Abstract
Aims
Diabetes‐related complications have declined during the past two decades. We aimed to examine whether mortality in people with diabetes improved over time in the 1999 to 2010 National Health and Nutrition Examination Survey (NHANES).
Methods
We conducted a prospective cohort study using 1999 to 2004 and 2005 to 2010 data from the NHANES. For primary analyses, we compared the unadjusted, age‐adjusted and multivariable‐adjusted hazard ratios (HR) for mortality outcomes (total, cardiovascular, cardiac and cancer deaths) of the participants with diabetes with those without diabetes using Cox proportional hazard models.
Results
For each mortality outcome, HR (95% confidence interval) in diabetic participants during the period 2005 to 2010 was lower than that during the period 1999 to 2004 (all‐cause death, 2.76 [1.87‐4.08] vs 4.23 [2.57‐6.98]; cardiovascular death, 2.70 [1.20‐6.04] vs 8.82 [3.28‐23.70]; cardiac death, 2.45 [0.98‐6.09] vs 15.55 [7.01‐34.50]; cancer death, 2.33 [0.87‐6.23] vs 3.03 [1.20‐7.65]). Compared with mortality outcome during the period 1999 to 2004, greater declines in mortality during the period 2005 to 2010 were observed for cardiovascular (−54.0%) and cardiac deaths (−64.8%). In age‐adjusted and multivariable‐adjusted models, the cumulative event rates for total, cardiovascular and cardiac deaths were not significantly different between participants with and without diabetes during the period 2005–2010; this was not the case during the period 1999–2004. The leading cause of death was malignant neoplasm during the period 2005–2010.
Conclusion
Considerably improved outcomes for total, cardiovascular and cardiac deaths were observed in people with diabetes during the 2005 to 2010 NHANES compared to the 1999 to 2004 NHANES.
Keywords: cancer, cardiovascular disease, cardiovascular risk, diabetes mellitus, mortality
1. INTRODUCTION
The number of patients with diabetes has grown worldwide and approximately 12% of the adult population in the USA has diabetes.1, 2, 3 Because diabetes negatively affects population health and health economics in various ways,4 multifaceted interventions should be conducted for different populations. That is, in addition to interventions aiming to prevent diabetes,5 it is important to control serious complications and prolong life expectancy. Diabetes is associated with an increased risk of cardiovascular events such as myocardial infarction and stroke.6 Although it has not been proven conclusively that intensive glycaemic control alone reduces cardiovascular events in patients with diabetes,7 intensive interventions with multiple drug combinations and behaviour modification have proven beneficial effects on cardiovascular mortality.8, 9 Patients with diabetes have reportedly become more successful in controlling their risk factors and have improved adherence to preventive practices.10 Moreover, rates of diabetes‐related complications have declined in the past 2 decades.10, 11 Although mortality rates in patients with diabetes remain higher compared to those without diabetes, reductions in all‐cause mortality in individuals with diabetes have occurred over time throughout the world.12, 13, 14, 15, 16 Therefore, we aimed to examine whether there were improved outcomes over time in individuals with diabetes in the 1999 to 2010 US NHANES. In particular, we compared survival rates between adults with diabetes during the periods 1999 to 2004 and 2005 to 2010. Additionally, we investigated whether the causes of death in the population with diabetes have changed during the last decade.
2. MATERIALS AND METHODS
2.1. Data source and study population
This was a prospective cohort study using data from the US National Health and Nutrition Examination Survey (NHANES) of adults and children. Written informed consent was obtained from all participants. The National Center for Health Statistics (NCHS) Research Ethics Review Board approved the NHANES protocols. NHANES is conducted by the NCHS, part of the Centers for Disease Control and Prevention, Hyattsyille, Maryland. It uses a stratified, multistage probability sampling design, which is intended to be representative of the US civilian, non‐institutionalized population. Data were collected at home and mobile examination centres. Blood specimens were collected during the mobile centre examination.
Among the population participating in the NHANES during the period 1999 to 2010, the unweighted response rate of household interviews was 80.6% and that of the mobile centre examinations was 77.1%. We focused on the participants in NHANES 1999 to 2010 (n = 62 160). Among them, we excluded participants younger than 20 years (n = 29 696). In addition, we excluded participants without fasting blood samples (n = 19 464) because these were necessary for the diagnosis of diabetes. The final main study size was 13 000 participants. To assess whether there were improved outcomes in the 2005 to 2010 surveys compared to the 1999 to 2004 surveys, participants were categorized into 2 groups, 1999 to 2004 (n = 6036) and 2005 to 2010 (n = 6964), and were followed up for a minimum of 2 years.
2.2. Definition of diabetes
We defined diabetes using 1 of the following 5 criteria: previous diagnosis of diabetes, intake of anti‐diabetic medications or insulin, glycated haemoglobin level of ≥6.5%, fasting glucose level of ≥126 mg/dL, or a 2‐h glucose level of ≥200 mg/dL after an oral glucose tolerance test.17 A total of 5462 (44%) study participants without intake of anti‐diabetic medications or insulin were checked for 2‐h glucose levels. Participants who did not satisfy any of these 5 criteria were defined as without diabetes.
2.3. Outcome measurements
Main outcome measurements in this study were total, cardiovascular, cardiac and cancer mortalities. Additionally, these mortality risks in the last decade were compared between participants with and without diabetes. We also investigated whether the causes of death in participants with or without diabetes changed.
We used publicly released mortality follow‐up data, provided in the Public‐use Linked Mortality Files.5. These files are available for NHANES 1999 to 2010 and have been updated through December 31, 2011. We prospectively followed study participants from the survey participation interview date until the date of death or until December 31, 2011. To identify causes of death in participants, NHANES used the International Classification of Diseases, Tenth Revision for deaths occurring in or after 1999. The specific codes used were as follows: I00–I09, I11, I13 and I20‐I51 for causes of death from diseases of the heart (cardiac death); C00‐C97 for causes of death from malignant neoplasms (cancer death); J40‐J47 for causes of death from chronic lower respiratory tract diseases; V01‐X59 and Y85‐Y86 for causes of death from accidents; and I60‐I69 for causes of death from cerebrovascular diseases. All other causes of death were residual. Cardiovascular death was defined as death resulting from cardiac and cerebrovascular diseases.
