Abstract
The study examined whether the benefit of deactivating stimulation sites estimated to have broad neural excitation was attributed to improved spectral resolution in cochlear implant users. The subjects' spatial neural excitation pattern was estimated by measuring low-rate detection thresholds across the array [see Zhou (2016). PLoS One 11, e0165476]. Spectral resolution, as assessed by spectral-ripple discrimination thresholds, significantly improved after deactivation of five high-threshold sites. The magnitude of improvement in spectral-ripple discrimination thresholds predicted the magnitude of improvement in speech reception thresholds after deactivation. Results suggested that a smaller number of relatively independent channels provide a better outcome than using all channels that might interact.
1. Introduction
Channel interaction has been one of the greatest obstacles that modern multichannel cochlear implant users face (Friesen et al., 2001; Fu and Nogaki, 2005). The factors that contribute to channel interaction include but are not limited to electrode impedance, electrode position relative to the modiolus, and survival pattern of the auditory nerve fibers (e.g., Long et al., 2014). High impedance, large electrode-neuron distance, or a significant loss of nerve fibers near the stimulation site, would result in an increased spread of neural excitation from the targeted tonotopic place. Forward-masked psychophysical tuning curves or forward masking patterns have been used to assess spatial selectivity of neural excitation in cochlear implant users (e.g., Boex et al., 2003; Chatterjee and Shannon, 1998; Kwon and van den Honert, 2006; Dingemanse et al., 2006; Nelson et al., 2011; McKay, 2012). These psychophysical procedures are, however, time consuming, which makes it impractical to evaluate neural excitation patterns across the whole electrode array.
Previous research has reported a potentially time efficient threshold measure that correlates with the traditional measures of spatial tuning (Zhou and Pfingst, 2016; Zhou, 2016). These studies showed that lower psychophysical detection thresholds for pulse trains at a low stimulation rate (80 pulses per second; pps) predicted narrower neural excitation. These studies have shown that the relationship between thresholds and spatial tuning is dependent on the parameters of the stimuli used for measuring thresholds. When the inter-pulse interval of the pulse train is sufficiently narrow (high stimulation rate) or stimulation repeats for a relatively long time (long stimulation duration), neural excitability reduces due to refractoriness, sub-threshold accommodation, or spike-rate adaptation (Boulet et al., 2016). Consequently, the response from a single auditory fiber does not always increase as stimulation rate or duration increases. It has been speculated that a broad spread of neural excitation might facilitate the detection of long-duration or high-rate pulse trains because the availability of a large number of excitable neurons can potentially offset the reduced excitability in any single unit. Previous results showed that the slope of the forward-masking patterns was negatively correlated with the slope of the threshold-versus-pulse-rate functions, with broader neural activation predicting a steeper threshold decrease with stimulation rate (Zhou and Pfingst, 2016). In extreme cases, it has been observed that detection thresholds for high-rate or long-duration pulse trains were lower (better) at stimulation sites with broad neural activation than those receiving narrow activation (Zhou and Pfingst, 2016; Zhou, 2016). Suffering very little from inter-pulse interactions, the low-rate detection thresholds were found to well reflect the estimated spatial neural activation patterns, with high thresholds predicting broad neural activation.
Zhou (2016) found that implant users' speech recognition improved when using low-rate thresholds to select simulation sites. The study showed that after the five stimulation sites with the highest low-rate thresholds were deactivated, subjects' speech recognition performance significantly improved in noise as well as in quiet compared to using the whole array. A control condition was also tested where five randomly chosen stimulation sites were deactivated. For the majority of the subjects, performance worsened with random deactivation when compared to using the whole array. For subjects who showed a benefit from random deactivation, the improvement was not as large as when deactivation was based on the threshold measure. The results suggested that the improvement seen with deactivation based on thresholds was not due to spatial separation between arbitrary electrodes. If the stimulation sites with the highest low-rate thresholds were indeed those that produced broad neural activation which in turn led to interaction between channels, removing those electrodes should improve spectral resolution. The present study aimed to test this hypothesis by comparing spectral-ripple discrimination thresholds (Aronoff and Landsberger, 2013) before and after the deactivation of stimulation sites with poor low-rate thresholds. It was predicted that a smaller number of relatively independent stimulation sites would provide better spectral resolution than a greater number of sites that interact. Speech recognition was also evaluated before and after deactivation. Any change in spectral-ripple discrimination thresholds due to deactivation was then compared to the corresponding change in speech recognition performance.
