Abstract
Aims
To compare the glycaemic outcomes of 2 glucose‐lowering treatment strategies in vulnerable (moderately ill and/or frail) patients aged ≥65 years with type 2 diabetes whose individual HbA1c targets were not met with diet/exercise and/or oral anti‐hyperglycaemic medications (OAMs).
Methods
The primary endpoint of this study was a composite of achieving/maintaining individualized HbA1c targets without “clinically significant” hypoglycaemia (severe hypoglycaemia or repeated hypoglycaemia causing interruption of patients’ activities or blood glucose <54 mg/dL). Strategy‐A comprised glucose‐dependent therapies (n = 99) with a non‐sulphonylurea OAM and a glucagon‐like peptide‐1 receptor agonist as the first injectable. Strategy‐B comprised non‐glucose‐dependent therapies (n = 93) with sulphonylurea as the preferred OAM and insulin glargine as the first injectable.
Results
There was no significant difference between Strategy‐A and Strategy‐B in percentages of patients achieving the primary endpoint (64.5% vs 54.9%; P = .190). Mean incidences (A vs B) of total (10.2% vs 53.8%), documented symptomatic (5.1% vs 36.6%), and asymptomatic (8.2% vs 32.3%) hypoglycaemia were lower for Strategy‐A (P < .001 each). Proportions of patients achieving/maintaining HbA1c target (A, 63.3% vs B, 55.9%) were similar.
Conclusion
Similar proportions of older, vulnerable aged ≥65 years patients with type 2 diabetes achieved/maintained glycaemic treatment goals without clinically significant hypoglycaemia with Strategies A or B. However, Strategy‐A resulted in lower risk of total, documented symptomatic, and asymptomatic hypoglycaemia. These results identify an approach of potential clinical benefit in this age group and will inform future clinical research in older patients with type 2 diabetes.
Keywords: GLP‐1 analogue , glycaemic control , hypoglycaemia , insulin delivery , sulphonylureas
1. INTRODUCTION
Diabetes global prevalence is ~20% in people aged 65 to 79 years,1 most have type 2 diabetes (T2D), and rates are increasing.2, 3, 4 Such patients are often vulnerable because of comorbid conditions and/or frailty.5, 6 Lowering blood glucose (BG) is a mainstay of treatment, regardless of age and functional status.7, 8 In older patients, the main objective is to avoid/minimize symptoms and potential complications of hyperglycaemia.6, 9 Older individuals are also prone to hypoglycaemia,10 consequences of which can be severe.11
Optimal management in this population is difficult to define, however, largely because patients with frailty and/or significant comorbidities are underrepresented in trials and, if included, functional status is typically not reported.8, 9, 12, 13 Therefore, it remains unclear whether the intensive glycaemic control benefits demonstrated in younger patients,9, 14 can be achieved in such older patients.
There is also uncertainty about appropriate glycaemic targets for each patient. When selecting individual targets, the relative importance of individual characteristics will vary depending on practitioner experience, patient characteristics and comorbidities, and specific clinical circumstances.15 Various guidelines recommend individualized glycaemic targets aligned with functional status for people with T2D7, 16, 17; however, recommended targets have not been validated in clinical trials involving older populations.
Sulphonylurea therapy continues to be commonly prescribed in monotherapy or in combination with other OAMs,18, 19, 20 and basal insulin continues to be recommended as first‐line injectable therapy to people with T2D.7, 16 Despite the importance of avoiding hypoglycaemia in older patients,6, 9 these therapies are still considered reasonably safe options in this population as reflected by studies examining treatment patterns.19, 20, 21 Sulfonylurea exerts its glucose‐lowering action primarily through stimulation of insulin secretion.22 This action does not depend on blood glucose levels and thus may cause excessive insulin secretion and trigger hypoglycaemia.23 New classes of anti‐hyperglycaemic medications (eg, dipeptidyl peptidase‐4 [DPP‐4] inhibitors, thiazolidinediones, injectable glucagon‐like peptide‐1 receptor agonists [GLP‐1 RA] and sodium‐glucose cotransporter‐2 [SGLT‐2] inhibitors), typically do not trigger excessive insulin secretion and do not increase the risk of hypoglycemia.7, 24 Hypersecretion of incretin hormones resulting in lowering blood glucose to hypoglycemic levels has been described in some pathophysiological states eg, in patients with Long‐QT syndrome25 or in patients after gastric by‐pass surgery.26 However in broad populations of people with T2D the risk of hypoglycemia with incretin‐based therapies has been shown to be low.27
In the present study, we evaluated effects of 2 anti‐hyperglycaemic treatment strategies in vulnerable (moderately ill and/or frail) older patients (≥65 years) with suboptimally controlled T2D. Strategy‐A used oral and injectable therapies that do not stimulate insulin secretion when BG reaches normal/low values (a glucose‐dependent mode of action). GLP‐1 RA27 was a preferred first‐line injectable. Sulphonylurea therapy22 and insulin were excluded. Strategy‐B used treatments that exert their glucose‐lowering effect irrespective of prevailing glycaemia; sulphonylurea22 as preferred OAM and insulin as preferred first‐line injectable therapy (non‐glucose‐dependent agents). We tested the hypothesis that more patients in Strategy‐A would achieve better results (ie, more patients reaching their individual glycaemic target without hypoglycaemia) than in Strategy‐B.
2. METHODS
2.1. Study design
This randomized, multinational, open‐label, in‐label, active‐controlled, parallel‐group study involved moderately ill and/or frail older patients. The primary objective was to assess relative success of 2 treatment strategies in achieving/maintaining glycaemic control without “clinically significant hypoglycaemia.” The study, which was planned to continue for 72 weeks (Figure S1), was preceded by an internal pilot phase (described in this paper) that included ~20% of the planned full study population who were treated for ≥24 weeks. Once this number was reached, enrollment in the full study was paused and an interim analysis was conducted. Enrollment was to resume if interim results indicated feasibility of the full study. Interim results (unblinded efficacy and safety data) were evaluated by an internal Data Monitoring Committee (DMC).
