Abstract
Sequestration of nerve growth factor (NGF) significantly attenuates skeletal pain in both animals and humans. However, relatively little is known about the specific cell types that express NGF or its cognate receptors tropomyosin receptor kinase A (TrkA) and p75 in the intact bone and articular cartilage. In the present study, antibodies raised against NGF, TrkA, and p75 (also known as CD271) were used to explore the expression of these antigens in the non-decalcified young mouse femur. In general, all three antigens displayed a remarkably restricted expression in bone and cartilage with less than 2% of all DAPI+ cells in the femur displaying expression of any one of the three antigens. Robust NGF immunoreactivity was found in mostly CD-31− blood vessel-associated cells, a small subset of CD-31+ endothelial cells, an unidentified group of cells located at the subchondral bone/articular cartilage interface, and a few isolated, single cells in the bone marrow. In contrast, p75 and TrkA were almost exclusively expressed by nerve fibers located nearby NGF+ blood vessels. The only non-neuronal expression of either p75 or TrkA in the femur was the expression of p75 by a subset of cells located in the deep and middle zone of the articular cartilage. Understanding the factors that tightly regulate the basal level of expression in normal bone and how the expression of NGF, TrkA, and p75 change in injury, disease, and aging may provide insights into novel therapies that can reduce skeletal pain and improve skeletal health.
Keywords: Decalcification, aging, skeleton, pain, sensory, sympathetic
Introduction
There continues to be a significant unmet need for safe and effective medications that attenuate skeletal pain.1–5 Opioids and nonsteroidal anti-inflammatory drugs (NSAIDs) still dominate the clinical landscape as they are the most frequently used therapies to relieve chronic skeletal pain despite their limited effectiveness and considerable side-effect profile.6,7 Thus, although there has been a myriad of pain targets proposed over the past several decades, the majority of these pharmacotherapies for treating skeletal pain have failed to come to market.8–10
In the last decade, nerve growth factor (NGF) and its interaction with its cognate receptor tropomyosin receptor kinase A (TrkA) have come to be recognized as important mediators of skeletal pain.11–13 Pharmacotherapies targeting this pathway are now considered promising targets for the treatment of a variety of skeletal pain conditions including osteoarthritis (OA),13–15, low back pain,16–19 bone fracture,20,21 orthopedic surgery,22 and bone cancer.23,24 Several methodologic approaches, including sequestration of free NGF,12 prevention of NGF binding and TrkA activation,25 and inhibition of TrkA function,26–28 have been investigated in the development of new pharmacotherapies. Among these, NGF-sequestering antibodies have exhibited significant clinical promise and results from these clinical trials have been extensively published in peer-reviewed journals.
However, in 2010, a small subset of OA patients who were receiving anti-NGF exhibited rapid joint destruction leading to earlier than expected joint replacement. This prompted the US Food and Drug Administration (FDA) to place a hold on all clinical trials involving anti-NGF antibodies. Although this hold was lifted in 2015, and clinical trials for anti-NGF are again moving forward, in 2012 the FDA commissioned an independent arthritis advisory committee to further investigate these claims and concluded that joint failures were probably related to anti-NGF treatment and represented a unique clinical form of rapidly progressive OA, citing rapid and considerable joint destruction typically within 6–12 months of exposure.29 These cases were characterized by pathological features including femoral head flattening and medial femoral condyle involvement with subchondral fractures, associated edema, joint effusions, and marked pain. The committee also determined that events were more likely to occur with higher doses, longer exposures, and concurrent NSAID use. Although the precise etiology is still not known, several plausible mechanisms were discussed including higher susceptibility in patients with atrophic and neuropathic forms of OA, subchondral bone pathology, possible drug toxicity with concurrent NSAID use, and simply excessive overuse of the now less painful arthritic joints.29
Given the evidence that NGF plays an important role in driving skeletal pain, one important data set that we still do not have is what specific cells in the in vivo bone express NGF, TrkA, or p75.4,30–32 In large part, this lack of knowledge may be because, for many antibodies, the decalcification process will denature the epitope recognized by the antibody making it difficult or impossible to detect any specific immunohistochemical staining. In the present report, this issue is addressed by also examining the young mouse femur which is both small and relatively non-calcified so that decalcification was not required, thus potentially preserving the antigenicity of NGF, TrkA, and p75 in a large load bearing bone.
Materials and methods
Animals
Experiments were performed on 20 young (10 days old), 10 adult (4 months old), and 10 aging (24 months old) male C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME). Since the present study was only examining normal mice, both femurs were obtained from each mouse providing 40 young, 20 adult, and 20 aging femurs that were used in the present study. The mice were housed in accordance with the National Institutes of Health guidelines under specific pathogen-free conditions in autoclaved cages maintained at 22℃ with a 12-h alternating light/dark cycle and access to food and water ad libitum. All procedures adhered to the guidelines of the Committee for Research and Ethical Issues of the International Association for the Study of Pain and were approved by the Institutional Animal Care and Use Committee at the University of Arizona (Tucson, AZ, USA) (Protocol Number: 08–005).
Preparation of tissue for immunohistochemistry and histology
Young mice (10 days old) were deeply anesthetized with CO2 delivered from a compressed gas cylinder then decapitated. Young hindlimbs were then excised and placed in 4% formaldehyde/12.5% picric acid solution in 0.1 M phosphate-buffered saline (PBS) (pH 6.9 at 4 ℃) overnight. Following fixation, the femurs were dissected out and either placed in PBS (pH 7.4) for 48 h and then cryoprotected in 30% sucrose at 4℃ for 2 weeks or the femurs were placed in a decalcification solution for 2 weeks (0.5 M ethylene-diamine-tetra-acetic acid (EDTA) in PBS, pH 7.4 at 4℃) and then cyroprotected in 30% sucrose at 4℃ for at least 48 h before sectioning. Thus, half of the young femurs (20 femurs) were placed in the same decalcification solution used to demineralize adult and aging femurs before tissue sectioning, while the other 20 young femurs were sectioned without going through the decalcification process.
The adult and aging mice were deeply anesthetized with ketamine/xylazine (0.01 ml/g, 100 mg/10 kg, s.c.) and perfused intracardially as described previously.33–35 Briefly, mice were perfused intracardially with a fixative solution of 4% formaldehyde/12.5% picric acid solution in 0.1 M PBS (pH 6.9 at 4℃). Following perfusion, hindlimbs were removed and postfixed in the same fixative solution overnight. Following fixation, the femurs were decalcified for two weeks in 0.5 M EDTA in PBS, pH 7.4 at 4℃. For all femurs that went through the decalcification protocol, the EDTA solution was changed daily. In the adult and aging mice, decalcification was monitored weekly radiographically with a Faxitron MX-20 digital cabinet X-ray system (Faxitron/Bioptics, Tucson, AZ, USA). Following the two-week decalcification procedure, each femur was cryoprotected in 30% sucrose at 4℃ for at least 48 h before being sectioned.
