Rhinovirus (RV) viremia was detected in 10% of RV-positive patients. Viremia was restricted to young children and was predominantly associated with RV species C. Viremia was significantly associated with an asthma history, wheezing, and chest retractions at clinical presentation.
Keywords: rhinovirus, community-acquired pneumonia, viremia
Abstract
Background
Rhinoviruses (RVs) are ubiquitous respiratory pathogens that often cause mild or subclinical infections. Molecular detection of RVs from the upper respiratory tract can be prolonged, complicating etiologic association in persons with severe lower respiratory tract infections. Little is known about RV viremia and its value as a diagnostic indicator in persons hospitalized with community-acquired pneumonia (CAP).
Methods
Sera from RV-positive children and adults hospitalized with CAP were tested for RV by real-time reverse-transcription polymerase chain reaction. Rhinovirus species and type were determined by partial genome sequencing.
Results
Overall, 57 of 570 (10%) RV-positive patients were viremic, and all were children aged <10 years (n = 57/375; 15.2%). Although RV-A was the most common RV species detected from respiratory specimens (48.8%), almost all viremias were RV-C (98.2%). Viremic patients had fewer codetected pathogens and were more likely to have chest retractions, wheezing, and a history of underlying asthma/reactive airway disease than patients without viremia.
Conclusions
More than 1 out of 7 RV-infected children aged <10 years hospitalized with CAP were viremic. In contrast with other RV species, RV-C infections were highly associated with viremia and were usually the only respiratory pathogen identified, suggesting that RV-C viremia may be an important diagnostic indicator in pediatric pneumonia.
Rhinoviruses (RVs), together with enteroviruses (EVs), are members of the Enterovirus genus, family Picornaviridae, and are further divided into 3 species (RV-A, -B, and -C) with >160 types [1]. Although RVs and EVs share a common genomic organization and both can cause acute respiratory disease, they exhibit significant differences in disease pathology. A hallmark of EV infections is their capacity for viremia and systemic infections [2], which has rarely been demonstrated for RVs using classical culture-based diagnostic methods [3, 4]. However, recent studies using sensitive molecular methods have reported detection of RV in the blood of children presenting with respiratory illness [5–7].
Although more often associated with mild upper respiratory tract infections, RVs are now recognized as a major cause of exacerbations of asthma and other chronic pulmonary diseases [8, 9] and a cause of severe lower respiratory tract infections, including pneumonia [10–13]. In the Centers for Disease Control and Prevention (CDC) Etiology of Pneumonia in the Community (EPIC) study, RV was found to be the most commonly detected pathogen in the upper respiratory tract of patients hospitalized with community-acquired pneumonia (CAP) [12, 13]. However, RVs were also detected in a high proportion of asymptomatic pediatric controls [12, 14] and were often codetected with other respiratory pathogens [12], complicating determination of their causal relationship with CAP. Thus, the causality of RV in CAP is unknown but may correlate with viremia as defined by detection of RV RNA in the blood. Nested within the EPIC study, we determined the prevalence of RV viremia in RV-infected patients hospitalized with CAP and assessed correlations between viremia and corresponding virologic, demographic, and clinical features.
METHODS
Patient and Specimens
From 1 January 2010 to 30 June 2012, children and adults admitted to 8 hospitals located in Chicago, Memphis, Nashville, and Salt Lake City were enrolled in the EPIC study [12, 13]. Enrollment criteria included evidence of CAP based on clinicians’ initial interpretation of chest radiographs obtained within 72 hours before or after hospital admission, and patients who were severely immunocompromised were excluded. Whereas previously published EPIC studies [12, 13] restricted incidence calculations to those patients whose chest radiographs were confirmed for evidence of pneumonia by independent board-certified radiologists, we included all enrolled RV-positive patients who had an acute serum specimen available. Study patients had acute serum and nasopharyngeal/oropharyngeal (NP/OP) swabs combined in viral transport media, with most (99.3%) collected within 72 hours of hospital admission; 42% of patients also had a convalescent serum specimen drawn 3−10 weeks later. Serum was separated from clotted blood, aliquoted, and stored frozen at −70°C or colder prior to testing. Written informed consent was obtained from all patients or their caregivers before enrollment. The study protocol was approved by the institutional review board at each participating institution and the CDC.
Rhinovirus Real-Time Reverse-Transcription Polymerase Chain Reaction Assay
Patients had previously been tested for multiple respiratory viral, bacterial, and fungal pathogens by molecular methods, serology, and culture at the study sites and CDC [12, 13]. For this study, we tested NP/OP specimens and corresponding acute serum specimens from RV-positive patients. Total nucleic acids were extracted from both serum and NP/OP specimens (200 µL) on a NucliSens EasyMAG (BioMerieux). The extracts were tested by a real-time reverse transcription-polymerase chain reaction (rRT-PCR) assay [15] with a modified forward primer (5’-CYALNAGCCTLNAGCGTGGY-3’) to expand RV-C type coverage and using the AgPath-ID One-Step RT-PCR Kit (Thermo Fisher Scientific). Cycle threshold (Ct) values <40 Ct were considered positive and were used as a proxy for virus load. The NP/OP and serum specimen extractions and rRT-PCR were performed on separate days to minimize potential for cross-contamination.