2.4. Other measurements
We extracted data on potential confounders, including age, sex, race and ethnicity, educational level, obesity, smoking status, and diagnoses of hypertension and hyperlipidaemia. Because individuals 80 years of age and over are top‐coded at 80 years of age in NHANES data, age was divided into 4 groups: 20–39, 40–59, 60–79 and ≥80 years. Additionally, we performed sensitivity analyses using a narrower age categorization by 5‐year intervals. Race and ethnicity were classified into non‐Hispanic white, non‐Hispanic black, Mexican American, and other, including other Hispanic and multi‐racial participants.
Obesity was defined as body mass index (BMI), calculated as weight in kilograms divided by height in meters‐squared of ≥30 kg/m2, based on measurements at the mobile examination centre examination. Low‐density lipoprotein (LDL) cholesterol, high‐density lipoprotein (HDL) cholesterol and triglyceride levels were evaluated in the morning after ≥8.5‐h fasting. LDL cholesterol was calculated using the Friedewald equation (total cholesterol − HDL cholesterol − triglycerides/5) for fasting participants examined in the morning, with triglyceride levels of ≤400 mg/dL (to convert triglycerides to millimoles per liter, multiply by 0.0113).18 Dyslipidaemia was defined as a previous diagnosis of hyperlipidaemia, intake of lipid‐lowering medications, LDL cholesterol levels of ≥160 mg/dL, HDL cholesterol levels of <40 mg/dL or triglyceride levels of ≥200 mg/dL.19
Systolic and diastolic blood pressure were measured 3 times after resting quietly in a sitting position for 5 minutes and representative values were calculated as suggested in the analytic guideline (average of the last 2 measurements were used in 90.5%). Hypertension was defined as either a previous diagnosis of hypertension or intake of anti‐hypertensive medications.
Diabetic retinopathy, chronic kidney disease, ischaemic heart disease and stroke were defined as diabetes‐related complications. The presence or absence of albuminuria was also assessed. Diabetic retinopathy was ascertained by self‐reporting. Chronic kidney disease was defined as impaired glomerular filtration rate (GFR) of <60 mL/min/1.73 m2.7, 17 Estimated GFR was calculated by the Modification of Diet in Renal Disease (MDRD): estimated GFR (mL/min/1.73 m2) = 175 × (Scr)−1.154 × (Age)−0.203 × 0.742 for female × 1.212 for African Americans.20 Serum creatinine, urine albumin and urine creatinine levels were measured during mobile centre examination. Ischaemic heart disease was defined as myocardial infarction or angina pectoris. Ischaemic heart disease and stroke were self‐reported during home interview.
2.5. Statistical analysis
Demographic data were presented as numbers with proportions (%) or means with standard deviations (SD). Participants during the period 1999 to 2004 were compared with those during the period 2005 to 2010 using t‐test for continuous variables and chi‐square test for categorical variables.
For primary analyses of the 4 outcomes (total, cardiovascular, cardiac and cancer mortalities), we analysed the unadjusted, age‐adjusted and multivariable‐adjusted hazard ratios (HR) in participants with diabetes in both the 1999 to 2004 and 200 to 2010 groups, compared to those without diabetes, using Cox proportional hazard models. Furthermore, we tested for interactions between diabetes and 2 time periods in the multivariable model. Kaplan–Meier survival curves for mortality outcomes in participants with and without diabetes were constructed with weighting provided by NCHS, to show the survival curves in the overall US population with and without diabetes. In the multivariable analysis, we included age, sex, race and ethnicity, educational level, smoking status, BMI (continuous), dyslipidaemia and hypertension for adjustment. In the multivariable analysis in participants with diabetes, we excluded those with missing information on education level (n = 5), smoking status (n = 4), BMI (n = 69), dyslipidaemia (n = 167) and hypertension (n = 5). We conducted additional analysis using the multivariable model, including history of ischaemic heart disease, stroke and cancer, and co‐morbidity of chronic kidney disease. We also performed Cox proportional hazard analyses to calculate HR in participants with diabetes in the 2005 to 2010 group compared with those in the 1999 to 2004 group. Survival rates of participants with diabetes in the 1999 to 2004 group were compared with those in the 2005 to 2010 group using the same method. All event rates were expressed as number of events per 1000 person years and differences in absolute rates between participants with diabetes in the 1999 to 2004 and 2005 to 2010 groups were calculated. Causes of death were assessed in participants both with and without diabetes.
To exclude the potential effect of a large increase in detection or diagnostic practices, we conducted sensitivity analyses limited to participants with ≥1 year duration of diabetes.
All statistical analyses were conducted using STATA software (version 14.1, Stata Corp, College Station, Texas), accounting for the complex survey design. Following recommendations of the Centers for Disease Control and Prevention, we used appropriate weighting for each analysis, based on the selected variables without including participants with missing information. The weights were provided by the NCHS and accounted for unequal probabilities of selection and non‐responses to provide unbiased national estimates representative of the US population.21 Taylor series linearization, which the NCHS currently recommends in all NHANES surveys, was used for variance estimation. In addition, for further understanding of the public health impact of diabetes, age‐standardized mortality ratios of the participants with diabetes in the 1999 to 2004 and 2005 to 2010 groups were also calculated, using census 2000 population data, as the recommended standard population for the continuous NHANES dataset.22 P values <.05 were considered statistically significant. Given the lack of statistical power inherent in the interaction tests, we used a P value cut‐off of <.2 for such tests.23, 24
3. RESULTS
3.1. Baseline characteristics of US participants with and without diabetes
The characteristics of participants aged ≥20 years are presented in Table 1. Among participants with and without diabetes during the period 1999 to 2010, distribution of individuals ≥60 years was 50.9% and 19.0%, and that of female sex was 49.5% and 52.3%, respectively. Prevalence of obesity was 55.7% and 29.3%, dyslipidaemia was 77.3% and 59.8%, hypertension was 60.4% and 25.0%, and cardiovascular disease was 18.0% and 5.0%, respectively. Among study participants, 724 (12.0%) and 1418 (20.4%) had diabetes during the periods 1999 to 2004 and 2005 to 2010. Among participants without diabetes, age, sex, race and ethnicity did not change between the periods 1999 to 2004 and 2005 to 2010, whereas the proportion with an above high‐school educational level and obesity prevalence were significantly higher in the 2005 to 2010 group than in the 1999 to 2004 group. Among participants with diabetes, the proportion of participants aged ≥80 years was significantly higher in the 2005 to 2010 group than in the 1999 to 2004 group. The proportion of those with a status of current smoking tended to be lower in all participants in the 2005 to 2010 group than in the 1999 to 2004 group. Obesity prevalence was higher in the 2005 to 2010 group than in the 1999 to 2004 group. Compared with those in the 1999 to 2004 group, participants in the 2005 to 2010 group had a significantly lower prevalence of dyslipidaemia and a significantly higher proportion of individuals using lipid‐lowering medications. LDL cholesterol and triglyceride levels were significantly lower and HDL cholesterol levels were significantly higher in all participants in the 2005 to 2010 group. Systolic and diastolic blood pressure levels were lower in all participants in the 2005 to 2010 group than those in the 1999 to 2004 group. Among participants with diabetes, HbA1c levels were significantly lower and the proportion of individuals who used insulin was significantly higher in the 2005 to 2010 group than in the 1999 to 2004 group. The prevalence of microvascular and macrovascular complications in all participants did not differ significantly between the 1999 to 2004 and 2005 to 2010 groups.
