Yang S, Monsma FJ, Gustafson EL. The KiSS-1 receptor GPR54 is essential for the development of the murine reproductive system. Biochem Biophys Res Commun. 2003;312:1357–1363. doi: 10.1016/j.bbrc.2003.11.066. [DOI] [PubMed] [Google Scholar]
- 43.Gilbeau P, Almirez RG, Holaday JW, Smith CG. Opioid effects on plasma concentrations of luteinizing hormone and prolactin in the adult male rhesus monkey. J Clin Endocrinol Metab. 1985;60:299–305. doi: 10.1210/jcem-60-2-299. [DOI] [PubMed] [Google Scholar]
- 44.Gonzalez LC, Pinilla L, Tena-Sempere M, Aguilar E. Leptin(116– 130) stimulates prolactin and luteinizing hormone secretion in fasted adult male rats. Neuroendocrinology. 1999;70:213–220. doi: 10.1159/000054479. [DOI] [PubMed] [Google Scholar]
- 45.Gonzalez M, Celis ME, Hole DR, Wilson CA. Interaction of oestradiol, alpha-melanotrophin and noradrenaline within the ventromedial nucleus in the control of female sexual behaviour. Neuroendocrinology. 1993;58:218–226. doi: 10.1159/000126536. [DOI] [PubMed] [Google Scholar]
- 46.Gottsch M, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145:4073–4077. doi: 10.1210/en.2004-0431. [DOI] [PubMed] [Google Scholar]
- 47.Grill H, Schwartz MW, Kaplan JM, Foxhall JS, Breininger J, Baskin DG. Evidence that the caudal brainstem is a target for the inhibitory effect of leptin on food intake. Endocrinology. 2002;143:239–246. doi: 10.1210/endo.143.1.8589. [DOI] [PubMed] [Google Scholar]
- 48.Guy J, Li S, Pelletier G. Studies on the physiological role and mechanism of action of neuropeptide Y in the regulation of luteinizing hormone secretion in the rat. Regul Pept. 1988;23:209–216. doi: 10.1016/0167-0115(88)90028-6. [DOI] [PubMed] [Google Scholar]
- 49.Hakansson M, Brown H, Ghilardi N, Skoda RC, Meister B. Leptin receptor immunoreactivity in chemically defined target neurons of the hypothalamus. J Neurosci. 1998;18:559–572. doi: 10.1523/JNEUROSCI.18-01-00559.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hill J, Levine JE. Abnormal response of the neuropeptide Y-deficient mouse reproductive axis to food deprivation but not lactation. Endocrinology. 2003;144:1780–1786. doi: 10.1210/en.2002-221024. [DOI] [PubMed] [Google Scholar]
- 51.Horvath T, Bechmann I, Naftolin F, Kalra SP, Leranth C. Heterogeneity in the neuropeptide Y-containing neurons of the rat arcuate nucleus: GABAergic and non-GABAergic subpopulations. Brain Res. 1997;756:283–286. doi: 10.1016/s0006-8993(97)00184-4. [DOI] [PubMed] [Google Scholar]
- 52.Huo L, Maeng L, Bjorbaek C, Grill HJ. Leptin and the control of food intake: neurons in the nucleus of the solitary tract are activated by both gastric distension and leptin. Endocrinology. 2007;148:2189–2197. doi: 10.1210/en.2006-1572. [DOI] [PubMed] [Google Scholar]
- 53.Kalra SP, Crowley WR. Neuropeptide Y: a novel neuroendocrine peptide in the control of pituitary hormone secretion, and its relation to luteinizing hormone. Front Neuroendocrinol. 1992;13:1–46. [PubMed] [Google Scholar]
