Abstract
Oxaliplatin-induced chronic painful neuropathy is the most common dose-limiting adverse event that negatively affects cancer patients’ quality of life. However, the underlying molecular mechanisms are still unclear. In the present study, we found that the intraperitoneal administration of oxaliplatin at 4 mg/kg for five consecutive days noticeably upregulated the expression of CXC motif ligand 12 (CXCL12) in the dorsal root ganglion, and the intrathecal injection of an anti-CXCL12 neutralizing antibody or CXCL12 siRNA attenuated the mechanical allodynia and thermal hyperalgesia induced by oxaliplatin. We also found that the signal transducers and transcription activator 3 (STAT3) was activated in the dorsal root ganglion, and inhibition of STAT3 with S3I-201 or the injection of AAV-Cre-GFP into STAT3flox/flox mice prevented the upregulation of CXCL12 expression in the dorsal root ganglion and chronic pain following oxaliplatin administration. Double-label fluorescent immunohistochemistry findings also showed that p-STAT3 was mainly localized in CXCL12-positive cells in the dorsal root ganglion. Furthermore, the results of a chromatin immunoprecipitation assay revealed that p-STAT3 might be essential for oxaliplatin-induced CXCL12 upregulation via binding directly to the specific position of the CXCL12 gene promoter. Finally, we found that cytokine TNF-α and IL-1β increases mediated the STAT3 activation following oxaliplatin treatment. Taken together, these findings suggested that the upregulation of CXCL12 via TNF-α/IL-1β–dependent STAT3 activation contributes to oxaliplatin-induced chronic pain.
Keywords: oxaliplatin, CXCL12, chronic pain, STAT3, DRG
Introduction
Oxaliplatin is a platinum-based chemotherapeutic drug and widely used to treat colon or rectal cancer. However, oxaliplatin-induced chronic painful neuropathy is a common dose-limiting side effect during chemotherapy and the major reason for the delay or discontinuation of chemotherapy. Because there are no well-established treatments to prevent or minimize oxaliplatin-induced persistent pain, the underlying molecular mechanisms remain to be elucidated.
The chemokine CXC motif ligand 12 (CXCL12), also named stromal cell-derived factor 1 (SDF-1), is widely expressed in various kinds of cells and involved in multiple functions of the nervous system.1 For example, CXCL12 plays a critical role in regulating synaptic plasticity via binding with its receptor, chemokine C-X-C motif receptor 4 (CXCR4).2,3 Moreover, studies have shown that inhibiting CXCL12 upregulation in the dorsal root ganglion (DRG) and spinal dorsal horn alleviated mechanical allodynia induced by spared nerve injury or bone cancer,4,5 which indicates that CXCL12 also participated in neuropathic pain. Importantly, recent studies showed that the increased CXCL12 in the spinal cord via enhanced nociceptive synaptic transmission contributes to the chronic pain induced by the anti-tubulin chemotherapeutic drugs paclitaxel or vincristine.6 However, whether the expression of CXCL12 in the DRG is involved in oxaliplatin-induced chronic pain remains unclear.
Signal transducers and transcription activator 3 (STAT3) is an important transcription factor involved in physiological and pathologic processes in the nervous system, including synaptic plasticity, memory formation, microglia inflammatory response, and neural degeneration.7 It has been reported that the activity of STAT3 is significantly enhanced after the injury of both central and peripheral nerves,8 and the increased STAT3 activity might be involved in chronic pain because blocking STAT3 activity significantly ameliorated neuropathic pain induced by nerve injury.9 However, whether STAT3 expressed in the DRG mediates oxaliplatin-induced chronic pain remains unknown. It is well known that STAT3 phosphorylation induces nuclear translocation and binding to specific promoter sequences on target genes, which results in gene transcription in response to extracellular signals.10 Furthermore, studies showed that activated STAT3 mediated the immune/inflammatory reaction and modulated the expression inflammatory factors.11,12 However, whether the expression of the chemokine CXCL12 in the DRG is regulated by STAT3 following oxaliplatin treatment deserves further investigation.
In the present study, we first examined the expression of CXCL12 in the DRG and its role in chronic pain after the administration of oxaliplatin. Importantly, we further illustrated whether the transcription factor STAT3 mediated the upregulation of CXCL12 following oxaliplatin treatment.
Materials and methods
Animals and surgery
Male Sprague–Dawley rats weighing 200–220 g were obtained from the Institute of Experimental Animals of Sun Yat-sen University. Mice used in this study were adult male animals with a C57BL/6 background (weight 20–30 g). STAT3flox/flox mice (ID: 016923) were purchased from Jackson Laboratory (USA). All animals were housed in separated cages with ad libitum access to food and water. The room was kept at 24℃ and 50–60% humidity, under a 12/12 h light/dark cycle. All experimental procedures were approved by the Institutional Animal Care Committee of Sun Yat-sen University and were carried out in accordance with the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize animal suffering and to reduce the number of animals used.
For the intrathecal injection of the drug, a polyethylene-10 (PE-10) catheter was implanted as previously described.13 In brief, rats were anesthetized intraperitoneally with sodium pentobarbital (50 mg/kg), a sterile catheter filled with saline was inserted into the subarachnoid space through the L5/L6 intervertebral space and was gently advanced caudally to the spinal lumbar enlargement level. Then, the catheter was fixed under the skin with paravertebral muscles and sutured at the head of rats. After the rats were completely recovered from anesthesia, the correct placement of the catheter was confirmed by observing the behavior of dragging or paralysis in the bilateral hind limbs following the injection of 2% lidocaine (10 μl). Finally, rats with catheter prolapse, infection, or neurological deficits were excluded from the subsequent experiments. All rats were observed for three days before the administration of drugs.
