Abstract
Introduction:
Renal cell carcinoma (RCC) is the most common malignancy to metastasize to the thyroid gland. The aims of this study are as follows: (1) to analyze the clinical characteristics of patients with thyroid involvement of RCC and (2) in patients with RCC thyroid metastasis, to determine whether RCC metastasis to glandular organs only portends a better prognosis compared with other patterns of RCC metastasis.
Methods:
Patients from Wake Forest Baptist Medical Center (WFBMC) diagnosed with thyroid metastasis from RCC were identified and medical records retrospectively examined. A systematic review of the literature for cases of RCC involving the thyroid gland was also performed. The clinical characteristics of the institutional cohort and the cases from the literature review were compared. Descriptive statistical analysis was performed, and overall survival (OS) was summarized using Kaplan-Meier methods.
Results:
The median OS for the WFBMC cohort was 56.4 months. In the literature review cohort, OS of patients with RCC thyroid metastasis was 213.6 months, and there was no statistically significant survival difference based on the site of metastasis. Median survival after thyroid metastasis from RCC for the WFBMC and literature cohort was 21.6 and 45.6 months, respectively.
Conclusions:
Metastatic RCC should be included in the differential of a new thyroid mass. Treatment directed at the thyroid metastasis results in prolonged survival in some cases. Further analysis into the genomic differences and mechanisms of thyroid metastasis is warranted.
Keywords: Renal cell carcinoma, thyroid gland, metastasis, cancer, kidney, glandular metastasis
Introduction
Cancer remains the second leading cause of death in the United States, although current trends show the incidence of many cancers are declining.1 The incidence of renal cell carcinoma (RCC), however, has been rising steadily, increasing 2% to 4% per year.2 Renal cell carcinoma is the most common type of kidney cancer and is the seventh most common cancer overall among men.1 Renal cell carcinoma is curable with surgery, but many organs including the lungs, liver, bone, and the thyroid gland have the propensity to house metastatic cells from the kidney. Approximately one-third of patients will have metastatic disease at diagnosis and a quarter of patients will develop metastasis after curative-intent nephrectomy. Unfortunately, despite numerous therapeutic advances in recent years, the prognosis for metastatic RCC is poor, with a median survival of only about 2 years and a 5-year survival rate of less than 10%.3 Early detection of RCC is paramount, but few patients present with recognizable symptoms, making an early diagnosis difficult.
Metastasis to the thyroid is relatively rare, despite its rich blood supply. Renal cell carcinoma, however, is one of the more common types of neoplasms to metastasize to the thyroid.4,5 Little is understood about the mechanism of metastasis or the potentially advantageous environment of the thyroid for RCC cells. Further barriers to understanding this relationship arise from the stochastic nature of disease progression, as some patients present with RCC and no signs of thyroid involvement until years later, whereas others present with thyroid metastasis before clinically appreciable kidney cancer.4 On a genomic level, there is mutational heterogeneity between the primary tumor and the metastatic sites.6 Furthermore, prognostic implications after thyroid metastasis are unclear. Studies attempting to determine prognosis after thyroid involvement have been limited by low numbers of patients and a variety of treatment approaches.5,7–11
In an effort to better characterize this patient population, we conducted a retrospective study analyzing patients with pathologically confirmed RCC metastasis to the thyroid gland at Wake Forest Baptist Medical Center (WFBMC). In addition, we performed a systematic review of the literature to identify additional cases of thyroid metastases from RCC. We hypothesized that patients with RCC metastasis to the thyroid gland would have improved survival compared with patients with other patterns of metastasis.
Materials and Methods
Institutional cohort
To capture all patients with RCC metastasis to the thyroid at WFBMC, we obtained Institutional Review Board (IRB) approval and searched the cancer registry and hospital tumor bank back to 2005. Our initial query produced 11 patients. After reviewing the patient charts, 4 cases were excluded as they had suggestive imaging but no pathologic confirmation of thyroid metastasis. From the remaining 7 patients, data were extracted regarding demographics, pathology, time course of disease and metastasis, site of metastasis, laboratory results, family history of cancer, and cancer treatment. Descriptive statistics were used summarize the WFBMC cohort. Overall survival (OS) was summarized using Kaplan-Meier methods.
