Abstract
Objective
To test the hypothesis that oral (PO) feeding at first neonatal intensive care unit (NICU) discharge is associated with less neurodevelopmental impairment and better feeding milestones, as compared with discharge with a gastrostomy tube (g-tube).
Study design
We studied outcomes for a retrospective cohort of 194 neonates < 37 weeks gestation referred for evaluation and management of feeding difficulties between July 2006–July 2012. Discharge milestones, length of hospitalization, and Bayley Scales of Infant Development scores at 18–24 months were examined. Chi-Square, Mann-Whitney U, or t-tests and multivariable logistic regression models were used.
Results
60% (n=117) of infants were discharged on PO feedings; of these, 96% remained PO-fed at 1-year. The remaining 40% (n=77) were discharged on g-tube feedings; of these, 31 (40%) remained g-tube dependent, 17 (22%) became PO-fed, and 29 (38%) were on PO and g-tube feedings at one year. Infants discharged on a g-tube had lower cognitive (p<0.01), communication (p=0.03), and motor (p<0.01) composite scores. The presence of a g-tube, younger gestation, bronchopulmonary dysplasia, or intraventricular hemorrhage was significantly associated with neurodevelopmental delay.
Conclusions
For infants referred for feeding concerns, g-tube evaluations and feeding management, the majority did not require a g-tube. Full PO feeding at first NICU discharge was associated with superior feeding milestones and less long-term neurodevelopmental impairment, relative to full or partial g-tube feeding. Evaluation and feeding management before and after g-tube placement may improve long-term feeding and neurodevelopmental outcomes.
Keywords: Feeding Difficulties, Infants, Neurodevelopmental Outcomes, Gastrostomy, Aerodigestive
Technological advances for premature infants have raised survival rates, but contributed to increased aerodigestive and neurodevelopmental morbidity (1) and high societal costs.(2) Prematurity negatively impacts attainment of feeding milestones,(3, 4) as 40% of infants referred to feeding clinics were born preterm.(5) Infections, growth failure, bronchopulmonary dysplasia, necrotizing enterocolitis, and neurological sequelae in the neonatal intensive care unit (NICU) are associated with neurodevelopmental and feeding dysfunctions in later childhood.(6–9) The relationship between concurrent post-discharge childhood feeding behaviors and neurodevelopmental status has been assessed.(10, 11) Postdischarge feeding difficulties in infancy are likely related to sensory or motor neurologic vulnerabilities, static or progressive neurological diseases, behavioral deficits, chronic lung disease, gastrointestinal causes, or most often a combination of all these etiologies.(12–15) Furthermore, FD, when fully apparent in later life, have deleterious consequences because the condition has already made an imprint on the developing sensory-motor neural architecture and aerodigestive reflex functionality.(16) Dysfunctional feeding behaviors at 18 months of age are associated with neurodevelopmental delays (assessed by the Bayley Scales of Infant Development–Third Edition; BSID-III).(10) However, to our knowledge, no studies have addressed the impact of personalized feeding methods attained at the first NICU discharge on later neurodevelopmental outcomes.
Diagnosis and management of neonatal FD is difficult because of individual heterogeneity, interplay between multiple target organs, regulatory and coordinating neurosensory/neuromotor processes, evolving pathophysiology, involvement of multiple disciplines, and empiric therapies.(12, 17–19) Therefore, we prospectively examined: (1) the proportion of NICU infants with complex FD, discharged with a gastrostomy tube (g-tube) from a Neonatal and Infant Feeding Disorders Program that included an individualized plan based on clinical and physiologic characteristics; (2) the feeding milestones attained by 1-year of age in those infants that received a g-tube prior to NICU discharge; and (3) the hypothesis that oral (PO) feeding at 1st NICU discharge is associated with less neurodevelopmental impairment and better feeding milestones at 2 years age, as compared with infants with g-tube.
METHODS
Participants were convalescing premature infants referred to our neonatal feeding disorders program for evaluation and management of severe FD, including evaluation for a g-tube placement. FD was characterized by an inability to consume adequate oral feeding, gavage-tube dependence, feeding or post-prandial related-cardio-respiratory spells, coughing, gagging, arching, refusal to feed, and/or poor sucking ability.
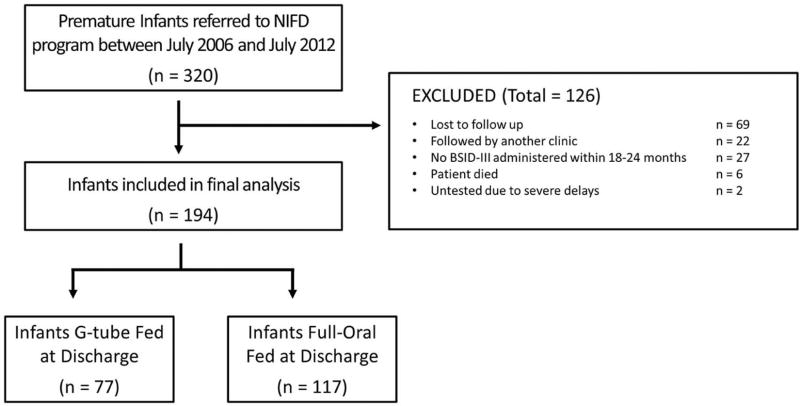
Inclusion criteria were: preterm birth (<37 weeks gestation), inpatient referral to the neonatal feeding program, hospital discharge, and neurodevelopmental evaluation at 18–24 months corrected age. Between July 2006 to July 2012, 320 infants were seen by our program, of which 194 met all four study criteria. Of 126 infants that did not meet the study criteria, , 91 were lost to follow-up, 6 died, and 29 did not have a BSID-III assessment (Figure 1; available at www.jpeds.com). Respiratory modalities, discharge diagnoses, and neurodevelopmental assessments at age 2-years were collected on all infants. Informed parental consent and the institutional review board approval were obtained.
