Abstract
Human adult skeletal muscle has a limited ability to regenerate after injury and therapeutic options for volumetric muscle loss are few. Technologies to enhance regeneration of tissues generally rely upon bioscaffolds to mimic aspects of the tissue extracellular matrix (ECM). In the present study, silk fibroins from four Lepidoptera (silkworm) species engineered into three‐dimensional scaffolds were examined for their ability to support the differentiation of primary human skeletal muscle myoblasts. Human skeletal muscle myoblasts (HSMMs) adhered, spread and deposited extensive ECM on all the scaffolds, but immunofluorescence and quantitative polymerase chain reaction analysis of gene expression revealed that myotube formation occurred differently on the various scaffolds. Bombyx mori fibroin scaffolds supported formation of long, well‐aligned myotubes, whereas on Antheraea mylitta fibroin scaffolds the myotubes were thicker and shorter. Myotubes were oriented in two perpendicular layers on Antheraea assamensis scaffolds, and scaffolds of Philosamia/Samia ricini (S. ricini) fibroin poorly supported myotube formation. These differences were not caused by fibroin composition per se, as HSMMs adhered to, proliferated on and formed striated myotubes on all four fibroins presented as two‐dimensional fibroin films. The Young's modulus of A. mylitta and B. mori scaffolds mimicked that of normal skeletal muscle, but A. assamensis and S. ricini scaffolds were more flexible. The present study demonstrates that although myoblasts deposit matrix onto fibroin scaffolds and create a permissive environment for cell proliferation, a scaffold elasticity resembling that of normal muscle is required for optimal myotube length, alignment, and maturation. © 2016 The Authors Journal of Tissue Engineering and Regenerative Medicine Published by John Wiley & Sons Ltd. StartCopTextStartCopText© 2016 The Authors Journal of Tissue Engineering and Regenerative Medicine Published by John Wiley & Sons Ltd.
Keywords: silk fibroin, extracellular matrix, primary human myoblasts, myotubes, skeletal muscle tissue engineering, biomaterials, elasticity
1. Introduction
When skeletal muscle is damaged and undergoes myofibre necrosis, the satellite (progenitor) cells of skeletal muscle are activated to proliferate, differentiate and fuse to form mature myotubes. However, when large amounts of muscle become necrotic or are lost, such as in battlefield injuries or car accidents, this classic regenerative response is not sufficient to repair the defect (Grogan et al., 2011). Current therapies such as muscle grafts to replace lost muscle mass are not ideal because of donor site morbidities, and strategies to enhance endogenous repair of muscle also have limitations (reviewed in Grounds, 2014). Recently, the efficacy of acellular bioscaffolds to stimulate new muscle formation in vivo, or the ex vivo formation of muscle‐like structures for subsequent implantation have been explored (Wang et al., 2014). At present, one of the most successful bioscaffolds reported in clinical skeletal muscle repair is decellularized porcine extracellular matrix (ECM) derived from small intestine or bladder. These scaffolds have been implanted in load‐bearing limb muscles and, in combination with physical therapy, have resulted in functional improvement in human patients, albeit in small studies (Mase et al., 2010; Gentile et al., 2014; Sicari et al., 2014). Although numerous studies have examined different bioscaffolds for their compatibility with muscle cells in vitro, these have generally used the murine myoblast cell line C2C12. The use of primary human skeletal muscle myoblasts (HSMMs) to test the cytocompatibility of different scaffolds is an essential first step to assess their viability for human muscle bioengineering.
Silk is a biopolymer produced by the silk glands of arthropods with the main source being the larvae of various Lepidoptera silkworms. Two major groups of silkworm are of commercial importance: Bombycidae (mulberry) and Saturniidae (non‐mulberry) (Mahendran et al., 2006). The domesticated mulberry species Bombyx mori is widely distributed, whereas the commercially available non‐mulberry varieties Antheraea mylitta (Tropical Tasar), Antheraea assamensis/assama (Muga) and Philosamia/Samia ricini (Eri) are all from the Indian subcontinent.
There are two types of silk proteins obtained from the cocoons of B. mori: a water‐soluble protein called sericin and a fibrous hydrophobic protein called fibroin. Fibroin is the key component of silk from B. mori and consists of a heavy (H) chain (390 kDa) and a light (L) chain (26 kDa) connected by a disulphide bond (Zhou et al., 2000). A 30 kDa glycoprotein (P25) is non‐covalently linked to the H–L chain complex through hydrophobic interactions (Tanaka et al., 1999). The H chain comprises a highly repetitive glycine‐rich core, flanked by non‐repetitive sequences. These glycine motifs form highly ordered β‐sheets which gives rise to the strength and toughness of silk, while the non‐crystalline regions contribute flexibility and elasticity (Fu et al., 2009).
Fibroin proteins from non‐mulberry silkworm species have slightly different structures. Antheraea mylitta is a homodimer containing 197 kDa subunits, A. assamensis fibroin is a heterodimer comprising chains of 220 kDa and 20 kDa, and fibroin from S. ricini consists of a heterodimer of chains of approximately 245 kDa and 210 kDa (Kundu et al., 2012b; Pal et al., 2013). Non‐mulberry silk also contains poly‐alanine rather than poly‐glycine repeats and is more hydrophobic than B. mori silk (Kundu et al., 2012a). In addition, fibroin from A. mylitta contains the integrin binding motif arginine–glycine–aspartic acid (RGD), whereas the others do not (Morgan et al., 2008).
Properties such as high tensile strength, elasticity, thermal stability, aqueous preparation, slow degradability and biocompatibility make silk a useful biomaterial. Silk proteins are used in implantable biomaterials, drug delivery vehicles and medical devices (Omenetto and Kaplan, 2010; Kundu et al., 2013, Yucel et al., 2014). Although silk from B. mori is the most commonly used in medical applications, attention is gradually focusing on silk produced by non‐mulberry species, as these fibroins can be easily extracted in aqueous solution (Patra et al., 2012; Kar et al., 2013; Pal et al., 2013). Silk biomaterials can be moulded into hydrogels, membranes, nets, sponges, micro and nanoparticles and nanofibrous mats, (Kundu et al., 2012b) and can be used for different tissue engineering applications including bone (Meinel et al., 2005; Kim et al., 2008; Meinel and Kaplan, 2012), cartilage (Bhardwaj et al., 2011; Talukdar et al., 2011), cardiac muscle (Patra et al., 2012), liver (Banani and Kundu, 2013) and skin repair (Bhardwaj et al., 2015). Silk scaffolds biodegrade without the release of toxic products both in vitro (Horan et al., 2005; Li et al., 2003) and in vivo (Wang et al., 2008; Zhou et al., 2010), and are associated with transient but not chronic inflammation when implanted into rats (Wang et al., 2008). In vitro studies have also shown that silk fibroin films can support growth of a large number of cell types, including C2C12 mouse myoblasts (Park et al., 2013a) and MG‐63 osteoblast like cells (Kar et al., 2013), whereas three‐dimensional (3D) porous silk fibroin scaffolds have been used to culture rat neonatal cardiomyocytes (Patra et al., 2012) and cortical neuronal cells (Tang‐Schomer et al., 2014).
Although a wide range of materials have been investigated as bioscaffolds for skeletal muscle formation and repair, few studies have used silk (see Table 1), and none have tested the response of primary human skeletal muscle cells. This aim of this study was to explore the capacity of 3D scaffolds engineered from different mulberry and non‐mulberry silk fibroins derived from four Lepidoptera species (B. mori, A. mylitta, A. assamensis and P ./S. ricini) to support myoblast proliferation, differentiation and myotube formation. It was hypothesized that human myoblasts would behave differently on the different silk scaffolds. The response of human myoblasts to solubilized silk fibroins in two‐dimensions (2D) was also examined, to investigate if differences in myoblast maturation resulted from the chemical composition of the fibroins or the 3D structural properties of the scaffolds. Striking differences were observed in the way the human muscle cells responded to the different silk fibroin substrates.
Table 1.
Previous in vitro studies of myoblast growth and differentiation using silk protein matrices
| Silk matrix type | Cell type | Experiment | Outcome | Reference |
|---|---|---|---|---|
| Fibroin*–polyurethane blended films | C2C12 mouse myoblasts | Compatibility and differentiation | Silk fibroin improved the cell compatibility of polyurethane films | Park et al. (2013b) |
| Fibroin* immobilized on polyurethane membranes | Cells from skeletal muscle biopsy (primary hypopharynx myoblasts) | Compatibility and differentiation | Cell proliferation, alignment and myofibre differentiation achieved in aligned microchannels | Shen et al. (2013) |
| Fibroin*–tropoelastin films | C2C12 cells and human bone marrow stem cells (hMSCs) | Cell attachment, proliferation and myogenic lineage differentiation | Roughness & stiffness favouring proliferation and differentiation of C2C12 cells or hMSCs determined | Hu et al. (2011) |
| Recombinant spider silk protein eADF4(C16) film | C2C12 mouse myoblasts | Cell adhesion and orientation | Myoblasts adhered to and proliferated best on structured, not unstructured films | Bauer et al. (2013) |
| Sericin* supplemented serum‐free medium | C2C12 mouse myoblasts | Myoblast differentiation | Myotubes formed with sarcomeres | Fujita et al., 2010 (Fujita et al., 2010) |
Silk protein obtained from Bombyx mori, a mulberry silkworm species.
2. Materials and methods
All reagents and chemicals were purchased from Sigma‐Aldrich (St Louis, MO, USA), unless otherwise stated.
2.1. Collection of mulberry and non‐mulberry silkworm species
Bombyx mori cocoons were collected from Debra Sericulture Farm, West Midnapore, West Bengal, India. Antheraea mylitta fifth instar mature larvae were collected from the Indian Institute of Technology (IIT) Kharagpur Farm. Both A .assamensis and S. ricini fifth instar larvae were collected from Coochbehar, West Bengal, India.