Serial tissue sections of young, adult, and aging femurs were cut at thickness of either 20 or 60 µm and thaw mounted with two sections of bone per gelatin-coated slide. Sections at 20 µm thickness were stained with Safranin O or hematoxylin and eosin (H&E), and the 60 -µm-thick sections were used for immunohistochemistry and confocal microscopy.
Immunohistochemistry and histology
To process the tissue for immunohistochemistry or histology, sectioned slides were dried at room temperature (RT) for 30 min and then washed in PBS for 3 × 10 min. For slides used in histology, following this PBS wash the slides were stained with Safranin O or H&E. For slides to be used for immunohistochemistry, the slides were blocked with 3% normal donkey serum (Jackson ImmunoResearch, Cat# 017-11-121; West Grove, PA, USA) in PBS with 0.3% Triton-X 100 (Sigma Chemical Co., Cat# X100; St. Louis, MO, USA) for 1 h. Afterwards, the slides were incubated overnight with primary antibodies made in 1% normal donkey serum and 0.1% Triton-X 100 in 0.1 M PBS at RT. NGF was labeled using an antibody from Santa Cruz (Dallas, TX, USA; polyclonal rabbit anti-mouse, 1:1000, Cat# SC-548). TrkA was labeled with a polyclonal goat anti-mouse (1:1000, Cat# AF1056; R&D Systems, Minneapolis, MN, USA) and p75 was labeled with a polyclonal rabbit anti-mouse (1:1000, Cat# AB1554; Millipore, Temecula, CA, USA). Peptide-rich sensory nerve fibers were labeled with an antibody against calcitonin gene-related peptide (CGRP; polyclonal rabbit anti-rat CGRP; 1:10,000; Cat #8198; Sigma Chemical Co.). Sympathetic nerve fibers were identified using an antibody against tyrosine hydroxylase (TH; polyclonal rabbit anti-mouse TH, 1:1000, Cat #AB152; Millipore). Endothelial cells were identified using an antibody raised against cluster differentiation-31, PECAM-1 (polyclonal rabbit anti-mouse CD31; 1:500, Cat #550274; BD Pharmingen, San Jose, CA, USA).
After primary antibody incubation, preparations were washed 3 × 10 min each in PBS and incubated for 3 h at RT with secondary antibodies conjugated to fluorescent markers (Cy3/Dylight 488/AlexaFluor; 1:600 and 1:400; Jackson ImmunoResearch). Preparations were then washed 3 × 10 min each in PBS. Cellular nuclei were labeled using 4′,6-diamidino-2-phenylindole (DAPI) (1:500; Cat#D21490; Invitrogen, Grand Island, NY, USA) followed by an additional 3 × 10 min wash in PBS. Slides were dehydrated through an alcohol gradient (2 min each; 70%, 80%, 90%, and 100%), cleared in xylene (2 × 2 min), and cover slipped with di-n-butylphthalate-polystyrene-xylene (Sigma Chemical Co., Cat#06522). Preparations were allowed to dry covered at RT for 12 h before imaging.
Bright field and laser confocal microscopy
Bright field images of histologically stained sections were acquired using an Olympus BX51 microscope fitted with an Olympus DP70 digital CCD. Confocal images of NGF, TrkA, p75 CGRP, TH, and CD31 were acquired using an Olympus FV1200 microscope (Olympus Life Sciences, Center Valley, PA) and a 60 × /1.42 PlanApo N objective using excitation beams of 488 and 599 nm, and emissions were detected using BA505-540 and B575-620 emission filters. Nuclear staining (DAPI) was visualized using an excitation beam of 405 nm and emissions were detected using a BA430-470 emission filter. Sequential acquisition mode was used to reduce bleed-through from fluorophores. The average volume of data that was collected was 211.7 µm × 211.7 µm × 60 µm, with each Z-axis slice being 1.0 µm/slice.
Assessment of immunohistochemical staining
To assess the effect of the decalcification process on immunohistochemical staining, we examined CGRP, TH, CD31, NGF, TrkA, and p75 staining in tissue sections obtained from non-decalcified young bone and decalcified young, adult, and aging bone. For every antibody we examined, tissues from the non-decalcified young femur and the decalcified young, adult, and aging femur were examined from at least six different animals. Sections were examined under an Olympus FV1200 confocal microscope using a 60 × objective, under which it was easily possible to obtain single cell resolution and to distinguish individual nerve fibers. The distal end of the femur was selected for evaluation, as this area of bone allows easy anatomical orientation and has significant articular cartilage in the femoral knee joint, a thick periosteum, and abundant and well vascularized bone marrow. To qualitatively assess the effect that the decalcification process had on young bones, and the quality of staining we were able to obtain in young, adult, and aging bones, a scale of − to +++ was used. The − indicates no detectable signal; (+) indicates signal at the very limit of detection, + indicates minimal detection, requires significant increase in laser power to visualize; ++ indicates average staining signal, requires minimal increase in laser power to visualize; +++ indicates maximum staining signal, requires no increase in laser power to visualize.
Results
The expression of NGF in bone and articular cartilage of the young femur
For every antibody used in this study (NGF, TrkA, p75, CGRP, TH, and CD-31), we examined tissues from the non-decalcified young femur and the decalcified young, adult, and aging femur in at least six different animals. In general, the staining was very similar in animals within each group, and at least in the non-decalcified young femurs, the immunostaining obtained for NGF, TrkA, and p75 was comparable to that obtained for CGRP, TH, and CD-31 in terms of being robust, highly specific, with very low background staining. Because the only group of bones that provided highly robust staining of NGF, TrkA, and p75 were the femurs that were not decalcified, the expression patterns of NGF, TrkA, and p75, which are described below and are presented in Figures 1 to 3 and Table 1, were all obtained from the non-decalcified young mouse femur.
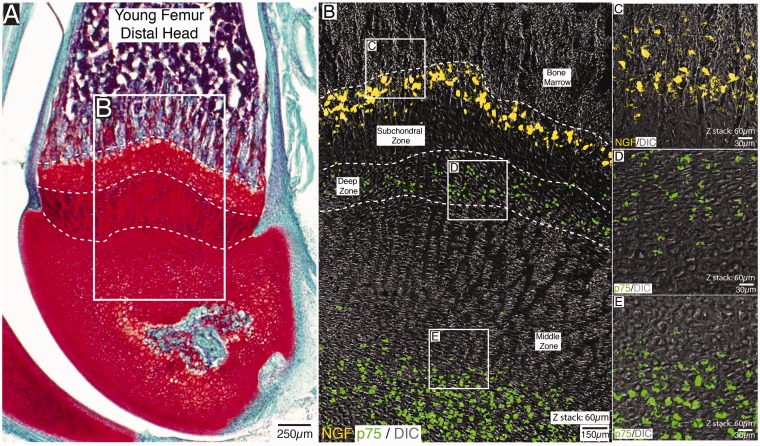
Figure 1.
