Abstract
Background
The past twenty years have seen distinct shifts in the way the participation of children and adolescents in research is viewed. This has been emphasized by the growing pediatric research enterprise. Additional information on children’s and adolescents’ experiences during research participation is needed to better inform researchers on the ethical conduct of research with this vulnerable population.
Aims
The objective of this analysis was to examine ethical issues in research with children and adolescents from their perspective as participants, including: assent, parental consent, risk perception, impact of research participation, and incentives.
Methods
This systematic review was conducted per the Long et al. framework by means of an iterative searching process. Using the key words ‘research ethics’ and ‘child or pediatric or adolescent’, PubMed, CINAHL, and EBSCOhost databases were searched to identify articles. Limitations placed on the original searches were: English language, year of publication between 2003–2014, humans, abstract available, and age birth–18 years.
Findings
Twenty-three empiric studies were identified and formed the sample. Included studies represented a diverse range of areas of research, methods, settings, sample demographics, authors, and journals.
Discussion
Even young children demonstrated the ability to understand essential elements of research, although there is variability in children’s level of understanding. Trust was a significant contributing factor to children’s and adolescents’ participation in research, and also shaped their assessments of risk. Research participation was mainly beneficial for children and adolescents. Incentives were mainly viewed positively, although concerns of possible undue influence were expressed.
Linking Evidence to Action
This systematic review highlights the importance of including the perspectives of children and adolescents and provides researchers and nurse clinicians with best practices for involving children in research.
Keywords: Ethics, Research, Participation, Participant, Child, Adolescent, Consent, Assent, Incentive, Risk
Introduction
In 2004, the Institute of Medicine published a report, ‘Ethical Conduct of Clinical Research Involving Children’, the purpose of which was to review federal regulations, reports and research and make recommendations about ethical research involving children (Institute of Medicine, 2004). Themes of the report included the need for:
Well-designed and executed research with children to improve the health of children and future generations worldwide;
Children to not be either burdened or excluded from participation in research;
A robust system for protecting child and adolescent research participants, including additional resources like experts in physiology and development, to recognize and address unique ethical issues.
In the ensuing years, researchers have continued to recognize the need to balance the inherent vulnerability of children and adolescents with the necessity to research their unique needs and perspectives (Broome, Kodish, Geller, & Siminoff, 2003; Hurst, 2008; Levine et al., 2004; Solomon, 2013). There have been new research investigations with child and adolescent participants, outside of traditional clinical research settings and those using more novel designs. More child/adolescent populations have been identified as vulnerable within the research context; indeed the current prevalence of vulnerable populations has compromised our understanding of the unique needs of any particular vulnerable population (Levine et al., 2004). As the research enterprise continues to evolve it is important to better inform researchers about the unique needs that should inform the ethical conduct of research with children and adolescents.
Much of our understanding of the conduct of ethical research with children and adolescents has been formulated based on general ethical principles, without consideration of the heterogeneity of children and adolescents research participants (Carter, 2009; Hurst, 2008; Levine et al., 2004). It is unclear what, if any, voice children and adolescents have had in the development of these ethical guidelines. The objective of this systematic review was to examine ethical issues surrounding research with children and adolescents from their perspective as participants. Specific questions that guided this review were:
What research methods have been used to understand children’s and adolescents’ experiences of participating in research?
What has been learned from children and adolescents about assent and parental consent for research participation?
How do children and adolescents perceive the risks inherent with research participation?
What impact have children and adolescents identified as a result of their research participation?
What has been learned from child and adolescent research participants regarding the use of incentives?
Methods
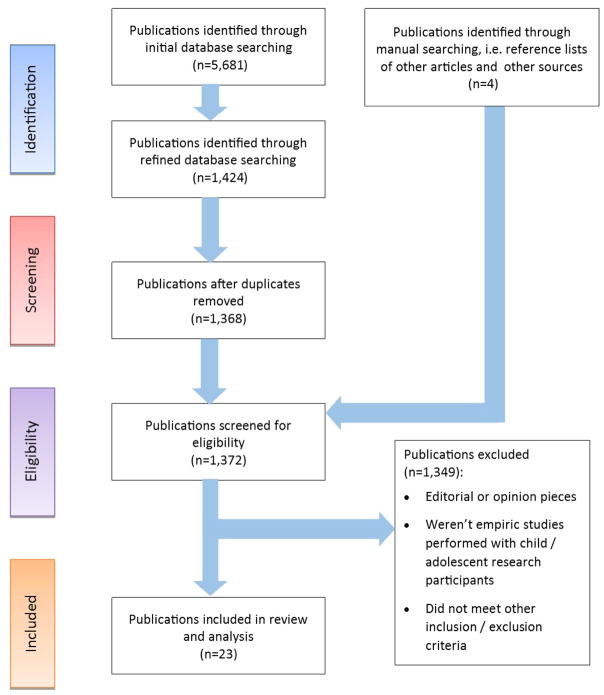
This systematic review was conducted using the framework described by Long, Godfrey, Randall, Brettle, and Grant (2002). An iterative searching process was used including three stages: scoping, refinement and confirmation (Long et al., 2002). After a broad search and relevance check on initially identified studies, inclusion and exclusion criteria were refined and questions refocused. Searches were then rerun. References cited in included studies were reviewed to identify further relevant studies. A PRISMA flowchart was used to graphically represent search procedures (Moher, Liberati, Tetzlaff, & Altman, 2009).