2.2. Processing of silk protein fibroin into 3D scaffolds
The methods used to prepare the regenerated fibroin solutions are outlined in Figure 1. Briefly, fibroin was isolated from B. mori cocoons using a protocol described elsewhere (Sofia et al., 2001). Fibroins from the non‐mulberry species were isolated by squeezing the glands of fifth instar larvae immediately prior to them spinning their cocoons following the steps described elsewhere (Datta et al., 2001a; Kar et al., 2013; Pal et al., 2013). Fibroin solutions (2% w/vol) were used to fabricate 3D porous sponges/scaffolds using the freeze drying technique described in Nazarov et al. (2004). The scaffolds (14 mm in diameter and 4 mm high) were fixed with 90% ethanol for 1 h and then, for 30 min each, with 70% and 50% ethanol. Scaffolds were washed twice in phosphate‐buffered saline (PBS) for 30 min, placed under ultraviolet (UV) light for 20 min and then into the wells of a 24‐well plate and preconditioned in Skeletal Muscle Growth Medium‐2 (SkGM‐2; Lonza, Basel, Switzerland) for 2 h at 37°C.
Figure 1.
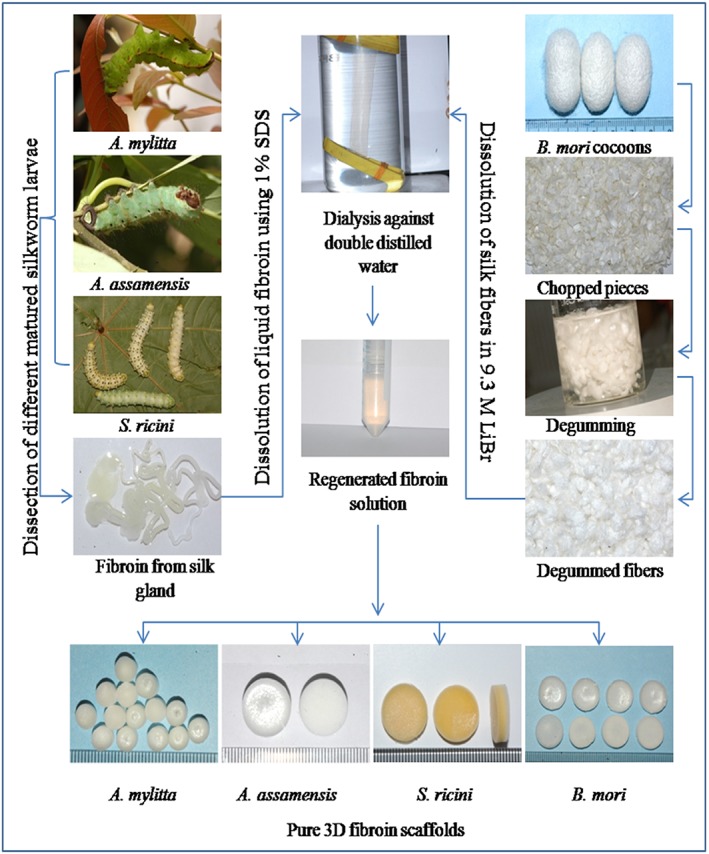
Schematic representation of silk protein fibroin isolation from mulberry and non‐mulberry silkworm species and the preparation of three‐dimensional (3D) fibroin scaffolds. SDS, sodium dodecyl sulphate. [Colour figure can be viewed at wileyonlinelibrary.com]
2.3. Solubilization of the silk fibroin from freeze dried scaffolds for 2D culture
All silk fibroin scaffolds were solubilized in PBS. Scaffolds were placed in 1.5 ml tubes, 500 μl of PBS was added and incubation proceeded at 42°C for 30 min with rocking. Supernatants collected after centrifugation at 9000 rpm for 10 min at 4° C were centrifuged again and their protein content measured using a BCA protein assay kit (Pierce Biotechnology, Rockford, IL, USA). The solubilized silk was stored at 4° C. Routinely, 10 μg/cm2 silk fibroin was used to coat tissue culture plastic or etched glass surfaces.
2.4. Antibodies
Rabbit polyclonal antibodies against fibronectin and glucose transporter 4 (GLUT4) were purchased from Abcam (Cambridge, UK). The anti‐perlecan rabbit polyclonal antibody was a gift from Prof. John Whitelock (University of NSW, Sydney, Australia). Anti‐myosin [slow skeletal heavy chain (MyHCB), clone NOQ7.5.4D] mouse monoclonal antibody (mAb) was purchased from Millipore (Temecula, CA, USA). Secondary antibodies were goat anti‐rabbit Alexa Fluor 546 (AF546), goat anti‐mouse Alexa Fluor 488 (AF488) and goat anti‐rabbit AF488 (all Molecular Probes, Life Technologies, Eugene, OR, USA). Primary antibodies were used at concentrations of 2–4 μg per test. The control antibodies were rabbit IgG and mouse IgG (Zymed; Life Technologies).
2.5. Cell culture
The HSMMs (derived from a single donor) were purchased from Lonza (catalogue number CC‐2580) and maintained in a humidified 37°C incubator equilibrated at 5% CO2 in SkGM‐2 (Lonza) containing human epidermal growth factor (EGF), dexamethasone, l‐glutamine and 10% fetal bovine serum (FBS). The HSMMs were passaged at 50–70% confluence and cells from less than five passages were used in experiments.
2.6. Proliferation and differentiation of HSMMs on 3D scaffolds
The HSMMs in SkGM‐2 medium (7.5 × 104 cells in 25 μl) were added to scaffolds and allowed to adhere at 37°C for 30 min, after which cells and scaffolds were transferred to new wells and 2 ml of SkGM‐2 medium was added. Cells were grown for 5 days with medium replenished on day 3. The scaffolds were fixed and processed either for scanning electron microscopy (SEM) or stained with rhodamine–phalloidin, 10 Units/ml (Molecular Probes), in PBS for confocal microscopy. To assess differentiation, HSMMs in SkGM‐2 medium (5 × 105 cells in 25 μl) were added to scaffolds as described above. After 4 days of culture, the medium was changed to Dulbecco's Modified Eagle Medium (DMEM)/F10/2% horse serum (HS) to trigger differentiation. Cells were cultured for a further 10 or 11 days with medium replacement every third day, then fixed, permeabilized and stained with anti‐MyHCB mAb as described previously.
2.7. Immunofluorescence of matrix proteins
The HSMMs in SkGM‐2 medium were seeded on 3D silk scaffolds (7.5 × 104 cells/cm2) and cultured for 3 days. The scaffolds were washed and fixed with 4% paraformaldehyde (PFA)/PBS, then incubated in blocking solution for 1 h at room temperature (RT). Following washing with PBS they were incubated for 2 h at RT in a polyclonal antibody recognizing either fibronectin or perlecan, then washed with PBS and incubated in secondary antibody (anti‐rabbit AF488). All antibodies were diluted in blocking solution. The scaffolds were washed and mounted in Vectashield (Vector Laboratories, Burlingame, CA, USA). The images were captured with a A1+ confocal microscope (Nikon, Tokyo, Japan) using NIS‐Elements AR analysis version 4.10 software (Nikon, Tokyo, Japan). All images were acquired at the same gain and exposure settings.
2.8. Confocal laser scanning microscopy of scaffolds
To generate a single image, 15–25 z‐stack images (2, 5 or 10 μm stacks) of cell‐laden scaffolds were taken using a Nikon A1+ confocal microscope and merged. Six images/scaffold were captured at different locations and representative images are presented. To image large areas of the scaffold 50 z‐stack images (2.5 μm stack) were taken using an UltraVIEW VoX Spinning Disc Confocal Microscope (PerkinElmer, Waltham, MA, USA) and the images were stitched together using the Volocity software (PerkinElmer). Myotube lengths were measured using the line tool measurement facility.
2.9. Scanning electron microscopy of scaffolds
The silk scaffolds (± HSMMs) were fixed in 4% PFA/PBS for 30 min at RT and then dehydrated in ethanol solutions of increasing concentration (30–100%) for 20 min per concentration, freeze dried using a ScanVac (LaboGene, Lynge, Denmark), mounted on aluminium stubs and sputter coated with platinum (5 nm) using a 208HR sputter coater (Cressington, Redding, CA, USA). The data were collected using either a Neon 40EsB scanning electron microscope (SEM) (Carl Zeiss, Oberkochen, Germany) or MIRA SEM (TESCAN, Brno, Czech Republic).
2.10. Rheological measurements of 3D silk scaffolds
Sample measurements were conducted using a HAAKE MARS III Modular Advanced Rheometer (Thermo Fisher Scientific, Waltham, MA, USA) with 35 mm diameter parallel plate geometry. Silk scaffolds (14 mm in diameter and 4 mm high) were fixed as described then hydrated in PBS. The gap height for the parallel plate arrangement was determined by monitoring the normal force, which was maintained at 0.3 ± 0.1 N during all measurements. The linear viscoelastic region, determined by performing stress sweep measurements, is the area where the moduli values were independent of the applied stress or strain. The moduli values: elastic modulus (G′) and the viscous modulus (G′′), were determined in the linear viscoelastic region by strain sweep measurements. The stress sweep was performed from 1 to 70 Pa, the strain sweep was performed from 0.01–3% strain. Both sweeps were done at a frequency of 1 Hz and a temperature of 37°C. The data was analysed using HAAKE RheoWin software (version 3.61). The stress and strain values measured during the strain sweep (which correspond to the linear viscoelastic region for the scaffolds) were used to calculate Young's modulus. The stress was plotted against the strain and a linear regression was applied to the data. The gradient of the regression curve is equal to Young's modulus; the average value of four replicates was plotted as mean ± SD.
2.11. Alamar Blue assay for HSMM proliferation
HSMMs were cultured in the wells of a 48‐well plate (Corning Inc., Corning, NY, USA) at a seeding density of 1 × 103cells/well in SkGM‐2 medium/10% FBS (500 μl). After 2, 5, 8 and 11 days of proliferation on 2D substrates of a silk fibroin (10 μg/cm2), or collagen I or fibronectin (10 μg/cm2); or tissue culture plastic, 50 μl of Alamar Blue reagent (Life Technologies, Carlsbad, CA, USA) was added. The plates were incubated in a CO2 incubator for 4 h and the fluorescence intensity measured using excitation and emission wavelengths of 560 nm and 590 nm, respectively, on an EnSpire Multimode plate reader (Perkin Elmer). Blank medium was the negative control and fluorescence units (RFU) relative to the control were calculated. Previous experiments indicated that the RFUs were in the linear range of an Alamar Blue standard curve obtained by titrating cell numbers.
