Abstract
Aims
To simplify administration of aqueous exenatide once weekly, which requires reconstitution, the exenatide microspheres have been reformulated in a ready‐to‐use autoinjector with a Miglyol diluent (exenatide QWS‐AI). This study compared the efficacy and safety of exenatide QWS‐AI with the first‐in‐class glucagon‐like peptide‐1 receptor agonist exenatide twice daily (BID).
Materials and Methods
This randomized, open‐label, controlled study in patients with type 2 diabetes using diet and exercise or taking stable oral glucose‐lowering medication randomized patients 3:2 to either exenatide QWS‐AI (2 mg) or exenatide BID (10 μg) for 28 weeks. The primary outcome was the 28‐week change in glycated haemoglobin (HbA1c). A subset of patients completed a standardized meal test for postprandial and pharmacokinetic assessments.
Results
A total of 375 patients (mean HbA1c, 8.5% [69 mmol/mol]; body mass index, 33.2 kg/m2; diabetes duration, 8.5 years) received either exenatide QWS‐AI (n = 229) or exenatide BID (n = 146); HbA1c was reduced by −1.4% and −1.0%, respectively (least‐squares mean difference, −0.37%; P = .0072). More patients achieved HbA1c <7.0% with exenatide QWS‐AI (49.3%) than with exenatide BID (43.2%; P = .225). Body weight was reduced in both groups (P = .37 for difference). Gastrointestinal adverse events (AEs) were reported in 22.7% (exenatide QWS‐AI) and 35.6% (exenatide BID) of patients; fewer patients in the exenatide QWS‐AI group withdrew because of AEs than in the exenatide BID group. Minor hypoglycaemia occurred most often with concomitant sulfonylurea use.
Conclusions
Exenatide QWS‐AI was associated with a greater reduction in HbA1c, similar weight loss and a favorable gastrointestinal AE profile compared with exenatide BID.
Keywords: autoinjector, exenatide, glucagon‐like peptide‐1 receptor agonist, type 2 diabetes
1. INTRODUCTION
Patients with type 2 diabetes require long‐term treatment to achieve and maintain glycaemic control; thus, the availability of therapeutic options that are effective and simple to administer is important. The initial formulation of exenatide, a first‐in‐class glucagon‐like peptide‐1 receptor agonist (GLP‐1RA) that has been used clinically for over 10 years, is still available but requires twice‐daily (BID) subcutaneous administration (exenatide BID). Subsequently, exenatide was packaged into biodegradable poly‐(d,l‐lactide‐co‐glycolide) microspheres that allow for once‐weekly (QW) administration but require reconstitution in an aqueous diluent before injection. A single dose of exenatide QW results in the gradual release of exenatide from the microspheres over approximately 10 weeks.1 When administered every 7 days, the therapeutic threshold is reached after 2 weeks, and steady‐state plasma concentrations are achieved after approximately 7 weeks.2 Patients receiving exenatide QW over time have demonstrated sustained reductions from baseline in glycated haemoglobin (HbA1c) and body weight.3, 4
To improve ease of use, development efforts were directed at simplifying the delivery of exenatide‐containing microspheres by eliminating the need for reconstitution in aqueous diluent before administration. Exenatide QW suspension by autoinjector (exenatide QWS‐AI) contains exenatide microspheres in a mixture of medium‐chain triglycerides (Miglyol 812), which is recognized as safe by the US Food and Drug Administration and is approved for use in a variety of drugs, foods and cosmetics in the USA and Europe. The new formulation allows for easier administration of exenatide QW via an autoinjector.
This study compared the efficacy, safety and tolerability of exenatide QWS‐AI with the original exenatide BID formulation over 28 weeks of treatment in patients with type 2 diabetes.
2. MATERIALS AND METHODS
2.1. Patients and study design
This phase III, randomized, controlled, open‐label study (DURATION‐NEO‐1; ClinicalTrials.gov identifier: NCT01652716), conducted at 65 sites in the USA, compared the efficacy, safety and tolerability of exenatide QWS‐AI with that of exenatide BID. The initial controlled treatment period was 28 weeks. A 52‐week extension will be reported separately. Patients were aged ≥18 years and had type 2 diabetes. They were treated with diet and exercise alone or with a stable regimen of metformin, sulfonylurea, pioglitazone or any combination of 2 of these agents. They had an HbA1c level of 7.1% to ≤11.0% (54‐97 mmol/mol). Patients were excluded if they had a clinically significant medical condition, including a history of pancreatitis or renal transplantation, current or past family history of medullary thyroid carcinoma or multiple endocrine neoplasia type 2, severe renal impairment (estimated glomerular filtration rate [eGFR] < 30 mL/min/1.73 m2) or were receiving dialysis, or active cardiovascular disease within 3 months of screening.
Patients were stratified by background diabetes therapy (diet and exercise alone, sulfonylurea use [± metformin and/or pioglitazone] or non–sulfonylurea use [metformin and/or pioglitazone]), HbA1c (<9.0% or ≥9.0% [<75 or ≥75 mmol/mol]) and renal function (normal [eGFR ≥90 mL/min/1.73 m2], mild renal impairment [eGFR 60‐89 mL/min/1.73 m2] or moderate renal impairment [eGFR 30‐59 mL/min/1.73 m2]) at screening. They were randomized in a 3:2 ratio to treatment with exenatide QWS‐AI or exenatide BID, respectively. Randomization was done centrally through an interactive web system by study‐site personnel.