Figure 1 (online only).
Flow chart of infants seen by our Neonatal and Infant Feeding Disorders (NIFD) program and screened for eligibility.
Because of heterogeneity among FD infants, our neonatal feeding and aerodigestive disorders management program provides both a targeted and individualized diagnostic approach and mechanisms-based feeding strategies for convalescing NICU neonates.
The feasibility of such an approach, validation of diagnostic methods and description of specific strategies has been reported earlier (17, 18, 20). In brief, neonatal nurses and nurse practitioners, neonatologists, pediatric surgeons, pediatric gastroenterologists, and parents requested referrals for evaluation of oropharyngeal dysphagia, gastroesophageal reflux disease (GERD), and for g-tube placement, and/or fundoplication. Every infant received a complete history and physical examination, with attention to observation of swallowing-breathing coordination during feeds, sucking and swallowing reflexes, potentially undiagnosed congenital aerodigestive anomalies, and cardio-respiratory effort during feeding. If clinically indicated, initial structural and functional evaluations of the aerodigestive tract were performed by video fluoroscopic swallow and/or upper gastrointestinal fluoroscopy studies. Consultation with otorhinolaryngology occurred for evidence of upper airway obstruction. The neuromotor mechanisms of feeding-related symptoms were evaluated via assessment of swallow-integrated esophageal motility, concurrent with cardiorespiratory observations at baseline and during provocation. Infants with suspected GERD were evaluated with a 24-hour pH Impedance study.
Findings were discussed with the primary care team and an individualized feeding management strategy was formulated to include feeding approach (type of milk, volume, feeding duration, feeding method, caloric density, and breastfeeding), feeding progression, nutrition, growth, related pathophysiology, and relevant pharmacological treatment. Common evidence-based strategies to manage functional oro-pharyngeal dysphagia were explained to the team, including pacing techniques, nipple selection, feeding position, gradual progression from continuous to bolus feeds, and advancement towards minimizing feeding duration per feed. Breastfeeding was encouraged and approaches were recommended to resolve FD during breastfeeding. Behavioral therapy was attempted with encouragement of pacifier-dips, alleviating infants’ stress with hand containment, facilitated tucking and kangaroo care with parents. Self-regulatory behaviors and tolerance to positional changes were encouraged before reacting to events. GERD was treated with pharmacologic therapy and decreasing feeding flow rates.(21) Rarely, poor gut motility was treated with short-term prokinetic agents (Erythromycin or Augmentin) to improve oral feeding and feeding intolerance.
Compliance with the individualized feeding plan was monitored by our feeding program and during multidisciplinary feeding rounds. Strategies included: a) education regarding factors that are helping or impeding feeding progress; b) monitoring nutrition and growth; and c) personalized guidance for feeding delays.(20) Feeding related education was provided to nurses, feeding therapists, and parents to ensure compliance to the directions. Each infant’s self-regulatory behaviors and tolerance to positional changes were noted and bedside providers were taught to respond to these behaviors.
Infants were followed in the outpatient follow-up program and by primary care providers, and infants with lung disease were followed in our chronic lung disease program. During clinic visits, infants were assessed for feeding, growth, and airway-related issues. BSID-III examinations were conducted at 18–24 months corrected gestational age by independent occupational and physical therapists, and results were stratified based on feeding method at discharge.
The primary metric was discharge feeding outcomes (full-PO or g-tube feeding). The secondary outcomes were post-discharge aerodigestive milestones and developmental follow-up studies at 18–24 months. Aerodigestive metrics were ventilation duration, first PO feed attainment, prevalence of g-tube or tracheostomy, and supplemental oxygen at 36 weeks postmenstrual age (PMA) and discharge. Feeding methods were categorized as follows: full-PO fed was considered as exclusive PO feeding, and those that were transitioning and partially tube-fed were categorized under g-tube feeding. We classified severity of BPD according to the NIH consensus definition at 36 weeks PMA.(22) Neurodevelopmental outcomes included composite scores of BSID-III comprised of cognitive, communication, and motor scores, corrected for gestational immaturity. Neurodevelopmental delay was defined as any composite score <80.(23) Infants were stratified based on feeding method at discharge, as the study aim was to characterize and compare the aerodigestive and neurodevelopmental outcomes at 18–24 months PMA between successful (full-PO) feeders vs. unsuccessful (g-tube) feeders.