The study was conducted according to the ethical principles of the Declaration of Helsinki and Council for International Organisations of Medical Science International Ethical Guidelines, the International Conference on Harmonisation Good Clinical Practice Guidelines, and applicable laws and regulations. Participating investigators are listed in Appendix S1.
2.2. Eligibility criteria
Eligible patients (male or female) were ≥65 years with T2D, HbA1c >7.3% (56 mmol/mol) and <10.9% (96 mmol/mol) and ≥0.4% higher than the upper limit of the individualized target range set at screening. Patients were assessed according to established frailty scales (Clinical Frailty Scale [CFS]),28 and comorbidities (Total Illness Burden Index [TIBI]),29 in older individuals, and were required to have a CFS score ≥4 and/or a TIBI score ≥5. Patients could enroll if before study entry they were treated with diet and exercise (if metformin contraindicated) or for ≥3 months received OAMs, as monotherapy/dual combination: sulphonylurea (any dose); maximally tolerated/effective doses of metformin (≥1500 mg/d), DPP‐4 inhibitor (any marketed dose), thiazolidinedione (≥30 mg/d of pioglitazone/≥4 mg/d of rosiglitazone), or acarbose (≥75 mg/d).
2.3. Selection of individualized HbA1c target
For each individual, treatment aimed at achieving and maintaining individualized, preset HbA1c target ranges, while avoiding hypoglycaemia. Individual HbA1c targets (7.5%‐7.9%, 7.0%‐7.4%, and <7%) were determined at screening. HbA1c targets were ultimately at investigator discretion, based on presence of comorbidities and complications, cognitive status, life expectancy, duration of diabetes, functional status, and hypoglycaemic risk, and were agreed between investigator and patient at screening. Guidelines for selection of individualized treatment targets are available online (Appendix S2).
2.4. Treatment
Investigators were provided general management rules and guidance for using study treatments according to locally‐approved product labels; however, within a given strategy, choice of specific treatments and their combinations was at their discretion. Self‐monitoring of BG was performed at the discretion of the investigator. Treatments were titrated throughout until maximally tolerated and/or approved doses or HbA1c target was reached. If maximally tolerated and/or approved doses were reached but individualized HbA1c targets were not met, next‐line therapy was initiated by adding another treatment.
Marketed OAMs and injectable glucose‐lowering treatments were used across different lines of treatment in each strategy, beginning with a single OAM and progressing to 3 OAMs, and first‐line injectable therapy.
Patients randomized to Strategy‐A (Figure S2) were excluded from sulphonylurea and insulin therapy. If OAMs were not effective and injectable treatment was indicated, Strategy‐A patients commenced available GLP‐1 RA therapy (exenatide twice daily, exenatide once weekly, or liraglutide). Patients randomized to Strategy‐B (Figure S3) were treated with glimepiride as part of any OAM treatment line (monotherapy, dual, or triple combination) and insulin glargine as first‐line injectable treatment. If appropriate, investigators could start next‐line therapy once the patient was receiving glimepiride 4 mg/d. Insulin glargine dosage was adjusted based on self‐monitored fasting BG (FBG) according to study titration algorithms (Appendix S3), with the aim of reaching HbA1c target without hypoglycaemia. Once injectable treatment was started, OAM could be continued in combination with GLP‐1 RA or insulin at the same/lower dose or could be discontinued, at investigator discretion.
Metformin, pioglitazone, DPP‐4 inhibitors, and acarbose could be used in either strategy; other anti‐hyperglycaemic medications, including SGLT‐2 inhibitors, were excluded from both strategies. Some pre‐study treatments were replaced at study entry: rosiglitazone was replaced with pioglitazone, and DPP‐4 inhibitors other than linagliptin or sitagliptin, were replaced with either of these. For patients randomized to Strategy‐B, sulphonylureas other than glimepiride, were replaced with this medication, while in Strategy‐A, sulphonylurea was stopped and replaced with other OAMs. These and other treatments were continued throughout the study.
2.5. Outcome measures
The primary outcome was a composite of achieving and maintaining individualized HbA1c targets without “clinically significant” hypoglycaemia. This was defined as any one of the following: (1) severe hypoglycaemia (signs and symptoms consistent with hypoglycaemia, requiring assistance of another person and associated with BG ≤70 mg/dL/prompt recovery after oral carbohydrate/glucagon/intravenous glucose); (2) within 1 month, ≥2 documented events of symptomatic hypoglycaemia (confirmed with BG ≤70 mg/dL) that forced the patient to interrupt what he/she was currently doing/or ≥2 nocturnal hypoglycaemic events (confirmed with BG ≤70 mg/dL) that woke the patient and forced him/her to act; (3) within 2 weeks, ≥2 events of asymptomatic documented hypoglycaemia (BG <54 mg/dL) detected by self‐monitoring.
Achievement of primary outcome and maintenance until study end was considered a treatment success. Conversely, treatment was considered a failure if any of the following occurred: (1) individualized HbA1c treatment target not reached/maintained at 2 consecutive determinations starting from week‐24 for patients with data beyond week‐24, or not reached at week‐24 for patients without data beyond week‐24; (2) “clinically significant” hypoglycaemia; (3) HbA1c target not achieved at study discontinuation.
Secondary efficacy outcomes included proportions of patients reaching treatment target at last available visit, proportions of patients requiring alternative treatment after glycaemic failure of first‐line injectable therapy, and change from baseline to endpoint in HbA1c.
Safety assessments included incidence and rate of hypoglycaemia including severe and nocturnal hypoglycaemia, presence of diabetic kidney disease (DKD) (estimated glomerular filtration rate [eGFR] <60 mL/min/1.73 m2 or albumin‐to‐creatinine ratio > 30 mg/g), progression of DKD (≥30% decrease in eGFR from baseline/increased albumin‐to‐creatinine ratio from ≤30 mg/g at baseline to >30‐300 mg/g post‐baseline),30, 31 and incidence of adverse events.
2.6. Statistical analysis
Demographic and baseline characteristics data were summarized using descriptive statistics. Categorical analyses were performed using chi‐squared or Fisher's exact test.
