Abstract
Purpose
To study the effect and time course of body position changes on IOP in nonhuman primates.
Methods
We recorded continuous bilateral IOP measurements with a wireless telemetry implant in three rhesus macaques in seven different body positions. IOP measurements were acquired in the seated-upright, standing, prone, supine, right and left lateral decubitus positions (LDPs), and head-down inverted positions. Continuous IOP was recorded for 90 seconds in each position before returning to a supine reference position until IOP stabilized; measurements were averaged after IOP stabilized at each position.
Results
Head-down inversion increased IOP an average of 8.9 mm Hg, compared to the supine reference. In the LDP, IOP decreased an average of 0.5 mm Hg in the nondependent eye (i.e., the higher eye), while the fellow dependent (i.e., lower) eye increased an average of 0.5 mm Hg, compared to supine reference. Standing and seated positions decreased IOP 1.5 and 2.2 mm Hg, respectively, compared with supine reference. IOP changes occurred within 4 to 15 seconds of a body position change, and timing was affected by the speed at which body position was changed. Compared to the IOP in the supine position, the IOP in the inverted, prone, and seated positions was significantly different (P = 0.0313 for all). The IOP in the standing position was not statistically different from the IOP in the supine position (P = 0.094). In addition, the IOP was significantly different between the nondependent eye and the dependent eye in the LDPs compared to the supine position (P = 0.0313).
Conclusions
Body position has a significant effect on IOP and those changes persist over time.
Keywords: intraocular pressure, body position, glaucoma, nonhuman primate, telemetry
Previous studies have associated asymmetric glaucomatous visual field loss with habitual sleep position.1 In said study, glaucomatous visual field loss is more severe in the dependent eye of patients that self-reported a preference for sleeping on one side versus the other when in the lateral decubitus position (LDP). Hence, it is important to understand the effects of body position on IOP, especially the LDP, as well as the time course and persistence of those changes.
Several studies have investigated this, but all are limited by periodic, single time point IOP measurement techniques available in the clinic, and therefore do not capture the complete time course of IOP change with body position change. Krieglstein et al.2 have shown that IOP changes with different body positions, and there is a nonlinear relationship between IOP increase and body position from 60° semi-upright tilt to 30° head-down tilt. In these studies, IOP is measured by using snapshot, single time point measurements, and no information on the time course is presented other than measurements that were taken approximately 3 minutes apart. Several other studies have shown the change in IOP with body position changes. These studies show a significant IOP difference between eyes in the LDP when the head is in a neutral position.3–9 Eklund et al.3 studied the postural influence on simultaneously measured IOP and intracranial pressure (ICP) and report similar results on IOP changes with body position in the sitting and supine positions. They calculated the trans–lamina cribrosa pressure difference (TLCPD) in different body positions by subtracting ICP from IOP. ICP is lowest in the sitting position, resulting in the largest TLCPD, compared to the supine and head-down tilt positions.3
There are no reports of IOP changes in body position when using continuous IOP measurement techniques that can accurately assess both the magnitude and time course of these changes. All previous studies report IOP changes with body position, using applanation or rebound tonometry, which can have an inherent measurement error of up to 3 mm Hg from true IOP, although longitudinal measurements within eyes should capture relative IOP change. Many clinical IOP measurement devices such as Goldmann Applanation Tonometer (GAT) (Haag-Streit, Essex, UK) cannot be used other than when the patients are in seated position. Although the Tonopen (Reichert Technologies, Depew, NY, USA) can estimate IOP in different positions, it too has an inherent IOP measurement variation of 2 to 3 mm Hg,4 which may not be accurate enough to measure even relative changes in IOP with certain body positions. The Perkins (Haag-Streit, Essex, UK) handheld applanation tonometer can also be used in different body positions, but with the same measurement reproducibility of GAT.5 The snapshot measurement techniques used in previous studies provide no IOP data during the time gaps between measurements, and can be inaccurate when performed in awake patients whose IOPs could be affected by second-to-second changes in ocular muscle tone.6,7
In this study, we measured the effect of changing body positions on IOP in nonhuman primates (NHPs) using an accurate, bilateral, continuous wireless IOP telemetry system. Further, the telemetry implant transducers are calibrated to true intracameral IOP with anterior chamber manometry. This allowed us to determine the magnitude, time course, and persistence of IOP changes due to alterations in body position under tightly controlled conditions.
Methods
Animals
Three male rhesus macaques (NHPs) aged 4 to 6 years were used in this study. All animal experiments were conducted in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, under Institutional Animal Care and Use Committee (IACUC) approval from the University of Alabama at Birmingham. This ancillary study was performed as part of a larger study to determine if transient IOP fluctuations contribute to glaucoma onset and/or progression.
Telemetry System
We have developed and validated a fully implanted wireless telemetry system (Konigsberg Instruments, Inc., Pasadena, CA, USA) that allows continuous monitoring of IOP in awake, behaving NHPs.7 The second-generation system used in this study provides bilateral IOP measurements, bilateral electro-oculogram (EOG) recordings, aortic blood pressure, and core body temperature measurement. Only the continuous IOP data stream was used for this study, which was transmitted wirelessly at 500 Hz from a battery-powered transceiver module implanted in the animals' abdominal wall to an antenna in the perch of the cage. Data are transferred from the receiving antenna to an analog base station wherein the digital signals are decoded and stored. A barometric pressure sensor at the base station recorder is used to offset atmospheric pressure in real time. Accuracy of the IOP transducers in this system is ±0.2 mm Hg. Data were recorded with the NOTOCORD-hem data acquisition system (version 4.3.0.67; NOTOCORD Systems, Croissy-sur-Seine, France).
