Abstract
Importance
Polycyclic aromatic hydrocarbons (PAH) are carcinogenic and neurotoxic combustion by-products commonly found in urban air. Exposure to PAH is disproportionately high in low income communities of color who also experience chronic economic stress.
Objective
In a prospective cohort study in New York City (NYC) we previously found a significant association between prenatal PAH exposure and Attention Deficit Hyperactivity Disorder (ADHD) behavior problems at age 9. Here, we have evaluated the joint effects of prenatal exposure to PAH and prenatal/childhood material hardship on ADHD behavior problems.
Materials and Methods
We enrolled nonsmoking African-American and Dominican pregnant women in New York City between 1998 and 2006 and followed their children through 9 years of age. As a biomarker of prenatal PAH exposure, PAH-DNA adducts were measured in maternal blood at delivery and were dichotomized at the limit of detection (to indicate high vs. low exposure). Maternal material hardship (lack of adequate food, housing, utilities, and clothing) was self-reported prenatally and at multiple time points through child age 9. Latent variable analysis identified four distinct patterns of hardship. ADHD behavior problems were assessed using the Conners Parent Rating Scale-Revised. Analyses adjusted for relevant covariates.
Results
Among 351 children in our sample, across all hardship groups, children with high prenatal PAH exposure (high adducts) generally had more symptoms of ADHD (higher scores) compared to those with low PAH exposure. The greatest difference was seen among the children with hardship persisting from pregnancy through childhood. Although the interactions between high PAH exposure and hardship experienced at either period (“persistent” hardship or “any” hardship) were not significant, we observed significant differences in the number of ADHD symptoms between children with high prenatal PAH exposure and either persistent hardship or any hardship compared to the others. These differences were most significant for combined high PAH and persistent hardship: ADHD Index (p<0.008), DSM-IV Inattentive (p=0.006), DSM-IV Hyperactive Impulsive problems (p=0.033), and DSM-IV Index Total (p=0.009).
Conclusion
The present findings add to existing evidence that co-exposure to socioeconomic disadvantage and air pollution in early life significantly increases the risk of adverse neurodevelopmental outcomes. They suggest the need for multifaceted interventions to protect pregnant mothers and their children.
Keywords: air pollution, PAH, ADHD, prenatal, adducts, material hardship
1: Introduction
Approximately 11% of children 4–17 years of age in the United States (U.S.) have been diagnosed with Attention-deficit/hyperactivity disorder (ADHD), making it one of the most commonly diagnosed behavioral disorders in children (Visser et al., 2014) ADHD symptoms of hyperactivity, impulsivity and inattention often persist and may lead to poor performance in the academic and occupational settings throughout the adult years (Biederman and Faraone 2005). The etiology of ADHD is complex; genetic, environmental and social factors all contribute to the emergence and severity of the disease (Biederman and Faraone 2005). Known or suggested risk factors include obstetrical complications, maternal smoking, alcohol use, lead exposure, polycyclic aromatic hydrocarbon (PAH) exposure, low socioeconomic status, and psychosocial adversity (Faraone et al. 2005; Grizenko et al. 2012; Sagiv et al. 2013).
As reviewed previously, exposure to PAH is prevalent in urban populations from combustion of fossil fuels and other organic material (Vishnevetsky et al. 2015). The sources of PAH include combustion of diesel, gasoline, coal, residential heating oil; tobacco smoking and chargrilling or broiling of foods (Bostrom et al. 2002; Larsen and Baker 2003). Exposures to ambient and indoor air pollutants tend to be disproportionately high in lower income communities or communities consisting largely of racial or ethnic minorities (Hou et al. 2012; Jerrett 2009; Mohai et al. 2009; Morello-Frosch et al. 2011; Woodruff et al. 2003), as is experience of material hardship, an indicator of economic stress. Racial disparities in self-rated health persist even after differences in socioeconomic status are controlled for (Morello-Frosch et al. 2011). Residents are also more likely to live in low quality housing and have inadequate educational and nutritional resources compared to higher income communities (Vishnevetsky et al. 2015). Socioeconomic and psychological stress experienced by the mother during pregnancy and/or stress in the early childhood years have been associated with child ADHD (Grizenko et al. 2012; Linnet et al. 2003; Russell et al. 2015). Socioeconomic stressors have been shown to exacerbate the neurodevelopmental impacts of toxic environmental exposures (Bellinger et al. 1987; Bellinger et al. 1989; Bellinger 2000; Darmon and Drewnowski 2008; Evans and Kantrowitz 2002; Lansdown et al. 1986; Vishnevetsky et al. 2015).
We previously showed that prenatal PAH was significantly associated with symptoms of ADHD (Hou et al. 2012). Here we tested the hypothesis that the observed effect of prenatal PAH exposure on ADHD behavior problems is increased in the presence of material hardship experienced by the mother during pregnancy and the child’s early years. Concern about prenatal exposures to PAH arises from their ready transfer across the placenta and the fetal blood brain barrier reviewed in (Brown et al. 2007; Hood et al. 2000). Moreover, the fetus is particularly susceptible to chemical insults due to the rapid, dynamic and complex brain development taking place during this period, as well as their inability to efficiently detoxify and clear chemicals and repair DNA damage (Grandjean and Landrigan 2006; Perera et al. 2004). Prenatal exposure to PAH can be measured by PAH-DNA adducts in maternal blood or cord blood donated at delivery. Since adducts reflect not only exposure but also absorption, metabolic activation, and DNA repair, they are considered an individual biologic dosimeter of PAH. Moreover, PAH-DNA adducts in white blood cells have the advantage of providing an integrated measure of exposure over the past 3–4 months (Mooney et al. 1995). This biomarker has previously been associated with multiple adverse neurodevelopmental outcomes in children (Hou et al. 2012; Perera et al. 2008; Perera et al. 2011; Perera et al. 2012).
Material hardship assesses an individual’s unmet basic needs in the areas of food, housing, and clothing (Mayer and Jencks 1989). Because prior studies have reported adverse effects of economic disadvantage and stress experienced both during pregnancy and childhood (Bolton et al. 2013; Schoon et al. 2012), as in our prior paper on child IQ, we evaluated material hardship experienced during both of these developmental periods. We addressed the following questions: Do PAH exposure and material hardship interact to influence ADHD symptoms? Does the co-occurrence of high prenatal PAH exposure and the pattern (trajectory) of prenatal and postnatal hardship though childhood impact ADHD scores? Is there a pattern of hardship that is worse in combination with high prenatal PAH exposure?
2: Methods
2.1: The Columbia Center for Children’s Environmental Health (CCCEH) cohort study
For a more detailed description of the CCCEH cohort and study design, see our prior report (Perera et al. 2006). Briefly, between 1998 and 2006 we recruited African-American and Dominican women who resided in Washington Heights, Harlem, or the South Bronx in New York City (NYC) through the local prenatal care clinics. Enrollment was restricted to women who were nonsmokers, between 18–35 years, non-users of other tobacco products or illicit drugs, free of diabetes, hypertension, or known HIV, and who had initiated prenatal care by the 20th week of pregnancy. The Institutional Review Board of Columbia University approved the study. The mothers provided informed consent for themselves and their younger children; beginning at age 7 children provided assent.
2.2: Personal interviews, home caretaking environment, maternal intelligence, maternal ADHD, child anxiety/depression at age 9
2.2.1: Prenatal interview
A trained bilingual interviewer administered a 45-minute questionnaire during the last trimester of pregnancy to elicit demographic information, residential history, health and environmental data such as active smoking (to confirm nonsmoking status as reported on the screening questionnaire) and exposure to environmental tobacco smoke (ETS). In the cohort, the mean cotinine measured in cord blood was significantly higher in newborns whose mothers reported ETS exposure during pregnancy (t-value=-3.08, p-value=-0.002). Additionally, information was collected on dietary PAH (specifically, consumption of broiled, fried, grilled or smoked meat), income and education.