Upon randomization, patients received injections of either exenatide QWS‐AI (2 mg) with a 23‐gauge needle1 or exenatide BID (5 μg for 4 weeks, 10 μg subsequently; administered 60 minutes before morning and evening meals) with a 29‐ to 31‐gauge needle.5 Prior concomitant glucose‐lowering medications were continued at pretrial doses, and patients continued prescribed diet and exercise regimens. Investigators initiated rescue medication for patients who experienced a loss of glucose control (2 consecutive visits with fasting plasma glucose [FPG] > 270 mg/dL [>15.0 mmol/L] from weeks 4‐16 or >240 mg/dL [>13.3 mmol/L] from weeks 16‐28). Drug choice was left to the investigator's discretion and followed local prescribing practices.
A subset of patients from select study sites also participated in a standardized meal test to assess postprandial glycaemic and pharmacokinetic measures. Meal test assessments were completed at baseline and at 16 weeks.
The study was conducted in accordance with the Declaration of Helsinki and was approved by the institutional review board for each study site. All patients provided written informed consent.
2.2. End points and assessments
The primary efficacy end point for the study was change in HbA1c from baseline (randomization) to week 28. Secondary efficacy end points included the proportion of patients achieving HbA1c < 7.0% (<53 mmol/mol) by 28 weeks, change in FPG and body weight from baseline to week 28, and change in 2‐hour postprandial glucose (PPG) from baseline to week 16 in a subgroup of patients enrolled in the meal test cohort. An exploratory analysis of the change in HbA1c, FPG and body weight from baseline to week 28 was also conducted in patients using sulfonylureas vs those not using sulfonylureas. Additional efficacy measures included the proportion of patients achieving HbA1c ≤ 6.5% (≤48 mmol/mol) after 28 weeks and changes from baseline to week 28 in fasting insulin, glucagon and lipid concentrations, systolic and diastolic blood pressure (BP) and other cardiovascular risk biomarkers.
The primary safety and tolerability measure was incidence of reported adverse events (AEs), which was summarized descriptively. A clinical events classification committee was responsible for blinded review and adjudication of certain events. AEs of interest for the GLP‐1RA class were also monitored for duration and time course and included thyroid neoplasms (adjudicated), pancreatic cancer (adjudicated), renal failure, hypersensitivity reactions, immune‐related AEs, injection site‐related AEs, gastrointestinal AEs, hypoglycaemia, pancreatitis (adjudicated) and cardiovascular events (adjudicated). Severe hypoglycaemia was defined as any event requiring third‐party assistance because of severe impairment in consciousness or behavior that resolved after administration of glucagon or glucose. Minor hypoglycaemia was classified by symptoms consistent with hypoglycaemia and a glucose concentration <54 mg/dL (<3 mmol/L).
Clinical laboratory tests, including measures of renal function and liver function, were evaluated throughout the 28‐week treatment period. Anti‐exenatide antibodies were measured with an enzyme‐linked immunosorbent assay.6
For the meal test cohort, patients consumed a standardized breakfast meal (~660 kcal [~2761.4 kJ], mixed solid foods) containing approximately 60% carbohydrates, 15% protein and 25% fat, within 15 minutes, Fasting measures, including glucose, glucagon, insulin and triglycerides, were obtained before meal consumption. Serial measurements of glucose, insulin and triglyceride concentrations were obtained over 5 hours. Change in 2‐hour PPG was also recorded. Pharmacokinetic analyses summarized plasma concentrations of exenatide, with the steady‐state concentration determined as the average plasma concentration from week 10 to week 28.
2.3. Statistical methods
The target sample size was approximately 375 patients, with an anticipated withdrawal rate of 15% by week 28. A sample size of 225 patients randomized to receive exenatide QWS‐AI and 150 patients randomized to receive exenatide BID was estimated to provide >95% power to demonstrate that exenatide QWS‐AI is noninferior to exenatide BID (within a 0.4% noninferiority upper margin) in the HbA1c change from baseline to week 28, assuming a common standard deviation (SD) of 1.15% and an expected mean difference of −0.1% or less (exenatide QWS‐AI − exenatide BID).
The sample size was estimated to provide 91% power to show superiority of exenatide QWS‐AI in HbA1c change from baseline to week 28, assuming a common SD of 1.15% and a mean difference of −0.4% (exenatide QWS‐AI − exenatide BID).
The modified intention‐to‐treat (mITT) population, which comprised all randomized patients who received at least 1 dose of study drug, was used for efficacy and pharmacodynamic analyses. A full description of the study populations used for analysis is available online in Appendix S1.
Efficacy measures were summarized descriptively by treatment, and comparisons were made using the mixed‐effects model for repeated measures, with the exception of analysis of the proportion of patients achieving HbA1c goals (7.0% and 6.5% [53 and 48 mmol/mol], respectively; Cochran–Mantel–Haenszel test) and change in 2‐hour PPG at week 16 (meal test cohort; general linear model). Further details on the models used for analysis are provided in Appendix S1. Change‐from‐baseline data are presented as the least‐squares mean and standard error of the least‐squares mean, and the difference in change between groups is reported as the least‐squares mean difference and standard error of the least‐squares mean difference, unless otherwise noted. A closed testing procedure was implemented to preserve the family‐wise type I error rate; noninferiority was evaluated first, and if achieved, superiority for the primary end point was assessed. For the primary end point, a 2‐sided significance level of 0.05 was used. Missing data were imputed using the last‐observation‐carried‐forward method. Incremental area under the curve was calculated to assess the postprandial response as a baseline‐subtracted measure of area under the curve for glucose, serum insulin and triglyceride measures.