2. Methods
2.1. Subjects and hardware
Six postlingually deafened subjects implanted with Cochlear Americas Nucleus® devices (Cochlear Corp., Englewood, CO) participated in the study. Two of the subjects were sequentially implanted bilaterally and both ears were tested (one at a time), resulting in a total of eight ears. These subjects were a subset of subjects who participated in the Zhou (2016) study. None of the subjects had measurable residual acoustic hearing. The use of human subjects was approved by the Institutional Review Board at East Carolina University.
2.2. Psychophysics and speech testing
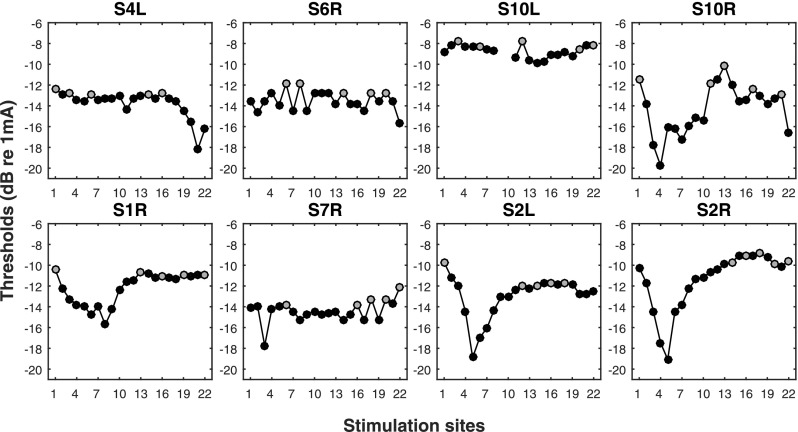
For each tested ear, the latest clinical every-day use map was downloaded from the subject's processor. An experimental map was then constructed using the subject's 80-pps threshold data published in Zhou (2016) (re-plotted in Fig. 1). In the experimental map, five stimulation sites with the highest thresholds that were not immediately next to each other were identified and deactivated from the clinical map. The maps were different from those tested in Zhou (2016), if the subject's clinical map had been updated since the participation in the Zhou study. The deactivated sites are shown in gray color in Fig. 1. Frequency allocation was automatically reassigned after deactivation. The experimental map with the deactivated stimulation sites was otherwise identical to the clinical map. The acute effect of deactivation on speech recognition was re-evaluated using the SRT (speech reception threshold) test, and the acute effect of spectral resolution was evaluated using the spectral-temporally modulated ripple test (SMRT). The testing order of the clinical and experimental maps was randomized and was kept blind to the subjects. Although subjects had significantly more experience with the clinical map, they were not able to consistently identify the order of the maps during testing. For both tests, stimuli were delivered acoustically via a loudspeaker in a double-walled sound booth.
Fig. 1.
Across-site variation in the 80-pps thresholds. The gray symbol indicates the poor-threshold stimulation sites that were deactivated in the experimental maps. This is a subset of data published in Zhou (2016).
SRT was defined as the signal-to-noise ratio (SNR) required for the subject to correctly recognize 50% of the CUNY (City University of New York) sentences (Boothroyd et al., 1985). The noise was a white noise amplitude modulated with a 100% modulation depth at 10 Hz. The level of the noise was kept at 55 dB (A), while the level of the CUNY sentences was adapted based on the subject's response following a one-down one-up rule. The subject had to repeat the entire sentence correctly for it to be counted as a correct response. The SNRs at the last six reversal points out of a total of twelve reversals were averaged and taken as the SRT. Different sentence lists were used for evaluating the clinical and experimental maps. Stimuli used in the SMRT test were non-harmonic tone complexes with spectral ripples of varying starting phases. The reference stimulus had a ripple density of 20 ripples per octave (RPO), and the probe stimulus started with a ripple density of 0.5 RPO. Using a three-interval forced choice paradigm, the number of RPO in the probe stimulus adapted based on the subject's response using a one-down one-up rule. The spectral ripple density at the last six reversal points out of a total of ten reversals was averaged and taken as the spectral-ripple discrimination threshold. Both thresholds were repeated at least twice for each map.