Rhinovirus Molecular Typing
Molecular typing of RVs detected from NP/OP specimens of patients enrolled at the 3 children’s hospitals was initially performed by the University of Utah Health Sciences Center (method available on request); RV genotypes were subsequently confirmed by the CDC for children with viremia. For adult patients, RV typing from NP/OP and serum specimens was performed at the CDC. Rhinovirus typing was based on RT-PCR and sequencing of the VP4/VP2 capsid gene region spanning nucleotide positions 626–1046 (numbering according to reference strain RV-A1, GenBank accession no. FJ445111.1). In brief, 5 µL of total nucleic acid extract was subjected to RT-PCR amplification with SuperScript III One-Step RT-PCR System with Platinum Taq DNA Polymerase (Thermo Fisher Scientific) in a final volume of 25 µL following manufacture’s protocol using external forward primer (5’-GGCCCCTGAATGYGGCTAA-3’) and reverse primer (5’-GCATCIGGYARYTTCCACCACCANCC-3’). The RT-PCR cycling conditions were 55°C for 30 minutes and 94°C for 2 minutes followed by 40 cycles of 94°C for 30 seconds, 52°C for 30 seconds, and 68°C for 50 seconds, with a final extension of 68°C for 5 minutes. One µL of the RT-PCR product was then added to a seminested reaction using internal forward primer (5’-ACTACTTTGGGTGTCCGTGTTTC-3’) and reverse primer in a final volume of 50 µL using Taq DNA Polymerase (Roche Applied Science). The PCR cycling conditions were 95°C for 2 minutes followed by 30 cycles of 95°C for 30 seconds, 55°C for 30 seconds, and 72°C for 40 seconds, with a final extension of 72°C for 5 minutes. Seminested amplicons were resolved on 1% agarose gels and purified using ExoSAP-IT (USB Corporation). Amplicon sequencing was performed in both directions using BigDye Terminator v1.1 Cycle Sequencing Kit on a 3730XL Genetic Analyzer (Thermo Fisher Scientific) using internal forward and reverse primers. Sequencher version 5.3 software (Gene Codes) was used for sequence assembly and editing. VP4/VP2 nucleotide sequences were aligned using Clustal X version 1.83 as implemented in BioEdit version 7.2.5. (Ibis Biosciences), and phylogenetic trees were constructed by the neighbor joining method using a maximum composite likelihood substitution model with pairwise deletion of gaps and missing data as implemented in MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 [16]. Rhinovirus species and provisional genotype assignments were based on phylogenetic congruence of VP4/VP2 sequences and pairwise nucleotide divergence criteria developed by McIntyre et al [1]. Sequences generated in this study were deposited in GenBank under the accession numbers MF579447–MF579503.
Statistical Analysis
Statistical analyses were performed using SAS version 9.3 (SAS Institute). Chi-square and Wilcoxon rank-sum tests were performed to assess clinical and demographic associations of children with and without RV viremia. The Mann–Whitney U test was used for pair-wise comparison of Ct values as a proxy for viral loads in respiratory specimens among RV species. A P value < .05 was considered statistically significant.
RESULTS
Study Population
Of 2638 children enrolled in the EPIC study, 723 (27.4%) had RV detected in their NP/OP specimens, among whom 416 (57.5%) had acute serum available; of these, 356 (85.6%) had radiographically confirmed pneumonia. Of 2488 enrolled adults, 209 (8.4%) had RV detected in their NP/OP specimens, among whom 154 (73.7%) had acute serum available; of these, 146 (94.8%) had radiographically confirmed pneumonia.