3. RESULTS
3.1. Patients
The study, including the extension phase, was conducted from January 28, 2013 through March 26, 2015. A total of 377 patients were randomized to receive exenatide QWS‐AI (n = 229) or exenatide BID (n = 148) (Figure 1). Two patients in the exenatide BID group withdrew before the first dose of study medication (mITT population: exenatide QWS‐AI, n = 229; exenatide BID, n = 146). The treated population was identical to the mITT population. Compliance was high for both groups (see Appendix S1 for more details).
Figure 1.
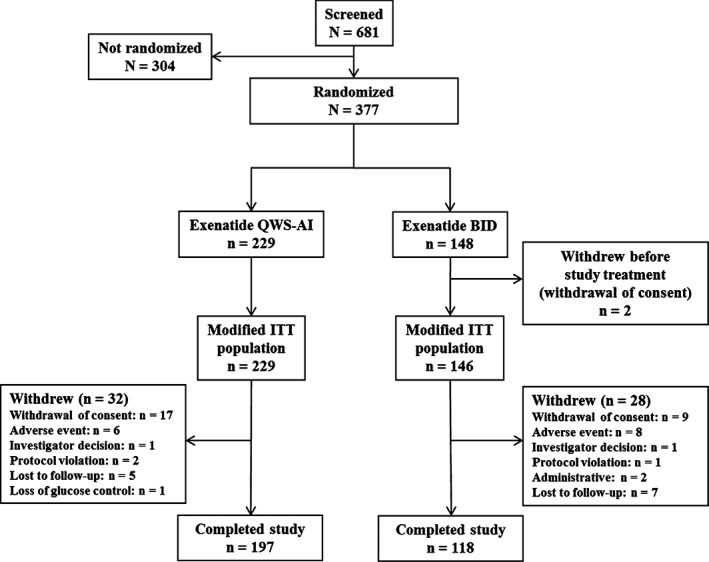
Patient disposition. Abbreviations: BID, twice daily; ITT, intention‐to‐treat; QWS‐AI, once‐weekly suspension by autoinjector
Baseline characteristics were similar between treatment groups (Table 1). Patients were primarily male and white, with a baseline HbA1c of 8.5% (69 mmol/mol). During the 28‐week assessment period, 23 patients (10%) in the exenatide QWS‐AI group and 22 patients (15%) in the exenatide BID group received new concomitant glucose‐lowering medication. This includes 5 patients (2.2%) in the exenatide QWS‐AI group and 6 patients (4.1%) in the exenatide BID group who met the protocol‐specified criteria for rescue medication.
Table 1.
Demographic and baseline characteristics (modified intention‐to‐treat population)
| Characteristic | Exenatide QWS‐AI (n = 229) | Exenatide BID (n = 146) |
|---|---|---|
| Male sex | 148 (65) | 92 (63) |
| Age, years | 56 ± 10 | 57 ± 9 |
| Race | ||
| White | 168 (73) | 110 (75) |
| Black | 38 (17) | 23 (16) |
| Asian | 17 (7) | 8 (6) |
| American Indian or Alaskan Native | 2 (1) | 3 (2) |
| Native Hawaiian or other Pacific Islander | 1 (<1) | 0 (0) |
| Other | 3 (1) | 2 (1) |
| Ethnicity (Hispanic or Latino) | 54 (24) | 34 (23) |
| Body weight, kg | 97 ± 23 | 97 ± 19 |
| Body mass index, kg/m2 | 33 ± 6 | 33 ± 5 |
| HbA1c, % | 8.5 ± 1.0 | 8.5 ± 1.0 |
| HbA1c, mmol/mol | 69 ± 11 | 69 ± 11 |
| Fasting plasma glucose, mg/dL | 181 ± 45 | 184 ± 47 |
| Fasting plasma glucose, mmol/L | 10.0 ± 2.5 | 10.2 ± 2.6 |
| Type 2 diabetes duration, years | 9 ± 6 | 8 ± 6 |
| Renal functiona | ||
| Normal | 85 (37) | 55 (38) |
| Mild impairment | 113 (49) | 76 (52) |
| Moderate impairment | 29 (13) | 15 (10) |
| Diabetes management method at screening | ||
| No use of sulfonylurea at screening | 140 (61) | 86 (59) |
| Diet and exercise | 31 (14) | 17 (12) |
| Metformin alone | 102 (45) | 65 (45) |
| Thiazolidinedione alone | 2 (1) | 0 (0) |
| Metformin + thiazolidinedione | 5 (2) | 4 (3) |
| Use of sulfonylurea at screening | 89 (39) | 60 (41) |
| Sulfonylurea alone | 8 (4) | 6 (4) |
| Sulfonylurea + metformin | 76 (33) | 52 (36) |
| Sulfonylurea + thiazolidinedione | 1 (<1) | 0 (0) |
| Sulfonylurea + metformin + thiazolidinedione | 4 (2) | 2 (1) |
Abbreviations: BID, twice daily; eGFR, estimated glomerular filtration rate; HbA1c, glycated haemoglobin; QWS‐AI, once‐weekly suspension by autoinjector.