Telemetry Calibration
Approximately every 2 weeks, each animal was placed under isoflurane anesthesia, and both eyes were cannulated with a 27-gauge needle inserted into the anterior chamber (AC) through the peripheral cornea. The needle was connected to a bottle of sterile isotonic saline solution via a sterile infusion set, and the connecting tube was fitted with an in-line, digital pressure gauge placed at the level of the AC manometer needle (model XP2i; Crystal Engineering, San Luis Obispo, CA, USA). The saline manometer bottle was lowered such that the in-line pressure gauge read 5 mm Hg and IOP was allowed to stabilize. The telemetric IOP reading from the implanted transducer was then recorded for comparison. The manometer-controlled IOP was raised from 5 to 40 mm Hg in increments of 5 mm Hg, and the telemetric IOP reading was compared to the in-line pressure gauge at each step. Calibration offsets were determined using comparisons at 15 mm Hg, which is in the center of the normal physiological IOP range for NHPs. The telemetric and gauge IOP were used to quantify IOP transducer signal accuracy and drift. The calibration file was updated on the signal processor (TD-14 Basestation; Konigsberg Instruments) after each IOP calibration check, such that the transducer IOP data accurately reflected intracameral IOP. Changes in barometric pressure were continuously offset in real time during IOP data acquisition via a NIST-traceable barometric pressure sensor in the NHP holding facility.
Software Compensation for IOP Transducer Drift
IOP calibration procedures occurred every 2 weeks, and the IOP data were continually offset by the drift calculated for that period, normalized to the linear drift calculated for that exact time in the calibration period. The IOP signal was assumed to have drifted linearly between transducer calibration tests. IOP transducer drift is typically <1 mm Hg per week. This approach assured that the telemetric IOP data were accurate to within 1 mm Hg of true IOP for the 2-year data collection period. We reported average IOP for each session that depends on this calibration correction, but the body position IOP data were based on relative change in IOP compared to a reference IOP collected just a few moments before, so transducer drift is not a factor.
Compensation for IOP Transducer Height Relative to AC Tube Opening Height
The IOP transducer is located in the orbital wall rather than inside the eye (Fig. 1B), so the small differences in hydrostatic column height between the tube opening in the AC and the transducer must be factored in to ensure accurate data. The pressure transducer is located 1 cm above, 1 cm laterally, and 1 cm posteriorly to the site of the aqueous transduction tube opening in the AC. Since the system is calibrated with the head in an upright position, all body positions in which the head is not in the upright position were offset in a position-dependent manner to compensate for the small hydrostatic column differences in the telemetry implant system.
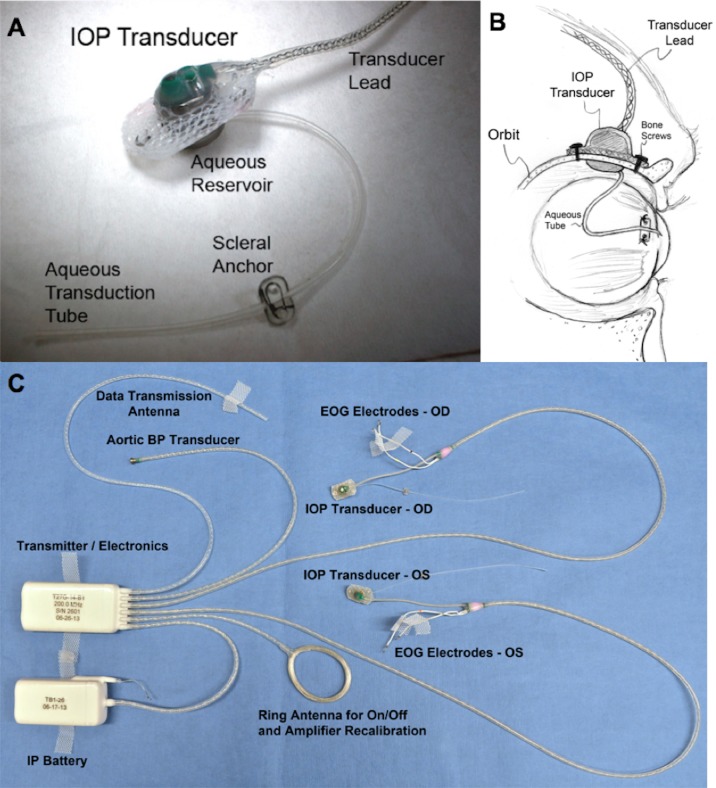
Figure 1.
(A) Photograph of the extraorbital surface of our custom IOP transducer housing that is secured within a ¼-inch hole in the lateral orbital wall with bone screws as shown in (B). A 23-gauge silicone tube delivers aqueous from the anterior chamber to a fluid reservoir on the intraorbital side of the transducer (partially hidden from view in [A]); the tube (with appropriate slack to allow for eye movement) is trimmed, inserted into the anterior chamber, sutured to the sclera by using the integral scleral tube anchor plate, and covered with a scleral patchgraft (not shown). Adapted from Downs et al.7 (C) Photograph of enhanced Konigsberg Instruments total implant system for continuous monitoring of bilateral IOP, bilateral EOG, aortic blood pressure, and body temperature.8
Since the pressure measurement system is relatively rigid and closed from the opening of the AC transduction tube to the orbital wall-mounted pressure transducer, we performed an experiment to test if it behaves similarly to an open hydrostatic water column system to ensure that the aforementioned compensations were accurate. In a closed, fluid-filled, rigid system with no gas (air), changes in water column height will not change pressure at a fixed-position transducer owing to the fluid vacuum in the system. Despite this, we hypothesized that IOP changes associated with transducer-to-AC height changes would be comparable to an open system, since the globe is not rigid, wherein a centimeter of fluid column height change elicits a 0.74 mm Hg change in pressure (1 cm H2O = 0.74 mm Hg). To test this, we cannulated the AC in an anesthetized NHP with a 27-G needle connected directly to a digital pressure gauge with a fluid-filled tube (no gas bubbles). Precise adjustments in the height of the pressure gauge from −5 to 5 cm resulted in a 0.71 mm Hg pressure measurement change per cm of gauge height change, which is 97% of the expected value of 0.74 mm Hg. This result confirms that our IOP telemetry implant system behaves nearly identically to an open hydrostatic column system, and we therefore compensated the transducer IOP by 0.71 mm Hg per cm of height differential as described above.