Statistical Analyses
Comparisons were made using Chi-Square, Mann-Whitney U, or t-tests comparing full-PO vs. g-tube feedings. We used multivariable logistic regression to adjust for measured risk factors that may serve as potential confounders of the effect of g-tube placement on neurodevelopmental outcomes. Only cases with complete data were included in the multivariable regression models.
RESULTS
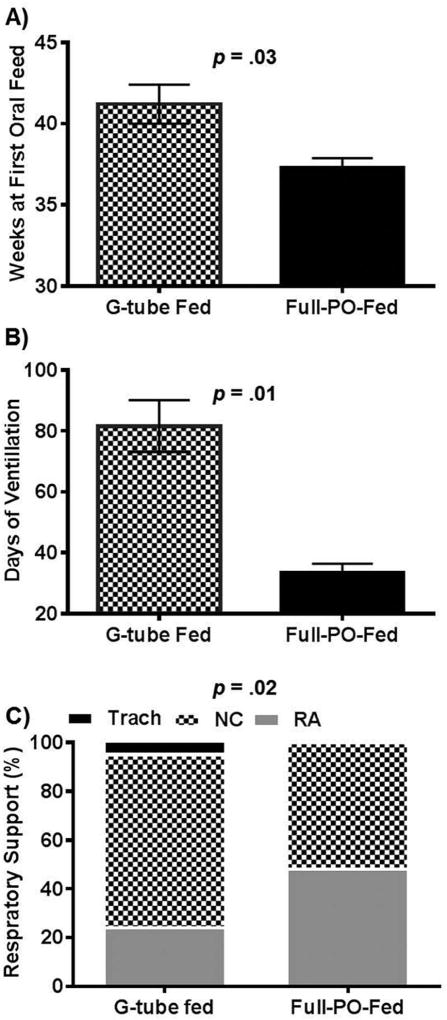
Infants (N=194) who had developmental assessments at 2-years follow-up were stratified based on feeding method during their first NICU discharge. There were 77 infants (40%) discharged on a g-tube and 117 infants (60%) discharged on PO feedings. G-tube placement occurred at 51.9 ± 8.6 weeks PMA (median 49.6 weeks). Infant characteristics were similar at birth (Table I; available at www.jpeds.com). However, morbidity characteristics during the NICU stay differed in the g-tube group, which had prolonged hospitalization (p< 0.0001), prolonged ventilation (p<0.01), and higher proportion of respiratory support at discharge (p =0.02) (Figure 2).
Table 1.
Clinical Characteristics Stratified by Feeding Method at Discharge
| Characteristics | G-tube-Fed (N = 77) |
Full-PO-Fed (N = 117) |
p-Value |
|---|---|---|---|
| Gestational age, weeks | 26 (25–28) | 28 (25–29) | 0.2 |
| Birth weight, grams | 780 (640–1015) | 875 (685–1230) | 0.1 |
| Length of hospitalization, days | 194 (152–255) | 114 (90–144) | <0.0001 |
| Small for gestational age, n (%) | 22 (28) | 23 (19) | 0.2 |
| Supplemental oxygen at 36 weeks PMA, n (%) | 60 (86) | 71 (60) | 0.02 |
| Supplemental oxygen at discharge, n (%) | 50 (63) | 62 (52) | 0.01 |
| PMA at g-tube placement, weeks | 49.6 (45.8–54.9) | -- | -- |
| PMA at NICU discharge, weeks | 52 (48–57) | 44 (41–46) | <0.0001 |
| Weight at NICU discharge, kg | 5.2 (4.2–6.6) | 3.7 (3.2–4.3) | <0.0001 |
| Intraventricular hemorrhage grade I-II, n (%) | 20 (25) | 27 (23) | 0.7 |
| Intraventricular hemorrhage grade III-IV, n (%) | 8 (10) | 8 (7) | 0.4 |
| Patent ductus arteriosus, n (%) | 49 (62) | 60 (50) | 0.2 |
| Medical necrotizing enterocolitis*, n (%) | 6 (8) | 14 (12) | 0.5 |
| Tracheostomy, n (%) | 5 (6) | 1 (1) | 0.04 |
| Fundoplication, n (%) | 5 (6) | 1 (1) | 0.08 |
Values presented as Median (IQR) or n (%); PMA: postmenstrual age; NICU: neonatal intensive care unit;
There were no cases of surgical necrotizing enterocolitis referred to the feeding program
Figure 2.
Respiratory and Feeding Milestones during Hospital Stay. A) The average PMA at first oral feed is increased among g-tube fed infants. B) The average duration of ventilation is increased among g-tube infants. C) G-tube fed infants had higher prevalence of tracheostomy and NC support. Tracheostomy tube (Trach), Nasal cannula (NC), Room air (RA).