Rhinovirus NP/OP and Viremia-Associated Species/Genotypes
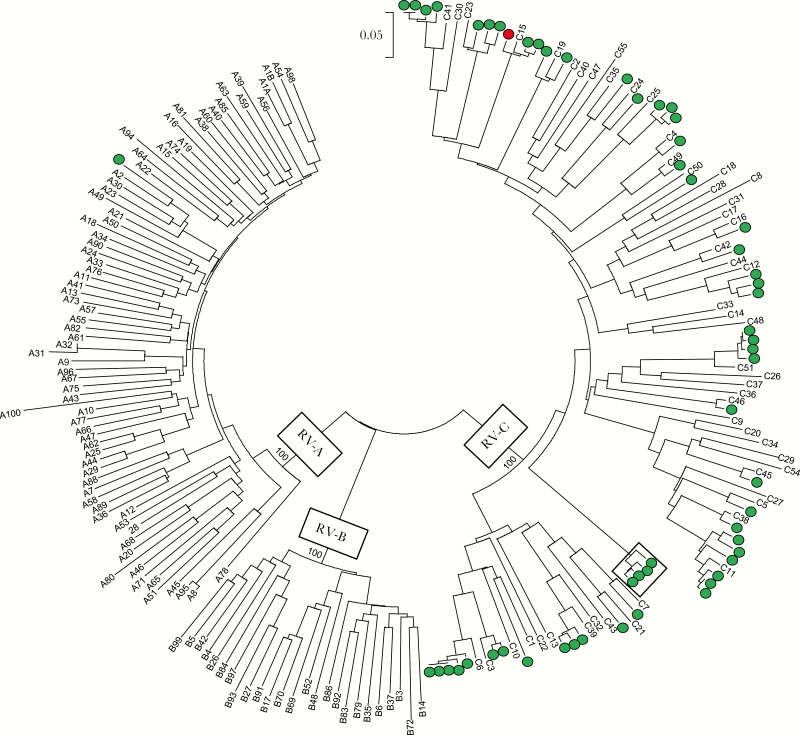
Of the 570 RV-NP/OP–positive patients from whom serum was available, RVs were all successfully typed from the NP/OP specimens. Rhinovirus A was identified in 278 (48.8%), RV-B in 39 (6.8%), and RV-C in 253 (44.4%) (Table 1). Rhinovirus viremia was detected by rRT-PCR in the acute serum of 57 (10%) of these patients. Overall, 56 of 57 (98.2%) viremias were associated with RV-C, and the remaining 1 viremia was associated with RV-A. Identical VP4/V2 sequences were obtained from both the respiratory and acute serum specimens of all viremic patients except for one 6-month-old child with RV-A identified in the respiratory specimen and RV-C in the serum. Convalescent sera collected a median of 27 days (range, 14–51 days) after the acute specimen from 29 viremic patients were all rRT-PCR negative for RV. Phylogenetic analysis of the RV-C VP4/VP2 sequences identified >25 different genotypes (Figure 1). In addition, a cluster of closely related RV-C sequences obtained from 4 patients enrolled at EPIC study sites in 3 different states diverged by >23% from other known RV-C genotypes, which exceeded the 10% threshold for new genotype assignment in the VP4/VP2 region [1] and therefore may represent a new genotype.
Table 1.
Rhinovirus Detections in Acute-Phase Serum (n) and Corresponding Nasopharyngeal/Oropharyngeal Specimens (N) Among 570 Rhinovirus-Positive Community-Acquired Pneumonia Patients by Age Group and Rhinovirus Species
| Age group | RV-A | RV-B | RV-C | Total RV n/N (%) |
|---|---|---|---|---|
| n/N (%) | n/N (%) | n/N (%) | ||
| <6 mo | 0/27 (0.0) | 0/4 (0.0) | 2/20 (10.0) | 2/51 (3.9) |
| 6–11 mo | 0/25 (0.0) | 0/2 (0.0) | 5/28 (17.9)a | 5/55 (9.1) |
| 12–23 mo | 1/25 (4.0) | 0/3 (0.0) | 20/52 (38.5) | 21/80 (26.3) |
| 24–59 mo | 0/38 (0.0) | 0/2 (0.0) | 20/58 (34.5) | 20/98 (20.4) |
| 5–9 y | 0/31 (0.0) | 0/7 (0.0) | 9/53 (17.0) | 9/91 (9.9) |
| 10–17 y | 0/28 (0.0) | 0/2 (0.0) | 0/11 (0.0) | 0/41 (0.0) |
| ≥18 y | 0/104 (0.0) | 0/19 (0.0) | 0/31 (0.0) | 0/154 (0.0) |
| Total | 1/278 (0.4) | 0/39 (0.0) | 56/253 (22.1) | 57/570 (10.0) |
Abbreviations: RV, rhinovirus.
aRV-C detected in the serum of 1 patient, and RV-A detected in the corresponding respiratory specimen.
Figure 1.
Neighbor-joining tree of rhinovirus (RV) VP4/VP2 nucleotide sequences from 57 viremic patients (open and solid circles); numbers are genotype designations of RV-A, -B, and -C reference strain sequences available from GenBank. Solid circle denotes patient with RV-C detected in the acute serum and RV-A detected in the respiratory specimen. Box highlights 4 similar sequences obtained from different patients that show >23% divergence from known RVs and may represent a new virus genotype. Scale bar shows genetic distance as nucleotide substitutions per site. Boostrap values (1000 replicates) are indicated at the RV species nodes.
Rhinovirus NP/OP and Age Group
Most RV detections from NP/OP specimens (65.8%) were in children aged <10 years, and proportionately more detections of RV-C (n/N = 211/253; 83.4%) were in this age group than detections of RV-A (n/N = 146/278; 52.5%; P < .0001) or RV-B (n/N = 18/39; 46.2%; P < .0001) (Table 1).