Search Methods
Using the key words ‘research ethics’ and ‘child or pediatric or adolescent’, PubMed, CINAHL, and EBSCOhost (including Academic Search Premier, Health Source, PsycINFO, SocINDEX, Family & Society Studies Worldwide, MasterFILE Premier, Biomedical Reference Collection, Applied Science & Technology Source, Historical Abstracts) databases were searched to identify potentially relevant published articles. Limitations placed on the original searches were: English language, year of publication between 2003 and 2014, humans, abstract available, and age birth – 18 years. Due to the high volume of articles initially retrieved (PubMed = 2,240 and EBSCOhost = 3,313) additional limitations were placed on searches. Explicitly, in PubMed a limitation was added for the MeSH search term ‘Ethics, research’ and in EBSCOhost a limitation was placed using ‘Research ethics’ as a major concept. Using this process, these searches yielded a combined total of 1,424 potentially relevant published articles (including 56 duplicates).
Search Outcomes
Articles were then reviewed to select those that met criteria for inclusion. Inclusion criteria included: 1) empiric research studies from the child/adolescent perspective; 2) articles considering ethical issues in research with children/adolescents or child/adolescent perspectives of research participation; and 3) ethical issues as the primary focus of the paper. Exclusion criteria included articles that focused solely on: 1) broad medical ethics or bioethics, or 2) research procedures or regulatory approval processes. From the original search 19 articles met these criteria. Manual searching yielded four further articles meeting the criteria for inclusion. In the end, 23 articles provided the sample for this analysis. Table 1 lists the included articles. Figure 1 illustrates the search results and screening procedures.
Table 1.
Articles Included in Sample
| Article | Purpose/Aims |
|---|---|
| (Bagley, Reynolds, & Nelson, 2007) | To evaluate factors that would influence children and adolescents’ decision-making for research participation, in terms of the impact of monetary and other incentives. |
| (Birnie, Noel, Chambers, von Baeyer, & Fernandez, 2011) | To describe the ethical challenges and acceptability of the cold pressor test from the perspective of researchers, children and parents. |
| (Brawner et al., 2013) | To determine adolescents’ perceptions of participation in research involving the collection of biomarkers via blood, saliva and/or urine samples. |
| (Bruzzese & Fisher, 2003) | To examine the capacity of 4th, 7th, and 10th graders, as well as college students, to understand their rights in research and the extent to which this capacity can be enhanced following exposure to The Research Participants’ Bill of Rights. |
| (Burke et al., 2005) | To maximize the amount of information children and adolescents understand about the risks and benefits associated with participation in a biomedical research study. |
| (Chu et al., 2008) | To compare the effects of research participation on children who have experienced traumatic events with children who have not, in their perception of the risks and benefits of research participation and their understanding of assenting to participate. |
| (Cohn et al., 2005) | To explore the factors that influenced adolescents’ decisions to participate in an ED-based research study about youth violence, and to determine the feelings elicited by being a research subject. |
| (Ellonen & Pösö, 2011) | To evaluate children’s perceptions of completing a research survey about their exposure to violence. |
| (Ensign, 2006) | To describe the experiences and perspectives of homeless young people as participants in research, including their perspectives and advice on how to handle ethical challenges posed by such research. |
| (Fernandez et al., 2009) | To define an appropriate process for providing research results to participants in pediatric oncology clinical trials, based on participants’ needs and attitudes. |
| (Fisher, 2003) | To empirically examine generational and ethnic variations about ethical issues in youth drug use and suicide survey research in order to: a) evaluate risks and benefits, b) establish guardian permission requirements, c) develop disclosure and confidentiality policies, and d) identify appropriate incentives for recruitment. |
| (Langhinrichsen-Rohling et al., 2006) | To examine distress related to answering personal survey questions about drug use, suicidal behavior, and physical and sexual abuse in multiple convenience samples of adolescents. |
| (Mayeux et al., 2007) | To investigate children’s and teachers’ perceptions of emotional responses to sociometric testing, and whether children understood their research rights as participants. Also to measure both quantitative and qualitative aspects of the sociometric experience. |
| (Moreno, Grant, Kacvinsky, Moreno, & Fleming, 2012) | To determine older adolescents’ responses after learning that they were participants in a research study that involved identification of participants using Facebook. |
| (O’Reilly et al., 2012) | To explore parent and children’s views of anonymity and the intrinsic link to the ethic of confidentiality with the objective of questioning the taken- for-granted nature of the ethic of anonymity. |
| (Reynolds & Nelson, 2007) | To increase our understanding of how diabetic and at-risk adolescents (i.e., those who are obese and/or have a family history of diabetes) and their parents perceive risks and make decisions about research participation. |
| (Swartling et al., 2011) | To explore what views children 10–12 years of age express about medical research and participation in such research. |
| (Swartling et al., 2014) | To explore 10- to 13-year-old children’s views on medical research, trust, information, decision making, and their views on data sampling and risk identification. |
| (Traube et al., 2013) | To examine factors influencing informed assent, initial involvement, and ongoing involvement in HIV- focused community based participatory research for African American children. |
| (Unguru et al., 2010) | To assess what children aged 7 to 18 with cancer understand about research, their research-related treatment, and their preferences for inclusion in decision-making. |
| (Vitiello et al., 2007) | To examine the extent to which parents and adolescents participating in the Treatment for Adolescents With Depression Study understood key aspects of the study. |
| (Wagner et al., 2006) | To prospectively assess youths’ and their parents’ attitudes and experiences about participation in clinical treatment research. |
| (Woodgate & Edwards, 2010) | To detail how parents as well as children view and assess the risks to involving children in health research. This paper focuses on one of the factors, a matter of trust, that shaped Canadian parents’ and children’s perceptions and assessments of risk in child health research. |
Figure 1.