Literature review cohort
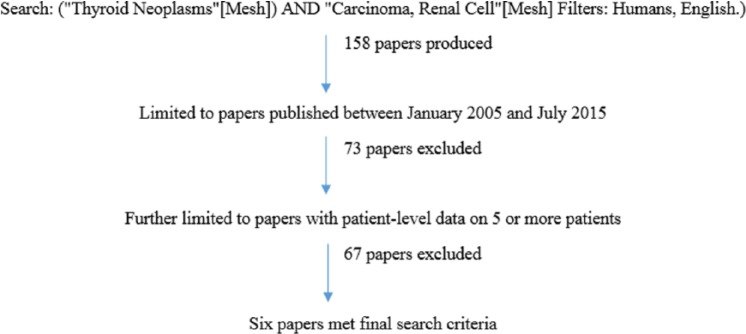
As shown in Figure 1, we conducted a literature search using PubMed with the following search criteria: (“Thyroid Neoplasms”[Mesh]) AND “Carcinoma, Renal Cell”[Mesh] Filters: Humans, English). The original search produced 158 papers. The search results were further limited to papers published between January 2005 and July 2015, to capture data from patients in the era of vascular endothelial growth factor–directed therapy. This excluded 73 papers. Bibliographies of all reviewed articles were searched to identify additional relevant titles. Only studies with 5 or more patients were included to limit reporting bias. Furthermore, only studies that reported patient-level data such as age at nephrectomy, interval between nephrectomy and thyroid metastasis, and OS were included. Applying these criteria, only 6 papers remained, reporting on a total of 72 patients.5,7–11 Descriptive statistics were used summarize the findings. The Kaplan-Meier method was used to calculate OS.
Figure 1.
PubMed search strategy for papers describing at least 5 patients with renal cell carcinoma metastasis to the thyroid gland.
Results
Institutional cohort
Seven patients with pathologically confirmed RCC metastasis to the thyroid gland were identified from the institutional query, 5 men and 2 women. Of all the patients, 6 were white, 1 was African American. Three patients had a first-degree relative with cancer, although none had cancer of the kidney or the thyroid gland. Two patients had a family history of nonneoplastic thyroid disease (hypothyroidism). Four patients underwent systemic therapy for treatment of RCC, and 5 of the 7 patients had either partial or total thyroidectomy after metastasis. The age range at initial RCC diagnosis for these patients was 39 to 61 years, with the average age being 52.4 years. The median interval between RCC diagnosis and thyroid metastasis was 58.1 months. Two patients had metastasis exclusively to glandular organs (adrenal, thyroid), whereas the others had metastasis to both glandular and nonglandular organs. All but one patient had metastasis to organs other than the thyroid. The median follow-up time from date of RCC diagnosis to date of last follow-up (n = 5) or death (n = 2) was 53 months.
The median OS for the WFBMC cohort was 56.4 months (95% confidence interval [CI]: 0.6-10.5). Median survival after RCC metastasis to the thyroid was 21.5 months. Overall survival for patients with glandular-only metastasis was prolonged compared with those with thyroid plus nonglandular metastasis; however, this was skewed by one outlier in a very small sampling of patients. That patient was a white woman who was diagnosed with RCC at age 39 and thyroid metastasis occurred 14.4 years later. When she was younger, she had been diagnosed with and treated for acute lymphoblastic leukemia. Her RCC treatment course included thyroidectomy and adrenalectomy with no systemic therapy. She was the only patient with the Xp11 translocation variant of RCC in the cohort. Translocation carcinoma is thought to be a more aggressive RCC variant12; however, she continues to do well, suggesting that location of metastasis may be more predictive than molecular subtype in some cases. Table 1 provides patient-level data from the WFBMC cohort.
Table 1.