Table 1.
Baseline characteristics of diabetic and non‐diabetic US participants aged ≥20 y during the period 1999 to 2010*
| Characteristics | Non‐DM | DM | ||||||
|---|---|---|---|---|---|---|---|---|
| Period | 1999 to 2010 | 1999 to 2004 | 2005 to 2010 | P value | 1999 to 2010 | 1999 to 2004 | 2005 to 2010 | P value |
| Unweighted sample | 10 858 | 5312 | 5546 | 2142 | 724 | 1418 | ||
| Age range, y | ||||||||
| 20 to 39 | 42.7% | 42.9% | 42.5% | .75 | 10.1% | 10.8% | 9.7% | .47 |
| 40 to 59 | 38.3% | 37.6% | 39.0% | .27 | 40.0% | 42.3% | 36.9% | .08 |
| 60 to 79 | 15.7% | 16.0% | 15.4% | .61 | 41.8% | 39.8% | 43.0% | .32 |
| ≥80 | 3.3% | 3.5% | 3.1% | .43 | 9.1% | 7.1% | 10.4% | .01 |
| Female sex | 52.3% | 52.6% | 52.0% | .52 | 49.5% | 46.7% | 51.2% | .10 |
| Race and ethnicity | ||||||||
| Non‐Hispanic white | 71.4% | 72.6% | 70.4% | .38 | 65.9% | 64.4% | 66.9% | .56 |
| Non‐Hispanic black | 10.5% | 10.3% | 10.7% | .81 | 13.7% | 14.1% | 13.5% | .76 |
| Mexican American | 7.8% | 7.3% | 8.2% | .50 | 8.5% | 7.1% | 9.3% | .27 |
| Othersa | 10.3% | 9.8% | 10.8% | .54 | 11.9% | 14.4% | 10.3% | .18 |
| Educational attainment | ||||||||
| Less than high school | 18.1% | 19.2% | 17.0% | .06 | 28.7% | 30.9% | 27.3% | .21 |
| High school or GED | 24.8% | 26.1% | 23.5% | .05 | 27.9% | 25.7% | 29.3% | .21 |
| More than high school | 57.1% | 54.7% | 59.5% | .01 | 43.4% | 43.4% | 43.4% | .98 |
| Current smoking | 23.5% | 24.4% | 22.7% | .22 | 18.9% | 21.7% | 17.1% | .06 |
| Body mass index (kg/m2)b | 27.8 (5.3) | 27.6 (5.0) | 28.0 (5.7) | .01 | 32.0 (7.3) | 31.8 (6.8) | 32.1 (7.6) | .53 |
| Obesityc | 29.3% | 27.9% | 30.7% | .02 | 55.7% | 52.7% | 57.5% | .16 |
| Dyslipidaemiad | 59.8% | 62.6% | 57.2% | <.001 | 77.3% | 81.4% | 74.7% | .004 |
| Lipid lowering medications | 20.7% | 17.4% | 24.3% | <.001 | 49.2% | 35.7% | 58.5% | <.001 |
| LDL cholesterol (mg/dL) | 119.0 (30.2) | 121.2 (29.6) | 116.9 (30.7) | <.001 | 111.3 (35.6) | 116.5 (30.9) | 108.2 (38.4) | <.001 |
| HDL cholesterol (mg/dL) | 53.8 (13.7) | 52.5 (13.1) | 55.0 (14.3) | <.001 | 49.0 (15.0) | 46.8 (12.9) | 50.3 (16.2) | .001 |
| Triglyceride (mg/dL) | 134 (96) | 141 (104) | 127 (86) | <.001 | 186 (191) | 209 (220) | 172 (163) | .002 |
| Hypertensione | 25.0% | 24.4% | 25.6% | .34 | 60.4% | 56.1% | 63.2% | .01 |
| Anti‐hypertensive medications | 85.3% | 85.2% | 85.3% | .94 | 93.4% | 92.6% | 93.8% | .57 |
| Systolic blood pressure (mm Hg) | 119.5 (14.3) | 120.9 (14.7) | 118.2 (13.7) | <.001 | 129.5 (19.7) | 130.9 (18.1) | 128.7 (20.6) | .07 |
| Diastolic blood pressure (mm Hg) | 70.2 (10.1) | 72.1 (9.6) | 68.6 (10.5) | <.001 | 68.8 (15.2) | 70.7 (13.7) | 67.6 (16.1) | .005 |
| HbA1c (%) | 5.2 (0.3) | 5.2 (0.2) | 5.3 (0.3) | <.001 | 6.9 (1.6) | 7.1 (1.5) | 6.8 (1.6) | .004 |
| Insulin use | – | – | – | 11.4% | 5.3% | 15.2% | <.001 | |
| Anti‐diabetic medications | – | – | – | 66.0% | 67.8% | 64.9% | .47 | |
| Microvascular diseases | ||||||||
| Diabetic retinopathy | – | – | – | 19.4% | 19.8% | 19.0% | .78 | |
| Chronic kidney diseasef | 5.7% | 5.1% | 6.2% | .09 | 18.0% | 15.5% | 19.5% | .15 |
| Urine albumin (mg/g creatinine) | ||||||||
| <30 | 93.1% | 93.2% | 93.1% | .90 | 73.7% | 72.3% | 74.6% | .38 |
| 30 to 299 | 6.1% | 6.2% | 6.0% | .77 | 20.6% | 21.9% | 19.8% | .38 |
| ≥300 | 0.8% | 0.6% | 0.9% | .14 | 5.7% | 5.8% | 5.6% | .91 |
| Macrovascular diseases | ||||||||
| Cardiovascular disease | 5.0% | 5.2% | 4.8% | .48 | 18.0% | 17.2% | 18.5% | .61 |
| Ischaemic heart disease | 3.6% | 3.8% | 3.7% | .68 | 13.4% | 13.8% | 13.1% | .75 |
| Stroke | 2.0% | 2.1% | 1.9% | .55 | 7.3% | 6.1% | 8.1% | .17 |
| Cancer | 7.6% | 7.5% | 7.7% | .84 | 15.9% | 14.1% | 17.0% | .18 |
Abbreviations: DM, diabetes; GED, General Educational Development; HDL, high density lipoprotein; HbA1c, glycated hemoglobin; LDL, low density lipoprotein.