Body Position Data Collection
We studied continuous IOP measurements collected 500 times per second in both eyes of three NHPs placed in seven different body positions. Anesthesia was initiated with an intramuscular injection of ketamine and xylazine and maintained with isoflurane gas for the experiment. Body position was recorded on time-synchronized video and time-stamped into the IOP data streams within NOTOCORD-hem. Experiments were repeated on 5 separate days at least 2 weeks apart in each NHP. Continuous IOP data were collected while the NHPs were placed in the following positions: supine, seated upright, standing, prone, head-down inverted, lying on the right shoulder in the LDP (right LDP), and lying on the left shoulder in the LDP (left LDP). Body positions were carefully changed by a technician and held stationary while acquiring data in the inverted, seated, and standing positions. For the supine, prone, and lateral decubitus positions, the animal was placed on a table until IOP stabilized. Furthermore, in the supine and lateral decubitus positions we placed a small soft pad under the NHPs' head to maintain a head-neutral position. IOP data were collected for ∼90 seconds in each position until IOP stabilized in both eyes for at least 30 seconds; the NHP was then returned to the reference supine position until IOP stabilized bilaterally before proceeding to the next body position. Stable IOP was defined as the time point at which the standard deviation of IOP measurements, as calculated continuously via NOTOCORD-hem, was <0.5 mm Hg for at least 30 seconds. Reported IOP data for each body position were the mean of these final 30 to 60 seconds of continuous, stable IOP data collected at each body position, referenced to the stable supine IOP acquired just before each body position change. For the LDP analysis, we examined IOP asymmetry between eyes. IOP asymmetry was calculated as the IOP difference from supine in the dependent eye minus the difference from supine in the nondependent eye; this method was chosen because baseline IOP asymmetry was variable, yet changes from supine were consistent. In one NHP, we performed an additional experiment by using only the LDP for 1 hour to assess the stability of the IOP difference between fellow eyes over time. Measurements were taken of eye-to-heart heights in all positions.
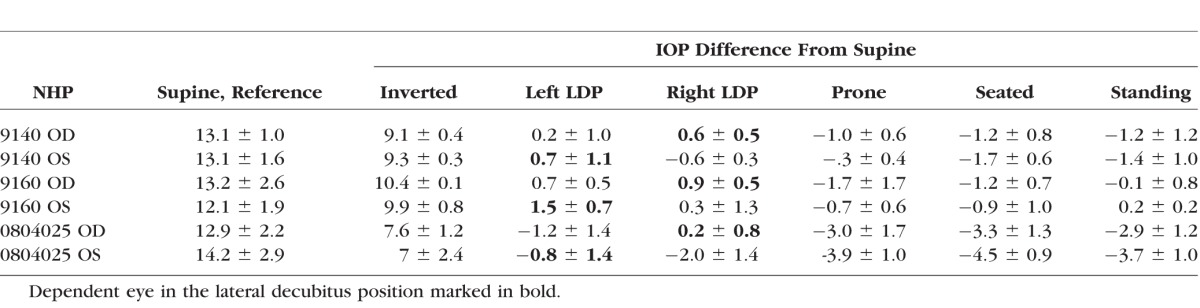
Table.
Mean Supine IOP ± Standard Deviation (Left) for Each NHP by Eye With IOP Difference From Supine Position Averaged Over Five Sessions ± Standard Deviation (Right)
Statistical Analyses
Measurements of IOP change at different body positions compared to the supine reference position were taken in seven different positions on 5 different days. The values obtained during each session were averaged and then used as the unit of analysis. The signed rank test was used to test whether the difference in IOP between each position and the supine position was different from zero. A signed rank test was used to determine if the intereye IOP difference in the LDP compared to supine reference position measurements was significantly different from zero.
Results
IOP changes relative to the supine reference position are presented in the Table for each NHP and averaged across all NHPs together by eye in Figure 2. Figure 3 shows the total IOP difference between fellow eyes while in the LDP, and Figures 4 and 5 show diagrams of the average IOP difference at each position relative to both the supine position and seated position, which are the two most common positions that IOP is measured clinically. Further, the approximate eye-to-heart height differentials are shown in Figures 5 and 6 (bottom).