Description of patients with RCC metastasis to the thyroid gland from WFBMC.
| Clinical characteristics of WFBMC cohort | |||||||
|---|---|---|---|---|---|---|---|
| Patient | Age (at RCC diagnosis) | Gender | Time from RCC diagnosis to thyroid metastasis, mo | Sites of metastasis | OS (from RCC diagnosis—mo) | Treatment | Status |
| 1 | 61 | M | 45 | Thyroid, lung | 56 | Thyroidectomy | Alive |
| 2 | 50 | M | 17 | Thyroid, brain | 38 | No thyroid treatment, radiation (brain), sorafenib, sunitinib, temsirolimus | Deceased |
| 3 | 57 | F | 2 | Thyroid, lung, pelvis | 7 | Radiation to thyroid, radiation to pelvis, temsirolimus, sunitinib | Deceased |
| 4 | 58 | M | 99 | Thyroid, lung, adrenal | 126 | Thyroidectomy, sunitinib, pazopanib, temsirolimus | Alive |
| 5 | 53 | M | 17 | Thyroid, pituitary, colon, lung, cavernous sinus | 42 | Thyroidectomy, radiation (clivus), pazopanib | Alive |
| 6 | 49 | M | 54 | Thyroid | 69 | Thyroidectomy, radiation (right flank) | Alive |
| 7 | 39 | F | 173 | Thyroid, adrenal | 195 | Thyroidectomy | Alive |
Abbreviations: WFBMC, Wake Forest Baptist Medical Center; OS, overall survival; RCC, renal cell carcinoma.
First metastasis bolded if known.
Literature review cohort
The systematic review resulted in 6 papers with a total of 72 unique patients with RCC thyroid metastasis. The average age at the time of nephrectomy (which in many patients signified RCC diagnosis) was 56.8 years. The average age at the occurrence of thyroid metastasis was 65.1 years. The median interval between initial RCC diagnosis and thyroid metastasis was 9.7 years. Overall survival for the entire cohort after RCC diagnosis was 17.8 years (95% CI: 13.1-19.5). Overall survival for patients with glandular-only metastasis was 18.8 years (n = 13) and OS for patients with thyroid and nonglandular metastasis was 19.5 years (n = 11), a nonsignificant difference. Median survival after RCC metastasis to the thyroid was 45.6 months or 3.8 years (95% CI: 2.0-5.2).
A comparison of age, sex, interval between RCC diagnosis and thyroid metastasis, as well as survival data between the WFBMC and literature cohorts is shown in Table 2. There was no statistically significant difference in survival between patients with glandular-only vs nonglandular sites of RCC metastasis.
Table 2.
Characteristics of patients with RCC metastatic to the thyroid gland from the institutional and literature cohorts.
| Comparison of clinical characteristics and survival between WFBMC cohort and literature review cohort | ||
|---|---|---|
| WFBMC cohort N = 7 | Literature review cohort N = 72 | |
| Median age at RCC diagnosis, y | 52.4 | 56.8 |
| Gender (M:F), % | 71.5: 28.5 | 42: 58 |
| Interval between RCC diagnosis and thyroid metastasis, y | 4.8 | 9.7 |
| OS: entire cohort, y | 4.7 | 17.8 |
| OS: glandular-only metastasis, y | 11.0 | 18.8 |
| OS: nonglandular metastasis, y | 3.5 | 19.5 |
| OS: after thyroid metastasis, y | 1.8 | 3.8 |
Abbreviations: WFBMC, Wake Forest Baptist Medical Center; OS, overall survival; RCC, renal cell carcinoma.
Discussion
Although metastasis to the thyroid remains uncommon, an increasing number of cases are being reported of nonthyroid cancer found in the thyroid gland. Renal cell carcinoma remains the most common cancer to metastasize to the thyroid, with nearly 25% of the cases of metastatic disease in the thyroid attributed to RCC. This can be difficult to identify, as thyroid metastasis in our data set occurred years after initial RCC diagnosis and in some cases after a prolonged disease-free interval, such as the patient case reported above. Furthermore, up to a quarter of patients with RCC treated with a tyrosine kinase inhibitor (TKI) develop thyroid dysfunction, related not to metastasis but instead due to changes in thyroid hormone metabolism and possibly also gland architecture.13 The interplay between thyroid metastasis and TKI-related thyroid dysfunction is not known. Treatment choice and timing of treatment relative to thyroid metastasis was highly variable in both our institutional cohort and in the literature, and thus, this study cannot address this question. Nonetheless, because surgical removal may be beneficial, it is important to consider thyroid metastasis in the differential diagnosis for any patient with a history of RCC and a thyroid nodule.