Data are presented as number of participants, percent, or mean (SD). P value was calculated by comparing variables during the period 1999 to 2004 with those during the period 2005 to 2010.
The category includes other Hispanics and other races including multi‐racial participants.
Body mass index was calculated as weight in kg divided by the square of height in meters.
Obesity was defined as body mass index ≥30 kg/m2.
Dyslipidaemia was defined as previous diagnosis of hyperlipidaemia, intake of lipid lowering medications, low‐density lipoprotein cholesterol levels of ≥160 mg/dL, high‐density lipoprotein cholesterol levels of <40 mg/dL, or triglyceride levels of ≥200 mg/dL.
Hypertension was defined as either a previous diagnosis of hypertension or documentation that the participant was taking anti‐hypertensive medications.
Chronic kidney disease was defined as impaired glomerular filtration rate of <60 mL/min/1.73 m2.
3.2. Mortality in participants with and without diabetes in the 1999 to 2010 group
Unadjusted Kaplan–Meier survival curves and event rates for total, cardiovascular, cardiac and cancer mortalities of all participants in the 1999 to 2010 group are shown in Figure S1 and Table S1, respectively. When all datasets from the 1999 to 2010 NHANES were used, mean follow‐up periods (±SD) were 6.6 ± 2.9 years in participants without diabetes and 5.5 ± 3.2 years in those with diabetes. The cumulative event rates for these 4 outcomes were significantly higher in participants with diabetes and the unadjusted HRs (95% confidence intervals [CI]) were 3.28 (2.73‐3.94) for all‐cause death, 4.47 (3.23‐6.18) for cardiovascular death, 4.41 (3.00‐6.50) for cardiac death and 2.51 (1.75‐3.61) for cancer death.
Age‐adjusted and multivariable‐adjusted HRs for total, cardiovascular and cardiac deaths were also significantly higher in participants with diabetes than in those without diabetes, although the results for cancer death were not significantly different between participants with and without diabetes. Causes of death in all participants are presented in Figure S2. The most common cause of death was heart disease (20.4%) in participants with diabetes and malignant neoplasm (26.0%) in participants without diabetes.
3.3. Risk of mortality in diabetic compared with non‐diabetic participants in the 1999 to 2004 and 2005 to 2010 groups
The unadjusted Kaplan–Meier survival curves for total, cardiovascular, cardiac and cancer deaths in all participants in the 1999 to 2004 and 2005 to 2010 groups are shown in Figure 1. A total of 89.9% completed 2 years of follow‐up. In the survival curves for 2005 to 2010, the differences between participants with and without diabetes narrowed considerably for cardiovascular and cardiac deaths. Compared with participants without diabetes during the same period, HRs were lower in participants with diabetes in the 2005 to 2010 group than those in the 1999 to 2004 group (all‐cause death, 2.76 [95% CI, 1.8‐4.08] vs 4.23 [95% CI, 2.57‐6.98]; cardiovascular death, 2.70 [95% CI, 1.20‐6.04] vs 8.82 [95% CI, 3.28‐23.70]; cardiac death, 2.45 [95% CI, 0.98‐6.09] vs 15.55 [95% CI, 7.01‐34.50]; cancer death, 2.33 [95% CI, 0.87‐6.23] vs 3.03 [95% CI, 1.20‐7.65]).
Figure 1.
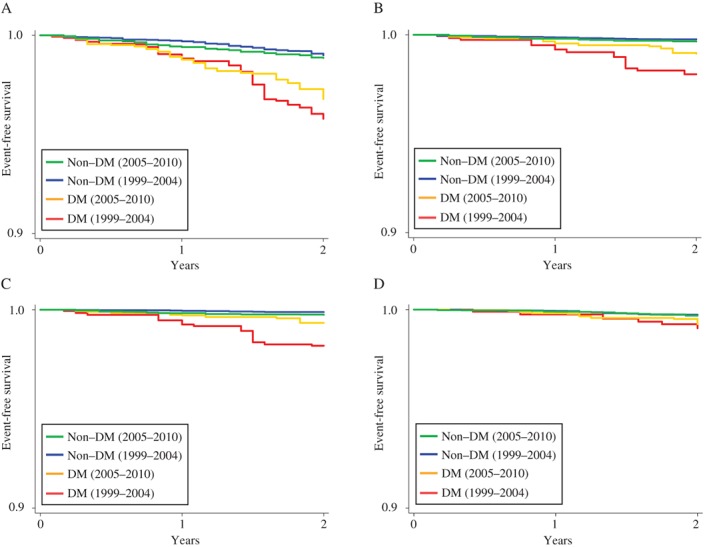
Rates of freedom from mortality in all participants during the periods 1999 to 2004 and 2005 to 2010. Rates of freedom from total death (A), cardiovascular death (B), cardiac death (C), and cancer death (D).
Age‐adjusted and multivariable‐adjusted HR in all participants in the 1999 to 2004 and 2005 to 2010 groups are presented in Table 2. Unlike those for participants in the 1999 to 2004 group, the cumulative event rates for total, cardiovascular and cardiac death did not significantly differ between participants with and without diabetes in the 2005 to 2010 group. These results did not change even when we used a narrower age categorization that spanned 5 years (Table S2) or limited participants to those who completed a 2‐year follow‐up (Table S3). Additional adjustment, including history of ischaemic heart disease, stroke and cancer, and co‐morbidity of chronic kidney disease, indicated that, compared with participants without diabetes during the same period, the HRs for all‐cause and cardiovascular death were lower in participants with diabetes in the 2005 to 2010 group than those in the 1999 to 2004 group (all‐cause death, 1.15 [95% CI, 0.67‐1.99] vs 2.20 [95% CI, 1.15‐4.19]; cardiovascular death, 1.31 [95% CI, 0.40‐3.45] vs 4.99 [95% CI, 1.42‐17.57]). Significant interaction was observed between diabetes and 2 time periods in the multivariable model (all‐cause mortality, P = .06 for the interaction term; cardiovascular mortality, P = .03 for the interaction term; and cardiac mortality, P = .001 for the interaction term).