Continuous variables are presented as mean ± standard deviation; categorical variables are presented as n (%).
Normal function: eGFR ≥90 mL/min/1.73 m2; mild impairment: eGFR 60 to 89 mL/min/1.73 m2; moderate impairment: eGFR 30 to 59 mL/min/1.73 m2; severe impairment: eGFR <30 mL/min/1.73 m2.
3.2. Primary and secondary efficacy end points
HbA1c was reduced with both exenatide QWS‐AI (−1.39% ± 0.09% [−15 ± 1 mmol/mol]) and exenatide BID treatment (−1.02% ± 0.11% [−11 ± 1 mmol/mol]) (Figure 2A). The reduction in HbA1c after 28 weeks was significantly greater with exenatide QWS‐AI (difference, −0.37% ± 0.13% [−4 ± 1 mmol/mol]; 95% confidence interval [CI], −0.63% to −0.10%; P = .0072). Thus, the superiority of exenatide QWS‐AI over exenatide BID was demonstrated.
Figure 2.
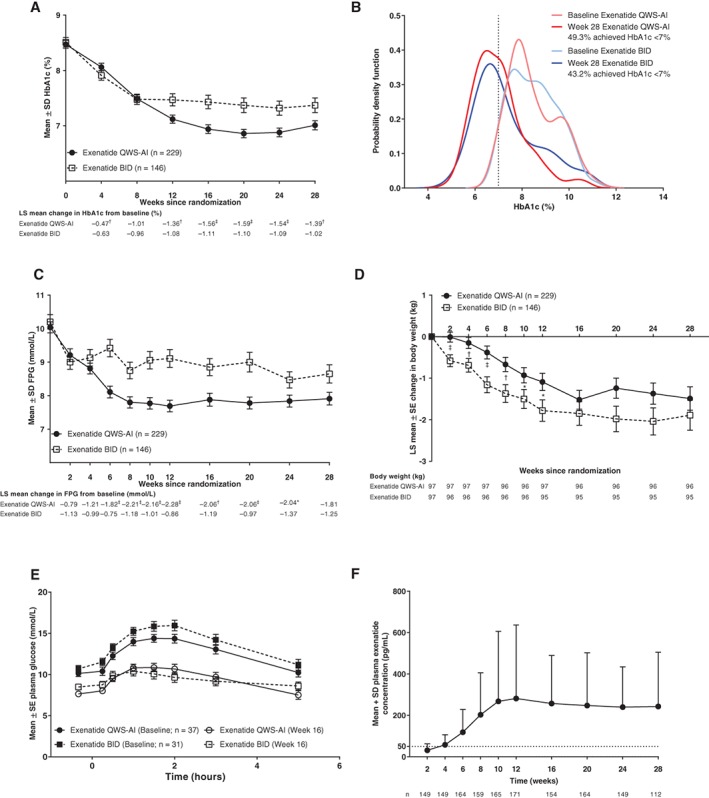
A, HbA1c over time (mITT population). B, Probability density function of HbA1c (mITT population). C, FPG over time (mITT population). D, Change in body weight over time (mITT population). E, Plasma glucose during meal test (meal test–evaluable population). F, Plasma exenatide concentration for patients with antibody titers ≤625. Dotted line indicates minimum effective concentration of exenatide for glucose lowering (~50 pg/mL). *P < .05 vs exenatide BID. † P ≤ .01 vs exenatide BID. ‡ P ≤ .001 vs exenatide BID. Abbreviations: BID, twice daily; FPG, fasting plasma glucose; HbA1c, glycated haemoglobin; LS, least‐squares; mITT, modified intention‐to‐treat; QWS‐AI, once‐weekly suspension by autoinjector; SD, standard deviation; SE, standard error
Certain patient demographic characteristics were tested for interaction with the treatment difference between exenatide QWS‐AI and exenatide BID. No treatment interaction was found for age or sex, but a significant treatment interaction was found for race (treatment‐by‐week‐by‐race, P = .0007). HbA1c was reduced by −1.5% and −1.0% (−16 and −11 mmol/mol; n = 278) in white patients, by −1.1% and −0.9% (−12 and −10 mmol/mol; n = 25) in Asian patients, and by −1.0% and −1.1% (−11 and −12 mmol/mol; n = 61) in black patients treated with exenatide QWS‐AI and exenatide BID, respectively.
After 28 weeks, the shift in probability density function suggested that more patients receiving exenatide QWS‐AI vs exenatide BID had HbA1c values between 4.0% and 8.0% (20 and 64 mmol/mol) and fewer patients had HbA1c values between 8.0% and 12.0% (64 and 108 mmol/mol) (Figure 2B). However, there was no statistical difference in the proportion of patients who achieved HbA1c <7.0% (<53 mmol/mol) with exenatide QWS‐AI (49.3%) or exenatide BID (43.2%; P = .225) and, although a greater proportion of patients achieved HbA1c ≤6.5% (≤ 48 mmol/mol; P = .051) with exenatide QWS‐AI (35.8%) than with exenatide BID (26.0%), this difference also did not reach significance.