2.2.2: Postnatal interviews and assessments
Postnatal interviews were administered in-person at 6 months and annually thereafter to determine changes in residence, ETS exposure, and health and environmental conditions. At child age 3, Caldwell and Bradley’s Home Observation for Measurement of the Environment (HOME) (Bradley 1994) was used to assess the quality of the proximal caretaking environment Maternal nonverbal intelligence was measured by the Test of Non-Verbal Intelligence-Third Edition (TONI-3) administered during the infant’s 6 month visit or a subsequent visit using. The TONI-3 is a 15-minute, language-free measure of general intelligence, that is relatively stable and free of cultural bias (Brown et al. 1997). To address the high heritability rate of ADHD (Todd et al. 2001), mothers completed the Conners Adult ADHD Rating Scales (CAARS) (Conners et al. 1999) at the child’s 7 year visit; and maternal ADHD symptoms were included as a covariate in our analyses. Because childhood ADHD and anxiety/depression are frequently comorbid conditions (Spencer et al. 2007), in order to isolate the impact of PAH and material hardship on ADHD symptoms, the continuous score for symptoms of anxiety/depression reported by the mother on the Child Behavior Checklist (CBCL) at child age 9 was also included as a covariate (Achenbach and Rescorla 2001).
2.3: Material hardship
A widely used measure of material hardship (Mayer and Jencks 1989) was used to assess the level of unmet basic needs in the areas of food, housing, and clothing. This was obtained prenatally and at child age 6 months, and at 1, 2, 3, 5, 7 and 9 years by asking “In the past year has there been a time when you: 1. Couldn’t afford to buy food?; 2. Couldn’t afford a place to stay?; 3.couldn’t afford gas/electricity?; or 4. Couldn’t afford clothing?” A positive response to each question was given a score of one point and a negative response was coded as zero. For each time point (6 months, 1, 2, 3, 5, 7 and 9 years), a scored sum of material hardships was created (range: 0–4 points).
2.4 Biomarker measurement for PAH
At the time of delivery, research staff collected umbilical cord blood and maternal blood and transported the bio-specimens to the CCCEH Molecular Epidemiology Laboratory within several hours of collection. The buffy coat, packed red blood cells, and plasma were separated and stored at –70°C. DNA adducts of the representative PAH, benzo[a]pyrene (B[a]P), were analyzed in extracted white blood cell DNA using a high performance chromatography (HPLC)/fluorescence method which detects B[a]P tetrols (Alexandrov et al. 1992; Perera et al. 2004). The adducts were dichotomized as detectable (“high adducts”) vs. non-detectable (“low adducts”) because 59% of maternal blood DNA samples had levels below the limit of detection (0.25 adducts per 108 adducts). Some children lacked data on maternal DNA adducts due to inadequate quantity or quality of DNA (n=144).
PAH metabolites were measured in spot urine samples collected at child age 3 and 5 at the Centers for Disease Control and Prevention (CDC) using automated liquid-liquid extraction and gas chromatography/isotope dilution high-resolution mass spectrometry, as previously described (Li et al. 2006; Li et al. 2008; Miller et al. 2010). PAH metabolites are a short-term biomarker (half-life of 6–35 hours) (Jongeneelen et al. 1990); however, in conditions of chronic exposure they are considered a useful measure of exposure to PAH from all exposure sources and pathways (Li et al. 2006; Li et al. 2008). To adjust for urinary dilution of the samples, metabolite levels were adjusted for specific gravity (SG) using the formula: freshweight metabolites for the subject*(mean SG-1)/(SG for that subject-1) (Hauser et al. 2004). The 5 year measures were used as the default, with 3 year values substituted when 5 year data were not available.
2.5 Outcomes
At child age 9 years, mothers completed the CPRS-Revised: Long Version (Conners 1997). The CPRS is a focused assessment of problems associated with childhood ADHD and its common comorbid disorders (Conners 1997; Conners et al. 1998). The instrument has been used in studies of a number of environmental contaminants including lead and PAH (Huang et al. 2016; Perera et al. 2014). The instrument provides scales derived from the DSM-IV (2000) that are intended to screen for ADHD-behavior problems and indicate those children requiring follow-up. Mothers completed the 80-item CPRS (Conners 1997) when their children were 9 years old, under the guidance of trained research workers. Outcomes analyzed included the CPRS ADHD Index and DSM-IV subscales (denoted as “Total”, “Inattentive”, and “Hyperactive-Impulsive”), with the “Total” DSM-IV measure comprising the “Inattentive” and “Hyperactive-Impulsive” subscales. The responses were scored and summed to a raw score that was treated continuously. In addition, T-scores were derived from the raw scores based on the normative comparison sample, as described in the administration manual, and used to determine the child’s classification (Conners 1997). The CPRS DSM-IV subscales and ADHD Index scores were dichotomized based on the classification of a T score > 65 as “moderately to markedly atypical” and a T score < 65 as “in the normal range”(Conners 1997).
2.6: Statistical analysis
As shown in Figure 1, a total of 581 children had available data on maternal PAH-DNA adducts. Of these, 362 had available data on maternal ADHD and child anxiety/depression at age 9; 351 of these children also had complete data on the Conners; and all 351 had data on material hardship from at least four of 8 possible time points. The covariates in the present analysis included child’s sex, ethnicity, maternal self-report of ETS exposure during pregnancy, maternal education (<high school, >high school), gestational age, maternal intelligence (treated as a continuous variable), maternal ADHD (treated as a continuous variable), quality of the early home caretaking environment assessed at child age 3 (treated as a continuous variable), child anxiety and depression at age 9 using the CBCL (treated as a continuous variable), child’s exact age at assessment (in months) and season (heating vs. non-heating) of the third trimester. We conducted multiple imputation for missing data on all covariates except for maternal ADHD and child anxiety and depression at age 9. In no case were data missing for more than 5% of children. For each continuous variable that had missing values, we randomly drew a value from a normal distribution with mean and variance estimated from the available data for that variable. Similarly, for each binary variable, we randomly drew a value from a binomial distribution with probability of event estimated from the available data for that variable. We repeated the procedure 10 times and obtained 10 imputed datasets. We then computed 10 different sets of point and variance estimates for the parameter of interest in our model, and used Rubin’s method to combine these results and generated valid inference about this parameter (Rubin et al,. 2004).
Figure 1.
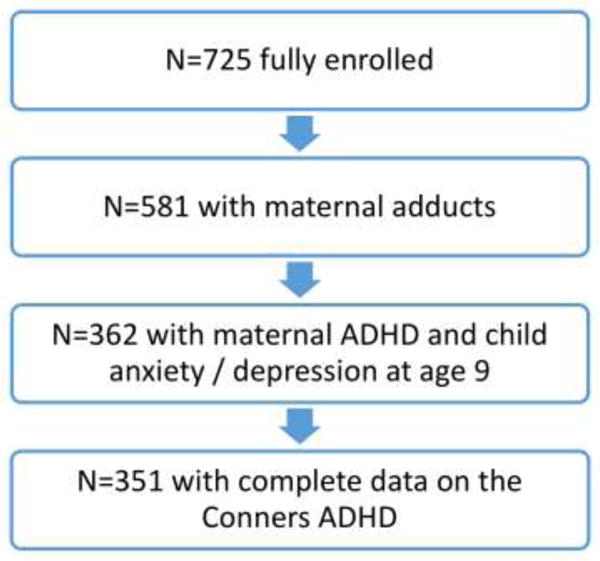
Selection of the sample analyzed
We used group-based trajectory analysis (Jones et al. 2001) with a Poisson distribution to identify groups of participants with shared experiences of material hardship between the prenatal period and 9 year follow-up visit. We fit models on all 351 children. We varied the number of groups from 1–5 and used Bayesian information criterion (BIC) values to evaluate fit (see Supplemental Table S1). The best fitting model revealed four unique trajectories of hardship over time as shown in Figure 2: latent class 4 or ‘persistent’ (i.e., constant prenatal and postnatal hardship, 22.3%); latent class 3 (increasing over time, 20.0%); latent class 2 (decreasing over time,15.3%); and latent class 1 (never/infrequent, 42.4%). The number of latent classes was chosen based on the BIC (Supplemental Table S1).