Localization of NGF and p75 expressing cells in the distal head of the non-decalcified young mouse femur. An image of a Safranin O-stained section of the distal end of the femur (A) and a confocal image (B) showing the localization of NGF (yellow) and p75 (green) expressing cells in a section that was serially adjacent to (A). Note that in the confocal image shown in (B), the same section has simultaneously been stained with antibodies raised against NGF and p75. The NGF expressing cells are confined to a subpopulation of cells located at the subchondral zone/articular cartilage interface (B), whereas cells that robustly express p75 are located in deep and middle zone of the articular cartilage. Note that the high-power image of NGF-expressing cells shown in (C), which were obtained in tissue sections adjacent to that shown in (B), suggests these NGF+ cells do not have the morphology of endothelial cells, blood vessel-associated cells, or chondrocytes, whereas the p75 expressing cells in the deep (D) and middle (E) zone of the articular cartilage have the location and morphology of chondrocytes. Also note that p75 expressing nerve fibers were not observed near the NGF+ cells in the subchondral bone. The H&E stained section in (A) is 20 µm thick and the z-stacks of the confocal images shown in (B–E)=60 µm.
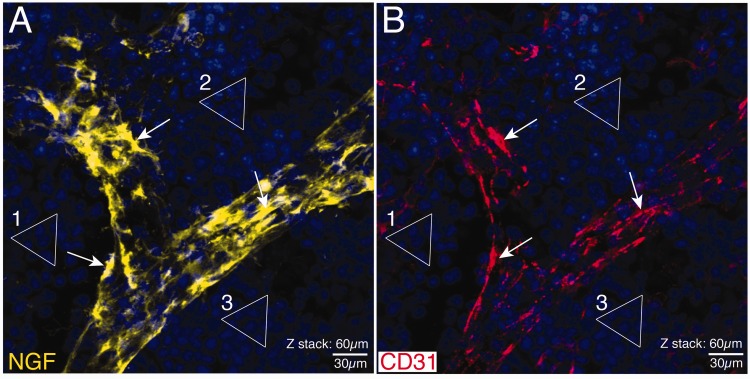
Figure 2.
Confocal images showing the localization of NGF and CD-31 immunoreactivity in a blood vessel in the young mouse femur. Note that in these confocal images, the same section has simultaneously been stained with antibodies raised against NGF (A) and CD-31 (B). The triangles (1–3) outline the clusters of DAPI+ nuclei to show that what is being shown in (A) is in exact register with (B). Note that there is at best only a partial co-localization (arrows) of NGF+ cells (A) and CD-31+ endothelial cells (B). These data suggest that another non-CD-31+ endothelial cells such as pericytes may be the primary NGF+ blood vessel-associated cells in bone. Similar NGF+ blood vessel-associated cells were observed throughout the marrow and periosteum but was not observed in cortical bone as the Haversian canals that vascularize cortical bone had yet to form in these young mice. These confocal images are taken from one 60 µm section where the total z-stack = 60 µm. Line bar = 30 µm.
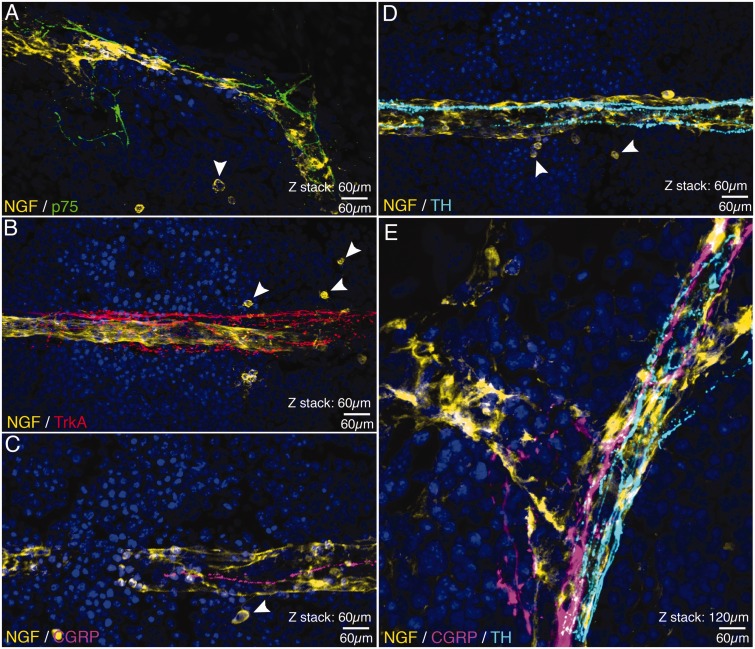
Figure 3.
Confocal images showing the association between cells NGF+ blood vessels and nerve fibers expressing p75, TrkA, CGRP, and TH. In these confocal images (all of which were obtained in the bone marrow), nerve fibers expressing p75 (A), Trk A (B), CGRP (C and E) and TH (D and E) appear to be closely associated with NGF+ blood vessels (yellow in A–D) that vascularize the bone marrow. Also note (arrowheads) that there are a few single NGF+ cells (A–D) that are spherical and have a very different morphology and do not appear to be closely associated with the NGF+ blood vessels. Confocal images shown in (A–D) are from 60 µm sections, whereas (E) is a merged image showing the relationship between a NGF+ blood vessel and CGRP+ sensory nerve fibers and TH+ post-ganglionic sympathetic nerve fibers with a total z-stack = 120 µm. Line bar = 60 µm.
Table 1.
Cellular localization of NGF, TrkA, and p75 immunoreactivity in the non-decalcified young mouse femur.
| Tissue | NGF | TrkA | p75 |
|---|---|---|---|
| Bone | |||
| CD31− blood vessel | √ | ||
| CD31+ blood vessel | √ | ||
| Subchondral bone | √ | ||
| Single cells in bone marrow | √ | ||
| Sensory nerve fibers | √ | √ | |
| Sympathetic nerve fibers | √ | √ | |
| Articular cartilage | |||
| Chondrocytes | √ |
Note. Cells that expressed robust NGF immunoreactivity were observed in CD-31− and CD31+ blood vessels, non-CD-31+ cells in subchondral bone and single or small clusters of non-blood vessel-associated cells in the bone marrow. Both TrkA and p75 were abundantly expressed by nerve fibers innervating NGF+ blood vessels in the marrow and periosteum. Robust expression of p75, but not TrkA, was also observed in cells in the deep and middle zone of the articular cartilage. NGF: nerve growth factor; TrkA: tropomyosin receptor kinase A.