FPG decreased comparably with both treatments by week 2 (the first post‐baseline measurement) and remained below baseline for the duration of the study (Figure 2C). After 28 weeks, the change in FPG was −32.7 ± 3.9 mg/dL (−1.81 ± 0.22 mmol/L) for exenatide QWS‐AI and −22.5 ± 4.9 mg/dL (−1.25 ± 0.27 mmol/L) for exenatide BID (difference, −10.2 ± 5.8 mg/dL [−0.57 ± 0.32 mmol/L]; P = .083).
Body weight was reduced from baseline with both exenatide QWS‐AI (−1.49 ± 0.28 kg) and exenatide BID (−1.89 ± 0.36 kg) (Figure 2D). After 28 weeks, there was no significant between‐group difference in change in body weight (difference, 0.4 ± 0.4 kg; P = .37).
For patients who participated in the meal test, 2‐hour PPG was reduced by week 16 with exenatide QWS‐AI (−87.0 ± 12.8 mg/dL [−4.83 ± 0.71 mmol/L]) and exenatide BID (−113.7 ± 14.8 mg/dL [−6.31 ± 0.82 mmol/L]; difference, 26.7 ± 15.9 [1.48 ± 0.88 mmol/L]; nominal P = .0999).
3.3. Additional efficacy end points
Fasting insulin concentrations increased by 6.8 ± 1.8 pmol/L with exenatide QWS‐AI and by 2.5 ± 2.2 pmol/L with exenatide BID (difference, 4.3 ± 2.7 pmol/L; nominal P = .11). Fasting glucagon concentrations increased by 7.5 ± 3.1 pmol/L with exenatide QWS‐AI and by 6.9 ± 3.8 pmol/L with exenatide BID (difference, 0.6 ± 4.6 pmol/L; nominal P = .89).
Both treatments reduced pre‐meal glucose and post‐meal excursion values after a standardized meal (Figure 2E). However, treatment with exenatide QWS‐AI resulted in a greater reduction in pre‐meal glucose, whereas treatment with exenatide BID resulted in a greater reduction in post‐meal excursions, although neither difference was statistically significant. Postprandial insulin was enhanced with exenatide QWS‐AI compared with exenatide BID after 16 weeks of treatment (Table S1, Appendix S1). Postprandial serum triglycerides decreased after 16 weeks with both exenatide QWS‐AI and exenatide BID, with a greater decrease in postprandial lipaemia with exenatide BID (Table S1, Appendix S1).
3.4. Efficacy with sulfonylurea use
The use of sulfonylurea (exenatide QWS‐AI, n = 89; exenatide BID, n = 60) vs no use of sulfonylurea (exenatide QWS‐AI, n = 140; exenatide BID, n = 86) was associated with a lesser reduction in HbA1c, FPG and body weight for both treatments (Figure S1, Appendix S1). A treatment‐by‐sulfonylurea use interaction was not significant for the change in HbA1c (P = .21) or body weight (P = .068) but was significant for the change in FPG over 28 weeks (P = .024), indicating that sulfonylurea use was associated with a lesser FPG response to exenatide.
3.5. Cardiovascular risk factors
Several cardiovascular risk factors were evaluated after 28 weeks (Table S2, Appendix S1). Both exenatide QWS‐AI (−0.8 ± 1.1 mm Hg) and exenatide BID (−1.6 ± 1.4 mm Hg) recipients had reductions in systolic BP (difference, 0.8 ± 1.6 mm Hg; nominal P = .61). Diastolic BP increased slightly with exenatide QWS‐AI and decreased with exenatide BID (difference, 2.0 ± 0.9 mm Hg; nominal P = .03). Heart rate increased slightly in both groups (mean ± SD: exenatide QWS‐AI, 1.9 ± 9.4 beats/min; exenatide BID, 1.6 ± 8.5 beats/min). There were no clinically meaningful changes in fasting lipids or other cardiovascular risk factors, including high‐sensitivity C‐reactive protein, brain natriuretic peptide or urinary albumin/creatinine ratio (data not shown).
3.6. Safety and tolerability results
The most common AEs were gastrointestinal in nature (Table 2) and occurred more often with exenatide BID than with exenatide QWS‐AI. The proportion of patients with gastrointestinal AEs was lower with exenatide QWS‐AI (n = 52/229; 22.7%) than with exenatide BID (n = 52/146; 35.6%); most gastrointestinal AEs in both groups were of mild or moderate intensity. Injection site‐related AEs were more frequent overall with exenatide QWS‐AI (n = 61/229; 26.6%) than with exenatide BID (n = 6/146; 4.1%). Nodules were the most common injection site‐related AE in the exenatide QWS‐AI group (n = 29/229; 12.7%) but were less frequent with exenatide BID (n = 1/146; 0.7%); in most cases, nodules were single events, of mild intensity, and were reported during the first 60 days of treatment. Other injection site‐related AEs, occurring in ≥3% of patients in the exenatide QWS‐AI and exenatide BID groups, respectively, were pruritus (4.4% [n = 10/229] vs 0.7% [n = 1/146]), erythema (3.5% [n = 8/229] vs 0.7% [n = 1/146]), injection‐site mass (3.1% [n = 7/229] vs 0.0% [n = 0/146]) and pain (3.1% [n = 7/229] vs 0.0% [n = 0/146]). Of note, there were fewer withdrawals because of AEs among patients treated with exenatide QWS‐AI (n = 6/229; 2.6%) than with exenatide BID (n = 8/148; 5.4%).