PRISMA Diagram of Search Results and Screening
Quality Appraisal
All articles in the sample were evaluated using an instrument to assess overall quality; the Evaluative Tool for Quantitative Research Studies (Long et al., 2002) or the Critical Review Form for Qualitative Research (Letts et al., 2007). Mixed methods studies were assessed using both instruments. These tools were used to ensure the overall quality of studies within the sample, to summarize study findings, and as a method for ensuring inter-rater agreement between the two authors. Both authors independently completed assessments of 14 articles in the sample, three articles at a time, comparing results until substantive agreement was achieved. The nine remaining articles were then assessed by the first author, with the second author performing a secondary confirmation. All articles included in the sample met a minimum of 80% of the instruments’ criteria. The potential for bias in the quality appraisal was minimal as both authors are trained in research ethics and experienced in conducting research with pediatric and adolescent participants.
Data Abstraction and Synthesis
Data abstracted from the articles included: journal, country of publication, purpose, approach, method, data collection techniques, research context, sample size, sample characteristics (ages, health, and research experience), method of child assent and parent consent, and findings.
Findings
For further details of the articles in the sample, including their findings, journal and country of publication, research methodologies used, and demographics of the children and adolescents studied in each article, see Tables S1 through S3 online.
Characteristics of Reviewed Studies
The sample reflected a variety of different methods and settings. Of the 23 articles, 11 used a descriptive, quantitative design, eight used a descriptive qualitative design, two used mixed methods, and two used a quantitative design that involved testing an intervention. Eleven of the articles involved retrospective reflection on a research experience and one involved a prospective, longitudinal design. The remaining 11 articles considered participants’ current views on research issues. In terms of setting, 10 of the articles involved studies conducted at or through a hospital, nine at schools, three were community based, and one did not indicate the study site.
Children and adolescents were asked to either reflect on their personal experience as a research participant, on research in general, or on a specific hypothetical research situation. Hypothetical scenarios reflected a variety of medical treatments, clinical trials, diagnostic procedures, collection of laboratory samples and/or descriptive research studies.. In other studies children were asked to reflect on their experiences as part of a study they just participated in including those studying exposure to violence, depression, oncology, pain, drug testing, sociometric testing, and health screening. In most cases these reflections were obtained at the end of their participation in the study. In five of the studies children were asked to reflect on their overall impressions of research participation.
Methods for data collection varied. Four of the studies involved either semi-structured or unstructured individual interviews, one involved a focus group, and two studies used a combination of focus groups and interviews. Eight of the studies used written instruments completed at home, school, hospital, or juvenile justice program settings. Seven studies involved structured quantitative instruments that were completed in individual interviews. One study involved a structured quantitative survey conducted orally in a classroom.
Obtaining Assent and Parental Consent
Per the Declaration of Helsinki, “when a potential research subject who is deemed incapable of giving informed consent is able to give assent to decisions about participation in research, the [researcher] must seek that assent in addition to the consent of the legally authorized representative” (World Medical Association, 2013). When conducting research with children and adolescents, when and how to obtain assent versus informed consent (for adolescents) and parental consent has been ethically debated (Giesbertz, Bredenoord, & van Delden, 2014; Lambert & Glacken, 2011). In general, literature and policies support researchers seeking assent from all child and adolescent participants in addition to parental consent. In this sample of 23 articles, seven indicated assent was obtained from the participating children/adolescents although the method was not discussed, seven obtained assent using a written form, and three obtained assent verbally. Two articles took the completion of written survey instruments as an indication of implied consent, and the remaining seven articles did not discuss assent procedures.
The majority of child and adolescent research participants were able to comprehend the purpose and nature of research, research risks and benefits, and the voluntary nature of research participation. Comprehension increased with grade level, with 15 – 16 year olds having a similar understanding of research as adults (Bruzzese & Fisher, 2003; Unguru, Sill, & Kamani, 2010). However, while even young children could understand complex concepts like research risks and benefits, there remained a significant minority of children under around the age of 10 who had difficulties understanding research concepts (Bruzzese & Fisher, 2003; Burke, Abramovitch, & Zlotkin, 2005). In addition, cultural factors, including race, influenced children’s interpretation of research information (Traube, Cederbaum, Kerkorian, Bhupali, & McKay, 2013; Unguru et al., 2010).
Noteworthy challenges to children’s comprehension, including their inability to specify any risks associated with previously collected data or samples, were found in a multi-year longitudinal study. This highlights the need to view assent as an ongoing process to be reaffirmed in studies with research activities at more than one time point (Swartling, Hansson, Ludvigsson, & Nordgren, 2011). In addition, challenges were found in children’s ability to understand clinical trials despite researchers’ explanations; with children’s expectations for clinical improvement as a result of clinical trial participation sometimes being unrealistically high (Unguru et al., 2010; Wagner, Martinez, & Joiner, 2006).
Most children preferred a shared decision-making model when deciding to participate in research, where they were actively involved and supported by parents, doctors, and researchers (Swartling, Helgesson, Ludvigsson, Hansson, & Nordgren, 2014; Unguru et al., 2010). One challenging situation, however, was indicated by children with cancer who did not feel free to dissent to clinical trial enrollment (Unguru et al., 2010). In another, homeless adolescents felt strongly that they should be able to independently consent to participate in research, without requiring parental approval (Ensign, 2006).