In the young mouse femur, there was robust and consistent NGF staining of a population of cuboidal shaped, non-CD-31 cells at the subchondral bone/articular cartilage interface. These cells are present in stacked grouping and robustly express NGF immunoreactivity (Figure 1(B) and (C)) and these cells may be mesenchymal progenitor cells or undifferentiated chondrocytes. In the bone marrow and periosteum, robust NGF+ immunoreactivity was observed in association with blood vessels (Figure 2(A) and (B)). When this staining was first observed, the assumption was that all or at least a significant majority of this staining was present in CD-31+ endothelial cells. However, upon close examination (as can be seen when comparing Figure 3(A) with (B)) while some of the NGF+ immunoreactivity may co-localize with CD-31+ endothelial cells (arrows), the great majority of NGF+ staining does not co-localize with CD-31+ cells, as these cells have the morphology of pericytes. One other cell type that was frequently found to express NGF in the bone marrow was NGF+ cells (which were not CD-31+) which appear as single cells or small groups of single cells (arrowheads in Figure 3(A) and (D)). Although these cells were usually found within 100 µm of NGF+ blood vessels, they do not appear to have any direct connection with the NGF+ blood vessel nor do they have the morphology of NGF+ blood vessels associated cells which are found in both the periosteum and bone marrow (Figure 3(A) and (D) and Table 1).
The expression of TrkA in bone
The expression of TrkA in bone appears to be restricted to nerve fibers which appear to have the morphology of either sensory (CGRP+) or sympathetic (TH+) nerve fibers (Figure 3(C) to (E)). In general, TrkA+ nerve fibers in the bone marrow and periosteum are nearly always found in close association with NGF+ blood vessels (Figure 3(B)). It should be noted that TrkA+ nerve fibers were not found near the NGF+ cells located near the subchondral bone/articular cartilage interface nor were any TrkA+ cells found in the articular cartilage (Table 1).
The expression of p75 in bone and articular cartilage
The expression of p75 in bone is, with one exception, very similar to the expression of TrkA in bone. In both the bone marrow and periosteum, p75+ (which in the mesenchymal progenitor cell literature is also known as CD271) is present in nerve fibers which have the morphology of sensory (CGRP+) and sympathetic (TH+) nerve fibers (Figure 3(C) to (E)) and like TrkA+ nerve fibers, p75+ immunoreactive nerve fibers were nearly always closely associated with NGF+ blood vessels. However, unlike TrkA, robust p75 expression was also observed in a subset of cells located in the deep and middle zones of the articular cartilage of the knee joint. The p75+ cells which are located in the middle zone of the articular cartilage (Figures 1(B) and (E)) clearly have the circular, isolated cell morphology characteristics of chondrocytes, whereas the p75+ cells in the deep zone of the articular cartilage have a much more flattened and less differentiated appearance (Figure 1(B) and (D) and Table 1).
The effect of decalcification on the immunohistochemical detection of NGF, TrkA, p75, CGRP, TH, and CD-31 in bone
To assess whether decalcification of bone affected our ability to immunohistochemically detect NGF, TrkA, and p75, we examined both non-decalcified vs. decalcified young bones (Figure 4). In all cases, immunostaining of NGF, TrkA, and p75 was reduced in the decalcified young bones when compared to the non-decalcified young bones. For example, in the young non-decalcified femur, robust NGF immunoreactivity was easily observed in blood vessels, an unidentified group of cells located at the subchondral bone/articular cartilage interface, and a few isolated, single cells in the bone marrow. However, in the decalcified young bone, NGF+ staining was at the limits of being detectable (Figure 4(G) vs. (H)). Similarly, the robust expression of TrkA and p75 that was observed in nerve fibers (Figure 4(C) and (E), respectively) significantly declined in decalcified bones (Figures 4(D) and (F), respectively). Notable, decalcification of bone did not affect all antigens equally. Thus, CGRP (Figure 4(A) and (B)), TH, and CD31 (which were run as positive controls in attempting to localize NGF, p75 and TrkA in adult and aging bones) exhibited similar robust staining in the non-decalcified young bone and the decalcified young, adult, and aging bone (Table 2).
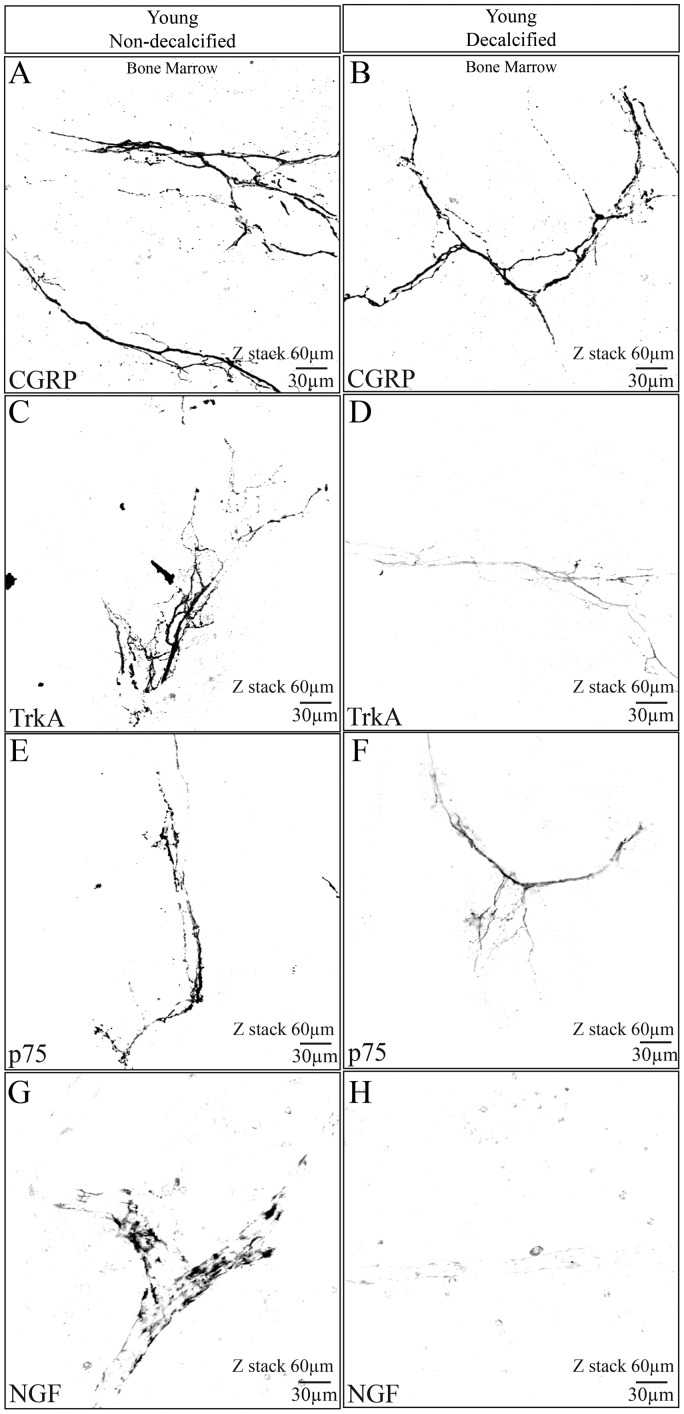
Figure 4.