Figure 2.
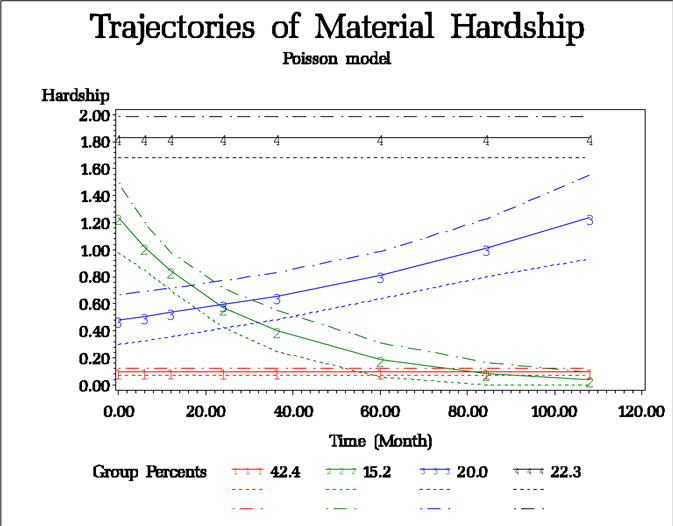
Trajectory analysis of material hardship
The associations between the dichotomized (“high”/“low”) PAH exposure (maternal PAH-DNA adducts) and/or material hardship (group/trajectory) and the continuous ADHD-related behavior scores or the classification of moderately or markedly atypical symptoms were analyzed using generalized linear models (with a scale parameter to fit for over-dispersed Poisson distributions) and binomial distributions for binary outcomes, respectively, including covariates described above. As per our a priori hypothesis, we assessed the potential interaction between PAH exposure and the four material hardship groups; that is, whether the effects of high vs. low prenatal PAH exposure on ADHD-related behavior outcomes differed by the four hardship trajectory classes, by including their product terms in the regression models. After examining the magnitude of the effects of prenatal PAH exposure on ADHD-related behavior scores within each of the four hardship trajectory classes based on this interaction model, we created two binary hardship groups by combining trajectories with similar prenatal PAH effects on ADHD. The resultant groups were: a) “persistent” hardship (hardship trajectory class 4) vs. all others (hardship trajectory classes 3, 2, and 1); and b) “any” hardship (hardship trajectory classes 4, 3, and 2) vs. never/infrequent hardship (hardship trajectory class 1). We then assessed the interaction between PAH exposure and each binary hardship variable. We further examined the effects of combined high PAH exposure and ‘persistent’ hardship as well as the effects of combined high PAH exposure and “any” hardship on ADHD symptoms by introducing a new categorical variable with 1 as having both high PAH exposure and persistent hardship and 0 otherwise, and a new categorical variable with 1 as having both high PAH exposure and any hardship and 0 otherwise.
In sensitivity analyses, we examined whether the inclusion of postnatal PAH exposure, represented by the sum of 9 urinary metabolites at age 5 or 3 years, substantially altered the association of adducts and hardship with ADHD outcomes. As in our prior analysis with age 7 IQ as the outcome, results did not differ before and after adjusting for postnatal PAH exposure; therefore this variable was not included in our final model. Further, in post-hoc sensitivity analyses, we applied the inverse probability weighting (IPW) technique (Curtis et al. 2007; Hernan et al. 2004; Robins et al. 2000) in order to account for potential bias due to loss to follow-up.
3: Results
Socio-demographic and exposure characteristics of the sample included in the analysis are presented in Table 1. Among the children with maternal adduct data, there were no significant differences between those who were included in the analysis and those not included due to lack of data on material hardship, required covariates, or ADHD (n=230), except that the mean for child anxiety and depression at age 9 was significantly higher in the sample analyzed (p=0.04). In the sample analyzed, the association between high prenatal material hardship and high cord adducts was not significant (X2=0.29, p=0.59). Supplemental Table S2 provides the descriptive statistics of the maternal adduct levels in each latent class of material hardship. Table S3 presents the ADHD scores and the number of children in the “moderately to markedly atypical” range and the number in the “normal” range, with respect to each ADHD outcome.
Table 1.
Characteristics of children included in the analysis (N=351)
| Variable1 | Subjects included in the analysis (N=351) |
|---|---|
| Gestational age at birth (weeks) | 39.34 ± 1.32 |
| Maternal TONI score | 20.28 ± 8.63 |
| HOME inventory | 39.74 ± 5.91 |
| Maternal ADHD | 38.31 ± 8.39 |
| Child anxiety/depression at age 9 | 2.65 ± 2.99 |
| Age at ADHD assessment (months) | 108.15 ± 2.29 |
| Percent female | 53.56 |
| Percent African American | 39.32 |
| Percent with prenatal ETS exposure | 34.1 |
| Percent >= high school education | 62.64 |
| Percent born in heating season | 57.56 |
Units are means+SD unless otherwise noted.
Among the 351 children in our sample, the interactions between PAH exposure (“high” vs. “low”) and material hardship latent classes (4 groups) on ADHD symptom scores were not statistically significant. However, across all four hardship groups, children with high prenatal PAH exposure (high adducts) generally had more symptoms of ADHD (higher scores) compared to those with low PAH exposure (Figure 3). The greatest difference between scores of children who had high vs. low PAH exposure was seen within the “persistent” hardship group (latent class 4) (Figure 3 and Supplemental Table S4). Although the interactions between PAH exposure (high vs. low) and each binary hardship variable were not statistically significant, they suggested that i) the effects of having both high PAH exposure and “persistent” hardship or ii) having both high PAH exposure and “any” hardship may be greater than the sum of their individual effects (Supplemental Figures S1 and S4, and Supplemental Table S5). Further analyses assessing the combined effects of a) having high PAH exposure and “persistent” hardship vs. everything else (Table 2) or b) having high PAH exposure and “any” hardship vs. everything else (Table 3) showed significant combined effects on multiple ADHD scores (more symptoms) and on a child’s classification of having moderately to markedly atypical scores. These associations were strongest for combined high PAH and “persistent” hardship: ADHD Index (p=0.008), DSM-IV Inattentive (p=0.006), DSM-IV Hyperactive-Impulsive problems (p=0.033), and DSM-IV Index Total (p=0.009). Increases in odds of having moderately to markedly atypical scores in the PAH/“persistent” hardship group were significant for ADHD Index (p=0.030), DSM-IV Inattentive (p=0.003) and DSM-IV Index Total (p=0.009). For combined high PAH and any” hardship, the corresponding p-values were: ADHD Index (p=0.037), DSM-IV Inattentive (p=0.047), DSM-IV Hyperactive-Impulsive problems (p=0.026), and DSM-IV Index Total (p=0.024). Increases in odds of having moderately to markedly atypical scores in the PAH/“persistent” hardship group were significant for DSM-IV Inattentive (p=0.046) and DSM-IV Index Total (p=0.024). Each of the exposures (adducts and material hardship) individually was associated with significant adverse impacts on ADHD scores (Supplemental Table S6 and S7). While the interaction term was not statistically significant, the effect of having both high maternal adducts and persistent maternal hardship on a number of outcomes appears to be greater than the sum of their separate effects, suggesting that the effect is greater than additive (as shown in Supplemental Figure S1). The measure of postnatal PAH exposure (child urinary metabolites) was not significantly associated with prenatal PAH (X2=1.16, p=0.28) or with any of the ADHD continuous scores. After adjustment for postnatal PAH exposure in the smaller sample with available child PAH metabolite data (N=330), the effects of prenatal exposure were similar to those without adjustment (Supplemental Tables S68 and S9). Repeating the analysis of combined high exposure (high adduct and “persistent” material hardship vs. other) and ADHD with IPW, we found that the direction and magnitude of associations were similar albeit less significant (see Supplemental Table S10). This result indicates that there is some selection bias; however, the inference remains the same.