An important consideration is the type of parental consent processes that should be used in research with children and adolescents. Three studies specified that parental consent was obtained but did not indicate a method, eight of the articles obtained consent from parents using a written form, three obtained verbal parental consent, and three articles did not discuss parental consent processes. Other processes used included passive parental consent (1), implied parental consent (1), and not obtaining any parental consent (2). Of the two articles that did not obtain any parental consent one was conducted with homeless adolescents, and the other involved a survey completed at school (Ellonen & Pösö, 2011; Ensign, 2006). For this latter study, the children aged 12–16 years decided whether they wished to participate in the study and parents were notified afterwards regarding their child’s participation. Participation rates were lower when active parental consent processes were used in school-based research (Langhinrichsen-Rohling, Arata, O’Brien, Bowers, & Klibert, 2006). One article demonstrated that an active vs. passive parental consent procedure did not reduce the risk of adolescents completing a survey of high-risk behavior feeling upset (Langhinrichsen-Rohling et al., 2006). However, other findings suggested that whether parents were present during the assent discussion impacted whether adolescents perceived their decision to participate in research to be autonomous (Cohn, Ginsburg, Kassam-Adams, & Fein, 2005).
Specific suggestions from children and adolescents on improving assent processes included: for researchers to speak directly to children about research participation – not simply through their parents, ensuring written materials are written in a way that is appealing and understandable, and providing written information instead of using e-mails or websites (Brawner, Volpe, Stewart, & Gomes, 2013; Burke et al., 2005; Swartling et al., 2011; Swartling et al., 2014; Unguru et al., 2010). Specific tools that were demonstrated to enhance children’s and adolescents’ comprehension of research included an assent quiz, and a specific lesson on research rights (Bruzzese & Fisher, 2003; Chu, DePrince, & Weinzierl, 2008).
Perception of Research Risks
The presence or absence of trust was perceived by children as a contributing factor to being involved in research, and also shaped their assessments of risk (Brawner et al., 2013; Traube et al., 2013; Woodgate & Edwards, 2010). Findings in the sample of articles suggested that children and adolescents are most willing to participate in research when they feel safe (Brawner et al., 2013; O’Reilly, Karim, Taylor, & Dogra, 2012; Swartling et al., 2011; Traube et al., 2013). Most children and adolescents expected that researchers and their parents would protect them during their research participation (Brawner et al., 2013; Woodgate & Edwards, 2010). Children tended to believe that if a researcher behaved unethically or caused harm, the researcher would suffer consequences professionally and personally (Traube et al., 2013). When asked in one study to identify factors that shaped their perceptions and assessments of risks in research children identified: the potential for harm to the child; the potential for good for the child and children in general; the burden to the child and family; and the trust experienced by the child and their parents (Woodgate & Edwards, 2010).
However, weighing of risks and benefits may be age dependent in children, with young children more likely to choose options they are familiar with, and older children paying more attention to the advantages and disadvantages of each option (Burke et al., 2005). In addition, Traube et al. (2013) found that both race and relationship of the researchers to participants impacted children’s trust of researchers, with African-American children having more trust in researchers who weren’t from their own community, and the most trust in Caucasian researchers who had no connection to their community.
There was also evidence that research participants interpreted and used risk information subjectively, based on their personal experiences, and may overlook risk probability information (Reynolds & Nelson, 2007). One article demonstrated that adolescents and adults evaluate risks using a similar process where first the magnitude of the risks are considered, followed by consideration of the probability of the risks (Reynolds & Nelson, 2007). If the magnitude was acceptable, adolescents were willing to tolerate the stated or perceived risks of a research procedure, regardless of probability.
Adolescents were very concerned regarding how their information and samples would be used, and in particular whether information would be shared with their parents (Brawner et al., 2013). Adolescents and parents were found to have different opinions about research disclosures; parents often wanted to receive their children’s research information, but adolescents reported wanting to withhold private and sensitive findings (Brawner et al., 2013). Specific suggestions adolescents made to researchers included allowing their parents and/or friends to attend data collection visits, being able to participate in research along with their friends, and in research where blood, urine, or other biological samples are taken to explicitly inform the adolescent whether or whether not pregnancy, sexually transmitted disease, or drug testing would be performed (Brawner et al., 2013).
Impact of Research Participation Experiences
Twelve studies examined children’s feelings about their own research participation. When asked how the participation affected them, overall the majority of ratings and reports were positive. Specific benefits reported by children/adolescents included: 1) learned something new, 2) helped others, 3) helped other people learn something new, 4) felt ‘empowered’, 5) liked talking about themselves to someone else, 6) enjoyed the procedures, 7) thought filling out forms was ‘fun’, 8) trusted in researchers, 9) thought they experienced ‘clinical improvement’, and 10) would be willing to participate in another study (Bruzzese & Fisher, 2003; Chu et al., 2008; Ensign, 2006; Fernandez et al., 2009; Reynolds & Nelson, 2007; Swartling et al., 2014; Wagner et al., 2006).