2.12. Etching of glass coverslips
Glass coverslips (13 mm diameter; ProScitech, Thuringowa, Australia) that had been stored in 100% ethanol were treated with etch solution [6.0 g NaOH dissolved in 24 ml double distilled (dd) H2O and the volume made up to 60 ml with 95% ethanol] for 30 min at RT. Coverslips were then washed with ddH2O, dried at RT and sterilized by UV light.
2.13. Immunofluorescent staining of myoblasts in 2D culture
The HSMMs in SkGM‐2 medium were seeded (15 × 103/cm2) on etched glass coverslips coated with fibroin, collagen type I or fibronectin (coating concentration 10 μg/cm2) and cultured for 3 days. The medium was changed to differentiation medium (DMEM/F12/2% HS). After day 7 or 10 of differentiation cells were fixed in 4% PFA/PBS, permeabilized with 0.1% Triton X‐100/PBS at RT and blocked using 10% FBS/1% BSA/PBS (blocking solution) for 1 h. Cells were incubated with the anti‐MyHCB mAb and the GLUT4 antibody diluted in blocking solution for 2 h at RT, washed with PBS and incubated for 1 h in AF488‐conjugated goat anti‐mouse antibody and AF546‐conjugated goat anti‐rabbit antibody diluted in blocking solution. The coverslips were washed and mounted in 4′,6‐diamidino‐2‐phenylindole (DAPI)‐containing Vectashield. Fluorescent images were captured using a Axioskop microscope (Carl Zeiss) with a × 40 objective and Spot Advanced software (SPOT imaging solutions, Sterling Heights, MI, USA). Nuclei and myotubes were counted in six random fields of view (>250 nuclei) and fusion index (FI) was calculated according to the formula: FI = (number of nuclei within myotubes containing ≥2 nuclei/total number of nuclei) × 100. Two independent experiments were performed and representative data are shown.
2.14. Quantitative real‐time reverse transcription polymerase chain reaction (qRT‐PCR) of muscle differentiation genes
Total mRNA was isolated using TRI Reagent (Sigma‐Aldrich) as per the manufacturer's instructions from HSMMs cultured on B. mori and A. mylitta scaffolds (seeded at 5 × 105 cells/scaffold) in SkGM‐2 media and differentiated in DMEM/F12/HS as described. mRNA concentration and purity was assessed using a Nanodrop spectrophotometer (Thermo Fisher Scientific). All mRNA samples had an A260/A280 ratio of >1.8. Reverse transcription was performed on 500 ng of mRNA using the Tetro cDNA synthesis kit (Bioline, Alexandria, Australia) as instructed by the manufacturer. A qRT‐PCR was performed on cDNA prepared from differentiation day 0 (day 4 of proliferation, immediately before triggering differentiation) and differentiation day 4, with gene expression levels normalized to the baseline expression at day 0, and from cDNA prepared on differentiation days 2 and 10, with gene expression on day 10 normalized to expression on day 2. The qRT‐PCR reactions were performed using SensiFAST SYBR Lo‐Rox kit (Bioline), with triplicate reactions containing 5 μl SYBR Green Lo‐RoX Mix, 2 μl template cDNA, 1 μl forward/reverse primer (25 ng/μl) and 2 μl RNase free H2O. The reactions were performed on a ViiA™ 7 Real‐Time PCR system (Applied Biosystems, Life Technologies) with fast 96‐well block using the cycling conditions: denaturation at 95°C for 2 min, 40 cycles of denaturation at 95°C for 5 s and annealing and extension together in one step at 60°C for 20 s followed by a melt step ranging from 55 to 95°C. Primers (Table 2) were selected for three reference genes [succinate dehydrogenase complex, subunit A (SDHA), beta actin (ACTAB), TATA‐binding protein (TBP)] and five genes of interest for muscle transcription factors [myogenic factor 5 (Myf5), myogenic determinant protein‐1 (MyoD1), heavy chain myosin (MYH1 and MYH7) and skeletal muscle alpha actin (ACTA1)] using the PrimerBank database (Spandidos et al., 2010) and purchased from Geneworks (Hindmarsh, Australia). Of the reference genes, TBP had the most stable expression according to Normfinder (Andersen et al., 2004) and Bestkeeper (Pfaffl et al., 2004) software and was used as the reference gene. TBP is a central regulatory eukaryotic transcription factor, used by cellular RNA polymerases and is a stable reference gene for HSMMs under differentiation conditions (Stern‐Straeter et al., 2009). The expression levels for MyoD1, Myf5, MYH1, MYH7 and ACTA1 were normalized to TBP expression values and fold‐change determined using the 2‐delta delta Ct method with baseline expression at differentiation day 0 set at 1. The mean ± standard error of four replicates are provided.
Table 2.
Primer Information
| Gene | Sequence (5′–3′) | NCBI ref. | Primer Bank ID | TM (°C) | Length (bp) |
|---|---|---|---|---|---|
| SDHA | F: TGG CAT TTC TAC GAC ACC GTG R: GCC TGC TCC GTC ATG TAG TG | NM_004168 | 156416002c3 | 54, 56 | 77 |
| ACTAB | F: CATGTACGTTGCTATCCAGGC R: CTCCTTAATGTCACGCACGAT | NM_001101 | 4501885a1 | 61, 60 | 250 |
| TBP | F: CCC GAA ACG CCG AAT R: ATA ATC C AAT CAG TGC CGT GGT TCG TG | NM_003194 | 285026518c2 | 55, 54 | 80 |
| MYOD1 | F: CGG CAT GAT GGA CTA CAG CG R: CAG GCA GTC TAG GCT CGA C | NM_002478 | 77695919c2 | 56, 55 | 133 |
| MYF5 | F: CTG CCA GTT CTC ACC TTC TGA R: AAC TCG TCC CCA AAT TCA CCC | NM_005593 | 156104905c1 | 54, 54 | 78 |
| ACTA1 | F: GGC ATT CAC GAG ACC ACC TAC R: CGA CAT GAC GTT GTT GGC ATA C | NM_002478 | 47078293c1 | 56, 55 | 84 |
| MYH1 | F: GGG AGA CCT AAA ATT GGC TCA A R: TTG CAG ACC GCT CAT TTC AAA | NM_005963 | 115527081c2 | 53, 50 | 106 |
| MYH7 | F: TGG ATG TGA GTG AAC TTG GGG R: GCA CCC AGA CTC GCT TCT T | NM_020884 | 291045202c2 | 54, 53 | 108 |
F, forward; R, reverse.
2.15. Statistical analysis
The data from three to four independent replicates per data point were collected from duplicate experiments and represented as means ± standard deviations. Analysis of cell proliferation experiments and rheology measurements was performed using one‐way analysis of variance (ANOVA) followed by Tukey's post‐hoc test, after the data were confirmed to have a normal distribution using the Shapiro–Wilk test. Statistical analyses of qRT‐PCR data were performed using the non‐parametric Mann–Whitney U‐test, because these data have a non‐normal distribution.
3. Results
3.1. The interaction of HSMMs with the silk 3D scaffolds
At 2 days after seeding HSMMs in proliferation medium onto the scaffolds, rhodamine–phalloidin staining of polymerized actin fibres revealed that these cells had adhered to all four scaffold types and were covering the scaffold surfaces (Figure 2). The F‐actin fibre arrangement indicated the cells were well spread on all scaffolds. On A. mylitta, S. ricini or A. assamensis scaffolds the cells appeared to be oriented around the scaffold pores (Figure 2f,j,n), whereas on B. mori scaffolds a different F‐actin arrangement was apparent, with actin fibres oriented in parallel and, in places, crossing over the pores (Figure 2b). Staining of unseeded scaffolds revealed their porosity (Figure 2a,e,i,m). Immunostaining of HSMMs on 3D scaffolds revealed deposition of both fibronectin and perlecan. The staining intensities suggested comparable deposition of these molecules on all four scaffolds (Figure 2). The organization of the matrix proteins largely mirrored the F‐actin fibre arrangement, although this is less apparent on A. assamensis scaffolds. A comparison of the fibronectin staining on the different scaffolds revealed fibronectin was deposited as parallel fibres on B. mori scaffolds (Figure 2c), whereas on A. mylitta and S. ricini scaffolds the fibronectin fibres were arranged in a circular pattern around the pores (Figure 2g,k).
Figure 2.
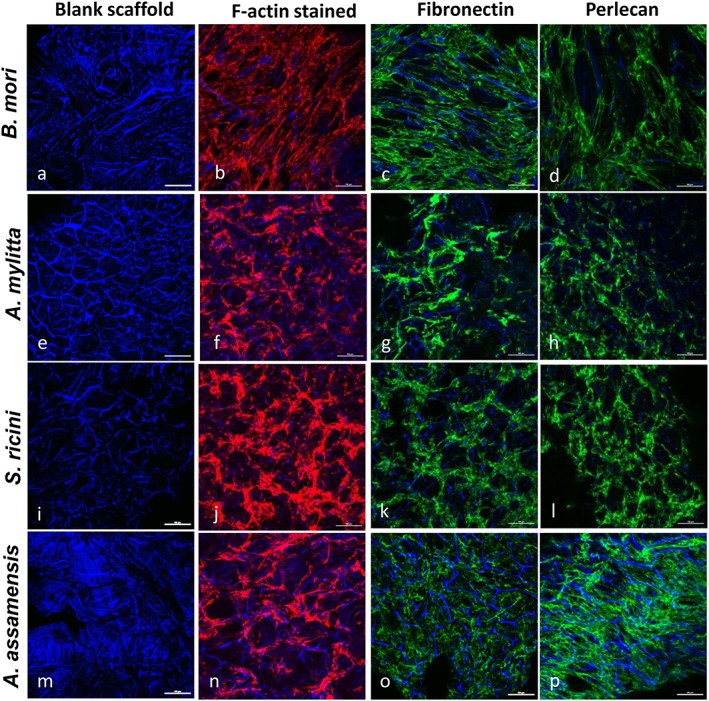
Human skeletal muscle myoblasts (HSMMs) adhere and secrete extracellular matrix (ECM) proteins on three‐dimensional (3D) silk scaffolds. The HSMMs were cultured on 3D silk scaffolds for 2 days in skeletal muscle growth medium‐2 proliferation medium, then fixed and stained with rhodamine–phalloidin (F‐actin staining; b,f,j,n), or antibodies recognizing either fibronectin (c,g,k,o), or perlecan (d,h,l,p). 4′,6‐diamidino‐2‐phenylindole (DAPI) stained the silk scaffolds (all panels). Images were captured using a Nikon A1 confocal laser scanning microscope. The merged images of several z‐stack images (5 μm each stack, 150–200 μm deep into the scaffold) are presented. A representative image of six fields of view is shown. Bar: 50 μm. [Colour figure can be viewed at wileyonlinelibrary.com]
3.2. HSMMs differentiation on 3D silk scaffolds
When stimulated with differentiation medium the HSMMs fused to form myotubes. Immunostaining with a mAb recognizing slow muscle myosin (MyHCB) revealed that multinucleated myotubes formed on all scaffolds (Figure 3), although there were markedly fewer myotubes on S. ricini scaffolds. Confocal imaging indicated that the myotubes were not necessarily confined to the same focal plane, suggesting they were formed by the fusion of cells at different depths in the scaffolds. The parallel alignment of the MyHCB‐positive myotubes on B. mori, A. mylitta and A. assamensis scaffolds was striking. On A. assamensis scaffolds there appeared to be two layers of myotubes, one at a 90° orientation to the other. Confocal microscopy revealed that the area covered by the myotubes on B. mori scaffolds was greater than that seen with the other scaffolds at identical cell seeding densities. In addition, as the longest myotubes formed on B. mori scaffolds, the length of these myotubes and the range in their lengths were determined. Measurements of 25 myotubes indicated an average length of 347 ± 7.2 μm and a range of 175–515 μm (Figure 4).