Figure 3.
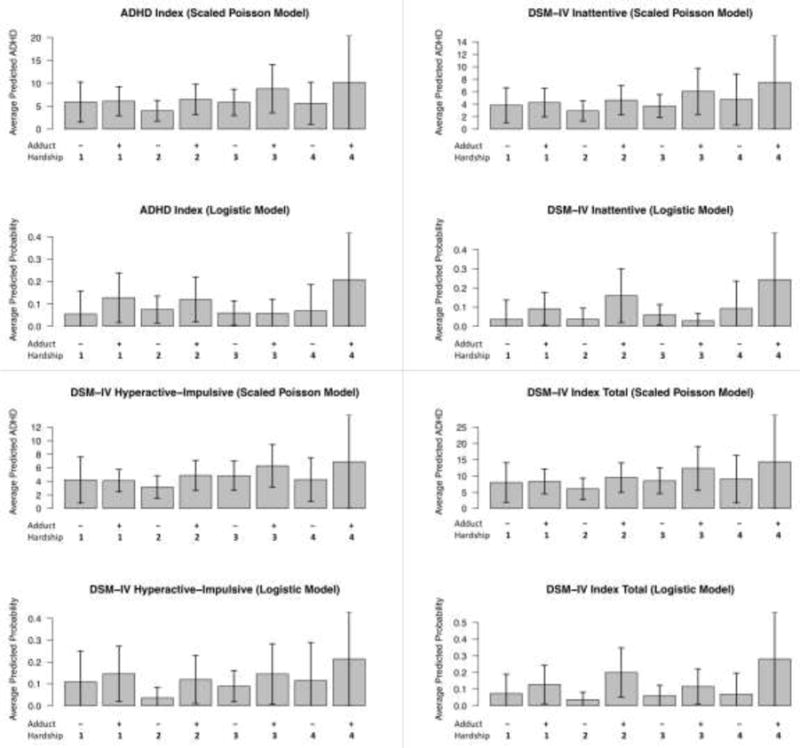
Averaged predicted ADHD scores in the low and high PAH (maternal adduct) groups stratified by material hardship latent classes
Table 2.
| ADHD symptoms | N | Estimate | Lower CL | Upper CL | P-value |
|---|---|---|---|---|---|
| ADHD analyzed continuously3 | |||||
| ADHD Index | 351 | 0.42 | 0.11 | 0.74 | 0.01 |
| DSM-IV Inattentive | 351 | 0.46 | 0.13 | 0.79 | 0.01 |
| DSM-IV Hyperactive-Impulsive | 351 | 0.33 | 0.03 | 0.62 | 0.03 |
| DSM-IV Index Total | 351 | 0.39 | 0.10 | 0.68 | 0.01 |
| ADHD analyzed dichotomously4 | |||||
| ADHD Index | 351 | 1.23 | 0.12 | 2.35 | 0.03 |
| DSM-IV Inattentive | 351 | 1.77 | 0.62 | 2.93 | <0.01 |
| DSM-IV Hyperactive-Impulsive | 351 | 0.50 | −0.61 | 1.61 | 0.38 |
| DSM-IV Index Total | 351 | 1.40 | 0.35 | 2.46 | 0.01 |
Covariates: gender, ethnicity, prenatal ETS, maternal education, gestational age, TONI, HOME, age at assessment, maternal ADHD, child anxiety/depression at age 9, heating season; analyses compared the children with combined high exposure to those who did not experience combined high exposure.
Twenty eight children were in the high PAH/persistent hardship group, 113 in the high PAH/non-persistent hardship group, 43 in the low PAH/persistent hardship group, and 167 in the low PAH/non-persistent hardship group.
ADHD analyzed continuously using scaled Poisson regression on ADHD raw scores
ADHD analyzed dichotomously using logistic regression on ADHD T-score (>65 v.s. <=65)
Table 3.
Association between combined exposure (high PAH and any hardship) and Conners ADHD1
| ADHD symptoms | N | Estimate | Lower CL | Upper CL | P-value |
|---|---|---|---|---|---|
| ADHD analyzed continuously2 | |||||
| ADHD Index | 351 | 0.25 | 0.02 | 0.48 | 0.04 |
| DSM-IV Inattentive | 351 | 0.25 | 0.00 | 0.49 | 0.047 |
| DSM-IV Hyperactive-Impulsive | 351 | 0.24 | 0.03 | 0.46 | 0.03 |
| DSM-IV Index Total | 351 | 0.25 | 0.03 | 0.46 | 0.02 |
| ADHD analyzed dichotomously3 | |||||
| ADHD Index | 351 | 0.40 | −0.47 | 1.28 | 0.37 |
| DSM-IV Inattentive | 351 | 0.93 | 0.01 | 1.85 | 0.046 |
| DSM-IV Hyperactive-Impulsive | 351 | 0.02 | −0.80 | 0.83 | 0.96 |
| DSM-IV Index Total | 351 | 0.92 | 0.12 | 1.72 | 0.02 |
Covariates: gender, ethnicity, prenatal ETS, maternal education, gestational age, TONI, HOME, age at assessment, maternal ADHD, child anxiety/depression at age 9, heating season; analyses compared the children with combined high exposure to those who did not experience combined high exposure.
ADHD analyzed continuously using scaled Poisson regression on ADHD raw scores
ADHD analyzed dichotomously using logistic regression on ADHD T-score (>65 v.s. <=65)
The results using the prenatally monitored concentration of PAH in air, rather than the biomarker of exposure, as the independent variable were generally nonsignificant, with the exception of the association of combined air PAH and persistent hardship with the DSM-IV Inattentive outcome (p=0.018). This finding is consistent with our previous report (Hou et al. 2012).
Finally, we note that the various measures of material hardship defined in the study were inversely associated with the quality of the home caretaking environment (HOME) and maternal IQ; and the binary hardship measure (any hardship vs. no hardship) was positively correlated with maternal ADHD.
4: Discussion
This is the first report of the effects of co-exposure to high prenatal exposure to PAH and chronic socioeconomic stress on children’s ADHD symptoms. The findings are consistent with our prior report on PAH, material hardship, and child IQ at age 7 (Vishnevetsky et al. 2015) and with studies showing modification of neurodevelopmental effects of lead by social class (Bellinger 2000). Our failure to observe statistically significant interactions between the two “stressors” may be due in part to the limited sample size. However, the findings indicate that high prenatal PAH exposure combined with persistent hardship or high prenatal PAH combined with any hardship significantly increased ADHD symptoms in children at age 9.