In the studies asking about positive aspects of participation there were always some children who did not report positive experiences. The percentages in those studies who reported negative experiences ranged from 4% – 6.1% (Cohn et al., 2005; Ellonen & Pösö, 2011). Negative reports associated with research participation included: anxiety, feeling upset, being bored, worry about being identified as high risk for disease, and inconvenient or painful (i.e. blood draws) procedures (Bruzzese & Fisher, 2003; Wagner et al., 2006). In some cases, those who rated aspects of research experiences more negatively also rated other aspects positively, indicating an overall positive cost-benefit ratio of research participation (Cohn et al., 2005). Interestingly, of the few studies that examined factors associated with negative or positive appraisals of research participation, only one found that a demographic variable, namely the child’s level of emotional problems, was associated with their appraisals (i.e. there was a positive association between emotional problems and negative feelings towards research participation) (Cohn et al., 2005; Ellonen & Pösö, 2011; Swartling et al., 2011). No other demographic variables –including age, gender, data collection strategy, type of precipitating event that lead to inclusion in the study (i.e. violence), or anxiety - were reported as significantly associated with children’s appraisals.
Use of Incentives
In seven of the articles a cash incentive of between $10 and $50 was provided to participants. Other incentives used included: small tokens or prizes, $10 phone cards, movie gift certificates, psychology course credits, and a raffle for a gift certificate). Three articles did not provide incentive to participants. Twelve articles did not discuss whether participants were provided with incentives. One article where studies were conducted at schools provided the school with $1 per participant recruited to the study.
There was an interesting range of opinions expressed by children and adolescents about the usefulness of incentives. In five of the studies, at least some of the participants thought cash incentives were a preferred form of incentive (Brawner et al., 2013; Bruzzese & Fisher, 2003; Ensign, 2006; Fernandez et al., 2009; Langhinrichsen-Rohling et al., 2006). Rationale for this included: justice, pleasure associated with receiving cash, and compensation for time spent, discomfort experienced, and effort expended. In a few studies the issue of ‘how much is too much’, in terms of cash incentives as a coercive factor, was explored with children and adolescents. In one study, some adolescents voiced concerns that disproportionately large amounts of cash could be coercive for homeless youth (Ensign, 2006). In another, ethnic minority children were concerned that financial incentives could potentially undermine altruistic motivations, or even tempt youth into providing false information (Mayeux, Underwood, & Risser, 2007). In another study, adolescents felt that cash was not coercive for older children who had a better understanding of the role of incentives (Vitiello et al., 2007).
Discussion
Obtaining Assent and Parental Consent
Although parental consent for participation remains the first step in involving children and adolescents in most research, researchers and human research ethics committees are now taking assent to participate in children aged 7–12 and consent from adolescents aged 13–18 far more seriously. This systematic review confirms that obtaining children’s and adolescents’ assent to participate in research is valid and important, as even young children have demonstrated the ability to understand the essential elements of research (Burke et al., 2005; Unguru et al., 2010). Some of the variability in levels of understanding reported in studies with children likely reflects how assent forms were written, as opposed to developmental differences (Burke et al., 2005). The challenge for researchers is to find better ways to get information across to children. This problem, while relevant for all research participants, is especially pertinent in younger children who have less life experience and are challenged with a less developed ability to understand new experiences (Brawner et al., 2013; Swartling et al., 2014). Assent processes and instruments need to be created with the assistance of child development specialists and piloted with children before being used in a research study (Burke et al., 2005). Researchers should also consider using quizzes to assess children’s understanding of assent information. In addition to setting an empirical standard for assessing understanding, assent quizzes also provide additional opportunities to interact with children about assent information (Chu et al., 2008).
The decision-making model used in research consent and assent processes needs to reflect the context. With the adolescent population, in particular, there is a need to balance the desire for privacy and autonomy with inclusion of parents (Cohn et al., 2005; Fisher, 2003). Both adolescents and parents are sympathetic to the ethical dilemmas researchers face when conducting sensitive research with adolescents (Fisher, 2003). A priori consultation with representative adolescents and parents can provide guidance for the selection of consent and assent procedures within challenging contexts (Fisher, 2003). While this type of consultation may seem burdensome for researchers, it demonstrates that researchers respect local norms regarding parental decision-making and do not wish to inadvertently undermine the parent–child relationship (Fisher, 2003).
A key gap in the findings of this review is consideration of whether children and adolescents, beyond simply understanding their research rights, are capable of applying this knowledge and of actually exerting their research rights (Bruzzese & Fisher, 2003; Unguru et al., 2010). For example, is a child truly capable of asserting and following through on their desire to stop participating in a research study or dissenting to participate?
Perception of Research Risks
Findings of this systematic review demonstrate that children and adolescents have a realistic perception of research risks, and that trust - towards both parents and researchers - is essential in establishing a safe environment for children and adolescents to participate in research. As a result, researchers must establish a mutual respect with child and adolescent participants; if their trust is eroded this could have implications for both the researcher-child and the parent-child relationship (Woodgate & Edwards, 2010). In addition, when performing sensitive research with adolescents, researchers should consider obtaining a federal-wide assurance to protect all data collected for use solely in research, and in particular to prevent data from being used in legal proceedings (Langhinrichsen-Rohling et al., 2006).
Researchers need to appreciate that the assessment of risk is an ongoing process throughout a research study, beyond simply explanations provided when obtaining assent, and that ethnicity and context play important roles in children’s and adolescents’ perceptions of risk. In a study of African-American children, it was found that children were more likely to trust researchers from outside their neighborhood, in particular Caucasian researchers, over and above researchers they knew from their own community (Traube et al., 2013). Children in this study seemed to be fearful that a researcher from their own neighborhood might tell their parents what they shared (Traube et al., 2013). This is particularly interesting as the same sample of children reported that during consent processes they believed the veracity of information provided from African-American researchers over that of Caucasian researchers (Traube et al., 2013).