Figure 3.
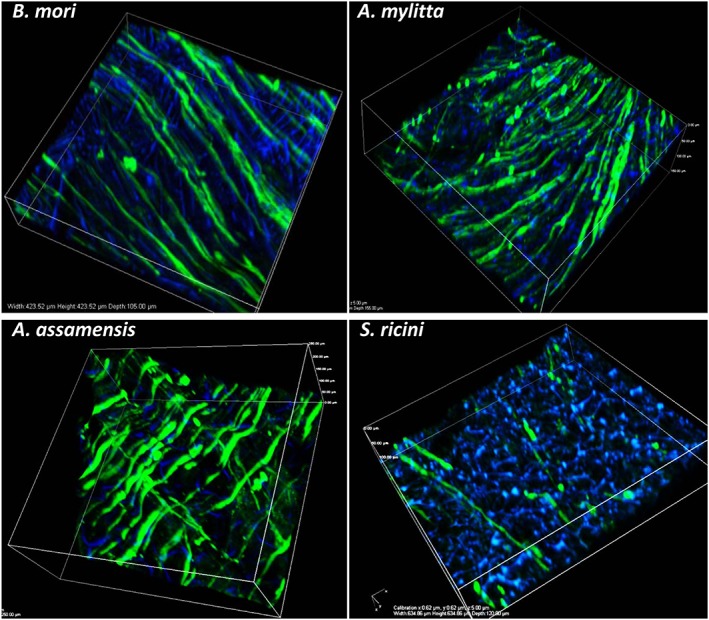
Immunostained images of differentiated human skeletal muscle myoblasts (HSMMs) on three‐dimensional (3D) silk scaffolds. The HSMMs were cultured on the silk scaffolds for 4 days in proliferation medium and a further 4 days in differentiation medium. After differentiation, the scaffolds were stained with anti‐myosin monoclonal antibody (NOQ7.5.4D) and goat anti‐mouse IgG‐AF488. The nuclei are stained with 4′,6‐diamidino‐2‐phenylindole (DAPI, blue). Images were captured using a Nikon A1 confocal microscope. The 3D merged images of several z‐stack images (5 μm each stack, 150–200 μm deep into scaffold) are represented. Bar: 100 μm. [Colour figure can be viewed at wileyonlinelibrary.com]
Figure 4.
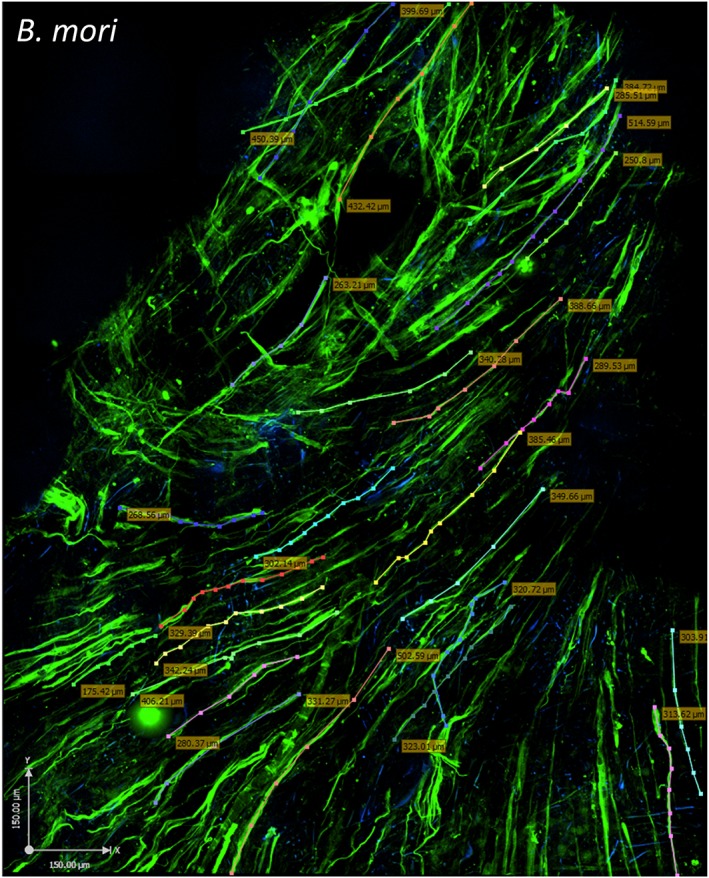
Human skeletal muscle myoblasts (HSMMs) form a three‐dimensional (3D) muscle‐like tissue on Bombyx mori scaffolds. The HSMMs were cultured for 4 days in skeletal muscle growth medium‐2 proliferation medium and 4 days in differentiation medium on B. mori silk scaffolds. Myotubes were stained with anti‐myosin monoclonal antibody (NOQ.5.4.D), followed by a goat anti‐mouse AF488 conjugated second antibody. The images were captured using an Ultraview spinning disc confocal microscope (PerkinElmer). To capture a large area and estimate the coverage of the myotubes on the scaffold surface, 50 z‐stack images (2.5 μm each stack) were stitched together using Volocity software. Muscle fibre length (coloured dotted lines and boxed numbers) was measured by tracing individual fibres using Volocity software. The area covered in this image is indicated by the following: x‐axis, 1.58 mm; y‐axis, 1.213 mm; z‐stack, 125 μm. Bar: 150 μm. [Colour figure can be viewed at wileyonlinelibrary.com]
3.3. Ultrastructure and elastic properties of the 3D scaffolds
To determine if the ultrastructure of the scaffolds could have contributed to the differences in myotube formation, SEM was performed. All scaffolds had interconnecting pores, with diameters ranging from 50–120 μm on A. mylitta, 30–110 μm on B. mori and 30–90 μm on S. ricini and A. assamensis scaffolds, although S. ricini had a larger number of smaller pores than A. assamensis (Figure 5A;a,c,e,g). Despite these minor differences in pore size the HSMMs formed a continuous layer on all scaffolds, virtually covering the scaffold after 5 days (Figure 5A;b,d,f,h). On A. assamensis scaffolds, this appeared to consist of multiple cell layers (Figure 5A;h). Scanning electron microscopy of HSMMs on an A. mylitta scaffold revealed the cells were well spread, flat and elongated, with many contact points with the scaffold visible at 2 days after seeding (Figure 5B;b). The deposition of fibrous material, probably ECM proteins, onto the scaffold surface was also evident (Figure 5B;c).
Figure 5.
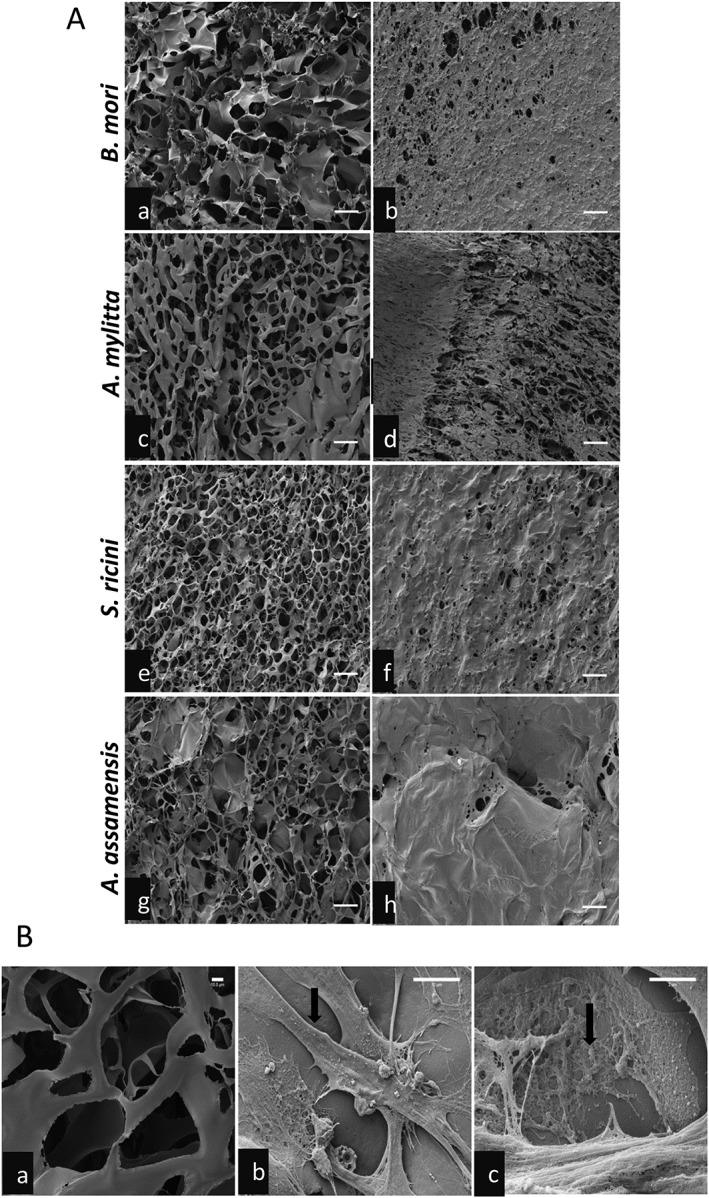
Scanning electron micrographs of three‐dimensional (3D) silk scaffolds with and without human skeletal muscle myoblasts (HSMMs). (A) The scaffolds without cells (a, c, e, g) and at day 5 after seeding with HSMMs (b,d,f,h) are shown. Bars: 100 μm. (B) Highly magnified scanning electron micrographs of Antheraea mylitta silk scaffolds. The blank scaffold (a) shows the interconnected pores. HSMMs are shown at day 2 after seeding with a spread cell (b) indicated by arrow, and (c) matrix proteins deposited onto the scaffold (arrow). Bar: 10 μm (a,b), 2 μm (c)
Rheology measurements revealed the elastic properties of the silk scaffolds were quite different (Figure 6). The B. mori silk scaffolds had the highest Young's modulus with a mean value of 16.53 kPa followed by A. mylitta scaffolds with a mean value of 10.67 kPa, both of which were higher than those of S. ricini and A. assamensis scaffolds, which had similar Young's moduli (3.74 kPa and 3.73 kPa, respectively). A similar pattern was observed in the viscous modulus of the scaffolds with B. mori scaffolds having the highest viscous modulus and S. ricini scaffolds the lowest modulus (Figure 6).