- 54.Kauffman A, Clifton DK, Steiner RA. Emerging ideas about kisspeptin-GPR54 signaling in the neuroendocrine regulation of reproduction. Trends Neurosci. 2007;30:504–511. doi: 10.1016/j.tins.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 55.Kennedy GC. The relation between the central control of appetite, growth and sexual maturation. Guys Hosp Rep. 1969;118:315–327. [PubMed] [Google Scholar]
- 56.Khorram O, Pau KY, Spies HG. Bimodal effects of neuropeptide Y on hypothalamic release of gonadotropin-releasing hormone in conscious rabbits. Neuroendocrinology. 1987;45:290–297. doi: 10.1159/000124743. [DOI] [PubMed] [Google Scholar]
- 57.Klentrou P, Plyley M. Onset of puberty, menstrual frequency, and body fat in elite rhythmic gymnasts compared with normal controls. Br J Sports Med. 2003;37:490–494. doi: 10.1136/bjsm.37.6.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kohsaka A, Watanobe H, Kakizaki Y, Habu S, Suda T. A significant role of leptin in the generation of steroid-induced luteinizing hormone and prolactin surges in female rats. Biochem Biophys Res Commun. 1999;254:578–581. doi: 10.1006/bbrc.1998.0112. [DOI] [PubMed] [Google Scholar]
- 59.Konner AC, Janoschek R, Plum L, Jordan SD, Rother E, Ma X, Xu C, Enriori P, Hampel B, Barsh GS, Kahn CR, Cowley MA, Ashcroft FM, Bruning JC. Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metab. 2007;5:438–449. doi: 10.1016/j.cmet.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 60.Kowalski TJ, Liu SM, Leibel RL, Chua SC., Jr Transgenic complementation of leptin-receptor deficiency. I. Rescue of the obesity/diabetes phenotype of LEPR-null mice expressing a LEPR-B transgene. Diabetes. 2001;50:425–435. doi: 10.2337/diabetes.50.2.425. [DOI] [PubMed] [Google Scholar]
- 61.Lapatto R, Pallais JC, Zhang D, Chan YM, Mahan A, Cerrato F, Le WW, Hoffman GE, Seminara SB. Kiss1−/− mice exhibit more variable hypogonadism than Gpr54−/− mice. Endocrinology. 2007;148:4927–4936. doi: 10.1210/en.2007-0078. [DOI] [PubMed] [Google Scholar]
- 62.Leadem C, Kalra SP. Reversal of beta-endorphin-induced blockade of ovulation and luteinizing hormone surge with prostaglandin E2. Endocrinology. 1985;117:684–689. doi: 10.1210/endo-117-2-684. [DOI] [PubMed] [Google Scholar]
- 63.Leranth C, MacLusky NJ, Shanabrough M, Naftolin F. Immunohistochemical evidence for synaptic connections between pro-opiomelanocortin-immunoreactive axons and LH-RH neurons in the preoptic area of the rat. Brain Res. 1988;449:167–176. doi: 10.1016/0006-8993(88)91035-9. [DOI] [PubMed] [Google Scholar]
- 64.Licinio J, Negrao AB, Mantzoros C, Kaklamani V, Wong ML, Bongiorno PB, Mulla A, Cearnal L, Veldhuis JD, Flier JS, McCann SM, Gold PW. Synchronicity of frequently sampled, 24-h concentrations of circulating leptin, luteinizing hormone, and estradiol in healthy women. Proc Natl Acad Sci USA. 1998;95:2541–2546. doi: 10.1073/pnas.95.5.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mercer J, Hoggard N, Williams LM, Lawrence CB, Hannah LT, Trayhurn P. Localization of leptin receptor mRNA and the long form splice variant (Ob-Rb) in mouse hypothalamus and adjacent brain regions by in situ hybridization. FEBS Lett. 1996;387:113–116. doi: 10.1016/0014-5793(96)00473-5. [DOI] [PubMed] [Google Scholar]