3. Results
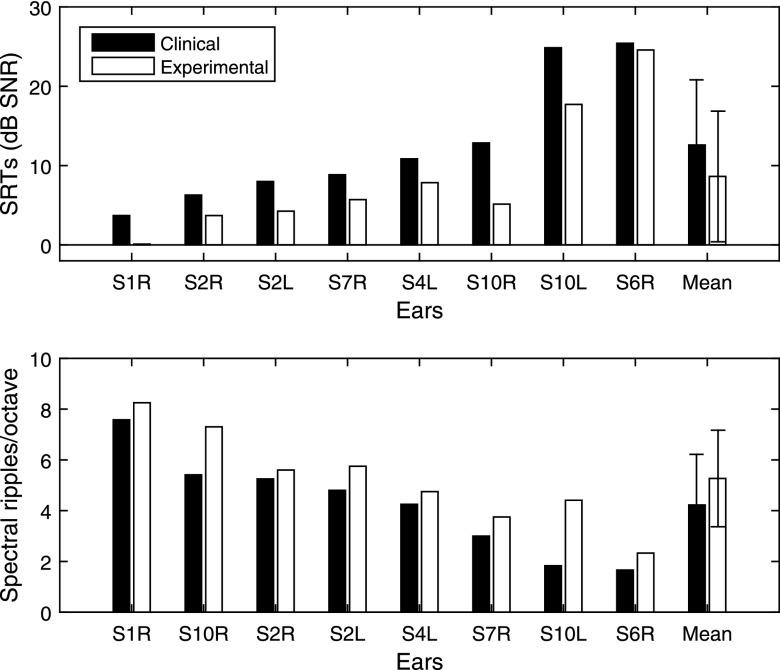
Figure 2 shows the individual SRTs and spectral-ripple discrimination thresholds with the clinical and experimental maps, rank ordered based on the subjects' SRT with the clinical map (i.e., black bar in the top panel). The mean SRT measured with the experimental map was significantly lower (better) compared to that with the subjects' clinical map [t(7) = 4.87, p = 0.001]. On average, SRT improved by 3.98 dB SNR. The magnitude of SRT improvement was not correlated with the across-site variance in the low-rate thresholds [r = −0.11, p = 0.79]. The mean spectral-ripple discrimination threshold measured using the experimental map also significantly improved (by 1.05 RPO) compared to that using the subjects' clinical map [t(7) = −3.80, p = 0.006]. Since data obtained from the two ears of the bilaterally implanted subjects might be correlated, the statistical analyses were re-run including just the left ears of S1 and S10. The differences between the clinical and the experimental maps remained significant for both the SRT [t(5) = 3.21, p = 0.02] and spectral-ripple discrimination thresholds [t(5) = −4.33, p = 0.007].
Fig. 2.
Speech reception and spectral ripple thresholds. Top panel: individual and group mean SRTs. Bottom panel: individual and group mean spectral-ripple discrimination thresholds. Performance measured using subjects' clinical map (before deactivation) is shown in black and performance measured using the experimental map with deactivation is shown in white. The thresholds were rank ordered based on the subjects' SRT scores with the clinical map (top panel black bars). Error bars represent standard deviation.