Figure 6.
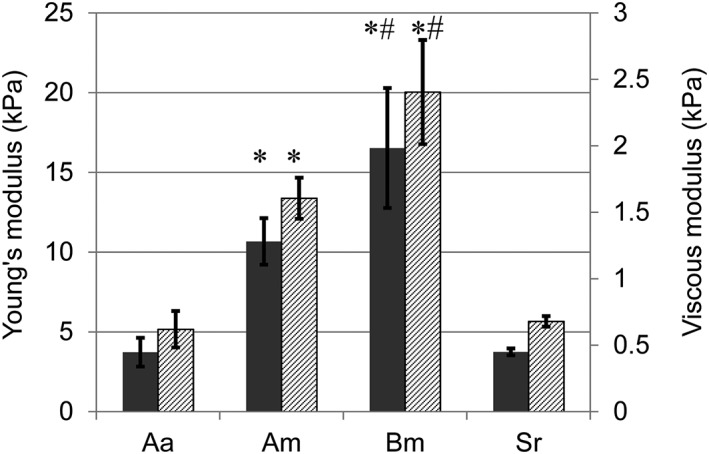
Young's modulus and viscous modulus of three‐dimensional silk scaffolds. Strain sweep was performed from 0.01–3% at 1 Hz and 37°C. Scaffold dimensions were 14 mm in diameter and 4 mm thick and four replicate scaffolds were measured. Grey bars are Young's modulus (YM), hatched bars are viscous modulus (VM). Data are mean ± standard deviation (n = 4). Am, Antheraea mylitta; Bm, Bombyx mori; Aa, Antheraea assamensis; Sr, Samia ricini. *YM and VM are significantly greater in Am and Bm compared with Aa and Sr (p ≤ 0.01); #YM and VM are significantly greater in Bm than Am (p ≤ 0.01)
3.4. Growth and differentiation of HSMMs on 2D silk fibroin substrates
To clarify whether amino acid composition differences in the fibroins could have affected the behaviour of the HSMMs, the proliferation and differentiation of these cells on 2D fibroin substrates was examined and compared with substrates of fibronectin and type I collagen. The 2D format was chosen to eliminate any confounding effects from scaffold structure differences. The growth and viability of HSMMs cultured for 11 days in SkGM‐2 proliferation medium on the 2D substrates were measured using the Alamar Blue assay, which detects metabolic activity. The time‐points chosen were days 2, 5, 8 and 11 as the doubling time of HSMMs was more than 42 h (data not shown). The HSMMs cultured on the silk fibroin and matrix protein substrates exhibited similar growth when subconfluent, at up to 8 days of culture (Figure 7). However, when the cells reached confluence (day 11), the assay measurements for HSMMs cultured on A. mylitta fibroin were significantly higher than those obtained from similarly cultured cells on the other substrates.
Figure 7.
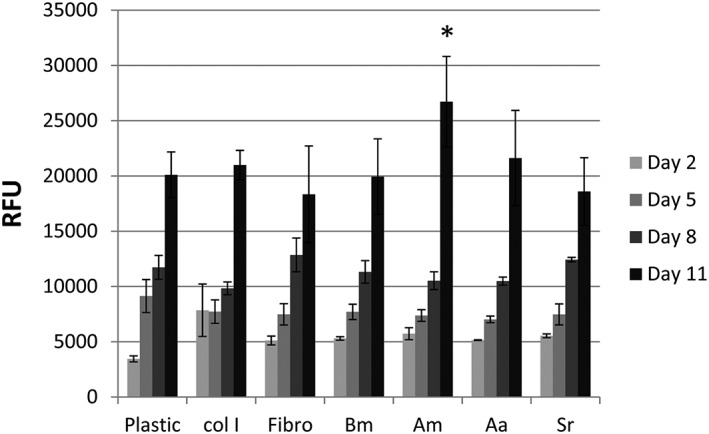
Human skeletal muscle myoblasts (HSMMs) are viable on silk fibroin substrates. Metabolic activity of HSMMs on four different silk fibroins, two extracellular matrix proteins and tissue culture plastic in Skeletal Muscle Growth Medium‐2 was assessed using the Alamar Blue assay. The days in culture when the Alamar Blue dye was added and absorbance measured are indicated. The relative fluorescence units (RFU) were calculated relative to the control (media, no cells). Data are mean ± standard deviation (n = 4). Am, Antheraea mylitta; Bm, Bombyx mori; Aa, Antheraea assamensis; Col I, collagen I; Fibro, Fibronectin; Sr, Samia ricini; *p ≤ 0.05
Immunostaining with an anti‐MyHCB mAb of cells differentiated on 2D silk fibroin substrates revealed multinucleated myotubes with elongated nuclei (Figure 8). These myotubes formed parallel to each other on all substrates by day 7 of differentiation and striations in the myotubes were visible by day 7 and remained at day 10. The staining pattern of an antibody to GLUT4, a glucose transporter protein and a marker of functional myotubes (Guillet‐Deniau et al., 1994) revealed that GLUT4 was present along the length of the myotubes, although some perinuclear accumulation also occurred on all silk substrates (Figure 8e–h). To quantify differentiation, fusion indices of MyHCB‐positive myotubes were determined (see the Supplementary material online, Figure A). The results show a similar percentage of nuclei within the myotubes, (77–81%) on collagen I, fibronectin and fibroins from B. mori, A. mylitta and S. ricini. However, on A. assamensis fibroin, the percentage of nuclei within the myotubes was significantly lower (57%) than that of HSMMs on the other substrates. The number of myotubes formed per field of view was also similar on collagen I, fibronectin and the fibroins from B. mori, A. mylitta and S. ricini, but on A. assamensis fibroin, fewer myotubes formed (see the Supplementary material online, Figure B).
Figure 8.
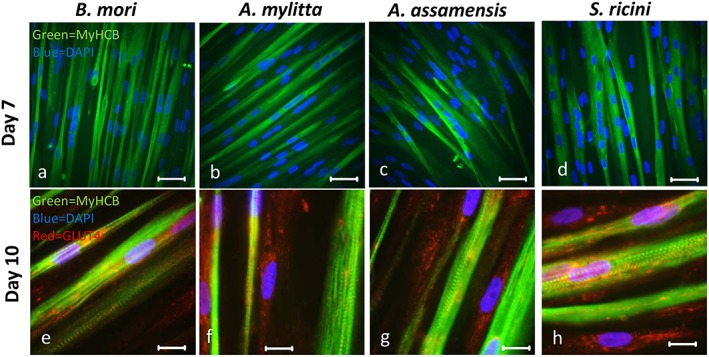
Human skeletal muscle myoblasts (HSMMs) differentiate to form myotubes on silk fibroin substrates. HSMMs were seeded on etched glass coated with the silk fibroins (10 μg/cm2) and cultured for 4 days in skeletal muscle growth medium‐2 proliferation medium and a further 7 or 10 days in differentiation medium. After 7 days of differentiation myotubes were stained with a mouse anti‐myosin heavy chain monoclonal antibody (mAb) (NOQ7.5.4D) and goat anti‐mouse IgG‐AF488 (a–d) (MyHCB, mAb recognizing slow muscle myosin); after 10 days, myotubes were stained with both the anti‐myosin mAb/goat anti‐mouse IgG‐AF488 combination and with rabbit anti‐glucose transporter 4 (GLUT4) antibody followed by goat anti‐rabbit IgG‐AF546 (e–h). The nuclei were stained with 4′,6‐diamidino‐2‐phenylindole (DAPI). Myotubes were imaged using a Zeiss Axioskop fluorescent microscope. Bar: 50 μm (a–d), 25 μm (e–h). [Colour figure can be viewed at wileyonlinelibrary.com]
3.5. Gene expression of myogenic markers in HSMMs on B. mori and A. mylitta 3D scaffolds
As the HSMMs readily formed myotubes on B. mori and A. mylitta fibroins in both the 3D scaffold and 2D film formats, cells grown on 3D scaffolds of these silk fibroins were selected for gene expression studies. The mRNA levels for muscle transcription factors (Myf5 and MyoD1), heavy chain myosins (MYH1 and MYH7), skeletal muscle alpha actin (ACTA1) and the reference gene (TBP) were determined by qRT‐PCR. Gene expression by HSMMs in differentiation medium was examined at two sets of time‐points to capture gene expression changes both early and late in myotube formation. Early gene expression was assessed at differentiation days 0 and 4, with day 4 data presented relative to expression levels at day 0 (Figure 9). On day 4, Myf5 mRNA levels decreased and MyoD1 expression increased on both scaffolds as the cells proceeded towards fusion and myotube formation (Figure 9a,b). A time‐dependent increase was observed in ACTA1, MYH1 and MYH7 levels, indicative of maturing myotubes. The expression of the ACTA1 gene was much higher on A. mylitta scaffolds than on B. mori scaffolds. The expression of these genes was also examined at differentiation day 10 relative to differentiation day 2, thereby detecting late gene expression changes. In this latter data set ACTA1 expression in HSMMs at day 10 on A. mylitta scaffolds was significantly lower than that at day 2, whereas for the other genes, expression remained stable at day 2 and day 10. In contrast, on B. mori scaffolds the expression of ACTA1 and MYH1 was higher at day 10 than at day 2 (Figure 9c,d) and Myf5 and MyoD1 expression levels had decreased from day 10 to that at day 2. Collectively, these data suggest that HSMMs began to differentiate earlier on A. mylitta scaffolds than on B. mori scaffolds, but the mature myotube gene expression pattern was lost at day 10, whereas on B. mori scaffolds it was maintained.