As noted earlier, PAH are a class of hazardous air pollutants, PAH include known carcinogens and neurotoxicants such as B[a]P which is considered a representative PAH and is highly correlated with most other members of the class (Perera et al. 2012). PAH are ubiquitous in the urban environment, however low-income communities tend to be disproportionately exposed due to the more frequent siting in those communities of highly trafficked roadways, depots for buses and trucks, fossil fuel generating power plants and industrial boilers; moreover there is a higher prevalence of smokers in low-income households (Chuang et al. 1999; Evans and Kantrowitz 2002).
Many studies have demonstrated the vulnerability of the developing fetus and young child to the toxic effects of environmental pollutants, including PAH, (Grandjean and Landrigan 2006; Perera et al. 2004) as well as to socioeconomic disadvantage (Laplante et al. 2008; Sandman et al. 2012; Singer et al. 1997) (for review see(Matthews and Gallo 2011)). A number of mechanisms have been described by which prenatal PAH exposure is able to exert harmful effects on the developing brain; and they are not necessarily mutually exclusive. As previously reviewed, they include binding to receptors for placental growth factors resulting in decreased exchange of oxygen and nutrients (Dejmek et al. 2000) and binding to the human Ah receptor to induce P450 enzymes (Manchester et al. 1987). PAH have also been shown to generate oxidative stress as a result of inhibition of the brain antioxidant scavenging system (Saunders et al. 2006). Genotoxic and epigenetic pathways are implicated as well: DNA damage from PAH can result in activation of apoptotic pathways (Meyn 1995; Nicol et al. 1995; Wood and Youle 1995) while epigenetic alterations (DNA methylation changes) by PAH may affect gene expression (Perera and Herbstman 2011; Wilson and Jones 1983) and alter expression of nuclear transcription factors that mediate the onset of neuronal cell differentiation (Hood et al. 2000).
Economic deprivation and related stress early in life are known risk factors for behavioral problems in children (Duncan et al. 1994). The observation of combined effects of exposure to PAH and material hardship is consistent with reports that the co-occurrence of environmental pollutants such as lead, traffic-related pollutants or ETS with adverse social conditions leads to more neurotoxic effects than either individually (Bellinger 2000; Clougherty et al. 2007; Dietrich et al. 1991; Rauh et al. 2004; Winneke and Kraemer 1984) (see (McEwen and Tucker 2011) for review). Mechanisms underlying these impacts are being elucidated; however, chronic psychosocial stress increases allostatic load, “interfering with the normal functioning of protective toxicokinetic and toxicodynamic processes” with resultant inflammation that can impair an individual’s resilience in the face of toxic insults (McEwen 1998; McEwen and Gianaros 2011; McEwen and Tucker 2011). Physical toxicants can also stimulate the production of inflammatory mediators (Bierhaus et al. 2003; Saxon and Diaz-Sanchez 2000). Thus both psychosocial and physical “toxicants” could potentiate each other through the common physiological pathways of inflammation (Bierhaus et al. 2003; Bierhaus et al. 2006; McEwen 2007; McEwen and Tucker 2011). Research in mice on the combined effect of maternal stress during pregnancy and prenatal air pollution showed that these stressors interact synergistically to induce neuroinflammation, leading to neurobehavioral disorders in the offspring (Bolton et al. 2013). In our analysis, prenatal PAH exposure elicited the strongest effect when material hardship persisted throughout early childhood. It is possible that the two factors act through the same pathway both when experienced concurrently and when hardship continues on to add to the earlier insult. Socioeconomic disadvantage, conceptualized as reported difficulty in affording basic necessities (e.g., food, shelter, clothing, utilities) may have both direct effects via psychosocial stress as outlined above or indirect impacts on a child’s risk of ADHD such as through lower levels of parent involvement (Russell et al. 2015).
Relevant to the interpretation of our results, it may be that PAH are equally toxic under different conditions of hardship, but that families without material hardship have resources not measured in this study or have better diets that positively affect the development of children and buffer the adverse impact of PAH exposure (Gershoff et al. 2007) (Ashiabi and O’Neal 2007; George et al. 2005). Families without material hardship may also have lower exposure to other chemical neurotoxicants such as lead (Brody et al. 1994; Lanphear et al. 2005), ETS (Rauh et al. 2004) and other air pollutants (Calderon-Garciduenas et al. 2008; Suglia et al. 2008; Wang et al. 2009) that contribute to the increased ADHD symptoms seen in the children in the present study.
We acknowledge a number of limitations. Although we have adjusted for the possible confounding effects of a number of covariates, there is always the possibility that some residual confounding remains. In addition, to decrease the possibility of confounding, we excluded active smokers, illicit drug users, and women with preexisting disease, thereby limiting generalizability. Our measure of PAH exposure (adducts in blood) does not allow us to distinguish between inhaled versus ingested PAH; however, in our cohort our measure of dietary PAH was not significantly associated with PAH-DNA adducts. Further, our overall limited sample size and small numbers of subjects in certain subgroups of PAH and hardship did not allow sufficient power for definitive analyses of interactions between the two stressors. Finally, our adjustment of heritability was based on the maternal ADHD; paternal data were not available. Among the strengths of the study are the longitudinal design and the ability to account for a number of factors other than PAH exposure known to affect child neurobehavioral development.
There is growing recognition of the continuing need to understand the combined effects of adverse social conditions/psychosocial stressors and environmental toxicants and to elucidate the mechanisms involved in order to guide effective intervention (Hernandez 2006; McEwen and Tucker 2011). The present results suggest that, although intervention on one of these exposures might reduce the risk of ADHD problems, additional benefit to the developing fetus and young child would come from a multifaceted approach to reduce PAH exposure and alleviate material hardship. Such an approach would combine economic assistance for women in need of material support with policy interventions to reduce air pollution exposure in urban areas, especially in low-income communities of color. Concentrations of PAH can be reduced using currently available pollution controls, greater energy efficiency, alternative energy sources, and regulatory intervention to control or remove highly polluting sources.
5. Conclusion
This study in a low income community of color provides the first evidence that material hardship combines with prenatal exposure to environmental PAH to increase child ADHD behavior problems. These results suggest the need for a multifaceted approach to prevention that incorporates more social support for this vulnerable population and greater environmental protection.
Supplementary Material
Highlights.
PAH are common carcinogenic and neurotoxic combustion-related air pollutants.
Exposure to PAH tends to be disproportionately high in low income communities.
Prenatal PAH exposure was measured by PAH-DNA adducts in maternal blood.
We evaluated the combined effects of prenatal PAH and material hardship on ADHD.
We observed significant effects of combined exposures on ADHD behavior problems.
Acknowledgments
PAH metabolites were measured by Andreas Sjodin, PhD and colleagues at the Centers for Disease Control and Prevention (CDC), National Center for Environmental Health, Division of Laboratory Sciences, 4770 Buford Highway, Atlanta, Georgia 30341, United States.