Metastatic RCC is not classically associated with thyroid involvement, thus studies evaluating this relationship have been limited to case reports and small cohorts. The institutional cohort presented here represents the largest cohort of RCC thyroid metastasis from the United States in the TKI era (2005 onward). Despite our more contemporary cohort, a single-center European cohort of 34 patients with RCC thyroid metastasis identified between 1978 and 2007 similarly reports a median of 6.5 years between initial RCC diagnosis and thyroid metastasis, and in some cases, prolonged survival after thyroid metastasis.14 Many other publications that include RCC thyroid metastasis focus on the types of cancers that metastasize to the thyroid gland, not the clinical characteristics of the patients with these cancers.15 For example, of more than a thousand patients undergoing thyroid surgery at Memorial Sloan Kettering Cancer Center from 1986 to 2005, 21 patients with thyroid metastasis were identified. Of these 21 patients, 10 had RCC, highlighting that solid tumor metastasis to the thyroid is extremely rare but most likely to be from RCC.16 Still other studies have been broad in their approach, for example, investigating RCC metastasis to the head and neck region but not specifically to the thyroid gland17 or investigating RCC metastasis to glandular organs in general.18 Renal cell carcinoma metastasis to the adrenal gland19 and to the pancreas20 has been more thoroughly explored than RCC metastasis to the thyroid, likely in part due to ease of detection of metastasis to these intra-abdominal sites on routine surveillance imaging. Although limited by its retrospective nature and small sample size, the data from the WFBMC cohort of patients with RCC metastasis to the thyroid gland are generally consistent with the data found in the literature. A detailed comparative analysis of the clinical characteristics between the institutional cohort and the patients in the literature, however, was not possible due to small numbers and incomplete or inconsistent data reported.
Why RCC has a propensity to metastasize to the thyroid is not understood. The thyroid gland is very vascular, and it has been suggested that disruption of the blood flow by goiter or thyroiditis predisposes for deposition of metastatic cells.21,22 In contrast, on-treatment thyroid dysfunction has been associated with improved outcomes, although inconsistently.23,24 There may be an explanation to be found in the genotypic analysis of RCC, as intratumor heterogeneity in RCC has been well described.6 Molecular analysis of RCC metastasis to the thyroid may elucidate specific tumor characteristics, and future studies comparing thyroid metastasis with the primary tumors will likely be revealing. Furthermore, there may be an underlying molecular tropism of RCC for glandular organs, especially the thyroid gland, or there may be an advantageous microenvironment in glandular organs for RCC cells. A recent retrospective study compared 138 patients with glandular RCC metastasis to 420 patients with nonglandular RCC metastasis and demonstrated in multivariate analysis that glandular metastasis was an independent prognostic factor for survival.18 Thus, further exploration into mechanisms of glandular metastasis is warranted.
Surgical resection of metastatic cancer is a common treatment option for oligometastatic disease and is often considered in patients who develop metastasis after nephrectomy or as an alternative treatment when systemic therapy fails.18,25,26 Prolonged survival has been suggested in patients with thyroidectomy after diagnosis of metastatic disease from any primary neoplasm.18 From a cohort of 97 patients, 41 had surgical excision of the thyroid after metastatic disease was diagnosed. The median survival for patients who underwent thyroid resection or resection with adjuvant therapy was 30 months, compared with 12 months in those without thyroid surgery (P = .09). Although not statistically significant, this does suggest that for patients with isolated metastasis to the thyroid, surgical excision should be considered.26 Furthermore, Alt et al reported that complete resection of all metastatic RCCs coincided with an improved cancer-specific survival of 4.3 years versus 1.3 years in those without complete resection. Interestingly, complete resection led to improved cancer-specific survival in patients who had >3 metastatic RCC lesions, regardless of whether they were synchronous or asynchronous.25 Prolonged survival after diagnosis of thyroid metastasis suggests that surgery on isolated metastasis may be warranted and necessitates further analysis. This is additionally supported by reports of prolonged survival after surgery for isolated pancreatic and adrenal metastasis from RCC.27,28 Although we did not compare outcomes of patients undergoing metastatectomy with those receiving systemic therapy alone in this analysis, we suggest that thyroidectomy be strongly considered in patients with metastatic disease to the thyroid.