Figure 9.
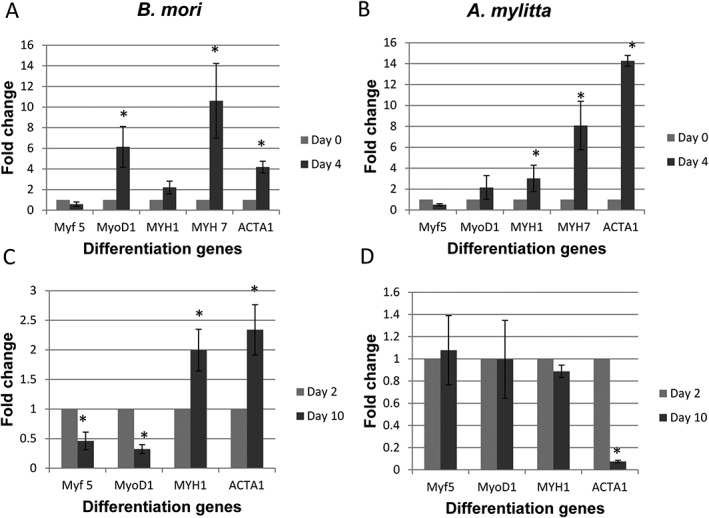
Quantitative real‐time reverse transcription polymerase chain reaction (qRT‐PCR) analysis reveals muscle differentiation on Bombyx mori and Antheraea mylitta three‐dimensional (3D) silk scaffolds. qRT‐PCR was used to determine the expression of five differentiation markers (Table 2) in HSMMs grown in skeletal muscle growth medium‐2 on Bombyx mori (a,c) and A mylitta (b,d) scaffolds at day 4 (a,b) and day 10 (c,d) of differentiation. Relative expression levels for MYF5, MYOD1, MYH1, MYH7 and ACTA1 are normalized to the Ct value of the reference gene (TBP) and fold‐change was determined using the using 2‐delta delta Ct method. Day 4 and day 10 differentiation expression levels were normalized to day 0 and day 2 differentiation, respectively. The mean ± standard error of four biological replicates are presented. The Mann–Whitney U‐test was performed and significance (p) values shown at each time‐point for each gene using *p < 0.05
4. Discussion
The present study used four silk fibroins originating from different silkworm species to investigate what features are likely to be important in a 3D biomaterial for use in human skeletal muscle formation during regeneration. Those scaffolds that more closely resembled muscle in their elasticity (approximately 12–16 kPa) were best able to support myotube formation and alignment. Scaffolds of fibroin from A. mylitta, which has an RGD motif, did not enhance HSMM fusion and myotube formation over that seen with scaffolds of B. mori fibroin, which does not have an RGD sequence. Rather, the HSMMs adhered to, and deposited extensive ECMs on all of the 3D scaffolds regardless of their fibroin composition. Nevertheless, the scaffolds did not equally support myotube formation and alignment, even though striated myotubes, expressing a functional marker, formed on 2D films of all fibroin types. When the length, alignment and gene expression pattern of the myotubes are considered, it is likely that scaffolds of B. mori fibroin will be the most useful for clinical applications involving skeletal muscle.
Other in vitro studies of silk as a biomaterial for skeletal muscle tissue engineering have only used fibroin from B. mori (Table 1) and most have used the immortalized murine myoblast cell line, C2C12 (Table 1). The present study used primary human myoblasts and four different silk fibroins and indicated that all the fibroins, when displayed in either a 2D or a 3D format, were compatible with HSMMs in tissue culture. On 3D silk scaffolds the HSMMs adhered to and spread on all scaffolds to form a cell sheet by day 5 in culture (Figures 2 and 5). Immunofluorescence analyses indicated that the HSMMs deposited ECM molecules on all of the silk scaffolds, a result supported by the SEM data of HSMMs on an A. mylitta scaffold (Figure 5B). These endogenous matrix proteins will interact with integrins on the cell surface of HSMMs to facilitate spreading and adhesion. Myoblasts express both the RGD binding integrins, α5β1, αvβ3 and αvβ5, as well as other non‐RGD binding integrins including α3β1, α4β1, α4β7, α6β1 and α7β1 (García et al., 1999; Mayer, 2003). The rapid secretion of matrix proteins probably compensates for the absence of RGD peptides in some of the silk fibroins (Datta et al., 2001b), particularly as stable interactions mediated by α5β1 require another site in fibronectin, located adjacent to RGD (minimal sequence: PHSRN), and the αv integrins also bind to another site in fibronectin in addition to RGD (Leiss et al., 2008). As fibronectin assembles into fibrils at the cell surface bound to α5β1 or the αv integrins (Leiss et al., 2008), it is likely all of the silk fibroins provide a surface to which fibronectin fibrils can adsorb and this is what stabilizes cell adhesion and myotube formation on the different silk scaffolds. Others have similarly concluded that what is required for cell‐self organization is a surface that facilitates cells to deposit and assemble their own, endogenous, adhesive ECM (Cerchiari et al., 2015).
These secreted ECM proteins are likely to have a role in myotube formation. A number of studies have reported a role for integrins in C2C12 cell myotube formation (García et al., 1999) and in an earlier study it was shown C2C12 cells very rapidly secreted fibronectin and perlecan under conditions of serum‐free culture and formed myotubes (Chaturvedi et al., 2015). Data from in vivo animal models of muscle injury and repair indicate that fibronectin levels increase in regenerating areas of damaged muscle and there is increased production of collagen IV and the laminin α2 chain as the muscle myotubes mature (Grounds, 2008). Recent reviews also highlight the importance of fibronectin and perlecan (with its glycosaminoglycan chains), for muscle satellite cell self‐renewal and skeletal muscle morphogenesis (Goody et al., 2015; Thomas et al., 2014). In a different system, silk/chitosan scaffolds modified by fibronectin and laminin deposited by Schwann cells initiated better repair of sciatic nerve injuries than non‐coated scaffolds (Gu et al., 2014). Hence, biomaterials that trigger the resident cells to synthesize and deposit their own matrix may better support tissue repair.
Although there are differences in the capabilities of the fibroins to support myotube formation and alignment, this seemed to be because of the way the fibroins were presented to the cells rather than the composition of the fibroins per se. When the fibroins were used as a coating on tissue culture plastic, they all supported HSMM proliferation and differentiation, with only slight differences being evident. For example, the rapidly increased metabolic activity measured by the Alamar Blue assay in cells plated on A. mylitta fibroin suggested that these cells may have differentiated earlier than cells on the other three silk substrates (Figure 7), and the fusion indices and myotube numbers indicated that myotubes formed less well on A. assamensis films, but similarly on B. mori, A. mylitta and S. ricini fibroin films (Figure 8, and see the Supplementary material online, Figure S1). Interestingly, cell proliferation and myotube formation on the fibroin films was comparable to that seen on fibronectin and collagen I used at the same concentration and coating conditions (Figure 7 and Figure S1).
In contrast to the 2D format, the different fibroin 3D scaffolds supported HSMM myotube formation to varying degrees and this was quite apparent following immunostaining with an anti‐MyHCB antibody (Figure 3). On B. mori scaffolds, HSMMs formed extremely long, well‐aligned myotubes, whereas the myotubes that formed on A. mylitta scaffolds were thicker and shorter. The parallel alignment of HSMMs on B. mori scaffolds occurred by day 2 of proliferation, as revealed by the similar orientation of most of the F‐actin fibres and by the orientation of the fibronectin fibrils (Figure 2). In contrast, on the other scaffolds the F‐actin fibres tended to follow the edges of the scaffold pores, indicating that HSMMs on these scaffolds were not spanning the pores. Myotubes on A. assamensis fibroin scaffolds were predominately oriented in two perpendicular layers in an arrangement that will not favour a functioning skeletal muscle. The SEM images suggested multiple layers of cells on A. assamensis (Figure 5), whereas the cell layer was thinner on A. mylitta and B. mori scaffolds. Myosin expression was much lower in HSMMs seeded on the S. ricini fibroin scaffolds (Figure 3), suggesting that the architecture of these scaffolds was not ideal for myotube maturation.
The degree to which the HSMMs differentiated on the different scaffolds was examined by comparing the expression patterns of genes associated with myogenesis. Myf5 is the first muscle‐specific regulatory factor to be expressed during mouse embryogenesis while MyoD1 promotes myoblasts cycle withdrawal and the induction of differentiation (Bentzinger et al., 2013). MYH1 and MYH7 are adult heavy chain myosin isoforms expressed in myotubes and myofibres, and ACTA1 is a muscle actin isoform (Stern‐Straeter et al., 2011). In the present study, TBP was the most stable reference gene of those tested, which is in agreement with the study by Stern‐Straeter et al. (2009).
