Abstract
Introduction
The Pitié Salpêtrière Hospital Hemobiotherapy Department, Paris, France, has been providing extracorporeal photopheresis (ECP) since November 2011, and started using the Therakos® CELLEX® fully integrated system in 2012. This report summarizes our single‐center experience of transitioning from the use of multi‐step ECP procedures to the fully integrated ECP system, considering the capacity and cost implications.
Materials and Methods
The total number of ECP procedures performed 2011–2015 was derived from department records. The time taken to complete a single ECP treatment using a multi‐step technique and the fully integrated system at our department was assessed. Resource costs (2014€) were obtained for materials and calculated for personnel time required. Time‐driven activity‐based costing methods were applied to provide a cost comparison.
Results
The number of ECP treatments per year increased from 225 (2012) to 727 (2015). The single multi‐step procedure took 270 min compared to 120 min for the fully integrated system. The total calculated per‐session cost of performing ECP using the multi‐step procedure was greater than with the CELLEX® system (€1,429.37 and €1,264.70 per treatment, respectively).
Conclusions
For hospitals considering a transition from multi‐step procedures to fully integrated methods for ECP where cost may be a barrier, time‐driven activity‐based costing should be utilized to gain a more comprehensive understanding the full benefit that such a transition offers. The example from our department confirmed that there were not just cost and time savings, but that the time efficiencies gained with CELLEX® allow for more patient treatments per year.
Keywords: cost comparison, photopheresis, time‐driven activity‐based costing
1. INTRODUCTION
Extracorporeal photopheresis (ECP) is a therapeutic procedure recommended for the treatment of patients with conditions associated with cutaneous T‐cell lymphoma (CTCL) as well as acute and chronic graft‐versus‐host disease (GvHD). ECP has also been reported to benefit patients with solid organ transplant rejection and autoimmune diseases such as scleroderma and Crohn's disease.1, 2, 3
ECP combines leukapheresis and photodynamic therapy, and can be performed using a multi‐step procedure or using a fully integrated system.4 The mechanism of photopheresis is not fully understood, however, it is theorized that T‐lymphocyte cells damaged by ultraviolet A (UVA) during the procedure activate the patient's immune system.1, 5
Multi‐step ECP procedures require separate components for leukocyte collection (including cell separation using an instrument such as the COBE® Spectra [Terumo BCT]), addition of the photosensitizing agent (methoxsalen/8‐methoxypsoralen, such as UVADEX® [Therakos®] or 8‐MOP [MacoPharma®] solutions), UVA radiation (performed using a system such as Theraflex [MacoPharma®]), and re‐infusion of treated cells.1, 6 UVADEX® is the only formulation of methoxsalen with widespread regulatory approval for the treatment of CTCL as part of ECP.7 8‐MOP solution, recommended for use with the Theraflex system, is only approved as a related therapeutic product in France.8 The European Guidelines for minimal cell manipulation state that multi‐step procedures should be performed in a class A laminar airflow cabinet located in a class D laboratory.9
Fully integrated ECP systems combine all the required processes, with the patient remaining connected to the system throughout the treatment.1 This ensures sterility, alleviates the need to cross match re‐infused materials, and reduces the risk of improper infusion, contamination, or infection.1 The duration of the ECP procedure with fully integrated systems is shorter than procedures requiring multiple steps.1, 10, 11, 12, 13, 14 In Europe, the only approved fully integrated instruments specifically designed for ECP are the Therakos® UVAR‐XTS and CELLEX® systems,1 which utilize UVADEX® photosensitizing solution.7 Fully integrated systems are associated with higher set up costs compared to the multi‐step procedures, and some reports suggest that the fully integrated systems may be less suitable for patients with restricted venous access.15, 16 Current estimates suggest that around 75% of apheresis departments in France perform ECP using integrated systems.17
Ensuring effective and efficient use of healthcare resources is a system‐wide priority. When seeking to optimize hospital services, particularly where there is an option to incur substantial acquisition costs related to new technologies, time‐driven, activity‐based costing (TDABC) methods can be useful as these go beyond methods which utilize traditional hospital cost accounting systems. TDABC provides a more comprehensive understanding of resource usage and its associated costs, while measuring processes and encouraging quality improvement.18, 19 A number of studies have investigated differences in procedure times and costs associated with the Therakos® CELLEX® fully integrated ECP system and other main alternatives, and have suggested advantages for the integrated system.12, 20, 21
The Hemobiotherapy Department at Pitié Salpêtrière Hospital in Paris, France, has focused on the mobilization and collection of hematopoietic stem cells (HSC) for allogeneic and autologous HSC transplantation (HSCT) since 1989. The department has been performing ECP procedures for patients presenting with GvHD after HSCT and patients with CTCL since November 2011, and for lung transplant patients since June 2013. At the time of the study, the department's resources included three hospital beds, two Spectra Optia® cell separators, two CELLEX® fully integrated ECP systems, two full‐time nurses, and one full‐time physician. Given its size and resource availability, the department sought to assess and address the increasing demand for ECP procedures while also maintaining the same number of necessary healthcare providers within the unit.