The effect of decalcification on CGRP, NGF, TrkA, and p75 immunostaining in the femur of the young mouse. These confocal images were taken from young bones that had been fixed and then placed for 14 days in either PBS solution (pH = 7.4, 4℃) (A, C, E, G) or a decalcification solution consisting of PBS + EDTA (pH = 7.4, 4℃) (B, D, F, H). After 14 days, both the decalcified and non-decalcified femurs were placed in a 30% sucrose solution for two days, frozen sections cut, and the sections then immunostained for CGRP, TrkA, p75, or NGF. Note that robust immunohistochemical staining is observed for CGRP, TrkA, p75, and NGF in bones that were not decalcified (A, C, E, G). In contrast, in bones that had been decalcified, CGRP showed no significant loss of signal following decalcification (B), whereas TrkA (D), p75 (F), and NGF (H) all showed significant loss of immunohistochemical signal. All images presented here were obtained in the bone marrow. z-stack = 60 µm, line bar = 30 µm.
Table 2.
Immunostaining in the non-decalcified young femur and the decalcified young, adult and aging femur.
| Non-decalcified young | Decalcified young | Decalcified adult | Decalcified aging | |
|---|---|---|---|---|
| NGF | +++ | (+) | — | — |
| TrkA | +++ | + | + | + |
| p75 | +++ | + | — | — |
| CD31 | +++ | +++ | +++ | +++ |
| CGRP | +++ | +++ | +++ | +++ |
| TH | +++ | +++ | +++ | +++ |
Note. Note that whereas robust immunostaining NGF, TrkA, and p75 were obtained in the non-decalcified young femur, immunostaining for p75, TrkA, and NGF were all markedly reduced when the young bone was processed for decalcification. Similarly, immunostaining for NGF, TrkA, and p75 in the decalcified adult and aging femurs was reduced from what is observed in the non-decalcified young bone. It should be noted all structures (blood vessels, nerve fibers, chondrocytes, etc.) that expressed NGF, TrkA, or p75 were equally affected by the decalcification process. In contrast, robust CGRP, TH, and CD-31 immunostaining was observed in the non-decalcified young femur as well as in the decalcified young, adult, and aging femur. These results suggest that comparison of immunostaining in a decalcified vs. non-decalcified young bone may provide insight into the potential impact decalcification has on the ability to detect an antigen of interest in decalcified skeletal tissues. NGF: nerve growth factor; TrkA: tropomyosin receptor kinase A; CGRP: calcitonin gene-related peptide; TH: tyrosine hydroxylase.
It should be noted that while the immunostaining in the present study is quite robust with low background, it is still subject to all of the limitations of the method. Thus, while antibody specificity is important, it is very difficult to prove in tissue sections. Similarly, while it is clear that the decalcification procedure introduces difficulties, what is not clear is whether the decalcification process induces actual loss of antigen from the tissue or simply the modification of an antigen that is still present, but no longer recognized by the antibody. While we believe it is the latter, we have also tried several forms of antigen retrieval (heating, microwave, etc.; data not shown) in the decalcified young, adult, and aging skeleton and no antigen retrieval protocol has yet to provide the robust staining for NGF, TrkA, or p75 which we were able to detect in the young, non-decalcified bones.
Discussion
Discovery of NGF and its involvement in pain
NGF was initially discovered in the 1950s as a tumor tissue-produced soluble factor that promotes the growth and differentiation of sensory and sympathetic ganglia.36 NGF was the first growth factor to be identified and its discovery represented a landmark achievement in developmental neurobiology.37 The illumination of NGF’s critical role in neuronal development eventually led to the creation of the “neurotrophic factor hypothesis” and the classical neurotrophic model in which NGF is synthesized and released by target tissues during embryonic development, promoting the growth, differentiation, and survival of neurons in a dose-dependent manner.38
By the postnatal period, TrkA expression and NGF sensitivity decline, and the role of NGF–TrkA signaling shifts from promoting neuron growth and survival to regulating the sensitivity of the peripheral nervous system to noxious stimuli.39 A role for NGF has been demonstrated in both acute, transient nociceptive responses, as well as in long-term, chronic pain. As early as 1977, a report that NGF exerts effects on mast cells suggested that the physiologic effects of NGF were not limited to neuronal development and maturation.12 Currently, the NGF-TrkA axis is thought to play a pivotal role in the early, intermediate, and long-term generation and maintenance of several types of acute and chronic pain.40 Thus, NGF concentrations are increased in chronic pain conditions such as interstitial cystitis, prostatitis, arthritis, pancreatitis, chronic headaches, cancer pain, diabetic neuropathy, and noncancer pain, suggesting that NGF-mediated signaling is an ongoing and active process in chronic nociceptive and neuropathic pain states.12,41–51 Given the evidence that NGF plays an important role in driving skeletal pain, one important data set that we still do not have is what specific sets of cells in the intact bone express NGF, TrkA, or p75.4,32,33,52
Decalcification of bone and the ability to immunohistochemically detect NGF, TrkA, and p75
Previous studies have suggested that the process of bone decalcification can significantly affect the antigenicity of peptides and proteins and the ability to detect the cellular localization of these antigens using immunohistochemistry.53,54 Relatively few studies attempted to simultaneously localize NGF, TrkA, and p75 in skeletal tissues and assess the impact of decalcification on the ability to detect these proteins.12,30 In the present study, we found robust NGF immunoreactivity in CD-31− and CD-31+ blood vessel-associated cells, CD-31− cells at the interface of subchondral bone and articular cartilage, and single NGF+ cells in bone marrow in non-decalcified bone tissue. In contrast, in young, adult, or aging bones which had been decalcified, robust NGF immunoreactivity became difficult to detect. Similarly, the robust expression of TrkA and p75 in nerve fibers and the p75 expression in chondrocytes observed in the non-decalcified young bone declined significantly in the decalcified young, adult, or aging bone. These results suggest that when exploring antigen expression in skeletal tissues, using a non-decalcified bone as a positive control may provide significant insight into whether the decalcification process is negatively affecting the ability to detect the antigen of interest.
Expression of NGF in normal young bone and articular cartilage
In the present report, the great majority of cells that express NGF in the young bone marrow and periosteum appear to be CD-31+ endothelial cells and CD-31− blood vessel-associated cells. These observations support previous studies showing that endothelial cells and pericytes in the rat heart can express NGF.55 Previous studies have also shown that depending on the vascular bed, endothelial cells can either be NGF+ or NGF− and a similar heterogeneity in NGF expression is also apparent for myocytes.55 What is clear in the present report is that not all CD-31+ endothelial cells in bone express NGF and that most NGF+ cells in bone do not express CD-31. These data suggest that non-CD31+ blood vessel-associated cells, including pericytes56 are probably the major cells in the blood vessel that are expressing NGF, rather than the endothelial cell. What is also clear is that in vascular beds in the periosteum and bone marrow, where there are NGF+ cells, there is usually also a significant number of TrkA+, p75+, CGRP+ sensory nerve fibers, and TH+ post-ganglionic sympathetic nerve fibers that are intimately associated with the NGF+ blood vessel.