Logistic regression with treatment target, country, and baseline HbA1c as covariates was applied to the comparison between treatment strategies of proportions of patients achieving treatment success, and to proportions of patients reaching individualized HbA1c target at last available visit. Hypothesis testing was two‐sided with an α = .05 significance level.
HbA1c changes from baseline (randomization) were analysed using a mixed‐model repeated measures approach that included treatment, baseline HbA1c, treatment target, country, pre‐study OAM use, pre‐study sulphonylurea use, and treatment‐by‐visit interaction as fixed effects.
A pre‐defined interim analysis was to be performed when ~142 patients completed 24 weeks of treatment. Conditional power (CP) was calculated to determine the feasibility of the full study and was defined according to prespecified protocol criteria. If the CP was “promising” (0.20‐0.95), the study was continued and sample size was increased to a maximum of 660 completed patients; if “favourable” (>0.95), the study was continued using the planned sample size of 500 completed patients; if “unfavourable” (<0.20), the study was terminated.
3. RESULTS
3.1. Patients
In total, 388 patients were enrolled/screened from February 2014 to October 2015 at 40 study sites in 4 countries (Austria, Germany, UK, USA [including Puerto Rico]), and 192 patients were entered/randomized to Strategy‐A (n = 99) and Strategy‐B (n = 93). Of these, 98 and 93 patients, respectively, were treated and included in the analysis (Figure 1). Baseline patient characteristics were generally similar between strategies (Tables 1 and S1).
Figure 1.
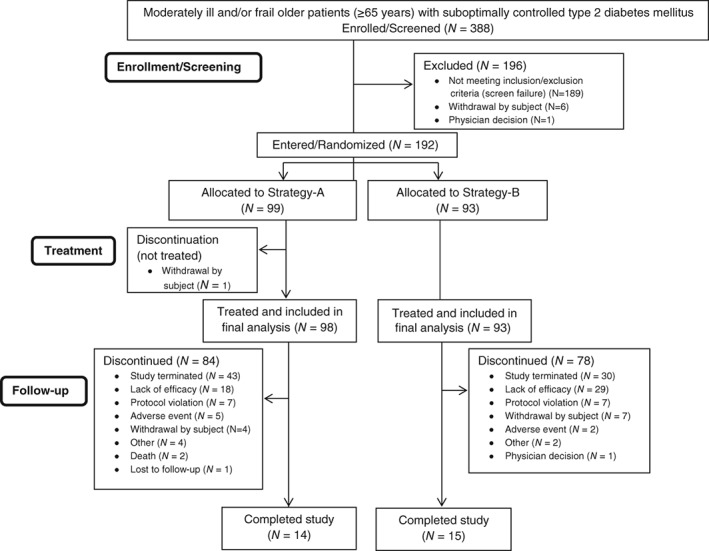
Patient flow chart of moderately ill and/or frail older patients (≥65 years) with suboptimally controlled type 2 diabetes mellitus included in the analysis
Table 1.
Baseline patient characteristics
| Strategy‐A,N = 99 | Strategy‐B,N = 93 | |
|---|---|---|
| Male | 56 (56.6) | 59 (63.4) |
| Age, years | ||
| Mean ± SD | 70.7 ± 5.3 | 70.7 ± 4.4 |
| Median (min, max) | 70.0 (65, 91) | 70.0 (65, 82) |
| ≥75 | 17 (17.2) | 18 (19.4) |
| Race | ||
| White | 92 (92.9) | 81 (87.1) |
| Black or African‐American | 7 (7.1) | 11 (11.8) |
| American Indian or Alaska Native | 0 (0.0) | 1 (1.1) |
| Ethnicity | ||
| Hispanic or Latino | 35 (35.4) | 32 (34.4) |
| Weight, kg | 86.2 ± 17.2 | 88.5 ± 16.4 |
| BMI, kg/m2 | 31.0 ± 5.7 | 31.3 ± 4.8 |
| HbA1c,% | ||
| Mean ± SD | 8.4 ± 0.9 | 8.2 ± 0.8 |
| <8 | 42 (42.4) | 41 (44.1) |
| ≥8 to <9 | 35 (35.4) | 38 (40.9) |
| ≥9 to <10 | 15 (15.2) | 12 (12.9) |
| ≥10 | 7 (7.1) | 2 (2.2) |
| HbA1c target,% | ||
| <7 | 47 (47.5) | 47 (50.5) |
| ≥7 to <7.5 | 39 (39.4) | 36 (38.7) |
| ≥7.5 to <8 | 13 (13.1) | 10 (10.8) |
| FBG, mg/dL | 174.6 ± 45.0 | 167.4 ± 43.2 |
| eGFR, mL/min/1.73 m2 | ||
| ≥30 to <60 | 22 (22.2) | 16 (17.6) |
| ≥60 to <90 | 51 (51.5) | 51 (56.0) |
| ≥90 | 26 (26.3) | 24 (26.4) |
| Prior antidiabetic treatment | ||
| Naïve | 3 (3.0) | 1 (1.1) |
| 1 OAM | 38 (38.4) | 40 (43.0) |
| 2 OAMs | 50 (50.5) | 44 (47.3) |
| >2 OAMs | 8 (8.1) | 8 (8.6) |
| Prior sulphonylurea use | 51 (51.5) | 38 (40.9) |
| TIBIa | ||
| Mean score ± SD | 3.9 ± 2.2 | 3.7 ± 2.5 |
| Total score ≥ 5 | 37 (37.8) | 31 (33.3) |
| CFSa | ||
| Mean score ± SD | 4.0 ± 0.5 | 4.1 ± 0.6 |
| Total score ≥ 4 | 91 (92.9) | 91 (97.8) |
| TIBI score ≥ 5 or CFS score ≥ 4 | 97 (99.0) | 93 (100) |
| TIBI score ≥ 5 and CFS score ≥ 4 | 31 (31.6) | 29 (31.2) |
| TIBI score < 5 and CFS score < 4 | 1 (1.0) | 0 |
Abbreviations: BMI, body mass index; CFS, Clinical Frailty Scale; eGFR, estimated glomerular filtration rate; FBG, fasting blood glucose; HbA1c, glycated haemoglobin; OAM, oral anti‐hyperglycaemic medication; SD, standard deviation; TIBI, Total Illness Burden Index.