Impact of Research Participation
Findings from this review provide evidence that research participation can be beneficial for children and adolescents. The overwhelming majority of participants confirmed a willingness to participate in research again. Children and adolescents also identified reasons for participation in research as including altruistic motivations to help others and their own learning.
This review confirmed that most children prefer to be involved in the decision-making of whether they will participate in research. In addition, soliciting children’s and adolescents’ opinions about their involvement in illness management decision-making has been found to be related to adherence to care regimens (Miller & Jawad, 2014). This decision-making involvement could also be important in intervention research where children’s participation is important to the integrity of the intervention.
All research with children and adolescents could benefit from inclusion of a short interview or survey with participants to gauge their degree of satisfaction with the study. If this were done it should be formative rather than summative. That is, the responses from participants early in the study could help shape aspects of the study to enhance the experience for future participants. Talking with children and adolescents about the process of research participation, beyond the actual study, may also be useful in enhancing engagement and future participation in research.
Use of Incentives
In this review, a common reason children and adolescents gave for participating in research was incentives. Incentives for the most part were viewed positively, although some children did share that the amount of cash incentives should be carefully considered by researchers. Based on findings from this review the age of the child and their vulnerability status needs to be considered when developing plans for incentives. Younger children do not have the same understanding of monetary incentives and could be better suited to more age appropriate incentives such as toys, books, and movie passes. Researchers could consider consulting parents or adolescents from the target population when designing a study to obtain their perceptions about the timing, type, and amount of incentives. Children and adolescents who are homeless or very financially disadvantaged require special consideration, as these participants may weigh incentives differently from those who are more advantaged. For all children and adolescents, per the findings of this integrative review, when obtaining assent/consent researchers should include non-monetary elements as a benefit of study participation including: helping other children learn things, learning something new themselves, and making others’ lives better.
Conclusions
Although there have been many studies on obtaining informed consent and assent when conducting research with ill children, historically there has been much less emphasis placed on children who are involved in research in other contexts. In addition, few studies have focused on the perspectives of children and adolescents themselves. The objective of this systematic review was to examine ethical issues in research with children and adolescents from their perspective as participants, related to assent, parental consent, risk perception, impact of research participation, and incentives. This systematic review highlights the importance of including the voice of children and adolescents in the debate regarding the ethical conduct of research. Children and adolescents are a vulnerable population in the research context, formed of diverse individuals with unique, varying needs. The wide variety of strategies used in the studies described herein exemplifies that in addition to primary research studies of children’s and adolescents’ perspectives of research participation, secondary objectives related to examining their experience as participants can feasibly be added into any pediatric research study. This analysis highlights how researchers and nurses working with children and adolescents enrolled in research can expand their voice and encourage the children to share their experiences in terms of benefits, risks and challenges. It is through linking the evidence found in these studies with their own practice that researchers can improve the experience of and benefits to child and adolescent research participants.
Supplementary Material
TABLE S1. Articles Included in Sample: Summary and Characteristics
TABLE S2. Articles Included in Sample: Methodologies Used
TABLE S3. Articles Included in Sample: Demographics of Participating Children and Adolescents
Linking Evidence to Action.
Assent processes and instruments need to be created with the assistance of child development specialists and piloted with children before being used.
A priori consultation with representative adolescents and parents can provide guidance for developing consent and assent procedures within challenging contexts.
A key gap is consideration of whether children and adolescents, beyond simply understanding their research rights, are capable of applying this knowledge and of actually exerting their research rights.
Researchers need to appreciate that the assessment of risk is an ongoing process throughout a research study, beyond simply explanations provided when obtaining assent and/or consent.
All research with children and adolescents could benefit from inclusion of a short, formative, off-study interview or survey with participants to gauge their experience in the study.
The age of the child and their vulnerability status needs to be considered when selecting incentives.
Contributor Information
Stacey Crane, Predoctoral Fellow, Indiana University School of Nursing, 1111 Middle Dr., NU345, Indianapolis, IN, 46202, 513-508-3936.
Marion E. Broome, Ruby F. Wilson Distinguished Professor and Dean, Vice-Chancellor for Nursing Affairs, Duke University School of Nursing, 307 Trent Drive, Durham, NC, 27710, 919-684-9444.