Drugs and administration
Oxaliplatin was purchased from Sigma (USA) and dissolved in 5% glucose/H2O as a stock solution of 1 mg/ml as a previous study reported.14 Rats were intraperitoneally administered oxaliplatin at 4 mg/kg once per day for five consecutive days to induce mechanical allodynia. The control rats received an intraperitoneal injection of an equivalent volume of 5% glucose/H2O. Intrathecal injection of the neutralizing antibody against CXCL12 (8 µg, 10 μl; Torrey Pines Biolabs, Secaucus, NJ), neutralizing antibody against TNF-α (10 µg, 10 μl; R&D systems, Minneapolis, MN), a isotype IgG (8 µg, 10 μl; Sigma, Bellevue, WA), the IL-1 receptor antagonist (IL-1ra; 50 µg, 10 μl; R&D Systems, Minneapolis, MN), a STAT3 inhibitor S3I-201 (100 µg, 10 μl; Selleckchem, Houston, TX), scramble siRNA (1 nmol, 10 μl; Ribobio, China), or CXCL12 small interfering RNA (siRNA; 1 nmol, 10 μl; Ribobio, China) for consecutive 10 days was performed 30 min before oxaliplatin administration. In addition, recombinant rat CXCL12 (2 µg, 10 μl; Signalway Antibody, College Park, USA) were intrathecally injected for consecutive 10 days.
Injection of adenovirus-associated vector
Recombinant adeno-associated virus (AAV) encoding Cre and a GFP marker (AAV8-Cre-GFP) and AAV encoding GFP (AAV8-GFP) were purchased from Beijing Vector Gene Technology Company Ltd. A total of four microliters of PBS containing 1 × 1013 vector genomes/ml AAV8-Cre-GFP was intrathecally injected into the subarachnoid space of L4-L6 spinal cord of STAT3flox/flox mice. Control mice were injected with the same amount of AAV encoding GFP (AAV8-GFP). The administration of oxaliplatin was performed on day 21 after the virus injection.
Behavioral test
The 50% withdrawal threshold was assessed with von Frey hairs as described previously.15 Briefly, each animal was loosely restrained beneath a plastic box on a metal mesh for at least 15 min once daily for three consecutive days. Mechanical allodynia was assessed with the hindpaw withdrawal threshold in response to probing with a series of von Frey filaments. Each von Frey hair was applied 10 times. A nociceptive response was defined as a brisk paw withdrawal or flinching of the paw following von Frey filament application. The responses to all filaments for both paws were tabulated as a single value, and 50% withdrawal threshold was defined as the lowest bending force that produced 10 or more responses.
Thermal hyperalgesia was tested by measuring the paw withdrawal latency in response to the radiant heat stimulation with a plantar test (7370, Ugo Basile Plantar Test Apparatus, Italy) according to previously standardized protocols.16 Briefly, a radiant heat source beneath a glass floor was aimed at the plantar surface of the hindpaw of animals. Three measurements of withdrawal latency were taken for each hindpaw in each test session. The hindpaw of each animal was tested alternately with greater than 5 min intervals between the consecutive tests. Three measurements of withdrawal latency per side were averaged for the result of each test. A 20-s cutoff was set to prevent tissue damage. The behavioral test was performed by an experimenter blinded to the treatment.
RNA extraction and quantitative polymerase chain reaction
Total RNA was extracted from the animal L4/6 DRG tissues with Trizol reagent (Invitrogen). Reverse transcription was performed with oligo-dT primers and M-MLV reverse transcriptase (Promega, USA) according to the manufacturer’s protocol with minor modifications as previously described.6 Specific primer sequences of the examined mRNA for PCR reactions are listed in Table 1. Real-time quantitative PCR was performed with a SYBR Green quantitative polymerase chain reaction (qPCR) SuperMix (Invitrogen) and the ABI PRISM 7500 Sequence Detection System (USA). The reactions were set up according to the manufacturer’s protocol. The PCR amplifications were performed at 95℃ for 3 min followed by 40 cycles of thermal cycling (10 s at 95℃, 20 s at 58℃, and 10 s at 72℃). The melting curves were established to validate the utility and specificity of each PCR product. The relative expression ratio of mRNA in the DRG tissues was evaluated via the Comparative CT Method (2−ΔΔCT).
Table 1.
Specific primer sequences.