Conclusions
The goal of this work was to describe clinical characteristics of patients with RCC metastatic to the thyroid gland. Due to an unexpectedly small institutional cohort and relatively little published data on this subject, the hypothesis that patients with RCC metastatic to the thyroid has longer survival than those with other patterns of metastasis could not be confirmed. However, the 7 patients described here make this the largest published cohort of patients with thyroid RCC metastases from the United States in the TKI era. Moving forward, it will be important to describe not only the clinical characteristics of the patients but also the molecular and genetic characteristics of both the primary tumor and the metastatic sites.
Footnotes
Author Contributions: GJ performed the literature search and patient data extraction. GJ and RLB analyzed and interpreted the data in the context of the literature. NF provided statistical analysis. All authors read and approved the final manuscript.
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work is supported by the Wake Forest Baptist Comprehensive Cancer Center’s NCI Cancer Center Support Grant P30CA012197.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. [DOI] [PubMed] [Google Scholar]
- 2. King SC, Pollack LA, Li J, King JB, Master VA. Continued increase in incidence of renal cell carcinoma, especially in young patients and high grade disease: United States 2001 to 2010. J Urol. 2014;191:1665–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Choueiri TK, Motzer RJ. Systemic therapy for metastatic renal-cell carcinoma. N Engl J Med. 2017;376:354–366. [DOI] [PubMed] [Google Scholar]
- 4. Heffess CS, Wenig BM, Thompson LD. Metastatic renal cell carcinoma to the thyroid gland: a clinicopathologic study of 36 cases. Cancer. 2002;95:1869–1878. [DOI] [PubMed] [Google Scholar]
- 5. Machens A, Dralle H. Outcome after thyroid surgery for metastasis from renal cell cancer. Surgery. 2010;147:65–71. [DOI] [PubMed] [Google Scholar]
- 6. Gerlinger M, Rowan AJ, Horswell S, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366:883–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bula G, Waler J, Niemiec A, Trompeta J, Steplewska K, Gawrychowski J. Unusual malignant thyroid tumours—a clinical study of 20 cases. Acta Chir Belg. 2008;108:702–707. [DOI] [PubMed] [Google Scholar]
- 8. Calzolari F, Sartori PV, Talarico C, et al. Surgical treatment of intrathyroid metastases: preliminary results of a multicentric study. Anticancer Res. 2008;28:2885–2888. [PubMed] [Google Scholar]
- 9. Cichon S, Anielski R, Konturek A, Barczynski M, Cichon W. Metastases to the thyroid gland: seventeen cases operated on in a single clinical center. Langenbecks Arch Surg. 2006;391:581–587. [DOI] [PubMed] [Google Scholar]
- 10. Iesalnieks I, Trupka A, Raab M, et al. Renal cell carcinoma metastases to the thyroid gland-8 cases reported. Thyroid. 2007;17:49–52. [DOI] [PubMed] [Google Scholar]
- 11. Iesalnieks I, Winter H, Bareck E, et al. Thyroid metastases of renal cell carcinoma: clinical course in 45 patients undergoing surgery. Assessment of factors affecting patients’ survival. Thyroid. 2008;18:615–624. [DOI] [PubMed] [Google Scholar]
- 12. Malouf GG, Camparo P, Molinie V, et al. Transcription factor E3 and transcription factor EB renal cell carcinomas: clinical features, biological behavior and prognostic factors. J Urol. 2011;185:24–29. [DOI] [PubMed] [Google Scholar]