At 1-year corrected age, among the 77 g-tube fed infants, 17 (22%) were PO-fed exclusively, 29 (38%) of these infants were fed transitionally (g-tube and PO), and 31 (40%) remained g-tube dependent. At 18–24 months follow-up, an additional 18 infants had achieved oral feeds. Among the 117 infants discharged on PO feedings, 112 (96%) remained PO-fed at 1-year of age, with the other 4% being transitionally fed. These infants also maintained oral feeding at 18–24 months, with 113 (97%) being orally fed at time of BSID-III examination. Of the infants discharged on oxygen, 33% of g-tube fed infants were weaned to room air at one year vs. 86% of the PO-fed infants (p<0.01). The rest of g-tube fed infants were provided supplemental oxygen via a nasal cannula (58%) at 0.03–2.0 liters per minute or via a tracheostomy-mist collar (10%).
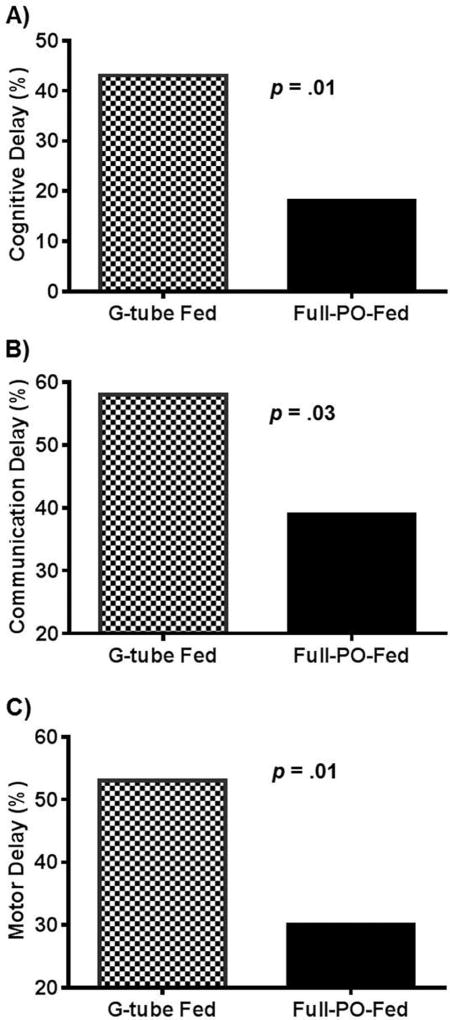
Neurodevelopmental Outcomes comparisons were made at 18–24 months (Table II). The average corrected age at the time of BSID-III evaluation was 18.3 ± 1.3 and 18.4 ± 1.8 months for g-tube and PO-fed infants, respectively (p=0.8). Infants discharged with a g-tube had lower composite scores in cognitive (p<0.01), communication (p=0.03), and motor (p<0.01) sub-categories and had higher proportions of neurodevelopmental delay (Table II and Figure 3). Multivariable logistic regression models identified a strong relationship between the presence of a g-tube at discharge and developmental delays, controlling for gestation and co-morbidities (Table III). Infants with a g-tube at discharge were more likely to have cognitive and motor delays at 18–24 months, and tended to have more communication delays (p=0.08). In addition, each week of increasing PMA at the time of initial g-tube placement was associated with an increased multivariable adjusted odds of cognitive delay (OR: 1.1; 95% confidence interval [CI]: 1.0–1.2; p=0.02), communication delay (OR 1.2; 95% CI: 1.0–1.3; p=0.02), and motor delay (OR 1.2; 95% CI: 1.1–1.3; p=0.01). G-tube placement that occurred before the median PMA (49.3 weeks) for placement was associated with a reduced, adjusted OR (OR 0.19; 95% CI 0.05–0.74; p=0.02) for cognitive delay. Similar trends were noted for communication (OR 0.20; 95% CI: 0.06–0.72; p=0.01) and motor delay (OR 0.20; 95% CI: 0.04–0.63; p=0.01).
Table 2.
BSID-III Scores Stratified by Feeding Method at Discharge
| Characteristics | G-tube-Fed (N = 77) |
Full-PO-Fed (N = 117) |
p-Value |
|---|---|---|---|
| CCA at time of BSID evaluation, months | 18.3 ± 1.3 | 18.4 ± 1.8 | 0.8 |
| Cognitive composite score | 80 (70–90) | 90 (80–100) | < 0.01 |
| Communication composite score | 77 (65–91) | 86 (71–94) | 0.03 |
| Receptive communication scaled score | 6 (5–9) | 8 (6–9) | 0.01 |
| Expressive communication scaled score | 6 (4–8) | 7 (5–9) | 0.06 |
| Motor composite score | 79 (64–91) | 88 (76–100) | < 0.01 |
| Fine motor scaled score | 7 (5–10) | 9 (7–11) | 0.01 |
| Gross motor scaled score | 5.0 (3–8) | 8 (6–9) | < 0.01 |
Values stated as mean ±SD and median (IQR). Corrected chronological age (CCA) was defined as the age of the child from the expected date of delivery.
Figure 3.
Association between Discharge Feeding Method and Neurodevelopmental Delay. Neurodevelopmental delay was defined as a BSID composite score < 80. G-tube at first hospital discharge is associated with a higher proportion of infants with cognitive, communication, and motor delays.
Table 3.