| Gene | Primer | Sequence |
|---|---|---|
| CXCL12 | Forward | 5′-GGGAAACGGAGAAAGCTACC-3′ |
| Reverse | 5′-CCCTCACCACACACACATCA-3′ | |
| STAT3 | Forward | 5′-TACCACAAAAGTCAGGTTGCTG-3′ |
| Reverse | 5′-ACATCCCCAGAGTCCTTATCAA-3′ | |
| IL-1β | Forward | 5′-GGATGATGACGACCTGCTA-3′ |
| Reverse | 5′-CACTTGTTGGCTTATGTTCTG-3′ | |
| IL-6 | Forward | 5′-CCACTGCCTTCCCTACTT-3′ |
| Reverse | 5′-TTGCCATTGCACAACTCT-3′ | |
| IL-8 | Forward | 5′-CAGAGACTTGGGAGCCACTC-3′ |
| Reverse | 5′-GCTGAAATTATCCACCCTGATT-3′ | |
| IL-10 | Forward | 5′-TGGACAACATACTGCTGACAG-3′ |
| Reverse | 5′-GGTAAAACTTGATCATTTCTGACAAG-3′ | |
| TNF-α | Forward | 5′-GGCCACCACGCTCTTCTGTC-3′ |
| Reverse | 5′-GGGCTACGGGCTTGTCACTC-3′ | |
| CCL2 | Forward | 5′-CATTGTGGCCAAGGAGATCTG-3 |
| Reverse | 5′-CTTCGGAGTTTGGGTTTGCTT-3′ | |
| BDNF | Forward | 5′-TCAGCAGTCAAGTGCCTTTGG-3′ |
| Reverse | 5′-CGCCGAACCCTCATAGACATG-3′ | |
| IGF-1 A | Forward | 5′-GTGCTCCGCTGAAGCCTAC-3′ |
| Reverse | 5′-CAAGTGTACTTCCTTCTGAGTCTTGG-3′ | |
| β-actin | Forward | 5′-AGGGAAATCGTGCGTGACAT-3′ |
| Reverse | 5′-GAACCGCTCATTGCCGATAG-3′ |
Western blot
Animals were anesthetized with sodium pentobarbital (50 mg/kg, i.p.) at different time points. The L4/6 DRG was immediately removed and homogenized on ice in 15 mmol/l Tris containing a cocktail of proteinase inhibitors and phosphatase inhibitors. Protein samples were separated by gel electrophoresis (SDS-PAGE) and transferred onto a PVDF membrane. The blots were placed in the blocking buffer for 1 h at room temperature and incubated with primary antibodies against CXCL12 (1:1000; Cell Signaling Technology, USA), phosphorylated STAT3 (1:1000, Abcam, USA), STAT3 (1:1000, Abcam, USA), or β-actin (1:2000, Cell Signaling Technology, USA) overnight at 4℃. The blots were then incubated with horseradish peroxidase-conjugated secondary antibody. ECL (Pierce, USA) was used to detect the immune complex. The band was quantified with a computer-assisted imaging analysis system (NIH ImageJ).
Immunohistochemistry
Immunohistochemistry was performed as previously described.13 Briefly, rats were anesthetized via an intraperitoneal injection of sodium pentobarbital (50 mg/kg) and immediately perfused through the ascending aorta with 4% paraformaldehyde. The L4/6 DRG was removed and postfixed in the same fixative overnight. Cryostat sections (16 µm) were cut and processed for immunohistochemistry with primary antibodies for CXCL12 (1:50; Cell Signaling Technology, USA), phosphorylated STAT3 (Tyr705) (1:1000, Abcam, USA), IB4-conjugated FITC (1:25, Sigma, USA), NF-200 (1:200, Chemicon, USA) and GFAP (1:200, Chemicon, USA). After incubation overnight at 4℃, the sections were incubated with cy3-conjugated and fluorescein isothiocyanate-conjugated secondary antibodies for 1 h at room temperature. The stained sections were then examined with a Leica (Leica, Germany) fluorescence microscope, and images were captured with a Leica DFC350 FX camera.
siRNA preparation and screening
Specific siRNAs were used to knockdown the expression of CXCL12. According to the previous screening test, the siRNA with the nucleotide sequence 5′-GCCGAUUCUUUGAGAGCCAdTdT-3′ (sense) and 3′-dTdT CGGCUAAGAAACUCUCGGU-5′ (antisense) prevented the expression of the CXCL12 subunit in spinal cord tissues in vivo.6 This siRNA targeting rat Rela (CXCL12) gene was designed and synthesized by Ribobio (China) for the subsequent experiments in vivo.
Chromatin immunoprecipitation assays
Chromatin immunoprecipitation (ChIP) assays were performed with the ChIP Assay kit (Thermo, USA). The L4/6 DRG samples were placed in 1% formaldehyde and sonicated on ice for 2 min. After DNA was fragmented and sheared with micrococcal nuclease, aliquots were kept for input DNA as controls or subjected to ChIP with 1 µg of either the goat STAT3 antibody (R&D, USA) or goat control IgG (Cell Signaling Technology, USA). Following incubation with precleared chromatin solution overnight, the complexes of antibody and DNA were captured, washed, and eluted, and then the cross-link was reversed. The DNA purified from the complexes and the input fractions was resuspended in nuclease-free water, and quantitative real-time PCR or semiquantitative PCR was conducted as described in the above methods. The relative ChIP/input ration was calculated. The primers (5′-GAGGTCCTGGCACCAAAGAA-3′ and 5′-GACTACAAATGCTCCCATCCTG-3′) were designed as previously described6 to amplify a −1667/−1685 region that contained a STAT3-binding site and related to the transcription start site of the rat CXCL12 promoter.
Statistical analysis
All data were expressed as the means ± SEM and analyzed with SPSS 13.0 (SPSS, USA). Western blot and qPCR data were analyzed via two-way analysis of variance (ANOVA) followed by a Tukey post-hoc test. For behavioral tests, one-way or two-way ANOVA with repeated-measures followed by a Tukey post-hoc test was carried out. The criterion for statistical significance was P < 0.05. While no power analysis was performed, the sample size was based on previous studies of painful behavior and pertinent molecular studies.