Funding: Support from the National Institute of Environmental Health Sciences (5P01ES09600, 5R01ES08977) the US EPA RD-83450901, RD832096), the John and Wendy Neu Family and the Blanchette Hooker Rockefeller Foundations, and the National Center for Advancing Translational Sciences (UL1TR000040).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions:
Conception and design of the work, acquisition of data, or analysis and interpretation of data: FPP, DT, YW, AM, GB, WC, RLM, VR, SW, JH. Drafted the article or revised it critically for important intellectual content: FPP, KW, JH. Final approval of the version to be published: FPP, DT, KW, YW, AM, RLM, VR, SW, JH
Conflicts of Interest: None
References
- Achenbach T, Rescorla L. Child behavior checklist for age 6–18. 6-1-01 Burlington VT: ASEBA; 2001. [Google Scholar]
- Alexandrov K, Rojas M, Geneste O, Castegnaro M, Camus AM, Pesruzzelli S, et al. An improved fluorometric assay for dosimetry of benzo(a)pyrene diol-epoxide-DNA adducts in smokers’ lung: Comparisons with total bulky adducts and aryl hydrocarbon hydroxylase activity. Cancer Res. 1992;52:6248–6253. [PubMed] [Google Scholar]
- American Psychiatric Association., American Psychiatric Association. Task Force on DSM-IV. Diagnostic and statistical manual of mental disorders: Dsm-iv-tr. 4th. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Ashiabi GS, O’Neal KK. Children’s health status: Examining the associations among income poverty, material hardship, and parental factors. Plos One. 2007;2:e940. doi: 10.1371/journal.pone.0000940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger D, Leviton A, Waternaux C, Needleman H, Rabinowitz M. Longitudinal analyses of prenatal and postnatal lead exposure and early cognitive development. New Engl J Med. 1987;316:1037–1043. doi: 10.1056/NEJM198704233161701. [DOI] [PubMed] [Google Scholar]
- Bellinger D, Leviton A, Waternaux C. Lead iq and social class. Int J Epidemiol. 1989;18:180–185. doi: 10.1093/ije/18.1.180. [DOI] [PubMed] [Google Scholar]
- Bellinger DC. Effect modification in epidemiologic studies of low-level neurotoxicant exposures and health outcomes. Neurotoxicology and teratology. 2000;22:133–140. doi: 10.1016/s0892-0362(99)00053-7. Review. [DOI] [PubMed] [Google Scholar]
- Biederman J, Faraone SV. Attention-deficit hyperactivity disorder. Lancet (London, England) 2005;366:237–248. doi: 10.1016/S0140-6736(05)66915-2. [DOI] [PubMed] [Google Scholar]
- Bierhaus A, Wolf J, Andrassy M, Rohleder N, Humpert PM, Petrov D, et al. A mechanism converting psychosocial stress into mononuclear cell activation. Proc Natl Acad Sci U S A. 2003;100:1920–1925. doi: 10.1073/pnas.0438019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierhaus A, Humpert PM, Nawroth PP. Linking stress to inflammation. Anesthesiol Clin. 2006;24:325–340. doi: 10.1016/j.atc.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Bolton JL, Huff NC, Smith SH, Mason SN, Foster WM, Auten RL, et al. Maternal stress and effects of prenatal air pollution on offspring mental health outcomes in mice. Environ Health Perspect; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostrom CE, Gerde P, Hanberg A, Jernstrom B, Johansson C, Kyrklund T, et al. Cancer risk assessment, indicators, and guidelines for polycyclic aromatic hydrocarbons in the ambient air. Environmental health perspectives. 2002;110(Suppl 3):451–488. doi: 10.1289/ehp.110-1241197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley RH. The home inventory: Review and reflections. Adv Child Dev Behav. 1994;25:241–288. doi: 10.1016/s0065-2407(08)60054-3. [DOI] [PubMed] [Google Scholar]
- Brody DJ, Pirkle JL, Kramer RA, Flegal KM, Matte TD, Gunter EW. Blood lead levels in the us population. Phase i of the national health and nutrition examination survey (nhanes iii, 1988 to 1991) JAMA. 1994;272:277–283. doi: 10.1001/jama.272.4.277. [DOI] [PubMed] [Google Scholar]
- Brown L, Sherbenou RJ, Johnsen SK. Test of nonverbal intelligence. Third. Bensenville, IL: Scholastic Testing Service, Inc.; 1997. [Google Scholar]
- Brown LA, Khousbouei H, Goodwin JS, Irvin-Wilson CV, Ramesh A, Sheng L, et al. Down-regulation of early ionotrophic glutamate receptor subunit developmental expression as a mechanism for observed plasticity deficits following gestational exposure to benzo(a)pyrene. Neurotoxicology. 2007;28:965–978. doi: 10.1016/j.neuro.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, Mora-Tiscareno A, Ontiveros E, Gomez-Garza G, Barragan-Mejia G, Broadway J, et al. Air pollution, cognitive deficits and brain abnormalities: A pilot study with children and dogs. Brain Cogn. 2008;68:117–127. doi: 10.1016/j.bandc.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Chuang JC, Callahan PJ, Lyu CW, Wilson NK. Polycyclic aromatic hydrocarbon exposures of children in low-income families. J Expo Anal Environ Epidemiol. 1999;9:85. doi: 10.1038/sj.jea.7500003. [DOI] [PubMed] [Google Scholar]
- Clougherty JE, Levy JI, Kubzansky LD, Ryan PB, Suglia SF, Canner MJ, et al. Synergistic effects of traffic-related air pollution and exposure to violence on urban asthma etiology. Environmental health perspectives. 2007;115:1140–1146. doi: 10.1289/ehp.9863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners C, Erhardt D, Sparrow E. Conners’ adult adhd rating scales: Technical manual. New York: Multi-Health Systems; 1999. [Google Scholar]
- Conners CK. Conners’ rating scales-revised (crs-r): User’s manual. Toronto, ON: Multi-Health Systems Inc.; 1997. [Google Scholar]
- Conners CK, Parker JDA, Sitarenios G, Epstein JN. The revised conners’ parent rating scale (cprs-r): Factor structure, reliability, and criterion validity. Journal of Abnormal Child Psychology. 1998;26:2567–2268. doi: 10.1023/a:1022602400621. [DOI] [PubMed] [Google Scholar]
- Curtis LH, Hammill BG, Eisenstein EL, Kramer JM, Anstrom KJ. Using inverse probability-weighted estimators in comparative effectiveness analyses with observational databases. Med Care. 2007;45:S103–S107. doi: 10.1097/MLR.0b013e31806518ac. [DOI] [PubMed] [Google Scholar]
- Darmon N, Drewnowski A. Does social class predict diet quality? Am J Clin Nutr. 2008;87:1107–1117. doi: 10.1093/ajcn/87.5.1107. [DOI] [PubMed] [Google Scholar]
- Dejmek J, Solansky I, Benes I, Lenicek J, Sram RJ. The impact of polycyclic aromatic hydrocarbons and fine particles on pregnancy outcome. Environ Health Perspect. 2000;108:1159–1164. doi: 10.1289/ehp.001081159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich KN, Succop PA, Berger OG, Hammond PB, Bornschein RL. Lead exposure and the cognitive development of urban preschool children: The cincinnati lead study cohort at age 4 years. Neurotoxicology and teratology. 1991;13:203–211. doi: 10.1016/0892-0362(91)90012-l. [DOI] [PubMed] [Google Scholar]
- Duncan GJ, Brooks-Gunn J, Klebanov PK. Economic deprivation and early childhood development. Child Dev. 1994;65:296–318. [PubMed] [Google Scholar]
- Evans GW, Kantrowitz E. Socioeconomic status and health: The potential role of environmental risk exposure. Annual Review of Public Health. 2002;23:303–331. doi: 10.1146/annurev.publhealth.23.112001.112349. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Perlis RH, Doyle AE, Smoller JW, Goralnick JJ, Holmgren MA, et al. Molecular genetics of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1313–1323. doi: 10.1016/j.biopsych.2004.11.024. [DOI] [PubMed] [Google Scholar]