- 66.Moenter S, DeFazio RA. Endogenous gamma-aminobutyric acid can excite gonadotropin-releasing hormone neurons. Endocrinology. 2005;146:5374–5379. doi: 10.1210/en.2005-0788. [DOI] [PubMed] [Google Scholar]
- 67.Morton G, Niswender KD, Rhodes CJ, Myers MG, Jr, Blevins JE, Baskin DG, Schwartz MW. Arcuate nucleus-specific leptin receptor gene therapy attenuates the obesity phenotype of Koletsky [fa(k)/fa(k)] rats. Endocrinology. 2003;144:2016–2024. doi: 10.1210/en.2002-0115. [DOI] [PubMed] [Google Scholar]
- 68.Mounzih K, Lu R, Chehab FF. Leptin treatment rescues the sterility of genetically obese ob/ob males. Endocrinology. 1997;138:1190–1193. doi: 10.1210/endo.138.3.5024. [DOI] [PubMed] [Google Scholar]
- 69.Nagatani S, Guthikonda P, Thompson RC, Tsukamura H, Maeda KI, Foster DL. Evidence for GnRH regulation by leptin: leptin administration prevents reduced pulsatile LH secretion during fasting. Neuroendocrinology. 1998;67:370–376. doi: 10.1159/000054335. [DOI] [PubMed] [Google Scholar]
- 70.Nagatani S, Zeng Y, Foster DL, Jaffe CA. Leptin regulates pulsatile luteinizing hormone and growth hormone secretion in the sheep. Endocrinology. 2000;141:3965–3975. doi: 10.1210/endo.141.11.7762. [DOI] [PubMed] [Google Scholar]
- 71.Niswender KD, Morrison CD, Clegg DJ, Olson R, Baskin DG, Myers MG, Jr, Seeley RJ, Schwartz MW. Insulin activation of phosphatidylinositol 3-kinase in the hypothalamic arcuate nucleus: a key mediator of insulin-induced anorexia. Diabetes. 2003;52:227–231. doi: 10.2337/diabetes.52.2.227. [DOI] [PubMed] [Google Scholar]
- 72.Pape J, Skynner MJ, Sim JA, Herbison AE. Profiling gamma-aminobutyric acid (GABAA) receptor subunit mRNA expression in postnatal gonadotropin-releasing hormone (GnRH) neurons of the male mouse with single cell RT-PCR. Neuroendocrinology. 2001;74:300–308. doi: 10.1159/000054697. [DOI] [PubMed] [Google Scholar]
- 73.Parfitt DB, Church KR, Cameron JL. Restoration of pulsatile luteinizing hormone secretion after fasting in rhesus monkeys (Macaca mulatta): dependence on size of the refeed meal. Endocrinology. 1991;129:749–756. doi: 10.1210/endo-129-2-749. [DOI] [PubMed] [Google Scholar]
- 74.Pasquali R, Gambineri A, Pagotto U. The impact of obesity on reproduction in women with polycystic ovary syndrome. BJOG. 2006;113:1148–1159. doi: 10.1111/j.1471-0528.2006.00990.x. [DOI] [PubMed] [Google Scholar]
- 75.Plum L, Ma X, Hampel B, Balthasar N, Coppari R, Munzberg H, Shanabrough M, Burdakov D, Rother E, Janoschek R, Alber J, Belgardt B, Koch L, Seibler J, Schwenk F, Fekete C, Suzuki A, Mak T, Krone W, Horvath T, Ashcroft F, Bruning JC. Enhanced PIP3 signaling in POMC neurons causes KATP channel activation and leads to diet-sensitive obesity. J Clin Invest. 2006;116:1886–1901. doi: 10.1172/JCI27123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rondini T, Baddini SP, Sousa LF, Bittencourt JC, Elias CF. Hypothalamic cocaine- and amphetamine-regulated transcript neurons project to areas expressing gonadotropin releasing hormone immunoreactivity and to the anteroventral periventricular nucleus in male and female rats. Neuroscience. 2004;125:735–748. doi: 10.1016/j.neuroscience.2003.12.045. [DOI] [PubMed] [Google Scholar]