Results
CXCL12 upregulation in the DRG contributes to oxaliplatin-induced chronic pain
In this study, we observed the paw withdrawal threshold in the rats following an intraperitoneal administration of oxaliplatin (4 mg/kg for five consecutive days, i.p.). The results showed that the application of oxaliplatin significantly induced mechanical allodynia and thermal hyperalgesia on day 5, which was maintained until the end of the experiment (day 10) (Figure 1(a) and (b)). Next, we investigated the expression of CXCL12 in the DRG of rats following oxaliplatin administration. The result revealed that oxaliplatin treatment significantly enhanced CXCL12 mRNA and protein expression in the DRG, which occurred on day 5 and was maintained until day 10 (Figure 1(c) and (d)). In addition, immunohistochemistry results showed that CXCL12 expression was exclusively located in IB4- and NF-200-positive cells but not in GFAP-positive cells in DRG on day 10 after oxaliplatin treatment (Figure 1(e)). Moreover, the consecutive intrathecal application of an anti-CXCL12 neutralizing antibody at a dose of 10 µg/10 μl for 10 days significantly attenuated mechanical allodynia and thermal hyperalgesia induced by oxaliplatin treatment (Figure 1(a) and (b)). To further confirm the role of CXCL12 in the DRG in oxaliplatin-induced chronic pain, we intrathecally injected CXCL12 siRNA (1 nmol/10 μl for 10 days), which was validated for the deletion of spinal cord CXCL12.6 The results showed that the intrathecal injection of CXCL12 siRNA for 10 consecutive days reduced CXCL12 mRNA expression in the DRG (Figure 1(h)) and ameliorated the mechanical allodynia and thermal hyperalgesia induced by oxaliplatin treatment (Figure 1(a) and (b)). In addition, as a previous study reported that intrathecal application of CXCL12 at 1 µg produced pain hyperalgesia,6 here we further found that intrathecal administration of CXCL12 at 1 µg/10 μl induced both mechanical allodynia and thermal hyperalgesia (Figure 1(f) and (g)). These findings suggested that the upregulation of CXCL12 in the DRG mediated the chronic pain induced by oxaliplatin treatment. However, the mechanism of CXCL12 upregulation in the DRG mediated by oxaliplatin treatment is still unclear.
Figure 1.
The increase in CXCL12 expression in the DRG contributes to oxaliplatin-induced mechanical allodynia and thermal hyperalgesia. (a, b) Continuous intrathecal delivery of anti-CXCL12 neutralizing antibody (10 µg/10 μl for 10 days) or CXCL12 siRNA (8 µg/10 μl for 10 days) attenuated mechanical allodynia and thermal hyperalgesia induced by oxaliplatin (**P < 0.01 versus the vehicle group; ##P < 0.01 versus the corresponding oxaliplatin group, n = 8 in each group). (c, d) Representative histogram and blots showed the upregulation of CXCL12 mRNA and protein induced by oxaliplatin treatment (**P < 0.01 versus the vehicle group, n = 6 in each group). (e) Double-staining results showed that CXCL12+ (red) cells were colocalized with NF200+ (green) cells and IB4+ (green) cells but not with GFAP+ (green) cells in the oxaliplatin group (n = 6, scale bar, 100 µm). (f, g) Continuous intrathecal administration of CXCL12 (1 µg/10 μl for 10 days) produced significant mechanical allodynia and thermal hyperalgesia (**P < 0.01 versus vehicle group, n = 8 in each group). (h) Continuous intrathecal injection of CXCL12 siRNA significantly reduced the mRNA level of CXCL12 in the DRG (**P < 0.01 versus the scramble group, n = 6 in each group).
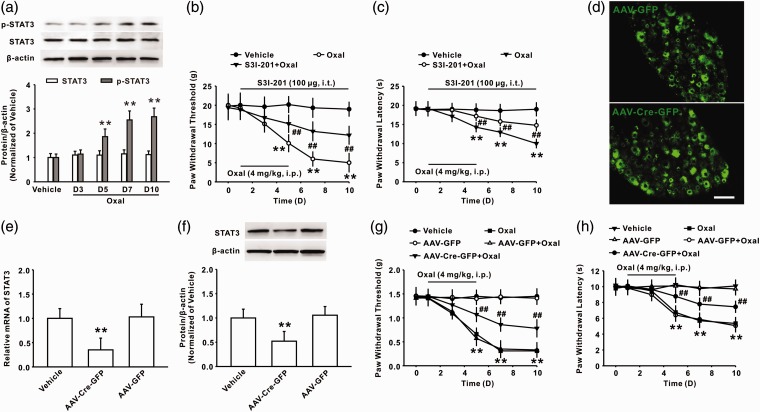
Activation of STAT3 in the DRG mediates mechanical allodynia induced by oxaliplatin
It is known that STAT3, an important transcriptional factor, contributes to chronic pain induced by nerve inflammation via the regulation of the expression of many cytokines and chemokines.12 To determine whether STAT3 signaling in the DRG participated in oxaliplatin-induced chronic pain, we first examined the level of phosphorylated STAT3 (p-STAT3) in the DRG following oxaliplatin treatment. The result showed that the expression of p-STAT3 in the DRG was significantly increased on days 5, 7, and 10 following oxaliplatin treatment (Figure 2(a)). Behavior study results showed that the continuous intrathecal administration of S3I-201 (100 µg/10 µl for 10 days) attenuated mechanical allodynia and thermal hyperalgesia induced by oxaliplatin (Figure 2(b) and (c)). To further confirm the role of p-STAT3 in the DRG in the pathogenesis of oxaliplatin-induced chronic pain, recombinant AAV that encoded Cre and GFP (AAV-Cre-GFP) was intrathecally injected into the subarachnoid space of the L4-L6 spinal cord of STAT3flox/flox mice. Control mice were injected with AAV that encoded GFP (AAV-GFP). Twenty-one days after virus injection, marked green fluorescence in the restricted DRG suggested a high efficiency of transfection (Figure 2(d)). The qPCR and Western blot analysis indicated that the expression of both STAT3 mRNA (Figure 2(e)) and protein (Figure 2(f)) in the DRG were significantly decreased on day 21 after the injection of AAV-Cre-GFP into STAT3flox/flox mice. Importantly, mechanical allodynia and thermal hyperalgesia was significantly ameliorated in AAV-Cre-GFP–injected STAT3flox/flox mice compared with AAV-GFP–injected STAT3flox/flox mice following oxaliplatin treatment (Figure 2(g) and (h)). Taken together, these results suggested that STAT3 activation in the DRG contributes to oxaliplatin-induced chronic pain.