Data are presented as mean ± SD or n (%) unless otherwise indicated.
The distribution of patients by TIBI and CFS scores by treatment strategy is provided in Table S1 in the online supporting information.
n = 98 for glucose‐dependent strategy.
3.2. Study treatment
At final analysis patients had received treatment for a median duration of 49.0 weeks (range 0.1‐78.6 weeks), with the most frequent treatment modality being 3 OAMs in Strategy‐A (32.7% of patients) and 2 OAMs in Strategy‐B (40.9% of patients), both without injectable therapy (Table 2). More than 40% (Strategy‐A) and ~20% (Strategy‐B) of patients started injectable (4th‐line) therapy in combination with 1 to 3 OAMs. Predominant OAMs were metformin (83.7%), DPP‐4 inhibitors (67.3%), and thiazolidinediones (pioglitazone, 60.2%) in Strategy‐A, and glimepiride (95.7%), metformin (89.2%), and DPP‐4 inhibitors (49.5%) in Strategy‐B.
Table 2.
Study treatmenta
| Strategy‐A,N = 98 | Strategy‐B,N = 93 | |
|---|---|---|
| Treatment modalities | ||
| 1 OAM | 2 (2.0) | 2 (2.2) |
| 2 OAMs | 23 (23.5) | 38 (40.9) |
| 3 OAMs | 32 (32.7) | 30 (32.3) |
| 1 OAM + injectable | 15 (15.3) | 1 (1.1) |
| 2 OAMs + injectable | 18 (18.4) | 7 (7.5) |
| 3 OAMs + injectable | 7 (7.1) | 14 (15.1) |
| Injectable only | 1 (1.0) | 1 (1.1) |
| Study drug | ||
| Biguanides: Metformin | 82 (83.7) | 83 (89.2) |
| DPP‐4 Inhibitors | 66 (67.3) | 46 (49.5) |
| Sitagliptin | 50 (51.0) | 34 (36.6) |
| Linagliptin | 16 (16.3) | 12 (12.9) |
| SUs: Glimepiride | 1 (1.0)b | 89 (95.7) |
| TZDs: Pioglitazone | 59 (60.2) | 7 (7.5) |
| α Glucosidase inhibitors: acarbose | 8 (8.2) | 0 |
| GLP‐1 RAs | 40 (40.8) | 0 |
| Liraglutide | 17 (17.3) | 0 |
| Exenatide QW | 22 (22.4) | 0 |
| Exenatide BID | 1 (1.0) | 0 |
| Insulin glargine | 2 (2.0)b | 23 (24.7) |
Abbreviations: BID, twice daily; DPP‐4, dipeptidyl peptidase‐4; GLP‐1 RAs, glucagon‐like peptide‐1 receptor agonists; OAM, oral anti‐hyperglycaemic medication; QW, once weekly; SUs, sulphonylureas; TZDs, thiazolidinediones.
Data are presented as n (%).
Maximum line of therapy.
These patients (protocol violations) were included in the analysis as the small numbers in each strategy were not anticipated to effect the study results.
3.3. Primary outcome
The primary outcome was not significantly different between treatment strategies (A, 68.9% vs B, 65.9% of patients; P = .666) at the pre‐defined interim analysis. This lack of a difference between strategies in treatment success was apparent in each of its components: failure to achieve and maintain individualized glycaemic targets and similar percentages of patients with “clinically significant” hypoglycaemia in both strategies. Further analysis yielded a CP of 0.05, indicating an “unfavourable” probability of demonstrating a difference between strategies in treatment success, even if the sample size was increased for the full study. On the basis of these results and recommendations from the DMC, the study was discontinued. Once the decision to stop the trial was made, patients were asked to come for their next scheduled study visit which was considered final.
For the primary outcome at final analysis (Table 3), there was no significant difference between treatment strategies (A, 64.5% vs B, 54.9% of patients; P = .190). Failure to achieve/maintain HbA1c target occurred in 36.7% of patients in Strategy‐A and 44.1% in Strategy‐B, and 34.7% and 38.7% of patients, respectively, did not achieve HbA1c target at study discontinuation. “Clinically significant” hypoglycaemia was reported in Strategy‐B only (1.1% of patients). Patients in Strategy‐A were more likely than those in Strategy‐B to be receiving injectable therapy at HbA1c target failure (ie, ≤3 OAMs + 1st‐line injectable; 21.4% vs 14.0%) (Table 3). There were no significant differences between treatment strategies in proportions of patients reaching/maintaining HbA1c target at last visit, or in HbA1c change from baseline to endpoint (Table 3).
Table 3.
Treatment outcomes
| Strategy‐A,N = 98 | Strategy‐B,N = 93 | |
|---|---|---|
| Relative success of treatment strategies | ||
| Successa, n (%) | 62 (63.3) | 52 (55.9) |
| Adjusted % (95% CI) | 64.5 (54.4, 73.4) | 54.9 (44.6, 64.9) |
| Adjusted % (95% CI)/P‐value,Strategy‐A vs Strategy‐B | 9.5 (−4.7, 23.2)/.190 | |
| Conditional powerb | 0.58 | |
| Failure, n (%) | 36 (36.7) | 41 (44.1) |
| Reason for failurec, n (%) | ||
| Clinically significant hypoglycaemia | 0 | 1 (1.1) |
| HbA1c target not reached/maintainedd | 36 (36.7) | 41 (44.1) |
| Treatment at HbA1c target failuree, n (%) | ||
| 1 OAM | 3 (3.1) | 1 (1.1) |
| 2 OAMs | 8 (8.2) | 12 (12.9) |
| 3 OAMs | 3 (3.1) | 15 (16.1) |
| ≤3 OAMs + first‐line injectable | 21 (21.4) | 13 (14.0) |
| HbA1c target not achieved at study discontinuation | 34 (34.7) | 36 (38.7) |
| HbA1c target at last visit | ||
| Success, n (%) | 64 (65.3) | 55 (59.1) |
| Adjusted % (95% CI) | 67.5 (57.3, 76.2) | 57.8 (47.2, 67.7) |
| Adjusted % (95% CI)/P‐value,Strategy‐A vs Strategy‐B | 9.7 (−4.5, 23.4)/0.182 | |
| Failure, n (%) | 34 (34.7) | 38 (40.9) |
| HbA1c from baseline to endpoint | ||
| Baseline, mean % (SD) | n = 98, 8.4 (0.9) | n = 93, 8.2 (0.8) |
| Endpoint, mean % (SD) | n = 96, 7.2 (1.3) | n = 92, 7.2 (0.8) |
| Change from baseline to endpoint, LS mean % (SE) | −1.17 (0.10) | −1.05 (0.10) |
| LS mean % (95% CI)/P‐value, A vs B | −0.12 (−0.40, 0.16)/0.390 | |
Abbreviations: CI, confidence interval; HbA1c, glycated haemoglobin; OAM, oral anti‐hyperglycaemia medication; LS, least square; SD, standard deviation; SE, standard error.