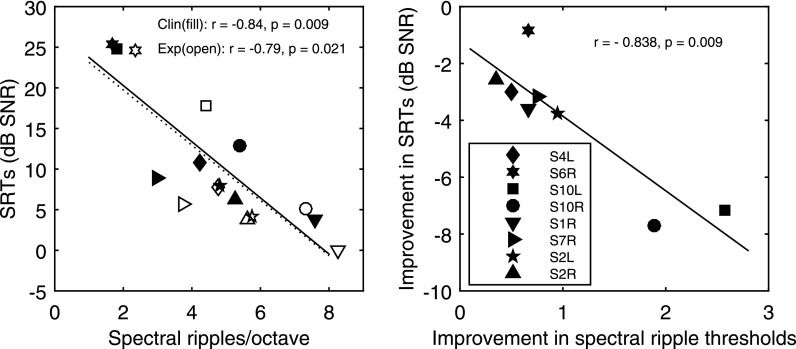
The left panel of Fig. 3 shows the scatter plot of SRTs against the spectral-ripple discrimination thresholds using the clinical map (filled symbols) overlaid on the scatter plot of the data using the experimental map (open symbols). These plots allow for a visual inspection of how speech performance changed before and after deactivation for individual subjects in relation to the corresponding change in measured spectral resolution. SRTs were significantly correlated with the spectral-ripple discrimination thresholds before [r = −0.84, p = 0.009] and after deactivation [r = −0.79, p = 0.021]. Again, including just the left ears of the bilateral subjects, the correlation remained significant before deactivation [r = −0.86, p = 0.03], and was marginally significant after deactivation [r = −0.80, p = 0.05]. The right panel shows the magnitude of improvement in spectral-ripple discrimination thresholds predicted the amount of SRT improvement as a result of deactivation. Because the magnitudes of improvement were not normally distributed for either set of thresholds, a nonparametric Spearman's rho was performed to examine the correlation between the improvements [r = −0.84, p = 0.009]. Again with just the left ears of the bilateral subjects, the correlation was marginally significant [r = −0.81, p = 0.05], which could be due to a reduced statistical power. Spearman's rho was then re-run with the removal of S10R, which was the data point with the highest leverage in the regression, and the result remained significant [r = −0.79, p = 0.03].
Fig. 3.
Correlations between SRTs and spectral-ripple discrimination thresholds. Symbols represent subjects and lines represent linear fit to the data. The left panel shows correlations between SRTs and the spectral-ripple discrimination thresholds before and after deactivation using the subjects' clinical (filled symbols and solid line) and experimental maps (open symbols and dashed line), respectively. The right panel shows the correlation between the improvement in SRTs and the spectral-ripple discrimination thresholds resulted from deactivation.
4. Discussion and conclusions
The results of the current study reproduced the benefits of deactivating stimulation sites based on low-rate thresholds for speech recognition in a subset of subjects who participated in the Zhou (2016) study. More importantly, results of the current study suggested that the improved speech recognition could be attributed to improved spectral resolution as a result of the deactivation.
Spatial selectivity of neural excitation was estimated by detection thresholds for pulse trains at a low stimulation rate (80 pps). The use of low stimulation rate ensures that thresholds minimally reflect the neural temporal response characteristics, and that the spread of neural excitation does not play a facilitative role in detection as it does for high-rate and long-duration stimuli. The benefit of electrode deactivation observed here and that reported in Zhou (2016) was not due to spatial separation between arbitrary electrodes, because Zhou (2016) showed that when five randomly chosen sites were deactivated for these subjects, performance either worsened or improved with a smaller magnitude compared to deactivation based on the threshold measure. The present data also showed that the magnitude of SRT improvement was not associated with the extent to which the thresholds varied across the sites. That is, deactivation performed on an array that showed greater across-site variation in the low-rate thresholds did not produce a greater benefit for speech recognition than deactivation from an array that showed a smaller threshold variation. Moreover, the tonotopic location of the deactivated stimulation sites also did not appear to contribute to how SRT improved after deactivation (Figs. 1 and 2).
With channel interaction, a subgroup of auditory fibers receives stimulation pulses from multiple electrodes causing spectral information to smear across places. Fu and Nogaki (2005) demonstrated that recognition of vocoded speech in a fluctuating noise measured in normal-hearing listeners was only comparable to that of the cochlear implant users when channel interaction was simulated where the analysis-band filters were manipulated to be shallowly sloped. Spatial summation of stimulation would not only cause spectral information to smear but also cause the subgroup of auditory fibers to be stimulated at a rate that is much higher than the single-electrode rate, increasing the probability of neural adaptation (Boulet et al., 2016). Because neural survival, electrode position, and other pathological variables are not homogeneous across the tonotopic axis in an implanted ear (Nadol, 1997; Long et al., 2014), some stimulation sites are expected to interact more than others. The hypothesis tested in the current study was whether removing the stimulation sites estimated to produce broad neural excitation would improve spectral resolution, which in turn would improve speech recognition. Our results showed that after deactivation of the poor-threshold stimulation sites, spectral-ripple discrimination thresholds improved relative to using the whole array. The results suggested that a smaller number of relatively independent electrodes could provide better spectral resolution than a greater number of electrodes that interact. It should be noted however that it remains to be tested whether random deactivation would produce a similar improvement in spectral resolution, although Zhou (2016) suggested that it would lead to improved speech recognition.