Figure 2.
Activated STAT3 in the DRG-mediated chronic pain induced by oxaliplatin. (a) Representative blots and histogram showed the upregulation of p-STAT3 following oxaliplatin treatment in rats (**P < 0.01 versus vehicle group, n = 6 in each group). (b, c) Intrathecal administration of S3I-201 (100 µg/10 μl for 10 days) attenuated mechanical allodynia and thermal hyperalgesia induced by oxaliplatin (**P < 0.01 versus vehicle group, ##P < 0.01 versus oxaliplatin group, n = 8 in each group). (d) A marked green fluorescence in the restricted DRG of mice was observed on day 21 following AAV-GFP or AAV-Cre-GFP injection. Scale bar, 100 µm. (e) The intrathecal injection of AAV-Cre-GFP significantly reduced the expression of STAT3 mRNA in the DRG of STAT3flox/flox mice (**P < 0.01 versus the corresponding AAV-GFP group, n = 6 in each group). (f) A representative blot and histogram showed that STAT3 expression in the DRG was significantly decreased on day 21 after the injection of AAV-Cre-GFP into STAT3flox/flox mice (**P < 0.01 versus the corresponding AAV-GFP group, n = 6 in each group). (g, h) The mechanical allodynia and thermal hyperalgesia were markedly ameliorated in AAV-Cre-GFP–injected STAT3flox/flox mice compared with that of AAV-GFP–injected STAT3flox/flox mice following oxaliplatin treatment (**P < 0.01 versus the corresponding AAV-GFP group, ##P < 0.01 versus the AAV-GFP + oxaliplatin group, n = 8 in each group).
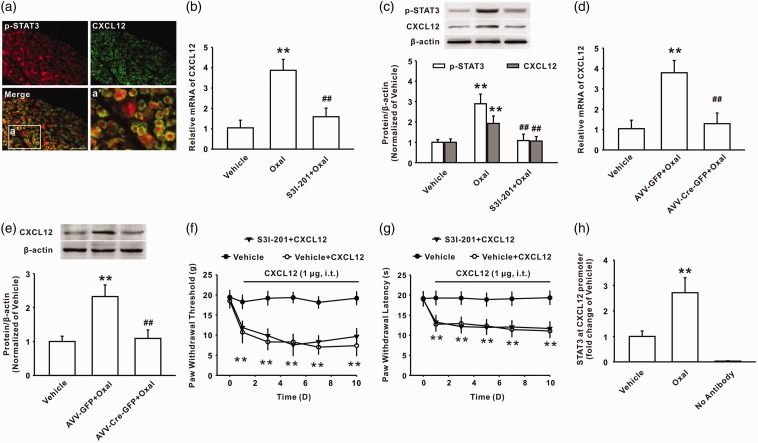
The enhanced recruitment of p-STAT3 to the CXCL12 gene promoter region modulated CXCL12 up-regulation in the DRG
Upon activation of the STAT3 pathway, p-STAT3 can bind to a target gene promoter and facilitate the expression of target genes.10 To determine whether p-STAT3 mediated the expression of CXCL12 in the DRG following oxaliplatin treatment, we first examined the location of p-STAT3 and CXCL12 in DRG cells. Immunohistochemistry results showed that p-STAT3 was localized in CXCL12-positive cells after oxaliplatin treatment (Figure 3(a)). Importantly, consistent with the inhibitory effect on the phosphorylation of STAT3 (Figure 3(c)), the intrathecal injection of S3I-201 reduced the increased expression of CXCL12 mRNA (Figure 3(b)) and protein (Figure 3(c)) in the DRG induced by oxaliplatin treatment. In addition, the local knockout of STAT3 via the intrathecal injection of AAV-Cre-GFP into STAT3flox/flox mice also significantly decreased the upregulation of CXCL12 mRNA and protein expression in the DRG following oxaliplatin treatment (Figure 3(d) and (e)). However, behavioral test showed that preintrathecal administration of S3I-201 had no effect on mechanical allodynia and thermal hyperalgesia induced by consecutive intrathecal injection of CXCL12 (Figure 3(f) and (g)). Next, we investigated whether p-STAT3 could bind to the CXCL12 gene and modulate the expression of the CXCL12 protein. Given the analysis of TFSEARCH and the JASPAR database that the position −1667/−1685 of the CXCL12 gene is a potent-binding site for p-STAT3, a ChIP-PCR assay was used to investigate the interaction of p-STAT3 and the CXCL12 gene. The results revealed that the recruitment of p-STAT3 to the CXCL12 gene promoter (a 163-bp fragment from −1608 to −1770) was noticeably increased following oxaliplatin administration on day 7 (Figure 3(h)). These findings suggested that the upregulation of CXCL12 in the DRG induced by oxaliplatin treatment was dependent on the activation of the STAT3 signaling pathway.
Figure 3.