Multivariable Adjusted odds of Neurodevelopmental Delay at 18 –24 Months PMA Following G-tube Placement Prior to NICU Discharge
| Cognitive Delay | Communication Delay | Motor Delay | ||||
|---|---|---|---|---|---|---|
| Variables | OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value |
| G-tube | 2.5 (1.2–5.2) | 0.01 | 1.8 (0.9–3.4) | 0.08 | 2.1 (1.1–4.1) | 0.03 |
| GA, weeks | 0.8 (0.7–1.0) | 0.02 | 0.9 (0.8–1.0) | 0.01 | 0.8 (0.8–1.0) | 0.01 |
| Moderate BPD | 0.5 (0.2–1.6) | 0.25 | 1.0 (0.4–2.4) | 0.92 | 1.4 (0.6–3.6) | 0.46 |
| Severe BPD | 1.5 (0.6–3.6) | 0.37 | 2.0 (0.9–4.5) | 0.08 | 2.6 (1.1–6.0) | 0.02 |
| IVH grade 3–4 | 5.0 (1.6–15.8) | 0.01 | 1.9 (0.6–6.1) | 0.28 | 3.3 (1.0–10.6) | 0.05 |
| PDA* | 1.2 (0.5–2.7) | 0.64 | 0.8 (0.4–1.6) | 0.59 | 0.8 (0.4–1.7) | 0.58 |
| HIE* | 11.7 (0.5–261.7) | 0.12 | 1.6 (0.2–15.2) | 0.69 | 1.1 (0.1–12.7) | 0.92 |
| Medical NEC | 1.0 (0.3–3.3) | 0.99 | 0.7 (0.2–1.8) | 0.42 | 0.6 (0.2–1.7) | 0.32 |
| Small for GA | 1.5 (0.7–3.5) | 0.33 | 0.7 (0.3–1.4) | 0.30 | 1.8 (0.8–3.9) | 0.14 |
| Large for GA | 0.4 (0.1–2.4) | 0.34 | 0.4 (0.1–1.6) | 0.20 | 0.5 (0.1–2.2) | 0.35 |
BPD: bronchopulmonary dysplasia; GA: gestational age; HIE: hypoxic-ischemic encephalopathy; IVH: intraventricular hemorrhage; NEC: necrotizing enterocolitis; PDA: Patent ductus arteriosus; PMA: postmenstrual age; OR: odds ratio; CI: confidence interval
Data was missing for one patient; only complete cases were used in final regression analysis.
DISCUSSION
Management of chronic complex FD and g-tube decisions among convalescing NICU neonates can be challenging. Due to heterogeneity among preterm infants with aerodigestive disorders and FD, we have adopted assessments and feeding strategy development targeted to each individual infant.(7, 17, 18) Our hypothesis was that following evaluation and development of personalized feeding methods, PO feeding at first NICU discharge would be associated with less neurodevelopmental impairment and better feeding milestones at ~2-years age relative to g-tube requirement. In the current study, 40% of the infants referred for FD were g-tube dependent at discharge (n=77).
The salient findings from this study are that, regardless of gestational immaturity and respiratory morbidity, the presence of a g-tube and inability to attain full-PO feeding milestones at discharge were associated with cognitive, motor and communicative neurodevelopmental delays at 18–24 months. The requirement of a g-tube at discharge appears to be an independent predictor of future neurodevelopmental delay, after controlling for gestational age and common clinical risk factors. The length of initial hospitalization and incidence of respiratory morbidity at discharge is significantly higher in the g-tube fed group.
The decision to perform gastrostomy in most centers is based on clinical decision-making, and parents often question the indications of gastrostomy and are reluctant to the procedure.(24, 25) Using an individualized approach, 40% of the complex infants referred with aerodigestive and severe FD ultimately needed a g-tube. Thus, patient-focused feeding programs may enhance decision-making and reduce economic and societal burden.(18, 26–28) We also found that PO-fed infants showed significant superior scores in all domains of BSID-III. It is likely that infants who require a g-tube for feeding at discharge are already predisposed to future neurological delay at the time of g-tube placement. However, we speculate that better developmental outcomes in PO-fed infants may be, in part, due to stimulatory effects of PO feeding, personalized oromotor interventions, and parental attention to infant feeding techniques. The rationale for this hypothesis is that the PO feeding process involves the functions of V, VII, IX, X, XI, and XII cranial nerves, and provides direct neurosensory and emotional support using tactile, proprioceptive, hunger-satiety modulatory behaviors, and swallowing-breathing reciprocal interactions.(29, 30) Some of these putative mechanisms stimulate the aerodigestive, neurosensory and neuromotor apparatus more than in g-tube fed infants. A longitudinal study may be needed to address the possibility that infants with a poorer neurodevelopmental outcome were less capable to learn how to feed by mouth. The PO-fed infants had less chronic lung disease of infancy. We speculate that PO skills and less chronic lung disease may have resulted from better development and adaptation of aerodigestive reflexes, facilitating airway protection mechanisms and development of endurance. Safe PO feeding involves integration and coordination of dynamic reflexes and reciprocal regulatory patterns and behaviors. In developing better feeding strategies, providers should consider all aspects of deglutition as well as airway protection mechanisms and feeding safety.(31–33)
There were limitations to our study. Gavage tubes, when dislodged, can result in a high choking and aspiration risk, but are associated with leaks, infections and reflux. Even though we prefer a g-tube placement to home gavage feeds, there are no data on trials of home gavage feeds vs. g-tube feeds. Such a comparative study could be difficult and ethically challenging due to difficulty randomizing to either intervention. The interval between g-tube insertion and discharge is variable and dependent on parent comfort with handling the g-tube.(24, 25) The exact volume, and number of PO feeding attempts in the g-tube categories were not well recorded. We do not have accurate records of dose, volume, duration, or additional nutritional supplements to these infants during infancy. Thus, the variability in the g-tube group may be in part due to variation in practice or to heterogeneity of the patients’ disease process. Teaching parents can be challenging and difficult to measure. Although not evaluated in our study, the value of breast milk and breastfeeding to infant health, development, and familial bonding cannot be overemphasized.(34, 35) As demographics for the two groups were similar at birth, the requirement for a g-tube appears to be an independent marker for neurodevelopmental delay after controlling for commonly measured clinical risk factors.