References
- Bagley SJ, Reynolds WW, Nelson RM. Is a “Wage-Payment” model for research participation appropriate for children? Pediatrics. 2007;119(1):46. doi: 10.1542/peds.2006-1813. [DOI] [PubMed] [Google Scholar]
- Birnie KA, Noel M, Chambers CT, von Baeyer CL, Fernandez CV. The cold pressor task: Is it an ethically acceptable pain research method in children? Journal of Pediatric Psychology. 2011;36(10):1071–1081. doi: 10.1093/jpepsy/jsq092. [DOI] [PubMed] [Google Scholar]
- Brawner BM, Volpe EM, Stewart JM, Gomes MM. Attitudes and beliefs toward biobehavioural research participation: Voices and concerns of urban adolescent females receiving outpatient mental health treatment. Annals of Human Biology. 2013;40(6):485–495. doi: 10.3109/03014460.2013.806590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broome ME, Kodish E, Geller G, Siminoff LA. Children in research: New perspectives and practices for informed consent. IRB: Ethics and Human Research, Suppl. 2003;25(5):S20–S25. [PubMed] [Google Scholar]
- Bruzzese JM, Fisher CB. Assessing and enhancing the research consent capacity of children and youth. Applied Developmental Science. 2003;7(1):13–26. Retrieved from http://search.ebscohost.com/login.aspx?direct=true&db=aph&AN=8699738&site=ehost-live. [Google Scholar]
- Burke TM, Abramovitch R, Zlotkin S. Children’s understanding of the risks and benefits associated with research. J Med Ethics. 2005;31(12):715–720. doi: 10.1136/jme.2003.003228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter B. Tick box for child? The ethical positioning of children as vulnerable, researchers as Barbarians and reviewers as overly cautious. International Journal of Nursing Studies. 2009;46(6):858–864. doi: 10.1016/j.ijnurstu.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Chu AT, DePrince AP, Weinzierl KM. Children’s perception of research participation: Examining trauma exposure and distress. Journal of Empirical Research on Human Research Ethics. 2008;3(1):49–58. doi: 10.1525/jer.2008.3.1.49. Retrieved from http://search.ebscohost.com/login.aspx?direct=true&db=psyh&AN=2008-03332-005&site=ehost-live; achu@du.edu. [DOI] [PubMed] [Google Scholar]
- Cohn JM, Ginsburg KR, Kassam-Adams N, Fein JA. Adolescent decisional autonomy regarding participation in an emergency department youth violence interview. American Journal of Bioethics. 2005;5(5):70–74. doi: 10.1080/15265160500246319. [DOI] [PubMed] [Google Scholar]
- Ellonen N, Pösö T. Children’s experiences of completing a computer-based violence survey: ethical implications. Children & Society. 2011;25(6):470–481. doi: 10.1111/j.1099-0860.2010.00292.x. [DOI] [Google Scholar]
- Ensign BJ. Perspectives and experiences of homeless young people. Journal of Advanced Nursing. 2006;54(6):647–652. doi: 10.1111/j.1365-2648.2006.03853.x. [DOI] [PubMed] [Google Scholar]
- Fernandez CV, Gao J, Strahlendorf C, Moghrabi A, Pentz RD, Barfield RC, … Kodish E. Providing research results to participants: Attitudes and needs of adolescents and parents of children with cancer. Journal of Clinical Oncology. 2009;27(6):878–883. doi: 10.1200/JCO.2008.18.5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher CB. Adolescent and parent perspectives on ethical issues in youth drug use and suicide survey research. Ethics Behav. 2003;13(4):303–332. doi: 10.1207/S15327019EB1304_1. Retrieved from http://search.ebscohost.com/login.aspx?direct=true&db=rzh&AN=2004184880&site=ehost-live. [DOI] [PubMed] [Google Scholar]
- Giesbertz NAA, Bredenoord AL, van Delden JJM. Clarifying assent in pediatric research. European Journal of Human Genetics. 2014;22(2):266–269. doi: 10.1038/ejhg.2013.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst SA. Vulnerability in research and health care; Describing the elephant in the room? Bioethics. 2008;22(4):191–202. doi: 10.1111/j.1467-8519.2008.00631.x. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine. The ethical conduct of clinical research involving children. Washington, DC: National Academies Press; 2004. [PubMed] [Google Scholar]
- Lambert V, Glacken M. Engaging with children in research: Theoretical and practical implications of negotiating informed consent/assent. Nurs Ethics. 2011;18(6):781–801. doi: 10.1177/0969733011401122. [DOI] [PubMed] [Google Scholar]
- Langhinrichsen-Rohling J, Arata C, O’Brien N, Bowers D, Klibert J. Sensitive research with adolescents: Just how upsetting are self-report surveys anyway? Violence & Victims. 2006;21(4):425–444. Retrieved from http://media.proquest.com/media/pq/classic/doc/1096430801/fmt/pi/rep/NONE?hl=&cit%3Aauth=Langhinrichsen-Rohling%2C+Jennifer%3BArata%2C+Catalina%3BO%27Brien%2C+Natalie%3BBowers%2C+David%3BKlibert%2C+Jeffrey&cit%3Atitle=Sensitive+Research+With+Adolescents%3A+Just+How+Upsetting+Are+Self-Report+Surveys+Anyway%3F&cit%3Apub=Violence+and+Victims&cit%3Avol=21&cit%3Aiss=4&cit%3Apg=425&cit%3Adate=Aug+2006&ic=true&cit%3Aprod=ProQuest&_a=ChgyMDE0MTAwOTIyMDI0MTM2Mjo5OTgwNjUSBjExNDA1MBoKT05FX1NFQVJDSCIOMTQwLjE4Mi42Ny4yMTcqBTQ1NjE5MgkyMDg1MzE2NTA6DURvY3VtZW50SW1hZ2VCATBSBk9ubGluZVoCRlRiA1BGVGoKMjAwNi8wOC8wMXIKMjAwNi8wOC8zMXoAggExUC0xMDA3MDI1LTczOTgtQ1VTVE9NRVItMTAwMDAwMzkvMTAwMDAxNDctMTA0NjkxMZIBBk9ubGluZcoBB0VuZE5vdGXSARJTY2hvbGFybHkgSm91cm5hbHOaAgdQcmVQYWlkqgIoT1M6RU1TLVBkZkRvY1ZpZXdCYXNlLWdldE1lZGlhVXJsRm9ySXRlbcoCD0FydGljbGV8RmVhdHVyZdICAVniAgFO6gIA8gIA&_s=ucBTFWGYfgRjWNZzoaaQAoiq3pQ%3D. [PubMed] [Google Scholar]
- Letts L, Wilkins S, Law M, Stewart D, Bosch J, Westmorland M. Guidelines for critical review form: Qualitative studies (version 2.0) 2007 Retrieved from http://www.srs-mcmaster.ca/Portals/20/pdf/ebp/qualreview_version2.0.pdf Retrieved from Retrieved from http://www.srs-mcmaster.ca/Portals/20/pdf/ebp/qualreview_version2.0.pdf.