The aim of this report is to summarize a single‐center experience of transitioning from the use of multi‐step ECP procedures to a fully integrated ECP system, considering the capacity and cost implications through the use of TDABC.
2. MATERIALS AND METHODS
2.1. Data acquisition
Total ECP procedures performed by the department were derived from a review of the department records from 2011 to 2015. A detailed review of procedures carried out in 2015 was also undertaken to investigate the reliability of the integrated ECP procedures.
To investigate the relative resource requirements of each ECP method, one patient undergoing ECP using the fully integrated system and one patient undergoing ECP using multi‐step procedures at Pitié Salpêtrière Hospital were observed during February 2014. Specifically, the fully integrated Therakos CELLEX® system was used, and the multi‐step procedures included cell separation using Spectra Optia®, and UVA irradiation using MacoPharma® Theraflex MB‐Plasma. Informed, written consent was obtained from both patients prior to the procedures.
Details of each activity's execution were recorded, including time spent per activity performed by hospital personnel. The time for pre‐ and post‐treatment observations (eg, blood pressure), cannulation time, treatment time, addition of methoxsalen, UVA irradiation, biological sampling, and the reinfusion of cells were measured. Total duration of patient retention time using both systems was compared. Results were reviewed by experienced system users to confirm that the observations were typical of each procedure method.
2.2. Time‐driven, activity‐based costing comparisons
TDABC methods were applied to provide a meaningful cost comparison between administration of ECP using multi‐step procedures and fully integrated systems.17, 18
Costs reviewed included unit costs of hardware and calculated costs of personnel based on timeline comparison results. The costs of individual activities used in both techniques were obtained from the purchasing department of the Pitié Salpêtrière Hospital. The costs of every activity and consumable associated with performing ECP using both the multi‐step procedure and fully integrated system were considered. It was assumed that the associated typical costs are independent of patients' disease type and other patient characteristics.
Bed retention times were calculated by taking the daily overhead cost incurred by the hospital while providing a bed. This cost includes all associated overheads but does not include personnel costs. Daily costs were prorated for each system based on the number of hours required to complete one treatment.
Personnel costs were calculated by taking the hourly rate of salary for each involved healthcare provider, plus all associated additional employment costs incurred by the hospital (pension, tax, insurance, etc). The number of hours and minutes that a provider (nurse and/or physician) needed to be present with a patient undergoing treatment was then calculated. The hourly rate was then multiplied by the time that the provider was in attendance with the patient to create an assigned personnel cost. In the case of the multi‐step procedures, the additional costs of porters (for transport of cells) and for laboratory technicians (for the offline treatment of cells) were also included in the calculation. Costs were recorded in 2014 euros, and not adjusted for inflation.
3. RESULTS
Initially, the hospital utilized multi‐step procedures for all ECP treatments. In the first full year of practice (2012), 225 treatments were carried out. A transition from the multi‐step procedures to the Therakos® CELLEX® fully integrated system began at the end of 2012. The following year, 397 ECP procedures (75.3% of all procedures) were carried out using the CELLEX® fully integrated system.