Compared with RV-C (median age, 3.0 y; interquartile range [IQR], 1–6 y), other RV species were more commonly detected in older patients (RV-A: median age, 8.0 y; IQR, 1–50 y, P < .0001; RV-B: median age, 16.0 y; IQR, 4–54 y, P < .0001).
Rhinovirus Viremia and Age Group
Rhinovirus viremia varied significantly with age (P < .0001) (Table 1). Overall, RV viremia was detected in 15.2% of 375 patients aged <10 years with RV detected from NP/OP specimens. The proportion of patients with viremia increased with age from 3.9% in children aged <6 months to a peak of 26.3% in children aged 12–23 months. Rhinovirus viremia then declined and was not detected in patients aged >10 years.
Rhinovirus Viremia and NP/OP Respiratory Viral Load
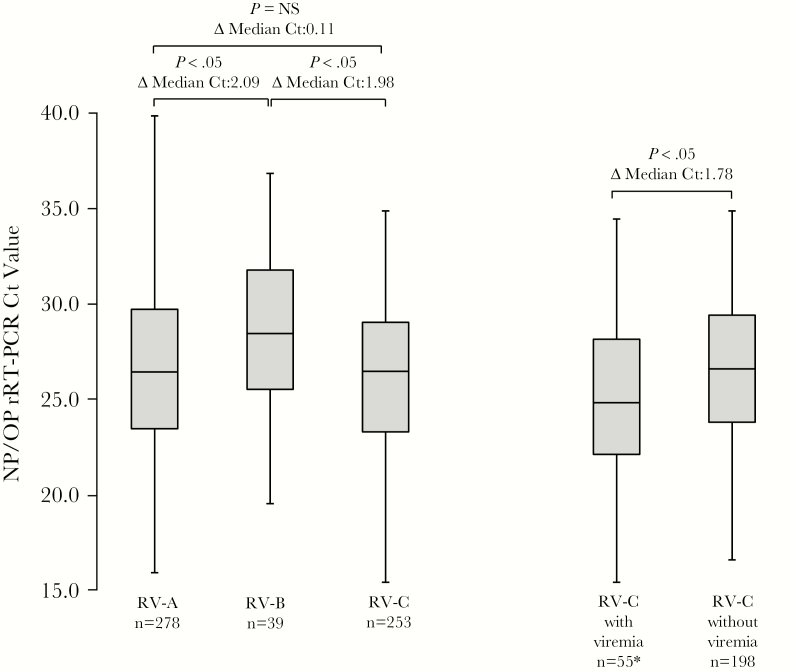
We compared median Ct values from NP/OP specimens for each RV species to determine whether virus load in the upper respiratory tract might account for differences among RV species associated with viremia (Figure 2). Median NP/OP Ct values for RV-A (median Ct, 26.4; IQR, 23.5–29.7) and RV-C (median Ct, 26.5; IQR, 23.3–29.0) did not differ significantly from each other but were significantly lower for both RV-A and RV-C (representing higher virus loads) than for RV-B (median Ct, 28.4; IQR, 25.5–31.8). Among patients with RV-C, median Ct values were significantly lower among those with a detectable viremia (median Ct, 24.8; IQR, 22.1–28.1) than those without viremia (median Ct, 26.6; IQR, 23.8–29.4).
Figure 2.
Box-and-whisker plots of rhinovirus (RV) real-time reverse-transcription polymerase chain reaction cycle threshold (Ct) values obtained from respiratory specimens from patients with RV-A, RV-B, and RV-C infections and comparing patients with RV-C with and without RV viremia. Note that of 56 patients with RV-C viremias, only 55 had corresponding RV-C detections from respiratory specimens (*). Whiskers mark the lowest and highest Ct values; boxes are bounded by the 25th and 75th percentiles; and box bisecting lines represent median Ct values. Sample size is indicated for each category. Differences (Δ) in median Ct values for each category are given as well as P values calculated by the Mann-Whitney U test. Abbreviations: Ct, cycle threshold; NP/OP, nasopharyngeal/oropharyngeal; NS, not significant, rRT-PCR, real-time reverse-transcription polymerase chain reaction; RV, rhinovirus.
Rhinovirus Viremia and Codetection With Other Respiratory Pathogens
We compared proportions of codetections in patients with and without RV viremia to determine whether patients with viremia were less likely to be coinfected with another respiratory pathogen that could account for the illness. Among 566 (99.3%) patients with RV-positive respiratory specimens and available test results for bacterial and other viral pathogens, 143 (25.3%) had another viral or bacterial respiratory pathogen infection detected. Among 372 patients aged <10 years with RV detected from the respiratory specimen and available test results for other pathogens, 7 of 57 (12.3%) with RV viremia also had another pathogen detected compared with 107 of 315 (34.0%) without viremia (P = .001). Interestingly, all codetections were viruses, including respiratory syncytial virus (n = 3), influenza virus (n = 2), parainfluenza virus (n = 1), and respiratory syncytial virus/adenovirus (n = 1).