In addition to NGF+ blood vessel-associated cells in the bone marrow and periosteum, there was a small subset of NGF+ cells that were located at the interface of the subchondral bone/articular cartilage. These cells did not have the morphology or organization of blood vessels, they did not express CD-31, nor did they have the very distinctive round morphology of chondrocytes. While these cells may secrete NGF that could bind to p75+ chondrocytes that are in the middle zone of the articular cartilage, it is difficult to understand how a large protein such as NGF could diffuse through the articular cartilage to bind to p75+ chondrocytes. One other possibility is that NGF released from these NGF+ cells could interact with TrkA+ sensory nerve fibers that other authors have suggested undergo sprouting in injured subchondral bone in OA.57 In the present study, there were few if any TrkA+ or p75+ sensory or sympathetic nerve fibers in the vicinity of these NGF+ cells. However, with the pathological deterioration of an osteoarthritic joint, there are frequently significant changes in subchondral bone58–60 and studies have suggested that sprouting of nerve fibers can occur into this newly remodeling subchondral bone. Whether these NGF+ cells, or an entirely new type of NGF expressing cells, play a role in the neuritogenesis in OA remains unclear but these NGF+ cells are clearly at the subchondral bone/articular cartilage interface and could release NGF in response to the increased strain and loading that occurs as the osteoarthritic cartilage losses its elasticity and strength with disease progression.61–63
Expression of TrkA and p75 in the normal young mouse femur
In the normal skeleton of the young mouse when TrkA+ immunoreactivity is in nerve fibers, these TrkA fibers are also usually in close proximity to NGF+ blood vessels and have either a rather linear morphology which is characteristic of CGRP+ sensory nerve fibers or a corkscrew like morphology which is frequently observed in TH+ post-ganglionic sympathetic nerve fibers.64–66 One notable observation is that we did not observe any TrkA immunoreactivity in non-neuronal cells in the mouse femur and all the TrkA immunoreactivity that was observed was present in nerve fibers.
Similar to TrkA, p75 immunoreactivity was also observed in many nerve fibers that were intimately associated with NGF+ blood vessels. However, unlike TrkA expression, there was also a consistent and robust labeling of p75 immunoreactive cells in the deep and middle zone of the articular cartilage. These p75+ cells have the morphology and location of chondrocytes as they had a round or spherical morphology. Previous studies in both experimental animals and humans have reported p75 immunoreactive chondrocytes in articular cartilage and these p75+ (also known as CD271) in the present study have a very similar morphology to that reported in human articular cartilage.67–69
Conclusions and limitations
In the young mouse femur, robust NGF immunoreactivity is found in CD-31− and CD-31+ blood vessel-associated cells, in CD-31− cells at the interface of subchondral bone and articular cartilage and occasionally as single or small clusters of cells in bone marrow. In contrast, the great majority of p75 and TrkA expression in bone is present in nerve fibers with p75 but not TrkA expression also being observed in chondrocytes in the articular cartilage. The present study also suggests that decalcification of a skeletal tissue can have a markedly negative impact on NGF, TrkA, and p75 immunostaining.
The limitations of the present study are that the results were obtained in one bone, obtained from one age, one gender, and one strain of mice. As it has been shown that there is very little, if any, effect of inhibiting NGF on baseline pain sensitivity in the absence of pathology,4,12–15,17,20–23,25,36,70 understanding what cells are NGF positive and where its receptors are expressed in pathological skeletal pain states would greatly extend our understanding. In the present study, the only bones that provided interpretable results in terms of being able to simultaneously provide robust staining of NGF, TrkA, and p75 expression in the bone were the young bones which were not decalcified. These data suggest that inclusion of a positive control such as a young non-decalcified bone will help in interpreting immunohistochemical results when using decalcified tissues. Additionally, developing better techniques that can decalcify human and non-human tissues without eliminating or denaturing the epitope recognized by the antibody will be needed to determine how disease, injury, and aging effects the expression of NGF and its receptors in adult and aging animals of both genders.
Author contributions
PWM conceived and designed this project. Tissue preparation, immunohistochemistry, and confocal microscopy was performed by SRC. Tissue perfusions were performed by LAM. Writing, organizing and editing of the manuscript was provided by PWM and SAM. All authors contributed to the interpretation of data, writing, reviewing and revision of the manuscript. Study supervision was led by PWM.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was financially supported by NIH Grants CA154550, CA157449, and NS023970 to Patrick Mantyh.
References
- 1.Chang DS, Hsu E, Hottinger DG, et al. Anti-nerve growth factor in pain management: current evidence. J Pain Res 2016; 9: 373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clinch J, Eccleston C. Chronic musculoskeletal pain in children: assessment and management. Rheumatology 2009; 48: 466–474. [DOI] [PubMed] [Google Scholar]
- 3.Gaskin DJ, Richard P. The economic costs of pain in the United States. The Journal of Pain 2012; 13: 715–724. [DOI] [PubMed] [Google Scholar]
- 4.Mantyh PW. The neurobiology of skeletal pain. Eur J Neurosci 2014; 39: 508–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bull World Health Organ 2003; 81: 646–656. [PMC free article] [PubMed] [Google Scholar]
- 6.Kissin I. The development of new analgesics over the past 50 years: a lack of real breakthrough drugs. Anesth Analg 2010; 110: 780–789. [DOI] [PubMed] [Google Scholar]
- 7.Woolf CJ, Max MB. Mechanism-based pain diagnosis: issues for analgesic drug development. Anesthesiology 2001; 95: 241–249. [DOI] [PubMed] [Google Scholar]
- 8.Cohen SP, Mao J. Neuropathic pain: mechanisms and their clinical implications. BMJ 2014; 348: f7656. [DOI] [PubMed] [Google Scholar]