To mitigate the limitations of our study we recommend: 1) future studies to develop parent education tools and facilitate interaction between parent and provider; 2) development and evaluation of a neurorehabilitation program to focus on dysphagia and upper aerodigestive adaptation skills following a g-tube insertion, with an emphasis on achieving future feeding milestones; 3) development of a specialized feeding rehabilitation program that pays attention to aerodigestive and pulmonary pathologies; 4) avoidance of infections and illnesses to minimize weight loss and regression of feeding milestones; 5) infant driven, cue-based feeding that targets quality of PO feeding sessions as opposed to increasing quantity of feeds via prolonged feeding sessions and force feedings.
We tested the hypothesis that full PO feeding at 1st NICU discharge is associated with better feeding milestones and less neurodevelopmental impairment at 2 years of age, when compared with full or partial g-tube feeding at discharge. Inability to attain full PO feeding milestones at discharge is associated with cognitive, motor and communicative neurodevelopmental delays at 18–24 months of age. Therefore, the presence of a g-tube is a potential marker of developmental vulnerability, even after adjusting for commonly measured clinical risk factors. Due to the complex interplay of neurologic and behavioral areas that control feeding and cognitive function, infants who present with delayed aerodigestive milestones are likely to have neurodevelopmental delay later on. Timely initiation of oromotor therapies and personalization of feeding strategies may provide opportunities for improved progression of feeding skills as well as improvement in neurodevelopmental outcomes. In infants fed with a g-tube, comprehensive aerodigestive, oromotor, and developmental rehabilitation and individualized interventions and educational strategies may improve long-term outcomes. Future studies to test the benefits from an individualized approaches vs. other interventions may merit a multi-center clinical trial.
Acknowledgments
We are grateful to the parents of infants who actively participated in the personalized feeding program. We are thankful to the nurses, occupational and physical therapists, nutritionists, lactation consultants, and neonatal nurse practitioners who helped us with adapting to the personalized feeding strategies and monitored compliance. We are grateful to Mohamed Elmahdy, PhD, for his critical review of the manuscript.
Supported by the National Institutes of Health (R01 DK 068158 [to S.J.] and 5K08HL121182 [to J.S.]) and the Neonatology Service Line at the Nationwide Children’s Hospital.
ABBREVIATIONS
- BPD
bronchopulmonary dysplasia
- BSID-III
Bayley Scales of Infant Development
- GA
gestational age
- GERD
gastroesophageal reflux disease
- HIE
hypoxic-ischemic encephalopathy
- IVH
intraventricular hemorrhage
- NEC
necrotizing enterocolitis
- NICU
neonatal intensive care unit
- PDA
Patent ductus arteriosus
- PMA
postmenstrual age
- PO
per oral
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
References
- 1.Patel RM. Short- and Long-Term Outcomes for Extremely Preterm Infants. American journal of perinatology. 2016;33:318–28. doi: 10.1055/s-0035-1571202. Epub 2016/01/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Behrman RE, Butler AS, editors. Preterm Birth: Causes, Consequences, and Prevention. Washington (DC): National Academies Press (US); 2007. Institute of Medicine (US) Committee on Understanding Premature Birth and Assuring Healthy Outcomes. 12, Societal Costs of Preterm Birth. Available from: http://www.ncbi.nlm.nih.gov/books/NBK11358/ [PubMed] [Google Scholar]
- 3.Jadcherla SR, Wang M, Vijayapal AS, Leuthner SR. Impact of prematurity and comorbidities on feeding milestones in neonates: a retrospective study. J Perinatol. 2010;30:201–8. doi: 10.1038/jp.2009.149. Epub 2009/10/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jadcherla SR, Vijayapal AS, Leuthner S. Feeding abilities in neonates with congenital heart disease: a retrospective study. J Perinatol. 2009;29:112–8. doi: 10.1038/jp.2008.136. Epub 2008/09/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lefton-Greif MA, Okelo SO, Wright JM, Collaco JM, McGrath-Morrow SA, Eakin MN. Impact of Children's Feeding/Swallowing Problems: Validation of a New Caregiver Instrument. Dysphagia. 