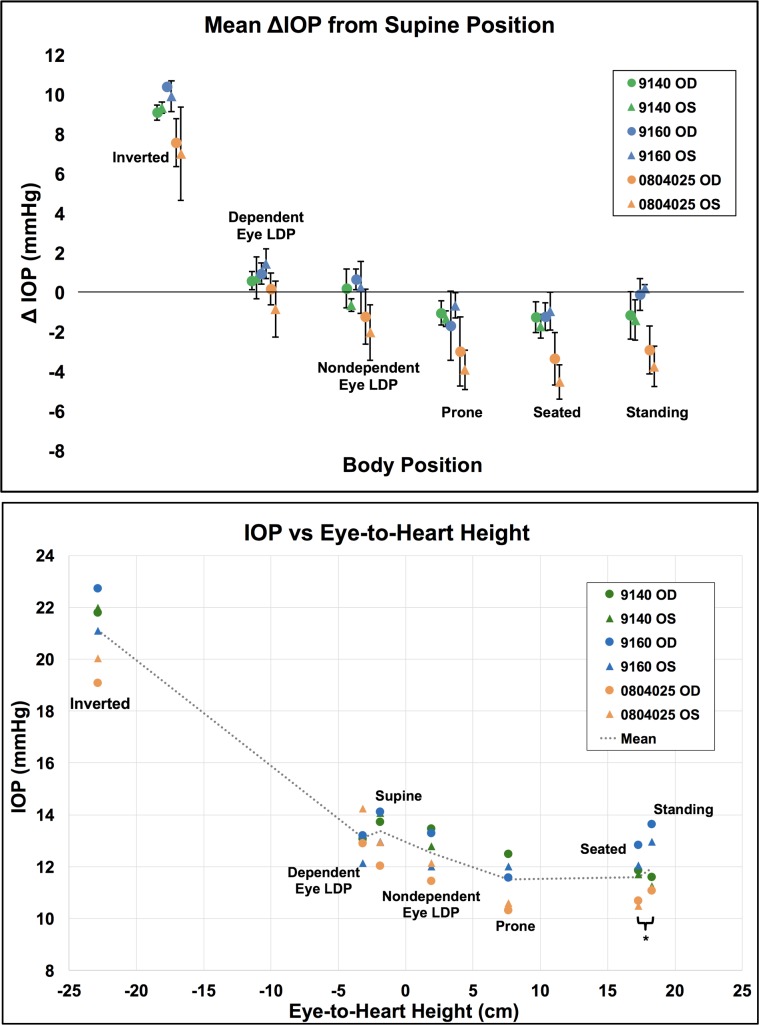
Figure 2.
Mean IOP from supine position in all animals (n = 3) across all sessions (n = 5). Error bars represent the standard deviation. *Compared to the IOP in the supine position, the IOP in the inverted, prone, and seated positions were significantly different (P = 0.0313 for all).
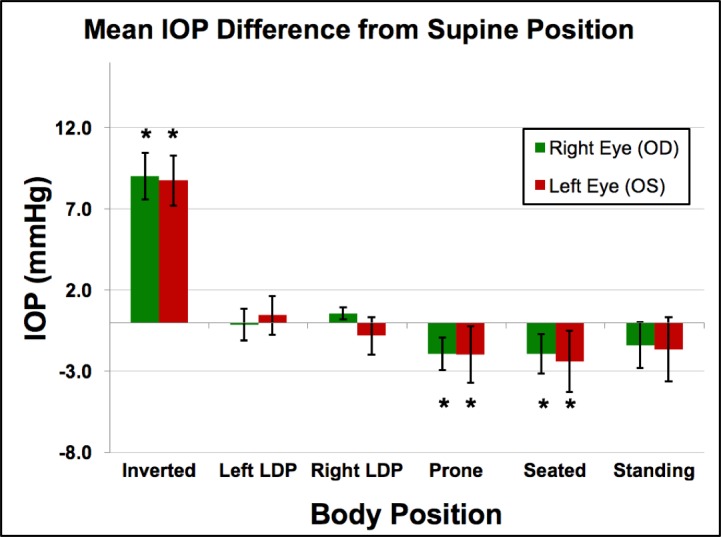
Figure 3.
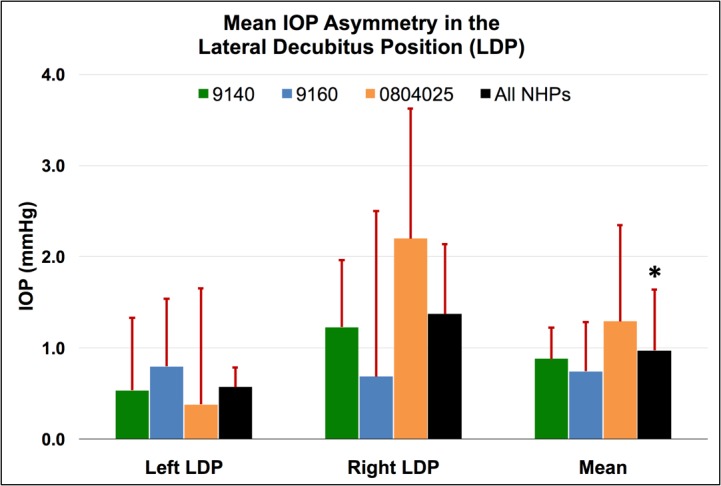
Mean IOP asymmetry between dependent and nondependent eyes in the LDP compared to the supine reference position for each NHP, as well as the total mean for all NHPs for all sessions. Error bars represent standard deviation. *The overall change in IOP in the dependent eye was significantly different from the change in IOP in the nondependent eye for all NHPs and both LDP positions combined (P = 0.0313).
Figure 4.
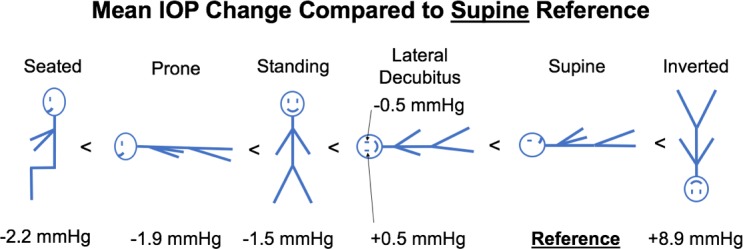
Diagram with different body positions and mean IOP change from supine position, ordered from negative change (left) to positive change (right).
Figure 5.