Rhinovirus Viremia and Clinical Features
We compared the clinical features of 375 patients aged <10 years with RV-positive NP/OP specimens with and without RV viremia. Patients with viremia were significantly more likely to have chest retractions (61.4% vs 45.9%; P < .05) or wheezing (86.0% vs 61.3%; P < .001) at enrollment and to have preexisting asthma/reactive airway disease (59.7% vs 41.2%; P < .05) than those without viremia. There were no differences in intensive care admission, mechanical ventilation, deaths, length of hospital stay, or chest radiographic features within 48 hours of admission between RV viremic and nonviremic children.
DISCUSSION
In this large, multisite study of CAP, we detected RV viremia in 10% of 570 hospitalized patients with RV detected from upper respiratory tract specimens. Our findings were similar to several international reports of RV viremia detected by RT-PCR in children with acute respiratory RV infections [5–7]. A study from Greece detected RV viremia in 10 of 88 (11.4%) children presenting with acute respiratory symptoms ranging from common colds to pneumonia [5]; a study from Italy detected RV viremia in 6 of 50 (12.0%) children with fever and acute respiratory symptoms [6]; and a study from Japan detected RV viremia in 30 of 243 (12.3%) children hospitalized in the Philippines with severe lower respiratory tract infections [7]. Although these studies examined RV respiratory tract infections of varied clinical severity among mostly young children, our study was larger, included all age groups, and focused on hospitalized patients diagnosed with CAP, of which nearly 90% of pneumonias were subsequently confirmed on radiographic review.
Before the advent of sensitive molecular diagnostic tests, RV infections were thought to remain localized to the mucosal epithelium of the upper respiratory tract [17]. Using culture-based detection methods, RV viremia had only been reported from a few children with sudden and unexpected death [3, 4]. Past failures to culture RV from blood might be explained by the predominance of RV-C in viremia (see below), which is known to not grow well in conventional cell lines [18]. With some recent exceptions [19, 20], acute RV infections have rarely been associated with disseminated disease, unlike EVs, where viremia can lead to infection of multiple sites with varied clinical manifestations [21]. However, blood detection of RV by RT-PCR may only represent identification of RV RNA and not true viremia with viable virus.
Routes of RV entry into the bloodstream are unknown. Experimental studies have shown that RV can infect lower respiratory tract airways [22, 23], and individual case reports have documented RV infection of the lung [24, 25]. Direct infection and viral-mediated injury of the lung might hypothetically lead to direct viral seeding of the blood. However, RV viremia has also been found in children presenting with only upper respiratory tract symptoms [4, 6], suggesting that infection of the lower respiratory tract may not be required for bloodstream access. Rhinovirus RNA and infected cells have been found in tonsils and adenoid tissues that facilitate antigen capture and immune cell recruitment [26, 27]. These tissues are drained by efferent lymphatic vessels that may serve to convey RV to the bloodstream.
Although there were more RV-A detections from respiratory specimens of study patients than RV-C detections, RV-C accounted for almost all viremias (98.2%). Differences in virus load in the upper respiratory tract did not appear to explain this finding; median Ct values were similar for RV-A and RV-C, although lower median Ct values (representing higher virus loads) for RV-C were associated with viremia. Our findings are consistent with other studies that compared proportions of viremias among children with acute RV respiratory tract infections by virus species [6, 7]. Of the 6 children with RV viremia detected by Esposito et al [6], 4 (66.7%) were identified as having RV-C. Of the 30 children with RV viremia detected by Fuji et al [7], 26 (86.7%) were found to have RV-C; interestingly, these authors found that the 4 patients with RV-A viremia all possessed RV-C-like 5’noncoding regions (NCRs) acquired through recombination, leading the authors to speculate that the RV-C 5’NCR might mediate viremia. However, the 5’NCR of the RV-A that we identified from a single viremic patient did not show evidence of recombination in this region (data not shown). Like Fuji et al [7], we found a wide range of RV-C genotypes without obvious phylogenetic clustering, suggesting that viremia is not genotype-specific.
We found young age to be strongly associated with RV viremia. Rhinovirus viremia peaked in children aged 1–2 years, with lower prevalence in infants aged <6 months and declining prevalence with increasing age after 2 years; viremia was not detected among patients aged >10 years. Clearance of RV from blood by maternal antibodies may explain the lower prevalence in infants aged <6 months [7, 28]. Because RV infections peak in early childhood [29], with reinfections occurring with increasing age, immune memory and cross-protective RV immunity may lead to more rapid virus clearance from blood and explain the subsequent decline in viremia. A further contributing factor may be that RV-C infections were proportionately more common in young children as compared with other RV species and more commonly associated with viremia [6, 7].