2014;29:671–7. doi: 10.1007/s00455-014-9560-7. Epub 2014/08/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hill J, Dierker D, Neil J, Inder T, Knutsen A, Harwell J, et al. A surface-based analysis of hemispheric asymmetries and folding of cerebral cortex in term-born human infants. J Neurosci. 2010;30:2268–76. doi: 10.1523/JNEUROSCI.4682-09.2010. Epub 2010/02/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shepherd EG, Knupp AM, Welty SE, Susey KM, Gardner WP, Gest AL. An interdisciplinary bronchopulmonary dysplasia program is associated with improved neurodevelopmental outcomes and fewer rehospitalizations. J Perinatol. 2012;32:33–8. doi: 10.1038/jp.2011.45. Epub 2011/05/07. [DOI] [PubMed] [Google Scholar]
- 8.Marlow N, Hennessy EM, Bracewell MA, Wolke D Group EPS. Motor and executive function at 6 years of age after extremely preterm birth. Pediatrics. 2007;120:793–804. doi: 10.1542/peds.2007-0440. Epub 2007/10/03. [DOI] [PubMed] [Google Scholar]
- 9.Dodrill P, McMahon S, Ward E, Weir K, Donovan T, Riddle B. Long-term oral sensitivity and feeding skills of low-risk pre-term infants. Early Hum Dev. 2004;76:23–37. doi: 10.1016/j.earlhumdev.2003.10.001. Epub 2004/01/20. [DOI] [PubMed] [Google Scholar]
- 10.Adams-Chapman I, Bann CM, Vaucher YE, Stoll BJ Eunice Kennedy Shriver National Institute of Child H, Human Development Neonatal Research N. Association between feeding difficulties and language delay in preterm infants using Bayley Scales of Infant Development-Third Edition. J Pediatr. 2013;163:680–5. doi: 10.1016/j.jpeds.2013.03.006. e1–3. Epub 2013/04/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aziz Q, Rothwell JC, Hamdy S, Barlow J, Thompson DG. The topographic representation of esophageal motor function on the human cerebral cortex. Gastroenterology. 1996;111:855–62. doi: 10.1016/s0016-5085(96)70053-7. Epub 1996/10/01. [DOI] [PubMed] [Google Scholar]
- 12.Rommel N, De Meyer AM, Feenstra L, Veereman-Wauters G. The complexity of feeding problems in 700 infants and young children presenting to a tertiary care institution. J Pediatr Gastroenterol Nutr. 2003;37:75–84. doi: 10.1097/00005176-200307000-00014. Epub 2003/06/27. [DOI] [PubMed] [Google Scholar]
- 13.Burklow KA, Phelps AN, Schultz JR, McConnell K, Rudolph C. Classifying complex pediatric feeding disorders. J Pediatr Gastroenterol Nutr. 1998;27:143–7. doi: 10.1097/00005176-199808000-00003. [DOI] [PubMed] [Google Scholar]
- 14.van den Engel-Hoek L, Erasmus CE, van Hulst KC, Arvedson JC, de Groot IJ, de Swart BJ. Children with central and peripheral neurologic disorders have distinguishable patterns of dysphagia on videofluoroscopic swallow study. Journal of child neurology. 2014;29:646–53. doi: 10.1177/0883073813501871. Epub 2013/09/12. [DOI] [PubMed] [Google Scholar]
- 15.van den Engel-Hoek L, van Hulst KC, van Gerven MH, van Haaften L, de Groot SA. Development of oral motor behavior related to the skill assisted spoon feeding. Infant behavior & development. 2014;37:187–91. doi: 10.1016/j.infbeh.2014.01.008. Epub 2014/02/28. [DOI] [PubMed] [Google Scholar]
- 16.Jadcherla SR. Pathophysiology of Aerodigestive Pulmonary Disorders in the Neonate. Clin Perinatol. 2012;39:639–54. doi: 10.1016/j.clp.2012.06.005. Epub 2012/09/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jadcherla SR, Stoner E, Gupta A, Bates DG, Fernandez S, Di Lorenzo C, et al. Evaluation and management of neonatal dysphagia: impact of pharyngoesophageal motility studies and multidisciplinary feeding strategy. J Pediatr Gastroenterol Nutr. 2009;48:186–92. doi: 10.1097/MPG.0b013e3181752ce7. Epub 2009/01/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jadcherla SR, Peng J, Moore R, Saavedra J, Shepherd E, Fernandez S, et al. Impact of Personalized Feeding Program in 100 NICU Infants: Pathophysiology-based Approach for Better Outcomes. J Pediatr Gastroenterol Nutr. 2012;54:62–70. doi: 10.1097/MPG.0b013e3182288766. Epub 2011/06/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malcolm WF, Gantz M, Martin RJ, Goldstein RF, Goldberg RN, Cotten CM, et al. Use of medications for gastroesophageal reflux at discharge among extremely low birth weight infants. Pediatrics. 2008;121:22–7. doi: 10.1542/peds.2007-0381. Epub 2008/01/02. [DOI] [PubMed] [Google Scholar]