- 13. Szymanski L, Matak D, Bartnik E, Szczylik C, Czarnecka AM. Thyroid hormones as renal cell cancer regulators. J Signal Transduct. 2016;2016:1362407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Beutner U, Leowardi C, Bork U, et al. Survival after renal cell carcinoma metastasis to the thyroid: single center experience and systematic review of the literature. Thyroid. 2015;25:314–324. [DOI] [PubMed] [Google Scholar]
- 15. Nakhjavani MK, Gharib H, Goellner JR, van Heerden JA. Metastasis to the thyroid gland. A report of 43 cases. Cancer. 1997;79:574–578. [DOI] [PubMed] [Google Scholar]
- 16. Nixon IJ, Whitcher M, Glick J, et al. Surgical management of metastases to the thyroid gland. Ann Surg Oncol. 2011;18:800–804. [DOI] [PubMed] [Google Scholar]
- 17. Bernicker EH, Khuri FR, Ellerhorst JA, et al. A case series of 65 patients with renal cell cancer presenting with metastases to the head and neck region. Am Soc Clin Oncol Proc. 1997;16:A1171. [Google Scholar]
- 18. Gravis G, Chanez B, Derosa L, et al. ; Renal Cross Channel Group. Effect of glandular metastases on overall survival of patients with metastatic clear cell renal cell carcinoma in the antiangiogenic therapy era. Urol Oncol. 2016;34:167.e117-123e117. [DOI] [PubMed] [Google Scholar]
- 19. Siemer S, Lehmann J, Kamradt J, et al. Adrenal metastases in 1635 patients with renal cell carcinoma: outcome and indication for adrenalectomy. J Urol. 2004;171:2155–2159; discussion 2159. [DOI] [PubMed] [Google Scholar]
- 20. Grassi P, Doucet L, Giglione P, et al. Clinical impact of pancreatic metastases from renal cell carcinoma: a multicenter retrospective analysis. PLoS ONE. 2016;11:e0151662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Linton RR, Barney JD. Metastatic hypernephroma of the thyroid gland. Surg Gynecol Obstet. 1946;83:493–498. [PubMed] [Google Scholar]
- 22. Chung AY, Tran TB, Brumund KT, Weisman RA, Bouvet M. Metastases to the thyroid: a review of the literature from the last decade. Thyroid. 2012;22:258–268. [DOI] [PubMed] [Google Scholar]
- 23. Schmidinger M, Vogl UM, Bojic M, et al. Hypothyroidism in patients with renal cell carcinoma: blessing or curse? Cancer. 2011;117:534–544. [DOI] [PubMed] [Google Scholar]
- 24. Nearchou A, Valachis A, Lind P, Akre O, Sandstrom P. Acquired hypothyroidism as a predictive marker of outcome in patients with metastatic renal cell carcinoma treated with tyrosine kinase inhibitors: a literature-based meta-analysis. Clin Genitourin Cancer. 2015;13:280–286. [DOI] [PubMed] [Google Scholar]
- 25. Alt AL, Boorjian SA, Lohse CM, Costello BA, Leibovich BC, Blute ML. Survival after complete surgical resection of multiple metastases from renal cell carcinoma. Cancer. 2011;117:2873–2882. [DOI] [PubMed] [Google Scholar]
- 26. Hegerova L, Griebeler ML, Reynolds JP, Henry MR, Gharib H. Metastasis to the thyroid gland: report of a large series from the Mayo Clinic. Am J Clin Oncol. 2015;38:338–342. [DOI] [PubMed] [Google Scholar]
- 27. Tosoian JJ, Cameron JL, Allaf ME, et al. Resection of isolated renal cell carcinoma metastases of the pancreas: outcomes from the Johns Hopkins Hospital. J Gastrointest Surg. 2014;18:542–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Antonelli A, Cozzoli A, Simeone C, et al. Surgical treatment of adrenal metastasis from renal cell carcinoma: a single-centre experience of 45 patients. BJU Int. 2006;97:505–508. [DOI] [PubMed] [Google Scholar]