Table 2.
Hazard ratios for mortality in participants with diabetes during the periods 1999 to 2004 and 2005 to 2010 compared with those without diabetesa
| 1999 to 2004 | 2005 to 2010 | |||
|---|---|---|---|---|
| Non‐DM (n = 5312) | DM (n = 724) | Non‐DM (n = 5546) | DM (n = 1418) | |
| Total mortality | ||||
| No. of deaths from any cause (%) | 86 (1.6%) | 36 (5.0) | 89 (1.6%) | 57 (4.0%) |
| Event rate (per 1000 person‐year) | 5.0 | 21.3 | 5.7 | 15.8 |
| Unadjusted HR (95% CI) | 1.00 [ref] | 4.23 (2.57 to 6.98) | 1.00 [ref] | 2.76 (1.87 to 4.08) |
| Age‐adjusted model | 1.00 [ref] | 2.31 (1.37‐3.91) | 1.00 [ref] | 1.21 (0.79‐1.86) |
| Multivariable‐adjusted modelb | 1.00 [ref] | 2.46 (1.18‐5.12) | 1.00 [ref] | 1.28 (0.74‐2.21) |
| Cardiovascular mortality | ||||
| No. of cardiovascular deaths (%) | 21 (0.4%) | 14 (1.9%) | 28 (0.5%) | 15 (1.1%) |
| Event rate (per 1000 person‐year) | 1.1 | 10.0 | 1.7 | 4.6 |
| Unadjusted HR (95% CI) | 1.00 [ref] | 8.82 (3.28‐23.70) | 1.00 [ref] | 2.70 (1.20‐6.04) |
| Age‐adjusted model | 1.00 [ref] | 5.66 (2.05‐15.62) | 1.00 [ref] | 1.15 (0.51‐2.60) |
| Multivariable‐adjusted model | 1.00 [ref] | 5.89 (1.47‐23.60) | 1.00 [ref] | 1.37 (0.51‐3.69) |
| Cardiac mortality | ||||
| No. of cardiac deaths (%) | 15 (0.3%) | 12 (1.7%) | 20 (0.4%) | 10 (0.7%) |
| Event rate (per 1000 person‐year) | 0.5 | 9.1 | 1.3 | 3.2 |
| Unadjusted HR (95% CI) | 1.00 [ref] | 15.55 (7.01‐34.50) | 1.00 [ref] | 2.45 (0.98‐6.09) |
| Age‐adjusted model | 1.00 [ref] | 9.94 (3.80‐25.94) | 1.00 [ref] | 1.08 (0.43‐2.71) |
| Multivariable‐adjusted model | 1.00 [ref] | 8.68 (2.45‐30.80) | 1.00 [ref] | 1.15 (0.37‐3.51) |
| Cancer mortality | ||||
| No. of cancer deaths | 27 (0.5%) | 7 (0.9%) | 23 (0.4%) | 12 (0.8%) |
| Event rate (per 1000 person‐year) | 1.5 | 4.6 | 1.5 | 3.5 |
| Unadjusted HR (95% CI) | 1.00 [ref] | 3.03 (1.20‐7.65) | 1.00 [ref] | 2.33 (0.87‐6.23) |
| Age‐adjusted model | 1.00 [ref] | 1.49 (0.60‐3.67) | 1.00 [ref] | 1.06 (0.43‐2.61) |
| Multivariable‐adjusted model | 1.00 [ref] | 0.95 (0.36‐2.46) | 1.00 [ref] | 0.93 (0.30‐3.03) |
Abbreviations: CI, confidence interval; HR, hazard ratio.
Data are presented as number, number (%), or hazard ratio (95% confidence interval).
Multivariable adjustment was made for age (20‐39, 40‐59, 60‐79, and ≥80 y), sex, race and ethnicity (non‐Hispanic white, non‐Hispanic black, Mexican American, and others), educational level (less than high school, high school graduation or General Education Development certificate, more than high school), current smoking status, body mass index, dyslipidaemia and hypertension.
All event rates were lower in participants with diabetes in the 2005 to 2010 group than those in the 1999 to 2004 group and the absolute changes (percent changes) between the 2 groups were −5.5 (−25.8%) for all‐cause death, −5.4 (−54.0%) for cardiovascular death, −5.9 (−64.8%) for cardiac death, and −1.1 (−23.9%) for cancer death. Notable decreases were for cardiovascular and cardiac deaths. In addition, age‐standardized mortality per 1000 person‐years (absolute change, percent) was lower in participants with diabetes in the 2005 to 2010 group than in those with diabetes in the 1999 to 2004 group (13.7 vs 20.9 [−7.2, −34.4%] for all‐cause death; 4.0 vs 10.0 [−6.0, −60.0%] for cardiovascular death; 2.8 vs 9.1 [−6.3, −69.2%] for cardiac death; and 3.1 vs 4.4 [−1.3, −29.5%] for cancer death).
3.4. Causes of death in participants with and without diabetes in the 1999 to 2004 and 2005 to 2010 groups
The common causes of death in all participants in the 1999 to 2004 and 2005 to 2010 groups are presented in Figure 2. In the 1999 to 2004 group, although the proportion of cancer deaths did not differ significantly between participants with and without diabetes, for cardiac death, it was significantly higher in participants with diabetes than in those without diabetes (42.8% vs 11.7%, P < .001). In the 2005 to 2010 group, the proportions of cardiac and cancer deaths were not significantly different between participants with and without diabetes (20.3% vs 22.9% for cardiac death, P = .78; 22.2% vs 26.3% for cancer death, P = .67). The leading cause of death in the 2005 to 2010 group was malignant neoplasm in all participants.
Figure 2.