Adjusted proportions and P ‐values were based on a logistic regression model with treatment target, country, and baseline HbA1c as covariates.
Treatment strategy was considered a success if HbA1c target was reached/maintained with no clinically significant hypoglycaemia.
Treatment (Strategy‐A vs Strategy‐B) was considered “promising” for a conditional power (CP) of 0.20‐0.95, “favourable” for a CP > 0.95, and “unfavourable” for a CP < 0.20.
Patients could have been counted in more than 1 category.
Individualized HbA1c treatment target was not reached/maintained upon 2 consecutive determinations starting from week 24 for patients with data beyond week 24, or not reached at week 24 for patients without data beyond week 24.
One patient in Strategy‐A did not have a treatment record as this patient was discontinued for a protocol violation (week 4) but was included as a treatment failure because the HbA1c target was not met at the last available visit.
3.4. Safety
Incidences of total, documented symptomatic, and asymptomatic hypoglycaemic events were significantly lower in Strategy‐A than in Strategy‐B (10.2% vs 53.8%; 5.1% vs 36.6%; 8.2% vs 32.3%, respectively; P < .001 for each), as were mean (SD) overall, documented symptomatic, and asymptomatic 30‐day rates: 0.05 (0.23) vs 0.31 (0.91); 0.03 (0.17) vs 0.22 (0.89); and 0.01 (0.09) vs 0.07 (0.15) events/patient/30 days, respectively; P < .001 for each). Differences between Strategy‐A and Strategy‐B in incidences of severe hypoglycaemia (0% vs 0%) and nocturnal hypoglycaemia (4.1% vs 10.8%) were not statistically significant.
Proportions of patients with ≥1 treatment‐emergent adverse event (TEAE) were similar for both strategies (A, 84.7%; B, 79.6%), while treatment‐related TEAEs occurred more frequently in Strategy‐A (27.6% vs 8.6%, P < .001). The most frequent TEAEs (≥5% of patients in either strategy) are summarized in Table 4. The gastrointestinal TEAEs, diarrhoea (12.2% vs 6.5%) and nausea (7.1% vs 3.2%), were more common for Strategy‐A. Falls resulting in TEAEs occurred in 9.2% and 9.7% of patients in Strategy‐A and Strategy‐B, respectively, but none were considered related to hypoglycaemia.
Table 4.
Treatment‐emergent adverse events (TEAEs; >5% in either arm)
| TEAE, n (%) | Strategy‐A,N = 98 | Strategy‐B,N = 93 |
|---|---|---|
| Any TEAE | 83 (84.7) | 74 (79.6) |
| Nasopharyngitis | 10 (10.2) | 16 (17.2) |
| Back pain | 9 (9.2) | 11 (11.8) |
| Diarrhoea | 12 (12.2) | 6 (6.5) |
| Vitamin D deficiency | 9 (9.2) | 3 (3.2) |
| Urinary tract infection | 3 (3.1) | 8 (8.6) |
| Nausea | 7 (7.1) | 3 (3.2) |
| Upper respiratory tract infection | 3 (3.1) | 7 (7.5) |
| Chikungunya virus infection | 4 (4.1) | 5 (5.4) |
| Hypertension | 5 (5.1) | 4 (4.3) |
| Headache | 7 (7.1) | 1 (1.1) |
| Oedema | 6 (6.1) | 0 (0.0) |
| Weight decrease | 5 (5.1) | 0 (0.0) |
Seven patients were discontinued because of an adverse event (AE) (A, n = 5 [5.1%]; B, n = 2 [2.2%]) but only 1 was considered to be related to treatment (BG increased [A]). Serious AEs occurred in 29 patients (A, n = 15 [15.3%]; B, n = 14 [15.1%]; P = .961), including 2 deaths in Strategy‐A (1 cerebrovascular accident; 1 acute myocardial infarction). None of these events was considered to be related to study treatment.
There was no difference between strategies in proportions of patients with kidney disease at study endpoint (A, 39.8%; B, 45.2%; P = .453), or in evidence of disease progression (A, 10.2%; B, 11.8%; P = .720).
4. DISCUSSION
A significant challenge in treating older patients with diabetes is achieving glucose targets without increased risk of hypoglycaemia. We report the results of the first study attempting to compare benefits and risks of treatment strategies available in a group representative of the older population. The study aimed at assessing whether a glucose‐dependent (Strategy‐A) versus a non‐glucose‐dependent (Strategy‐B) anti‐hyperglycaemic strategy may achieve better results in the primary composite endpoint of achieving and maintaining individualized glycaemic treatment goals without “clinically significant” hypoglycaemia in older, vulnerable patients with T2D. The novelty of this study is the primary endpoint, selected to comprise avoidance of both hyperglycaemia and hypoglycaemia. Other novel features include involvement of a well characterized (from a functional standpoint) older population who were frail and/or had comorbidities together with evaluation of treatment strategies across multiple lines of therapy and use of individualized glycaemic targets.
Both strategies resulted in improved glycaemic control and clinically meaningful HbA1c reductions of ~1.1%. At endpoint, mean HbA1c was 7.2% in each cohort. Improved glucose control was achieved with no clinically significant hypoglycaemia. However, it was evident from the interim results that Strategy‐B confers a significantly greater hypoglycaemia risk pertaining to categories other than clinically significant episodes (ie, total, symptomatic, asymptomatic).