- 9.Hsu E, Murphy S, Chang D, et al. Expert opinion on emerging drugs: chronic low back pain. Expert Opin Emerg Drugs 2015; 20: 103–127. [DOI] [PubMed] [Google Scholar]
- 10.Kissin I. Scientometrics of drug discovery efforts: pain-related molecular targets. Drug Des Devel Ther 2015; 9: 3393–3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hefti FF, Rosenthal A, Walicke PA, et al. Novel class of pain drugs based on antagonism of NGF. Trends Pharmacol Sci 2006; 27: 85–91. [DOI] [PubMed] [Google Scholar]
- 12.Mantyh PW, Koltzenburg M, Mendell LM, et al. Antagonism of nerve growth factor-TrkA signaling and the relief of pain. Anesthesiology 2011; 115: 189–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shelton DL, Zeller J, Ho WH, et al. Nerve growth factor mediates hyperalgesia and cachexia in auto-immune arthritis. Pain 2005; 116: 8–16. [DOI] [PubMed] [Google Scholar]
- 14.Brown MT, Murphy FT, Radin DM, et al. Tanezumab reduces osteoarthritic knee pain: results of a randomized, double-blind, placebo-controlled phase III trial. J Pain 2012; 13: 790–798. [DOI] [PubMed] [Google Scholar]
- 15.Schnitzer TJ, Marks JA. A systematic review of the efficacy and general safety of antibodies to NGF in the treatment of OA of the hip or knee. Osteoarthritis Cartilage 2015; 23(Suppl 1): S8–S17. [DOI] [PubMed] [Google Scholar]
- 16.Gimbel JS, Kivitz AJ, Bramson C, et al. Long-term safety and effectiveness of tanezumab as treatment for chronic low back pain. Pain 2014; 155: 1793–1801. [DOI] [PubMed] [Google Scholar]
- 17.Katz N, Borenstein DG, Birbara C, et al. Efficacy and safety of tanezumab in the treatment of chronic low back pain. Pain 2011; 152: 2248–2258. [DOI] [PubMed] [Google Scholar]
- 18.Kivitz AJ, Gimbel JS, Bramson C, et al. Efficacy and safety of tanezumab versus naproxen in the treatment of chronic low back pain. Pain 2013; 154: 1009–1021. [DOI] [PubMed] [Google Scholar]
- 19.Sanga P, Polverejan E, Wang S, et al. Efficacy, safety, and tolerability of fulranumab as an adjunctive therapy in patients with inadequately controlled, moderate-to-severe chronic low back pain: a randomized, double-blind, placebo-controlled, dose-ranging, dose-loading phase II study. Clin Ther 2016; 38: 1435–1450. [DOI] [PubMed] [Google Scholar]
- 20.Jimenez-Andrade JM, Martin CD, Koewler NJ, et al. Nerve growth factor sequestering therapy attenuates non-malignant skeletal pain following fracture. Pain 2007; 133: 183–196. [DOI] [PubMed] [Google Scholar]
- 21.Koewler NJ, Freeman KT, Buus RJ, et al. Effects of a monoclonal antibody raised against nerve growth factor on skeletal pain and bone healing after fracture of the C57BL/6J mouse femur. J Bone Miner Res 2007; 22: 1732–1742. [DOI] [PubMed] [Google Scholar]
- 22.Majuta LA, Longo G, Fealk MN, et al. Orthopedic surgery and bone fracture pain are both significantly attenuated by sustained blockade of nerve growth factor. Pain 2015; 156: 157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sevcik MA, Ghilardi JR, Peters CM, et al. Anti-NGF therapy profoundly reduces bone cancer pain and the accompanying increase in markers of peripheral and central sensitization. Pain 2005; 115: 128–141. [DOI] [PubMed] [Google Scholar]
- 24.Sopata M, Katz N, Carey W, et al. Efficacy and safety of tanezumab in the treatment of pain from bone metastases. Pain 2015; 156: 1703–1713. [DOI] [PubMed] [Google Scholar]
- 25.Ghilardi JR, Freeman KT, Jimenez-Andrade JM, et al. Sustained blockade of neurotrophin receptors TrkA, TrkB and TrkC reduces non-malignant skeletal pain but not the maintenance of sensory and sympathetic nerve fibers. Bone 2011; 48: 389–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen B, Zhao L, Li X, et al. Syntaxin 8 modulates the post-synthetic trafficking of the TrkA receptor and inflammatory pain transmission. J Biol Chem 2014; 289: 19556–19569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Owolabi JB, Rizkalla G, Tehim A, et al. Characterization of antiallodynic actions of ALE-0540, a novel nerve growth factor receptor antagonist, in the rat. J Pharmacol Exp Ther 1999; 289: 1271–1276. [PubMed] [Google Scholar]
- 28.Winston JH, Toma H, Shenoy M, et al. Acute pancreatitis results in referred mechanical hypersensitivity and neuropeptide up-regulation that can be suppressed by the protein kinase inhibitor k252a. J Pain 2003; 4: 329–337. [DOI] [PubMed] [Google Scholar]
- 29.Hochberg MC. Serious joint-related adverse events in randomized controlled trials of anti-nerve growth factor monoclonal antibodies. Osteoarthritis Cartilage 2015; 23(Suppl 1): S18–21. [DOI] [PubMed] [Google Scholar]
- 30.Asaumi K, Nakanishi T, Asahara H, et al. Expression of neurotrophins and their receptors (TRK) during fracture healing. Bone 2000; 26: 625–633. [DOI] [PubMed] [Google Scholar]
- 31.Driscoll C, Chanalaris A, Knights C, et al. Nociceptive sensitizers are regulated in damaged joint tissues, including articular cartilage, when osteoarthritic mice display pain behavior. Arthritis Rheumatol 2016; 68: 857–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nencini S, Ringuet M, Kim DH, et al. Mechanisms of nerve growth factor signaling in bone nociceptors and in an animal model of inflammatory bone pain. Mol Pain 2017; 13: 1744806917697011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chartier SR, Thompson ML, Longo G, et al. Exuberant sprouting of sensory and sympathetic nerve fibers in nonhealed bone fractures and the generation and maintenance of chronic skeletal pain. Pain 2014; 155: 2323–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thompson ML, Chartier SR, Mitchell SA, et al. Preventing painful age-related bone fractures: anti-sclerostin therapy builds cortical bone and increases the proliferation of osteogenic cells in the periosteum of the geriatric mouse femur. Mol Pain 2016; 12: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thompson ML, Jimenez-Andrade JM, Mantyh PW. Sclerostin immunoreactivity increases in cortical bone osteocytes and decreases in articular cartilage chondrocytes in aging mice. J Histochem Cytochem 2016; 64: 179–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aloe L. Rita Levi-Montalcini: the discovery of nerve growth factor and modern neurobiology. Trends Cell Biol 2004; 14: 395–399. [DOI] [PubMed] [Google Scholar]
- 37.Aloe L, Rocco ML, Bianchi P, et al. Nerve growth factor: from the early discoveries to the potential clinical use. J Transl Med 2012; 10: 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yuen EC, Howe CL, Li Y, et al. Nerve growth factor and the neurotrophic factor hypothesis. Brain Dev 1996; 18: 362–368. [DOI] [PubMed] [Google Scholar]