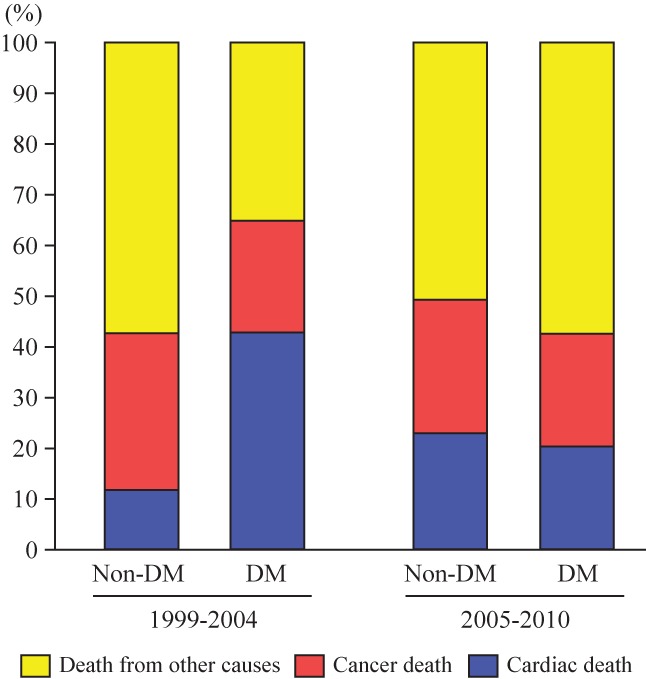
Causes of death in all participants in 1999 to 2004 and 2005 to 2010
3.5. Risk of mortality in participants with diabetes in the 1999 to 2004 group compared with those in the 2005 to 2010 group
Compared with participants with diabetes in the 1999 to 2004 group, the cumulative event rates for cardiovascular and cardiac deaths were significantly lower in participants with diabetes in the 2005 to 2010 group (Table 3). These results did not change using a narrower age categorization that spanned 5 years (Table S4). Sensitivity analyses in which the participants with diabetes were limited to those having 1 year or longer duration of diabetes, also showed declines in rates of total, cardiovascular and cardiac deaths among participants with diabetes in the 2005 to 2010 group compared with the 1999 to 2004 group (Tables S5 and S6).
Table 3.
Hazard ratios for mortality in participants with diabetes during the period 2005 to 2010 compared with those with diabetes during the period 1999 to 2004a
| DM | |||
|---|---|---|---|
| 1999 to 2004 | 2005 to 2010 | P value | |
| Total mortality | |||
| Unadjusted HR (95% CI) | 1.00 [ref] | 0.76 (0.45‐1.28) | .30 |
| Age‐adjusted model | 1.00 [ref] | 0.67 (0.41‐1.09) | .11 |
| Multivariable‐adjusted modelb | 1.00 [ref] | 0.62 (0.37‐1.03) | .07 |
| Cardiovascular mortality | |||
| Unadjusted HR (95% CI) | 1.00 [ref] | 0.47 (0.20‐1.05) | .06 |
| Age‐adjusted model | 1.00 [ref] | 0.42 (0.19‐0.93) | .03 |
| Multivariable‐adjusted model | 1.00 [ref] | 0.41 (0.16‐0.99) | .04 |
| Cardiac mortality | |||
| Unadjusted HR (95% CI) | 1.00 [ref] | 0.35 (0.13‐0.91) | .03 |
| Age‐adjusted model | 1.00 [ref] | 0.32 (0.12‐0.81) | .01 |
| Multivariable‐adjusted model | 1.00 [ref] | 0.29 (0.10‐0.87) | .02 |
| Cancer mortality | |||
| Unadjusted HR (95% CI) | 1.00 [ref] | 0.77 (0.28‐2.13) | .62 |
| Age‐adjusted model | 1.00 [ref] | 0.66 (0.24‐1.83) | .42 |
| Multivariable‐adjusted model | 1.00 [ref] | 0.53 (0.16‐1.76) | .29 |
Abbreviations: CI, confidence interval; HR, hazard ratio.
Data are presented as hazard ratio (95% confidence interval).
bMultivariable adjustment was made for age (20‐39, 40‐59, 60‐79, and ≥80 y), sex, race and ethnicity (non‐Hispanic white, non‐Hispanic black, Mexican American, and others), educational level (less than high school, high school graduation or General Education Development certificate, more than high school), current smoking status, body mass index.
We performed another sensitivity analysis by repeating the analysis for all‐cause mortality in 3 time intervals: 1999 to 2002, 2003 to 2006 and 2007 to 2010. In participants with diabetes, all‐cause mortality was highest in the 1999 to 2002 group and lowest in the 2007 to 2010 group (Table S7). HRs for all‐cause mortality in those with diabetes compared to those without gradually decreased from the periods 1999 to 2002 to 2007 to 2010, and all‐cause mortality in the period 2007–2010 did not differ significantly between those with and without diabetes.
4. DISCUSSION
Considerably improved outcomes for total, cardiovascular and cardiac deaths were observed over time in the 1999 to 2010 US NHANES. Greater declines in cardiovascular and cardiac mortality were observed in the 2005 to 2010 US NHANES compared to the 1999 to 2004 US NHANES. Although mortality was significantly different between populations with and without diabetes in the 1999 to 2004 group, it did not differ significantly in the 2005 to 2010 group. Additionally, the leading cause of death in the 2005 to 2010 group changed from heart disease to malignant neoplasms compared to the 1999 to 2004 group.
Previously, the risk of myocardial infarction was similarly high in both diabetic populations without previous myocardial infarction and non‐diabetic populations with previous myocardial infarction.25 However, the number of deaths resulting from coronary heart disease between 1980 and 2000 in the USA markedly decreased because of advances in medical technology and pharmacologic treatment, along with substantial public health efforts to reduce cardiovascular risk factors.26 Moreover, in the past decade, patients with diabetes have experienced a disproportionate reduction in in‐hospital mortality over time and a complete reversal in risk of mortality relative to patients without diabetes.27 In this study, mortality in the population with diabetes also decreased, particularly for cardiovascular and cardiac deaths. Increase in the prevalence of diabetes since 1999 might explain the increase in the proportion of patients with mild diabetes. However, the sensitivity analysis, which was limited to patients with diabetes of 1 year or longer duration, also showed lower total, cardiovascular and cardiac mortality among those in the 2005 to 2010 group compared with those in the 1999 to 2004 group. Therefore, we believe that outcomes in the US population with diabetes have certainly improved.