Multiple epidemiologic studies that compared RV species found RV-C to be more frequently associated with severe respiratory infections, particularly in children with asthma [8, 9, 30–32]. Although we found no clear differences in severity of RV infections between patients with and without viremia, patients with viremia were more likely to have chest retractions or wheezing symptoms at enrollment and were more likely to have preexisting asthma/reactive airway disease. In those studies of RV viremia in children hospitalized with severe respiratory illnesses, viremia was more often associated with increased severity measures suggestive of lower respiratory infection, including decreased oxygen saturation levels [6, 7] and required oxygen therapy [6]. Personal history of asthma and proportion of asthma exacerbations were significantly associated with RV viremia [5], as was wheezing [7]. Together, these findings suggest that the pathophysiology of infection with RV-C may differ from other RV species. A unique feature of RV-C is its use of the cadherin-related family member 3 (CDHR3) protein for host cell entry [33], whereas RV-A and RV-B use intercellular adhesion molecule 1 or low-density lipoprotein receptor family members. Interestingly, CDHR3 has been identified as a susceptibility locus for early childhood asthma [34]. Moreover, cells expressing genetic variants of CDHR3 show enhanced binding efficiency for RV-C and increased virus production, suggesting that these variants could be a risk factor for RV-C–associated childhood wheezing and asthma hospitalization [33, 35]. Further studies will be needed to determine the relationship between receptor utilization and pathophysiology of RV-C infection and whether viremia might be an indicator of or contributor to this process.
Table 2.
Comparison of Clinical Characteristics of 375 Hospitalized Community-Acquired Pneumonia Patients Aged <10 Years With and Without Rhinovirus Viremia
| Characteristic | RV viremia | ||
|---|---|---|---|
| Positive, no. (%) | Negative, no. (%) | P value | |
| Total | 57 (15.2) | 318 (84.8) | |
| Female sex | 28 (49.1) | 139 (43.7) | NS |
| Age, y, median (IQR) | 2.0 (1–4) | 2.0 (0–5) | NS |
| Symptoms at presentation | |||
| Cough | 52 (91.2) | 292 (91.8) | NS |
| Wheezing | 49 (86.0) | 195 (61.3) | <.05 |
| Shortness of breath | 47 (82.5) | 222 (69.8) | NS |
| Fever/feverish | 42 (73.7) | 256 (80.5) | NS |
| Rhinorrhea | 42 (73.7) | 234 (73.6) | NS |
| Anorexia | 38 (66.7) | 224 (70.4) | NS |
| Chest retractions | 35 (61.4) | 146 (45.9) | <.05 |
| Patient interview | |||
| Respiratory illness in the past month | 16 (28.1) | 80 (25.2) | NS |
| Ever diagnosed with pneumonia | 16 (28.1) | 69 (21.7) | NS |
| Antibiotics prior to hospitalization | 7 (12.3) | 58 (18.2) | NS |
| Supplemental oxygen at home | 3 (5.3) | 12 (3.8) | NS |
| Influenza antivirals prior to hospitalization | 0 (0.0) | 4 (1.3) | NS |
| Social history | |||
| Lives in household with indoor or outdoor smoking | 23 (40.4) | 119 (37.4) | NS |
| Household member with respiratory illness in past month | 21 (36.8) | 107 (33.7) | NS |
| Attend daycare (among persons aged <6 y) | 14/50 (28.0) | 72/260 (27.7) | NS |
| Medical conditions | |||
| Any 1 condition | 42 (73.7) | 174 (54.7) | <.05 |
| Asthma/reactive airway disease | 34 (59.7) | 131 (41.2) | <.05 |
| Congenital heart disease | 6 (10.5) | 17 (5.4) | NS |
| Neurologic disorder | 6 (10.5) | 17 (5.4) | NS |
| Preterm birth among persons aged <2 y | 7/28 (25) | 30/158 (19) | NS |
| Hospitalization | |||
| Intensive care unit admission | 13 (22.8) | 70 (22.0) | NS |
| Mechanical ventilation | 4 (7.0) | 21 (6.6) | NS |
| Death | 0 (0.0) | 1 (0.3) | NS |
| Hospital stay, d, median (IQR) | 2/1–3 | 2/1–4 | NS |
| Chest radiograph features (within 48 h of admission)a | |||
| Alveolar and/or interstitial infiltrate | 28 (50.0) | 156 (52.7) | NS |
| Consolidation | 26 (46.4) | 138 (46.6) | NS |
| Complicated bronchiolitis | 22 (39.3) | 114 (38.5) | NS |
| Pleural effusion | 0 (0.0) | 28 (9.5) | NS |
Abbreviations; IQR, interquartile range; NS, not significant; RV, rhinovirus.
aAmong patients with an abnormal initial chest radiograph
Rhinovirus infections in young children are often subclinical or are codetected with other respiratory pathogens, complicating disease attribution [36]. In the original EPIC study [12], >42% of all RV-positive pediatric patients had another respiratory pathogen detected, which could account for patient illness. Some studies that compared RV species in persons with acute respiratory infections have found codetection prevalence to be significantly lower in those with RV-C [10, 31]. We found significantly fewer codetections among RV-C infected patients with RV viremia, suggesting that viremic RV-C infections may be more indicative of pediatric pneumonia than nonviremic episodes.