- 39.Bennett DL. Neurotrophic factors: important regulators of nociceptive function. Neuroscientist 2001; 7: 13–17. [DOI] [PubMed] [Google Scholar]
- 40.Watson JJ, Allen SJ, Dawbarn D. Targeting nerve growth factor in pain: what is the therapeutic potential? BioDrugs 2008; 22: 349–359. [DOI] [PubMed] [Google Scholar]
- 41.Abe Y, Akeda K, An HS, et al. Proinflammatory cytokines stimulate the expression of nerve growth factor by human intervertebral disc cells. Spine (Phila Pa 1976) 2007; 32: 635–642. [DOI] [PubMed] [Google Scholar]
- 42.Falcini F, Matucci Cerinic M, Lombardi A, et al. Increased circulating nerve growth factor is directly correlated with disease activity in juvenile chronic arthritis. Ann Rheum Dis 1996; 55: 745–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Halliday DA, Zettler C, Rush RA, et al. Elevated nerve growth factor levels in the synovial fluid of patients with inflammatory joint disease. Neurochem Res 1998; 23: 919–922. [DOI] [PubMed] [Google Scholar]
- 44.Krock E, Currie JB, Weber MH, et al. Nerve growth factor is regulated by toll-like receptor 2 in human intervertebral discs. J Biol Chem 2016; 291: 3541–3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lowe EM, Anand P, Terenghi G, et al. Increased nerve growth factor levels in the urinary bladder of women with idiopathic sensory urgency and interstitial cystitis. Br J Urol 1997; 79: 572–577. [DOI] [PubMed] [Google Scholar]
- 46.Martins LB, Teixeira AL, Domingues RB. Neurotrophins and Migraine. Vitam Horm 2017; 104: 459–473. [DOI] [PubMed] [Google Scholar]
- 47.Miller LJ, Fischer KA, Goralnick SJ, et al. Nerve growth factor and chronic prostatitis/chronic pelvic pain syndrome. Urology 2002; 59: 603–608. [DOI] [PubMed] [Google Scholar]
- 48.Montagnoli C, Tiribuzi R, Crispoltoni L, et al. beta-NGF and beta-NGF receptor upregulation in blood and synovial fluid in osteoarthritis. Biol Chem 2017; 398: 1045–1054. [DOI] [PubMed] [Google Scholar]
- 49.Pezet S, McMahon SB. Neurotrophins: mediators and modulators of pain. Annu Rev Neurosci 2006; 29: 507–538. [DOI] [PubMed] [Google Scholar]
- 50.Safieh-Garabedian B, Poole S, Allchorne A, et al. Contribution of interleukin-1 beta to the inflammation-induced increase in nerve growth factor levels and inflammatory hyperalgesia. Br J Pharmacol 1995; 115: 1265–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Theodosiou M, Rush RA, Zhou XF, et al. Hyperalgesia due to nerve damage: role of nerve growth factor. Pain 1999; 81: 245–255. [DOI] [PubMed] [Google Scholar]
- 52.Nencini S, Ivanusic JJ. The physiology of bone pain. How much do we really know? Front Physiol 2016; 7: 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arnold W. Immunohistochemical investigation of the human inner ear. Limitations and prospects. Acta Otolaryngol 1988; 105: 392–397. [DOI] [PubMed] [Google Scholar]
- 54.Jiang X, Kalajzic Z, Maye P, et al. Histological analysis of GFP expression in murine bone. J Histochem Cytochem 2005; 53: 593–602. [DOI] [PubMed] [Google Scholar]
- 55.Hiltunen JO, Laurikainen A, Vakeva A, et al. Nerve growth factor and brain-derived neurotrophic factor mRNAs are regulated in distinct cell populations of rat heart after ischaemia and reperfusion. J Pathol 2001; 194: 247–253. [DOI] [PubMed] [Google Scholar]
- 56.Ishitsuka K, Ago T, Arimura K, et al. Neurotrophin production in brain pericytes during hypoxia: a role of pericytes for neuroprotection. Microvasc Res 2012; 83: 352–359. [DOI] [PubMed] [Google Scholar]
- 57.Walsh DA, McWilliams DF, Turley MJ, et al. Angiogenesis and nerve growth factor at the osteochondral junction in rheumatoid arthritis and osteoarthritis. Rheumatology (Oxford) 2010; 49: 1852–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Burr DB. The importance of subchondral bone in osteoarthrosis. Curr Opin Rheumatol 1998; 10: 256–262. [DOI] [PubMed] [Google Scholar]
- 59.Burr DB, Schaffler MB. The involvement of subchondral mineralized tissues in osteoarthrosis: quantitative microscopic evidence. Microsc Res Tech 1997; 37: 343–357. [DOI] [PubMed] [Google Scholar]
- 60.Lane LB, Villacin A, Bullough PG. The vascularity and remodelling of subchondrial bone and calcified cartilage in adult human femoral and humeral heads. An age- and stress-related phenomenon. J Bone Joint Surg Br 1977; 59: 272–278. [DOI] [PubMed] [Google Scholar]
- 61.Felson DT, Lawrence RC, Dieppe PA, et al. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med 2000; 133: 635–646. [DOI] [PubMed] [Google Scholar]
- 62.Vincent TL, Hermansson MA, Hansen UN, et al. Basic fibroblast growth factor mediates transduction of mechanical signals when articular cartilage is loaded. Arthritis Rheum 2004; 50: 526–533. [DOI] [PubMed] [Google Scholar]
- 63.Vincent TL, McLean CJ, Full LE, et al. FGF-2 is bound to perlecan in the pericellular matrix of articular cartilage, where it acts as a chondrocyte mechanotransducer. Osteoarthritis Cartilage 2007; 15: 752–763. [DOI] [PubMed] [Google Scholar]
- 64.Gajda M, Litwin JA, Cichocki T, et al. Development of sensory innervation in rat tibia: co-localization of CGRP and substance P with growth-associated protein 43 (GAP-43). J Anat 2005; 207: 135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mach DB, Rogers SD, Sabino MC, et al. Origins of skeletal pain: sensory and sympathetic innervation of the mouse femur. Neuroscience 2002; 113: 155–166. [DOI] [PubMed] [Google Scholar]
- 66.Tabarowski Z, Gibson-Berry K, Felten SY. Noradrenergic and peptidergic innervation of the mouse femur bone marrow. Acta Histochem 1996; 98: 453–457. [DOI] [PubMed] [Google Scholar]
- 67.Gigante A, Bevilacqua C, Pagnotta A, et al. Expression of NGF, Trka and p75 in human cartilage. Eur J Histochem 2003; 47: 339–344. [PubMed] [Google Scholar]
- 68.Grimsholm O, Guo Y, Ny T, et al. Expression patterns of neurotrophins and neurotrophin receptors in articular chondrocytes and inflammatory infiltrates in knee joint arthritis. Cells Tissues Organs 2008; 188: 299–309. [DOI] [PubMed] [Google Scholar]
- 69.Pecchi E, Priam S, Gosset M, et al. Induction of nerve growth factor expression and release by mechanical and inflammatory stimuli in chondrocytes: possible involvement in osteoarthritis pain. Arthritis Res Ther 2014; 16: R16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McKelvey L, Shorten GD, O’Keeffe GW. Nerve growth factor-mediated regulation of pain signalling and proposed new intervention strategies in clinical pain management. J Neurochem 2013; 124: 276–289. [DOI] [PubMed] [Google Scholar]