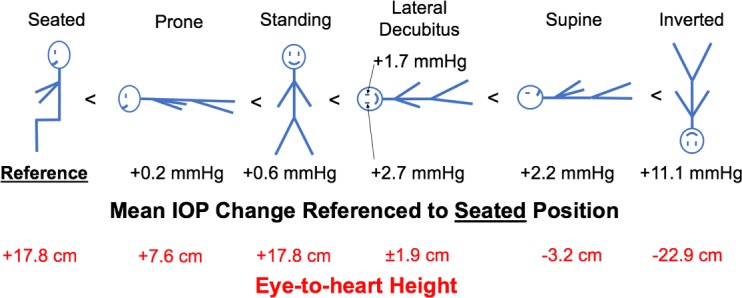
Mean IOP changes with body position normalized to the seated position and the eye-to-heart height at each position.
Figure 6.
Top: Mean IOP difference from supine position data for each eye (n = 6) by position. Error bars represent standard deviation. Bottom: Mean IOP per eye in each body position versus the approximate eye-to-heart distance for all NHP eyes (n = 6). Legend shows NHP ID and eye. *Seated and standing positions have identical eye-to-heart heights; data points are slightly offset for easier viewing.
IOP increased an average of 8.9 mm Hg compared to supine reference in the inverted head-down position. In the LDP, IOP decreased an average of 0.5 mm Hg in the nondependent eye (i.e., the higher eye; OS while lying in the right LDP and OD while in the left LDP), while the fellow dependent eye increased an average of 0.5 mm Hg, compared to supine reference. IOP decreased 1.5 and 2.2 mm Hg, compared with supine reference, while in the standing and seated positions, respectively. IOP changes occurred within 4 to 15 seconds (Fig. 7) of body position change, although this was affected by the speed at which body position was changed.
Figure 7.
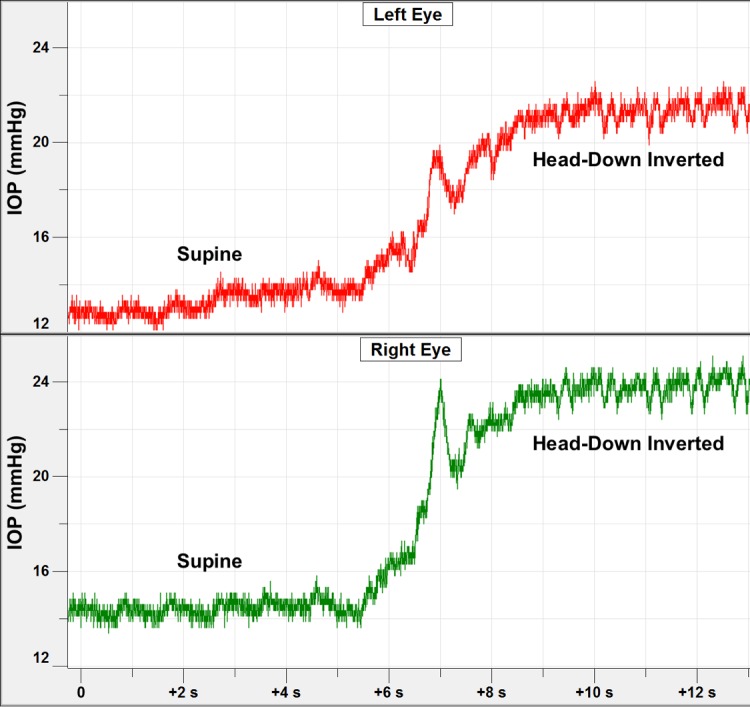
Short duration time course of IOP changes in both eyes of one NHP during the process of moving the animal into the inverted position. The IOP transient at ∼7-second mark was due to momentary neck flexion that occurs in the process of picking the NHP up off the table.
Compared to the IOP in the supine position, the IOP in the inverted, prone, and seated positions was significantly different (P = 0.0313 for all). The IOP in the standing position was not statistically different from the IOP in the supine position (P = 0.094). In addition, the difference in the IOP between the nondependent eye (from right or left LDP) and the dependent eye (from right or left LDP), compared to the supine position, was significantly different (P = 0.0313).
Discussion
Accurate bilateral IOPs were recorded via continuous telemetry in three NHPs as they were shifted from supine position to one of six different body positions. Change in body position causes a significant, rapid change in IOP that occurred as the NHP was moved, and stabilized within ∼4 to 15 seconds once the final body position was reached. IOP changes with body position are relevant for ocular diseases such as glaucoma wherein higher IOPs in the dependent eye in LDP (habitual side sleepers that favor one side) could result in asymmetric disease progression.