- 20.Jadcherla SR, Dail J, Malkar MB, McClead R, Kelleher K, Nelin L. Impact of Process Optimization and Quality Improvement Measures on Neonatal Feeding Outcomes at an All-Referral Neonatal Intensive Care Unit. JPEN J Parenter Enteral Nutr. 2015 doi: 10.1177/0148607115571667. Epub 2015/03/04. [DOI] [PubMed] [Google Scholar]
- 21.Jadcherla SR, Chan CY, Moore R, Malkar M, Timan CJ, Valentine CJ. Impact of feeding strategies on the frequency and clearance of Acid and nonacid gastroesophageal reflux events in dysphagic neonates. JPEN J Parenter Enteral Nutr. 2012;36:449–55. doi: 10.1177/0148607111415980. Epub 2011/11/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ehrenkranz RA, Walsh MC, Vohr BR, Jobe AH, Wright LL, Fanaroff AA, et al. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics. 2005;116:1353–60. doi: 10.1542/peds.2005-0249. Epub 2005/12/03. [DOI] [PubMed] [Google Scholar]
- 23.Moore T, Johnson S, Haider S, Hennessy E, Marlow N. Relationship between test scores using the second and third editions of the Bayley Scales in extremely preterm children. The Journal of pediatrics. 2012;160:553–8. doi: 10.1016/j.jpeds.2011.09.047. Epub 2011/11/04. [DOI] [PubMed] [Google Scholar]
- 24.Brotherton A, Abbott J, Hurley M, Aggett PJ. Home enteral tube feeding in children following percutaneous endoscopic gastrostomy: perceptions of parents, paediatric dietitians and paediatric nurses. Journal of human nutrition and dietetics : the official journal of the British Dietetic Association. 2007;20:431–9. doi: 10.1111/j.1365-277X.2007.00811.x. Epub 2007/09/12. [DOI] [PubMed] [Google Scholar]
- 25.Pedron-Giner C, Calderon C, Martinez-Costa C, Borraz Gracia S, Gomez-Lopez L. Factors predicting distress among parents/caregivers of children with neurological disease and home enteral nutrition. Child: care, health and development. 2014;40:389–97. doi: 10.1111/cch.12038. Epub 2013/03/07. [DOI] [PubMed] [Google Scholar]
- 26.Heyman MB, Harmatz P, Acree M, Wilson L, Moskowitz JT, Ferrando S, et al. Economic and psychologic costs for maternal caregivers of gastrostomy-dependent children. The Journal of pediatrics. 2004;145:511–6. doi: 10.1016/j.jpeds.2004.06.023. Epub 2004/10/14. [DOI] [PubMed] [Google Scholar]
- 27.Naiditch JA, Lautz T, Barsness KA. Postoperative complications in children undergoing gastrostomy tube placement. Journal of laparoendoscopic & advanced surgical techniques Part A. 2010;20:781–5. doi: 10.1089/lap.2010.0191. Epub 2010/08/14. [DOI] [PubMed] [Google Scholar]
- 28.Malcolm WF, Do BT, Smith PB, et al. Gastrostomy Tube Feeding in Extremely Low Birth Weight Infants; Pediatric Academic Societies’ Annual Meeting; 2016 April 30-May 3; Baltimore, MD. E-PAS2016:2891.714. [Google Scholar]
- 29.Lang IM. Brain stem control of the phases of swallowing. Dysphagia. 2009;24:333–48. doi: 10.1007/s00455-009-9211-6. Epub 2009/04/29. [DOI] [PubMed] [Google Scholar]
- 30.Lang IM, Dean C, Medda BK, Aslam M, Shaker R. Differential activation of medullary vagal nuclei during different phases of swallowing in the cat. Brain Res. 2004;1014:145–63. doi: 10.1016/j.brainres.2004.03.061. Epub 2004/06/24. [DOI] [PubMed] [Google Scholar]
- 31.Jadcherla SR. Upstream effect of esophageal distention: effect on airway. Curr Gastroenterol Rep. 2006;8:190–4. doi: 10.1007/s11894-006-0074-9. Epub 2006/06/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jadcherla SR, Duong HQ, Hoffmann RG, Shaker R. Esophageal body and upper esophageal sphincter motor responses to esophageal provocation during maturation in preterm newborns. J Pediatr. 2003;143:31–8. doi: 10.1016/S0022-3476(03)00242-7. Epub 2003/08/14. [DOI] [PubMed] [Google Scholar]
- 33.Jadcherla SR, Hoffmann RG, Shaker R. Effect of maturation of the magnitude of mechanosensitive and chemosensitive reflexes in the premature human esophagus. J Pediatr. 2006;149:77–82. doi: 10.1016/j.jpeds.2006.02.041. Epub 2006/07/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maas C, Wiechers C, Bernhard W, Poets CF, Franz AR. Early feeding of fortified breast milk and in-hospital-growth in very premature infants: a retrospective cohort analysis. BMC pediatrics. 2013;13:178. doi: 10.1186/1471-2431-13-178. Epub 2013/11/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neu J. Gastrointestinal development and meeting the nutritional needs of premature infants. Am J Clin Nutr. 2007;85:629S–34S. doi: 10.1093/ajcn/85.2.629S. Epub 2007/02/08. [DOI] [PubMed] [Google Scholar]