Hypoglycaemic events are typically assigned different degrees of clinical importance, but information continues to emerge that suggests all such episodes may be relevant. For example, recent data have shown increased risk of cardiac arrhythmias in association with both asymptomatic episodes with interstitial glucose levels <3.1 mmol/L (“severe episodes”)32 and nocturnal33 hypoglycaemia. Given that any hypoglycaemic event may be considered “clinically relevant,” particularly in older, vulnerable populations, hindsight leads us to question whether our choice of primary endpoint was too stringent or restrictive in this study. Moreover, it is unlikely that, had “any hypoglycaemic episode” been used in the primary endpoint, the study would have been stopped on the basis of futility; however, this can only be confirmed in future studies designed to investigate benefits and risks of these 2 strategies in vulnerable, older patients. Given the frequent occurrence of asymptomatic hypoglycaemia in older patients, use of continuous glucose monitoring (CGM) in all patients in this study may have yielded some valuable data. Indeed, a subset of patients in this study were monitored by CGM and the results have been reported separately.34 The major value of this study lies in the lessons learned that can be applied to the conduct of future studies. As such, we examined this study's limitations.
The first important point relates to the study population. Enrolled patients were generally younger and less frail than anticipated for Strategy‐A and Strategy‐B (mean CFS, 4.0 and 4.1; mean TIBI, 3.9 and 3.7, respectively). Future trials may use more stringent enrollment criteria to ensure a greater proportion of ill/frail patients and/or include older patients, though enrolling very frail patients and/or very old patients in such a trial can be challenging. Furthermore, even in this population, differences between strategies in total, symptomatic, and asymptomatic hypoglycaemia were striking. Conversely, one may argue that enrolling a more vulnerable population, and thus setting less aggressive HbA1c targets, may produce outcomes similar to those of the current study. The difference between number of enrolled/screened (388) and number of entered/randomized (192) patients also suggests that the study population may not be generalizable to a broader, more clinically relevant population. A second limitation, as discussed earlier, relates to choice of the hypoglycaemic component in the composite primary outcome.
The definition of “clinically significant hypoglycaemia” applied to this study, is to some extent, consistent with what we attempted to define as “moderate hypoglycaemia,” which is based on the concept that hypoglycaemic episodes cause significant disruption to lifestyle and are associated with both increased morbidity and mortality. This is a view increasingly shared by others. The American Diabetes Association (ADA) and European Association for the Study of Diabetes (EASD) have recently published a position statement that argues that future clinical trials should include an additional hypoglycaemic outcome level of 3 mmol/L (54 mg/dL) or below which they categorize as “serious” hypoglycaemia.35
Hypoglycaemia (whichever definition is used) incidence is likely to be closely related to the glycaemic targets assigned to patients and aggressiveness in pursuing those targets. There has been a gradual move in recent years towards less aggressive HbA1c targets, particularly in older patients.7 Although the lowest HbA1c target in this study (<7%) was chosen for approximately half the patients in each group, about one‐third of patients in each group did not achieve treatment success (the primary outcome) because of failure to achieve the target HbA1c. This suggests that, despite glycaemic targets being agreed between clinician and patient, these were not aggressively pursued in many individuals. Stronger encouragement by study investigators to achieve HbA1c target might have led to a higher success rate. However, clinicians’ reluctance to pursue relatively low HbA1c values may have been influenced by a number of factors including guideline recommendations,7, 16, 17 data showing better functional outcomes in older patients with higher HbA1c,36 and emergence of data showing a J‐shaped relationship between all‐cause mortality and HbA1c in T2D, with a nadir at an HbA1c of ~7.5%.37 This may have led physicians to be more cautious. However, notably, despite failure to show a difference between strategies in treatment success, both resulted in clinically meaningful HbA1c reductions of ~1.1%. In terms of study outcome, if glycaemic targets had been pursued more aggressively, it is likely that the hypoglycaemic component of the primary endpoint would have played a more substantial role in outcome, and a greater difference in “treatment success rate” between strategies might have been evident.
The protocol‐mandated treatment titration may also have contributed to failure to achieve glycaemic control. The protocol allowed a substantial degree of treatment discretion regarding titration and escalation of therapies, resulting in variations in individual interpretation of optimal treatment regimens and/or in primary outcome. Further, in hindsight, we feel that medication choices should have been better differentiated between the strategies (eg, we permitted the use of DPP‐4 inhibitors in both groups) and that guidance given to investigators in relation to drug choice and titration could have been clearer. The difference between groups in progression to use of injectable therapy (>40% in Strategy‐A, ~20% in Strategy‐B) may be related to the difference between GLP‐1 RAs and insulin hypoglycaemia risk,38 resulting in greater willingness to use injectable therapy in Strategy‐A. Further, the role of SGLT‐2 inhibitors, which were not used in the current study because of limited experience with their use in older, vulnerable patients, remains to be seen in such a population.
The planned treatment period of the interim analysis (24 weeks) is another factor that may have contributed to failure to achieve glycaemic control in some patients. Given the recommendation that anti‐hyperglycaemic medication titration should be initiated at low doses in older patients and titrated slowly, allowing up to 3 months between changes in medication,16 it is possible that the minimum study duration (ie, 24 weeks) was not long enough for clinicians to achieve the desired degree of glycaemic control, although the median duration was 42 weeks. Notably, per the final protocol for the full study, 36 weeks was considered sufficient time to reach the HbA1c target, even for those patients requiring multiple dose adjustments and changes of therapy (ie, additions of OAMs).
In conclusion, when “success” is defined as a combination of glycaemic control and absence of “clinically significant” hypoglycaemia (as defined in this study), treatment strategies involving glucose‐dependent and non‐glucose‐dependent anti‐hyperglycaemic medications result in similar success rates in vulnerable older patients with T2D. However, total, documented symptomatic, and asymptomatic hypoglycaemia were significantly less frequent in Strategy‐A, a finding that, upon reflection, is clinically relevant in a patient population in which hypoglycaemia can have serious implications. The results of this study will also inform future clinical research in older patients with T2D.