Several studies have investigated IOP changes with body position, but all are limited by periodic, single time point IOP measurement techniques available in the clinic, and therefore do not capture the complete time course of IOP change with body position change. Krieglstein et al.2 have shown that IOP changes with different body positions, and there is a nonlinear relationship between IOP increase and body position from 60° semi-upright tilt to 30° head-down tilt when using a Perkins applanation tonometer and the Alcon Pneumatonograph (Alcon, Fort Worth, TX, USA). In these studies, IOP is measured by using snapshot, single time point measurements, and no information on the time course is presented other than measurements that are taken approximately 3 minutes apart. Lee and colleagues9 have taken measurements 5 minutes and 30 minutes after changing position, using Tonopen XL to study the effect of the LDP on IOP in healthy young subjects. Subjects switched from the seated to supine to lateral decubitus position and exhibited significant changes in IOP between the dependent eye in LDP versus supine position. Lee et al.10 have also studied the effects of different sleeping postures on IOP and ocular perfusion pressure (OPP) in 20 healthy young subjects. They have found a 1.4 mm Hg difference between eyes in the LDP and similar results in the prone position with head turned to the side, also noting that IOPs are higher in the prone position with head turned versus LDP. They also report IOP in the LDP with different head positions,11 and a difference of 1.0 and 1.3 mm Hg between eyes is found in the head-neutral LDP. Finally, they have studied the effect of LDP on IOP in patients with untreated and treated glaucoma and report differences ranging from 1.2 to 1.6 mm Hg between eyes in the LDP.12,13 Hwang and colleagues14 have found more pronounced results and report that the mean differences in IOP between the eyes in the LDP range from 2.9 to 4.1 mm Hg in 20 patients, a greater difference between eyes than reported in other studies, potentially due to the duration spent in the LDP (5 to 150 minutes) or possibly due to large interpupillary distances of 7.0 ± 0.4 cm, creating a slightly larger hydrostatic column difference between eyes. They conclude that IOP is higher in the dependent eye and that IOPs in anesthetized patients are higher than in alert patients. Carlson and colleagues15 have measured changes in IOP in response to changes in body position (±15° and ±50° tilt from horizontal) while also looking at aqueous turnover as measured by fluorophotometry. They conclude that while aqueous formation is relatively insensitive to IOP, IOP changes 2.4 ± 1.2 mm Hg for ±15° from horizontal and 11.2 ± 2.7 mm Hg for ±50° from horizontal (mean ± SD), further demonstrating the significant changes in IOP with different body positions. Malihi and Sit16 have shown that IOP is lowest when measured while sitting with the neck in the neutral position. All other head and body positions result in an elevation of IOP, compared with the seated, head-neutral position typically used in clinical practice to measure IOP. Furthermore, this study also confirms a difference between eyes in the LDP. Eklund et al.3 have studied the postural influence on simultaneously measured IOP and intracranial pressure (ICP) and report similar results on IOP changes with body position in the sitting and supine positions. They have calculated the trans–lamina cribrosa pressure difference (TLCPD) in different body positions by subtracting ICP from IOP. ICP (in millimeters of mercury) is lowest in the sitting position (−0.8 ± 3.8), resulting in the largest TLCPD as compared to the supine and head-down tilt positions (19.8 vs. 12.3 [supine] and 6.6 [head-down tilt]).3
There are no reports of IOP changes in body position, using continuous IOP measurement techniques that can accurately assess both the magnitude and time course of these changes. All previous studies report IOP changes with body position, using applanation or rebound tonometry, which can have an inherent measurement error of up to 3 mm Hg from true IOP, although longitudinal measurements within eyes should capture relative IOP change. Many clinical IOP measurement devices such as Goldmann cannot be used other than when the patients are in seated position. Although the Tonopen can estimate IOP in different positions, it too has an inherent IOP measurement variation of 2 to 3 mm Hg,4 which may not be accurate enough to measure even relative changes in IOP with certain body positions. The Perkins handheld applanation tonometer can also be used in different body positions, but with the same measurement reproducibility of GAT.5 The single time point (snapshot) measurement techniques used in previous studies provide no IOP data during the time gaps between measurements, and can be inaccurate when performed in awake patients whose IOPs could be affected by second-to-second changes in ocular muscle tone.6,7
Other studies have reported changes in IOP with body position in humans, using applanation tonometry (Krieglstein et al.,2 Carlson et al.,15 and others), but only provide periodic instantaneous measurements at each body position, using tonometers with 1 to 3 mm Hg of inherent measurement error.2,3,9,10,12,15,16 The time course of IOP change immediately after body position change has not been addressed in prior work. Our results showed that IOP asymmetry in LDP in NHPs is similar to the results reported for humans.9–12,16 We reported results for additional body positions (prone, seated, standing, and inverted) that provided additional context and supporting data that are helpful in elucidating the mechanisms underlying these changes. In addition, results reported herein indicate that IOP changes very rapidly with body position, generally within 4 to 15 seconds; those changes persist for long periods in LDP (Fig. 8), similar to previously reported results in humans.14
Figure 8.
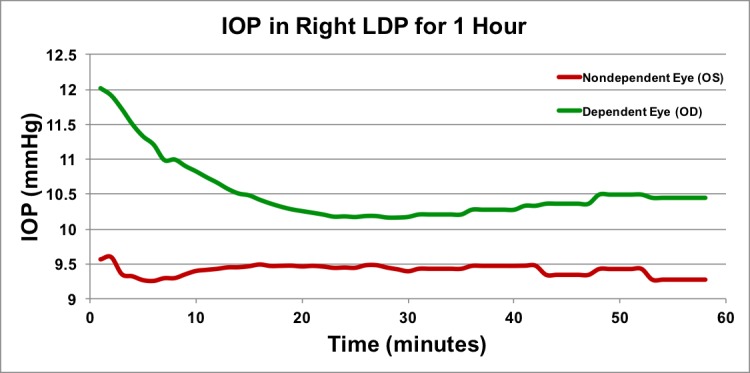
Long duration time course of IOP in the lateral decubitus position. Approximately 1 hour of IOP data in the LDP in one animal in one session. Data points are shown as 1-minute averages for first 30 minutes, and as 5-minute averages during the second 30 minutes. To keep the animal sedated for an hour, isoflurane maintenance was performed; this may have generated a temporary rise and fall in the first few minutes of data acquisition.