- George GC, Hanss-Nuss H, Milani TJ, Freeland-Graves JH. Food choices of low-income women during pregnancy and postpartum. Journal of the American Dietetic Association. 2005;105:899–907. doi: 10.1016/j.jada.2005.03.028. [DOI] [PubMed] [Google Scholar]
- Gershoff ET, Aber JL, Raver CC, Lennon MC. Income is not enough: Incorporating material hardship into models of income associations with parenting and child development. Child Dev. 2007;78:70–95. doi: 10.1111/j.1467-8624.2007.00986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P, Landrigan PJ. Developmental neurotoxicity of industrial chemicals. Lancet. 2006;368:2167–2178. doi: 10.1016/S0140-6736(06)69665-7. [DOI] [PubMed] [Google Scholar]
- Grizenko N, Fortier ME, Zadorozny C, Thakur G, Schmitz N, Duval R, et al. Maternal stress during pregnancy, adhd symptomatology in children and genotype: Gene-environment interaction. J Can Acad Child Adolesc Psychiatry. 2012;21:9–15. [PMC free article] [PubMed] [Google Scholar]
- Hauser R, Meeker JD, Park S, Silva MJ, Calafat AM. Temporal variability of urinary phthalate metabolite levels in men of reproductive age. Environmental health perspectives. 2004;112:1734–1740. doi: 10.1289/ehp.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernan MA, Hernandez-Diaz S, Robins JM. A structural approach to selection bias. Epidemiology. 2004;15:615–625. doi: 10.1097/01.ede.0000135174.63482.43. [DOI] [PubMed] [Google Scholar]
- Hernandez LM. Genes, behavior, and the social environment: Moving beyond the nature/nurture debate. National Academy Press; 2006. [PubMed] [Google Scholar]
- Hood DB, Nayyar T, Ramesh A, Greenwood M, Inyang F. Modulation in the developmental expression profile of sp1 subsequent to transplacental exposure of fetal rats to desorbed benzo[a]pyrene following maternal inhalation. Inhal Toxicol. 2000;12:511–535. doi: 10.1080/089583700402897. [DOI] [PubMed] [Google Scholar]
- Hou L, Zhang X, Gawron AJ, Liu J. Surrogate tissue telomere length and cancer risk: Shorter or longer? Cancer letters. 2012;319:130–135. doi: 10.1016/j.canlet.2012.01.028. [DOI] [PubMed] [Google Scholar]
- Huang S, Hu H, Sanchez BN, Peterson KE, Ettinger AS, Lamadrid-Figueroa H, et al. Childhood blood lead levels and symptoms of attention deficit hyperactivity disorder (adhd): A cross-sectional study of mexican children. Environmental health perspectives. 2016;124:868–874. doi: 10.1289/ehp.1510067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerrett M. Global geographies of injustice in traffic-related air pollution exposure. Epidemiology. 2009;20:231–233. doi: 10.1097/EDE.0b013e31819776a1. 210.1097/EDE.1090b1013e31819776a31819771. [DOI] [PubMed] [Google Scholar]
- Jones BL, Nagin DS, Roeder K. A sas procedure based on mixture models for estimating developmental trajectories. Sociological Methods & Research. 2001;29:374–393. [Google Scholar]
- Jongeneelen FJ, van Leeuwen FE, Oosterink S, Anzion RB, van der Loop F, Bos RP, et al. Ambient and biological monitoring of cokeoven workers: Determinants of the internal dose of polycyclic aromatic hydrocarbons. Br J Ind Med. 1990;47:454–461. doi: 10.1136/oem.47.7.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanphear BP, Hornung R, Khoury J, Yolton K, Baghurst P, Bellinger DC, et al. Low-level environmental lead exposure and children’s intellectual function: An international pooled analysis. Environmental health perspectives. 2005;113:894–899. doi: 10.1289/ehp.7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansdown R, Yule W, Urbanowicz MA, Hunter J. The relationship between blood-lead concentrations, intelligence, attainment and behaviour in a school population: The second london study. International archives of occupational and environmental health. 1986;57:225–235. doi: 10.1007/BF00405790. [DOI] [PubMed] [Google Scholar]
- Laplante DP, Brunet A, Schmitz N, Ciampi A, King S. Project ice storm: Prenatal maternal stress affects cognitive and linguistic functioning in 5 1/2-year-old children. J Am Acad Child Psy. 2008;47:1063–1072. doi: 10.1097/CHI.0b013e31817eec80. [DOI] [PubMed] [Google Scholar]
- Larsen RK, Baker JE. Source apportionment of polycyclic aromatic hydrocarbons in the urban atmosphere: A comparison of three methods. Environ Sci Technol. 2003;37:1873–1881. doi: 10.1021/es0206184. [DOI] [PubMed] [Google Scholar]
- Li Z, Romanoff LC, Trinidad DA, Hussain N, Jones RS, Porter EN, et al. Measurement of urinary monohydroxy polycyclic aromatic hydrocarbons using automated liquid-liquid extraction and gas chromatography/isotope dilution high-resolution mass spectrometry. Anal Chem. 2006;78:5744–5751. doi: 10.1021/ac0606094. [DOI] [PubMed] [Google Scholar]
- Li Z, Sandau CD, Romanoff LC, Caudill SP, Sjodin A, Needham LL, et al. Concentration and profile of 22 urinary polycyclic aromatic hydrocarbon metabolites in the us population. Environmental research. 2008;107:320–331. doi: 10.1016/j.envres.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Linnet KM, Dalsgaard S, Obel C, Wisborg K, Henriksen TB, Rodriguez A, et al. Maternal lifestyle factors in pregnancy risk of attention deficit hyperactivity disorder and associated behaviors: Review of the current evidence. Am J Psychiatry. 2003;160:1028–1040. doi: 10.1176/appi.ajp.160.6.1028. [DOI] [PubMed] [Google Scholar]
- Manchester DK, Gordon SK, Golas CL, Roberts EA, Okey AB. Ah receptor in human placenta: Stabilization by molybdate and characterization of binding of 2,3,7,8-tetrachlorodibenzo-p-dioxin, 3-methylcholanthrene, and benzo(a)pyrene. Cancer Res. 1987;47:4861–4868. [PubMed] [Google Scholar]
- Matthews KA, Gallo LC. Psychological perspectives on pathways linking socioeconomic status and physical health. Annu Rev Psychol. 2011;62:501–530. doi: 10.1146/annurev.psych.031809.130711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer SE, Jencks C. Poverty and the distribution of material hardship. The Journal of Human Resources. 1989;24:88–114. [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators. New England Journal of Medicine. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Gianaros PJ. Stress- and allostasis-induced brain plasticity. Annu Rev Med. 2011;62:431–445. doi: 10.1146/annurev-med-052209-100430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Tucker P. Critical biological pathways for chronic psychosocial stress and research opportunities to advance the consideration of stress in chemical risk assessment. Am J Public Health. 2011;101(Suppl 1):S131–139. doi: 10.2105/AJPH.2011.300270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyn MS. Ataxia-telangiectasia and cellular responses to DNA damage. Cancer Res. 1995;55:5991–6001. [PubMed] [Google Scholar]
- Miller RL, Garfinkel R, Lendor C, Hoepner L, Li Z, Romanoff L, et al. Polycyclic aromatic hydrocarbon metabolite levels and pediatric allergy and asthma in an inner-city cohort. Pediatr Allergy Immunol. 2010;21:260–267. doi: 10.1111/j.1399-3038.2009.00980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohai P, Lantz PM, Morenoff J, House JS, Mero RP. Racial and socioeconomic disparities in residential proximity to polluting industrial facilities: Evidence from the americans’ changing lives study. American Journal of Public Health. 2009;99:S649–656. doi: 10.2105/AJPH.2007.131383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney LA, Santella RM, Covey L, Jeffrey AM, Bigbee W, Randall MC, et al. Decline of DNA damage and other biomarkers in peripheral blood following smoking cessation. Cancer Epidemiol Biomarkers Prev. 1995;4:627–634. [PubMed] [Google Scholar]