At the start of 2014, the Hemobiotherapy Department was faced with capacity constraints. A complete transition to the fully integrated system was completed in 2014, with a total of 686 ECP procedures performed via the CELLEX® system that year. In order to optimize the delivery of ECP procedures with the new system, a ProcEx Solutions Limited work‐flow assessment was undertaken.15 The aim was to improve ways of managing the increasing demand while maintaining the same numbers of personnel necessary to operate the unit. The combination of improved efficiencies in work‐flow and the CELLEX® fully integrated system enabled a patient capacity increase of 223% from 2012 (225 procedures) to 2015 (727 procedures).
In 2015, all ECP procedures were completed with the CELLEX® system, with approximately 5% experiencing transient problems (resolved through a system stop followed by resuming the procedure) and two cases of failed procedures due to mechanical errors. There were no recorded cases of failed procedures due to patient complications. The CELLEX® systems required an average of three machine stops a year for maintenance.
3.1. Timeline comparison
The patient treated using the multi‐step procedure was retained for more than double the time of the patient treated with the fully integrated system (270 min vs. 120 min, respectively) (Figure 1). Given the assumption that there are only 450 min (7.5 h) in a working day, these findings translate to the ability to treat only one patient per bed per day using multi‐step procedures compared to three patients per bed per day using the fully integrated system.
Figure 1.
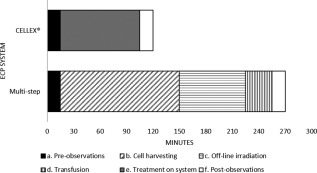
Timeline comparison of the multi‐step procedures and the fully integrated CELLEX® ECP system
3.2. Time‐driven activity‐based costing comparison
Following the timeline analysis and comparison, time‐driven costs were obtained for each of the steps required under both the multi‐step procedure and CELLEX® fully integrated system (Table 1). The cost of performing ECP using the multi‐step procedures was greater than with the CELLEX® system (€1,429.37 and €1,264.70 per treatment, respectively, Table 1). While the CELLEX® system had substantially higher costs up‐front, the additional steps and personnel costs required to perform ECP using multi‐step procedures had a higher total cost.
Table 1.
Time‐driven activity‐based cost comparison of the multi‐step procedures and the fully integrated CELLEX® ECP systema
| Phases in treatment cycle | Multi‐step | CELLEX® |
|---|---|---|
| Collection of leukocyte concentrate (PTC) | €173.75 | €1,009.20 |
| Biological analysis (patient) | €59.00 | €59.00 |
| Biological analysis (cells collection) | €31.05 | €0.00 |
| Transportation of cells to cell treatment area | €7.76 | €0.00 |
| Handling of cell product by cell manipulation facilityb | €616.55 | €0.00 |
| Biological analysis (irradiated cells) | €76.00 | €0.00 |
| Transportation of cells to ward | €7.76 | €0.00 |
| Injection (triple access) | €12.00 | €0.00 |
| Personnel costs | €108.00 | €40.50 |
| Bed retention cost/treatment (per hours used) | €337.50 | €156.00 |
| Total Cost | €1,429.37 | €1,264.70 |
The total cost is per one treatment cycle, values are in 2014 EUR.
Includes labeling, changing bags, sampling, and irradiation of leukocyte concentrate.
A total cost comparison for the number of treatments carried out in 2015 (based on 727 treatments) revealed cost savings of €119,715 associated with performing all ECP treatments on the CELLEX® system.
4. DISCUSSION
Previous studies within our department reported that the use of plerixafor to aid peripheral blood stem cell mobilization in patients undergoing autologous stem cell transplantation reduced the mean number of apheresis time‐slots lost per patient from 1.39 to 0.89 and increased the predictability of available time‐slots.22 This allowed the department to dedicate resources for ECP procedures. However, as the demand for ECP increased, it was necessary to increase the efficiency of ECP administration.