- 77.Sabatino F, Murnane JM, Hoffman RA, McDonald JK. Distribution of neuropeptide Y-like immunoreactivity in the hypothalamus of the adult golden hamster. J Comp Neurol. 1987;257:93–104. doi: 10.1002/cne.902570107. [DOI] [PubMed] [Google Scholar]
- 78.Salvi R, Castillo E, Voirol MJ, Glauser M, Rey JP, Gaillard RC, Vollenweider P, Pralong FP. Gonadotropin-releasing hormone-expressing neurons immortalized conditionally are activated by insulin: implication of the mitogen-activated protein kinase pathway. Endocrinology. 2006;147:816–826. doi: 10.1210/en.2005-0728. [DOI] [PubMed] [Google Scholar]
- 79.Schwartz M, Marks JL, Sipols AJ, Baskin DG, Woods SC, Kahn SE, Porte D., Jr Central insulin administration reduces neuropeptide Y mRNA expression in the arcuate nucleus of food-deprived lean (Fa/Fa) but not obese (fa/fa) Zucker rats. Endocrinology. 1991;128:2645–2647. doi: 10.1210/endo-128-5-2645. [DOI] [PubMed] [Google Scholar]
- 80.Schwartz MW, Sipols AJ, Marks JL, Sanacora G, White JD, Scheurink A, Kahn SE, Baskin DG, Woods SC, Figlewicz DP, et al. Inhibition of hypothalamic neuropeptide Y gene expression by insulin. Endocrinology. 1992;130:3608–3616. doi: 10.1210/endo.130.6.1597158. [DOI] [PubMed] [Google Scholar]
- 81.Scimonelli T, Medina F, Wilson C, Celis ME. Interaction of alphamelanotropin (alpha-MSH) and noradrenaline in the median eminence in the control of female sexual behavior. Peptides. 2000;21:219–223. doi: 10.1016/s0196-9781(99)00191-6. [DOI] [PubMed] [Google Scholar]
- 82.Seminara S, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O’Rahilly S, Carlton MBL, Crowley WF, Jr, Aparicio SAJR, Colledge WH. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- 83.Smith J, Acohido BV, Clifton DK, Steiner RA. KiSS-1 neurones are direct targets for leptin in the ob/ob mouse. J Neuroendocrinol. 2006a;18:298–303. doi: 10.1111/j.1365-2826.2006.01417.x. [DOI] [PubMed] [Google Scholar]
- 84.Smith J, Clay CM, Caraty A, Clarke IJ. KiSS-1 messenger ribonucleic acid expression in the hypothalamus of the ewe is regulated by sex steroids and season. Endocrinology. 2007;148:1150–1157. doi: 10.1210/en.2006-1435. [DOI] [PubMed] [Google Scholar]
- 85.Smith J, Clifton DK, Steiner RA. Regulation of the neuroendocrine reproductive axis by kisspeptin-GPR54 signaling. Reproduction. 2006b;131:623–630. doi: 10.1530/rep.1.00368. [DOI] [PubMed] [Google Scholar]
- 86.Smith J, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005a;146:3686–3692. doi: 10.1210/en.2005-0488. [DOI] [PubMed] [Google Scholar]
- 87.Smith J, Dungan HM, Stoll EA, Gottsch ML, Braun RE, Eacker SM, Clifton DK, Steiner RA. Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology. 2005b;146:2976–2984. doi: 10.1210/en.2005-0323. [DOI] [PubMed] [Google Scholar]
- 88.Thompson R, Canteras NS, Swanson LW. Organization of projections from the dorsomedial nucleus of the hypothalamus: a PHA-L study in the rat. J Comp Neurol. 1996;376:143–173. doi: 10.1002/(SICI)1096-9861(19961202)376:1<143::AID-CNE9>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 89.Thompson R, Swanson LW. Organization of inputs to the dorsomedial nucleus of the hypothalamus: a reexamination with Fluorogold and PHAL in the rat. Brain Res Brain Res Rev. 1998;27:89–118. doi: 10.1016/s0165-0173(98)00010-1. [DOI] [PubMed] [Google Scholar]