Table 2.
Incidence of AEs and hypoglycaemia (all treated population)
| Exenatide QWS‐AI (n = 229) | Exenatide BID (n = 146) | |
|---|---|---|
| All patients with AEs | 162 (70.7) | 108 (74.0) |
| AEs occurring in ≥5% of patients | ||
| Nausea | 22 (9.6) | 31 (21.2) |
| Injection‐site nodule | 29 (12.7) | 1 (0.7) |
| Diarrhea | 12 (5.2) | 17 (11.6) |
| Headache | 13 (5.7) | 9 (6.2) |
| Upper respiratory tract infection | 13 (5.7) | 5 (3.4) |
| Vomiting | 8 (3.5) | 9 (6.2) |
| Patients with serious AEs | 6 (2.6) | 7 (4.8) |
| Patients with AEs leading to withdrawal | 11 (4.8) | 11 (7.5) |
| Hypoglycaemia | ||
| With concomitant sulfonylurea usea | n = 89 | n = 60 |
| Major hypoglycaemia | 0 (0.0) | 0 (0.0) |
| Minor hypoglycaemia | 22 (24.7) | 11 (18.3) |
| Symptoms of hypoglycaemia | 24 (27.0) | 15 (25.0) |
| Without concomitant sulfonylurea usea | n = 140 | n = 86 |
| Major hypoglycaemia | 0 (0.0) | 0 (0.0) |
| Minor hypoglycaemia | 3 (2.1) | 3 (3.5) |
| Symptoms of hypoglycaemia | 12 (8.6) | 5 (5.8) |
Abbreviations: AE, adverse event; BID, twice daily; QWS‐AI, once‐weekly suspension by autoinjector.
Data are given as n (%).
At screening.
No severe hypoglycaemia occurred during the study. Minor hypoglycaemia occurred most often with concomitant sulfonylurea use. Six patients (2.6%) receiving exenatide QWS‐AI and 7 patients (4.8%) receiving exenatide BID experienced serious AEs; most serious AEs were considered not related to study treatment by the investigator. Of interest, 1 patient treated with exenatide QWS‐AI experienced a serious AE of myocardial infarction and 1 experienced pancreatitis, both of which were confirmed by adjudication. One patient treated with exenatide BID experienced a serious AE of basal cell carcinoma (adjudicated), 1 patient experienced uterine leiomyoma and 1 patient experienced acute renal failure (while also experiencing serious AEs of lactic acidosis, septic shock and toxic encephalopathy on the same day). There were no meaningful changes in laboratory safety parameters.
Among patients with available antibody data at week 28, 53.9% of patients treated with exenatide QWS‐AI and 28.8% of patients treated with exenatide BID were positive for exenatide antibodies. The majority of these patients displayed low titers, and antibody status had no observable impact on the change in HbA1c over 28 weeks (Table S3, Appendix S1).
3.7. Pharmacokinetics
The pharmacokinetic‐evaluable population consisted of 191 patients treated with exenatide QWS‐AI. Samples with the highest antibody titers (>625) were excluded because of the potential for antibodies to interfere with the assay. Plasma exenatide concentrations increased from baseline through week 10, at which point steady state was achieved and concentrations remained relatively stable (Figure 2F).
4. DISCUSSION
Longer‐acting GLP‐1RAs developed after exenatide BID, the first‐in‐class GLP‐1RA, have been administered with reduced injection frequency or with simpler injection devices to improve ease of use and patient satisfaction. This study compared administration of a long‐acting, soluble formulation of exenatide dosed QW using an autoinjector (exenatide QWS‐AI) vs the short‐acting, BID formulation (exenatide BID) over 28 weeks. Although HbA1c was significantly reduced with both formulations, the HbA1c reduction was significantly greater with exenatide QWS‐AI than with exenatide BID. A comparable number of patients achieved HbA1c targets with exenatide QWS‐AI and exenatide BID. Although there were clinically relevant reductions from baseline in FPG, body weight and 2‐hour PPG with both treatments, there were no significant differences between formulations. Fasting insulin and glucagon concentrations increased and postprandial triglycerides decreased with both formulations after 16 weeks. Gastrointestinal AEs were the most common AE overall, but fewer events occurred in the exenatide QWS‐AI group. Injection site‐related AEs occurred more often with exenatide QWS‐AI than with exenatide BID, as anticipated because of the microspheres in the long‐acting formulation. Compliance was high among both groups and was therefore unlikely to have affected the HbA1c differences between groups. Thus, exenatide QWS‐AI is a potential alternative to exenatide BID for the treatment of type 2 diabetes.
The differences in treatment effects for exenatide QWS‐AI and exenatide BID are consistent with their different pharmacokinetic profiles. Exenatide QWS‐AI, which releases exenatide from microspheres over 10 weeks, is long acting and stimulates insulin secretion between doses, improving FPG.7 In contrast, short‐acting exenatide BID (half‐life, 2.1 hours), which is administered before the 2 largest meals each day, affects PPG by temporarily stimulating insulin secretion and delaying gastric emptying.7, 8 The DURATION‐1 and DURATION‐5 studies, which compared exenatide QW and exenatide BID, found that exenatide QW resulted in significantly greater reductions in HbA1c and FPG.9, 10 In DURATION‐1, exenatide BID resulted in a significantly greater reduction in 2‐hour PPG (data not reported for DURATION‐5). Although the reductions in FPG and 2‐hour PPG were not significantly different between exenatide QWS‐AI and exenatide BID for the current study, the trends are consistent with the DURATION‐1 and DURATION‐5 findings.