Our study had several limitations. First, not all RV-positive patients had acute serum specimens available for testing, and specimen collection times from onset of patient reported symptoms varied and may have been suboptimal in some patients for viremia detection. We were restricted to specimens collected from the upper respiratory tract, preventing assessment of possible correlation of RV viremia with lower respiratory tract viral loads. Others have shown that sample collection timing and sample type (serum vs whole blood) might impact the ability to detect virus [5, 7]. Finally, because this study was restricted to patients hospitalized with more severe respiratory illness, further studies of RV viremia that include healthy controls and cases with milder respiratory symptoms are needed.
In conclusion, we detected RV viremia in hospitalized CAP patients aged <10 years with RV-positive upper respiratory tract specimens. Compared with other RV species, RV-C was almost exclusively associated with viremia and was more often the only respiratory pathogen identified in viremic patients. Although causality cannot be conclusively inferred, these findings strongly suggest that RV-C is an important cause of pediatric pneumonia and that viremia may be a useful diagnostic indicator.
Notes
Acknowledgments. We thank the patients who graciously enrolled in the EPIC study.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC).
Financial support. This work was supported by funds from the CDC.
Potential conflicts of interest. All reported support was received outside of the submitted work. W. H. S. reports grants from BioMerieux, Affinium Pharmaceuticals, Astute Medical, BRAHMS GmbH/Thermo Fisher, Pfizer, Rapid Pathogen Screening, Venaxis, BioAegis Inc, Sphingotec GmbH, and Ferring Pharmaceuticals, and personal fees from BioFire Diagnostics, Venaxis, Abbott Point of Care, and Cempra Pharmaceuticals. E. J. A. reports personal fees from AbbVie, grants and nonfinancial support from MedImmune, and grants from Regeneron and Novavax. C. G. G. reports serving as a consultant for Pfizer. R. G. W. reports grants from the CDC, funds to conduct clinical research from BioMerieux and Vertex Pharmaceuticals, and personal fees from BioMerieux and Visterra Inc. D. J. W. reports grants from the CDC. K. M. E. reports serving on a data and safety monitoring board for Novartis for which her institution receives fees. W. H. S. reports grants from the CDC and personal fees from BioFire Diagnostics, Venaxis, Abbott Point of Care and Cempra Pharmaceuticals. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: Sixth International Meeting on Emerging Diseases and Surveillance, Vienna, Austria, November 2016. Poster number: 20.016.
References
- 1. McIntyre CL, Knowles NJ, Simmonds P. Proposals for the classification of human rhinovirus species A, B and C into genotypically assigned types. J Gen Virol 2013; 94:1791–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Horstmann DM, McCollum RW, Mascola AD. Viremia in human poliomyelitis. J Exp Med 1954; 99:355–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Urquhart GE, Grist NR. Virological studies of sudden, unexplained infant deaths in Glasgow 1967–70. J Clin Pathol 1972; 25:443–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Urquhart GE, Stott EJ. Rhinoviraemia. Br Med J 1970; 4:28–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xatzipsalti M, Kyrana S, Tsolia M et al. . Rhinovirus viremia in children with respiratory infections. Am J Respir Crit Care Med 2005; 172:1037–40. [DOI] [PubMed] [Google Scholar]
- 6. Esposito S, Daleno C, Scala A et al. . Impact of rhinovirus nasopharyngeal viral load and viremia on severity of respiratory infections in children. Eur J Clin Microbiol Infect Dis 2014; 33:41–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fuji N, Suzuki A, Lupisan S et al. . Detection of human rhinovirus C viral genome in blood among children with severe respiratory infections in the Philippines. PLoS One 2011; 6:e27247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Khetsuriani N, Lu X, Teague WG, Kazerouni N, Anderson LJ, Erdman DD. Novel human rhinoviruses and exacerbation of asthma in children. Emerg Infect Dis 2008; 14:1793–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Miller EK, Edwards KM, Weinberg GA et al. ; New Vaccine Surveillance Network A novel group of rhinoviruses is associated with asthma hospitalizations. J Allergy Clin Immunol 2009; 123:98–104.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Iwane MK, Prill MM, Lu X et al. . Human rhinovirus species associated with hospitalizations for acute respiratory illness in young US children. J Infect Dis 2011; 204:1702–10. [DOI] [PubMed] [Google Scholar]