The qRT‐PCR data were consistent with the immunostaining data and indicated that HSMMs differentiated well on A. mylitta and B. mori scaffolds. On both scaffolds, a decrease in Myf5 mRNA levels and an increase in MyoD1 levels indicated that most myoblasts had begun to differentiate after 4 days in differentiation medium. The levels of MYH7 mRNA were 8‐ to 10‐fold higher at day 4, compared with day 0, which correlated with the strong myosin immunostaining of myotubes on both scaffolds (Figure 9). In contrast, ACTA1 levels were much higher on A. mylitta than on B. mori at day 4 in differentiation medium, but by day 10 ACTA1 mRNA levels in cells on A. mylitta scaffolds decreased relative to the levels at day 2, while the levels of expression of the other genes were similar to at both day 2 and day 10 (Figure 9). These data were quite different from those of cells on B. mori scaffolds and from the gene expression studies of human myoblast differentiation conducted by Stern‐Straeter et al. (2011). The expression levels of all the genes examined at both the early and late differentiation stages on B. mori scaffolds were as expected from the earlier study (Stern‐Straeter et al., 2011). Collectively these data suggest that although HSMMs started to differentiate more quickly on A. mylitta than on B. mori scaffolds, it appeared the myotubes that formed on A. mylitta scaffolds did not maintain their phenotype at the latter time‐point. Possibly, the formation of long, well‐aligned myotubes on B. mori scaffolds (Figure 8), occurred because on this scaffold, the gene expression pattern was characteristic of mature human muscle.
Pore size and the mechanical properties of scaffolds have been described as factors regulating cell proliferation and differentiation. Zhang et al. (2010) reported that human bone marrow mesenchymal stromal cells seeded on silk fibroin scaffolds showed greatest proliferation on scaffolds, with a pore size of 100–300 μm (Zhang et al., 2010). All the scaffolds used here have similar porosities, but the SEM indicated that the pore size of S. ricini (Figure 5A) was smaller than the other three scaffolds. Substrate stiffness or elasticity can also determine cell fate by altering mechanical signal transduction pathways (Engler et al., 2004, 2006). Possibly the HSMMs differentiated best on B. mori and A. mylitta scaffolds as these two scaffolds had a Young's modulus (E) of around 10–16 kPa, which is very close to the elastic modulus of human skeletal muscles (gastrocnemius and soleus) at rest (16.5 kPa and 14.5 kPa, respectively) (Shinohara et al., 2010) (Figure 6). There are no data on the Young's modulus of cultured HSMMs but for C2C12 myoblasts the Young's modulus is between 12 and 15 kPa (Collinsworth et al., 2002). The findings of the present study are consistent with those of Engler et al. (2004) who investigated the elastic properties of C2C12 myoblasts cultured on different substrates of varying stiffness (Engler et al., 2004). They found that although the myoblasts fused into myotubes irrespective of substrate flexibility, actin/myosin striations appeared only in myotubes cultured on gels with a stiffness close to normal muscle (Young's modulus: E ~ 12 kPa), indicating that substrate stiffness is a critical factor regulating myotube maturation (Engler et al., 2004). Later, the same group demonstrated the significance of matrix elasticity on stem cell fate (Engler et al., 2006). When mesenchymal stem cells (MSCs) were cultured on collagen‐coated gels of varying elasticity that mimicked brain, muscle and bone matrix in their stiffness, they differentiated into myogenic cells on medium stiffness gels (E ~ 12 kPa), osteocytes on high‐stiffness gels, and displayed neuronal morphology when cultured on matrices mimicking brain elasticity (Engler et al., 2006). Here it was shown that the viscous moduli of the two scaffolds that best supported myotube formation were similar and quite different from that of scaffolds from S. ricini and A. assamensis. These last two scaffolds either did not support differentiation or the myotubes that formed were not aligned in a single direction.
For some adult human skeletal muscles, individual myofibres may extend up to lengths of 35 cm, in parallel with the longitudinal orientation of the ECM (Paul, 2001; Harris et al., 2005). To form a functional new muscle in vivo, the implanted regenerated myofibres on the bioscaffold should fuse with the existing myofibres and become innervated. The in vitro data of the present study demonstrate that 3D fibroin scaffolds from B. mori supported HSMM differentiation into long, well‐aligned myotubes, and are encouraging in this context. In contrast, short, thick myotubes formed on A. mylitta scaffolds suggesting that rapid differentiation may not produce the best myotubes.
5. Conclusion
Silk fibroins from four different silkworm species, under 2D and 3D culture conditions, effectively supported HSMM proliferation, myotube formation and maturation, although their efficacy differed. All the scaffolds stimulated the HSMMs to deposit an endogenous ECM and so the presence of an RGD motif in A. mylitta fibroin did not confer an advantage. Of greater importance for myotube formation and maturation was a scaffold elasticity that resembled that of normal muscle. This comparative study suggested that silk fibroin from the domesticated mulberry species, B. mori, when processed into a scaffold with elasticity similar to skeletal muscle, could be a useful biomaterial for skeletal muscle bioengineering, although fibroin from A. mylitta similarly prepared may also be useful. Bombyx mori silk fibroin materials are widely used and well tolerated in clinical situations, and this demonstration of the suitability of B. mori fibroin bioscaffolds, is a necessary first step for potential bioengineering of human skeletal muscle cells for future clinical applications.
Conflict of interest
The authors have declared that there is no conflict of interest.
Supporting information
Figure S1. Supporting info item
Acknowledgments
The authors thank Prof. John Whitelock and Dr Megan Lord for the gift of the anti‐perlecan antibody, CCN‐1, and the assistance of Corey Giles in the preparation of Figure 7. This work was supported by a grant to DED and DRC from Defence Health Foundation, Australia and a Curtin University Early Career Research Fellowship to D.E.D. V.C. was supported by a Curtin University Strategic International Research Scholarship. The work was also supported by the Indian Council of Medical Research, Department of Biotechnology, and Government of India (S.C.K.). S.C.K. is grateful to Curtin University for providing all facilities during his visit to the School of Biomedical Science, Faculty of Health Sciences, Curtin University. The authors acknowledge the provision of research facilities and the scientific and technical assistance of the staff of both CHIRI Biosciences Research Precinct Core Facility and the Curtin University Electron Microscope Facility, which was partly funded by Curtin University, State and Commonwealth Governments. SC Kundu presently holds ERA Chair Full Professor of European Commission Programme (FoReCaST) at 3Bs Research Group, University of Minho, Portugal.
Chaturvedi, V. , Naskar, D. , Kinnear, B. F. , Grenik, E. , Dye, D. E. , Grounds, M. D. , Kundu, S. C. , and Coombe, D. R. (2017) Silk fibroin scaffolds with muscle‐like elasticity support in vitro differentiation of human skeletal muscle cells. J Tissue Eng Regen Med, 11: 3178–3192. doi: 10.1002/term.2227.
Contributor Information
Subhas C. Kundu, Email: kundu@hijli.iitjgp.erne
Deirdre R. Coombe, Email: d.coombe@curtin.edu.au
References
- Andersen CL, Jensen JL, Ørntoft TF. 2004; Normalization of real‐time quantitative reverse transcription‐PCR data: a model‐based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res 64: 5245–5250. [DOI] [PubMed] [Google Scholar]
- Banani K, Kundu SC. 2013; Bio‐inspired fabrication of fibroin cryogels from the muga silkworm Antheraea assamensis for liver tissue engineering. Biomed Mater 8: 055003. DOI: 10.1088/1748-6041/8/5/. [DOI] [PubMed] [Google Scholar]
- Bauer F, Wohlrab S, Scheibel T. 2013; Controllable cell adhesion, growth and orientation on layered silk protein films. Biomater Sci 1: 1244–1249. [DOI] [PubMed] [Google Scholar]
- Bentzinger CF, Wang YX, Dumont NA, et al 2013; Cellular dynamics in the muscle satellite cell niche. EMBO Rep 14: 1062–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj N, Nguyen QT, Chen AC, et al 2011; Potential of 3‐D tissue constructs engineered from bovine chondrocytes/silk fibroin‐chitosan for in vitro cartilage tissue engineering. Biomaterials 32: 5773–5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj N, Sow WT, Devi D, et al 2015; Silk fibroin‐keratin based 3D scaffolds as a dermal substitute for skin tissue engineering. Integr Biol (Camb) 7: 53–63. [DOI] [PubMed] [Google Scholar]
- Cerchiari AE, Garbe JC, Jee NY, et al 2015; A strategy for tissue self‐organization that is robust to cellular heterogeneity and plasticity. Proc Natl Acad Sci 112: 2287–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi V, Dye DE, Kinnear BF, et al 2015; Interactions between skeletal muscle myoblasts and their extracellular matrix revealed by a serum free culture system. PLoS One 10: e0127675. DOI: 10.1371/journal.pone. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinsworth AM, Zhang S, Kraus WE, Truskey, GA . 2002; Apparent elastic modulus and hysteresis of skeletal muscle cells throughout differentiation. Am J Physiol Cell Physiol 283: C1219–C1227. [DOI] [PubMed] [Google Scholar]
- Datta A, Ghosh AK, Kundu CS. 2001a; Purification and characterization of fibroin from the tropical Saturniid silkworm, Antheraea mylitta . Insect Biochem Mol Biol 31: 1013–1018. [DOI] [PubMed] [Google Scholar]
- Datta A, Ghosh AK, Kundu, SC . 2001b; Differential expression of the fibroin gene in developmental stages of silkworm, Antheraea mylitta (Saturniidae). Comp Biochem Physiol B Biochem Mol Biol 129: 197–204. [DOI] [PubMed] [Google Scholar]
- Engler AJ, Griffin MA, Sen S, et al 2004; Myotubes differentiate optimally on substrates with tissue‐like stiffness: pathological implications for soft or stiff microenvironments. J Cell Biol 166: 877–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, et al 2006; Matrix elasticity directs stem cell lineage specification. Cell 126: 677–689. [DOI] [PubMed] [Google Scholar]