- Levine C, Faden R, Grady C, Hammerschmidt D, Eckenwiler L, Sugarman J The Consortium to Examine Clinical Research Ethics. The limitations of “vulnerability” as a protection for human research participants. The American Journal of Bioethics. 2004;4(3):44–49. doi: 10.1080/15265160490497083. [DOI] [PubMed] [Google Scholar]
- Long AF, Godfrey M, Randall T, Brettle A, Grant M. Developing evidence based social care policy and practice. Part 3: Feasibility of undertaking systematic reviews in social care. 2002 Retrieved from http://usir.salford.ac.uk/13071/ Retrieved from Retrieved from http://usir.salford.ac.uk/13071/
- Mayeux L, Underwood MK, Risser SD. Perspectives on the ethics of sociometric research with children. Merrill-Palmer Quarterly. 2007;53(1):53–78. Retrieved from http://search.ebscohost.com/login.aspx?direct=true&db=psyh&AN=2007-08527-003&site=ehost-live; undrwd@utdallas.ed; lmayeux@ou.ed. [Google Scholar]
- Miller VA, Jawad AF. Relationship of youth involvement in diabetes-related decisions to treatment adherence. Journal of clinical psychology in medical settings. 2014;21(2):183–189. doi: 10.1007/s10880-014-9388-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Annals of internal medicine. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- Moreno MA, Grant A, Kacvinsky L, Moreno P, Fleming M. Older adolescents’ views regarding participation in Facebook research. Journal of Adolescent Health. 2012;51(5):439–444. doi: 10.1016/j.jadohealth.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly M, Karim K, Taylor H, Dogra N. Parent and child views on anonymity: ‘I’ve got nothing to hide’. International Journal of Social Research Methodology: Theory & Practice. 2012;15(3):211–223. Retrieved from http://search.ebscohost.com/login.aspx?direct=true&db=psyh&AN=2012-12510-003&site=ehost-live; Mjo14@le.ac.uk. [Google Scholar]
- Reynolds WW, Nelson RM. Risk perception and decision processes underlying informed consent to research participation. Social Science & Medicine. 2007;65(10):2105–2115. doi: 10.1016/j.socscimed.2007.06.021. [DOI] [PubMed] [Google Scholar]
- Solomon SR. Protecting and respecting the vulnerable: Existing regulations or further protections? Theor Med Bioeth. 2013;34(1):17–28. doi: 10.1007/s11017-013-9242-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartling U, Hansson MG, Ludvigsson J, Nordgren A. “My parents decide if I can. I decide if I want to.” Children’s views on participation in medical research. Journal of Empirical Research on Human Research Ethics. 2011;6(4):68–75. doi: 10.1525/jer.2011.6.4.68. [DOI] [PubMed] [Google Scholar]
- Swartling U, Helgesson G, Ludvigsson J, Hansson MG, Nordgren A. Children’s views on long-term screening for type 1 diabetes. Journal of Empirical Research on Human Research Ethics. 2014;9(4):1–9. doi: 10.1177/1556264614544456. [DOI] [PubMed] [Google Scholar]
- Traube DE, Cederbaum JA, Kerkorian D, Bhupali C, McKay MM. African American children’s perceptions of HIV-focused community-based participatory research. Journal of Empirical Research on Human Research Ethics. 2013;8(1):79–90. doi: 10.1525/jer.2013.8.1.79. [DOI] [PubMed] [Google Scholar]
- Unguru Y, Sill AM, Kamani N. The experiences of children enrolled in pediatric oncology research: Implications for assent. Pediatrics. 2010;125(4):e876–e883. doi: 10.1542/peds.2008-3429. Retrieved from http://search.ebscohost.com/login.aspx?direct=true&db=aph&AN=49753789&site=ehost-live. [DOI] [PubMed] [Google Scholar]
- Vitiello B, Kratochvil CJ, Silva S, Curry J, Reinecke M, Pathak S, … May DE. Research knowledge among the participants in the Treatment for Adolescents with Depression Study (TADS) Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46(12):1642–1650. doi: 10.1097/chi.0b013e318153f8c7. [DOI] [PubMed] [Google Scholar]
- Wagner KD, Martinez M, Joiner T. Youths’ and their parents’ attitudes and experiences about participation in psychopharmacology treatment research. Journal of Child & Adolescent Psychopharmacology. 2006;16(3):298–307. doi: 10.1089/cap.2006.16.298. [DOI] [PubMed] [Google Scholar]
- Woodgate RL, Edwards M. Children in health research: A matter of trust. J Med Ethics. 2010;36(4):211–216. doi: 10.1136/jme.2009.031609. Retrieved from http://ovidsp.ovid.com/ovidweb.cgi?T=JS&CSC=Y&NEWS=N&PAGE=fulltext&D=ovftk&AN=00005004-201004000-00006; http://jme.bmj.com/content/36/4/211.full.pdf. [DOI] [PubMed] [Google Scholar]
- World Medical Association. Declaration of Helsinki: Ethical principles for medical research involving human subjects. Journal of the American Medical Association. 2013;310(20):2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1. Articles Included in Sample: Summary and Characteristics
TABLE S2. Articles Included in Sample: Methodologies Used
TABLE S3. Articles Included in Sample: Demographics of Participating Children and Adolescents