Previous research reported direct links between spectral resolution assessed by spectral-ripple discrimination thresholds and speech recognition in cochlear implant subjects (Won et al., 2007; Holden et al., 2016). Our results showed a similar correlation between spectral-ripple discrimination thresholds and the subjects' speech performance both before and after site deactivation, consistent with the notion that spectral resolution is an important factor for cochlear implant speech recognition outcomes. Further, our results indicated that the amount of improvement in the spectral-ripple discrimination threshold as a result of site deactivation predicted the amount of SRT improvement that the subjects could achieve with fewer but more independent channels. After deactivation, the subjects could on average discriminate a stimulus with 5.26 spectral RPO from the reference that had essentially a flat spectral envelope. This corresponded to an improvement of 1 RPO relative to that prior to deactivation. It is conceivable that with reduced spectral smearing across channels, the spectral ripples would be more easily detectable. With the ability to discriminate denser spectral ripples, listeners would be better able to discriminate spectral peaks in a denser spectrum, such as one with multiple sources of sounds. An improved spectral resolution therefore would be advantageous for extracting information at frequencies with a favorable SNR.
Several site-removal strategies have been reported in the literature. Some were based on psychophysical measures such as amplitude modulation detection thresholds (e.g., Garadat et al., 2013), and others used image-guided techniques to identify stimulation sites that produce excessive current spread (Labadie et al., 2016). The exact relationship between the low-rate threshold measure used here and those that have proven beneficial in the literature warrants future research. Current focusing is another effective method for improving spectral resolution (e.g., Berenstein et al., 2008; Smith et al., 2013), however, it remains unclear whether this method would produce similar effects on speech recognition as electrode deactivation does.
Acknowledgments
We thank our dedicated subjects who participated in the study. Work was supported by the Emerging Research Grant from Hearing Health Foundation and NIH NIDCD Grant No. R03 DC014771-01A1.
References and links
- 1. Aronoff, J. M. , and Landsberger, D. M. (2013). “ The development of a modified spectral ripple test,” J. Acoust. Soc. Am. 134, EL217–EL222. 10.1121/1.4813802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Berenstein, C. K. , Mens, L. H. , Mulder, J. J. , and Vanpoucke, F. J. (2008). “ Current steering and current focusing in cochlear implants: Comparison of monopolar, tripolar, and virtual channel electrode configurations,” Ear Hear. 29, 250–260. 10.1097/AUD.0b013e3181645336 [DOI] [PubMed] [Google Scholar]
- 3. Boex, C. , Kos, M. I. , and Pelizzone, M. (2003). “ Forward masking in different cochlear implant systems,” J. Acoust. Soc. Am. 114, 2058–2065. 10.1121/1.1610452 [DOI] [PubMed] [Google Scholar]
- 4. Boothroyd, A. , Hanin, L. , and Hnath, T. (1985). A Sentence Test of Speech Perception: Reliability, Set Equivalence, and Short Term Learning ( City University of New York, New York: ). [Google Scholar]
- 5. Boulet, J. , White, M. , and Bruce, I. C. (2016). “ Temporal considerations for stimulating spiral ganglion neurons with cochlear implants,” J. Assoc. Res. Otolaryngol. 17, 1–17. 10.1007/s10162-015-0545-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chatterjee, M. , and Shannon, R. V. (1998). “ Forward masked excitation patterns in multielectrode electrical stimulation,” J. Acoust. Soc. Am. 103, 2565–2572. 10.1121/1.422777 [DOI] [PubMed] [Google Scholar]
- 7. Dingemanse, J. G. , Frijns, J. H. , and Briaire, J. J. (2006). “ Psychophysical assessment of spatial spread of excitation in electrical hearing with single and dual electrode contact maskers,” Ear. Hear. 27, 645–657. 10.1097/01.aud.0000246683.29611.1b [DOI] [PubMed] [Google Scholar]