Possible mechanisms underlying the reported IOP changes with body position include synchronous changes in venous and arterial circulation. The Goldmann Equation states that IOP is a function of aqueous production, outflow facility, and the episcleral venous pressure (EVP). Significant changes in aqueous production or outflow facility are not likely to occur on the timescale of the IOP changes with body position reported herein. Hence, the most likely mechanism underlying IOP changes with body position is acute, hydrostatic changes in EVP and/or choroidal volume due to changes in the eye-to-heart distance. Most studies point toward EVP changes as the principle mechanism behind changes in conventional aqueous outflow resistance and IOP on short timescales,17–19 although choroidal engorgement has also been implicated as a possible mechanism for transient IOP changes.16 Only one study in rabbits has investigated this relationship carefully, wherein EVP was measured via direct vessel cannulation while changing body positions. In rabbits, EVP and IOP changes due to body tilt are identical, although EVP and IOP responses to tilt do not seem to be passive in response to eye-to-heart height.17
The notion of active EVP autoregulation and/or a functional EVP floor (or lower limit) is supported by two observations apparent in our results. First, the magnitudes of change in IOP when body position is changed from supine to prone, seated, or standing should be much larger than we measured if EVP changes solely on the basis of eye-to-heart height. The eye-to-heart or eye-to-hydrostatic indifference point height differential between the supine and seated/standing positions is ∼21 cm in NHPs, which translates to 15.5 mm Hg EVP differential (21 cm water = 15.5 mm Hg), but we measured only ∼2 mm Hg IOP difference between the supine and the seated/standing/prone positions. Also, the eye-to-heart height differential is approximately 11 cm between the supine and prone positions, equating to ∼8 mm Hg EVP differential, and yet IOP only changed ∼2 mm Hg when body position was changed from supine to prone (Fig. 4).
Second, if IOP change with body position is driven by EVP alone, and the EVP is driven solely by the eye-to-heart height differential, then we would expect IOP to be much lower in the seated and standing positions than in the prone position because the eye-to-heart height differentials are very different between the supine-to-prone (11 cm) and the supine-to-seated/standing (21 cm) positions. We would also expect that the IOP change when body position is changed from supine to seated/standing would be much larger than when position is changed from supine to prone (Fig. 6); IOP change was ∼2 mm Hg when changing from supine to either prone or seated/standing. Hence, our data suggest that once the eye is approximately 7.5 cm above the heart (or the hydrostatic indifference point), EVP does not decrease further as eye-to-heart height increases further, which limits the IOP changes to ∼2 mm Hg as compared to supine. Thus, our data suggest that EVP has a functional lower limit, and therefore must be actively regulated.
Consideration of the prone position in terms of eye-to-heart height alone bears discussion. In this study, the animal's neck is in full flexion in the prone position (chin resting on padded table in face-forward position), but the neck is straight in the supine, seated, and standing positions, which may lead to different positional venous flow characteristics. The animal was also lying on its chest in the prone position, which could create additional pressure on the veins in the thorax. These two factors may contribute to the EVP and IOP in the prone position, so this result should be confirmed in future experiments wherein IOP is measured when the neck is straight for all body positions. Other mechanisms other than EVP could contribute to IOP stability when eye-to-heart height is >7.5 cm, such as pressure-dependent compression in the conventional outflow pathway or rapid choroidal volume change in response to body position. However, these mechanisms do not seem likely to come into play in this scenario given the relatively small ocular volume change associated with Schlemm's canal compression20 and the choroidal flux/volume autoregulation measured in previous physiology studies.21
In the LDP, IOP asymmetry varied greatly both between animals, and by position depending on whether the animal was in the left or right LDP (Fig. 3). There is no obvious explanation for these differences, but we speculate they may be due to animal- or eye-specific differences in EVP and/or outflow facility that are revealed only when acute differential IOPs are analyzed.
Our study is limited by the following considerations. First, our cohort size was limited to only three animals, and the reported results may not represent the wider population. However, our results were very consistent both between animals and within eyes over five sessions in each NHP, and statistical analyses showed significant differences in IOP by body position in spite of our limited cohort size. Second, results in NHPs may not represent the effects that would be present in humans owing to differences in body size and eye-to-heart distances. That said, the data from NHPs presented herein are similar to the limited data available from human studies using periodic, single time point IOP measurement techniques,2,3,9,10,12,15,16 and the relative differences between positions within and between NHPs are valid. Third, the study was performed while the animals were under anesthesia. It is possible that anesthesia affects IOP and/or EVP. For the current study, individual measurements of body position effects on IOP were obtained within 3 minutes, including supine baseline IOP measurement, and measurement of subsequent IOP after body position change. This is a short period during which anesthesia-related effects on IOP or EVP are unlikely to manifest. Hence, while it is possible that anesthesia has some effect on IOP and/or EVP, those effects are not likely to impact our results or conclusions. We also report differences relative to the seated position in Figure 4, which is clinically more applicable in terms of standard clinical IOP measurement position, although again, these measures may not reflect human responses due to body size differences.
Previous clinical trials have indicated that IOP is a major risk factor for visual field damage in glaucoma at all baseline IOPs. This epidemiologic result is not necessarily applicable to individual patients however, as patients have exhibited a wide range of eye-specific susceptibility to IOP-related glaucomatous damage. Cohort studies have indicated that glaucomatous visual field loss is significantly larger in the dependent eye of patients who report sleeping in the LDP on one side more than the other, a result that is clinically translatable to the individual. Our results suggest that the IOP in the dependent eye is ∼1 mm Hg higher than the IOP in the nondependent eye, which suggests that relatively small differences (or increases) in IOP, if persistent, could possibly be injurious in glaucoma.
Conclusions
We can conclude that (1) body position had a significant effect on IOP, and (2) IOP change occurred very rapidly after positional change, and those changes persisted over time in the LDP. Further, IOP differences followed changes in eye-to-heart height from −23 cm to +7.5 cm; body position changes in which eye-to-heart height is increased above +7.5 cm did not elicit additional significant IOP change. This indicates that EVP has a functional floor wherein EVP does not decrease further when eye-to-heart distance increases above approximately +7.5 cm, and/or there are other mechanisms such as pressure-dependent tissue motion in the conventional outflow pathway or rapid choroidal volume change that contribute to IOP stability when eye-to-heart height is >7.5 cm.