- 90.Thornton J, Cheung CC, Clifton DK, Steiner RA. Regulation of hypothalamic proopiomelanocortin mRNA by leptin in ob/ob mice. Endocrinology. 1997;138:5063–5066. doi: 10.1210/endo.138.11.5651. [DOI] [PubMed] [Google Scholar]
- 91.Titolo D, Cai F, Belsham DD. Coordinate regulation of neuropeptide Y and agouti-related peptide gene expression by estrogen depends on the ratio of estrogen receptor (ER)alpha to ERbeta in clonal hypothalamic neurons. Mol Endocrinol. 2006;20:2080–2092. doi: 10.1210/me.2006-0027. [DOI] [PubMed] [Google Scholar]
- 92.Tortoriello D, McMinn J, Chua SC. Increased expression of hypothalamic leptin receptor and adiponectin accompany resistance to dietary-induced obesity and infertility in female C57BL/6J mice. Int J Obes (Lond) 2007;31:395–402. doi: 10.1038/sj.ijo.0803392. [DOI] [PubMed] [Google Scholar]
- 93.Wang J, Leibowitz KL. Central insulin inhibits hypothalamic galanin and neuropeptide Y gene expression and peptide release in intact rats. Brain Res. 1997;777:231–236. doi: 10.1016/s0006-8993(97)00963-3. [DOI] [PubMed] [Google Scholar]
- 94.Wardlaw S, Ferin M. Interaction between beta-endorphin and alpha-melanocyte-stimulating hormone in the control of prolactin and luteinizing hormone secretion in the primate. Endocrinology. 1990;126:2035–2040. doi: 10.1210/endo-126-4-2035. [DOI] [PubMed] [Google Scholar]
- 95.Welt CK, Chan JL, Bullen J, Murphy R, Smith P, DePaoli AM, Karalis A, Mantzoros CS. Recombinant human leptin in women with hypothalamic amenorrhea. N Engl J Med. 2004;351:987–997. doi: 10.1056/NEJMoa040388. [DOI] [PubMed] [Google Scholar]
- 96.Woller M, Terasawa E. Estradiol enhances the action of neuropeptide Y on in vivo luteinizing hormone-releasing hormone release in the ovariectomized rhesus monkey. Neuroendocrinology. 1992;56:921–925. doi: 10.1159/000126325. [DOI] [PubMed] [Google Scholar]
- 97.Woods SC, Lotter EC, McKay LD, Porte D., Jr Chronic intracerebroventricular infusion of insulin reduces food intake and body weight of baboons. Nature. 1979;282:503–505. [Google Scholar]
- 98.Xu A, Kaelin CB, Takeda K, Akira S, Schwartz MW, Barsh GS. PI3K integrates the action of insulin and leptin on hypothalamic neurons. J Clin Invest. 2005;115:951–958. doi: 10.1172/JCI24301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xu M, Hill JW, Levine JE. Attenuation of luteinizing hormone surges in neuropeptide Y knockout mice. Neuroendocrinology. 2000;72:263–271. doi: 10.1159/000054595. [DOI] [PubMed] [Google Scholar]
- 100.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 101.Ziotopoulou M, Erani DM, Hileman SM, Bjorbaek C, Mantzoros CS. Unlike leptin, ciliary neurotrophic factor does not reverse the starvation-induced changes of serum corticosterone and hypothalamic neuropeptide levels but induces expression of hypothalamic inhibitors of leptin signaling. Diabetes. 2000;49:1890–1896. doi: 10.2337/diabetes.49.11.1890. [DOI] [PubMed] [Google Scholar]