- 11. Daleno C, Piralla A, Scala A, Senatore L, Principi N, Esposito S. Phylogenetic analysis of human rhinovirus isolates collected from otherwise healthy children with community-acquired pneumonia during five successive years. PLoS One 2013; 8:e80614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jain S, Williams DJ, Arnold SR et al. ; CDC EPIC Study Team Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med 2015; 372:835–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jain S, Self WH, Wunderink RG et al. ; CDC EPIC Study Team Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med 2015; 373:415–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Self WH, Williams DJ, Zhu Y et al. . Respiratory viral detection in children and adults: comparing asymptomatic controls and patients with community-acquired pneumonia. J Infect Dis 2016; 213:584–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lu X, Holloway B, Dare RK et al. . Real-time reverse transcription-PCR assay for comprehensive detection of human rhinoviruses. J Clin Microbiol 2008; 46:533–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 2016; 33:1870–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Arruda E, Boyle TR, Winther B, Pevear DC, Gwaltney JM Jr, Hayden FG. Localization of human rhinovirus replication in the upper respiratory tract by in situ hybridization. J Infect Dis 1995; 171:1329–33. [DOI] [PubMed] [Google Scholar]
- 18. Arden KE, Mackay IM. Newly identified human rhinoviruses: molecular methods heat up the cold viruses. Rev Med Virol 2010; 20:156–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tapparel C, L’Huillier AG, Rougemont AL, Beghetti M, Barazzone-Argiroffo C, Kaiser L. Pneumonia and pericarditis in a child with HRV-C infection: a case report. J Clin Virol 2009; 45:157–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lupo J, Schuffenecker I, Morel-Baccard C et al. . Disseminated rhinovirus C8 infection with infectious virus in blood and fatal outcome in a child with repeated episodes of bronchiolitis. J Clin Microbiol 2015; 53:1775–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lugo D, Krogstad P. Enteroviruses in the early 21st century: new manifestations and challenges. Curr Opin Pediatr 2016; 28:107–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gern JE, Galagan DM, Jarjour NN, Dick EC, Busse WW. Detection of rhinovirus RNA in lower airway cells during experimentally induced infection. Am J Respir Crit Care Med 1997; 155:1159–61. [DOI] [PubMed] [Google Scholar]
- 23. Papadopoulos NG, Bates PJ, Bardin PG et al. . Rhinoviruses infect the lower airways. J Infect Dis 2000; 181:1875–84. [DOI] [PubMed] [Google Scholar]
- 24. Imakita M, Shiraki K, Yutani C, Ishibashi-Ueda H. Pneumonia caused by rhinovirus. Clin Infect Dis 2000; 30:611–2. [DOI] [PubMed] [Google Scholar]
- 25. Craighead JE, Meier M, Cooley MH. Pulmonary infection due to rhinovirus type 13. N Engl J Med 1969; 281:1403–4. [DOI] [PubMed] [Google Scholar]
- 26. Rihkanen H, Carpén O, Roivainen M, Vaheri A, Pitkäranta A. Rhinovirus in adenoid tissue. Int J Pediatr Otorhinolaryngol 2004; 68:903–8. [DOI] [PubMed] [Google Scholar]
- 27. Suvilehto J, Roivainen M, Seppänen M et al. . Rhinovirus/enterovirus RNA in tonsillar tissue of children with tonsillar disease. J Clin Virol 2006; 35:292–7. [DOI] [PubMed] [Google Scholar]
- 28. Kieninger E, Fuchs O, Latzin P, Frey U, Regamey N. Rhinovirus infections in infancy and early childhood. Eur Respir J 2013; 41:443–52. [DOI] [PubMed] [Google Scholar]
- 29. Blomqvist S, Roivainen M, Puhakka T, Kleemola M, Hovi T. Virological and serological analysis of rhinovirus infections during the first two years of life in a cohort of children. J Med Virol 2002; 66:263–8. [DOI] [PubMed] [Google Scholar]
- 30. Bizzintino J, Lee WM, Laing IA et al. . Association between human rhinovirus C and severity of acute asthma in children. Eur Respir J 2011; 37:1037–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wisdom A, Kutkowska AE, McWilliam Leitch EC et al. . Genetics, recombination and clinical features of human rhinovirus species C (HRV-C) infections; interactions of HRV-C with other respiratory viruses. PLoS One 2009; 4:e8518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Han TH, Chung JY, Hwang ES, Koo JW. Detection of human rhinovirus C in children with acute lower respiratory tract infections in South Korea. Arch Virol 2009; 154:987–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bochkov YA, Watters K, Ashraf S et al. . Cadherin-related family member 3, a childhood asthma susceptibility gene product, mediates rhinovirus C binding and replication. Proc Natl Acad Sci U S A 2015; 112:5485–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bønnelykke K, Sleiman P, Nielsen K et al. . A genome-wide association study identifies CDHR3 as a susceptibility locus for early childhood asthma with severe exacerbations. Nat Genet 2014; 46:51–5. [DOI] [PubMed] [Google Scholar]
- 35. Bochkov YA, Gern JE. Rhinoviruses and their receptors: implications for allergic disease. Curr Allergy Asthma Rep 2016; 16:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fry AM, Lu X, Olsen SJ et al. . Human rhinovirus infections in rural Thailand: epidemiological evidence for rhinovirus as both pathogen and bystander. PLoS One 2011; 6:e17780. [DOI] [PMC free article] [PubMed] [Google Scholar]