Supporting information
Appendix S1. List of participating investigators.
Appendix S2. Guidelines provided to clinicians relating to selection of individualised treatment targets.
Appendix S3. Basal insulin treatment algorithms.
Table S1. Distribution of TIBI and CFS scores
Figure S1. Study design.
Figure S2. Treatment options for Strategy‐A.
Figure S3. Treatment options for Strategy‐B.
ACKNOWLEDGEMENTS
The authors thank Michelle Carey, PhD, (inVentiv Health Clinical) for writing and editorial assistance.
Conflict of interest
S. R. H. has undertaken consultancy for Novo‐Nordisk, Eli Lilly and Company, Sanofi, Boehringer‐Ingelheim, Merck Sharp & Dohme, and Takeda. He has received research support from Medtronic and has been a member of speaker bureaus for Novo‐Nordisk, Eli Lilly and Company, Sanofi, Takeda, and Merck Sharp & Dohme.
R. E. P. consulted for AstraZeneca, Boehringer‐Ingelheim, GlaxoSmithKline, Hanmi Pharmaceutical Co., Janssen Pharmaceuticals, Ligand Pharmaceuticals, Eli Lilly and Company, Merck, Novo‐Nordisk and Takeda. He has received research support from Eli Lilly and Company, Merck Sharp & Dohme, Novo‐Nordisk, Sanofi, and Takeda and has spoken on behalf of AstraZeneca and Novo‐Nordisk. All honoraria and fees from these activities have been directed to a non‐profit. R. E. P. does not receive any direct or indirect compensation for these services. A.S. has received consultancy fees from Merck, Takeda, Novartis and Eli Lilly and Company. A. F., J. K., C. S. B., R. D. and R. J. H. are full‐time employees and minor stockholders of Eli Lilly and Company.
Author contributions
S. R. H and R. E. P. were involved in the conception and design of the study, helped with the acquisition, analysis and interpretation of the data, critically revised the manuscript for important intellectual content, provided administrative, technical and material support, and assisted with drafting and supervising the development of the manuscript. A. S. was involved in the conception and design of the study, helped with the acquisition, analysis and interpretation of the data, and assisted with drafting the manuscript. A. F. was involved in the conception and design of the study, helped with the acquisition, analysis and interpretation of the data, obtained funding for the study, provided administrative, technical and material support, and assisted with drafting and supervising the development of the manuscript. J. K. was involved in the conception and design of the study, helped with the acquisition, analysis and interpretation of the data, critically revised the manuscript for important intellectual content, provided administrative, technical and material support, provided expertise as a study physician, and assisted with drafting and supervising the development of the manuscript. C. S. B. helped with the acquisition, analysis and interpretation of the data, provided administrative, technical and material support, and critically revised the manuscript for important intellectual content. R. D. was involved in the conception and design of the study, helped with the acquisition, analysis and interpretation of the data, performed statistical analysis, and assisted with drafting the manuscript. R. J. H. was involved in the conception and design of the study, helped with the acquisition, analysis and interpretation of the data, and assisted with drafting and supervising the development of the manuscript.
Heller SR, Pratley RE, Sinclair A, et al. Glycaemic outcomes of an Individualized treatMent aPproach for oldER vulnerable patIents: A randomized, controlled stUdy in type 2 diabetes Mellitus (IMPERIUM). Diabetes Obes Metab. 2018;20:148–156. https://doi.org/10.1111/dom.13051
Funding information This work was funded by Eli Lilly and Company.
REFERENCES
- 1. International Diabetes Federation . IDF Diabetes Atlas. 7th ed. 2015. http://www.diabetesatlas.org/. Accessed October 14, 2016. [Google Scholar]
- 2. Gambert SR, Pinkstaff S. Emerging epidemic: diabetes in older adults: demography, economic impact, and pathophysiology. Diabetes Spectrum. 2006;19:221. [Google Scholar]
- 3. Sloan FA, Bethel MA, Ruiz D Jr, Shea AM, Feinglos MN. The growing burden of diabetes mellitus in the US elderly population. Arch Intern Med. 2008;168:192–199. [DOI] [PubMed] [Google Scholar]
- 4. Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94:311–321. [DOI] [PubMed] [Google Scholar]
- 5. Sinclair A, Morley J. Frailty and diabetes. Lancet. 2013;382:1386–1387. [DOI] [PubMed] [Google Scholar]
- 6. Sinclair A, Dunning T, Rodriguez‐Mañas L. Diabetes in older people: new insights and remaining challenges. Lancet Diabetes Endocrinol. 2015;3:275–285. [DOI] [PubMed] [Google Scholar]
- 7. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient‐centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140–149. [DOI] [PubMed] [Google Scholar]
- 8. Lee SJ, Eng C. Goals of glycemic control in frail older patients with diabetes. JAMA. 2011;305:1350–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults. Diabetes Care. 2012;35:2650–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chelliah A, Burge MR. Hypoglycaemia in elderly patients with diabetes mellitus: causes and strategies for prevention. Drugs Aging. 2004;21:511–530. [DOI] [PubMed] [Google Scholar]
- 11. Du YF, Ou HY, Beverly EA, Chiu CJ. Achieving glycemic control in elderly patients with type 2 diabetes: a critical comparison of current options. Clin Interv Aging. 2014;9:1963–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pratley RE, Heller SR, Miller MA. Treatment of type 2 diabetes mellitus in the older adult: a review. Endocr Pract. 2014;20:722–736. [DOI] [PubMed] [Google Scholar]
- 13. Sinclair A, Morley JE, Rodriguez‐Mañas L, et al. Diabetes mellitus in older people: position statement on behalf of the International Association of Gerontology and Geriatrics (IAGG), the European Diabetes Working Party for Older People (EDWPOP), and the International Task Force of Experts in Diabetes. J Am Med Dir Assoc. 2012;13:497–502. [DOI] [PubMed] [Google Scholar]
- 14. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10‐year follow‐up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–1589. [DOI] [PubMed] [Google Scholar]
- 15. Cahn A, Raz I, Kleinman Y, et al. Clinical assessment of individualized glycemic goals in patients with type 2 diabetes: formulation of an algorithm based on a survey among leading worldwide diabetologists. Diabetes Care. 2015;38:2293–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. International Diabetes Federation . Managing older people with type 2 diabetes: global guideline. 2013. https://www.idf.org/our‐activities/advocacy‐awareness/resources‐and‐tools/78:global‐guideline‐for‐managing‐older‐people‐with‐type‐2‐diabetes.html. Accessed May 31, 2017.