Oxaliplatin treatment upregulated the expression of CXCL12 by increasing the recruitment of p-STAT3 to the CXCL12 gene promoter region. (a) Double immunostaining showed that p-STAT3 (red) was expressed on CXCL12-positive cells (green) in the oxaliplatin group (n = 6, scale bar, 100 µm). (b, c) The intrathecal injection of S3I-201 (100 µg/10 μl for 10 days) decreased the upregulated CXCL12 mRNA and protein level in the DRGs of rats on day 10 following oxaliplatin treatment (**P < 0.01 versus the vehicle group, ##P < 0.01 versus the corresponding oxaliplatin group, n = 6 in each group). (d, e) Intrathecal injection of AAV-Cre-GFP into STAT3flox/flox mice reduced the increased CXCL12 mRNA and protein level in the DRG induced by oxaliplatin treatment (**P < 0.01 versus vehicle group, ##P < 0.01 versus the corresponding AAV-GFP + oxaliplatin group, n = 6 in each group). (f, g) Preintrathecal application of S3I-201 (100 µg/10 μl for 10 days) did not inhibit mechanical allodynia and thermal hyperalgesia induced by continuous intrathecal administration of CXCL12 (1 µg/10 μl for 10 days) (**P < 0.01 versus vehicle group, n = 8 in each group). (h) ChIP analysis showed the increased recruitment of p-STAT3 to the CXCL12 gene promoter following oxaliplatin administration (**P < 0.01 versus vehicle group, n = 6 in each group).
The enhanced cytokine production of TNF-α and IL-1β mediated the STAT3 activation in DRG following oxaliplatin treatment
To explore the mechanism underlying the STAT3 activation following oxaliplatin treatment, we examined expression of several cytokines by performing quantitative PCR. The results showed that oxaliplatin induced an increase in the RNA of several of the examined cytokines in the DRG at different time points. Among these cytokines, TNF-α and IL-1β underwent marked dynamic changes, the patterns of which were consistent with chronic pain induced by oxaliplatin (Figure 4(a)). To determine whether TNF-α or IL-1β contributed to the STAT3 activation following oxaliplatin, we intrathecally injected neutralizing antibody against TNF-α (10 µg/10 µl) or the interleukin-1 receptor antagonist IL-1ra (50 µg/10 µl) for 10 consecutive days. The Western blot results showed that either intrathecal injection of TNF-α neutralizing antibody (Figure 4(b)) or IL-1ra (Figure 4(c)) significantly prevented the activation of STAT3 induced by the oxaliplatin treatment on day 10. These results suggested that the upregulation of cytokines TNF-α and IL-1β might mediate the STAT3 activation following oxaliplatin treatment.
Figure 4.
Cytokine TNF-α and IL-1β upregulation might contribute to the activation of STAT3 following oxaliplatin treatment. (a) The messenger RNA (mRNA) levels of BDNF, CCL2, interleukin (IL)-10, TNF-α, IGF-1 A, IL-1β, IL-6, and IL-8 were surveyed in DRG of the rats at days 1, 5, and 10 after treatment with oxaliplatin (*P < 0.05, **P < 0.01 versus the vehicle group, n = 6 in each group). (b, c) TNF-α neutralizing antibody (10 µg/10 μl for 10 days) or IL-1 receptor antagonist IL-1ra (50 µg/10 μl for 10 days) prevented activation of STAT3 in the DRG on day 10 following oxaliplatin treatment (**P < 0.01 versus vehicle group, ##P < 0.01 versus corresponding oxaliplatin group, n = 8 in each group).
Discussion
Herein, we demonstrated the critical role of the STAT3/CXCL12 pathway in the DRG in oxaliplatin-induced chronic pain. Our results revealed that the intraperitoneal injection of oxaliplatin at 4 mg/kg for five consecutive days significantly increased CXCL12 protein and mRNA expression in the DRG of rats, and the inhibition of CXCL12 with a neutralizing antibody or siRNA attenuated mechanical allodynia and thermal hyperalgesia following oxaliplatin treatment. Consecutive intrathecal injection of CXCL12 also induced persistent mechanical allodynia and thermal hyperalgesia. In addition, oxaliplatin treatment also increased the phosphorylation of STAT3 in the CXCL12-expressing DRG cells. The inhibition of STAT3 activity or the local knockdown of STAT3 in the DRG significantly reduced the increased CXCL12 expression and alleviated the mechanical allodynia and thermal hyperalgesia induced by oxaliplatin administration. However, intrathecal administration of S3I-201 had no effect on mechanical allodynia and thermal hyperalgesia induced by consecutive intrathecal injection of CXCL12. ChIP assay results further revealed that oxaliplatin treatment enhanced the recruitment of STAT3 to the CXCL12 gene promoter in the DRG. Taken together, the above findings revealed that the activation of STAT3 via the regulation of CXCL12 expression in the DRG contributed to chronic pain following oxaliplatin treatment.