The results of this study reflect the findings of similar studies conducted at different institutions comparing the CELLEX® fully integrated system and multi‐step procedures used for ECP.12, 20, 21 One study reported a reduction in overall procedural times from 221 min to 145 min, while another reported a reduction from 435 min to 125 min.12, 21 The CELLEX® treatment time observed (120 min) was also similar to those recorded by a number of other units across the world (range: 91‐135 min).15
In terms of procedural time, the CELLEX® fully integrated system benefits from a double‐needle mode, providing more rapid treatment times (110 min vs. 135 min using the single‐needle mode).15 However, some patients, particularly those with sclerotic skin and reduced joint movement around the elbows, have been reported to find the double‐needle mode to be uncomfortable and impractical. Some centers consider the single‐needle mode, which is still more rapid than performing ECP using a multi‐step procedure, more suitable for these patients.15 However, our protocol of anxiolytic medication, heating covers, and a local anesthetic applied 15 min prior to the ECP procedure ensures a good fit even in patients with poor venous access, and we have not found the single‐needle mode necessary in practice.
Our study had a number of limitations. Due to pragmatic reasons, the findings are heavily reliant on expert opinion as only one patient per therapy option was observed. However, the authors believe that the experience of the Hemobiotherapy Department at Pitié Salpêtrière Hospital is representative of departments elsewhere, particularly considering similar findings observed in other institutions.12, 21 The analysis demonstrates that the fully integrated ECP system would increase the volume and capacity of procedures completed, however, these efficiencies were only fully realized as a result of administrative structures in place to ensure that freed bed time is used by other patients. As multi‐step procedures are no longer routinely performed in our department, it was also not possible to directly compare the maintenance and failure rates of ECP procedures performed in this way to those of the CELLEX® system. Nevertheless, we found the CELLEX® system sufficiently reliable (2 failures/727 procedures in 2015) to justify a full transition to this system within the department.
5. CONCLUSIONS
For hospitals considering a transition from multi‐step procedures to fully integrated methods for ECP where cost may be a barrier, time‐driven activity‐based costing should be applied to gain a more comprehensive understanding of the full costs of such a transition. The example from Pitié Salpêtrière Hospital confirmed that time efficiencies gained with CELLEX® allow for more patient treatments per year.
ACKNOWLEDGMENTS
The authors acknowledge Costello Medical Consulting, UK, for writing and editorial assistance, which was funded by Mallinckrodt Pharmaceuticals. This study was funded by Mallinckrodt Pharmaceuticals. Publication of this article was not contingent upon approval by Mallinckrodt Pharmaceuticals.
Azar N, Leblond V, Ouzegdouh M, Button P. A transition from using multi‐step procedures to a fully integrated system for performing extracorporeal photopheresis: A comparison of costs and efficiencies. J Clin Apher. 2017;32:474–478. https://doi.org/10.1002/jca.21542
Funding information Mallinckrodt Pharmaceuticals
REFERENCES
- 1. Knobler R, Berlin G, Calzavara‐Pinton P, et al. Guidelines on the use of extracorporeal photopheresis. J Eur Acad Dermatol Venereol. 2014;28 (Suppl 1):1–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Scarisbrick JJ, Taylor P, Holtick U, et al. U.K. consensus statement on the use of extracorporeal photopheresis for treatment of cutaneous T‐cell lymphoma and chronic graft‐versus‐host disease. Br J Dermatol. 2008;158(4):659–678. [DOI] [PubMed] [Google Scholar]
- 3. Willemze R, Hodak E, Zinzani PL, Specht L, Ladetto M. Primary cutaneous lymphomas: ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2013;24(Suppl 6):vi149–vi154. [DOI] [PubMed] [Google Scholar]
- 4. Worel N, Leitner G. Clinical results of extracorporeal photopheresis. Transfus Med Hemother. 2012;39(4):254–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Edelson RL. Mechanistic insights into extracorporeal photochemotherapy: efficient induction of monocyte‐to‐dendritic cell maturation. Transfus Apher Sci. 2014;50(3):322–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hart JW, Shiue LH, Shpall EJ, Alousi AM. Extracorporeal photopheresis in the treatment of graft‐versus‐host disease: evidence and opinion. Ther Adv Hematol. 2013;4(5):320–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Therakos (UK) Limited . UVADEX™ 20 micrograms/ml solution for blood fraction modification—full prescribing information; 2014. http://www.therakos.co.uk/full-prescribing-information. Last accessed: 17 Jan 2017
- 8. ANSM . Liste des PTA autorisés en France dont l'AMM est active; 2014. http://ansm.sante.fr/. http://ansm.sante.fr/Activites/Autorisations-de-Mise-sur-le-Marche-AMM/Produit-therapeutiques-annexe-PTA/(offset)/9. Last accessed: 17 Jan 2017
- 9. European Parliament . European Guidelines for Minimal Cell Manipulation (Directive 2006/86/EC; Regulation N° 1394/2007/EC) Official Journal of the European Union; 2007.