These favourable changes in the 1999 to 2010 group probably reflect improvements in the healthcare system, efforts for health promotion in patients with diabetes and early diagnosis of diabetes.26, 28, 29 Compared with the population with diabetes in the 1999 to 2004 group, those with diabetes in the 2005 to 2010 group had better glycaemic control. In the 2005 to 2010 group, the proportion of current smokers and of those with cardiovascular risk factors decreased. Although the definition of dyslipidaemia in this study may not match recent standards in the USA, the management of lipids has certainly improved. One possible explanation is that statins became widely accepted and their use has progressively increased. Because statin therapy has a strong benefit in primary and secondary prevention of cardiovascular disease,30 statins may have had an important role in the improved outcomes in this study population with diabetes. The intensive statin therapy recommended by current guidelines may lead to further risk reduction in populations with diabetes. Improved outcomes might have been attributed to a higher proportion of patients with diabetes who met recommended goals for diabetes care.10 Furthermore, because the leading cause of death in the population with diabetes was malignant neoplasm, a greater emphasis on early detection and treatment of cancer may be needed for the US population with diabetes.31
This study has several limitations. First, it was a short‐term follow‐up study and the number of events was small. Although our analyses were performed using nationally representative data from the USA, these limitations could influence the results. Unfortunately, the participants in the period 2005 to 2010 included those in the 2009 to 2010 NHANES, in which follow‐up was only 2 years. To fully compare mortality in the participants in the 2005 to 2010 group with those in the 1999 to 2004 group, the follow‐up for study participants was a maximum of 2 years. Therefore, larger numbers of participants and longer follow‐up periods are needed to confirm the outcomes and prognosis of diabetes. Second, we could not fully specify whether cause of death was underlying or immediate. Therefore, our results need to be verified in prospective studies with clearly pre‐defined causes of death. In addition, missing data may have influenced the results. Because the present study could not sufficiently evaluate several important issues such as cancer risk in a population with diabetes using insulin,32, 33 additional large‐scale studies that would include such data are required to confirm the results of the present study.
In conclusion, this study showed improved outcomes for total, cardiovascular and cardiac deaths in those with diabetes over time in the 1999 to 2010 US NHANES. Continuous nationwide evaluation of diabetes care and long‐term follow‐up studies are needed.
Acknowledgements
This research was supported by a Grant‐in‐Aid for Scientific Research from the Japan Society for the Promotion of Science (Grant Number: 26860701) and in part by a Grant of National Center for Global Health and Medicine.
Conflict of interest
All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. No financial disclosures were reported.
Author contributions
T. T. was responsible for study concept and design. T. T. and T. S. were responsible for acquisition of data. T. T. and T. S. were responsible for analysis and interpretation of data.
T. T., H. K. and T. S. were responsible for drafting the manuscript. T. T. and T. S. were responsible for statistical analysis. T. T. had full access to all of the data in the study and takes responsibility for the integrity and accuracy of data analysis.
Supporting information
Figure S1. Rates of freedom from mortality in all participants in 1999 to 2010. Rates of freedom from total death (A), cardiovascular death (B), cardiac death (C), and cancer death (D).
Figure S2. Causes of death in all participants in 1999 to 2010.
Table S1. Hazard ratios for mortality in all participants in 1999 to 2010.*
Table S2. Hazard ratios for mortality in participants with diabetes in 1999 to 2004 and 2005 to 2010 compared with those without diabetes.*
Table S3. Hazard ratios for mortality in participants with diabetes who completed 2‐year follow‐up in 1999 to 2004 and 2005 to 2010 compared with those without diabetes.*
Table S4. Hazard ratios for mortality in participants with diabetes in 2005 to 2010 compared with those with diabetes in 1999 to 2004.*
Table S5. Hazard ratios for mortality in participants with diabetes who had 1 year or more duration of diabetes in 1999 to 2004 and 2005 to 2010 compared with those without diabetes.*
Table S6. Hazard ratios for mortality in participants with diabetes who had 1 year or more duration of diabetes in 2005 to 2010 compared with those with diabetes in 1999 to 2004.*
Table S7. Hazard ratios for all‐cause mortality in participants with diabetes in 1999 to 2002, 2003 to 2006, and 2007 to 2010 compared with those without diabetes.*
Tsujimoto T., Kajio H., Sugiyama T. Favourable changes in mortality in individuals with diabetes: US NHANES 1999–2010. Diabetes Obes Metab. 2018;20:85–93. https://doi.org/10.1111/dom.13039
Funding information This research received the JSPS KAKENHI Grant Number 26860701 and the Grant of National Center for Global Health and Medicine.
Abbreviations: BMI, body mass index; GFR, glomerular filtration rate; HbA1c, glycated haemoglobin; HR, hazard ratio; MDRD, Modification of Diet in Renal Disease; NCHS, National Center for Health Statistics; NHANES, National Health and Nutrition Examination Survey; SD, standard deviation.