In the nervous system, chemokines function as neuromodulators and regulate neurodevelopment, neuroinflammation, and synaptic transmission. Recently, chemokines were proposed to serve critical roles in chronic pain by mediating the spread of nociceptive signaling from neurons to glial cells.17,18 Our results revealed that the expression of CXCL12 in the DRG increases during the pathogenesis of oxaliplatin-induced chronic pain and the intrathecal administration of anti-CXCL12 antibody or siRNA attenuated the mechanical allodynia and thermal hyperalgesia, which is supported by a previous study that demonstrated that the upregulation of CXCL12 in the DRG was involved in nerve injury–induced neuropathic pain.4 Continuous intrathecal administration of CXCL12 produced persistent mechanical allodynia and thermal hyperalgesia further confirmed CXCL12 was involved in the chronic pain induced by oxaliplatin. Growing evidence indicates that CXCL12 synthesized by DRG neurons regulates the properties and number of ion channels in nociceptive sensory neurons to mediate neuronal sensitization.19,20 For example, increased CXCL12 expression via the regulation of Nav1.8 or Nav1.9 expression excited DRG neurons and contributes to chronic pancreatitis pain.19,21 Therefore, we suspect that the altered expression of CXCL12 may mediate the function of ion channels in DRG cells, thereby contributing to oxaliplatin-induced chronic pain. Studies also showed that CXCL12 expression in the spinal cord is involved in chronic pain. For example, increased CXCL12 expression in the spinal cord, via the sensitization of neurons and the activation of astrocytes and microglia, participates in the maintenance of bone cancer pain.5 Moreover, it is reported that the upregulation of CXCL12 in the spinal cord associated with spinal neuronal sensitization participates in paclitaxel or vincristine-induced neuropathic pain.6 Considering the potential dispersible effect of intrathecal injection of drug on both DRG and spinal cord, the present study cannot completely exclude the effect of intrathecal injection of the reagents on the spinal cord. However, our results suggested that CXCL12 might be an important molecule in chemotherapy-induced chronic pain.
Serving as a key signaling molecule in neurons and glial cells, STAT3 is prone to phosphorylation in nervous system diseases22,23 and plays an active role in the genesis of nerve injury–induced chronic pain through different cellular mechanisms.9,12. Studies also showed that proinflammatory cytokines such as IL-6 play a critical role in STAT3 activation,24 and oxaliplatin treatment significantly increased proinflammatory cytokines expression in the spinal cord.25 In this study, we observed that the expressions of p-STAT3, cytokine TNF-α, and IL-1β were persistently upregulated in the DRG following oxaliplatin treatment. It is well known that IL-6-mediated STAT3 activation contributes to chronic inflammatory diseases, autoimmunity, and cancer.26 In the present study, intrathecal injection of cytokine TNF-α neutralizing antibody or interleukin-1 receptor antagonist IL-1ra prevented the STAT3 activation induced by oxaliplatin. The difference between the present study and other studies may be due to the different model. Furthermore, it is supported by a previous study that IL-1β and TNF-α–initiated IL-6–STAT3 pathway is critical in mediating inflammatory cytokines in inflammatory arthritis.27 Therefore, it is possible that the increased proinflammatory cytokines following oxaliplatin application contributes to STAT3 activation.
Furthermore, we also found that the inhibition of STAT3 activity with S3I-201 or the local deficiency of STAT3 robustly suppressed oxaliplatin-induced mechanical allodynia and thermal hyperalgesia. These findings are supported by a previous report that demonstrated that the blockade of STAT3 activity significantly attenuated LPS induced-mechanical allodynia.12 It is shown that STAT3 activation mediated cytokine or chemokine expression to enhance neuronal excitability, which contributed to chronic pain.6,12 In the present study, we also observed the colocalization of p-STAT3 and CXCL12 in DRG cells. Furthermore, intrathecal injection of S3I-201 via inhibiting STAT3 activation prevented the upregulation of CXCL12 in the DRG following oxaliplatin administration, but had no effect on the increase of CXCL12 induced by consecutive intrathecal injection of exogenous CXCL12. It is possible that exogenous CXCL12 via directly acting on its receptor CXCR4 mediated the chronic pain, which has been reported by other previous studies.6 The difference of the present results further confirmed that the transcription factor STAT3 was an upstream molecular of CXCL12, and CXCL12 played a critical role in chronic pain. In addition, our data unequivocally showed that STAT3 directly bound to the CXCL12 promoter region to upregulate CXCL12 expression in the DRG. In support of our findings, recent studies revealed that CXCL12 expression was upregulated by increased STAT3 binding and H4 acetylation in the CXCL12 promoter region, thus contributing to paclitaxel- or vincristine-induced mechanical allodynia.6 In summary, we provide the first evidence for the participation of STAT3 signaling in oxaliplatin-induced chronic pain through simultaneously increasing CXCL12 expression in the DRG. This study indicates that CXCL12 might be a prognostic biomarker and a promising target for oxaliplatin chemotherapy-induced chronic pain.
Author Contributions
Yong-Yong Li, He Li, and Ze-Long Liu contributed equally to this work. Yong-Yong Li, He Li, Ze-Long Liu, Xiang-Zhong Zhang, and Zhen-Yu Li conceived of the project and designed the experiments. Yong-Yong Li, He Li, Ze-Long Liu, Qiong Li, and Hua-Wen Qiu carried out the experiments. Yong-Yong Li, He Li, Ze-Long Liu, Qiong Li, Hua-Wen Qiu, Li-Jin Zeng, and Wen Yang analyzed the data and prepared the figures. Xiang-Zhong Zhang and Zhen-Yu Li supervised the overall experiment. All authors read and approved the final manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by the National Natural Science Foundation of China (81500948), Natural Science Foundation of Guangdong (2014A030313138), and Public health research project of Futian District in Shenzhen (FTWS2017013).