- Morello-Frosch R, Zuk M, Jerrett M, Shamasunder B, Kyle AD. Understanding the cumulative impacts of inequalities in environmental health: Implications for policy. Health Affairs. 2011;30:879–887. doi: 10.1377/hlthaff.2011.0153. [DOI] [PubMed] [Google Scholar]
- Nicol CJ, Harrison ML, Laposa RR, Gimelshtein IL, Wells PG. A teratologic suppressor role for p53 in benzo[a]pyrene-treated transgenic p53-deficient mice. Nat Genet. 1995;10:181–187. doi: 10.1038/ng0695-181. [DOI] [PubMed] [Google Scholar]
- Perera F, Li TY, Zhou ZJ, Yuan T, Chen YH, Qu L, et al. Benefits of reducing prenatal exposure to coal burning pollutants to children’s neurodevelopment in china. Environ Health Perspect. 2008;116:1396–1400. doi: 10.1289/ehp.11480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera F, Herbstman J. Prenatal environmental exposures, epigenetics, and disease. Reproductive Toxicology. 2011;31:363–373. doi: 10.1016/j.reprotox.2010.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Tang D, Jedrychowski W, Hemminki K, Santella RM, Cruz LA, et al. Biomarkers in maternal and newborn blood indicate heightened fetal susceptibility to procarcinogenic DNA damage. Environ Health Perspect. 2004;112:1133–1136. doi: 10.1289/ehp.6833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Rauh V, Whyatt RM, Tsai WY, Tang D, Diaz D, et al. Effect of prenatal exposure to airborne polycyclic aromatic hydrocarbons on neurodevelopment in the first 3 years of life among inner-city children. Environmental health perspectives. 2006;114:1287–1292. doi: 10.1289/ehp.9084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Wang S, Vishnevetsky J, Zhang B, Cole KJ, Tang D, et al. Pah/aromatic DNA adducts in cord blood and behavior scores in new york city children. Environmental health perspectives. 2011;119:1176–1181. doi: 10.1289/ehp.1002705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Tang D, Wang S, Vishnevetsky J, Zhang B, Diaz D, et al. Prenatal polycyclic aromatic hydrocarbon (pah) exposure and child behavior at age 6–7. Environmental health perspectives. 2012 doi: 10.1289/ehp.1104315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Chang H, Tang D, Roen EL, Herbstman J, Camann D, et al. Early-life exposure to polycyclic aromatic hydrocarbons and adhd behavior problems. 2014 doi: 10.1371/journal.pone.0111670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh VA, Whyatt RM, Garfinkel R, Andrews H, Hoepner L, Reyes A, et al. Developmental effects of exposure to environmental tobacco smoke and material hardship among inner-city children. Neurotoxicology and teratology. 2004;26:373–385. doi: 10.1016/j.ntt.2004.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11:550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: J. Wiley & Sons; 1987. [Google Scholar]
- Russell AE, Ford T, Russell G. Socioeconomic associations with adhd: Findings from a mediation analysis. Plos One. 2015;10:e0128248. doi: 10.1371/journal.pone.0128248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagiv SK, Epstein JN, Bellinger DC, Korrick SA. Pre- and postnatal risk factors for adhd in a nonclinical pediatric population. Journal of attention disorders. 2013;17:47–57. doi: 10.1177/1087054711427563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandman CA, Davis EP, Buss C, Glynn LM. Exposure to prenatal psychobiological stress exerts programming influences on the mother and her fetus. Neuroendocrinology. 2012;95:8–21. doi: 10.1159/000327017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders CR, Das SK, Ramesh A, Shockley DC, Mukherjee S. Benzo(a)pyrene-induced acute neurotoxicity in the f-344 rat: Role of oxidative stress. J Appl Toxicol. 2006;26:427–438. doi: 10.1002/jat.1157. [DOI] [PubMed] [Google Scholar]
- Saxon A, Diaz-Sanchez D. Diesel exhaust as a model xenobiotic in allergic inflammation. Immunopharmacology. 2000;48:325–327. doi: 10.1016/s0162-3109(00)00234-4. [DOI] [PubMed] [Google Scholar]
- Schoon I, Jones E, Cheng H, Maughan B. Family hardship, family instability, and cognitive development. J Epidemiol Community Health. 2012;66:716–722. doi: 10.1136/jech.2010.121228. [DOI] [PubMed] [Google Scholar]
- Singer L, Arendt R, Farkas K, Minnes S, Huang J, Yamashita T. Relationship of prenatal cocaine exposure and maternal postpartum psychological distress to child developmental outcome. Development and Psychopathology. 1997;9:473–489. doi: 10.1017/s0954579497001259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer TJ, Biederman J, Mick E. Attention-deficit/hyperactivity disorder: Diagnosis, lifespan, comorbidities, and neurobiology. Journal of pediatric psychology. 2007;32:631–642. doi: 10.1093/jpepsy/jsm005. [DOI] [PubMed] [Google Scholar]
- Suglia SF, Gryparis A, Wright RO, Schwartz J, Wright RJ. Association of black carbon with cognition among children in a prospective birth cohort study. American journal of epidemiology. 2008;167:280–286. doi: 10.1093/aje/kwm308. [DOI] [PubMed] [Google Scholar]
- Todd RD, Rasmussen ER, Neuman RJ, Reich W, Hudziak JJ, Bucholz KK, et al. Familiality and heritability of subtypes of attention deficit hyperactivity disorder in a population sample of adolescent female twins. Am J Psychiatry. 2001;158:1891–1898. doi: 10.1176/appi.ajp.158.11.1891. [DOI] [PubMed] [Google Scholar]
- Visser SN, et al. Trends in the parent-report of health care provider-diagnosed and medicated attention-deficit/hyperactivity disorder: United States, 2003–2011. J Am Acad Child Adolesc Psychiatry. 2014;53(1):34–46.e32. doi: 10.1016/j.jaac.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishnevetsky J, Tang D, Chang H-W, Roen EL, Wang Y, Rauh V, et al. Combined effects of prenatal polycyclic aromatic hydrocarbons and material hardship on child iq. Neurotoxicology and teratology. 2015;49:74–80. doi: 10.1016/j.ntt.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Zhang J, Zeng X, Zeng Y, Wang S, Chen S. Association of traffic-related air pollution with children’s neurobehavioral functions in quanzhou, china. Environmental health perspectives. 2009;117:1612–1618. doi: 10.1289/ehp.0800023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson VL, Jones PA. Inhibition of DNA methylation by chemical carcinogens in vitro. Cell. 1983;32:239–246. doi: 10.1016/0092-8674(83)90514-7. [DOI] [PubMed] [Google Scholar]
- Winneke G, Kraemer U. Neuropsychological effects of lead in children: Interactions with social background variables. Neuropsychobiology. 1984;11:195–202. doi: 10.1159/000118077. [DOI] [PubMed] [Google Scholar]
- Wood KA, Youle RJ. The role of free radicals and p53 in neuron apoptosis in vivo. J Neuroscience. 1995;15:5851–5857. doi: 10.1523/JNEUROSCI.15-08-05851.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff TJ, Parker JD, Kyle AD, Schoendorf KC. Disparities in exposure to air pollution during pregnancy. Environmental health perspectives. 2003;111:942–946. doi: 10.1289/ehp.5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