- Fu C, Shao Z, Fritz V. 2009; Animal silks: their structures, properties and artificial production. Chem Commun 43: 6515–6529. [DOI] [PubMed] [Google Scholar]
- Fujita H, Endo A, Shimizu K, Nagamori, E . 2010; Evaluation of serum‐free differentiation conditions for C2C12 myoblast cells assessed as to active tension generation capability. Biotechnol Bioeng 107: 894–901. [DOI] [PubMed] [Google Scholar]
- García AJ, Vega MD, Boettiger D. 1999; Modulation of cell proliferation and differentiation through substrate‐dependent changes in fibronectin conformation. Mol Biol Cell 10: 785–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentile NE, Stearns KM, Brown EH, et al 2014; Targeted rehabilitation after extracellular matrix scaffold transplantation for the treatment of volumetric muscle loss. Am J Phys Med Rehabil 93: S79–S87. [DOI] [PubMed] [Google Scholar]
- Goody MF, Sher RB, Henry CA. 2015; Hanging on for the ride: adhesion to the extracellular matrix mediates cellular responses in skeletal muscle morphogenesis and disease. Dev Biol 401: 75–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grogan BF. Hsu JR, Skeletal Trauma Research, C . 2011; Volumetric muscle loss. J Am Acad Orthop Surg 19(Suppl 1): S35–S37. [DOI] [PubMed] [Google Scholar]
- Grounds, MD . 2008; Complexity of extracellular matrix and skeletal muscle regeneration In Skeletal Muscle Repair and Regeneration (Schiaffino SS, Partridge TT. eds). Springer; Amsterdam. [Google Scholar]
- Grounds MD. 2014; Therapies for sarcopenia and regeneration of old skeletal muscles. BioArchitecture 4: 81–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Zhu J, Xue C, et al 2014; Chitosan/silk fibroin‐based, Schwann cell‐derived extracellular matrix‐modified scaffolds for bridging rat sciatic nerve gaps. Biomaterials 35: 2253–2263. [DOI] [PubMed] [Google Scholar]
- Guillet‐Deniau I, Leturque A, Girard J. 1994; Expression and cellular localization of glucose transporters (GLUT1, GLUT3, GLUT4) during differentiation of myogenic cells isolated from rat foetuses. J Cell Sci 107: 487–496. [PubMed] [Google Scholar]
- Harris AJ, Duxson MJ, Butler JE, et al 2005; Muscle fiber and motor unit behavior in the longest human skeletal muscle. J Neurosci 25: 8528–8533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan RL, Antle K, Collette AL, et al 2005; In vitro degradation of silk fibroin. Biomaterials 26: 3385–3393. [DOI] [PubMed] [Google Scholar]
- Hu X, Park S‐H, Gil ES, et al 2011; The influence of elasticity and surface roughness on myogenic and osteogenic‐differentiation of cells on silk‐elastin biomaterials. Biomaterials 32: 8979–8989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kar S, Talukdar S, Pal S, et al 2013; Silk gland fibroin from indian muga silkworm Antheraea assama as potential biomaterial. Tissue Eng Regen Med 10: 200–210. [Google Scholar]
- Kim HJ, Kim U‐J, Kim HS, et al 2008; Bone tissue engineering with premineralized silk scaffolds. Bone 42: 1226–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu B, Rajkhowa R, Kundu SC, Wang, X . 2013; Silk fibroin biomaterials for tissue regenerations. Adv Drug Deliv Rev 65: 457–470. [DOI] [PubMed] [Google Scholar]
- Kundu SC, Kundu B, Talukdar S, et al 2012a; Invited review nonmulberry silk biopolymers. Biopolymers 97: 455–467. [DOI] [PubMed] [Google Scholar]
- Kundu SC, Kundu B, Talukdar S, et al 2012b; Nonmulberry silk biopolymers. Biopolymers 97: 455–467. [DOI] [PubMed] [Google Scholar]
- Leiss M, Beckmann K, Girós A, et al 2008; The role of integrin binding sites in fibronectin matrix assembly in vivo . Curr Opin Cell Biol 20: 502–507. [DOI] [PubMed] [Google Scholar]
- Li M, Ogiso M, Minoura, N . 2003; Enzymatic degradation behavior of porous silk fibroin sheets. Biomaterials 24: 357–365. [DOI] [PubMed] [Google Scholar]
- Mahendran B, Ghosh SK, Kundu, SC . 2006; Molecular phylogeny of silk‐producing insects based on 16S ribosomal RNA and cytochrome oxidase subunit I genes. J Genet 85: 31–38. [DOI] [PubMed] [Google Scholar]
- Mase VJ Jr, Hsu JR, Wolf SE, et al 2010; Clinical application of an acellular biologic scaffold for surgical repair of a large, traumatic quadriceps femoris muscle defect. Orthopedics 33: 511. [DOI] [PubMed] [Google Scholar]
- Mayer, U. 2003; Integrins: redundant or important players in skeletal muscle? J Biol Chem 278: 14587–14590. [DOI] [PubMed] [Google Scholar]
- Meinel L, Kaplan, DL . 2012; Silk constructs for delivery of musculoskeletal therapeutics. Adv Drug Deliv Rev 64: 1111–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinel L, Fajardo R, Hofmann S, Langer R, Chen J, Snyder B, Vunjak‐Novakovic G, Kaplan, D . 2005; Silk implants for the healing of critical size bone defects. Bone 37: 688–698. [DOI] [PubMed] [Google Scholar]
- Morgan AW, Roskov KE, Lin‐Gibson S, et al 2008; Characterization and optimization of RGD‐containing silk blends to support osteoblastic differentiation. Biomaterials 29: 2556–2563. [DOI] [PubMed] [Google Scholar]
- Nazarov R, Jin, H.‐J , Kaplan, DL . 2004; Porous 3‐D scaffolds from regenerated silk fibroin. Biomacromolecules 5: 718–726. [DOI] [PubMed] [Google Scholar]
- Omenetto FG, Kaplan, DL . 2010; New opportunities for an ancient material. Science 329: 528–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal S, Kundu J, Talukdar S, et al 2013; An emerging functional natural silk biomaterial from the only domesticated non‐mulberry silkworm Samia ricini . Macromol Biosci 13: 1020–1035. [DOI] [PubMed] [Google Scholar]
- Park H‐S, Gong M‐S, Park J‐H, et al 2013a; Silk fibroin–polyurethane blends: physical properties and effect of silk fibroin content on viscoelasticity, biocompatibility and myoblast differentiation. Acta Biomater 9: 8962–8971. [DOI] [PubMed] [Google Scholar]
- Park H‐S, Gong M‐S, Park J‐H, et al 2013b; Silk fibroin–polyurethane blends: physical properties and effect of silk fibroin content on viscoelasticity, biocompatibility and myoblast differentiation. Acta Biomater 9: 8962–8971. [DOI] [PubMed] [Google Scholar]
- Patra C, Talukdar S, Novoyatleva T, et al 2012; Silk protein fibroin from Antheraea mylitta for cardiac tissue engineering. Biomaterials 33: 2673–2680. [DOI] [PubMed] [Google Scholar]
- Paul AC. 2001; Muscle length affects the architecture and pattern of innervation differently in leg muscles of mouse, guinea pig, and rabbit compared to those of human and monkey muscles. Anat Rec 262: 301–309. [DOI] [PubMed] [Google Scholar]
- Pfaffl M, Tichopad A, Prgomet C, et al 2004; Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper – Excel‐based tool using pair‐wise correlations. Biotechnol Lett 26: 509–515. [DOI] [PubMed] [Google Scholar]
- Shen Z, Guo S, Ye D, et al 2013; Skeletal muscle regeneration on protein‐grafted and microchannel‐patterned scaffold for hypopharyngeal tissue engineering. Biomed Res Int 2013: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara M, Sabra K, Gennisson J‐L, et al 2010; Real‐time visualization of muscle stiffness distribution with ultrasound shear wave imaging during muscle contraction. Muscle Nerve 42: 438–441. [DOI] [PubMed] [Google Scholar]
- Sicari BM, Rubin JP, Dearth CL, et al 2014; An acellular biologic scaffold promotes skeletal muscle formation in mice and humans with volumetric muscle loss. Sci Transl Med 6: 234ra58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofia S, McCarthy MB, Gronowicz G, et al 2001; Functionalized silk‐based biomaterials for bone formation. J Biomed Mater Res 54: 139–148. [DOI] [PubMed] [Google Scholar]
- Spandidos A, Wang X, Wang H, et al 2010; PrimerBank: a resource of human and mouse PCR primer pairs for gene expression detection and quantification. Nucleic Acids Res 38: D792–D799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern‐Straeter J, Bonaterra G, Hormann K, et al 2009; Identification of valid reference genes during the differentiation of human myoblasts. BMC Mol Biol 10: 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern‐Straeter J, Bonaterra GA, Kassner SS, et al 2011; Characterization of human myoblast differentiation for tissue‐engineering purposes by quantitative gene expression analysis. J Tissue Eng Regen Med 5: e197–e206. [DOI] [PubMed] [Google Scholar]
- Talukdar S, Nguyen QT, Chen AC, et al 2011; Effect of initial cell seeding density on 3D‐engineered silk fibroin scaffolds for articular cartilage tissue engineering. Biomaterials 32: 8927–8937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Inoue S, Mizuno S. 1999; Hydrophobic interaction of P25, containing Asn‐linked oligosaccharide chains, with the H–L complex of silk fibroin produced by Bombyx mori . Insect Biochem Mol Biol 29: 269–276. [DOI] [PubMed] [Google Scholar]
- Tang‐Schomer MD, White JD, Tien LW, et al 2014; Bioengineered functional brain‐like cortical tissue. Proc Natl Acad Sci 111: 13811–13816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas K, Engler AJ, Meyer, GA . 2014; Extracellular matrix regulation in the muscle satellite cell niche. Connect Tissue Res 56: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Cao L, Shansky J, et al 2014; Minimally invasive approach to the repair of injured skeletal muscle with a shape‐memory scaffold. Mol Ther 22: 1441–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Rudym DD, Walsh A, et al 2008; In vivo degradation of three‐dimensional silk fibroin scaffolds. Biomaterials 29: 3415–3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yucel T, Lovett ML, Kaplan DL. 2014; Silk‐based biomaterials for sustained drug delivery. J Control Release 190: 381–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Fan W, Ma Z, et al 2010; The effects of pore architecture in silk fibroin scaffolds on the growth and differentiation of mesenchymal stem cells expressing BMP7. Acta Biomater 6: 3021–3028. [DOI] [PubMed] [Google Scholar]
- Zhou C‐Z, Confalonieri F, Medina N, et al 2000; Fine organization of Bombyx mori fibroin heavy chain gene. Nucleic Acids Res 28: 2413–2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Cao C, Ma X, et al 2010; In vitro and in vivo degradation behavior of aqueous‐derived electrospun silk fibroin scaffolds. Polymer Degrad Stabil 95: 1679–1685. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Supporting info item