References
- 1.Mithal DS, Banisadr G, Miller RJ. CXCL12 signaling in the development of the nervous system. J Neuroimmun Pharmacol 2012; 7: 820–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luo X, Wang XM, Xia ZY, et al. CXCL12/CXCR4 axis: an emerging neuromodulator in pathological pain. Rev Neuroscience 2016; 27: 83–92. [DOI] [PubMed] [Google Scholar]
- 3.Li MZ, Ransohoff RA. Multiple roles of chemokine CXCL12 in the central nervous system: a migration from immunology to neurobiology. Prog Neurobiol 2008; 84: 116–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bai L, Wang X, Li Z, et al. Upregulation of chemokine CXCL12 in the dorsal root ganglia and spinal cord contributes to the development and maintenance of neuropathic pain following spared nerve injury in rats. Neurosci Bull 2016; 32: 27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shen W, Hu XM, Liu YN, et al. CXCL12 in astrocytes contributes to bone cancer pain through CXCR4-mediated neuronal sensitization and glial activation in rat spinal cord. J Neuroinflamm 2014; 11: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu T, Zhang XL, Ou-Yang HD, et al. Epigenetic upregulation of CXCL12 expression mediates anti-tubulin chemotherapeutics-induced neuropathic pain. Pain 2017; 158: 637–648. [DOI] [PubMed] [Google Scholar]
- 7.Nicolas CS, Amici M, Bortolotto ZA, et al. The role of JAK-STAT signaling within the CNS. Jak-Stat 2013; 2: e22925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamauchi K, Osuka K, Takayasu M, et al. Activation of JAK/STAT signalling in neurons following spinal cord injury in mice. J Neurochem 2006; 96: 1060–1070. [DOI] [PubMed] [Google Scholar]
- 9.Tsuda M, Kohro Y, Yano T, et al. JAK-STAT3 pathway regulates spinal astrocyte proliferation and neuropathic pain maintenance in rats. Brain 2011; 134: 1127–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Becker S, Groner B, Muller CW. Three-dimensional structure of the Stat3beta homodimer bound to DNA. Nature 1998; 394: 145–151. [DOI] [PubMed] [Google Scholar]
- 11.Hillmer EJ, Zhang H, Li HS, et al. STAT3 signaling in immunity. Cytokine Growth Factor Rev 2016; 31: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu XD, Tian YY, Lu N, et al. Stat3 inhibition attenuates mechanical allodynia through transcriptional regulation of chemokine expression in spinal astrocytes. Plos One 2013; 8: e75804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li ZY, Zhang YP, Zhang J, et al. The possible involvement of JNK activation in the spinal dorsal horn in bortezomib-induced allodynia: the role of TNF-alpha and IL-1beta. J Anest 2016; 30: 55–63. [DOI] [PubMed] [Google Scholar]
- 14.Huang ZZ, Li D, Ou-Yang HD, et al. Cerebrospinal fluid oxaliplatin contributes to the acute pain induced by systemic administration of oxaliplatin. Anesthesiology 2016; 124: 1109–1121. [DOI] [PubMed] [Google Scholar]
- 15.Chaplan SR, Bach FW, Pogrel JW, et al. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Meth 1994; 53: 55–63. [DOI] [PubMed] [Google Scholar]
- 16.Hargreaves K, Dubner R, Brown F, et al. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 1988; 32: 77–88. [DOI] [PubMed] [Google Scholar]
- 17.Gao YJ, Zhang L, Samad OA, et al. JNK-induced MCP-1 production in spinal cord astrocytes contributes to central sensitization and neuropathic pain. J Neurosci 2009; 29: 4096–4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.White FA, Jung H, Miller RJ. Chemokines and the pathophysiology of neuropathic pain. Proc Natl Acad Sci USA 2007; 104: 20151–20158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qiu F, Li Y, Fu Q, et al. Stromal cell-derived factor 1 increases tetrodotoxin-resistant sodium currents Nav1.8 and Nav1.9 in rat dorsal root ganglion neurons via different mechanisms. Neurochem Res 2016; 41: 1587–1603. [DOI] [PubMed] [Google Scholar]
- 20.Yang F, Sun W, Yang Y, et al. SDF1-CXCR4 signaling contributes to persistent pain and hypersensitivity via regulating excitability of primary nociceptive neurons: involvement of ERK-dependent Nav1.8 up-regulation. J Inflamm 2015; 12: 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu HY, Liu X, Miao X, et al. Up-regulation of CXCR4 expression contributes to persistent abdominal pain in rats with chronic pancreatitis. Mol Pain 2017; 13: 1744806917697979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stromberg H, Svensson SP, Hermanson O. Distribution of the transcription factor signal transducer and activator of transcription 3 in the rat central nervous system and dorsal root ganglia. Brain Res 2000; 853: 105–114. [DOI] [PubMed] [Google Scholar]
- 23.Ben Haim L, Ceyzeriat K, Carrillo-de Sauvage MA, et al. The JAK/STAT3 pathway is a common inducer of astrocyte reactivity in Alzheimer’s and Huntington’s diseases. J Neurosci 2015; 35: 2817–2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu X, Adebiyi MG, Luo JL, et al. Sustained elevated adenosine via ADORA2B promotes chronic pain through neuro-immune interaction. Cell Rep 2016; 16: 106–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jung Y, Lee JH, Kim W, et al. Anti-allodynic effect of Buja in a rat model of oxaliplatin-induced peripheral neuropathy via spinal astrocytes and pro-inflammatory cytokines suppression. BMC Complement Altern Med 2017; 17: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neurath MF, Finotto S. IL-6 signaling in autoimmunity, chronic inflammation and inflammation-associated cancer. Cytokine Growth Factor Rev 2011; 22: 83–89. [DOI] [PubMed] [Google Scholar]
- 27.Mori T, Miyamoto T, Yoshida H, et al. IL-1beta and TNFalpha-initiated IL-6-STAT3 pathway is critical in mediating inflammatory cytokines and RANKL expression in inflammatory arthritis. Int Immunol 2011; 23: 701–712. [DOI] [PubMed] [Google Scholar]