- 17. Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults: a consensus report. J Am Geriatr Soc. 2012;60:2342–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brouwer ES, West SL, Kluckman M, et al. Initial and subsequent therapy for newly diagnosed type 2 diabetes patients treated in primary care using data from a vendor‐based electronic health record. Pharmacoepidemiol Drug Saf. 2012;21:920–928. [DOI] [PubMed] [Google Scholar]
- 19. Fu H, Curtis BH, Schuster DP, Festa A, Kendall DM. Treatment patterns among older patients with type 2 diabetes in the United States: a retrospective cohort study. Diabetes Technol Ther. 2014;16:833–839. [DOI] [PubMed] [Google Scholar]
- 20. Payk SL, Drew RH, Smith JD, Jiroutek MR, Holland MA. Sulfonylurea prescribing patterns after the introduction of DPP‐4 inhibitors and GLP‐1 receptor agonists. Clin Ther. 2015;37:1477–1482. [DOI] [PubMed] [Google Scholar]
- 21. Bhattacharya R, Zhou S, Wei W, Ajmera M, Sambamoorthi U. A real‐world study of the effect of timing of insulin initiation on outcomes in older Medicare beneficiaries with type 2 diabetes mellitus. J Am Geriatr Soc. 2015;63:893–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ashcroft FM. Mechanisms of the glycaemic effects of sulfonylureas. Horm Metab Res. 1996;28:456–463. [DOI] [PubMed] [Google Scholar]
- 23. Sola D, Rossi L, Schianca GP, et al. Sulfonylureas and their use in clinical practice. Arch Med Sci. 2015;11:840–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tahrani AA, Barnett AH, Bailey CJ. SGLT inhibitors in management of diabetes. Lancet Diabetes Endocrinol. 2013;1:140–151. [DOI] [PubMed] [Google Scholar]
- 25. Hyltén‐Cavallius L, Iepsen EW, Wewer Albrechtsen NJ, et al. Patients with long‐QT syndrome caused by impaired hERG‐encoded Kv11.1 potassium channel have exaggerated endocrine pancreatic and incretin function associated with reactive hypoglycemia. Circulation. 2017;135:1705–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Craig CM, Liu LF, Deacon CF, Holst JJ, McLaughlin TL. Critical role for GLP‐1 in symptomatic post‐bariatric hypoglycaemia. Diabetologia. 2017;60:531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nauck M. Incretin therapies: highlighting common features and differences in the modes of action of glucagon‐like peptide‐1 receptor agonists and dipeptidyl peptidase‐4 inhibitors. Diabetes Obes Metab. 2016;18:203–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Greenfield S, Billimek J, Pellegrini F, et al. Comorbidity affects the relationship between glycemic control and cardiovascular outcomes in diabetes: a cohort study. Ann Intern Med. 2009;151:854–860. [DOI] [PubMed] [Google Scholar]
- 30. American Diabetes Association . Standards of medical care in diabetes—2014. Diabetes Care. 2014;37(suppl 1):S14–S80. [DOI] [PubMed] [Google Scholar]
- 31. National Kidney Foundation . Clinical practice guidelines for chronic kidney disease: evaluation, classification and stratification. 2002. https://www.kidney.org/sites/default/files/docs/ckd_evaluation_classification_stratification.pdf. Accessed October 14, 2016.
- 32. Stahn A, Pistrosch F, Ganz X, et al. Relationship between hypoglycemic episodes and ventricular arrhythmias in patients with type 2 diabetes and cardiovascular diseases: silent hypoglycemias and silent arrhythmias. Diabetes Care. 2014;37:516–520. [DOI] [PubMed] [Google Scholar]
- 33. Chow E, Bernjak A, Williams S, et al. Risk of cardiac arrhythmias during hypoglycemia in patients with type 2 diabetes and cardiovascular risk. Diabetes. 2014;63:1738–1747. [DOI] [PubMed] [Google Scholar]
- 34. Pratley RE, Rosenstock J, Heller SR, Sinclair A, Heine RJ. Similar glucose control and hypoglycemia but reduced glucose variability with glucose dependent and independent therapies in older patients with type 2 diabetes mellitus (T2DM). Diabetes. 2016;65(suppl 1):A229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. International Hypoglycaemia Study Group . Glucose concentrations of less than 3.0 mmol/L (54 mg/dL) should be reported in clinical trials: a joint position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2017;40:155–157. [DOI] [PubMed] [Google Scholar]
- 36. Yau CK, Eng C, Cenzer IS, Boscardin WJ, Rice‐Trumble K, Lee SJ. Glycosylated hemoglobin and functional decline in community‐dwelling nursing home‐eligible elderly adults with diabetes mellitus. J Am Geriatr Soc. 2012;60:1215–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Arnold LW, Wang Z. The HbA1c and all‐cause mortality relationship in patients with type 2 diabetes is J‐shaped: a meta‐analysis of observational studies. Rev Diabet Stud. 2014;11:138–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang Y, Li L, Yang M, Liu H, Boden G, Yang G. Glucagon‐like peptide‐1 receptor agonists versus insulin in inadequately controlled patients with type 2 diabetes mellitus: a meta‐analysis of clinical trials. Diabetes Obes Metab. 2011;13:972–981. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. List of participating investigators.
Appendix S2. Guidelines provided to clinicians relating to selection of individualised treatment targets.
Appendix S3. Basal insulin treatment algorithms.
Table S1. Distribution of TIBI and CFS scores
Figure S1. Study design.
Figure S2. Treatment options for Strategy‐A.
Figure S3. Treatment options for Strategy‐B.