REFERENCES
- 1. Cowie CC, Rust KF, Ford ES, et al. Full accounting of diabetes and pre‐diabetes in the U.S. population in 1988–1994 and 2005–2006. Diabetes Care. 2009;32:287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yang W, Lu J, Weng J, et al. Prevalence of diabetes among men and women in China. N Engl J Med. 2010;362:1090–1101. [DOI] [PubMed] [Google Scholar]
- 3. Cheng YJ, Imperatore G, Geiss LS, et al. Secular changes in the age‐specific prevalence of diabetes among U.S. adults: 1988–2010. Diabetes Care. 2013;36:2690–2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gregg EW, Zhuo X, Cheng YJ, Albright AL, Narayan KM, Thompson TJ. Trends in lifetime risk and years of life lost due to diabetes in the USA, 1985–2011: a modelling study. Lancet Diabetes Endocrinol. 2014;2:867–874. [DOI] [PubMed] [Google Scholar]
- 5. Albright AL, Gregg EW. Preventing type 2 diabetes in communities across the U.S.: the National Diabetes Prevention Program. Am J Prev Med. 2013;44:S346–S351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Woodward M, Zhang X, Barzi F, et al. The effects of diabetes on the risks of major cardiovascular diseases and death in the Asia‐Pacific region. Diabetes Care. 2003;26:360–366. [DOI] [PubMed] [Google Scholar]
- 7. ADVANCE Collaborative Group , Patel A, MacMahon S, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572. [DOI] [PubMed] [Google Scholar]
- 8. Gaede P, Lund‐Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580–591. [DOI] [PubMed] [Google Scholar]
- 9. Buse JB, Ginsberg HN, Bakris GL, et al. Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Diabetes Care. 2007;30:162–172. [DOI] [PubMed] [Google Scholar]
- 10. Ali MK, Bullard KM, Saaddine JB, Cowie CC, Imperatore G, Gregg EW. Achievement of goals in U.S. diabetes care, 1999–2010. N Engl J Med. 2013;368:1613–1624. [DOI] [PubMed] [Google Scholar]
- 11. Gregg EW, Li Y, Wang J, et al. Changes in diabetes‐related complications in the United States, 1990–2010. N Engl J Med. 2014;370:1514–1523. [DOI] [PubMed] [Google Scholar]
- 12. Gregg EW, Gu Q, Cheng YJ, Narayan KM, Cowie CC. Mortality trends in men and women with diabetes, 1971 to 2000. Ann Intern Med. 2007;147:149–155. [DOI] [PubMed] [Google Scholar]
- 13. Gregg EW, Cheng YJ, Saydah S, et al. Trends in death rates among U.S. adults with and without diabetes between 1997 and 2006: findings from the National Health Interview Survey. Diabetes Care. 2012;35:1252–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Preis SR, Hwang SJ, Coady S, et al. Trends in all‐cause and cardiovascular disease mortality among women and men with and without diabetes mellitus in the Framingham Heart Study, 1950 to 2005. Circulation. 2009;119:1728–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. The Emerging Risk Factors Collaboration , Seshasai SR, Kaptoge S, et al. Diabetes mellitus, fasting glucose, and risk of cause‐specific death. N Engl J Med. 2011;364:829–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Luk AOY, Hui EMT, Sin MC, et al. Declining trends of cardiovascular‐renal complications and mortality in type 2 diabetes: The Hong Kong Diabetes Database. Diabetes Care. 2017;40:928–935. [DOI] [PubMed] [Google Scholar]
- 17. American Diabetes Association . Standards of medical care in diabetes‐‐2014. Diabetes Care. 2014;37(suppl 1):S14–S80. [DOI] [PubMed] [Google Scholar]
- 18. Fukuyama N, Homma K, Wakana N, et al. Validation of the Friedewald Equation for evaluation of plasma LDL‐cholesterol. J Clin Biochem Nutr. 2008;43:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults . Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 20. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. [DOI] [PubMed] [Google Scholar]
- 21. Zipf G, Chiappa M, Porter KS, Ostchega Y, Lewis BG, Dostal J. National health and nutrition examination survey: plan and operations, 1999–2010. Vital Health Stat. 1. 2013;56:1–37. [PubMed] [Google Scholar]
- 22. Klein RJ, Schoenborn CA. Age Adjustment Using the 2000 Projected U.S. Population. Healthy People 2010 Stat Notes. 2001;20:1–10. [PubMed] [Google Scholar]
- 23. Robinson WR, Gordon‐Larsen P, Kaufman JS, Suchindran CM, Stevens J. The female–male disparity in obesity prevalence among black American young adults: contributions of sociodemographic characteristics of the childhood family. Am J Clin Nutr. 2009;89:1204–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Obasaju C, Bowman L, Wang P, et al. Identifying the target NSCLC patient for maintenance therapy: an analysis from a placebo‐controlled, phase III trial of maintenance pemetrexed (H3E‐MC‐JMEN). Ann Oncol. 2013;24:1534–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–234. [DOI] [PubMed] [Google Scholar]
- 26. Ford ES, Ajani UA, Croft JB, et al. Explaining the decrease in U.S. deaths from coronary disease, 1980–2000. N Engl J Med. 2007;356:2388–2398. [DOI] [PubMed] [Google Scholar]
- 27. Butala NM, Johnson BK, Dziura JD, et al. Decade‐long trends in mortality among patients with and without diabetes mellitus at a major academic medical center. JAMA Intern Med. 2014;174:1187–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tricco AC, Ivers NM, Grimshaw JM, et al. Effectiveness of quality improvement strategies on the management of diabetes: a systematic review and meta‐analysis. Lancet. 2012;379:2252–2261. [DOI] [PubMed] [Google Scholar]
- 29. Palta P, Huang ES, Kalyani RR, Golden SH, Yeh HC. Hemoglobin A1c and mortality in older adults with and without diabetes: Results from the National Health and Nutrition Examination Surveys (1988–2011). Diabetes Care. 2017;40:453–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cholesterol Treatment Trialists (CTT) Collaboration , Baigent C, Blackwell L, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta‐analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tsilidis KK, Kasimis JC, Lopez DS, Ntzani EE, Ioannidis JP. Type 2 diabetes and cancer: umbrella review of meta‐analyses of observational studies. BMJ. 2015;350:g7607. [DOI] [PubMed] [Google Scholar]
- 32. Johnson JA, Gale EA. Diabetes, insulin use, and cancer risk: are observational studies part of the solution‐or part of the problem? Diabetes. 2010;59:1129–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Home P. Insulin therapy and cancer. Diabetes Care. 2013;36(suppl 2):S240–S244. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Rates of freedom from mortality in all participants in 1999 to 2010. Rates of freedom from total death (A), cardiovascular death (B), cardiac death (C), and cancer death (D).
Figure S2. Causes of death in all participants in 1999 to 2010.
Table S1. Hazard ratios for mortality in all participants in 1999 to 2010.*
Table S2. Hazard ratios for mortality in participants with diabetes in 1999 to 2004 and 2005 to 2010 compared with those without diabetes.*
Table S3. Hazard ratios for mortality in participants with diabetes who completed 2‐year follow‐up in 1999 to 2004 and 2005 to 2010 compared with those without diabetes.*
Table S4. Hazard ratios for mortality in participants with diabetes in 2005 to 2010 compared with those with diabetes in 1999 to 2004.*
Table S5. Hazard ratios for mortality in participants with diabetes who had 1 year or more duration of diabetes in 1999 to 2004 and 2005 to 2010 compared with those without diabetes.*
Table S6. Hazard ratios for mortality in participants with diabetes who had 1 year or more duration of diabetes in 2005 to 2010 compared with those with diabetes in 1999 to 2004.*
Table S7. Hazard ratios for all‐cause mortality in participants with diabetes in 1999 to 2002, 2003 to 2006, and 2007 to 2010 compared with those without diabetes.*