- 8. Friesen, L. M. , Shannon, R. V. , Baskent, D. , and Wang, X. (2001). “ Speech recognition in noise as a function of the number of spectral channels: Comparison of acoustic hearing and cochlear implants,” J. Acoust. Soc. Am. 110, 1150–1163. 10.1121/1.1381538 [DOI] [PubMed] [Google Scholar]
- 9. Fu, Q. J. , and Nogaki, G. (2005). “ Noise susceptibility of cochlear implant users: The role of spectral resolution and smearing,” J. Assoc. Res. Otolaryngol. 6, 19–27. 10.1007/s10162-004-5024-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Garadat, S. N. , Zwolan, T. A. , and Pfingst, B. E. (2013). “ Using temporal modulation sensitivity to select stimulation sites for processor maps in cochlear implant listeners,” Audiol. Neurootol. 18, 247–260. 10.1159/000351302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Holden, L. K. , Firszt, J. B. , Reeder, R. M. , Uchanski, R. M. , Dwyer, N. Y. , and Holden, T. A. (2016). “ Factors affecting outcomes in cochlear implant recipients implanted with a perimodiolar electrode array located in Scala Tympani,” Otol. Neurotol. 37, 1662–1668. 10.1097/MAO.0000000000001241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kwon, B. J. , and van den Honert, C. (2006). “ Effect of electrode configuration on psychophysical forward masking in cochlear implant listeners,” J. Acoust. Soc. Am. 119, 2994–3002. 10.1121/1.2184128 [DOI] [PubMed] [Google Scholar]
- 13. Labadie, R. F. , Noble, J. H. , Hedley-Williams, A. J. , Sunderhaus, L. W. , Dawant, B. M. , and Gifford, R. H. (2016). “ Results of postoperative, CT-based, electrode deactivation on hearing in prelingually deafened adult cochlear implant recipients,” Otol. Neurotol. 37, 137–145. 10.1097/MAO.0000000000000926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Long, C. J. , Holden, T. A. , McClelland, G. H. , Parkinson, W. S. , Shelton, C. , Kelsall, D. C. , and Smith, Z. M. (2014). “ Examining the electro-neural interface of cochlear implant users using psychophysics, CT scans, and speech understanding,” J. Assoc. Res. Otolaryngol. 15, 293–304. 10.1007/s10162-013-0437-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McKay, C. M. (2012). “ Forward masking as a method of measuring place specificity of neural excitation in cochlear implants: A review of methods and interpretation,” J. Acoust. Soc. Am. 131, 2209–2224. 10.1121/1.3683248 [DOI] [PubMed] [Google Scholar]
- 16. Nadol, J. B., Jr. (1997). “ Patterns of neural degeneration in the human cochlea and auditory nerve: Implications for cochlear implantation,” Otolaryngol. Head Neck Surg. 117, 220–228. 10.1016/S0194-5998(97)70178-5 [DOI] [PubMed] [Google Scholar]
- 17. Nelson, D. A. , Kreft, H. A. , Anderson, E. S. , and Donaldson, G. S. (2011). “ Spatial tuning curves from apical, middle, and basal electrodes in cochlear implant users,” J. Acoust. Soc. Am. 129, 3916–3933. 10.1121/1.3583503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Smith, Z. M. , Parkinson, W. S. , and Long, C. J. (2013). “ Multipolar current focusing increases spectral resolution in cochlear implants,” Conf. Proc. IEEE Eng. Med. Biol. Soc. 2013, 2796–2799. [DOI] [PubMed] [Google Scholar]
- 19. Won, J. H. , Drennan, W. R. , and Rubinstein, J. T. (2007). “ Spectral-ripple resolution correlates with speech reception in noise in cochlear implant users,” J. Assoc. Res. Otolaryngol. 8, 384–392. 10.1007/s10162-007-0085-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhou, N. (2016). “ Monopolar detection thresholds predict spatial selectivity of neural excitation in cochlear implants: Implications for speech recognition,” PLoS One 11, e0165476. 10.1371/journal.pone.0165476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhou, N. , and Pfingst, B. E. (2016). “ Evaluating multipulse integration as a neural-health correlate in human cochlear-implant users: Relationship to spatial selectivity,” J. Acoust. Soc. Am. 140, 1537–1547. 10.1121/1.4962230 [DOI] [PMC free article] [PubMed] [Google Scholar]