Exenatide BID is a well‐established comparator or “standard” for new members of the GLP‐1RA class. Other studies comparing exenatide BID with long‐acting or continuously acting GLP‐1RAs, including liraglutide,11 albiglutide12 and dulaglutide,13 have also demonstrated generally greater efficacy in reducing HbA1c and FPG, with lesser effects on PPG, for long‐acting GLP‐1RAs compared with exenatide BID. Consistent with the known weight‐loss effects of the GLP‐1RA class,14, 15 weight loss was similar between long‐acting GLP‐1RAs and exenatide BID. Thus, the findings of the current study are consistent with those of others comparing long‐acting GLP‐1RAs with exenatide BID.
GLP‐1RAs differ in safety profiles. Similar to the current study, in a pooled analysis comparing long‐acting (exenatide QW, liraglutide) and short‐acting (exenatide BID) GLP‐1RAs, upper gastrointestinal AEs, most commonly nausea and vomiting, appeared less frequently with exenatide QW or liraglutide than with exenatide BID.16 Diarrhea occurred at a similar frequency. The improved tolerability of the long‐acting formulations of exenatide may be related to the gradual increase in plasma concentration from the microspheres, which provides a natural titration process.2 However, the microsphere formulation contributed to the increased frequency of injection‐site reactions reported in the current study with exenatide QWS‐AI compared with exenatide BID, because the drug being administered was the same. The DURATION‐19 and DURATION‐510 studies also found injection‐site reactions to occur more frequently with exenatide QW than with exenatide BID.
In addition to increasing the incidence of hypoglycaemia, the current study found concomitant sulfonylurea use to be associated with reduced efficacy, of both exenatide QWS‐AI and exenatide BID. The EUREXA study, which compared exenatide BID with glimepiride (a sulfonylurea), found that treatment failure was less common with exenatide BID than with glimepiride (41% with exenatide BID, 54% with glimepiride; P = .002).17 HbA1c was also reduced significantly more with exenatide BID treatment than with a sulfonylurea. It is possible that sulfonylurea use in combination with a GLP‐1RA, as well as in monotherapy, negatively affects treatment response over time. Alternatively, patients taking a sulfonylurea may have more advanced type 2 diabetes than patients undergoing monotherapy, or may experience a more rapid decline in beta‐cell function.18
This study has limitations. The open‐label design may have contributed to bias in the study conduct and patient behaviors. Proportionately, more patients withdrew from the exenatide BID group. In addition, the sample size was too small for conclusions to be made on potential interactions between demographic characteristics and response to therapy, and the imbalance in the randomization ratio may have affected the power for primary and secondary end points. Diet and exercise behaviors were not recorded.
Overall, this study found that both the new formulation (exenatide QWS‐AI) and exenatide BID improved glycaemic control and reduced body weight in patients with type 2 diabetes. However, treatment with exenatide QWS‐AI resulted in a greater improvement in HbA1c. Although not statistically significant, there was a greater reduction in FPG with exenatide QWS‐AI and a greater reduction in 2‐hour PPG with exenatide BID. This pattern of efficacy is characteristic for a long‐acting GLP‐1RA. Additionally, exenatide QWS‐AI was associated with an improved AE profile and fewer treatment discontinuations than was exenatide BID.
Supporting information
Appendix S1.
ACKNOWLEDGEMENTS
This study was supported by Bristol‐Myers Squibb and AstraZeneca. Mollie Marko PhD, of inScience Communications, Springer Healthcare (New York, New York, USA), provided medical writing support funded by AstraZeneca.
This work was presented previously at the International Congress of Endocrinology (ICE), June 21 to 24, 2014, San Francisco, California: Wysham CH, Rosenstock J, Malloy J, Vetter M, He Y, Öhman P, Iqbal N. DURATION‐NEO‐1: Greater A1C reductions with exenatide suspension once weekly by autoinjector pen vs exenatide twice daily in inadequately controlled type 2 diabetes.
Conflict of interest
C. H. W. has received research support and served as a consultant, advisor and speaker for AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Novo Nordisk and Sanofi. J. R. has received research support from AstraZeneca, Boehringer Ingelheim, Bristol‐Myers Squibb, Daiichi Sankyo, Eli Lilly, GlaxoSmithKline, Hanmi, Intarcia, Janssen, Lexicon, Merck, Novo Nordisk, Pfizer and Sanofi, and has served on advisory boards of, or received consulting honoraria from, Boehringer Ingelheim, Daiichi Sankyo, Eli Lilly, Intarcia, Janssen, Merck, Novo Nordisk and Sanofi. M. L. V. was an employee of Bristol‐Myers Squibb during the conduct of the study. N. I. and P. Ö. are employees of AstraZeneca. F. D. was an employee of AstraZeneca during the development of this manuscript.
Author contributions
C. H. W., N. I. and F.D. contributed to the acquisition, analysis and interpretation of data and the outline and revision of the manuscript. J. R., M. L. V. and N. I. contributed to the outline of the manuscript, and were involved in the writing and discussion of the manuscript, analyses and interpretation of the data, and all critical revisions of the manuscript. P. Ö. is the guarantor of the work and takes full responsibility for the contents.