Acknowledgments
The authors thank Lisa Hethcox for her help in all aspects of the animal experiments, Chester Calvert for help in IOP data filtering and processing, and Ryan Whitley for his work in database programming and data retrieval.
Presented in part at the annual meeting of the Association for Research in Vision and Ophthalmology, Orlando, Florida, United States, May 2014.
Supported by National Institutes of Health Grants R01-EY024732 (JCD), R01-EY026035 (JCD), P30-EY003039 (UAB NEI Core Facilities Grant); EyeSight Foundation of Alabama, Birmingham, Alabama; Research to Prevent Blindness, New York, New York, United States.
Disclosure: D.C. Turner, None; B.C. Samuels, None; C. Huisingh, None; C.A. Girkin, None; J.C. Downs, None
References
- 1. Kim KN, Jeoung JW, Park KH, Kim DM, Ritch R. . Relationship between preferred sleeping position and asymmetric visual field loss in open-angle glaucoma patients. Am J Ophthalmol. 2014; 157: 739– 745. [DOI] [PubMed] [Google Scholar]
- 2. Krieglstein GK, Waller WK, Leydhecker W. . The vascular basis of the positional influence on the intraocular pressure. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1978; 206: 99– 106. [DOI] [PubMed] [Google Scholar]
- 3. Eklund A, Johannesson G, Johansson E,et al. The pressure difference between eye and brain changes with posture. Ann Neurol. 2016; 80: 269– 276. [DOI] [PubMed] [Google Scholar]
- 4. Cook JA, Botello AP, Elders A,et al. Systematic review of the agreement of tonometers with Goldmann applanation tonometry. Ophthalmology. 2012; 119: 1552– 1557. [DOI] [PubMed] [Google Scholar]
- 5. Arora R, Bellamy H, Austin M. . Applanation tonometry: a comparison of the Perkins handheld and Goldmann slit lamp-mounted methods. Clin Ophthalmol. 2014; 8: 605– 610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Coleman DJ, Trokel S. . Direct-recorded intraocular pressure variations in a human subject. Arch Ophthalmol. 1969; 82: 637– 640. [DOI] [PubMed] [Google Scholar]
- 7. Downs JC, Burgoyne CF, Seigfreid WP, Reynaud JF, Strouthidis NG, Sallee V. . 24-hour IOP telemetry in the nonhuman primate: implant system performance and initial characterization of IOP at multiple timescales. Invest Ophthalmol Vis Sci. 2011; 52: 7365– 7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Downs JC. . IOP telemetry in the nonhuman primate. Exp Eye Res. 2015; 141: 91– 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee JY, Yoo C, Jung JH, Hwang YH, Kim YY. . The effect of lateral decubitus position on intraocular pressure in healthy young subjects. Acta Ophthalmol. 2012; 90: e68– e72. [DOI] [PubMed] [Google Scholar]
- 10. Lee TE, Yoo C, Kim YY. . Effects of different sleeping postures on intraocular pressure and ocular perfusion pressure in healthy young subjects. Ophthalmology. 2013; 120: 1565– 1570. [DOI] [PubMed] [Google Scholar]
- 11. Seo H, Yoo C, Lee T-E, Lin S, Kim YY. . Head position and intraocular pressure in the lateral decubitus position. Optom Vis Sci. 2015; 92: 95– 101. [DOI] [PubMed] [Google Scholar]
- 12. Lee JY, Yoo C, Kim YY. . The effect of lateral decubitus position on intraocular pressure in patients with untreated open-angle glaucoma. Am J Ophthalmol. 2013; 155: 329– 335.e2. [DOI] [PubMed] [Google Scholar]
- 13. Lee TE, Yoo C, Lin SC, Kim YY. . Effect of different head positions in lateral decubitus posture on intraocular pressure in treated patients with open-angle glaucoma. Am J Ophthalmol. 2015; 160: 929– 936.e4. [DOI] [PubMed] [Google Scholar]
- 14. Hwang JW, Jeon YT, Kim JH, Oh YS, Park HP. . The effect of the lateral decubitus position on the intraocular pressure in anesthetized patients undergoing lung surgery. Acta Anaesthesiol Scand. 2006; 50: 988– 992. [DOI] [PubMed] [Google Scholar]
- 15. Carlson KH, McLaren JW, Topper JE, Brubaker RF. . Effect of body position on intraocular pressure and aqueous flow. Invest Ophthalmol Vis Sci. 1987; 28: 1346– 1352. [PubMed] [Google Scholar]
- 16. Malihi M, Sit AJ. . Effect of head and body position on intraocular pressure. Ophthalmology. 2012; 119: 987– 991. [DOI] [PubMed] [Google Scholar]
- 17. Lavery WJ, Kiel JW. . Effects of head down tilt on episcleral venous pressure in a rabbit model. Exp Eye Res. 2013; 111: 88– 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Linder BJ, Trick GL, Wolf ML. . Altering body position affects intraocular pressure and visual function. Invest Ophthalmol Vis Sci. 1988; 29: 1492– 1497. [PubMed] [Google Scholar]
- 19. Friberg TR, Sanborn G, Weinreb RN. . Intraocular and episcleral venous pressure increase during inverted posture. Am J Ophthalmol. 1987; 103: 523– 526. [DOI] [PubMed] [Google Scholar]
- 20. Xin C, Wang RK, Song S,et al. Aqueous outflow regulation: optical coherence tomography implicates pressure-dependent tissue motion. Exp Eye Res. 2017; 158: 171– 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kiel JW. . Choroidal myogenic autoregulation and intraocular pressure. Exp Eye Res. 1994; 58: 529– 544. [DOI] [PubMed] [Google Scholar]