- 10. Pierelli L, Perseghin P, Marchetti M, et al. Extracorporeal photopheresis for the treatment of acute and chronic graft‐versus‐host disease in adults and children: best practice recommendations from an Italian Society of Hemapheresis and Cell Manipulation (SIdEM) and Italian Group for Bone Marrow Transplantation (GITMO) consensus process. Transfusion. 2013;53(10):2340–2352. [DOI] [PubMed] [Google Scholar]
- 11. Klassen J. The role of photopheresis in the treatment of graft‐versus‐host disease. Curr Oncol. 2010;17(2):55–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Adorno GLA, Fiorelli E, et al. An efficiency study comparing an open system and Therakos® Cellex® for extracorporeal photopheresis procedures. In: European Group for Blood and Marrow Transplantation 39th Annual Meeting; 2013; London, UK.
- 13. Martino MCG, Pucci G, Irrera G, et al. Extracorporeal photoimmune therapy: a therapeutic alternative treatment of cutaneous T‐cell lymphoma and immunological diseases. Cancer Therapy. 2004;2:177–186. [Google Scholar]
- 14. Bisaccia E, Vonderheid EC, Geskin L. Safety of a new, single, integrated, closed photopheresis system in patients with cutaneous T‐cell lymphoma. Br J Dermatol. 2009;161(1):167–169. [DOI] [PubMed] [Google Scholar]
- 15. Rushton C, Robertson L, Taylor T, Taylor P, Button P, Alfred A. Improving the service for patients receiving extracorporeal photopheresis using Lean principles. Br J Nurs. 2016;25(16):917–921. [DOI] [PubMed] [Google Scholar]
- 16. Flommersfeld S, Wollmer E, Marschall R, Bein G, Sachs UJ. Single‐needle off‐line extracorporal photopheresis: feasibility and side‐effects. Bone Marrow Transplant. 2016;51:S381 [Google Scholar]
- 17. Therakos (UK) Ltd . Internal Data; 2017. [Google Scholar]
- 18. McLaughlin N, Burke MA, Setlur NP, et al. Time‐driven activity‐based costing: a driver for provider engagement in costing activities and redesign initiatives. Neurosurg Focus. 2014;37(5):E3. [DOI] [PubMed] [Google Scholar]
- 19. Kaplan RS, Witkowski M, Abbott M, et al. Using time‐driven activity‐based costing to identify value improvement opportunities in healthcare. J Healthc Manag. 2014;59(6):399–412. [PubMed] [Google Scholar]
- 20. de Waure C, Capri S, Veneziano MA, et al. Extracorporeal photopheresis for second‐line treatment of chronic graft‐versus‐host diseases: results from a Health Technology Assessment in Italy. Value Health. 2015;18(4):457–466. [DOI] [PubMed] [Google Scholar]
- 21. Bobhat AAN, Adorno G, Fiorelli E, Button PM. A multi‐centre cost comparison of integrated versus “off‐line” systems for performing extracorporeal photopheresis procedures. In: European Hematology Association 20th Annual Congress; June 2015; Vienna, Austria.
- 22. Azar N, Ouzegdouh M, Choquet S, Leblond V. Impact of plerixafor on hospital efficiency: a single center experience. In: European Hematology Association, 21st Congress, 2016; Copenhagen, Denmark: EHA Learning Center, p E1542.