Wysham CH, Rosenstock J, Vetter ML, Dong F, Öhman P, Iqbal N. Efficacy and tolerability of the new autoinjected suspension of exenatide once weekly versus exenatide twice daily in patients with type 2 diabetes. Diabetes Obes Metab. 2018;20:165–172. https://doi.org/10.1111/dom.13056
Funding information This study was supported by Bristol‐Myers Squibb and AstraZeneca.
REFERENCES
- 1.BYDUREON (Exenatide Extended‐Release) for Injectable Suspension: US Prescribing Information. Wilmington, Delaware: AstraZeneca Pharmaceuticals LP; 2017. https://www.azpicentral.com/bydureon/pi_bydureon.pdf#page=1. Accessed June 28, 2017.
- 2. Fineman M, Flanagan S, Taylor K, et al. Pharmacokinetics and pharmacodynamics of exenatide extended‐release after single and multiple dosing. Clin Pharmacokinet. 2011;50:65–74. [DOI] [PubMed] [Google Scholar]
- 3. Wysham CH, MacConell LA, Maggs DG, Zhou M, Griffin PS, Trautmann ME. Five‐year efficacy and safety data of exenatide once weekly: long‐term results from the DURATION‐1 randomized clinical trial. Mayo Clin Proc. 2015;90:356–365. [DOI] [PubMed] [Google Scholar]
- 4. Henry RR, Klein EJ, Han J, Iqbal N. Efficacy and tolerability of exenatide once weekly over 6 years in patients with type 2 diabetes: an uncontrolled open‐label extension of the DURATION‐1 study. Diabetes Technol Ther. 2016;18:677–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.BYETTA (Exenatide) Injection: US Prescribing Information. Wilmington, Delaware: AstraZeneca Pharmaceuticals, LP; 2015. https://www.azpicentral.com/byetta/pi_byetta.pdf#page=1. Accessed June 28, 2017.
- 6. Fineman MS, Mace KF, Diamant M, et al. Clinical relevance of anti‐exenatide antibodies: safety, efficacy and cross‐reactivity with long‐term treatment. Diabetes Obes Metab. 2012;14:546–554. [DOI] [PubMed] [Google Scholar]
- 7. Meier JJ. GLP‐1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol. 2012;8:728–742. [DOI] [PubMed] [Google Scholar]
- 8. Marathe CS, Rayner CK, Jones KL, Horowitz M. Relationships between gastric emptying, postprandial glycemia, and incretin hormones. Diabetes Care. 2013;36:1396–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Drucker DJ, Buse JB, Taylor K, et al. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open‐label, non‐inferiority study. Lancet. 2008;372:1240–1250. [DOI] [PubMed] [Google Scholar]
- 10. Blevins T, Pullman J, Malloy J, et al. DURATION‐5: exenatide once weekly resulted in greater improvements in glycemic control compared with exenatide twice daily in patients with type 2 diabetes. J Clin Endocrinol Metab. 2011;96:1301–1310. [DOI] [PubMed] [Google Scholar]
- 11. Buse JB, Rosenstock J, Sesti G, et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26‐week randomised, parallel‐group, multinational, open‐label trial (LEAD‐6). Lancet. 2009;374:39–47. [DOI] [PubMed] [Google Scholar]
- 12. Rosenstock J, Reusch J, Bush M, Yang F, Stewart M. Albiglutide study group. Potential of albiglutide, a long‐acting GLP‐1 receptor agonist, in type 2 diabetes: a randomized controlled trial exploring weekly, biweekly, and monthly dosing. Diabetes Care. 2009;32:1880–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wysham C, Blevins T, Arakaki R, et al. Efficacy and safety of dulaglutide added onto pioglitazone and metformin versus exenatide in type 2 diabetes in a randomized controlled trial (AWARD‐1). Diabetes Care. 2014;37:2159–2167. [DOI] [PubMed] [Google Scholar]
- 14. Sun F, Chai S, Li L, et al. Effects of glucagon‐like peptide‐1 receptor agonists on weight loss in patients with type 2 diabetes: a systematic review and network meta‐analysis. J Diabetes Res. 2015;2015:157201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Potts JE, Gray LJ, Brady EM, Khunti K, Davies MJ, Bodicoat DH. The effect of glucagon‐like peptide 1 receptor agonists on weight loss in type 2 diabetes: a systematic review and mixed treatment comparison meta‐analysis. PLoS One. 2015;10:e0126769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Horowitz M, Aroda VR, Han J, Hardy E, Rayner CK. Upper and/or lower gastrointestinal adverse events with glucagon‐like peptide‐1 receptor agonists: incidences and consequences. Diabetes Obes Metab. 2017;19:672–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gallwitz B, Guzman J, Dotta F, et al. Exenatide twice daily versus glimepiride for prevention of glycaemic deterioration in patients with type 2 diabetes with metformin failure (EUREXA): an open‐label, randomised controlled trial. Lancet. 2012;379:2270–2278. [DOI] [PubMed] [Google Scholar]
- 18. Shin MS, JH Y, Jung CH, et al. The duration of sulfonylurea treatment is associated with beta‐cell dysfunction in patients with type 2 diabetes mellitus. Diabetes Technol Ther. 2012;14:1033–1042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1.


